Abstract
The current COVID-19 epidemic is a sobering reminder that human susceptibility to infectious diseases remains even in our modern civilization. After all, infectious diseases are still the major reason of death globally. Healthcare authorities have often underestimated and ignored the threat posed by “microbial dangers,” although they put millions of lives at risk every year. Overlooked developing diseases including fungal infections (FIs) contribute to roughly 1.7 million fatalities per year. As many as 150 million cases of severe and potentially life-threatening FIs are reported each year. In the last few years, the number of instances has steadily increased. Most of them are invasive fungal infections that require specialized treatment and hospital care. In recent years herbal antifungal compounds have been explored to acquire effective and safe therapy against fungal infections. However, potential therapeutic effects are hampered by the poor solubility, stability, and bioavailability of these important chemicals as well as the gastric degradation that occurs in the gastrointestinal tract. To get around this issue, researchers have turned to novel drug delivery systems such as nanoemulsions, ethosomes, metallic nanoparticles, liposomes, lipid nanoparticles, transferosomes, etc by improving their limits, nanocarriers can enhance the medicinal effects of herbal oils and extracts. The present review article focuses on the available antifungal agents and their characteristics, mechanism of antifungal drugs resistance, herbal oils and extract as antifungal agents, challenges in the delivery of herbal drugs, and application of nano-drug delivery systems for effective delivery of antifungal herbal compounds.
Keywords: Fungal infection, Antifungal compounds, Herbal extract, Essential oils, And nanocarriers
Graphical abstract
1. Introduction
The existing COVID-19 outbreak serves as a depressing reminder that infectious disease vulnerability exists even in modern civilization [1]. After all, infectious diseases remain the leading cause of death worldwide. Even though “microbial hazards” put millions of lives at risk every year, healthcare authorities have frequently underestimated and neglected the damage they pose [2]. FIs are one of the most commonly overlooked emerging diseases, accounting for nearly 1.7 million deaths each year. Every year, up to 150 million cases of severe and potentially fatal FIs are documented. The number of cases has progressively climbed during the last few years. The majority of them are invasive fungi that necessitate specific treatment and hospitalization [3,4]. FIs can range in severity from superficial (affecting only the skin) to systemic (affecting multiple organs).
The high mortality and morbidity rates associated with invasive fungal infections place a major strain on healthcare systems [5]. Rates of opportunistic infections in immunocompromised patients, such as AIDS, those whose immune systems have been suppressed to avoid organ rejection, and those undergoing immunosuppressive chemotherapy or immunosuppressive therapy for autoimmune conditions, are particularly concerning [6,7]. In terms of mechanism of action, there are four broad types of currently available drugs for treating invasive fungal infections: echinocandins, polyenes, azoles, allylamines, and allylamines [8,9]. Azoles prevent the fungal cell wall to form sterol (ergosterol) by obstructing the oxidative enzymes of the fungal cell membrane. This leads to incomplete synthesis and enhanced permeability of the fungal cell wall. While, echinocandins prevent the formation of key polysaccharides (1,3-β-glucan) of the fungal cell wall, and polyenes directly interact with the ergosterol and pass inside the fungal cell by creating pores, leading to leakage of cellular organelles from the cell causing cell death [10]. However, there are a variety of side effects associated with the use of topical antifungal medications for the treatment of fungal infections, such as burning and redness at the site of application [11]. In some cases, treatment may need to be continued due to the rapid drug release causing limited drug penetration. Likely, these drugs won't go to where they're supposed to, leaving the illness unattended. Additionally, antifungal resistance is again the cornerstone of new therapeutic methods for antifungal infections because it affects all of the currently available antifungal treatments [12].
Antifungal medicines derived from natural plant extracts and oils may be a viable solution to this problem. Antifungal properties of many plants, including Vangueria infausta, Bucida buceras, Olinia ventosa, Breonadia salicina, Harpephyllum caffrum, and Xylotheca kraussiana, have been studied [13]. Cinnamon, peppermint, anise, citronella, pepper, clove, and camphor EOs, on the other hand, have been used in the creation of antimycotic medications because of their potent antifungal properties [14]. However, potential therapeutic effects are hampered by the poor solubility, stability, and bioavailability of these important chemicals as well as the gastric degradation that occurs in the gastrointestinal tract. To get over herbal extracts' drawbacks, new drug delivery systems have been developed as adaptable assemblies. Additionally, the resistance mechanisms could be avoided by encapsulating antimicrobial bioactive inside nanoparticles [15].
To get around this issue, researchers have turned to novel drug delivery systems such as nanoemulsions, ethosomes, metallic nanoparticles, liposomes, lipid nanoparticles, transfersomes, etc by improving their limits, nanocarriers can enhance the medicinal effects of herbal oils and extracts. The present review article focuses on the available antifungal agents and their characteristics, mechanism of antifungal drugs resistance, herbal oils and extract as antifungal agents, challenges in the delivery of herbal drugs, and application of nano-drug delivery systems for effective delivery of antifungal herbal compounds.
2. Available antifungals agents and their characteristics
Antifungal agents comprise azoles and polyenes, as well as benzylamines and methylamines. To treat fungal infections other than trioconazole, ketoconazole, econazole, miconazole, and terbinafine, clotrimazole, and amorolfine are also commonly prescribed. Shampoo containing ketoconazole is used for fungal infections of the scalp. Depending on the kind of fungal infection, amphotericin, micafungin itraconazole, flucytosine, anidulationfungin, voriconazole, caspofungin, and injections are also available [16].
Both imidazoles and triazoles are antifungal azoles. Both function by blocking the enzyme lanosterol 14-alpha-demethylase, which stops the translation of lanosterol into ergosterol, causing the fungal cell wall to become porous. Both groups reveal their activity at distinct locations in the spectrum and have structural differences: imidazole is made up of two nitrogen atoms, whereas triazole has three [10]. Aspergillosis and mucormycosis are among the most prevalent fungal infections that can be treated with polyene antifungals such as nystatin and amphotericin B. Because ergosterol is the primary membrane sterol, polyene antifungals generate a polyene–ergosterol complex, which opens up pores and increases cell permeability. Despite this, amphotericin B has fungicidal efficacy against Histoplasma capsulatum, Candida species, Blastomyces, Coccidioides immitis, and Cryptococcus neoformans, however active therapy depends on criteria such as the medication dosage and pH. (6.0–7.5). It falls under the polyene class and is efficient against candida infections on the mucous membrane, but less effective against dermatophytes on the skin [8,17].
Dermatophytosis is treated with butenafine and allylamines, two forms of benzylamine medicines. Ergosterol production is disrupted by the inhibition of the production of the squalene epoxidase enzyme, which inhibits ergosterol production. Although allylamines are considered to be less effective antifungal drugs, they have a distinct benefit in the treatment of tineapedis. As a second-line therapy option, oral antifungal medicines such as fluconazole, griseofulvin, and terbinafine are commonly utilized, with allitraconazole being found to be particularly beneficial [18,19].
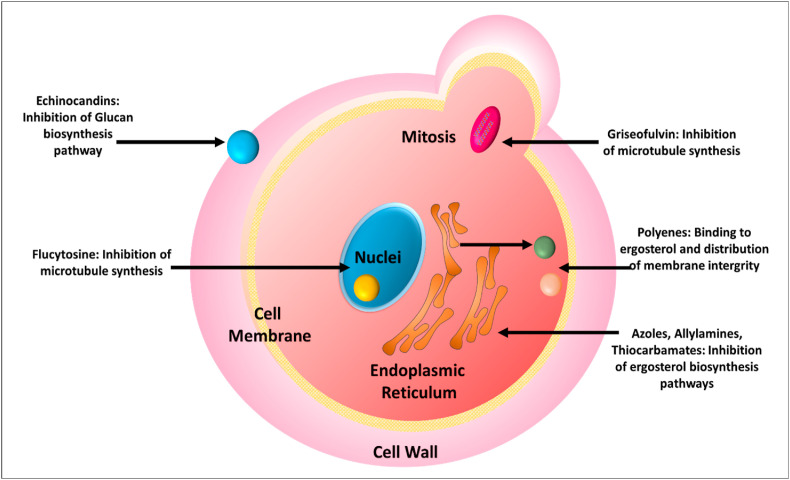
Various antifungal drugs and their mode of action have been depicted in Fig. 1 .
Fig. 1.
Mechanism of action of various antifungal drugs.
3. Mechanism of antifungal drugs resistance
Clinical and microbiologic resistance, or any combination of the two, are two ways to characterize antifungal resistance. Infectious organisms and pathogens are stated to be resistant to antimicrobial agents if their growth is hindered by concentrations higher than those seen in wild-type strains. When an antimicrobial agent is inhibiting the infecting organism at a concentration that is linked with a high risk of treatment failure, clinical resistance is said to exist [19,20]. Antibiotic resistance can be defined as the ability of an organism to evade typical concentrations of an antibiotic and/or to demonstrate a minimum inhibitory concentration (MIC) that falls within the range where particular mechanisms of resistance are likely and clinical benefit has not been reliably demonstrated in treatment studies [21,22]. Fungi have adopted a variety of ways for drug resistance and the main mechanisms against azoles, echinocandins and polyenes have been discussed below in detail.
3.1. Mechanisms of resistance to azoles
Azoles interfere with the cellular synthesis of ergosterol, a key element of the fungal cell membrane. Azole drugs prevent lanosterol 14-α-sterol-demethylase and stop the translation of lanosterol into ergosterol. As a result, the level of ergosterol in the cell membrane drops, and ultimately cellular structure and function are altered, which inhibits fungal growth [22].
Candida species are resistant to azole antibiotics in a variety of ways. To begin with, the stimulation of efflux pumps within the fungal cell reduces drug concentration at the enzyme target, decreasing Candida's susceptibility to azole antifungals or its ability to overcome its resistance to them [23]. Azole resistance in Candida albicans and Candida dubliniensis has been linked to increased expression of efflux pumps expressed by the MDR or CDR genes. Mutations in the ERG11 encoding gene are another characteristic pathway of resistance in Candida species, resulting in a changed target with reduced affinity for or incapability to bind azoles. Candida azole resistance could also be caused by an increase in the expression or upregulation of the changed target enzyme. In some cases, this might lead to azole medicines binding poorly to certain targets [24]. The establishment of bypass routes, which negate the membrane-disrupting effects of azole medications and are associated with decreased fungal growth, is the final possible mechanism of azole resistance in Candida species. In some resistant Candida strains, the ERG3 gene has been mutated, which may explain this [25].
3.2. Mechanisms of resistance to echinocandin
Echinocandins block the manufacture of 1,3-D-glucan, a critical component of the fungal cell wall, by interfering with the function of 1,3-D-glucan synthase. As a result, yeasts develop a faulty cell wall, which can lead to cell instability and lysis, while molds develop abnormal hyphal development. Candida resistance to echinocandins has been linked to mutations in a gene that encodes components of the 1,3-D-glucan synthase complex. Point mutations in the gene encoding the main and presumed catalytic component of 1,3-D-glucan synthase have been associated with lower susceptibility or resistance of Candida to echinocandins. C krusei, C tropicalis, C glabrata, and C dubliniensis have shown this resistance mechanism [22,26]. Echinocandin resistance in C glabrata has been linked to FKS2 gene mutations. Glucophage synthase enzyme activity is altered by FKS mutation, which results in a higher 50% inhibitory concentration (IC50) [10,27].
3.3. Polyene resistance
Polyenes, which include amphotericin B and nystatin, are the earliest antifungal drugs. When polyene drugs interact with fungal-specific ergosterol in the plasma membrane of fungi, they produce concentration-dependent channels that kill cells and allow ions to escape, resulting in cell death. Cells in extramembranous aggregates may be killed through the extraction of ergosterol from the lipid bilayers by amphotericin B [22,28].
Resistance to Amphotericin B is frequently selected during therapy for species that are essentially less vulnerable. In most circumstances, Amphotericin B is fungicide. Fusarium spp, Trichosporon spp, A. nidulans, Sporothrix schenckii, Scedosporium spp, A terreus, A calidoustus, A flavus, and A lentulus are all amphotericin B-resistant species [29]. Resistance to polyenes is mediated by a decrease in cellular ergosterol concentration. Polyene resistance can be conferred by treatment with an azole antifungal that reduces cellular sterol concentrations [30,31].
4. Herbal oils and extract as antifungal agents
4.1. Herbal extracts
Plant extract is a general term used for a product of natural origin whose chemical constituents have not been expounded. A variety of extracts have exhibited explicit antifungal activity including Curcuma zedoaria [32], Plectranthus barbatus, Hydrocotyle bonariensis, Lippia alba, Aristolochia cymbifera, Hydrocotyle bonariensis, Plectranthus amboinicus, Herreria salsaparilha [33,34], Mentha X piperita, Justicia pectoralis, Calamintha adscendens, Eleutherine bulbosa, Albizia inundata, Baccharis trimera, Plectranthus grandis, Cymbopogon citratus, Bauhinia forficate [35,36] and Euphorbia hirta L. [37].
The initial discovery of phytochemicals in Carya illinoensis leaves by Bottari et al. led to the development of plant extracts with antifungal properties against Candida species with MIC values ranging from 25 mg/mL to 6.25 mg/mL. Some of the efficacy against Candida strains was probably a result of the phenolic acids, flavonoid (rutin), and tannin compounds (catechin and epicatechin) [38]. In vitro biofilms developed by Candida tropicalis (fluconazole-resistant) significantly reduced when the berberine was isolated from natural herbs like Coptis chinensis, Phellodendron amurense, Berberis aristata, Berberis vulgaris, Berberis aquifolium, and Tinospora cordifolia's roots, rhizomes and treated with the test fungal strain [39].
Some plants in the Lamiaceae family were studied by Waller and his colleagues for their antifungal properties. Antifungal activity was found in the extracts and EOs of 55 botanical species belonging to 27 genera. Plants belonging to the Lamiaceae family were found to be in vitro susceptible to pathogenic fungus of Candida spp, Aspergillus spp., Malassezia and Cryptococcus spp., Sporothrix spp, Epidermophyton spp., Microsporum spp, Trichophyton Spp [40]. Recently, Barros and their group reported that ethanolic extracts of stems, leaves, and rhizomes of Chamaecostus cuspidatus against Candida and Trichophyton species and reported good antifungal activity [41]. In another study, Terças found antifungal effects in the leaf extract of Terminalia catappa when tested against Candida spp [42].
In vitro studies by Akroum et al. indicated that Vicia faba acetylic extract had antifungal activity against C. albicans (MIC 0.010 mg/mL). Moreover, the extract (20 g/mL) reduced mortality in mice with candidiasis after administration [43]. Todorovic et al. examined polyphenols of powders of Theobroma cacao against C. albicans. They reported good antifungal activity by the extract with a MIC of 5.0 mg/mL [44]. Numerous more plants, in addition to those listed above, are being studied for antifungal properties. Even though screening plant extracts can speed up the discovery process, research has focused on the identification of chemical components. Crude extract research may be the first stage in discovering a new promising drug, which is followed by an identification of the chemical components responsible for the antifungal action.
4.2. Essential oils (EOs)
Essential oils (EOs) are combinations of volatile components that can be found in various plant sections (flowers, bark, leaves, fruits, and rhizomes). Steam distillation is the most common method for extracting these oils. Mono- and sesquiterpenes and phenylpropanoids are the main components of EOs, which are responsible for the plant's olfactory qualities, as well as its ability to fight off microbes [45]. EOs cause yeast cell wall damage by creating a potential gradient across the cell wall and disrupting ATP synthesis [46]. The ability of EOs to infiltrate and tear fungal cell walls and protoplasm membranes facilitates the disintegration of mitochondrial membranes. In the electron transport chain, a modification in the flow of electrons can lead to this. Cells infected with fungi may have their lipids, proteins, and supermolecules damaged as a result of this. The microbe and fungal cell walls as well as the living material membrane are destroyed by the oil elements, resulting in an overly run protoplasm and its actions [47]. The EOs have been employed for a wide variety of plant pathogens. The EOs extracted from several plants such as Allium cepa, Eugenia cariophyllata, Curcuma longa, Ocimum basilicum, Moringa olifera, Cymbopogon, Thymus vulgaris and Salvia rosmarinus have shown significant antifungal activity against a large variety of fungi [48].
Some EOs, such as yarrow oil, pepper oil, cinnamon oil, and carrot oil, are more potent against fungi than bacteria. C. suvabenium essential oil was reported effective against Trichophyton mentagrophytes, Microsporum canis, and T. rubrum, along with C. albicans and C. glabrata with a MIC of 0.47–2.52 g/ml [49]. E. citriodora EOs from Algerian E. citriodora leaves was shown to have a stronger antifungal potential against the investigated microorganisms, with MFC ranging from (0.6–5L/mL and 1.25–5L/mL) respectively [50]. Antifungal properties of Rosmarinus officinalis EOs (REO) against A. flavus have recently been studied by da Silva and his team. MIC and MFC were both reported to be 500 g/mL. At a dosage of 250 g/mL, REO also repressed the growth of A. flavus [51].
Maccioni and the group explored the antifungal activity of essential oil from Teucrium capitatum L. and reported that the sample significantly inhibited C. albicans [52]. Ghasemi and colleagues tested the antifungal potential of Artemisia Siebert's EOs composition against Botrytis cinerea. When tested at concentrations of 1000 and 1500 ll-1, they found that A. Siebert's essential oil considerably slowed the test microorganism's mycelial development. They concluded that the tested essential oil has good antifungal properties [53]. Apart from the above discussed antifungal herbal extracts and EOs other antifungal herbal constituents showing effectiveness against various fungi have been elaborated in Table 1 .
Table 1.
List of herbs and their antifungal potential against various fungi.
| S·N. | Name of the plant | Family | Parts used | Chemical compound | Microorganism tested | References |
|---|---|---|---|---|---|---|
| Eugenia uniflora | Myrtaceae | Leaves | Sesquiterpenes, Monoterpene, hydrocarbons | C. albicans, C. dubliniensis, C. glabrata, C. krusei | [54] | |
| Psidium guajava | Myrtaceae | Leaves | beta-caryophyllene and caryophyllene oxide | Fusarium moniliforme, Rhizoctonia solani and Helminthosporium oryzae | [55] | |
| Curcuma longa | Zingiberaceae | Rhizome | Turmeric oil | Cladosporium cladosporioides, F. graminearum, Alternaria alternate, F. tricinctum, F.chlamydosporum, Botrytis cinerea, Sclerotinia sclerotiorum, F. culmorum, Rhizopus oryzae, | [56] | |
| Cassia occidentalis | Fabaceae | seed | Hydroxy anthraquinone | M. gypseum, Trichophyton mentagrophytes Microsporum nanum, and T. terrestre. | [57] | |
| Asparagus racemosus | Asparagaceae | leaves | Saponin | M. gypseum, M. nanum, T. mentagrophytes and T. terrestre. | [57] | |
| Schinus terebinthifolius | Anacardiaceae | Stem bark | Extract | C. glabrata, C.albicans, C. krusei; and C.tropicalis | [58] | |
| Persea americana | Lauraceae | Leaves | Chromene | Candida spp, Cryptococcus neoformans and Malassezia pachydermatis | [59] | |
| Parapiptadenia rigida | Fabaceae | Stem bark | Pyrrolidine amide | C. albicans | ||
| Ajania fruticulosa | Asteraceae | Leaves | camphene, 1,8-cineole,α- and β-thujone | A. carbonarius and Aspergillus niger | [60] | |
| Mimosa tenuiflora | Mimosaceae | wood | Sesquiterpene lactone | C.albicans and Cryptococcus neoformans | [61] | |
| P. regnellii | Piperaceae | Leaves | Extract | Trichophyton mentagrophyte | ||
| Tithonia diversifolia | Asteraceae | Whole plant | saponins, Polyphenols | Chlorella fusca and M. violaceum, | [62] | |
| Vernonanthura | Asteraceae | Root | Extracts | T. mentagrophytes | [63] | |
| Zingiber officinale | Zingiberaceae | Rhizomes | Steroidal saponin | F.verticillioides | [64] | |
| Datura metel | Solanaceae | Whole plant | Diterpenoid, Alkaloids | M. canis, T. longifusus, C. albicans, A. flavus, and F. solani, | [65] | |
| Cassia tora | Leguminosae | Seeds | Anthraquinone | B. cinerea, Phytophthora infestans, | [66] | |
| Rubia tinctorum | Rubiaceae | Root | Triterpene | P. Alternaria, A. niger, Mucor mucedo | [67] | |
| Chamaecyparis pisifera | Cupressaceae | Leaves and Twigs | Isoflavone | P. oryzae | [68] | |
| Prunus yedoensis | Rosaceae | Leaves | Diterpenes | C. herbarum | [69] | |
| Calea uniflora | Asteraceae | Underground parts | senecioyl | Candida spp. | [70] | |
| Clinacanthus nutans | Acanthaceae | Ariel parts | Megastigmanes 1, 2, 7, and 8 | Candida albicans | [71] | |
| Phytolacca tetramera | Phytolaccaceae | Berry, leaf and root extracts | Phytolaccagenin and phytolaccoside B | Candida albicans and Candida glabrata | [72] | |
| Thevetia peruviana | Apocynaceae | Leaf extract | Alkaloids, steroids, volatile oils, flavonoids, and tannins | Alternaria solani | [73] | |
| Vitis vinifera | Vitaceae | Berry Skins, Seeds, Leaves, and Stems extract | Phenols and Polyphenols | Candida albicans, Candida glabrata, Candida tropicalis, Candida parapsilosis and Candida dubliniens | [74] | |
| Scutellaria baicalensis | Lamiaceae | Root extract | Baicalein | Trichophyton rubrum, Trichophyton mentagrophytes, Aspergillus fumigatus, and Candida albicans | [75] | |
| Primula macrocalyx Bunge | Primulaceae | Candidaalbicans, Aspergillus niger, Saccharomyces kudriavzevii, Penicillium chrysogenum, Candida parapsilosis, Candida rugosa, Candida tropicalis and Rhizopus stolonifer | [76] |
5. Challenges in the delivery of herbal drugs
Because of their delicate structure, most herbal extracts are vulnerable to exposure to moisture, light, air, heat, metal ions, and pH variations. Therefore, they are unable to continue their operations for the duration of their designated shelf life. Because of their sensitivity to light, heat, and oxygen bioactive quickly oxidized and damaged, and hence lose their active properties. The main drawbacks of herbal extracts are related to their ability to penetrate and reach their target cells and organs in the active form [[77], [78], [79], [80]]. To use herbal extracts in a drug delivery system, their low permeability and low solubility must be taken into consideration. Permeability and solubility issues of herbal extracts have been the subject of numerous investigations [80,81]. Many herbal bioactives such as resveratrol, curcumin, naringenin, etc have limited solubility in water and lipophilic solutions, meaning that it has low bioavailability and cannot reach the organ or cell it is intended to benefit. As a solution, new drug delivery carriers have been created to address these issues and constraints, as well as to increase bioavailability and therapeutic efficacy [82].
6. Herbal NDDS against fungal infection
To solve the shortcomings of conventional drug delivery methods, NDDS employs a revolutionary approach to drug delivery. Many unique herbal formulations have been described using proactive and plant choices, including nanocapsules, liposomal delivery systems, microsphere-based delivery systems, and ethosome-based delivery systems. The major advantages of the novel formulation over conventional formulations, include enhanced solubility, enhanced bioavailability, protection from toxicity, improved cellular distribution, constant delivery, shielding from physical and chemical breakdown, enhanced pharmacological activity, and improved stability [83]. Incorporating natural medicines into modern dose forms allows them to be administered more appropriately and effectively. Novel herbal medicine delivery systems employing liposomes, SLNs, polymeric nanoparticles, NLCs, metallic nanoparticles, etc can be developed to achieve this specifically for the treatment of fungal infections (Fig. 2 ) [84]. Various novel drug delivery systems used for encapsulating herbal extracts and EOs for antifungal treatment have been elaborated below.
Fig. 2.
Nanocarriers employed for the delivery of herbal bioactive and essential oils.
6.1. Liposomes
For the preservation of natural antimicrobials, liposomes are among the most widely investigated lipid-based nanostructures. Liposomes are self-assembled closed vesicular structures constituted from one or more lipid bilayers that distinguish them from the adjacent aqueous milieu [[85], [86], [87]]. Liposomes have an amphiphilic character, and as a result, these structures can be utilized to encapsulate chemicals of different polarities [88,89].
In this context, Dave and the team formulated liposomal gel enriched with neem extract and ketoconazole for the effectual treatment of seborrheic dermatitis. They developed liposomes by thin-film hydration method 88.9 ± 0.7% drug entrapment with 141.6 nm particle size of optimized liposomes. The anti-fungal activity of liposomal formulation exhibited good antifungal activity against A. niger and C. tropicalis with an inhibition zone of 8.9 and 10.2 mm, respectively. Overall, they concluded that the developed novel gel could have great antifungal potential and synergetic effect on seborrheic dermatitis [90]. Mittal and group developed PEGylated Curcumin nanoliposomes and reported a 1000-fold enhancement in curcumin hydrophilicity and a tenfold increase in drug stability. Overall findings confirmed enhanced bioactivity of nano curcumin than plain curcumin suggesting curcumin nanoliposomes to be an effective modality to treat fungal and other infectious diseases [91].
6.2. Solid lipid nanoparticles (SLNs)
SLNs are nanoparticle constituted from one or more solid lipids. Their diameter ranges from 50 to 1000 nm, they don't require organic solvents for synthesis, they are inexpensive, and they can be easily scaled up. They are fascinating lipid-based carriers for several reasons [92]. Because of this, SLNs were created to circumvent the drawbacks of previous lipid-based nanocarriers. Substitution of liquid oil was done with solid oil, which had an ordered crystal structure and so allowed the bioactive components to be contained inside the matrix of lipids, thereby making the emulsion more stable [93,94]. In this regard, Lima and the group developed phytol-loaded solid lipid nanoparticles (SLN) and assessed the antifungal efficacy of the formulation against different strains of Candida species. Phytol's MIC against 15 Candida species strains was significantly improved by the encapsulating phytol inside SLN. Finally, they suggested, that the developed SLN could be an efficient cargo for phytol transport in anticandidal therapy [95].
6.3. Nanostructured lipid carriers (NLCs)
NLCs are the carriers made from a mixture of solid lipids and liquid lipids, which boost loading capacity and limit bioactive ingredient ejection [[96], [97], [98]]. Lipids distributed in water provide the basis of these nanostructures, which have a solid to liquid lipid ratio of 70:30. Liquid lipids in NLCs, as opposed to SLNs, prevent the particle from merging with the solid matrix, allowing bioactive substances to be encapsulated and better solubilized. NLCs, on the other hand, is created from a mixture of lipid molecules that are spatially distinct, resulting in a matrix that is more prone to encapsulating bio compounds than SLNs [[99], [100], [101]]. In recent research, Baldim et al., encapsulated Lippia sidoides essential oil (LSEO) in NLCs and examined its antifungal activity against C. auris. Both the LSEO-loaded NLC and plain LSEO showed strong activity against the yeast and did not exhibit any toxicity in the in vivo model [102].
6.4. Ethosomes
It's a non-intrusive vesicular carrier made up of the following components: ethanol; phospholipids; and water. Because of the high quantity of ethanol contained in the carrier, the skin's lipid bilayer is easily disrupted by a soft, flexible carrier. Ethosomes, on the other hand, has been widely used in topical medication delivery for antifungal agents [103]. Shetty et al., developed clove oil-enriched ethosomal gel and assessed its effectiveness for the treatment of cutaneous candidiasis. Compared to pure clove oil, the ethosomal gel has acceptable antifungal efficacy against the pathogen C. albicans. Based on the results, the new formulation for clove oil administration could be a promising one for treating cutaneous candidiasis [104].
6.5. Nanoemulsions
Several active substances may be made more bioavailable through the use of a colloidal dispersion called a nanoemulsion. Nanoemulsions have excellent stability, rapid digestion, protection from degradation, controlled release, and a high ability to enhance the bioavailability of drugs. Because nanoemulsions are highly flexible, they can be used to transport a variety of different drug moieties. To create nanoemulsions with various physicochemical and biological features, lipids and oils such as triglycerides and EOs can be used to construct the oily phase. It is also possible to alter the aqueous portion by introducing various water-soluble components [105]. Das et al., developed chamomile EO (CPe) enriched pickering nanoemulsion and examined the antifungal potential of the antifungal nanoemulsion against the Candida species. CPe showed significantly higher antifungal activities and overall findings suggested that the applicability of CPe nanoemulsion as a potential delivery system to fight against fungal infections [106].
In research, Mahajan et al., obtained essential oil obtained from fresh leaves of Ocimum gratissimum and developed essential oil-based nanoformulations to explore its antifungal potential against P. digitatum. Stable O. gratissimum. With an average droplet diameter of 259.4 nm and sonication period of 10 min, essential oil-enriched nanoemulsions with a 1:1 (v/v) ratio of essential oil and surfactant were produced. P. digitatum spore germination and hyphal extension were suppressed by O. gratissimum essential oil nanoemulsions more effectively than by pure oil, according to the results [107].
6.6. Transfersomes
There has been a significant advance in the development of vesicular drug delivery systems using deformable liposomes (Transfersomes), which are lipid bilayer-enclosed, water-repellent nanoparticles with an edge activator. Recently, Kammoun and their group developed an in-situ gel loaded with voriconazole‒clove oil nano-transfersomes (VRC–CO–NT) and evaluated its antifungal activity against A flavus. After 12 h, 82.5% of VRC was out from the optimized in situ gel, which resulted in a 5.4-fold improvement in drug penetration. An in-situ gel with VRC–CO–Transfersome loaded in it is a fundamental innovation in vesicular drug delivery devices that have one inner aqueous compartment enclosed by an edge activator lipid bilayer [108].
6.7. Metallic nanoparticles
When it comes to biological applications, metallic nanocarriers have been widely tested. Because of their small size, high surface area, ability to be surface modified, and high responsiveness towards living cells, they have achieved an extraordinary position in the field of diagnosis and drug delivery. When it comes to antifungal drug delivery, silver nanoparticles (AgNPs) are a common choice [109,110].
Mohammadi et al., investigated the potential of green synthesized silver AgNPs against Candida albicans. They adsorbed the plant extract on the surface of the pre-prepared AgNPs. To treat superficial fungal infections, the created AgNPs showed greater suppression of the test pathogen than fluconazole (FLZ), showing that the developed formulation is a suitable alternative to FLZ [111]. Nguyen and group developed AgNPs of leaf extracts of Pouzolzia zeylanica, Scoparia dulcis and Phyllanthus urinaria, namely P. zey.AgNPs, S. dul.AgNPs and P. uri.AgNPs, demonstrated antifungal capacity. P. zey.AgNPs, S. dul.AgNPs and P. uri.AgNPs, exhibited a good efficacy against A. niger, Fusarium oxysporum, and A. flavus, demonstrating their potential as antifungals [112].
Paul and their group developed, CUR-AgNPs to examine antifungal activity against fluconazole-resistant Candida spp. Isolated from HIV patients. Compared to silver nitrate and free CUR, CUR-AgNPs had a stronger antifungal activity against C. glabrata; C. albicans, C. tropicalis, C. parapsilosis, C. kefyr; and C. krusei [113]. In another research, Karkhane and team developed Zinc oxide nanoparticles (ZnO-NPs) using aqueous extract of Sargassum vulgare (SVE) as a reducing and capping agent. The particle size of ZnO NPs ranged from 50 to 150 nm (diameter) with spherical configuration. The ZnO-NPs demonstrated wide-spectrum antifungal action against Aspergillus, Candida and saccharomyces cerevisiae (Fig. 3 ). However, the antifungal activity against S. cerevisiae was found to be moderate. The results of the well-diffusion assay established highest efficacy of developed formulation on Candida and Aspergillus species [114].
Fig. 3.
Antifungal activity of ZnO NPs of five different fungal species. The results are presented as mean ± standard deviation of zone of inhibition on agar plates (Adopted from Ref. [114]).
6.8. Polymeric nanoparticle
A polymeric nanoparticle is one in which the medicine has been dissolved, trapped, or somehow attached to the nanoparticle matrix. Nanocapsules (matrix-like structure) and nanospheres (core-shell morphology) can be formed depending on the organic phase's composition and manufacturing procedure [115]. Increasing the safety profile of these nanoparticles is their ability to transport proteins, DNA, and medications such as antifungals to cells and particular target organs in the body. Antifungal herbal ingredients have also been successfully delivered using PNPs.
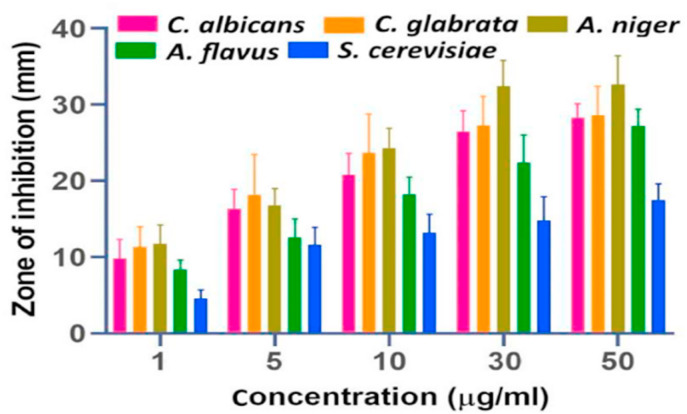
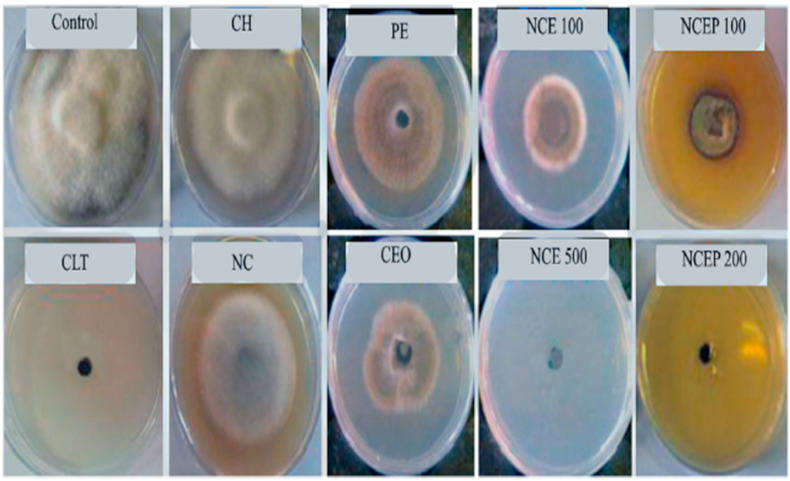
In this context, Izadi and group developed Carum copticum essential oil (CEO) and Peganum harmala extract (PE) loaded chitosan nanoparticles in inprove antifungal activity against A. alternata. The developed nanoparticles were spherical in shape having a mean size of 100 nm. CEO-PE enriched nanoparticles presented inhibitory at a concentration of 200 ppm, while plain essential oils and extracts exhibited inhibitory concentrations at 500 and 750 ppm, respectively. In-vivo tests demonstrated that free chitosan solution, CEO-PE, CEO, PE, also exhibited good antifungal but significantly higher activity was reported for CEO-PE enriched nanoformulation (see Fig. 4 ). These results showed that CEO-PE loaded chitosan nanoparticles may successfully suppress pathogenic fungus in both in vivo and in vitro circumstances [116].
Fig. 4.
Antifungal activity (against A. Alternaria) of control group, chitosan solution (CH), Chitosan nanoparticles, Peganum extract (PE), CEO loaded nanoparticles (NCE), PE-CEO loaded nanoparticles (NCEP), Carum essential oil (CEO), Chlorothalonil (CLT), Negative Control (Adopted from Reference [116]).
Using an olive leaf extract-loaded chitosan nanoparticle, Muzzalupo and coworkers recently tested the antifungal effect of the nanoparticles against the fungus Fusarium proliferatum (in vitro) (AACC0215). The larger concentration of extract-loaded nanoparticles tested against the target species proved to be highly effective, according to their findings. They found that both germination and growth were inhibited by 87.96 and 58.13%, respectively, according to their findings. The final thoughts were that these discoveries would allow for a reduction in the dosage of fungicides that might otherwise be detrimental to humans [117]. AgNPs were synthesized by Suwan and colleagues using an aqueous extract of Psidium guajava (PE). Poloxamer 407 polymeric micelles were used to further cover the AgNPs. They found that AgNPs-loaded polymeric micelles had great stability and good inhibitory efficacy against C. albicans [118].
Apart from the above-discussed nanocarriers, other nanocarriers used for the delivery of antifungal herbal constituents have been presented in Table 2 .
Table 2.
Novel drug delivery carriers employed to encapsulate herbal antifungal extracts and essential oils.
| SN | Nanocarrier | Herbal Constituent/Extract | Test Organism | Comment | References |
|---|---|---|---|---|---|
| Liposomes | Garlic extract |
P. expansum, P. herquei, F. graminearum, A. niger, and A. flavus |
Enhanced antifungal potential of extract loaded liposme than free extract | [119] | |
| Liposomes | α-Bisabolol in combination with Fluconazole | Candida albicans, Candida krusei, Candida tropicalis | Liposomal bisabolol potentiated the antifungal effect of fluconazole against the test organism | [120] | |
| Liposomes | Essential Oil of Eucalyptus camaldulensis Leaf | M. canis, M. gypseum, Trichophyton rubrum and T. verrucosum, | Enhanced antifungal potential of oil loaded liposme than free oil. | [121] | |
| Nanoliposomes | Artemisia annua L. essential oil (AEO) | C. parapsilosis, C. krusei, C. albicans, C. glabrata, C. albicans, and C. dubliniensis | Minimum fungicidal concentration (MFC) of pure AEO was significantly higher than AEO-loaded nanoliposomes. | [122] | |
| SLNs | Z. multiflora essential oil-loaded solid lipid nanoparticles (ZE-SLNs) | A. niger, A. ochraceus, A. flavus, Alternaria solani, Rhizopus stoloniferaand Rhizoctonia solani. | ZE-SLNs exhibited higher antifungal efficacy than ZEO. | [123] | |
| SLNs | Copaiba oil and allantoin | Candida krusei and Candida parapsilosis, Trichophyton rubrum and Microsporum canis | Improved antifungal activity of copaiba oil due to nanoencapsulation. | [124] | |
| NLCs | Palmarosa essential oil (PEO) | Aspergillus nomius. | 100% of inhibition of fungal growth was reported. | [125] | |
| NLCs | Cinnamon Essential Oil | Penicillium Citrinum and Penicillium Expansum | Significant reduction in antifungal activity | [126] | |
| AgNPs | Fruit extract of Prunus cerasifera | X. citri, P. syringae, A. niger, A. flavus, A. fumigatus, A. terreus, P. chrysogenum, F. solani and L. theobromae. | Broad spectrum inhibition by test formulation in comparison to standard antimicrobial drugs against organism | [127] | |
| AgNPs | Rhizophora mucronate leaves extract | C. albicans, A. fumigatus, A. flavus and Cryptococcus neoformans | Enhanced antifungal activity of fluconazole in presence of extract loaded AgNPs | [128] | |
| AgNPs | Lawsonia Inermis extract |
Candida albicans, Microsporum canis, Propioniabacterium acne and Trichophyton mentagrophytes | Phenolic compounds showed strong fungicidal activity. |
[129] | |
| AgNPs | Tropaeolum majus. | Penicilium notatum | Good antifungal activity against Penicilium notatum with MIC value 31.2 μg/ml. | [130] | |
| AgNPs Copper nanoparticles (CuNPs) and Iron nanoparticles (FeNPs) |
Green and black tea leaves extract | Aspergillus flavus and A. parasiticus | Green tea or black tea leaves extracts enriched Ag-NPs showed excellent antifungal property than FeNPs and CuNPs. |
[131] | |
| Polymeric nanoparticle | Curcumin | S. cerevisiae, A. niger, and Penicillium notatum | Broad-spectrum antifungal activity was reported | [132] | |
| Lipid nanoparticle | Lippia sidoides essential oil | C. albicans | Enhanced antifungal activity was reported | [102] |
7. Conclusions and future perspectives
Multidrug-resistant fungal strains are spreading, which necessitates the development of novel natural antifungal classes as well as a new and effective drug delivery technology. Due to the existence of active phytoconstituents, recent research on medicinal plants has revealed their significant pharmacological relevance. Due to high death rates associated with invasive mycoses and the need to create better fungicidal medications and thus reduce treatment lengths and costs, novel drug delivery vehicles encapsulating plant-based antifungals are being designed and developed.
As encapsulation technology and materials science have advanced, antifungal chemicals can be transported and released in a range of lipid, polymer, and metal nano-drug delivery systems. Natural antifungal chemicals can be effectively transported in these nanostructures because they can be manufactured to have different compositions, surface characteristics, and membrane fluidity. Nanostructures can contain hydrophobic, hydrophilic, and amphiphilic chemicals, making them excellent carriers for antifungal extracts and essential oils. Different researchers have been able to successfully encapsulate various antimicrobial peptides, enzymes, EOs, and antimicrobial phytoconstituents within lipid nanostructures. Different polysaccharides and proteins from biopolymers can also be incorporated into nanocarrier formulations, as well as metals such as silver and gold, resulting in increased stability and release properties and compatibility with various delivery systems. The development of nanocarriers that can be used in conjunction with traditional methods and/or that encourage prompted antifungal release is also useful to encourage microbial protection in healthcare and other sectors, even though herbal extracts and oils have been extensively studied using nanoencapsulation.
Funding
None.
Declaration of competing interest
None.
References
- 1.Pradhan M., Shah K., Alexander A., Ajazuddin, Minz S., Singh M.R., Singh D., Yadav K., Chauhan N.S. COVID-19: clinical presentation and detection methods. J. Immunoassay Immunochem. 2022;43 doi: 10.1080/15321819.2021.1951291. [DOI] [PubMed] [Google Scholar]
- 2.Kainz K., Bauer M.A., Madeo F., Carmona-Gutierrez D. Fungal infections in humans: the silent crisis. Microb. Cell (Graz, Austria) 2020;7:143–145. doi: 10.15698/mic2020.06.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Lilienfeld-Toal M., Wagener J., Einsele H., Cornely O.A., Kurzai O. Invasive fungal infection. Dtsch. Arztebl. Int. 2019;116:271–278. doi: 10.3238/arztebl.2019.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bongomin F., Gago S., Oladele R.O., Denning D.W. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi (Basel, Switzerland) 2017;3:57. doi: 10.3390/jof3040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanternier F., Cypowyj S., Picard C., Bustamante J., Lortholary O., Casanova J.-L., Puel A. Primary immunodeficiencies underlying fungal infections. Curr. Opin. Pediatr. 2013;25:736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Enoch D.A., Yang H., Aliyu S.H., Micallef C. The changing epidemiology of invasive fungal infections. Methods Mol. Biol. 2017;1508:17–65. doi: 10.1007/978-1-4939-6515-1_2. [DOI] [PubMed] [Google Scholar]
- 7.Yadav K., Pradhan M., Singh D., Singh M.R. Macrophage Target. Deliv. Syst. Springer; 2022. Macrophage-associated disorders: pathophysiology, treatment challenges, and possible solutions. [Google Scholar]
- 8.Hasim S., Coleman J.J. Targeting the fungal cell wall: current therapies and implications for development of alternative antifungal agents. Future Med. Chem. 2019;11:869–883. doi: 10.4155/fmc-2018-0465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aneshwari R.K., Yadav K., Banjara R.A., Kumar A., Kujur A., Jain V. Standardization and comparative evaluation of phytochemical content and antioxidant activity of Alocasia indica and Tephrosia purpurea. Int. J. Health Sci. 2022:2241–2251. doi: 10.53730/ijhs.v6ns2.5485. [DOI] [Google Scholar]
- 10.Mazu T.K., Bricker B.A., Flores-Rozas H., Ablordeppey S.Y. The mechanistic targets of antifungal agents: an overview. Mini Rev. Med. Chem. 2016;16:555–578. doi: 10.2174/1389557516666160118112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abd Rashed A., Rathi D.-N.G., Ahmad Nasir N.A.H., Abd Rahman A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal infections: conventional and nonconventional approaches. Molecules. 2021;26:1093. doi: 10.3390/molecules26041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fairlamb A.H., Gow N.A.R., Matthews K.R., Waters A.P. Drug resistance in eukaryotic microorganisms. Nat. Microbiol. 2016;1 doi: 10.1038/nmicrobiol.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Powers C.N., Osier J.L., McFeeters R.L., Brazell C.B., Olsen E.L., Moriarity D.M., Satyal P., Setzer W.N. Antifungal and cytotoxic activities of sixty commercially-available essential oils. Molecules. 2018;23:1549. doi: 10.3390/molecules23071549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mutlu-Ingok A., Devecioglu D., Dikmetas D.N., Karbancioglu-Guler F., Capanoglu E. Antibacterial, antifungal, antimycotoxigenic, and antioxidant activities of essential oils: an updated review. Molecules. 2020;25:4711. doi: 10.3390/molecules25204711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laffleur F., Keckeis V. Advances in drug delivery systems: work in progress still needed? Int. J. Pharm. X. 2020;2 doi: 10.1016/j.ijpx.2020.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reginatto P., Bergamo V.Z., Berlitz S.J., Guerreiro I.C.K., de Andrade S.F., Fuentefria A.M. Rational selection of antifungal drugs to propose a new formulation strategy to control Candida biofilm formation on venous catheters. Braz. J. Microbiol. 2020;51:1037–1049. doi: 10.1007/s42770-020-00242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mota Fernandes C., Dasilva D., Haranahalli K., McCarthy J.B., Mallamo J., Ojima I., Del Poeta M. The future of antifungal drug therapy: novel compounds and targets. Antimicrob. Agents Chemother. 2021;65:e01719–e01720. doi: 10.1128/AAC.01719-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abonia R., Garay A., Castillo J.C., Insuasty B., Quiroga J., Nogueras M., Cobo J., Butassi E., Zacchino S. Design of two alternative routes for the synthesis of naftifine and analogues as potential antifungal agents. Molecules. 2018;23:520. doi: 10.3390/molecules23030520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghannoum M.A., Rice L.B. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999;12:501–517. doi: 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berkow E.L., Lockhart S.R., Ostrosky-Zeichner L. Antifungal susceptibility testing: current approaches. Clin. Microbiol. Rev. 2020;33 doi: 10.1128/CMR.00069-19. e00069-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parente-Rocha J.A., Bailão A.M., Amaral A.C., Taborda C.P., Paccez J.D., Borges C.L., Pereira M. Antifungal resistance, metabolic routes as drug targets, and new antifungal agents: an overview about endemic dimorphic fungi. Mediat. Inflamm. 2017 doi: 10.1155/2017/9870679. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaller M.A. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012;125 doi: 10.1016/j.amjmed.2011.11.001. S3-13. [DOI] [PubMed] [Google Scholar]
- 23.Sanguinetti M., Posteraro B., Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58(Suppl 2):2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 24.Berman J., Krysan D.J. Drug resistance and tolerance in fungi. Nat. Rev. Microbiol. 2020;18:319–331. doi: 10.1038/s41579-019-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pristov K.E., Ghannoum M.A. Resistance of Candida to azoles and echinocandins worldwide. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2019;25:792–798. doi: 10.1016/j.cmi.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 26.Shields R.K., Nguyen M.H., Clancy C.J. Clinical perspectives on echinocandin resistance among Candida species. Curr. Opin. Infect. Dis. 2015;28:514–522. doi: 10.1097/QCO.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perlin D.S. Echinocandin resistance, susceptibility testing and prophylaxis: implications for patient management. Drugs. 2014;74:1573–1585. doi: 10.1007/s40265-014-0286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hull C.M., Bader O., Parker J.E., Weig M., Gross U., Warrilow A.G.S., Kelly D.E., Kelly S.L. Two clinical isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B both harbor mutations in ERG2. Antimicrob. Agents Chemother. 2012;56:6417–6421. doi: 10.1128/AAC.01145-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmad S., Joseph L., Parker J.E., Asadzadeh M., Kelly S.L., Meis J.F., Khan Z. ERG6 and ERG2 are major targets conferring reduced susceptibility to amphotericin B in clinical Candida glabrata isolates in Kuwait. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.01900-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carolus H., Pierson S., Lagrou K., Van Dijck P. Amphotericin B and other polyenes-discovery, clinical use, mode of action and drug resistance. J. Fungi (Basel, Switzerland) 2020;6:321. doi: 10.3390/jof6040321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakhani P., Patil A., Majumdar S. Challenges in the polyene- and azole-based pharmacotherapy of ocular fungal infections. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2019;35:6–22. doi: 10.1089/jop.2018.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson B., Abraham G., Manju V.S., Mathew M., Vimala B., Sundaresan S., Nambisan B. Antimicrobial activity of Curcuma zedoaria and Curcuma malabarica tubers. J. Ethnopharmacol. 2005;99:147–151. doi: 10.1016/j.jep.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Khan H., Mubarak M.S., Amin S. Antifungal potential of alkaloids as an emerging therapeutic target. Curr. Drug Targets. 2017;18:1825–1835. doi: 10.2174/1389450117666160719095517. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A., Khan F., Saikia D. Exploration of medicinal plants as sources of novel anticandidal drugs. Curr. Top. Med. Chem. 2019;19:2579–2592. doi: 10.2174/1568026619666191025155856. [DOI] [PubMed] [Google Scholar]
- 35.Arif T., Bhosale J.D., Kumar N., Mandal T.K., Bendre R.S., Lavekar G.S., Dabur R. Natural products--antifungal agents derived from plants. J. Asian Nat. Prod. Res. 2009;11:621–638. doi: 10.1080/10286020902942350. [DOI] [PubMed] [Google Scholar]
- 36.Liu Q., Luyten W., Pellens K., Wang Y., Wang W., Thevissen K., Liang Q., Cammue B.P.A., Schoofs L., Luo G. Antifungal activity in plants from Chinese traditional and folk medicine. J. Ethnopharmacol. 2012;143:772–778. doi: 10.1016/j.jep.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Rajeh M.A.B., Zuraini Z., Sasidharan S., Latha L.Y., Amutha S. Assessment of Euphorbia hirta L. leaf, flower, stem and root extracts for their antibacterial and antifungal activity and brine shrimp lethality. Molecules. 2010;15:6008–6018. doi: 10.3390/molecules15096008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bottari N.B., Lopes L.Q.S., Pizzuti K., Filippi Dos Santos Alves C., Corrêa M.S., Bolzan L.P., Zago A., de Almeida Vaucher R., Boligon A.A., Giongo J.L., Baldissera M.D., Santos R.C.V. Antimicrobial activity and phytochemical characterization of Carya illinoensis. Microb. Pathog. 2017;104:190–195. doi: 10.1016/j.micpath.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 39.de Andrade Monteiro C., dos Santos J.R.A. In: Phytochem. Hum. Heal. Rao V., Mans D., Rao L., editors. IntechOpen; Rijeka: 2020. Phytochemicals and their antifungal potential against pathogenic yeasts. [DOI] [Google Scholar]
- 40.Waller S.B., Cleff M.B., Serra E.F., Silva A.L., Gomes A.D.R., de Mello J.R.B., de Faria R.O., Meireles M.C.A. Plants from Lamiaceae family as source of antifungal molecules in humane and veterinary medicine. Microb. Pathog. 2017;104:232–237. doi: 10.1016/j.micpath.2017.01.050. [DOI] [PubMed] [Google Scholar]
- 41.Barros Cota B., Batista Carneiro de Oliveira D., Carla Borges T., Cristina Catto A., Valverde Serafim C., Rogelis Aquiles Rodrigues A., Kohlhoff M., Leomar Zani C., Assunção Andrade A. Antifungal activity of extracts and purified saponins from the rhizomes of Chamaecostus cuspidatus against Candida and Trichophyton species. J. Appl. Microbiol. 2021;130:61–75. doi: 10.1111/jam.14783. [DOI] [PubMed] [Google Scholar]
- 42.Terças A.G., Monteiro A. de S., Moffa E.B., Dos Santos J.R.A., de Sousa E.M., Pinto A.R.B., Costa P.C. da S., Borges A.C.R., Torres L.M.B., Barros Filho A.K.D., Fernandes E.S., Monteiro C. de A. Phytochemical characterization of Terminalia catappa linn. Extracts and their antifungal activities against Candida spp. Front. Microbiol. 2017;8:595. doi: 10.3389/fmicb.2017.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akroum S. Antifungal activity of acetone extracts from Punica granatum L., Quercus suber L. and Vicia faba L. J. Mycol. Med. 2017;27:83–89. doi: 10.1016/j.mycmed.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 44.Todorovic V., Milenkovic M., Vidovic B., Todorovic Z., Sobajic S. Correlation between antimicrobial, antioxidant activity, and polyphenols of alkalized/nonalkalized cocoa powders. J. Food Sci. 2017;82:1020–1027. doi: 10.1111/1750-3841.13672. [DOI] [PubMed] [Google Scholar]
- 45.Plant R.M., Dinh L., Argo S., Shah M. The essentials of essential oils. Adv. Pediatr. 2019;66:111–122. doi: 10.1016/j.yapd.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Elaissi A., Rouis Z., Ben Salem N.A., Mabrouk S., ben Salem Y., Salah K.B.H., Aouni M., Farhat F., Chemli R., Harzallah-Skhiri F., Khouja M.L. Chemical composition of 8 eucalyptus species' essential oils and the evaluation of their antibacterial, antifungal and antiviral activities. BMC Compl. Alternative Med. 2012;12:81. doi: 10.1186/1472-6882-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tariq S., Wani S., Rasool W., Shafi K., Bhat M.A., Prabhakar A., Shalla A.H., Rather M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019;134 doi: 10.1016/j.micpath.2019.103580. [DOI] [PubMed] [Google Scholar]
- 48.Uma K., Huang X., Kumar B.A. Antifungal effect of plant extract and essential oil. Chin. J. Integr. Med. 2017;23:233–239. doi: 10.1007/s11655-016-2524-z. [DOI] [PubMed] [Google Scholar]
- 49.Kalemba D., Kunicka A. Antibacterial and antifungal properties of essential oils. Curr. Med. Chem. 2003;10:813–829. doi: 10.2174/0929867033457719. [DOI] [PubMed] [Google Scholar]
- 50.Tolba H., Moghrani H., Benelmouffok A., Kellou D., Maachi R. Essential oil of Algerian Eucalyptus citriodora: chemical composition, antifungal activity. J. Mycol. Med. 2015;25:e128–e133. doi: 10.1016/j.mycmed.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 51.da Silva Bomfim N., Kohiyama C.Y., Nakasugi L.P., Nerilo S.B., Mossini S.A.G., Romoli J.C.Z., Graton Mikcha J.M., de Abreu Filho B.A., Machinski M.J. Antifungal and antiaflatoxigenic activity of rosemary essential oil (Rosmarinus officinalis L.) against Aspergillus flavus. Food Addit. Contam. Part A, Chem. Anal. Control. Expo. Risk Assess. 2020;37:153–161. doi: 10.1080/19440049.2019.1678771. [DOI] [PubMed] [Google Scholar]
- 52.Maccioni A., Falconieri D., Porcedda S., Piras A., Gonçalves M.J., Alves-Silva J.M., Salgueiro L., Maxia A. Antifungal activity and chemical composition of the essential oil from the aerial parts of two new Teucrium capitatum L. chemotypes from Sardinia Island. Italy., Nat. Prod. Res. 2021;35:6007–6013. doi: 10.1080/14786419.2020.1813136. [DOI] [PubMed] [Google Scholar]
- 53.Ghasemi G., Alirezalu A., Ishkeh S.R., Ghosta Y. Phytochemical properties of essential oil from Artemisia sieberi Besser (Iranian accession) and its antioxidant and antifungal activities. Nat. Prod. Res. 2021;35:4154–4158. doi: 10.1080/14786419.2020.1741576. [DOI] [PubMed] [Google Scholar]
- 54.Santos K.K.A., Matias E.F.F., Tintino S.R., Souza C.E.S., Braga M.F.B.M., Guedes G.M.M., Costa J.G.M., Menezes I.R.A., Coutinho H.D.M. Enhancement of the antifungal activity of antimicrobial drugs by Eugenia uniflora L. J. Med. Food. 2013;16:669–671. doi: 10.1089/jmf.2012.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jassal K., Kaushal S., Rashmi R. Rani. Antifungal potential of guava (Psidium guajava) leaves essential oil, major compounds: beta-caryophyllene and caryophyllene oxide. Arch. Phytopathol. Plant Protect. 2021;54:2034–2050. doi: 10.1080/03235408.2021.1968287. [DOI] [Google Scholar]
- 56.Chen C., Long L., Zhang F., Chen Q., Chen C., Yu X., Liu Q., Bao J., Long Z. Antifungal activity, main active components and mechanism of Curcuma longa extract against Fusarium graminearum. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194284. –e0194284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savarirajan D., Ramesh V.M., Muthaiyan A. In vitro antidermatophytic activity of bioactive compounds from selected medicinal plants. J. Anal. Sci. Technol. 2021;12:53. doi: 10.1186/s40543-021-00304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torres K.A. de M., Lima S.M.R.R., Ueda S.M.Y. Activity of the aqueous extract of Schinus terebinthifolius Raddi on strains of the Candida genus. Rev. Bras. Ginecol. e Obstet. Rev. Da Fed. Bras. Das Soc. Ginecol. e Obstet. 2016;38:593–599. doi: 10.1055/s-0036-1597694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leite J.J.G., Brito E.H.S., Cordeiro R.A., Brilhante R.S.N., Sidrim J.J.C., Bertini L.M., de Morais S.M., Rocha M.F.G. Chemical composition, toxicity and larvicidal and antifungal activities of Persea americana (avocado) seed extracts. Rev. Soc. Bras. Med. Trop. 2009;42:110–113. doi: 10.1590/s0037-86822009000200003. [DOI] [PubMed] [Google Scholar]
- 60.Sampietro D.A., Gomez A. de L.A., Jimenez C.M., Lizarraga E.F., Ibatayev Z.A., Suleimen Y.M., Catalán C.A. Chemical composition and antifungal activity of essential oils from medicinal plants of Kazakhstan. Nat. Prod. Res. 2017;31:1464–1467. doi: 10.1080/14786419.2016.1258560. [DOI] [PubMed] [Google Scholar]
- 61.de Souza Araújo E., Pimenta A.S., Feijó F.M.C., Castro R.V.O., Fasciotti M., Monteiro T.V.C., de Lima K.M.G. Antibacterial and antifungal activities of pyroligneous acid from wood of Eucalyptus urograndis and Mimosa tenuiflora. J. Appl. Microbiol. 2018;124:85–96. doi: 10.1111/jam.13626. [DOI] [PubMed] [Google Scholar]
- 62.Yemele Bouberte M., Krohn K., Hussain H., Dongo E., Schulz B., Hu Q. Tithoniamarin and tithoniamide: a structurally unique isocoumarin dimer and a new ceramide from Tithonia diversifolia. Nat. Prod. Res. 2006;20:842–849. doi: 10.1080/14786410500462892. [DOI] [PubMed] [Google Scholar]
- 63.Portillo A., Vila R., Freixa B., Ferro E., Parella T., Casanova J., Cañigueral S. Antifungal sesquiterpene from the root of Vernonanthura tweedieana. J. Ethnopharmacol. 2005;97:49–52. doi: 10.1016/j.jep.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 64.Castro J.C., Pante G.C., Centenaro B.M., De Almeida R.T.R., Pilau E.J., Dias Filho B.P., Mossini S.A.G., De Abreu Filho B.A., Matioli G., Machinski Junior M. Antifungal and antimycotoxigenic effects of Zingiber officinale, Cinnamomum zeylanicum and Cymbopogon martinii essential oils against Fusarium verticillioides. Food Addit. Contam. Part A, Chem. Anal. Control. Expo. Risk Assess. 2020;37:1531–1541. doi: 10.1080/19440049.2020.1778183. [DOI] [PubMed] [Google Scholar]
- 65.Bawazeer S., Rauf A. Vitro antibacterial and antifungal potential of amyrin-type triterpenoid isolated from datura metel linnaeus. BioMed Res. Int. 2021 doi: 10.1155/2021/1543574. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim Y.-M., Lee C.-H., Kim H.-G., Lee H.-S. Anthraquinones isolated from Cassia tora (Leguminosae) seed show an antifungal property against phytopathogenic fungi. J. Agric. Food Chem. 2004;52:6096–6100. doi: 10.1021/jf049379p. [DOI] [PubMed] [Google Scholar]
- 67.Manojlovic N.T., Solujic S., Sukdolak S., Milosev M. Antifungal activity of Rubia tinctorum, Rhamnus frangula and Caloplaca cerina. Fitoterapia. 2005;76:244–246. doi: 10.1016/j.fitote.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 68.Kang T.H., Il Hwang E., Yun B.S., Shin C.S., Kim S.U. Chitin synthase 2 inhibitory activity of O-methyl pisiferic acid and 8,20-dihydroxy-9(11),13-abietadien-12-one, isolated from Chamaecyparis pisifera. Biol. Pharm. Bull. 2008;31:755–759. doi: 10.1248/bpb.31.755. [DOI] [PubMed] [Google Scholar]
- 69.Mishra K.K., Kaur C.D., Sahu A.K., Panik R., Kashyap P., Mishra S.P., Dutta S. In: Med. Plants. Hassan B.A.R., editor. IntechOpen; Rijeka: 2020. Medicinal plants having antifungal properties. [DOI] [Google Scholar]
- 70.do Nascimento A.M., Salvador M.J., Candido R.C., de Albuquerque S., de Oliveira D.C.R. Trypanocidal and antifungal activities of p-hydroxyacetophenone derivatives from Calea uniflora (Heliantheae, Asteraceae) J. Pharm. Pharmacol. 2004;56:663–669. doi: 10.1211/0022357023231. [DOI] [PubMed] [Google Scholar]
- 71.Xu W., Li J., Li D., Tan J., Ma H., Mu Y., Wen Y., Gan L., Huang X., Li L. Chemical characterization, antiproliferative and antifungal activities of Clinacanthus nutans. Fitoterapia. 2021;155 doi: 10.1016/j.fitote.2021.105061. [DOI] [PubMed] [Google Scholar]
- 72.Butassi E., Svetaz L.A., Zhou S., Wolfender J.-L., Cortés J.C.G., Ribas J.C., Díaz C., Palacio J.P.-D., Vicente F., Zacchino S.A. The antifungal activity and mechanisms of action of quantified extracts from berries, leaves and roots of Phytolacca tetramera. Phytomedicine. 2019;60 doi: 10.1016/j.phymed.2019.152884. [DOI] [PubMed] [Google Scholar]
- 73.Meena B.R., Meena S., Chittora D., Sharma K. Antifungal efficacy of Thevetia peruviana leaf extract against Alternaria solani and characterization of novel inhibitory compounds by Gas Chromatography-Mass Spectrometry analysis. Biochem. Biophys. Reports. 2021;25 doi: 10.1016/j.bbrep.2021.100914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Simonetti G., Brasili E., Pasqua G. Antifungal activity of phenolic and polyphenolic compounds from different matrices of Vitis vinifera L. Against human pathogens. Molecules. 2020;25 doi: 10.3390/molecules25163748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Da X., Nishiyama Y., Tie D., Hein K.Z., Yamamoto O., Morita E. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci. Rep. 2019;9:1683. doi: 10.1038/s41598-019-38916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li X., Wang X., Li C., Khutsishvili M., Fayvush G., Atha D., Zhang Y., Borris R.P. Unusual flavones from primula macrocalyx as inhibitors of OAT1 and OAT3 and as antifungal agents against Candida rugosa. Sci. Rep. 2019;9:9230. doi: 10.1038/s41598-019-45728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armendáriz-Barragán B., Zafar N., Badri W., Galindo-Rodríguez S.A., Kabbaj D., Fessi H., Elaissari A. Plant extracts: from encapsulation to application. Expet Opin. Drug Deliv. 2016;13:1165–1175. doi: 10.1080/17425247.2016.1182487. [DOI] [PubMed] [Google Scholar]
- 78.Tirkey R., Adeepkujur, Yadav K., Tripathi V., Dewangan D., Saraf S. Potential of neoteric phytoactives and herbs for targeting pathophysiological modules of arthritis. Bull. Environ. Pharmacol. Life Sci. 2021;10:273–281. [Google Scholar]
- 79.Yadav K., Singh M.R., Rai V.K., Srivastava N., Prasad Yadav N. Adv. Ave. Dev. Nov. Carriers Bioact. Biol. Agents. Elsevier; 2020. Commercial aspects and market potential of novel delivery systems for bioactives and biological agents; pp. 595–620. [DOI] [Google Scholar]
- 80.Yadav K., Chauhan N.S., Saraf S., Singh D., Singh M.R. Adv. Ave. Dev. Nov. Carriers Bioact. Biol. Agents. Elsevier; 2020. Challenges and need of delivery carriers for bioactives and biological agents: an introduction; pp. 1–36. [DOI] [Google Scholar]
- 81.Yadav K., Pawar J., Singh D., Singh M.R. Promising phytoactives candidates for efficacious treatment of psoriasis and other skin disorders. J. Ravishankar Univ. 2019;31:10–22. doi: 10.52228/jrub.2018-31-1-2. [DOI] [Google Scholar]
- 82.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahman H.S., Othman H.H., Hammadi N.I., Yeap S.K., Amin K.M., Abdul Samad N., Alitheen N.B. Novel drug delivery systems for loading of natural plant extracts and their biomedical applications. Int. J. Nanomed. 2020;15:2439–2483. doi: 10.2147/IJN.S227805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Devi V.K., Jain N., Valli K.S. Importance of novel drug delivery systems in herbal medicines. Pharmacogn. Rev. 2010;4:27–31. doi: 10.4103/0973-7847.65322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pandey R., Bhairam M., Shukla S.S., Gidwani B. Colloidal and vesicular delivery system for herbal bioactive constituents. Daru. 2021;29:415–438. doi: 10.1007/s40199-021-00403-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yadav K., Singh D., Singh M.R. Nanovesicles delivery approach for targeting steroid mediated mechanism of antipsoriatic therapeutics. J. Drug Deliv. Sci. Technol. 2021;65 doi: 10.1016/j.jddst.2021.102688. [DOI] [Google Scholar]
- 87.Yadav K., Singh D., Singh M.R., Pradhan M. Multifaceted targeting of cationic liposomes via co-delivery of anti-IL-17 siRNA and corticosteroid for topical treatment of psoriasis. Med. Hypotheses. 2020;145 doi: 10.1016/j.mehy.2020.110322. [DOI] [PubMed] [Google Scholar]
- 88.Jahangir M.A., Anand C., Muheem A., Gilani S.J., Taleuzzaman M., Zafar A., Jafar M., Verma S., Barkat M.A. Nano phytomedicine based delivery system for CNS disease. Curr. Drug Metabol. 2020;21:661–673. doi: 10.2174/1389200221666200523161003. [DOI] [PubMed] [Google Scholar]
- 89.Ahmed H.M., Nabavi S., Behzad S. Herbal drugs and natural products in the light of nanotechnology and nanomedicine for developing drug formulations. Mini Rev. Med. Chem. 2021;21:302–313. doi: 10.2174/1389557520666200916143240. [DOI] [PubMed] [Google Scholar]
- 90.Dave V., Sharma S., Yadav R.B., Agarwal U. Herbal liposome for the topical delivery of ketoconazole for the effective treatment of seborrheic dermatitis. Appl. Nanosci. 2017;7:973–987. doi: 10.1007/s13204-017-0634-3. [DOI] [Google Scholar]
- 91.Mittal A., Kumar N., Chauhan N.S. Curcumin encapsulated PEGylated nanoliposomes: a potential anti-infective therapeutic agent. Indian J. Microbiol. 2019;59:336–343. doi: 10.1007/s12088-019-00811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao Y., Chang Y.-X., Hu X., Liu C.-Y., Quan L.-H., Liao Y.-H. Solid lipid nanoparticles for sustained pulmonary delivery of Yuxingcao essential oil: preparation, characterization and in vivo evaluation. Int. J. Pharm. 2017;516:364–371. doi: 10.1016/j.ijpharm.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 93.Feng N., Zhao J.-H., Liu Y., Wang Z., Zhang Y.-T., Feng N.-P. Preparation and characterization of solid lipid nanoparticles loaded with frankincense and myrrh oil. Int. J. Nanomed. 2012;7:2033. doi: 10.2147/IJN.S30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yadav K., Singh D., Singh M.R. Development and characterization of corticosteroid loaded lipid carrier system for psoriasis. Res. J. Pharm. Technol. 2021;14:966–970. doi: 10.5958/0974-360X.2021.00172.4. [DOI] [Google Scholar]
- 95.Lima T.L.C., Souza L.B.F.C., Tavares-Pessoa L.C.S., Dos Santos-Silva A.M., Cavalcante R.S., de Araújo-Júnior R.F., Cornélio A.M., Fernandes-Pedrosa M.F., Chaves G.M., da Silva-Júnior A.A. Phytol-loaded solid lipid nanoparticles as a novel anticandidal nanobiotechnological approach. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12090871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shen C.-Y., Dai L., Shen B.-D., Zhou X., Bai J.-X., Xu H., Lv Q.-Y., Han J., Yuan H.-L. Nanostructured lipid carrier based topical gel of Ganoderma Triterpenoids for frostbite treatment. Chin. J. Nat. Med. 2015;13:454–460. doi: 10.1016/S1875-5364(15)30039-X. [DOI] [PubMed] [Google Scholar]
- 97.Pradhan M., Alexander A., Singh M.R., Singh D., Saraf S., Saraf S., Yadav K. Ajazuddin, Statistically optimized calcipotriol fused nanostructured lipid carriers for effectual topical treatment of psoriasis. J. Drug Deliv. Sci. Technol. 2021;61 doi: 10.1016/j.jddst.2020.102168. [DOI] [Google Scholar]
- 98.Pradhan M., Yadav K., Singh D., Singh M.R. Topical delivery of fluocinolone acetonide integrated NLCs and salicylic acid enriched gel: a potential and synergistic approach in the management of psoriasis. J. Drug Deliv. Sci. Technol. 2021;61 doi: 10.1016/j.jddst.2020.102282. [DOI] [Google Scholar]
- 99.Lacatusu I., Istrati D., Bordei N., Popescu M., Seciu A.M., Panteli L.M., Badea N. Synergism of plant extract and vegetable oils-based lipid nanocarriers: emerging trends in development of advanced cosmetic prototype products. Mater. Sci. Eng. C. Mater. Biol. Appl. 2020;108 doi: 10.1016/j.msec.2019.110412. [DOI] [PubMed] [Google Scholar]
- 100.Yadav K., Singh D., Singh M.R. Novel archetype in psoriasis management bridging molecular dynamics in exploring novel therapies. Eur. J. Pharmacol. 2021;907 doi: 10.1016/j.ejphar.2021.174254. [DOI] [PubMed] [Google Scholar]
- 101.Singh M.R., Singh D., Sahu K.K., Pradhan Krishna M.Y. 2021. A Method of Preparation of Triamcinolone Acetonide Encapsulated Nanostructured Lipid Carriers for Psoriasis Treatment. [Google Scholar]
- 102.Baldim I., Tonani L., von Zeska Kress M.R., Pereira Oliveira W. Lippia sidoides essential oil encapsulated in lipid nanosystem as an anti-Candida agent. Ind. Crop. Prod. 2019;127:73–81. doi: 10.1016/j.indcrop.2018.10.064. [DOI] [Google Scholar]
- 103.Bhalaria M.K., Naik S., Misra A.N. Ethosomes: a novel delivery system for antifungal drugs in the treatment of topical fungal diseases. Indian J. Exp. Biol. 2009;47:368–375. [PubMed] [Google Scholar]
- 104.Shetty S., Jose J., Kumar L., Charyulu R.N. Novel ethosomal gel of clove oil for the treatment of cutaneous candidiasis. J. Cosmet. Dermatol. 2019;18:862–869. doi: 10.1111/jocd.12765. [DOI] [PubMed] [Google Scholar]
- 105.Singh Y., Meher J.G., Raval K., Khan F.A., Chaurasia M., Jain N.K., Chourasia M.K. Nanoemulsion: concepts, development and applications in drug delivery. J. Contr. Release. 2017;252:28–49. doi: 10.1016/j.jconrel.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 106.Das S., Horváth B., Šafranko S., Jokić S., Széchenyi A., Kőszegi T. Antimicrobial activity of chamomile essential oil: effect of different formulations. Molecules. 2019;24 doi: 10.3390/molecules24234321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahajan R., Tandon R., Kalia A., Mahajan B.V.C. Nanoemulsion formulation of Ocimum gratissimum essential oil and its antifungal activity against Penicillium digitatum. J. Nanosci. Nanotechnol. 2021;21:3556–3565. doi: 10.1166/jnn.2021.19008. [DOI] [PubMed] [Google Scholar]
- 108.Kammoun A.K., Khedr A., Hegazy M.A., Almalki A.J., Hosny K.M., Abualsunun W.A., Murshid S.S.A., Bakhaidar R.B. Formulation, optimization, and nephrotoxicity evaluation of an antifungal in situ nasal gel loaded with voriconazole‒clove oil transferosomal nanoparticles. Drug Deliv. 2021;28:2229–2240. doi: 10.1080/10717544.2021.1992040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vangijzegem T., Stanicki D., Laurent S. Magnetic iron oxide nanoparticles for drug delivery: applications and characteristics. Expet Opin. Drug Deliv. 2019;16:69–78. doi: 10.1080/17425247.2019.1554647. [DOI] [PubMed] [Google Scholar]
- 110.Yadav K., Pradhan M., Singh D., Singh M.R. In: Targeting Autoimmune Disorders through Metal Nanoformulation in Overcoming the Fences of Conventional Treatment Approaches. Rezaei N., editor. Academic Press; 2022. pp. 361–393. (Transl. Autoimmun.). [DOI] [Google Scholar]
- 111.Mohammadi M., Shahisaraee S.A., Tavajjohi A., Pournoori N., Muhammadnejad S., Mohammadi S.R., Poursalehi R., Delavari H H. Green synthesis of silver nanoparticles using Zingiber officinale and Thymus vulgaris extracts: characterisation, cell cytotoxicity, and its antifungal activity against Candida albicans in comparison to fluconazole. IET Nanobiotechnol. 2019;13:114–119. doi: 10.1049/iet-nbt.2018.5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nguyen D.H., Lee J.S., Park K.D., Ching Y.C., Nguyen X.T., Phan V.H.G., Hoang Thi T.T. Green silver nanoparticles formed by Phyllanthus urinaria, Pouzolzia zeylanica, and Scoparia dulcis leaf extracts and the antifungal activity. Nanomaterials. 2020:10. doi: 10.3390/nano10030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paul S., Mohanram K., Kannan I. Antifungal activity of curcumin-silver nanoparticles against fluconazole-resistant clinical isolates of Candida species. Ayu. 2018;39:182–186. doi: 10.4103/ayu.AYU_24_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Karkhane M., Lashgarian H.E., Mirzaei S.Z., Ghaffarizadeh A., cherghipour K., Sepahvand A., Marzban A. Antifungal, antioxidant and photocatalytic activities of zinc nanoparticles synthesized by Sargassum vulgare extract. Biocatal. Agric. Biotechnol. 2020;29 doi: 10.1016/j.bcab.2020.101791. [DOI] [Google Scholar]
- 115.Yadav K., Soni A., Singh D., Singh M.R. Polymers in topical delivery of anti-psoriatic medications and other topical agents in overcoming the barriers of conventional treatment strategies. Prog. Biomater. 2021;10:1–17. doi: 10.1007/s40204-021-00154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Izadi M., Moosawi Jorf S.A., Nikkhah M., Moradi S. Antifungal activity of hydrocolloid nano encapsulated Carum copticum essential oil and Peganum harmala extract on the pathogenic fungi Alternaria alternata. Physiol. Mol. Plant Pathol. 2021;116 doi: 10.1016/j.pmpp.2021.101714. [DOI] [Google Scholar]
- 117.Muzzalupo I., Badolati G., Chiappetta A., Picci N., Muzzalupo R. In vitro antifungal activity of olive (olea europaea) leaf extracts loaded in chitosan nanoparticles. Front. Bioeng. Biotechnol. 2020;8:151. doi: 10.3389/fbioe.2020.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suwan T., Khongkhunthian S., Okonogi S. Antifungal activity of polymeric micelles of silver nanoparticles prepared from Psidium guajava aqueous extract. Drug Discov. Ther. 2019;13:62–69. doi: 10.5582/ddt.2019.01024. [DOI] [PubMed] [Google Scholar]
- 119.Pinilla C.M.B., Thys R.C.S., Brandelli A. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int. J. Food Microbiol. 2019;293:72–78. doi: 10.1016/j.ijfoodmicro.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 120.Bezerra C.F., Júnior J.G. de A., Honorato R. de L., dos Santos A.T.L., da Silva J.C.P., da Silva T.G., de Freitas T.S., Vieira T.A.T., Bezerra M.C.F., Lima Sales D., V Rodrigues J.P., Filho J.M.B., Peixoto L.R., Pinheiro A.P., Coutinho H.D.M., Morais-Braga M.F.B., da Silva T.G. Antifungal effect of liposomal α-bisabolol and when associated with fluconazole. Cosmet. 2021;8 doi: 10.3390/cosmetics8020028. [DOI] [Google Scholar]
- 121.Moghimipour E., Aghel N., Mahmoudabadi A.Z., Ramezani Z., Handali S. Preparation and characterization of liposomes containing essential oil of Eucalyptus camaldulensis leaf. Jundishapur J. Nat. Pharm. Prod. 2012;7:117–122. doi: 10.5812/jjnpp.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Risaliti L., Pini G., Ascrizzi R., Donato R., Sacco C., Bergonzi M.C., Salvatici M.C., Bilia A.R. Artemisia annua essential oil extraction, characterization, and incorporation in nanoliposomes, smart drug delivery systems against Candida species. J. Drug Deliv. Sci. Technol. 2020;59 doi: 10.1016/j.jddst.2020.101849. [DOI] [Google Scholar]
- 123.Nasseri M., Golmohammadzadeh S., Arouiee H., Jaafari M.R., Neamati H. Antifungal activity of Zataria multiflora essential oil-loaded solid lipid nanoparticles in-vitro condition, Iran. J. Basic Med. Sci. 2016;19:1231–1237. https://pubmed.ncbi.nlm.nih.gov/27917280 [PMC free article] [PubMed] [Google Scholar]
- 124.Svetlichny G., Külkamp-Guerreiro I.C., Cunha S.L., Silva F.E.K., Bueno K., Pohlmann A.R., Fuentefria A.M., Guterres S.S. Solid lipid nanoparticles containing copaiba oil and allantoin: development and role of nanoencapsulation on the antifungal activity. Pharmazie. 2015;70:155–164. [PubMed] [Google Scholar]
- 125.Essential P., Uchida D.T., Siqueira G.F., Marques E., Hegeto L., Neto A.M., Reis A.V., Bruschi M.L., Nova V., Machinski M. 2021. Design of Nanostructured Lipid Carriers Containing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Radi M., Ahmadi H., Amiri S. Effect of cinnamon essential oil-loaded nanostructured lipid carriers (NLC) against penicillium citrinum and penicillium expansum involved in tangerine decay. Food Bioprocess Technol. 2022;15:306–318. doi: 10.1007/s11947-021-02737-5. [DOI] [Google Scholar]
- 127.Jaffri S.B., Ahmad K.S. Augmented photocatalytic, antibacterial and antifungal activity of prunosynthetic silver nanoparticles. Artif. Cell Nanomed. Biotechnol. 2018;46:127–137. doi: 10.1080/21691401.2017.1414826. [DOI] [PubMed] [Google Scholar]
- 128.Singh M., Kumar M., Kalaivani R., Manikandan S., Kumaraguru A.K. Metallic silver nanoparticle: a therapeutic agent in combination with antifungal drug against human fungal pathogen. Bioproc. Biosyst. Eng. 2013;36:407–415. doi: 10.1007/s00449-012-0797-y. [DOI] [PubMed] [Google Scholar]
- 129.Gupta A., Bonde S.R., Gaikwad S., Ingle A., Gade A.K., Rai M. Lawsonia inermis-mediated synthesis of silver nanoparticles: activity against human pathogenic fungi and bacteria with special reference to formulation of an antimicrobial nanogel. IET Nanobiotechnol. 2014;8:172–178. doi: 10.1049/iet-nbt.2013.0015. [DOI] [PubMed] [Google Scholar]
- 130.Valsalam S., Agastian P., Arasu M.V., Al-Dhabi N.A., Ghilan A.-K.M., Kaviyarasu K., Ravindran B., Chang S.W., Arokiyaraj S. Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65–74. doi: 10.1016/j.jphotobiol.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 131.Asghar M.A., Zahir E., Shahid S.M., Khan M.N., Asghar M.A., Iqbal J., Walker G. Iron, copper and silver nanoparticles: green synthesis using green and black tea leaves extracts and evaluation of antibacterial, antifungal and aflatoxin B1 adsorption activity. LWT. 2018;90:98–107. doi: 10.1016/j.lwt.2017.12.009. [DOI] [Google Scholar]
- 132.Wang W., Zhu R., Xie Q., Li A., Xiao Y., Li K., Liu H., Cui D., Chen Y., Wang S. Enhanced bioavailability and efficiency of curcumin for the treatment of asthma by its formulation in solid lipid nanoparticles. Int. J. Nanomed. 2012;7:3667–3677. doi: 10.2147/IJN.S30428. [DOI] [PMC free article] [PubMed] [Google Scholar]