Abstract
In continuation of research conducted on species of the spontaneous flora of Sicily (Italy) belonging to the Brassicaceae family, Brassica fruticulosa subsp. fruticulosa was selected. It is an edible species utilized in Sicilian traditional medicine. In this study, for the first time, the phenolic and the volatile compounds and the antioxidant properties of the hydroalcoholic extract obtained from the leaves of B. fruticulosa subsp. fruticulosa were characterized. Through HPLC-PDA/ESI-MS analysis, a total of 22 polyphenolic compounds (20 flavonoids and 2 phenolic acids) were identified, with 3-hydroxiferuloylsophoroside-7-O-glucoside (1.30 mg/g ± 0.01) and kaempferol-3-O-feruloylsophoroside-7-O-glucoside (1.28 mg/g ± 0.01) as the most abundant compounds. Through SPME-GC/MS several volatiles belonging to different chemical classes were characterized, with nitriles and aldehydes accounting for more than 54% of the whole volatile fraction. The extract of B. fruticulosa subsp. fruticulosa showed moderate activity in the DPPH assay (IC50 = 1.65 ± 0.08 mg/mL), weak reducing power (17.47 ± 0.65 ASE/mL), and good chelating properties (IC50 = 0.38 ± 0.02 mg/mL), reaching approximately 90% activity at the highest tested concentration. Lastly, the extract was non-toxic against Artemia salina, indicating its potential safety. According to the findings, it can be stated that B. fruticulosa subsp. fruticulosa represents a new valuable source of bioactive compounds.
Keywords: Brassica fruticulosa subsp. fruticulosa, edible plant, phenolic compounds, volatile compounds, antioxidant activity, Artemia salina Leach
1. Introduction
The Brassicaceae family (also called Cruciferae), order Brassicales, consisting of more than 300 genera and about 3500 species, includes a large number of vegetable crops recognized as rich sources of health-promoting phytochemicals [1,2].
Brassica is the economically most important genus within the tribe Brassiceae. Most of the Brassica species are cultivated throughout the world due to their economic, nutritional, medicinal, and pharmaceutical value. Nevertheless, the current increasing demand of medicinal plants for pharmaceuticals, nutraceuticals, cosmetics, and other products, represents an opportunity for the valorization of wild species of Brassica so far little or no investigated.
Some wild Brassica species have been used for centuries as important sources of food as part of the Mediterranean diet, and various studies have documented the nutritional and medicinal properties of the edible wild plants with respect to the cultivated crops [3].
Wild Brassica species have great potential as sources of bioactive compounds; indeed, the adaptation to challenging environmental conditions has led the plants to direct greater resources to the synthesis of specialized secondary metabolites as a chemical defense mechanism [4].
In the last few years, the species belonging to the Brassicaceae family that grow spontaneously in Sicily have been investigated by our research team to unearth new valuable plant sources of bioactive compounds. Recently, our team reported the characterization of the phenolic components, as well as the antioxidant and cytotoxic properties, of the leaf and flowering top extracts of Brassica incana Ten. [5]. In continuation of our studies, we selected Brassica fruticulosa Cyr. subsp fruticulosa, a species not fully studied so far.
Brassica fruticulosa subsp fruticulosa (Mediterranean cabbage) is a species with Mediterranean distribution, but with smaller representation in Europe, Southwest Asia, Central and Southern Africa, and the eastern coast of North America [6,7]. This species is widespread in southern Italy, and it grows in untilled lands, as well as on walls and debris, from 0 to 1200 m above the sea level [8,9].
Brassica fruticulosa Cyr. subsp. fruticulosa, included in the subgen. Brassica, sect. Micropodium DC. [10], is an herbaceous species, usually biennial to perennial, 20–60 cm high; it presents a suffruticose aspect with a woody stem at the base. The basal leaves are long-petiolate, lirate, and arranged to form a rosette; the cauline leaves are smaller, pinnate-lobed to entire. It blooms from January to December, and it has flowers gathered in racemes with violet sepals and yellow petals. The fruit is a siliqua constricted at intervals, stipitate, with a beak of 2–7 mm [11,12].
This species is widely diffused in Sicily (Italy), where its use in traditional medicine is reported; indeed, the leaf decoction of B. fruticulosa subsp. fruticulosa is utilized to raise blood pressure and as an antidiabetic [13,14].
Furthermore, B. fruticulosa subsp. fruticulosa is an edible plant; this species has been eaten since ancient times both raw and cooked. The edible portion is represented by young shoots and leaves which are picked up until they are tender, before flowering, and commercialized in local markets during October–April. In southern Italy, especially in Sicily, cooked leaves and young shoots of B. fruticulosa subsp. fruticulosa are utilized to prepare traditional dishes [8]. Typical dishes include shoots boiled and dressed with olive oil and lemon juice or stir-fried with garlic and chili pepper, as a side dish to pork sausages [15,16]. Its use for the preparation of a typical Sicilian polenta, known as “Frascatula”, together with Brassica incana and other wild herbs, is reported in Sicily [5].
Concerning phytochemical composition, some studies have been carried out on the leaves, roots, and seeds of this species [8,17,18,19,20]. To the best of our knowledge, no investigations about the biological properties of B. fruticulosa subsp. fruticulosa are reported.
The present work was undertaken to characterize the phenolic and volatile constituents, and to investigate the antioxidant properties and potential toxicity of a hydroalcoholic extract obtained from the leaves of B. fruticulosa subsp. fruticulosa grown wild in Sicily (Italy). In particular, the qualitative–quantitative profile of the phenolic and volatile constituents contained in the extract was obtained by HPLC-PDA/ESI-MS and SPME-GC/MS analyses. The antioxidant properties were examined by means of different in vitro systems: DPPH (2,2-diphenyl-1-picrylhydrazyl) scavenging, reducing power, and ferrous ion (Fe2+)-chelating activity. Lastly, the toxicity of the extract was assessed by the brine shrimp (Artemia salina Leach) lethality bioassay.
2. Results and Discussion
2.1. Phytochemical Investigations
2.1.1. Determination of Total Phenolic Content
The Folin–Ciocâlteu assay is a recognized, widely used procedure for quantification of total phenolic compounds in plant extracts. It is a colorimetric method based on electron transfer reactions between the Folin–Ciocâlteu reagent and phenolics, giving rise to the formation of a blue chromophore with the maximum absorption at 765 nm. Generally, gallic acid is used as the reference standard compound, and the results are usually expressed as gallic acid equivalent [21]. In most cases, the antioxidant properties of plant extracts are explained by their total phenolic content with good correlation, confirming the value of this assay. Therefore, the determination of their total amount in the extract used for this study was performed.
The results of the Folin–Ciocâlteu assay showed that the total phenolic content of B. fruticulosa subsp. fruticulosa leaf extract was equal to 32.63 ± 1.11 mg gallic acid equivalent (GAE)/g extract. This content was close to that of the B. incana leaf extract previously investigated (37.20 ± 0.93 mg GAE/g extract) [5].
2.1.2. Identification of Phenolic Compounds by HPLC-PDA/ESI-MS
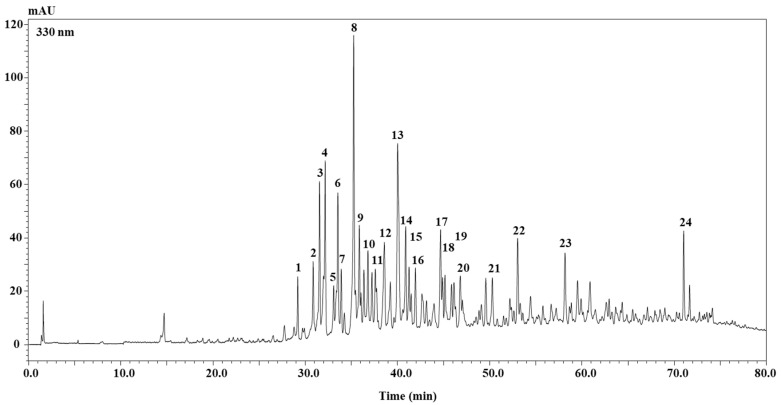
For the first time, the phenolic profile of the hydroalcoholic extract of the leaf of B. fruticulosa subsp. fruticulosa is reported. The HPLC-PDA chromatogram (λ = 330 nm) of the polyphenolic compounds occurring in the extract is shown in Figure 1. A total of 24 compounds were detected and, among them, according to retention times, as well as PDA, MS and MS/MS, and literature data, 22 were tentatively identified (Table 1) [5,22,23,24,25,26,27,28]. Notably, most of them belonged to the flavonoid class, whereas only two were phenolic acids. Among flavonoids, 10 were kaempferol derivates, nine were quercetin derivates, and only two were isorhamnetin derivates. With regard to the two phenolic acids, they were sinapic and ferulic hydroxycinnamic acids in conjugation with a gentiobiose moiety.
Figure 1.
HPLC-PDA chromatograms of the polyphenolic compounds, extracted at 330 nm wavelength, of B. fruticulosa subsp. fruticulosa leaf hydroalcoholic extract. For peak identification, see Table 1.
Table 1.
HPLC-PDA/ESI-MS (negative ionization mode) polyphenolic fingerprint of B. fruticulosa subsp. fruticulosa leaf hydroalcoholic extract. Results are expressed as mg/g extract ± SD (n = 3).
| No. | tR(min) | UV max (nm) |
[M-H]− | Compound | mg/g ± %RSD | Ref. |
|---|---|---|---|---|---|---|
| 1 | 29.19 | 254, 352 | 787, 625 | Quercetin-3-O-sophoroside-7-O-glucoside | 0.36 ± 0.001 | [5,21] |
| 2 | 30.87 | 340 | 979, 625 | Quercetin-3-O- hydroxyferuoyl-sophoroside-7-O-D-glucoside | 0.51 ± 0.002 | [5,21] |
| 3 | 31.57 | 264, 344 | 773, 609 | Kaempferol-3-O-diglucoside-7-O-glucoside | 0.75 ± 0.011 | [5,21] |
| 4 | 32.16 | 338 | 949, 301 | Quercetin-3-caffeoylsophoroside-7-glucoside | 0.86 ± 0.016 | [5] |
| 5 | 33.08 | 330 | 1111, 787 | Quercetin-3-triglucoside-7-diglucoside | 0.31 ± 0.001 | [22] |
| 6 | 33.53 | 328 | 963, 801 | Kaempferol-3-O-hydroxyferuloylsophoroside-7-O-glucoside | 0.53 ± 0.001 | [5,21] |
| 7 | 33.91 | 345 | 1125, 801 | Kaempferol-3-O-hydroxyferuloyl diglucoside-7-O-diglucoside | 0.32 ± 0.002 | [23] |
| 8 | 35.23 | 267, 331 | 933 | Kaempferol-3-hydroxyferuloylsophoroside-7-O-glucoside | 1.30 ± 0.003 | [21] |
| 9 | 35.83 | 334 | 1155, 831 | Quercetin-3-sinapoyltriglucoside-7-glucoside | 0.54 ± 0.012 | [5,21] |
| 10 | 36.76 | 338 | 963, 801 | Quercetin-3-Oferuloyldiglucoside-7-O-glucoside | 0.63 ± 0.006 | [23] |
| 11 | 37.20 | 334 | 963, 801 | Quercetin-3-O-feruloyldiglucoside-7-O-glucoside isomer | 0.45 ± 0.011 | [23] |
| 12 | 38.53 | 268, 331 | 977, 815 | Kaempferol-3-O-sinapoyldiglucoside-7-O-glucoside | 0.65 ± 0.015 | [23] |
| 13 | 39.95 | 268, 331 | 947, 609 | Kaempferol-3-O-feruloylsophoroside-7-O-glucoside | 1.28 ± 0.011 | [21] |
| 14 | 40.82 | 267, 330 | 1019 | Unknown | - | - |
| 15 | 41.18 | 268, 318 | 917 | Kaempferol-3-O-coumaroyl-sophoroside-7-O-d-glucoside | 0.53 ± 0.002 | [24] |
| 16 | 41.88 | 349 | 639, 417, 315 | Isorhamnetin-3-glucoside-7-glucoside | 0.36 ± 0.005 | [5,21] |
| 17 | 44.61 | 326 | 753 | Disinapoylgentiobiose | Nq | [5,21] |
| 18 | 45.07 | 263, 343 | 625, 301 | Quercetin-dihexoside | 0.53 ± 0.021 | [21,25] |
| 19 | 46.06 | 324 | 723, 529 | Sinapoylferuloylgentiobiose | Nq | [21,25] |
| 20 | 46.70 | 335 | 787, 301 | Quercetin-3-caffeoyisophoroside-7-glucoside | 0.50 ± 0.001 | [25] |
| 21 | 50.17 | 264, 343 | 609, 285 | Kaempferol-3-glucoside-7-glucoside | 0.10 ± 0.001 | [25] |
| 22 | 52.91 | 266, 331 | 771, 285 | Kaempferol-3-triglucoside | 0.11 ± 0.001 | [26] |
| 23 | 58.00 | 268, 334 | 785, 285 | Kaempferol-feruloyldihexoside | 0.48 ± 0.004 | [27] |
| 24 | 70.92 | 327 | 1121 | Unknown | - | - |
Nq: Not quantified.
As can be seen from Table 1, among the phenolic compounds identified, flavonols represented the most abundant constituents (11.1 mg/g extract), while phenolic acids were not quantified. Many of the compounds identified were previously reported to be constituents of Brassica juncea L. or B. incana [5,22].
Regarding quantification, since none of the compounds identified were commercially available, three selected reference standards were considered, namely, quercetin-3-O-glucoside, kaempferol-3-O-glucoside, and isorhamnetin-3-O-glucoside, for the determination of quercetin, kaempferol, and isorhamnetin derivates, respectively. Results were expressed as standard mg/g extract (dw) ± relative standard deviation (% RDS). Notably, peak no. 8, namely, kaempferol 3-hydroxyferuloylsophoroside-7-O-glucoside, turned out to be the most abundant (1.30 mg/g ± 0.01), followed by peak no. 13, kaempferol-3-O-feruloylsophoroside-7-O-glucoside (1.28 mg/g ± 0.01).
In a previous study, we characterized the polyphenol compounds contained in the leaves of another Brassica wild species from Sicily, namely, B. incana, utilizing the same procedure of extraction reported here. By comparing the polyphenol profile of the leaf hydroalcoholic extract of B. fruticulosa subsp. fruticulosa with that of B. incana, a similar flavonoid pattern could be appreciated between the two species, with derivatives of the flavonols quercetin, kaempferol, and isorhamnetin, together with the hydroxycinnamic acids sinapic acid and ferulic acid. Nonetheless, some differences among the two species were highlighted; indeed, a greater number of flavonoid derivatives were detected in the B. fruticulosa subsp fruticulosa leaf extract, whereas the hydroxycinnamic acids identified in the B. incana extract were found to be more numerous and in conjugation with malic acid and glucose moieties, in addition to gentiobiose [5].
2.1.3. Identification of Volatile Compounds by SPME-GC/MS
The volatile composition of the hydroalcoholic extract of the aerial parts of B. fruticolosa subsp. fruticulosa is reported in Table 2. Many compounds, such as esters, alcohols, acids, ketones, aldehydes, terpenes, hydrocarbons, sulfur compounds, and nitriles, were determined. Nitriles (35.08%) and aldehydes (19.67%) constituted more than 54% of the whole volatile fraction; terpenoids (12.11%) and ketones (11.06%) were also quantitatively well represented. Among sulfur compounds, no isothiocyanates were detected, with only dimethyl disulfide and dimethyl trisulfide identified.
Table 2.
Composition as volatile constituents and classes of substances of B. fruticolosa subsp. fruticulosa leaf hydroalcoholic extract.
| Compounds | LRI * on DB-5ms | LRI * on VF-WAXms | Amount ** | Percentage |
|---|---|---|---|---|
| Sulfur compounds | ||||
| Dimethyl disulfide | 735 | 1078 | 460.369 | 2.77 |
| Dimethyl trisulfide | 957 | 1380 | 656.201 | 3.95 |
| All | 1116.569 | 6.72 | ||
| Nitriles | ||||
| 3-Methyl-3-butenenitrile | 747 | - | 3487.559 | 20.98 |
| 5-Methylhexanenitrile | 934 | 1349 | 438.854 | 2.64 |
| Heptanenitrile | 968 | 1406 | 924.253 | 5.56 |
| Benzenepropane nitrile | 1226 | 2041 | 980.772 | 5.90 |
| All | 5831.437 | 35.08 | ||
| Aldehydes | ||||
| 3-Methylbutanal | 656 | 911 | 325.842 | 1.96 |
| 2-Methylbutanal | 662 | 897 | 552.183 | 3.32 |
| Hexanal | 790 | 1082 | 708.226 | 4.26 |
| (E)-2-Heptenal | 948 | 1327 | 450.252 | 2.71 |
| Benzaldehyde | 951 | 1530 | 214.505 | 1.29 |
| Octanal | 994 | 1284 | 128.366 | 0.77 |
| (E,E)-2,4-Heptadienal | 1005 | 1508 | 128.722 | 0.77 |
| Phenylacealdehyde | 1033 | 1645 | 535.453 | 3.22 |
| Nonanal | 1094 | 1390 | 147.754 | 0.89 |
| Decanal | 1195 | 1491 | 77.683 | 0.47 |
| All | 3268.984 | 19.67 | ||
| Ketones | ||||
| 2,2,6-trimethylcyclohexanone | 1049 | 1296 | 249.598 | 1.50 |
| 2-Methyl-2-nonen-4-one | 1202 | - | 1158874 | 6.97 |
| Hexahydrofarnesyl acetone | 1825 | 2121 | 430.662 | 2.59 |
| All | 1839.133 | 11.06 | ||
| Alcohols | ||||
| 2-Ethyl-1-hexanol | 1020 | 1483 | 126.165 | 0.76 |
| (E)-2-Octen-1-ol | 1059 | 1611 | 167.066 | 1.01 |
| All | 293.231 | 1.76 | ||
| Acids | ||||
| Octanoic acid | 1161 | 2062 | 503.754 | 3.03 |
| Nonanoic acid | 1257 | 2165 | 93.800 | 0.56 |
| Decanoic acid | 1355 | 2266 | 622.338 | 3.74 |
| All | 1219.892 | 7.34 | ||
| Esters | ||||
| Ethyl octanoate | 1186 | 1439 | 78.014 | 0.47 |
| Ethyl decanoate | 1382 | 1639 | 129.706 | 0.78 |
| Ethyl dodecanoate | 1580 | 1840 | 109.245 | 0.66 |
| Methy tridecanoate | 1612 | 1910 | 79.540 | 0.48 |
| Ethyl tetradecanoate | 1778 | 2040 | 26.940 | 0.16 |
| Methyl hexadecanote | 1905 | 2216 | 209.279 | 1.26 |
| All | 632.724 | 3.81 | ||
| Terpenoids | ||||
| Safranal | 1189 | 1649 | 520.036 | 3.13 |
| β-Cyclocitral | 1209 | 1626 | 400.886 | 2.41 |
| 10-(Acetyl methyl)-(+)-3-carene | 1374 | - | 1030.412 | 6.20 |
| (E)-β Ionone | 1467 | 1928 | 62.218 | 0.37 |
| All | 2013.551 | 12.11 | ||
| Hydrocarbons | ||||
| 4,8-Dimethyl-1,7-nonadiene | 1041 | - | 205.520 | 1.24 |
| 1,1,5-Trimethyl-1,2-dihydronaphthalene | 1341 | - | 201.675 | 1.21 |
| All | 407.194 | 2.45 |
* Linear retention indices calculated according to the van den Dool and Kratz equation. ** Peak area arbitrary scale.
The main volatile compounds were 3-methyl-3-butenenitrile, heptanenitrile and benzenepropane nitrile, hexanal, 2-methyl-2-nonen-4-one, and 10-(acetylmethyl)-(+)-3-carene.
The identified compounds are well known secondary metabolites of plants [27] and, in particular, nitriles are common in Brassica species. Indeed, nitriles, as well as isothiocyanates, originate from the hydrolysis of glucosinolates, a group of compounds typical of Brassicaceae, Capparaceae, and Caricaceae families. The enzyme myrosinase, released upon tissue damage, hydrolyzes the β-d-S-glycosidic bonds of glucosinolates, releasing the sulfur-containing aglycone moieties that are unstable and undergo the Lossen rearrangement to form various breakdown products such as isothiocyanates, thiocyanates, nitriles, epithionitriles, and oxazolidine-2-thiones. The products of glucosinolate hydrolysis depend on various factors, such as the glucosinolate substrate, the reaction conditions, the presence of substances which can modify the action of the enzyme, and the plant pretreatments. It has been demonstrated that, if the hydrolytic reaction occurs under acidic conditions, low temperature, and low water levels, nitrile formation is favored. Nitriles are also favored by autolysis, rather than by the action of an exogenous source of the enzyme and by the presence of ferrous ion. Moreover, in fresh or freeze–thawed leaves, the glucosinolate hydrolysis produced mainly nitriles, whereas dry heating of the leaves decreased the proportion of nitrile formation and increased the proportion of isothiocyanate formation [29].
Although few data are present in the literature on the glucosinolate composition of B. fruticolosa [30], with none referring to leaves, the nitriles here identified are consistent with the structure of glucosinolates previously reported in Brassicaceae [31].
Among the minor constituents, safranal, β-cyclocitral, and β-ionone originate from the enzymatic breakdown of carotenoids. These terpenoids have been detected in the flowering top extract of B. incana [32] and in the hydroalcoholic extract of the aerial part of different Matthiola species, such as M. fruticolosa [28] and M. tricuspidata (our unpublished data). Furthermore, hexahydrofarnesyl acetone or phytone was detected in our previous studies on hydroalcoholic extract of B. incana and Matthiola spp. [28,32,33]; this ketone very common in plants arises from the oxidative degradation of (E)-phytol, a diterpene alcohol that occurs as a side-chain of chlorophyll a [34].
The results here reported are quite different from those described in our previous study on the volatiles of B. fruticolosa leaves [8]. This can be explained considering that we previously applied the SPME technique directly to the plant leaves, whereas, in this case, a hydroalcoholic extract of the leaves was considered.
Considering the volatile profile of the leaf hydroalcoholic extract of another Brassica species, namely, B. incana, only a few qualitative similarities emerged, whereas, from a quantitative point of view, in the volatile profile of B. incana leaf extract, isothiocyanates prevailed vs. nitriles [32].
2.2. Antioxidant Activity
Oxidative stress has been identified as the root cause of the development and progression of many diseases. In recent years, several epidemiological studies have indicated that a high intake of plant products is associated with a reduced risk of a number of chronic diseases, such as atherosclerosis and cancer, partly attributed to the compounds which possess antioxidant activity [35]. Bibliographic data show that many species belonging to the genus Brassica contain phenolic compounds, widely considered to be the most important specialized metabolites with antioxidant activity [24].
Antioxidant activity should be evaluated by the use of various methods in order to acquire a more complete antioxidant profile. In these assays, plant extracts are generally assessed for their function as reducing agents, hydrogen donors, singlet oxygen quenchers, or metal chelators [36]. When they react with free radicals by producing less reactive species or by interrupting the radical chain reaction, they are classified as primary (chain breaking) antioxidants; on the contrary, when they act by suppressing the formation of radicals and protecting against oxidative damage, they are defined as secondary (preventive) antioxidants [37]. Thus, three in vitro assays based on different approaches and mechanisms were used in order to determine the antioxidant capacity of B. fruticulosa subsp. fruticulosa extract. The primary antioxidant properties were examined using the DPPH assay, based on the hydrogen atom transfer (HAT) and electron transfer (ET) mechanisms, and the reducing power, an ET-based assay. The secondary antioxidant ability was determined by measuring the ferrous ion (Fe2+)-chelating activity.
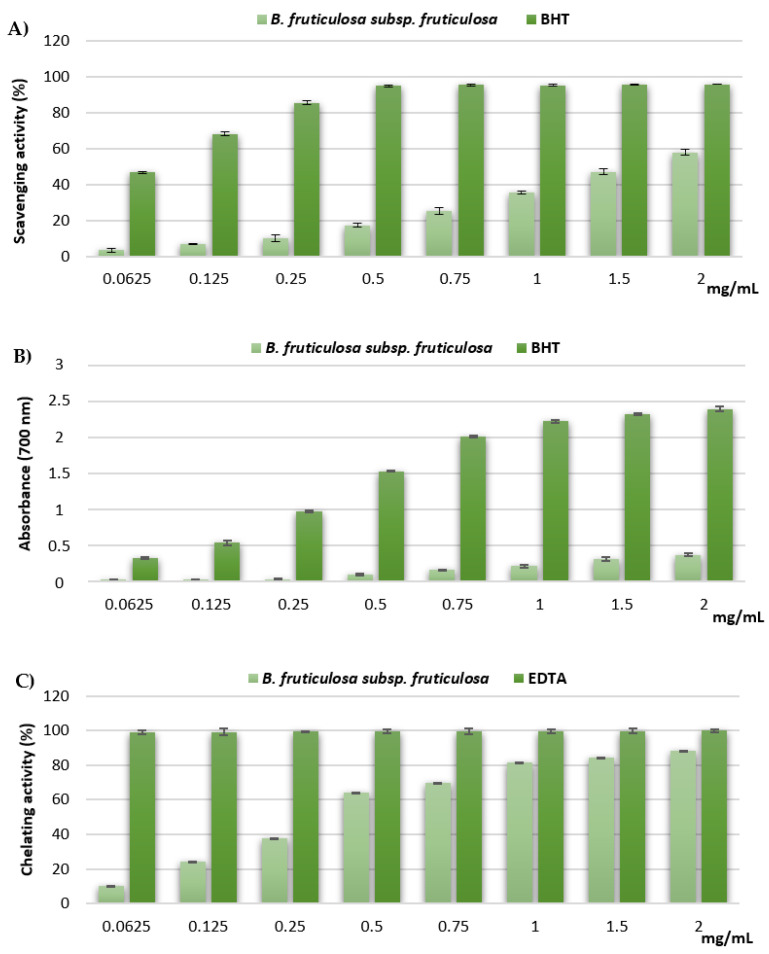
The results of the DPPH test, utilized to establish the free-radical-scavenging properties of the extract, are shown in Figure 2A. Compared with the reference standard BHT, the hydroalcoholic leaf extract of B. fruticulosa subsp. fruticulosa exhibited moderate scavenging activity, dose-dependently, in the range of concentrations assayed (0.0625–2 mg/mL), reaching about 60% inhibition of the DPPH radical at the highest concentration tested. The IC50 values confirmed the lower activity of the extract with respect to the standard BHT (1.65 ± 0.08 mg/mL and 0.07 ± 0.01 mg/mL, respectively).
Figure 2.
Free-radical-scavenging activity (DPPH assay) (A), reducing power (B), and ferrous ion-chelating activity (C) of B. fruticulosa subsp. fruticulosa leaf hydroalcoholic extract. Values are expressed as the mean ± SD (n = 3).
From the comparison of the scavenging activity of the extract with that highlighted for B. incana leaf extract (IC50 = 1.31 ± 0.05 mg/mL), previously investigated under the same experimental conditions, it is evident that B. fruticulosa subsp. fruticulosa extract had a slightly lower activity [5].
The reducing power reflects the ability to stop the radical chain reaction. In this assay, the presence of antioxidant compounds in the sample determines the reduction of Fe3+ to the ferrous form (Fe2+); this reduction is highlighted by spectrophotometric measurement (700 nm) of the change of yellow color of the test solution to various shades of green and blue, depending on the reducing power of the antioxidant sample [38].
Figure 2B shows the results of the reducing power of the hydroalcoholic leaf extract of B. fruticulosa subsp. fruticulosa; the extract exhibited mild, concentration-dependent, reducing power, as compared with the standard BHT. This was confirmed also by the ASE/mL values (17.47 ± 0.65 and 0.89 ± 0.06 ASE/mL, respectively). This result agrees with that previously reported for the extract of B. incana leaves [5].
The method of Fe2+-chelating activity utilized the reagent ferrozine, which can quantitatively form complexes with Fe2+; in the presence of chelating agents, the complex formation is inhibited, with the result that the red color of the complex is decreased. Measurement of color reduction, therefore, allows the estimation of the chelating activity of the coexisting chelator [38].
Brassica fruticulosa subsp. fruticulosa extract exhibited strong and dose-dependent chelating properties (Figure 2C), reaching approximately 90% activity at the highest tested concentration. Nevertheless, the extract was not as effective as the reference standard EDTA (IC50 = 0.38 ± 0.02 and 0.007 ± 0.001 mg/mL, respectively). In comparison with B. incana leaf extract, previously investigated under the same experimental conditions (IC50 = 1.147 ± 0.016 mg/mL), B. fruticulosa subsp. fruticulosa extract exhibited much higher chelating properties [5].
The results of the in vitro antioxidant tests showed that B. fruticulosa subsp. fruticulosa extract acts as moderate primary antioxidant and possesses strong secondary antioxidant properties.
Taking into consideration that flavonoids are known to display metal-chelating effects [39], the good chelating activity of the extract may depend to some extent on the presence of flavonol derivatives, mostly of quercetin and kaempferol, detected by HPLC-PDA/ESI-MS analysis; however, the involvement of other polar constituents present in the phytocomplex cannot be excluded.
2.3. Artemia salina Leach Lethality Bioassay
The toxicity of the extract was assessed by the Artemia salina Leach bioassay. The brine shrimp lethality bioassay is extensively utilized as an alternative model for toxicity evaluation because it offers numerous advantages such as rapidity, cost-effectiveness, continuous availability of cysts (eggs), and ease of handling and maintenance under laboratory conditions [40]. It represents a simple technique for predicting the toxicity of plant extracts in order to consider their safety. The results of the bioassay showed the absence of toxicity against brine shrimp larvae for the extract of B. fruticulosa subsp. fruticulosa. Indeed, the median lethal concentration values were found to be above 1000 µg/mL, thus indicating their potential safety according to Clarkson’s toxicity criterion [41]. These data are in agreement with those observed for the extracts of B. incana investigated in our previous work [5].
3. Materials and Methods
3.1. Chemicals and Reagents
LC–MS-grade water (H2O), acetonitrile (ACN), isorhamnetin-3-O-glucoside, quercetin-3-O-glucoside, and kaempferol-3-O-glucoside were obtained from Merck Life Science (Merck KGaA, Darmstadt, Germany). LC–MS-grade formic acid was purchased from Riedel-de Haën (Seelze, Germany). Methanol (MeOH) was purchased from Carlo Erba (Milan, Italy). Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (Milan, Italy).
3.2. Plant Material and Extraction Procedure
The leaves of the Brassica fruticulosa subsp. fruticulosa were collected in the locality of Massa San Giorgio, on the Peloritani Mountains (Messina, Sicily, Italy), in October 2019. The taxonomic identification was confirmed by Prof. S. Ragusa, Department of Health Sciences, University Magna Graecia of Catanzaro (Catanzaro, Italy). A voucher specimen (1016/19) was deposited in the same Department.
After harvesting, the plant material was washed, blended, frozen, and then lyophilized. Subsequently, the leaves, finely ground, were subjected to a preventive maceration at 25 °C with 70% MeOH (1:10 w/v) for 1 h. The extraction was performed with 70% MeOH (1:10 w/v) in an ultrasonic bath at 50 °C for 15 min, repeated three times; then, the filtrates were combined and evaporated to dryness by a rotavapor. The yield of the leaf extract, referring to 100 g of lyophilized plant material, was 22.99%.
3.3. Phytochemical Investigation
3.3.1. Determination of Total Phenolic Content
The total phenolic content of B. fruticulosa subsp. fruticulosa leaf extract was determined by the Folin–Ciocâlteu colorimetric method, using gallic acid as a standard phenolic compound [42]. An aliquot of 0.1 mL of each sample solution was mixed with 0.2 mL Folin–Ciocâlteu reagent, 2 mL of distilled water, and 1 mL of 15% Na2CO3. A linear calibration curve of gallic acid, in the range 125–500 µg/mL, was constructed. The absorbance was measured at 765 nm, after a 2 h incubation at room temperature, with a UV-1601 spectrophotometer (Shimadzu, Milan, Italy). The total phenolics were expressed as mg GAE/g of extract (dw) ± standard deviation (SD). The data were obtained from the average of three independent determinations.
3.3.2. Identification of Phenolic Compounds by HPLC-PDA/ESI-MS
The analyses were carried out using a Shimadzu HPLC system (Milan, Italy) equipped with a CBM-20A controller, LC-20AD pumps, a DGU-20A3 degasser, a SIL-20AC autosampler, an SPD-M20A photo diode array detector (PDA), and a triple-quadrupole mass analyzer (LCMS-8050, Shimadzu, Kyoto, Japan), equipped with an ESI interface, in positive and negative ionization mode. Data acquisition was performed by Shimadzu LabSolution software ver. 5.91.
Samples and Sample Preparation
B. fruticulosa subsp. fruticulosa leaf extract (30.5 mg) was dissolved in 100 µL of MeOH.
Chromatographic Conditions
Analyses were carried out on a Ascentis Express C18, 15 cm × 4.6 mm internal diameter (i.d.), with particle size of 2.7 µm (Merck Life Science, Merck KGaA, Darmstadt, Germany). The injection volume was 5 µL, and the mobile phase consisted of water/formic acid (99.9:0.1, v/v) (solvent A) and ACN/formic acid (99.9:0.1, v/v) (solvent B); the linear gradient profile was as follows: 0 min, 0% B; 15 min, 5% B; 65 min, 20% B; 95 min, 35% B; 100 min, 100% B; 101 min, 0% B. The flow rate for separation and detection was 1 mL/min, and it was split to 0.2 mL/min prior to MS detection.
PDA Conditions
The wavelength range was 200–400 nm, and the chromatograms were extracted at 280 nm. The time constant was 0.08 s, and the sample frequency was 40 Hz.
MS Conditions
The MS acquisition was performed using the ESI interface in negative ionization mode. Mass detection was performed in full scan mode in the spectral range 100–1400 m/z, with an interval of 0.5 s. Nitrogen (N2) was used as a nebulizing gas at a flow rate of 3 L/min. The following settings were applied to the instrument: interface temperature, 300 °C; heat block, 400 °C; DL temperature, 250 °C; DL voltage, −34 V; probe voltage, 4.5 kV; Q-array voltage, 1.0 V; RF voltage, 90 V; detection gain, 1.0 kV.
Quantitative determination was carried using calibration curves of three standards, representative of the chemical classes under study, namely, isorhamnetin-3-O-glucoside (y = 14948x − 2966.9; limit of detection (LOD) = 0.032, limit of quantification (LOQ) = 0.098), quercetin-3-O-glucoside (y = 13424x + 898.59; LOD = 0.013, LOQ = 0.043), and kaempferol-3-O-glucoside (y = 17660x − 10681; LOD = 0.023, LOQ = 0.072). Standard calibration curves were prepared in a concentration range 0.1–1000 mg/L with five different concentration levels.
3.3.3. Identification of Volatile Compounds by SPME-GC/MS
Extraction (HS-SPME)
The leaf extract of B. fruticolosa subsp. fruticulosa was analyzed for its volatile composition by HS-SPME-GC/MS as previously reported [28,33,43].
The dried extract was solubilized in saturated sodium chloride solution to a final concentration of 10 mg/mL; then, 3 ± 0.1 mL of each extract solution was transferred to a 7 mL vial closed with a ‘mininert’ valve (Supelco, Bellefonte, PA, USA). For the volatile extraction, the sample was equilibrated for 15 min at 40 °C, and a DVB/CAR/PDMS fiber, 50/30 μm film thickness (Supelco, Bellefonte, PA, USA), was exposed for 15 min to the headspace of the sample maintained at 40 °C under continuous magnetic stirring. Finally, the SPME fiber was placed for 3 min into the injector port of the GC/MS, held at 260 °C, for the thermal desorption of the analytes onto the capillary GC column.
Analysis (GC/MS)
The volatiles were analyzed by a Shimadzu GC 2010 Plus gas chromatograph coupled to a TQMS 8040 triple-quadrupole mass spectrometer (Shimadzu, Milan, Italy). Two capillary columns of different polarity were used: (1) a VF-WAXms, 60 m, 0.25 mm i.d., 0.25 μm film thickness polar column (Agilent Technologies Italia S.p.A., Milan, Italy); (2) a DB-5 ms, 30 m, 0.25 mm i.d., 0.25 μm film thickness apolar column (Agilent Technologies Italia S.p.A., Milan, Italy).
The conditions were as follows: injection mode, splitless; oven temperature (1) 45 °C held for 5 min, then increased to 80 °C at a rate of 10 °C/min and to 240 °C at 2 °C/min, held at 240 °C for 5 min for polar column, (2) 45 °C increased to 160 °C at a rate of 3 °C/min and to 260 °C at 10 °C/min, held at 260 °C for 5 min for apolar column; carrier gas, helium at a constant flow of 1 mL/min; transfer line temperature, 250 °C; acquisition range, 40 to 360 m/z; scan speed, 1250. For the identification of the volatiles, mass spectral data, NIST’ 14 (NIST/EPA/NIH Mass Spectra Library, version 2.0, Gaithersburg, MD, USA) and FFNSC 3.0 database, and linear retention indices (LRI) were used.
3.4. Antioxidant Activity
3.4.1. Free-Radical-Scavenging Activity
The free-radical-scavenging activity of the hydroalcoholic leaf extract of B. fruticulosa subsp. fruticulosa was evaluated using the DPPH (2,2-diphenyl-1-picrylhydrazyl) test [44]. DPPH is a stable radical in methanol with violet color because of delocalization of the spare electron throughout the molecule. When a proton is accepted in the reaction with the oxygen atom of a radical scavenger’s OH group, the reduced DPPH-H (2,2-diphenyl-1-picrylhydrazine) is formed, which is yellow. The degree of discoloration indicates the amount of DPPH scavenged; a greater bleaching action indicates higher antioxidant activity, as reflected in a lower IC50 value.
The extract was tested at different concentrations (0.0625–2 mg/mL) using butylated hydroxytoluene (BHT) as a reference compound. A volume of 0.5 mL of each sample solution was mixed with 3 mL of daily prepared methanol DPPH solution (0.1 M) and incubated for 20 min at room temperature in the dark. Then absorbance was measured at 517 nm using a model UV-1601 spectrophotometer (Shimadzu, Milan, Italy). The scavenging activity was measured as the decrease in absorbance of the samples versus DPPH standard solution. Results were obtained from the average of three independent experiments, and they were expressed as the mean radical-scavenging activity percentage (%) ± SD and mean 50% inhibitory concentration (IC50) ± SD.
3.4.2. Reducing Power Assay
The reducing power of the hydroalcoholic leaf extract of B. fruticulosa subsp. fruticulosa was determined according to the Fe3+–Fe2+ transformation method [45]. The extract was tested in the range of 0.0625–2 mg/mL. A volume of 1 mL of each sample was mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of 1% potassium ferricyanide (K3Fe(CN)6). Following incubation at 50 °C for 20 min and rapid cooling, 2.5 mL of 10% trichloroacetic acid was added, and the mixture was centrifuged (3000 rpm, 10 min). Finally, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of 0.1% ferric chloride (FeCl3). After incubation for 10 min of at room temperature in the dark, the color change of the sample was estimated by measuring absorbance at 700 nm. The increased absorbance of the reaction mixture indicates an increase in reducing power. Ascorbic acid and butylated hydroxytoluene (BHT) were used as reference compounds. The results were obtained from the average of three independent experiments, and they were expressed as the mean absorbance values (700 nm) ± SD and ascorbic acid equivalent/mL of extract (ASE/mL) ± SD.
3.4.3. Ferrous Ion (Fe2+)-Chelating Activity Assay
The Fe2+-chelating activity of the hydroalcoholic leaf extract of B. fruticulosa subsp. fruticulosa was estimated by measuring the formation of the Fe2+–ferrozine complex [46]. The extract was tested in the range of 0.0625–2 mg/mL, and ethylenediaminetetraacetic acid (EDTA) was used as positive control. A volume of 1 mL of each sample was mixed with 0.5 mL of MeOH and 50 µL of 2 mM FeCl2. Then, 0.1 mL of 5 mM ferrozine was added to initiate the reaction; the mixture was shaken vigorously and incubated at room temperature in the dark for 10 min. The control contained FeCl2 and ferrozine, which are complex formation molecules. The color change of the solutions was estimated by measuring absorbance spectrophotometrically at 562 nm. The results were obtained from the average of three independent experiments, and they were expressed as the mean inhibition of the ferrozine–Fe2+ complex formation (%) ± SD and IC50 ± SD.
3.5. Artemia Salina Leach Lethality Bioassay
The Artemia salina Leach (brine shrimp) lethality bioassay was employed to predict the toxicity of the leaf hydroalcoholic extract of B. fruticulosa subsp. fruticulosa [47]. Brine shrimp eggs were hatched in artificial seawater (33 g sea salt/L deionized water) by incubation under a 60 W lamp, providing direct light and warmth (24–26 °C). After hatching, 10 brine shrimp larvae were incubated at 25–28 °C in 5 mL of artificial seawater mixed with different amounts of the extract (10, 100, 500, and 1000 µg/mL). After 24 h, the numbers of surviving nauplii were counted using a magnifying glass. The experiments were conducted in triplicate for each concentration, and the median lethal concentration (LC50) values were determined by Litchfield and Wilcoxon’s method. The toxicity level of the extract was assessed according to the toxicity scale reported by Clarkson et al. [41]. Extractis considered non-toxic if the LC50 is higher than 1000 µg/mL.
4. Conclusions
This work described the results of the phytochemical characterization and the antioxidant properties of the leaf hydroalcoholic extract of B. fruticulosa subsp. fruticulosa growing wild in Sicily (Italy), never investigated before. An in-depth overview of the qualitative–quantitative composition of the phenolic and volatile constituents of the leaves was attained. On the basis of the in vitro antioxidant assays performed, it can be stated that the B. fruticulosa subsp. fruticulosa leaf extract had much higher secondary than primary antioxidant properties. Furthermore, the extract was found to be non-toxic against brine shrimp larvae, indicative of its potential safety.
The obtained results provide a substantial contribution to the knowledge of B. fruticulosa subsp. fruticulosa so far little studied, indicating this wild edible species as a new valuable source of antioxidant compounds with potential health-promoting effects.
Acknowledgments
Federica Davì thanks the Foundation “Prof. Antonio Imbesi” for the fellowship. The authors are thankful to Shimadzu and Merck Life Science Corporations for the continuous support and to Salvatore Ragusa for plant collection and identification.
Author Contributions
Conceptualization, M.F.T. and N.M.; investigation, M.F.T., F.D., E.C., M.R., F.C., Y.O.E.M., C.C., M.M. and N.M.; data curation, M.F.T., F.C., L.M., C.C., A.V. and N.M.; writing—original draft preparation, M.F.T., F.D., E.C., F.C., C.C. and N.M.; writing—review and editing, M.F.T., L.M., A.V., and N.M. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cartea M.E., Francisco M., Soengas P., Velasco P. Phenolic compounds in Brassica vegetables. Molecules. 2011;16:251–280. doi: 10.3390/molecules16010251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branca F., Cartea E. Wild Crop Relatives: Genomic and Breeding Resources: Oilseeds. Springer; Heidelberg/Berlin, Germany: 2011. Brassica; pp. 17–36. [Google Scholar]
- 3.Malfa G.A., Acquaviva R., Bucchini A.A.E., Ragusa S., Raimondo F.M., Spadaro V. The Sicilian wild cabbages as biological resources: Taxonomic update and a review on chemical constituents and biological activities. Fl. Medit. 2020;30:245–260. [Google Scholar]
- 4.Sánchez-Mata M.C., Cabrera Loera R.D., Morales P., Fernández-Ruiz V., Cámara M., Díez Marqués C., Pardo-de-Santayana M., Tardío J. Wild vegetables of the Mediterranean area as valuable sources of bioactive compounds. Genet. Resour. Crop. Evol. 2012;59:431–443. doi: 10.1007/s10722-011-9693-6. [DOI] [Google Scholar]
- 5.Miceli N., Cavò E., Ragusa M., Cacciola F., Mondello L., Dugo L., Acquaviva R., Malfa G.A., Marino A., D’Arrigo M., et al. Brassica incana Ten. (Brassicaceae): Phenolic constituents, antioxidant and cytotoxic properties of the leaf and flowering top extracts. Molecules. 2020;25:1461. doi: 10.3390/molecules25061461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marhold K. Brassicaceae. Euro+Med Plantbase-the Information Resource for Euro-Mediterranean Plant Diversity. [(accessed on 25 February 2022)]. Available online: http://www.emplantbase.org/home.html.
- 7.BrassiBase. [(accessed on 25 February 2022)]. Available online: https://brassibase.cos.uni-heidelberg.de/
- 8.Tripodi G., Verzera A., Dima G., Condurso C., Ragusa S. Brassica fruticulosa Cyr. and Brassica incana Ten. (Brassicaceae) as Mediterranean traditional wild vegetables: A valuable source of bioactive compounds. J. Essent. Oil Res. 2012;24:539–545. doi: 10.1080/10412905.2012.730492. [DOI] [Google Scholar]
- 9.Branca F., Fisichella A. Response of Brassica fruticulosa Cyr. to greenhouse cultivation. Acta Hortic. 2003;614:89–93. doi: 10.17660/ActaHortic.2003.614.11. [DOI] [Google Scholar]
- 10.Gómez-Campo C. Biology of Brassica Coenospecies. Volume 4. Elsevier; Amsterdam, The Netherlands: 1999. Taxonomy; pp. 3–32. [Google Scholar]
- 11.Pignatti S. Brassica L. In: Edagricole-New Business Media, editor. Flora d’Italia. Volume 2. Edagricole; Milano, Italia: 2017. pp. 1016–1028. [Google Scholar]
- 12.Tutin T.G., Burges N.A., Chater A.O., Edmondson J.R., Heywood V.H., Moore D.M., Valentine D.H., Walters S.M., Webb D.A. Flora Europaea. Volume 1 Cambridge University Press; London, UK: 1993. [Google Scholar]
- 13.Barbagallo C., Meli R., Savoca F., Nicotra M. Indagine sugli usi popolari delle piante medicinali nella Sicilia centro-orientale. Boll. Acc. Gioenia C. Nat. 2004;37:83–157. [Google Scholar]
- 14.Guarrera P.M. Le piante nelle tradizioni popolari della Sicilia. Erbor. Domani. 2009;46:46–55. [Google Scholar]
- 15.Romano D., Tribulato A., Toscano S., Scuderi D. Ethnobotanical uses of Brassicaceae in Sicily. Acta Hortic. 2013;1005:197–204. doi: 10.17660/ActaHortic.2013.1005.20. [DOI] [Google Scholar]
- 16.Lentini F., Venza F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007;3:15. doi: 10.1186/1746-4269-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellani L.M., Salvini L., Scialabba A. Characterization of Brassica fruticulosa seeds. Bocconea. 2009;23:451–457. [Google Scholar]
- 18.Singh B.K., Bala M., Rai P.K. Fatty Acid Composition and Seed Meal Characteristics of Brassica and Allied Genera. Natl. Acad. Sci. Lett. 2014;37:219–226. doi: 10.1007/s40009-014-0231-x. [DOI] [Google Scholar]
- 19.Cutillo F., D’Abrosca B., Della Greca M., Fiorentino A., Zarrelli A. Lignans and neolignans from Brassica fruticulosa: Effects on seed germination and plant growth. J. Agric. Food Chem. 2003;51:6165–6172. doi: 10.1021/jf034644c. [DOI] [PubMed] [Google Scholar]
- 20.Cutillo F., Dellagreca M., Previtera L., Zarrelli A. C13 Norisoprenoids from Brassica fruticulosa. Nat. Prod. Res. 2005;19:99–103. doi: 10.1080/14786410410001686409. [DOI] [PubMed] [Google Scholar]
- 21.Munteanu I.G., Apetrei C. Analytical methods used in determining antioxidant activity: A review. Int. J. Mol. Sci. 2021;22:3380. doi: 10.3390/ijms22073380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oulad El Majdoub Y., Alibrando F., Cacciola F., Arena K., Pagnotta E., Matteo R., Micalizzi G., Dugo L., Dugo P., Mondello L. Chemical Characterization of Three Accessions of Brassica juncea L. Extracts from Different Plant Tissues. Molecules. 2020;25:5421. doi: 10.3390/molecules25225421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen H., Aaby K., Borge G.I. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. Convar. acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009;57:2816–2825. doi: 10.1021/jf803693t. [DOI] [PubMed] [Google Scholar]
- 24.Picchi V., Lo Scalzo R., Tava A., Doria F., Argento S., Toscano S., Treccarichi S., Branca F. Phytochemical Characterization and In Vitro Antioxidant Properties of Four Brassica Wild Species from Italy. Molecules. 2020;25:3495. doi: 10.3390/molecules25153495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martínez-Sánchez A., Gil-Izquierdo A., Gil M.I., Ferreres F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008;56:2330–2340. doi: 10.1021/jf072975+. [DOI] [PubMed] [Google Scholar]
- 26.Sun J., Xiao Z., Lin L.Z., Lester G.E., Wang Q., Harnly J.M., Chen P. Profiling polyphenols in five Brassica species microgreens by UHPLC-PDA-ESI/HRMS(n.) J. Agric. Food Chem. 2013;61:10960–10970. doi: 10.1021/jf401802n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzales G.B., Raes K., Vanhoutte H., Coelus S., Smagghe G., Van Camp J. Liquid chromatography-mass spectrometry coupled with multivariate analysis for the characterization and discrimination of extractable and nonextractable polyphenols and glucosinolates from red cabbage and Brussels sprout waste streams. J. Chromatogr. A. 2015;1402:60–70. doi: 10.1016/j.chroma.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Taviano M.F., Cavò E., Spadaro V., Raimondo F.M., Musolino V., Cacciola F., Oulad El Majdoub Y., Mondello L., Condurso C., Cincotta F., et al. Phytochemical constituents, antioxidant activity, and toxicity assessment of the aerial part extracts from the infraspecific taxa of Matthiola fruticulosa (Brassicaceae) endemic to Sicily. Molecules. 2021;26:4114. doi: 10.3390/molecules26144114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fenwick G.R., Heaney R.K. Glucosinolates and their breakdown products in cruciferous crops, foods and feedingstuffs. Food Chem. 1983;11:249–271. doi: 10.1016/0308-8146(83)90074-2. [DOI] [Google Scholar]
- 30.Velasco L., Becker H.C. Analysis of total glucosinolate content and individual glucosinolates in Brassica spp. by near-infrared reflectance spectroscopy. Plant Breed. 1998;117:97–102. doi: 10.1111/j.1439-0523.1998.tb01459.x. [DOI] [Google Scholar]
- 31.Fahey J.W., Zalcmann A.T., Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry. 2001;56:5–51. doi: 10.1016/S0031-9422(00)00316-2. [DOI] [PubMed] [Google Scholar]
- 32.Speranza J., Taviano M.F., Ragusa S., Condurso C., Cincotta F., Verzera A., Day-Walsh P., Kroon P., Miceli N. Characterization of volatile components and in vitro inhibitory effect on gut microbial TMA production of the leaf hydroalcoholic extract of Brassica incana Ten. (Brassicaceae) growing wild in Sicily (Italy); Proceedings of the 115th Congresso della Società Botanica Italiana; Online. 9–11 September 2020; p. 194. [Google Scholar]
- 33.Miceli N., Cavò E., Spadaro V., Raimondo F.M., Ragusa S., Cacciola F., Oulad El Majdoub Y., Arena K., Mondello L., Condurso C., et al. Phytochemical Profile and Antioxidant Activity of the Aerial Part Extracts from Matthiola incana subsp. rupestris and subsp. pulchella (Brassicaceae) Endemic to Sicily. Chem. Biodivers. 2021;18:e2100167. doi: 10.1002/cbdv.202100167. [DOI] [PubMed] [Google Scholar]
- 34.Schulz S., Yildizhan S., Van Loon J.J. The biosynthesis of hexahydrofarnesylacetone in the butterfly Pieris brassicae. J. Chem. Ecol. 2011;37:360–363. doi: 10.1007/s10886-011-9939-y. [DOI] [PubMed] [Google Scholar]
- 35.Jahangir M., Kim H.K., Choi Y.H., Verpoorte R. Health-affecting compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009;8:31–43. doi: 10.1111/j.1541-4337.2008.00065.x. [DOI] [Google Scholar]
- 36.Chua L.S., Hidayathulla S. Phytochemical profile of fresh and senescent leaves due to storage for Ficus deltoidea. Plant Biosyst. 2017;151:74–83. [Google Scholar]
- 37.Kasote D.M., Katyare S.S., Hegde M.V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gülçin I., Elmastaş M., Aboul-Enein H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007;21:354–361. doi: 10.1002/ptr.2069. [DOI] [PubMed] [Google Scholar]
- 39.Mladěnka P., Macáková K., Filipský T., Zatloukalová L., Jahodář L., Bovicelli P., Silvestri I.P., Hrdina R., Saso L. In vitro analysis of iron chelating activity of flavonoids. J. Inorg. Biochem. 2011;105:693–701. doi: 10.1016/j.jinorgbio.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 40.Libralato G., Prato E., Migliore L., Cicero A.M., Manfra L. A review of toxicity testing protocols and endpoints with Artemia spp. Ecol. Indic. 2016;69:35–49. doi: 10.1016/j.ecolind.2016.04.017. [DOI] [Google Scholar]
- 41.Clarkson C., Maharaj V.J., Crouch N.R., Grace O.M., Pillay P., Matsabisa M.G., Bhagwandin N., Smith P.J., Folb P.I. In vitro antiplasmodial activity of medicinal plants native to or naturalised in South Africa. J. Ethnopharmacol. 2004;92:177–191. doi: 10.1016/j.jep.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Gao X., Ohlander M., Jeppsson N., Bjork L., Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruit of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 43.Miceli N., Cavò E., Ragusa S., Cacciola F., Dugo P., Mondello L., Marino A., Cincotta F., Condurso C., Taviano M.F. Phytochemical characterization and biological activities of a hydroalcoholic extract obtained from the aerial parts of Matthiola incana (L.) R. Br. subsp. incana (Brassicaceae) growing wild in Sicily (Italy) Chem. Biodivers. 2019;16:e1800677. doi: 10.1002/cbdv.201800677. [DOI] [PubMed] [Google Scholar]
- 44.Ohnishi M., Morishita H., Iwahashi H., Shitzuo T., Yoshiaki S., Kimura M., Kido R. Inhibitory effects of chlorogenic acid on linoleic acid peroxidation and haemolysis. Phytochemistry. 1994;36:579–583. doi: 10.1016/S0031-9422(00)89778-2. [DOI] [Google Scholar]
- 45.Oyaizu M. Studies on products of browning reaction: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- 46.Decker E.A., Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J. Agric. Food Chem. 1990;38:674–677. doi: 10.1021/jf00093a019. [DOI] [Google Scholar]
- 47.Meyer B.N., Ferrigni N.R., Putnam J.E., Jacobson L.B., Nichols D.E., McLaughlin J.L. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45:31–34. doi: 10.1055/s-2007-971236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.