Abstract
We describe a reactor-scale cultivation protocol for the fastest-growing and only known thermophilic member of the family Chlorobiaceae, Chlorobium tepidum. We discovered that C. tepidum would grow with sulfide as the sole electron source at rates and with final cell yields comparable to those found with thiosulfate only if the sulfide concentration was maintained below 0.1 mM and the culture redox potential was at −300 ± 20 mV. Such was also the requirement for growth in a photobioreactor when thiosulfate (optimum level, 12 mM) was used as the preferred electron source. For cultivation of C. tepidum on a 5- to 500-ml scale, we used the system of Balch and Wolfe (Appl. Environ. Microbiol. 32:781–791, 1976) using stopper-sealed serum tubes and bottles as an alternative to the methods commonly used for the cultivation of phototrophic anaerobes and obtained consistent results.
Chlorobium tepidum is the only known thermophilic green sulfur bacterium (12). With thiosulfate as an electron donor, it grows rapidly and to high cell densities, whereas with sulfide as the sole electron source, it grows poorly. In contrast, all other members of the family Chlorobiaceae grow vigorously with sulfide and most of them do not use thiosulfate for growth (11, 12). The lipid composition of C. tepidum is unusual compared to that of other chlorobia, and this distinction could be related to the thermophilic nature of the host (12). It is also the fastest-growing green sulfur bacterium, with a doubling time of 2 h (12), and it could be adapted to form colonies on agar plates with efficiencies as high as 100% (13). C. tepidum is amenable to genetic analysis by transformation with replicable plasmid vectors delivered from Escherichia coli by conjugation (13), and its entire genome is currently being sequenced by The Institute for Genomic Research (Rockville, Md.) (http://www.tigr.org/tdb/mdb/mdb.html). All of these attributes of this organism are attracting an increasing number of investigators to the study of C. tepidum.
We describe here the protocols for the cultivation of C. tepidum in a 22-liter photobioreactor under well-defined and -monitored conditions. These protocols helped us to provide an explanation for the reported inability of C. tepidum to grow well with sulfide as the sole electron source (12) and to establish the conditions under which this green sulfur bacterium would grow well with this most-reduced sulfur compound. Also described is an alternative method, based on the techniques of Balch and Wolfe (2), for cultivating this organism on smaller scales (5 to 500 ml).
Organism and medium compositions.
C. tepidum TLS (12) was obtained from M. T. Madigan (Southern Illinois University, Carbondale, Ill.) and maintained in sealed bottles and tubes (see below) containing medium 1. This medium was a modification of the maintenance medium of Wahlund et al. (12) and was composed of the following components (millimolar): EDTA, 0.034; MgSO4 · 7H2O, 0.811; NaCl, 6.84; KH2PO4, 3.67; NH4Cl, 7.48; NH4OOCCH3, 6.49; NaHCO3, 24; Na2S2O3 · 5H2O, 12; Na2SeO4, 0.002; Na2WO4 · 2H2O, 0.002; cyanocobalamin, 0.000015; Na2S · 9H2O, 1.5 mM; and 10 ml of a 100-fold-concentrated mineral solution for Methanococcus thermolithotrophicus (5) per liter of medium. For growth under autotrophic conditions, ammonium acetate was replaced with an equivalent amount of NH4Cl. Other modifications, that had been introduced for various growth studies, have been indicated below. However, regardless of whether thiosulfate or sulfide served as the electron source for growth, Na2S (for tubes, bottles, and a bioreactor) or H2S (for a bioreactor only) was used to reduce the medium and as the obligatory reduced-sulfur source (12). Whenever used, H2S was supplied as a mixture of N2 and H2S (90:10, vol/vol; henceforth referred to as H2S).
Protocols for tube- and bottle-scale cultivation.
The general techniques used for the preparation of medium for small-scale cultivation of C. tepidum were those of Balch and Wolfe (2). All medium components, except NH4Cl, NH4OOCCH3, Na2S2O3, and Na2S, were dissolved in distilled water, and the pH of the solution was adjusted to 6.8 with HCl. The solution was then made anaerobic by boiling under N2-CO2 (80:20, vol/vol). The anaerobic medium was cooled under N2-CO2 and placed inside an anaerobic chamber (Coy Laboratories, Grass Lake, Mich.) maintained under either N2-H2 (95:5, vol/vol) or N2-H2-CO2 (75:5:20, vol/vol/vol), and the required amounts of NH4Cl, NH4OOCCH3, Na2S2O3, and Na2S (taken into the chamber as solids) were dissolved in it. The medium was then dispensed into tubes or bottles: 5 to 20 ml per serum tube (catalog no. 2048-00150; Bellco Glass Inc., Vineland, N.J.; 2); 50 to 150 ml/160-ml serum bottle (catalog no. 223748; Wheaton Science Products, Millville, N.J.); and 250 to 500 ml/530-ml serum bottle (catalog no. 223952; Wheaton Science Products). Each tube or bottle containing medium was sealed with a solid rubber stopper and an aluminum crimp (a no. 2048-11800 stopper from Bellco Glass Inc. was used for each tube or 160-ml serum bottle [2]; a no. 1 solid black rubber stopper with one-third of the bottom portion cut off was used for each 530-ml serum bottle [5]). The gas phase of each sealed tube or bottle was exchanged with N2-CO2 (80:20, vol/vol; 2 × 104 Pa), and the medium was autoclaved. The sterile and cooled medium was inoculated by using the techniques of Balch and Wolfe (2). For studies on the effect of thiosulfate concentration on growth or for growth on sulfide, the medium was prepared without thiosulfate, and prior to inoculation, the desired amount of thiosulfate or an additional amount of Na2S was added to it from a sterile anaerobic stock. The inoculated bottles and tubes were placed in an incubator maintained at 47°C. Each culture was illuminated at 40 μmol of quanta s−1 m−2 with a fluorescent bulb (15 W; F15T18/CW; Philips) situated at a 30-cm distance from the bottles or tube.
Photobioreactor and accessories.
A 25-liter (22-liter working volume) glass bioreactor (UE206; B. Braun Biotech, Allentown, Pa.) with an inner diameter of 22 cm and fitted with three six-bladed Rushton-type turbine impellers (diameter, 10.5 cm) on the central agitator shaft was used. The bottom-most impeller was situated 11.5 cm above the vessel bottom, and the distance between two consecutive impellers was 17.8 cm. The top impeller was 12.7 cm below the liquid surface (when the vessel content was not being stirred). For certain experiments, four vertical stainless steel baffles (each 56 cm long, 2.5 cm wide, and 0.3 cm thick) were installed equally spaced inside the vessel (each at a distance of 1 cm from the wall and 0.5 cm below the liquid surface). The headspace in the bioreactor was 3.8 liters in volume. The vessel was fitted with a gel-filled sterilizable pH probe and a similar type of redox probe (Broadly James Corp., Santa Ana, Calif.) for the measurement of culture pH and redox potential, respectively, in situ. Wherever indicated, an additional redox probe was used for automatic control of the H2S flow; a controller (model 3677; Jenco Instruments Inc., San Diego, Calif.) connected to the probe and a solenoid valve (model 450156D; Bürkert Contromatic Corp., Irvine, Calif.) on the H2S supply line were used for this purpose. The culture was illuminated with a vertical array of 16 incandescent bulbs (60 W; Lumiline; General Electric). The filament (43 cm long) of each bulb was situated 6.5 cm from the inside surface of the glass vessel (wall thickness, 12 mm). The following light intensity values (in micromoles of quanta per second per square meter) were recorded inside the empty vessel: 50 at the inner surface of the vessel, 67.5 at the center of the vessel, and 81.5 at a location midway between those two locations. The culture temperature was maintained at 47°C by using heated water flow through the immersed stainless steel heat transfer coils. Each manual addition of sterile solution to the sterile medium or to the culture was performed through the rubber septum of one of the addition ports on the head plate by using either a sterile syringe fitted with 22-gauge needles or the double-needle (22 gauge) system of Baresi and Wolfe (for addition from a sealed, pressurized bottle; 3). For automatic control of culture pH, sterile and aerobic solutions of 2 M NaOH and 2 M H3PO4 were added by using the control and addition system of the bioreactor. The inoculum was transferred to the sterile medium from 530-ml serum bottles by using the double-needle system (3). Unless otherwise indicated, N2 and CO2 were supplied from the top of the vessel as overlays and H2S was bubbled from the bottom through a sparger. The N2 and CO2 streams were made oxygen free by passage through a common heated bed of copper turnings that was regenerated before use by passing H2 gas through it (2). The flow rates of gases were measured and controlled by using valved rotameters (model 641 BSV; Matheson Co., Joliet, Ill.). Each flow rate reported corresponds to a pressure of 1 atm or 1.01 × 105 Pa. The bioreactor was maintained at 5 × 103 Pa of overpressure throughout the culturing period. The cells were harvested anaerobically by using a Sharples centrifuge (type AS-14), frozen quickly in liquid nitrogen, and stored at −70°C.
Protocols for photobioreactor-scale cultivation.
For preparation of medium, all components except sodium thiosulfate, sodium bicarbonate, and sodium sulfide (whenever used) were dissolved in distilled, deionized water. The pH of this solution was usually 5.4, except that for medium in which NH4Cl was used as the sole nitrogen source (omitting NH4OOCCH3), the pH was 4.6. This solution was sterilized at 121°C for 40 min and then cooled to 47°C under N2. To this cooled medium, thiosulfate was added as an aerobic, filter-sterilized solution (∼20 ml) in water to the desired final concentration. An aerobic presterilized sodium bicarbonate solution (∼300 ml) was then added. The bicarbonate solution was prepared by autoclaving dry powder and 0.5 ml of water in a sealed 530-ml serum bottle and then dissolving the salt in sterile water added to it from another sealed bottle by using the double-needle system (3). After these additions, flows of CO2 (80 ml min−1) and H2S (where H2S was used as the reductant) to the reactor were initiated. For cultures that were raised with Na2S as the reductant and electron and sulfur source, Na2S was injected from an anaerobic and sterile stock solution (0.8 to 1.2 M) to the medium to the desired final concentration immediately after initiation of the CO2 flow, and H2S was not used. After the medium had been reduced, it was inoculated with cultures (a total of 1 liter) grown in 530-ml serum bottles. The content of each of these bottles was pressurized to 2 × 105 Pa with sterile N2 and then transferred into the photobioreactor by using the double-needle system. The strategies of maintaining the desired redox potential and supplying sulfide (H2S or Na2S) throughout the cultivation period were two of the topics addressed in this work and are discussed below. The pH of the cultures was continuously monitored and controlled at 6.8 ± 0.2.
Analytical methods.
The optical density of a culture sample at 600 nm (OD600) was measured by using a Lambda 3B dual-beam UV-visible spectrophotometer (Perkin-Elmer Corporation, Norwalk, Conn.) and a cuvette with a 1-cm light path. The cell protein content per milliliter of culture was determined as follows. The cells from each culture sample were pelleted by centrifugation at ca. 14,000 × g in a microcentrifuge and digested with 0.4 N NaOH (0.25 ml of NaOH per ml of culture centrifuged) in a boiling water bath for 15 min. Each digest was neutralized with 0.4 N HCl and assayed for protein content as described by Bradford (4) by using the dye reagent purchased from Pierce (Rockford, Ill.) and bovine serum albumin (as the standard). Since the culture samples often contained sulfur, all of the growth rate values reported below were calculated from the protein content data and not from OD600 data. The sulfide concentration in the culture liquid was determined by the methylene blue method of Pachmayr (7) as detailed by Trüper and Schlegel (10); prior to assays, the culture samples were filtered through 0.2-μm-pore-size filters to remove cells and sulfur. Light intensities were measured by using a model LI-190SB quantum sensor attached to a model LI-185B quantum/radiometer/photometer (LI-COR, Inc., Lincoln, Nebr.).
Growth of C. tepidum in tubes and bottles.
In our small-scale cultivation experiments, the methods of Balch and Wolfe (2), employing stopper-sealed serum tubes and bottles (see above), yielded consistent results. This system has been used in our laboratory for many years for the cultivation of phototrophic anaerobes (14), and it provides an alternative to the commonly used protocols (8, 11, 12). In serum tubes with 10 to 20 ml of medium 1 containing 8 mM thiosulfate, the maximum specific growth rates were about 0.23 to 0.35 h−1 (a minimum doubling time of 2 to 3 h) and the cell yields ranged from 0.21 to 0.28 mg of pellet protein ml of culture−1 (0.8 to 1.1 mg of dry cells ml of culture−1). These values were comparable to the corresponding published values (minimum doubling time, 2 h; cell yield, 1.45 mg of dry cells ml of culture−1 [12]) determined by using the conventional system (8, 11, 12). Similar to the conclusions of Wahlund et al. (12), in the stopper-sealed system, C. tepidum grew poorly when sulfide served as the sole electron source. Compared to the tubes, the bottles provided less illuminated surface area per unit of culture volume (∼4 cm2 ml of culture−1 for a tube and ∼0.7 cm2 ml−1 for a 530-ml bottle) and, consequently, lower growth rates and cell yields. However, a 500-ml culture in a 530-ml bottle provided ample volume that allowed withdrawal of samples for monitoring of growth and for analysis of culture liquid without significantly changing the growth conditions and provided sufficient cell mass (ca. 265 mg of dry cells in medium 1) that, in many cases, would be suitable for enzyme and cofactor level measurements. A collection of such bottles can also be used to generate gram quantities of cells, and both growth rates and cell yields could be improved by stirring the culture (as demonstrated for bioreactor cultures; see below).
Optimization of mixing rate and gassing mode in a photobioreactor for growth of C. tepidum.
We studied the growth of C. tepidum in medium 1 at various impeller rotational speeds in the presence and absence of baffles. The objective was to find the mixing conditions that maximize the exposure of cells to light at a fixed illumination without exceeding their limit of shear tolerance. The best growth rate was achieved at 300 rpm in the presence of baffles, (impeller Reynolds no. [NRe], 55,125; impeller tip speed, 165 cm s−1) and at 500 rpm in the absence of baffles (NRe, 91,875; impeller tip speed, 275 cm s−1). An impeller rotational speed of 500 rpm with baffles or 700 rpm (NRe, 128,625; impeller tip speed, 385 cm s−1) with or without baffles caused cell lysis. Hence, all subsequent experiments were carried out in the presence of baffles at an impeller speed of 300 rpm. The NRe values were calculated from the relationship NRe = [(revolutions per second)(impeller diameter in centimeters)2(density of culture liquid in grams per cubic centimeter)]/(viscosity of culture liquid in grams per centimeter per second) and by assuming the density and viscosity of the culture liquid to be 1 g cm−3 and 0.01 g cm−1 s−1, respectively. Each NRe value reported here corresponded to a turbulent mixing regimen (9). The relative shear rates were judged from the values of impeller tip speed, which is given by (π)(impeller diameter)(impeller rotational speed).
In our early experiments, we observed that the cultures foamed when all gases were supplied by sparging at the bottom. We found that overlaying the culture with N2 (to maintain a positive pressure of 5 × 103 Pa in the vessel) and CO2 (a component of the buffer system) instead of sparging not only eliminated the foaming problem but also improved the growth rate and cell yield (data not shown). Thus, unless otherwise mentioned, for rest of the study, N2 and CO2 were supplied as overlays. To facilitate dissolution of H2S, we continued to supply this gas (N2-H2S, 90:10, vol/vol) by sparging.
Growth of C. tepidum on thiosulfate in a photobioreactor.
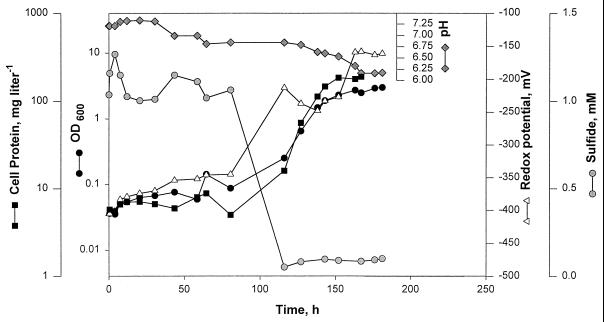
For growth on thiosulfate in the photobioreactor, we used H2S as the obligatory sulfide source (12) and medium reductant. Our preliminary experiments showed that with thiosulfate (in medium 1 with or without acetate), C. tepidum did not grow well or at all if the H2S flow rate was such that the culture redox potential dropped below −330 mV. The following protocol for H2S supply alleviated this problem and provided the highest growth rate and final cell yield for a given starting thiosulfate concentration. After the introduction of CO2 to the pH-adjusted and bicarbonate- and thiosulfate-containing sterilized medium (see above), the redox potential of the medium was lowered to −320 mV by using manually controlled H2S flow to the vessel. The H2S flow control system (see above) was then brought into action to maintain the culture redox potential at −300 ± 20 mV, and the culture was inoculated. The control on the H2S flow remained in effect for the entire cultivation period, and the culture was continuously overlaid with N2 and CO2 at flow rates of 560 and 80 ml min−1, respectively. Under these conditions, the optimal thiosulfate concentration for the growth of C. tepidum was found to be 12 mM and the corresponding maximum specific growth rate and final cell yield were, respectively, 0.22 h−1 and 376 mg of cell pellet protein liter of culture−1. Figure 1 shows the parameter profiles for such a culture. Regardless of the starting concentration of thiosulfate used (4, 8, 12, or 16 mM), at the early stage of culture, the changes in OD600 and cell pellet protein content did not follow the same pattern. This incongruence was due to the accumulation of sulfur, which occurred in significant amounts starting almost immediately after inoculation, as the cells rapidly oxidized sulfide and/or thiosulfate to sulfur. From about 30 h of culture age, addition of NaOH was needed to maintain the culture pH at 6.8. This consumption was due to the liberation of protons accompanying the oxidation of reduced sulfur species. Also, from about this time, sulfur was no longer visible in cell pellets, except in those from cultures started with 16 mM thiosulfate, where sulfur was present for the entire cultivation period.
FIG. 1.
Parameter profiles in a photobioreactor culture of C. tepidum with thiosulfate (12 mM) as the preferred electron source. Medium 1 with 12 mM thiosulfate was used for this culture. H2S gas was used primarily as the medium reductant and the obligatory required reduced-sulfur source. It was supplied by using the following strategy. Before inoculation, the redox potential of the medium was lowered to −320 mV by using manually controlled flow of H2S into the vessel. From then on, the H2S flow rate was regulated by a control system with a set point of −300 ± 20 mV for the culture redox potential. NaOH (2 M) was added via a control system to maintain the pH at 6.8 ± 0.2.
In an autotrophic culture started with 12 mM thiosulfate, the maximum specific growth rate was 0.15 h−1, whereas the corresponding value with 6.5 mM acetate (medium 1) in the medium was 0.22 h−1 (see above). However, similar to the observations of Wahlund et al. (12), with a non-growth-limiting level of thiosulfate (12 mM), elimination of acetate from the medium did not affect the final cell yield. If the concentrations of MgSO4, EDTA, cyanocobalamin, and minerals (added as a 100× mineral solution; 5) in medium 1 were doubled, the cell yield improved substantially (562 mg of cell protein liter−1 for the modified medium compared to 376 mg of cell protein liter−1 for unmodified medium 1), and it corresponded to 3.35 g of packed wet cells and 0.94 g of dry cells per liter.
Growth of C. tepidum on sulfide in a photobioreactor.
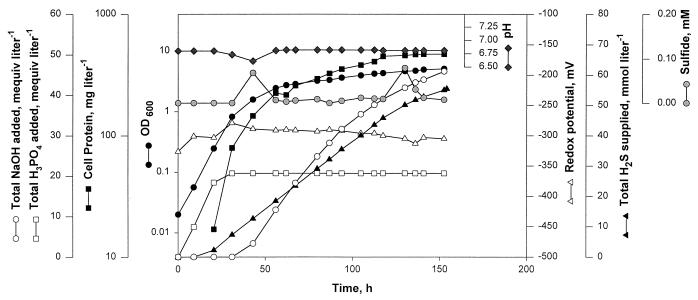
It was reported previously (12) that C. tepidum grows poorly with sulfide, the preferred electron source for all other members of the family Chlorobiaceae (11). We describe here the results from three experiments that provided the reason for this unusual observation with a green sulfur bacterium and established the conditions under which this organism would grow on sulfide as well as it does on thiosulfate. These experiments were carried out by using medium 1 devoid of added thiosulfate. In experiment 1, we used sodium sulfide as the sulfide source. The sterilized anaerobic medium was placed under a headspace of N2-CO2 (80:20; 3 × 104 Pa), the exhaust line of the vessel was closed, sodium sulfide was added to the medium to a final concentration of 2.5 mM (calculated based on the amount of sulfide added and the medium volume and not considering that some sulfide will escape into the headspace), and then the culture was inoculated. Under these conditions, no growth occurred, but the culture slowly bleached to a brown color and the culture redox potential remained below −350 mV (Fig. 2). At 80 h of culture age, in an attempt to flush out excess sulfide from the culture, the vessel exhaust was opened, and N2-CO2 was allowed to pass through the headspace at a rate of 750 ml min−1. The result was a drop in the dissolved-sulfide level which was coincident with a rise in redox potential, return of the culture to a green color, and the onset of growth (Fig. 2). In experiment 2, we added sodium sulfide as in experiment 1, but from a culture age of 1.5 h, the vessel exhaust line was left open and N2 and CO2 were allowed to flow into the vessel as overlays at rates of 560 and 80 ml min−1, respectively. As a result, the sulfide level in the culture remained below 0.1 mM and the redox potential remained above −330 mV. These conditions permitted growth of C. tepidum at a rate and with a final cell yield (data not shown) similar to those recorded with 12 mM thiosulfate as the electron source, as shown in Fig. 1. Experiment 3 provided the best cell yield with sulfide as the sole electron source (Fig. 3). Here, H2S served as the sulfide source, and it was supplied continuously to the culture by using the control system described above and a culture redox potential set point of −300 ± 20 mV (Fig. 3). Also, the pH of the culture was controlled at 6.8 ± 0.2. In such a setup, at the very early age of the culture, dissolved sulfide was rapidly converted into sulfur, which was seen in pellets from centrifugation of culture samples. This conversion led to alkalinization of the medium, which was reflected in the consumption of acid for maintenance of the medium pH at 6.8 (Fig. 3). At the later stage, the accumulated sulfur (presumably along with sulfide that was supplied continuously) was further oxidized, and this conversion led to liberation of protons and consumption of base for pH control (Fig. 3). Throughout this culturing period, the sulfide level in the liquid remained very near the limit of detection of the assay method (the methylene blue method) used in this work (7, 10). From the results of these three experiments, we concluded that C. tepidum would grow with sulfide as the sole electron source at a rate and with a final cell yield comparable to those found when thiosulfate serves as the electron source. However, to achieve these results, the culture redox potential must be maintained at about −300 mV and the sulfide level must be below 0.1 mM. In this context, it should be noted that the methylene blue method for determination of the sulfide concentration (7, 10) is not very sensitive at a level of ∼0.1 mM; it has a maximum error range of ±0.06 mM (10). Experiment 3 established the best conditions for growth of C. tepidum on sulfide. Experiment 2 provided a simpler alternative, where Na2S was added manually as the sulfide source.
FIG. 2.
Parameter profiles in a photobioreactor culture of C. tepidum with sodium sulfide (2.5 mM) added as the sole electron source at the start and no gas flow through the vessel until 80 h of culture age. Medium 1 without thiosulfate was used for this culture. Before inoculation, the sterilized anaerobic medium was placed under a headspace of N2-CO2 (80:20; 3 × 104 Pa), the vessel exhaust was closed, and sodium sulfide was added to the medium. At 80 h of culture age, the vessel exhaust was opened and N2-CO2 flow at a rate of 750 ml min−1 (as an overlay) was initiated. Except for the first few hours, in the period until 80 h of culture age, the culture was brown in color. The culture pH was not controlled. See the legend to Fig. 1 for other details.
FIG. 3.
Parameter profiles in a photobioreactor culture of C. tepidum with a regulated flow of H2S as the sole electron source. Medium 1 without thiosulfate was used for this culture. Before inoculation, the redox potential of the medium was lowered to −320 mV by using manually controlled H2S flow to the vessel. From then on, the H2S flow was regulated by a control system set to maintain the culture redox potential at −300 ± 20 mV. For pH control, 2 M H3PO4 and 2 M NaOH were used. See the legend to Fig. 1 for other details.
At small scales using the conventional systems (8, 11, 12), C. tepidum grows in tubes and bottles with sulfide as the electron source, albeit poorly (12), and the conclusion for our stopper-sealed system was similar, whereas in a well-monitored and -controlled closed system (see experiment 1 above), the organism did not grow with sulfide. Such a system even affected the growth on thiosulfate in the presence of sulfide as an obligatory reduced-sulfur source. It is possible that the protocols for preparing medium for bottles and tubes (those described in this work or those of others [8, 11, 12]) would allow substantial loss of sulfide as H2S at a medium pH of 6.8 (the pKa for H2S/HS− is 7.04 [1]), hence the growth of the organism in medium prepared in such a way.
In most of our reactor-scale growth experiments, we observed that the first logarithmic phase was followed by another with a much-reduced growth rate. The data in Fig. 1 show that the culture switched from a logarithmic growth rate of 0.22 to 0.03 h−1 at about 20 h. This change was most likely due to light limitation in a turbid culture and suggests the scope of further improvement in the growth rate, the length of the fast growth phase, and the cell yield. This improvement could be brought about by optimizing the amount of light received by each cell, which, in turn, depends on two factors: (i) the hydrodynamics in the reactor (see above) and (ii) the quantum flux (micromoles per second) delivered to the culture. By using a commercially available reactor, we have optimized the first factor, and the reported values for NRe and impeller tip speed would be useful in scale-up efforts or in developing protocols for getting the most out of an available system. This approach also provides a more homogeneous population of cells in a culture in terms of the extent of exposure to light. Our work has set the stage for optimization of the second factor.
In summary, we have described an alternative method for small-scale growth of C. tepidum; developed protocols for reactor-scale cultivation of this phototroph under defined, monitored, and controlled chemical and physical conditions; and put forward an explanation for the previously documented behavior of C. tepidum toward sulfide (12), the most common substrate for members of the family Chlorobiaceae (11). Our data would be very useful in designing careful experiments intended to explain the physiological properties of C. tepidum, in generating larger quantities of cells with better-defined characteristics for studying the biochemical and biophysical aspects of this organism, for optimization of the methods for the removal of H2S from gases that employ chlorobia (6), and in efforts to scale up the culture of C. tepidum in larger reactors.
Acknowledgments
We thank Lien Ly and Cindy Kreder for excellent technical assistance and Ralph S. Wolfe for support, encouragement, and comments on the manuscript. We thank M. T. Madigan for the gift of a culture of C. tepidum.
This work was supported by Department of Energy grant DE-FG02-87ER13651 to Ralph S. Wolfe. M.A. received a Deboer Summer Undergraduate Research Fellowship from the Department of Microbiology, University of Illinois at Urbana-Champaign. All reactor culture experiments were conducted at the Department of Microbiology Fermentor Facility, University of Illinois at Urbana-Champaign.
REFERENCES
- 1.Appleby C A. Inhibitors of respiratory enzymes, photosynthesis and phosphorylation: uncoupling reagents. In: Dawson R M C, Elliott D C, Elliott W H, Jones K M, editors. Data for biochemical research. 2nd ed. London, England: Oxford University Press; 1969. pp. 380–387. [Google Scholar]
- 2.Balch W E, Wolfe R S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol. 1976;32:781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baresi L, Wolfe R S. Levels of coenzyme F420, coenzyme M, hydrogenase, and methylcoenzyme M methylreductase in acetate-grown Methanosarcina. Appl Environ Microbiol. 1981;41:388–391. doi: 10.1128/aem.41.2.388-391.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Daniels L, Belay N, Rajagopal B S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol. 1986;51:703–709. doi: 10.1128/aem.51.4.703-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen A B, Webb C. Treatment of H2S containing gases: a review of microbiological alternatives. Enzyme Microb Technol. 1995;17:2–10. [Google Scholar]
- 7.Pachmayr F. Vorkommen und Bestimmung von Schwefelverbindungen in Mineralwasser. Ph.D. thesis. Munich, Germany: University of Munich; 1960. [Google Scholar]
- 8.Pfennig N, Trüper H G. The family Chromatiaceae. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3200–3221. [Google Scholar]
- 9.Rushton J H, Costich E W, Everett H J. Power characteristics of mixing impellers. Part 2. Chem Eng Prog. 1950;46:467–476. [Google Scholar]
- 10.Trüper H G, Schlegel H G. Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii. Antonie Leeuwenhoek. 1964;30:225–238. doi: 10.1007/BF02046728. [DOI] [PubMed] [Google Scholar]
- 11.Trüper H G, Pfennig N. The family Chlorobiaceae. In: Balows A, Trüper H G, Dworkin M, Harder W, Schleifer K-H, editors. The prokaryotes. 2nd ed. New York, N.Y: Springer-Verlag; 1992. pp. 3583–3592. [Google Scholar]
- 12.Wahlund T M, Woese C R, Castenholz R W, Madigan M T. A thermophilic green sulfur bacterium from New Zealand hot springs, Chlorobium tepidum sp. nov. Arch Microbiol. 1991;156:81–90. [Google Scholar]
- 13.Wahlund T M, Madigan M T. Genetic transfer by conjugation in the thermophilic green sulfur bacterium Chlorobium tepidum. J Bacteriol. 1995;177:2583–2588. doi: 10.1128/jb.177.9.2583-2588.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wolfe, R. S. Personal communication.