Abstract
The presence, but not expression, of homologs of three structural genes and a regulatory gene necessary for aflatoxin biosynthesis in Aspergillus parasiticus and A. flavus was shown for A. oryzae and A. sojae. Homologs of the regulatory gene aflR were cloned and sequenced from A. oryzae and A. sojae.
Although aflatoxin biosynthesis has been documented for Aspergillus flavus and A. parasiticus (14), the closely related species A. oryzae and A. sojae, used in food and ingredient manufacture, have no history of producing aflatoxins. Despite this lack of aflatoxin production, Woloshuk et al. (16) reported the presence of the aflatoxin pathway regulatory gene, aflR, in single strains of A. oryzae and A. sojae. Klich et al. (8, 9) reported the presence of aflR and omtA in several strains of A. oryzae and A. sojae, and Chang et al. (4) reported sequence variability of part of aflR in strains of A. parasiticus, A. flavus, A. oryzae, and A. sojae.
Our objectives were (i) to determine the presence and expression of several genes involved in aflatoxin biosynthesis in strains of A. parasiticus, A. oryzae, and A. sojae and (ii) to clone the gene encoding the A. oryzae homolog of aflR, the transcriptional regulator of the aflatoxin and sterigmatocystin biosynthesis gene clusters (1, 3, 18).
For both DNA and RNA preparation, fungi were grown in 100 ml of YES medium (15% sucrose, 5% yeast extract) in 250-ml conical flasks with shaking at 150 rpm and 25°C. Genomic DNA was prepared from freeze-dried ground mycelium either by multiple phenol extractions followed by cesium chloride gradient centrifugation or by use of a DNeasy extraction kit (Qiagen, Crawley, United Kingdom). Total RNA was isolated with an RNeasy plant RNA isolation kit (Qiagen) according to the manufacturer’s instructions.
Genomic DNA for Southern blotting was digested with EcoRI and transferred onto a Hybond-N+ membrane (Amersham International, High Wycombe, United Kingdom) under vacuum. For Northern blot analysis, 10 μg of total RNA was electrophoresed through a 1% formaldehyde (2.2 M)–MOPS (3-[N-morpholino]-propanesulfonic acid) gel at <3 V/cm against an RNA standard (Life Technologies, Paisley, United Kingdom) by standard protocols (11). The gel was washed through five changes of diethyl pyrocarbonate-treated H2O before transfer onto a Hybond-N+ membrane under vacuum. Probes used to analyze both Southern and Northern blots are detailed below. All of the probes were labeled with [32P]dATP with a Megaprime labeling kit (Amersham International) according to the manufacturer’s instructions. The nor-1 probe used was a 700-bp PstI/ClaI fragment produced by restriction of plasmid pNA17 (2), while the ver-1 probe was a 600-bp SacI/KpnI fragment isolated after restriction of plasmid pBSV2 (12). Probes for omtA (739 bp, coordinates +318 to +1056) and aflR (813 bp, coordinates +436 to +1248) were made by PCR amplification of portions of the genes with primers designed by reference to previously published sequences (3, 17). A 736-bp fragment corresponding to coordinates +727 to +1463 of the A. nidulans γ-actin gene (7) was used as a probe to normalize RNA loadings and confirm transfer across all lanes blotted. Prehybridization and hybridization of blots were carried out by standard protocols (11). Blots were hybridized at 65°C overnight and then washed twice in 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–1% sodium dodecyl sulfate for 10 min and once in 0.1× SSC–1% sodium dodecyl sulfate for 30 min at 65°C. Detection was with X-ray film (Fuji) or a Fuji BAS-1500 phosphorimager.
A genomic library of partial EcoRI-cut A. oryzae ATCC 14895 DNA was constructed in a λZapII vector and packaged with Gigapack Gold II packaging extract. PCR primers designed from the A. parasiticus aflR gene (3) were used to amplify a fragment of the predicted size from A. oryzae ATCC 14895 genomic DNA. aflR homology was verified by sequencing before the fragment was used to probe the A. oryzae library. Fifty thousand plaques were screened by hybridization after transfer onto a Hybond-N+ membrane.
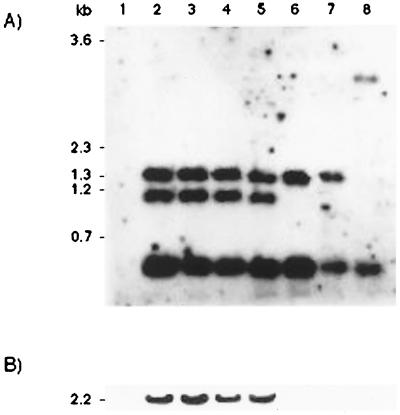
Results from the analysis with the aflR probe are shown in Fig. 1. Southern blot analysis (Fig. 1A) clearly showed the presence of sequences homologous to aflR in strains of A. parasiticus, A. oryzae, and A. sojae. In some strains, e.g., A. parasiticus ATCC 24690, three bands can be observed; those at 0.56 and 1.33 kb represent the predicted signals. The band at 1.14 kb is possibly due to a gene duplication in which, in one gene copy, an EcoRI* (star activity) site between the stop codon and the normal EcoRI site has mutated into an authentic EcoRI site. Quantification of signals from strains possessing this extra band supports this hypothesis, although differences in fragment transfer efficiencies mean that the data are not conclusive (data not shown). A. oryzae ATCC 16507 appears to have lost the common EcoRI site downstream of the aflR stop codon altogether. Results of the complete Southern blot analysis with all probes are summarized in Table 1. Sequences homologous to the aflatoxin biosynthesis genes nor-1, ver-1, and omtA were found in A. oryzae and A. sojae as well as in A. parasiticus. The identity of all these hybridization signals, like, those for aflR, was confirmed for A. oryzae ATCC 14895 and A. sojae ATCC 42251 by sequencing PCR fragments bearing DNA encoding 60 to 90% of all three gene products. In every case, identities of >95% were obtained with the A. parasiticus sequence. The presence of homologs of both omtA and aflR in the A. oryzae strains examined is in contrast to hybridization data obtained with three A. oryzae strains by Klich et al. (8). This difference may merely reflect the use of different strains in each study. However, using a range of primer pairs, Chang et al. (4) showed by PCR that aflR homologs exist in at least two of the A. oryzae strains used by Klich et al. (8), the third not having been tested; their study also showed that generation of PCR products from A. oryzae was dependent on strain type and the primer pair used. No attempt to reduce the stringency of the PCR cycling conditions in order to obtain products was reported. Given the sensitivity of PCR to the degree and positioning of base mismatches within primers, the most effective means of assessing sequence variability between different members of the Aspergillus Section Flavi is to sequence PCR fragments generated from the conserved coding region as has been done here. The genome of A. niger is the only one of those examined here which contains no homologs to genes encoding proteins involved in aflatoxin biosynthesis. This result agrees with the data of Kozlowski and Stepien (10), which suggests that A. niger has diverged significantly from the other species analyzed. Actin signals on all blots provided a positive control for all hybridizations (data not shown).
FIG. 1.
Southern and Northern blot analyses of Aspergillus strains with an aflR probe. (A) Southern blot analysis of EcoRI-cut genomic DNAs from eight strains of Aspergillus. Lanes: 1, A. niger ATCC 9029; 2, A. parasiticus ATCC 24690; 3, A. parasiticus ATCC 36537; 4, A. parasiticus ATCC 56774; 5, A. parasiticus ATCC 56775; 6, A. oryzae ATCC 14895; 7, A. sojae ATCC 42251; 8, A. oryzae ATCC 16507. (B) Northern blot analysis of the same strains.
TABLE 1.
Presence and expression of genes for aflatoxin biosynthesis in Aspergillus spp.
| Strain | Presence of:
|
Expression of:
|
||||||
|---|---|---|---|---|---|---|---|---|
| nor-1 | ver-1 | omtA | aflR | nor-1 | ver-1 | omtA | aflR | |
| A. niger ATCC 9029 | − | − | − | − | − | − | − | − |
| A. parasiticus ATCC 24690 | + | + | + | + | + | + | + | + |
| A. parasiticus ATCC 36537 | + | + | + | + | + | + | + | + |
| A. parasiticus ATCC 56774 | + | + | + | + | + | + | + | + |
| A. parasiticus ATCC 56775 | + | + | + | + | + | + | + | + |
| A. oryzae ATCC 14895 | + | + | + | + | − | − | − | − |
| A. sojae ATCC 42251 | + | + | + | + | − | − | − | − |
| A. oryzae ATCC 16507 | + | + | + | + | − | − | − | − |
Expression of aflatoxin genes is, however, confined to the A. parasiticus strains under the culture conditions used (Fig. 1B). Northern blot analyses of nor-1, ver-1, and aflR revealed transcript sizes that correlate well with those previously described for these sequences in A. parasiticus and A. flavus (13, 15, 16). No transcripts for any of the probes tested were seen in any of the A. oryzae or A. sojae strains examined (Table 1). These results were confirmed for all eight strains by reverse transcription-PCR from total RNA with primers specific for omtA, nor-1, and ver-1 transcripts. Reverse transcription-PCR products were observed only from those A. parasiticus strains tested (data not shown). Since there has never been any record of aflatoxin production in A. oryzae or A. sojae, it seems unlikely that transcription of the genes was occurring. Recently, however, Klich et al. (9) have shown evidence for the transcription of aflR and uvm8 in certain strains of A. sojae. Their findings are consistent with our data, since they also found that A. sojae ATCC 42251 (=SRRC 1126) produced no aflR transcript. We have not tested their A. sojae strains which produced transcript. These data also suggest that a nonaflatoxigenic phenotype may develop by more than one mechanism as Cotty and Bhatnagar (6) have already hypothesized. With strains in which no transcription of any of the genes can be found it is likely that the overall cause of the nonaflatoxigenic phenotype is a regulatory malfunction, possibly at the level of the positive regulator aflR.
The translated gene sequences of aflR from A. flavus, A. parasiticus, and A. oryzae are virtually identical (Fig. 2). The similarity between the A. flavus, A. parasiticus, and A. nidulans AFLR homologs has been noted previously (4, 18). Also, the similarity of short PCR-derived fragments of aflR homologs from A. oryzae, A. sojae, A. flavus, and A. parasiticus has been reported (4) although a full sequence of an AFLR homolog from A. oryzae or A. sojae has not previously been published.
FIG. 2.
Sequence comparison of the C termini of the AFLR homologs translated from GenBank database entries for A. flavus (L32576 [16]) and A. parasiticus (L26220 [5]) with the A. oryzae ATCC 14895 (A. oryzae 2; Y16967 [this paper]) sequence. Sequence data from PCR fragments obtained from A. oryzae CBS 108.24 (A. oryzae 1) and A. sojae ATCC 42551 are also included. The A. parasiticus AFLR sequence is modified to correct a sequencing error at base 1121, codon 378; the A. flavus AFLR is corrected at two sites as outlined in the text. Translation of the A. oryzae ATCC 14895 and A. sojae ATCC 42551 aflR sequences beyond their predicted stop codons is included for comparative purposes.
The sequence from A. oryzae has two interesting features. Unlike the AFLR proteins from both A. flavus and A. parasiticus, the A. oryzae protein has extra histidine and alanine residues at coordinates 111 and 112 to produce a HAHA motif. This motif is also seen in a PCR-derived fragment of the aflR homolog from A. sojae ATCC 42251 (unpublished data). In addition, there are potentially important differences at the C termini. The A. oryzae AFLR is truncated by ca. 60 residues because the AGA codon, which encodes Arg383 in both A. flavus and A. parasiticus, has been mutated to a stop codon through an A→T transversion (Fig. 2). Since no frameshift occurs, translation of the sequence beyond the stop codon continues the identity of the A. oryzae AFLR homolog with those from A. flavus and A. parasiticus. The A. sojae AFLR homolog sequence is also truncated at this point through an identical mutation (data not shown). Although the published sequence (5) of the A. parasiticus aflR gene suggests that its gene product is truncated in comparison to that of the A. flavus gene, our sequence data from A. parasiticus ATCC 56775 indicate that a C had been omitted from position 1121 of the published sequence (5), introducing a frameshift and a premature stop codon. Our sequence data also show a Val residue at position 388 in the A. parasiticus AFLR protein, in line with the A. flavus and A. oryzae aflR gene products, rather than the Ala in the published sequence (5). When these corrections are included, the revised C-terminal sequence is almost identical to that of the published A. flavus AFLR sequence with the exception of the last few residues (16). We have also sequenced two separate type strains of A. flavus, CBS 110.55 and CBS 485.65, and our results indicate that both aflR genes have an extra C residue between bases 1693 and 1694 and also 1704 and 1705 compared to the published A. flavus aflR sequence (16). Translation of this amended sequence provides a carboxyl terminus identical to that of the A. parasiticus AFLR (Fig. 2). The stop codon we found in the A. oryzae aflR homolog has been confirmed by repeated sequencing in both directions with two independently acquired cultures from the American Type Culture Collection. Thus, the aflR sequences from A. oryzae, A. flavus, and A. parasiticus are virtually identical but the A. oryzae aflR has an amber mutation at Arg 383 which, upon translation, would give rise to a truncated protein.
We do not yet know if the truncation affects the functionality of the protein. AFLR is thought to up-regulate its own expression through a GAL4-type binuclear zinc finger DNA-binding domain (residues 29 to 56) and an acid patch (residues 349 to 380 [5]). However, the amber mutation lies downstream of both proposed domains in the A. oryzae and A. sojae isolates we have tested. This finding suggests that either the carboxyl-terminal region is required for AFLR function or there is a second mutation outside the coding sequence which prevents transcription from the aflR locus. Our data do not allow us to distinguish between these possibilities.
Comparison of the A. oryzae ATCC 14895 aflR sequence with data from strains of A. oryzae, A. sojae, A. flavus, and A. parasiticus suggests that this strain may be a misclassified strain of A. sojae. Of over 25 strains we have examined, the HAHA duplication and premature stop codon appear to be linked and confined to aflR sequences from strains of A. sojae (data not shown). The exceptions to this rule are the aflR sequence from A. oryzae ATCC 14895 presented here (Fig. 2) and an A. parasiticus strain, CBS 126.49, which is used in the miso brewing industry and is nonaflatoxigenic in our hands.
In conclusion, the presence, but not expression, of four genes necessary for aflatoxin biosynthesis has been shown for strains of A. oryzae and A. sojae. The entire aflR gene from A. oryzae ATCC 14895 has also been cloned and sequenced. On the basis of sequence comparison of aflR genes from A. oryzae and A. sojae strains, A. oryzae ATCC 14895 clusters with A. sojae rather than with A. oryzae. In addition, corrections to published sequences for the aflR genes from A. parasiticus and A. flavus have revealed a greater degree of sequence similarity between the four AFLR proteins than has previously been reported.
Nucleotide sequence accession number.
The DNA sequence of the A. oryzae ATCC 14895 aflR gene, which encodes a 384-amino-acid protein, can be obtained from GenBank (accession no. Y16967).
Acknowledgments
This research was supported by a grant from the Department for International Development of the United Kingdom. The project (R6772) was funded under the Crop Post-Harvest research program.
We thank John Linz (University of Michigan) for the kind gift of plasmids pNA17 and pBSV2 and Susan Seal, Ray Coker, and Peter Wareing (NRI) for discussions.
REFERENCES
- 1.Brown D W, Yu J-H, Kelkar H S, Fernandes M, Nesbitt T C, Keller N P, Adams T H, Leonard T J. Twenty-five coregulated transcripts define a sterigmatocystin gene cluster in Aspergillus nidulans. Proc Natl Acad Sci USA. 1996;93:1418–1422. doi: 10.1073/pnas.93.4.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang P-K, Skory C D, Linz J E. Cloning of a gene associated with aflatoxin B1 biosynthesis in Aspergillus parasiticus. Curr Genet. 1992;21:231–233. doi: 10.1007/BF00336846. [DOI] [PubMed] [Google Scholar]
- 3.Chang P-K, Cary J W, Bhatnagar D, Cleveland T E, Bennett J W, Linz J E, Woloshuk C P, Payne G A. Cloning of the Aspergillus parasiticus apa-2 gene associated with the regulation of aflatoxin biosynthesis. Appl Environ Microbiol. 1993;59:3273–3279. doi: 10.1128/aem.59.10.3273-3279.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang P-K, Bhatnagar D, Cleveland T E, Bennett J W. Sequence variability in homologs of the aflatoxin pathway gene aflR distinguishes species in the Aspergillus Section Flavi. Appl Environ Microbiol. 1995;61:40–43. doi: 10.1128/aem.61.1.40-43.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang P-K, Ehrlich K C, Yu J, Bhatnagar D, Cleveland T E. Increased expression of Aspergillus parasiticus aflR, encoding a sequence-specific DNA-binding protein, relieves nitrate inhibition of aflatoxin biosynthesis. Appl Environ Microbiol. 1995;61:2372–2377. doi: 10.1128/aem.61.6.2372-2377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotty P J, Bhatnagar D. Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl Environ Microbiol. 1994;60:2248–2251. doi: 10.1128/aem.60.7.2248-2251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fidel S, Doonan J H, Morris N R. Aspergillus nidulans contains a single actin gene which has unique intron locations and encodes a γ-actin. Gene. 1988;70:283–293. doi: 10.1016/0378-1119(88)90200-4. [DOI] [PubMed] [Google Scholar]
- 8.Klich M A, Yu J, Chang P-K, Mullaney E J, Bhatnagar D, Cleveland T E. Hybridisation of genes involved in aflatoxin biosynthesis to DNA of aflatoxigenic and non-aflatoxigenic aspergilli. Appl Microbiol Biotechnol. 1995;44:439–443. doi: 10.1007/BF00169941. [DOI] [PubMed] [Google Scholar]
- 9.Klich M A, Montalbano B, Ehrlich K. Northern analysis of aflatoxin biosynthesis genes in Aspergillus parasiticus and Aspergillus sojae. Appl Microbiol Biotechnol. 1997;47:246–249. [Google Scholar]
- 10.Kozlowski M, Stepien P P. Restriction enzyme analysis of mitochondrial DNA of members of the genus Aspergillus as an aid in taxonomy. J Gen Microbiol. 1982;128:471–476. doi: 10.1099/00221287-128-3-471. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 12.Skory C D, Chang P-K, Cary J, Linz J E. Isolation and characterization of a gene for Aspergillus parasiticus associated with the conversion of versicolorin A to sterigmatocystin in aflatoxin biosynthesis. Appl Environ Microbiol. 1992;58:3527–3537. doi: 10.1128/aem.58.11.3527-3537.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skory C D, Chang P-K, Linz J E. Regulated expression of the nor-1 and ver-1 genes associated with aflatoxin biosynthesis. Appl Environ Microbiol. 1993;59:1642–1646. doi: 10.1128/aem.59.5.1642-1646.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trail F, Mahanti N, Linz J. Molecular biology of aflatoxin biosynthesis. Microbiology. 1995;141:755–765. doi: 10.1099/13500872-141-4-755. [DOI] [PubMed] [Google Scholar]
- 15.Trail F, Mahanti N, Rarick M, Mehigh R, Liang S-H, Zhou R, Linz J E. Physical and transcriptional map of an aflatoxin gene cluster in Aspergillus parasiticus and functional disruption of a gene involved early in the aflatoxin pathway. Appl Environ Microbiol. 1995;61:2665–2673. doi: 10.1128/aem.61.7.2665-2673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woloshuk C P, Foutz K R, Brewer J F, Bhatnagar D, Cleveland T E, Payne G A. Molecular characterization of aflR, a regulatory locus for aflatoxin biosynthesis. Appl Environ Microbiol. 1994;60:2408–2414. doi: 10.1128/aem.60.7.2408-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Cary J W, Bhatnagar D, Cleveland T E, Keller N P, Chu F S. Cloning and characterization of a cDNA from Aspergillus parasiticus encoding an O-methyltransferase involved in aflatoxin biosynthesis. Appl Environ Microbiol. 1993;59:3564–3571. doi: 10.1128/aem.59.11.3564-3571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu J-H, Butchko R A E, Fernandes M, Keller N P, Leonard T J, Adams T H. Conservation of structure and function of the aflatoxin regulatory gene aflR from Aspergillus nidulans and A. flavus. Curr Genet. 1996;29:549–555. doi: 10.1007/BF02426959. [DOI] [PubMed] [Google Scholar]