Abstract
Carbon isotope distribution of [13C]citrinin from Monascus ruber incubated with [13C]acetate revealed that the biosynthesis of the toxin originated from a tetraketide, instead of a pentaketide as has been shown for Penicillium and Aspergillus species. The production of polyketide red pigments and citrinin by M. ruber may therefore be regulated at the level of the tetraketide branch point.
In filamentous fungi, the polyketide pathway is the major route for the formation of secondary metabolites (4, 16), including various mycotoxins (15). Citrinin is a typical toxin, isolated first in Penicillium citrinum and later in Aspergillus species (6) and Monascus ruber (3). It exhibits antibiotic activity against gram-positive bacteria, but its nephrotoxic properties prevent its use as a therapeutic drug (14). The production of citrinin together with red pigments (named “Anka”) rules out the use of M. ruber (3) as a producer of natural colorants for food technology (8). However, the fact that other fungi, like Aspergillus and Penicillium, do not produce these pigments but do synthesize mycotoxins suggests possible variations in the biosynthetic pathway of citrinin in M. ruber. It is known that in Aspergillus, citrinin is formed by the condensation of one acetyl coenzyme A (acetyl-CoA) molecule with four malonyl-CoA molecules, followed by the addition of three methyl units (2, 5, 9). We have therefore investigated the biosynthetic pathway of citrinin in M. ruber by 13C nuclear magnetic resonance (NMR) analysis of carbon isotope distribution of 13C-enriched citrinin after feeding the cultures with 13C-labeled acetate, and we found that this pathway is different from the one previously identified in Aspergillus terreus. This finding may lead to new strategies to selectively control the production of pigments and uncouple the formation of them from that of citrinin.
A strain of M. ruber (ATCC 96218) was grown at 28°C in a chemically defined medium as described previously (8). A suspension of 108 spores was used to inoculate a 1-liter baffled Erlenmeyer flask containing 200 ml of glucose medium. The presence of red pigments and citrinin in the culture broth was determined spectrophotometrically as described previously (3, 8). For the 13C labeling of citrinin, 1 ml of an aqueous solution (99.2% enriched with [1-13C] [2-13C]sodium acetate [20 mg · ml−1] or 98.6% enriched with [1,2-13C]sodium acetate [10 mg · ml−1]) was added after 3, 4, 5, 6, and 7 days to a 200-ml culture. Citrinin was isolated from the medium by filtration of the mycelium cultures on M14 membranes (pore size of 0.8 μm; Tech-sep, Bollene, France). The filtrate was lyophilized, resuspended in 60 ml of water, and extracted three times with water saturated with n-butanol. The organic phase was dried and vacuum concentrated, and the residue was dissolved in 50 ml of acidified water (pH 2.0). This solution was treated twice with 120 ml of ethyl acetate, and the retained organic phase was extracted twice with 150 ml of 0.4% NaHCO3. The aqueous phase was adjusted to a pH of 3.0 with HCl and again extracted twice with 120 ml of ethyl acetate. The organic phase containing citrinin was evaporated to dryness and resuspended in a minimal volume of water. The toxin was isolated by thin-layer chromatography in chloroform-methanol-water (65/25/4, vol/vol/vol), and the band containing the toxin was solubilized in chloroform which was evaporated. 13C and 1H NMR spectra were recorded on a Bruker ARX 400-MHz spectrophotometer with CDCl3 (99.6%) as a solvent. Spectra were referenced internally to the solvent for 13C NMR and to trimethylsilyl for 1H NMR. Minimization of the relative molecular energy of intermediates 2 and 3 (see Fig. 2) was carried out with the molecular mechanics programs Biosym and Discover (version 2.9.7-95.0-3.0.0 cvff force field) on a Silicon Graphics machine.
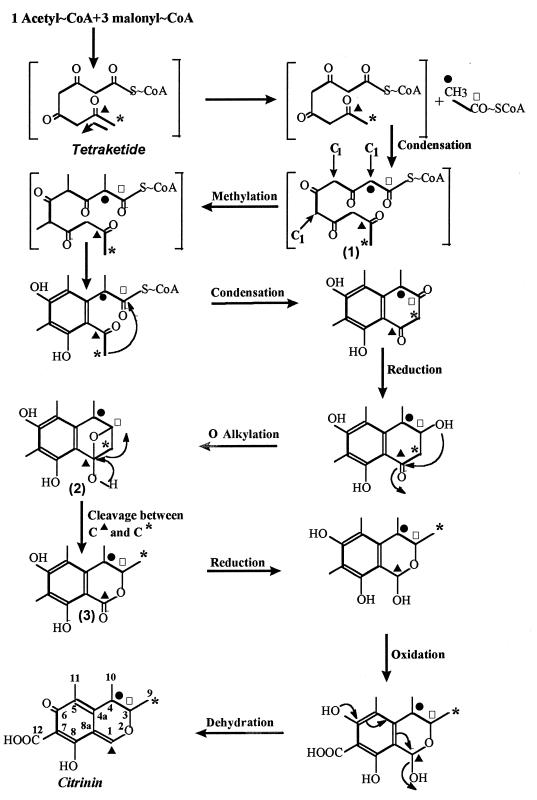
FIG. 2.
Scheme of the biosynthesis of citrinin by M. ruber. The start of the condensing reaction is indicated by the bent arrow in the upper left panel. Intermediates are numbered. Enrichment of C-1 (▴), C-3 (□), C-9 (*), and C-4 (•) is indicated.
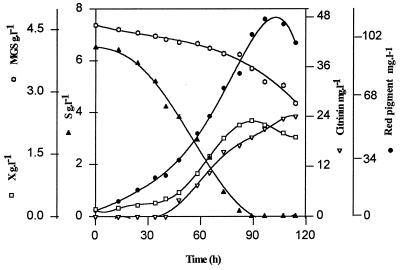
The kinetics of pigment and citrinin production during the growth of M. ruber in the presence of 6.5 g of glucose · liter−1 and 4.5 g of monosodium glutamate · liter−1 are presented in Fig. 1. After a lag of about 20 h, cell biomass and red pigments increased in parallel to reach 2.3 g (dry weight) · liter−1 and 112 mg · liter−1, respectively, at the time of glucose exhaustion. The maximal rate of pigment production was observed 20 to 60 h after the initiation of the fermentation. In contrast, the production of citrinin started after about 45 h of cultivation and did not appear to stop when the glucose from the medium was consumed. The uncoupling in the production of these two polyketide derivatives suggested that they might not follow the same metabolic pathway. Feeding the culture medium with [1-13C]acetate resulted in the enrichment of C-1, C-8, C-6, C-4a, and C-3 (Table 1), while the enrichment of C-8a, C-7, C-5, C-4, and C-9 was obtained with [2-13C]acetate (Table 1). These experiments confirmed that citrinin arose from the polyketide pathway, by a route apparently similar to that found in P. citrinum and A. terreus (1, 14). Another experiment carried out with [1,2-13C]acetate resulted in the enrichment of all the carbons coming from the acetate pool, except for C-10, C-11, and C-12, which came from the endogenous C1 pool (most likely CO2). However, we found that the spectra from labeled citrinin were strikingly different from those obtained for P. citrinum and A. terreus, incubated under the same conditions (1, 14) (results not shown). The relative enrichment of 13C peaks was estimated with respect to its natural abundance. Since C-10 on the citrinin molecule came from the endogenous C1 (methyl) pool and could be considered an internal reference, the relative enrichment (RE) of each carbon was measured in reference to that of C-10. It can be seen in Table 1 that the RE value of C-1 (Fig. 2) in the citrinin structure was the highest, while that of C-3 (Fig. 2) was the lowest, which was exactly the opposite to what has been observed in A. terreus (14). Similar to C-1 from acetate moieties, the condensation reaction progressed from C-1, as the most enriched, to C-8, C-6, C-4a, and then C-3. With carbon arising from the CH3 unit of acetate, the enrichment increased from C-9 (Fig. 2) to C-5 (Table 1). Such an enrichment towards the polyketide condensing reactions has been reported previously for the biosynthesis of botryodiplodine (13). The last two carbons which closed the ring were C-4 (Fig. 2) and C-3, which had comparable but very low levels of isotopic enrichment. This situation contrasted with the high and uniform enrichment of the same carbon obtained in Penicillium and Aspergillus (1, 14).
FIG. 1.
Culture development of M. ruber in a 2-liter fermentor at 28°C. X, biomass; S, glucose; MGS, monosodium glutamate.
TABLE 1.
Chemical shifts and coupling constants for [13C]citrinin and RE with labeled [1,2-13C2]acetatea
| Carbon atom | Chemical shift (ppm) | 1J(13C-13C) (Hz) | Enrichment | REb |
|---|---|---|---|---|
| 1 | 162.9 | 69.6 | a | 3.1 |
| 3 | 81.8 | 37.6 | a | 0.6 |
| 4 | 34.7 | 40.7 | b | 0.7 |
| 4a | 139.2 | 40.7 | a | 1.3 |
| 5 | 123.2 | 57.0 | b | 2.1 |
| 6 | 183.9 | 57.0 | a | 2.4 |
| 7 | 100.4 | 63.9 | b | 2.0 |
| 8 | 177.3 | 63.9 | a | 2.5 |
| 8a | 107.5 | 69.6 | b | 1.6 |
| 9 | 18.3 | 37.6 | b | 1.1 |
| 10 | 9.6 | s | 1.0 | |
| 11 | 18.4 | s | ||
| 12 | 174.7 | s |
a, incorporation of [1-13C]acetate; b, incorporation of [2-13C]acetate; s, singlet.
RE was calculated by the following equation: α · IC-XE/IC-XN, where α is determined by the equation IC-10N/IC-10E, C-X is the carbon number, C-10 is the reference carbon, IC-XN is the intensity of the NMR signal of carbon X naturally enriched, and IC-XE is the intensity of the NMR signal of carbon X enriched in the presence of [1,2-13C2]acetate. The calculation takes into account the experimental 13C NMR conditions.
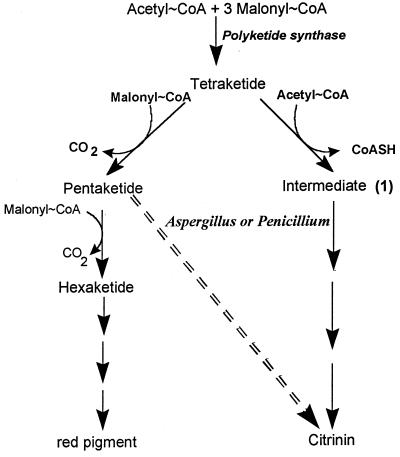
To account for these striking differences, we suggest in Fig. 2 that the precursor for citrinin formation is a tetraketide arising from the condensation of one acetyl-CoA molecule with three malonyl-CoA molecules instead of a pentaketide (one acetyl-CoA molecule and four malonyl-CoA molecules). Then, an additional acetyl-CoA molecule is added to the tetrakedite to form intermediate 1. Furthermore, one cannot exclude the possibility that a malonyl-CoA molecule condenses to the tetraketide and that this is accompanied by a decarboxylation. Subsequent reactions include O alkylation and the cleavage of the single bond between C-1 and C-9 (Fig. 2) in a way similar to that which occurs in the formation of bovilactone or gomphilactone (10, 11). This cleavage also agrees with the proximity of C-3 and C-9. In addition, intermediates 2 and 3 were energetically acceptable, with molecular energy levels of 100.6 and 93.8 kcal · mol−1, respectively. In summary, the occurrence of a tetraketide as the precursor for both citrinin and red pigments may account for the differential production of these two polyketides during the growth of M. ruber. It will be interesting to further characterize the enzymatic reactions at the tetraketide branch point (Fig. 3) in order to develop strategies aimed at a selective production of red pigments.
FIG. 3.
Biosynthesis of citrinin and red pigment in M. ruber. The toxin pathway in Aspergillus and Penicillium is indicated by the dashed arrow.
Acknowledgments
H. Hajjaj acknowledges INRA (Institut National de la Recherche Agronomique), France, for financial support.
We thank N. D. Lindley for a critical reading of the manuscript.
REFERENCES
- 1.Barber J, Staunton J. New insights into polyketide metabolism; the use of protium as a tracer in the biosynthesis of citrinin by Penicillium citrinum. J Chem Soc Perkin Trans I. 1980;1980:2244–2248. [Google Scholar]
- 2.Birch A J, Fitton P, Pride F, Ryan A J, Smith H, Whalley W B. Studies in relation to biosynthesis. Part XVII. Sclerotiorin, citrinin, and citromycetin. J Chem Soc. 1958;1958:4576–4581. [Google Scholar]
- 3.Blanc P J, Laussac J P, Le Bars J, Le Bars P, Loret M O, Pareilleux A, Prome D, Prome J C, Santerre A L, Goma G. Characterization of monascidin A from Monascus as citrinin. Int J Food Microbiol. 1995;27:201–213. doi: 10.1016/0168-1605(94)00167-5. [DOI] [PubMed] [Google Scholar]
- 4.Chandler M, McIntyre C R, Simpson T J. Biosynthesis of LL-D253a, a polyketide chromanone metabolite of Phoma pigmentivora: incorporation of 13C, 2H and 18O labelled precursors. J Chem Soc Perkin Trans I. 1992;1992:2285–2293. [Google Scholar]
- 5.Colombo L, Gennari C, Potenza D, Scolastico C. Biosynthesis of citrinin and synthesis of its biogenetic precursors. J Chem Soc Perkin Trans I. 1981;1981:2594–2597. [Google Scholar]
- 6.Deruiter J, Jacyno J M, Davis R A, Cutler H G. Studies on aldose reductase inhibitors from fungi. I. Citrinin and related benzopyran derivatives. J Enzyme Inhibition. 1992;6:201–210. doi: 10.3109/14756369209020170. [DOI] [PubMed] [Google Scholar]
- 7.Hadfield J R, Holker J S E, Stanway D N. The biosynthesis of fungal metabolites. Part II. The β-oxo-lactone equivalents in rubropunctatin and monascorubrin. J Chem Soc Sect C. 1967;1967:751–755. [Google Scholar]
- 8.Hajjaj H, Klaébé A, Loret M O, Tzédakis T, Goma G, Blanc P J. Production and identification of N-glucosylrubropunctamine and N-glucosylmonascorubramine from Monascus ruber and occurrence of electron donor-acceptor complexes in these red pigments. Appl Environ Microbiol. 1997;63:2671–2678. doi: 10.1128/aem.63.7.2671-2678.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hill R A, Carter R H, Staunton J. Biosynthesis of fungal metabolites. Terrein, a metabolite of Aspergillus terreus Thom1. J Chem Soc Perkin Trans I. 1981;1981:2570–2576. [Google Scholar]
- 10.Jagers E, Steglich W. Polyamide-catalyzed dimerization of 2,5-dihydroxybenzoquinones to 4-ylidenetetronic acid, a model for the biosynthesis of bovilactone-4,4. Angew Chem Int Ed Engl. 1981;20:1016–1017. [Google Scholar]
- 11.Jagers E, Stefan B, Ardenne R, Steglich W. Stoffwechselprodukte des 1.2.4-Trihydroxybenzols aus Fruchtkörpern von Gomphidius maculatus and G. glutinosus (Boletales) Z Naturforsch Teil C. 1981;36:488. [Google Scholar]
- 12.O’Hagan D, Rogers S V, Duffin G R, Edwards R L. Biosynthesis of the fungal polyketide, cubensic acid from Xylaria cubensis. Tetrahedron Lett. 1992;33:5585–5588. [Google Scholar]
- 13.Renauld F, Moreau S. Biosynthèse de la botryodiplodine, mycotoxine de Penicillium roqueforti: incorporations d’acétate [1-13C], [2-13C], [1-213C] et d’acide orsellinique [2-13C-carboxyle 13C], [3-4 13C] Tetrahedron. 1984;40:1823–1834. [Google Scholar]
- 14.Sankawa U, Ebizuka Y, Noguchi H, Isikawa Y, Kitaghawa S, Yamamoto Y, Kobayashi T, Iitak Y. Biosynthesis of citrinin in Aspergillus terreus. Tetrahedron. 1983;39:3583–3591. [Google Scholar]
- 15.Simpson T J. Studies of polyketide chain-assembly processes. In: Steyn P S, Vleggaar R, editors. Mycotoxins and phycotoxins. Amsterdam, The Netherlands: Elsevier Science Publishers B. V.; 1986. pp. 85–96. [Google Scholar]
- 16.Turner W B. Fungal metabolites. 15. Polyketides. London, England: Academic Press; 1971. pp. 445–476. [Google Scholar]