Abstract
Purpose
To analyze the relationship between monocyte count and preoperative deep venous thrombosis (DVT) in older patients with hip fracture.
Methods
Consecutive older patients with hip fracture undergoing surgery were included from January 2014 to December 2021. Monocyte count was measured on admission, and Doppler ultrasonography was performed for DVT screening prior to surgery. Univariate and multivariate logistic regression analyses were used to assess the association between monocyte count and DVT.
Results
A total of 674 patients were finally included, and 128 patients (19.0%) were diagnosed with preoperative DVT. Patients with DVT exhibited a higher monocyte count than patients without DVT [0.55 (0.43-0.72) × 109/L versus 0.49 (0.38-0.63) × 109/L, P = 0.007]. Multivariate logistic regression analysis showed that a high monocyte count (> 0.6 × 109/L) was independently associated with a higher risk of DVT (OR = 1.705, 95% CI: 1.121-2.593, P = 0.013), and for every 0.1 × 109/L increase in monocyte count, the risk of DVT increased by 8.5% (OR = 1.085, 95% CI: 1.003-1.174, P = 0.041). Other risk factors associated with DVT included intertrochanteric fracture (OR = 1.596, 95% CI: 1.022-2.492, P = 0.040), and elevated fibrinogen level (OR = 1.236, 95% CI: 1.029-1.484, P = 0.023).
Conclusion
A high monocyte count is associated with an increased risk of DVT in older patients with hip fracture. Future studies should evaluate the potential role of monocyte in the prevention and treatment of thrombosis.
Keywords: monocyte, deep venous thrombosis, hip fracture, older adults
Introduction
Venous thromboembolism (VTE), including both deep venous thrombosis (DVT) and pulmonary embolism (PE), is a common and potentially fatal disease that affects nearly 10 million people every year worldwide. 1 Although there have been substantial advances in thromboprophylaxis, VTE still leads to high healthcare costs, morbidity and mortality.2–4 For these reasons, VTE is a major global public health burden. 1
As is well known, thrombus formation is a complex process that involves the interaction of various blood cells, such as platelets and leukocytes. 1 Recently, growing evidences from basic researches support that peripheral monocytes may play an important role in thrombus formation.5–8 Even more, Von Brühl et al 9 clearly observed that monocytes and neutrophils were the first circulating cells that actively accumulated at the vascular surface during DVT development. However, to our knowledge, clinical studies on the association between monocyte count and VTE risk are limited,10–13 and these results are not always consistent.12,14,15 Besides this, the incidence of VTE increases dramatically with age, but this relationship has not been explored in older people. Moreover, recent studies included almost all blood cells for the prediction of VTE, except for monocyte count, suggesting that clinicians did not actually consider monocytes as a potential risk factor for VTE occurrence.16–18
Hip fracture is a common type of fracture in older adults. Due to the rapid global aging, it is predicted that the number of hip fracture will be dramatically increased worldwide from 1.66 million in 1990 to 6.26 million in 2050. 19 Also, hip fracture is associated with excess mortality, great risk of disability, and heavy economic burden. 20 Notably, DVT is one of the most common complications after hip fracture that should not be ignored. Many studies have reported a high incidence of preoperative DVT after acute hip fracture, ranging from 10.2% to 35.0%.14,16,21–23 On the basis of the hip fracture database that established by our team, 24 we aimed to investigate the relationship between monocyte count and preoperative DVT in these patients.
Materials and Methods
We conducted a retrospective case-control study based on the data available from our own hip fracture database, which has been described in detail in our previous studies.24–26 From January 2014, patients were consecutively enrolled in this clinical database if they meet the following inclusion criteria: (1) diagnosis of hip fracture according to the radiographic image, including femoral neck fracture and intertrochanteric fracture; (2) not pathological fracture; (3) age ≥ 60 years; (4) caused by low-energy fall; (5) fresh fracture ≤ 3 weeks. Until now, the database contains 1240 older patients with hip fracture. In this study, the following patients were excluded: (1) non-surgical treatment; (2) no screening for DVT by lower-extremity ultrasonography; (3) postoperative DVT; (4) missing monocyte count on admission. The study protocol was approved by the Institutional Ethics Committee of Deyang People's Hospital (approval number: 2021-04-019-K01), and performed in accordance with the Declaration of Helsinki. As this was a retrospective study, and data were analyzed anonymously, informed consent was therefore waived by the committee.
Prior to surgery, patients’ both lower limbs were screened for DVT using Doppler ultrasonography (iU 22, Philips, The Netherlands), which were conducted by an experienced radiologist, and the results were reviewed by another senior radiologist. The scanning was routinely performed at iliac vein, common femoral vein, superficial femoral vein, popliteal vein, anterior tibial vein, posterior tibial vein, fibular vein and calf muscle vein. DVT was diagnosed according to Robinov group's criteria. 27 Proximal DVT was defined as thrombosis occurring in the popliteal vein and / or above, whereas below the popliteal vein as distal DVT. Patients with both proximal and distal thrombosis were classified as mixed DVT.
The following data were extracted from the hip fracture database, including demographics [age, sex, and body mass index (BMI), and smoking], comorbidities (hypertension, diabetes, pulmonary disease, atrial fibrillation, renal disease, liver disease, cancer and heart failure), fracture information (time from injury to hospital, hip fracture type), ultrasonography result (with / without DVT, thrombosis location), and laboratory results within 24 h after admission (monocyte, neutrophil, hemoglobin, platelet, albumin, fibrinogen and D-dimer). The reference range of monocyte at our institution is (0.1–0.6) × 109/L, and a high monocyte was defined as above the upper limit of normal (> 0.6 × 109/L).
Statistical Analysis
Before statistical analysis, normality of continuous variables was checked by the Shapiro-Wilk test, and described as mean ± standard deviation (SD) or median (interquartile range, IQR). Differences between mean or median were compared by Student's t-test or Wilcoxon rank-sum test as appropriate. Categorical variables were expressed as counts (percentages), and analyzed by chi-square test or Fisher exact test. Logistic regression analyses were performed to examine the association between factors and DVT, and variables that were significant in univariate analysis were further included in the multivariate analysis. Moreover, monocyte count was also entered into the multivariate analysis model as a continuous variable (per 0.1 × 109/L increase) instead of a categorical variable. Odds ratio (OR) and 95% confidence interval (CI) were calculated. All reported P values are two-sided, and P < 0.05 was considered statistically significant. All analyses were performed using JMP Pro software (version 13.2.1; SAS Institute Inc., Cary, NC, USA).
Results
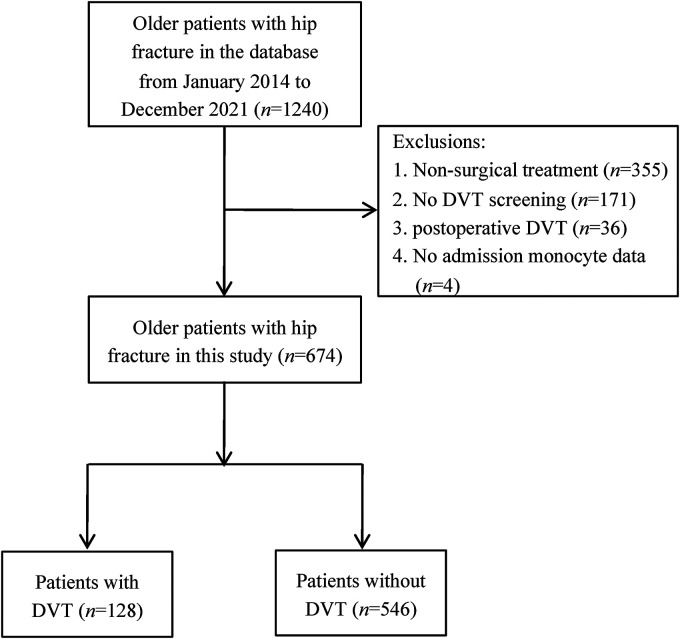
A total of 674 older patients with hip fracture were finally included in this study (Figure 1). Among them, 128 patients (19.0%) were diagnosed with preoperative DVT, of whom 88 (13.1%) had distal DVT, 29 (4.3%) had proximal DVT, and 11 (1.6%) had mixed DVT. During hospitalization, two patients developed PE. The median time from admission to the diagnosis of DVT was 2.0 days, ranging from 1.0 to 16.0 days.
Figure 1.
Flowchart of inclusion and exclusion process
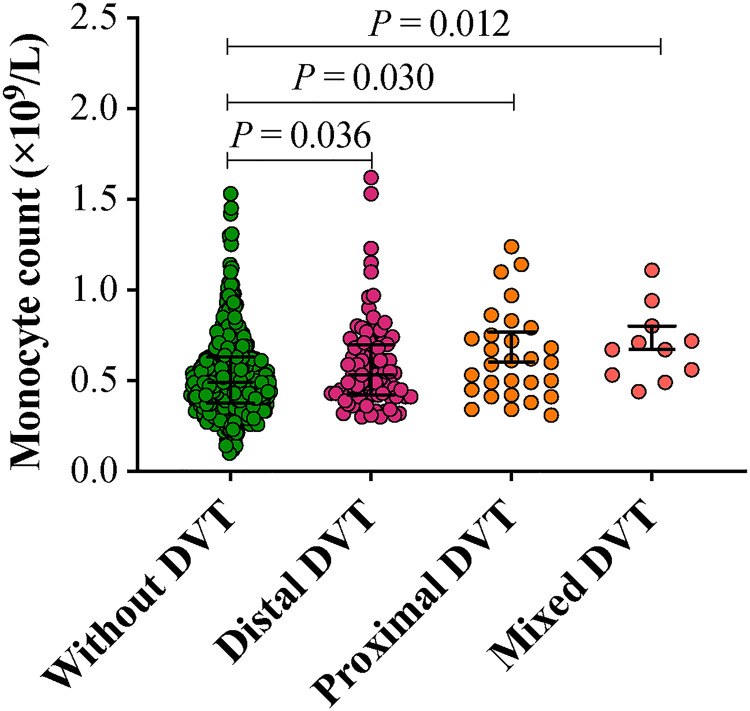
Demographic characteristics and laboratory data of the patients are listed in Table 1. When compared with patients without DVT, patients with DVT were older, and had a longer time from injury to admission, and a higher proportion of intertrochanteric fracture (P < 0.05). Laboratory findings showed that the median monocyte count was 0.49 × 109/L [IQR: (0.38–0.64) × 109/L], and 155 patients (23.0%) had a high monocyte count over 0.6 × 109/L. Patients with DVT were more likely to have higher levels of monocyte, platelet and fibrinogen, but lower levels of hemoglobin and albumin (P < 0.05). Regarding the DVT localization, patients with distal DVT [0.53 (0.42–0.70) × 109/L], proximal DVT [0.60 (0.44–0.77) × 109/L], and mixed DVT [0.67 (0.53–0.80) × 109/L] also exhibited a higher monocyte count than patients without DVT (Figure 2, P < 0.05). However, no significant differences were observed in sex, BMI, smoking, comorbidities, neutrophil count and D-dimer level.
Table 1.
Demographic characteristics and laboratory data of patients with DVT and without DVT.
| Variables | With DVT (n = 128) | Without DVT (n = 546) | P value |
|---|---|---|---|
| Age (years) | 80.5 ± 7.7 | 78.7 ± 8.8 | 0.035 |
| Sex, n (%) | 0.509 | ||
| Male | 42 (32.8) | 196 (35.9) | |
| Female | 86 (67.2) | 350 (64.1) | |
| Body mass index (Kg/m2) | 22.1 ± 3.6 | 21.8 ± 3.2 | 0.324 |
| Smoking, n (%) | 23 (18.0) | 102 (18.7) | 0.852 |
| Comorbidities, n (%) | |||
| Hypertension | 55 (43.0) | 202 (37.0) | 0.211 |
| Diabetes | 31 (24.2) | 108 (19.8) | 0.264 |
| Pulmonary disease | 26 (20.3) | 97 (17.8) | 0.502 |
| Atrial fibrillation | 11 (8.6) | 46 (8.4) | 0.951 |
| Renal disease | 10 (7.8) | 37 (6.8) | 0.679 |
| Liver disease | 3 (2.3) | 17 (3.1) | 0.644 |
| Cancer | 2 (1.6) | 22 (4.0) | 0.175 |
| Heart failure | 2 (1.6) | 2 (0.4) | 0.165 |
| Time from injury to admission (hours) | 24.0 (6.0–96.0) | 10.5 (4.0–48.0) | 0.001 |
| Hip fracture type, n (%) | 0.008 | ||
| Femoral neck fracture | 49 (38.3) | 280 (51.3) | |
| Intertrochanteric fracture | 79 (61.7) | 266 (48.7) | |
| Admission laboratory data | |||
| Monocyte ( × 109/L) | 0.55 (0.43–0.72) | 0.49 (0.38–0.63) | 0.007 |
| Neutrophil ( × 109/L) | 6.9 (4.9–8.9) | 7.2 (5.4–9.5) | 0.226 |
| Hemoglobin (g/L) | 107.7 ± 19.5 | 112.6 ± 20.6 | 0.014 |
| Platelet ( × 109/L) | 161.0 (126.3–210.8) | 148.0 (109.0–243.0) | 0.011 |
| Albumin (g/L) | 38.6 ± 4.7 | 40.1 ± 4.3 | 0.001 |
| Fibrinogen (g/L) | 3.6 ± 1.2 | 3.3 ± 1.1 | 0.001 |
| D-dimer (mg/L) | 5.0 (3.1–8.0) | 4.4 (2.3–8.0) | 0.172 |
DVT deep venous thrombosis
Figure 2.
Comparison of monocyte count between patients with and without deep venous thrombosis (DVT)
The details of logistic regression analysis are described in Table 2. Univariate analysis results showed that age, time from injury to admission, hip fracture type, monocyte, hemoglobin, platelet, albumin and fibrinogen were associated with DVT occurrence (P < 0.05). In terms of DVT sites, patients with a high monocyte count had a higher risk of distal DVT (OR = 1.603, 95% CI: 1.004–2.559, P = 0.048), proximal DVT (OR = 2.376, 95% CI: 1.120–5.039, P = 0.024), and mixed DVT (OR = 4.455, 95% CI: 1.286–15.432, P = 0.018). when adjusted for all the above factors, a high monocyte count remained an independent risk factor for DVT (OR = 1.705, 95% CI: 1.121–2.593, P = 0.013). Other risk factors associated with DVT included intertrochanteric fracture (OR = 1.596, 95% CI: 1.022–2.492, P = 0.040), and elevated fibrinogen level (OR = 1.236, 95% CI: 1.029–1.484, P = 0.023). After adjusting for the same factors, every 0.1 × 109/L increase in monocyte count was significantly associated with a 8.5% higher risk of DVT (OR = 1.085, 95% CI: 1.003–1.174, P = 0.041). Due to the limited patient number in the distal, proximal and mixed DVT, we did not perform multivariate analyses.
Table 2.
The univariate and multivariate logistic regression analysis of factors associated with DVT.
| Variable | Univariate | Multivariate | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Age (per 1 year increase) | 1.025 (1.002–1.048) | 0.036 | 1.006 (0.982–1.031) | 0.610 |
| Sex (female vs male) | 1.467 (0.766–1.737) | 0.511 | ||
| BMI (per 1.0 Kg/m2 increase) | 1.031 (0.973–1.092) | 0.304 | ||
| Time from injury to admission (per 10 hours increase) | 1.022 (1.005–1.040) | 0.014 | 1.012 (0.990–1.034) | 0.285 |
| Hip fracture type (Intertrochanteric vs Neck) | 1.697 (1.145–2.516) | 0.009 | 1.596 (1.022–2.492) | 0.040 |
| Monocyte (> 0.6 vs ≤ 0.6 × 109/L) | 1.918 (1.290–2.851) | 0.001 | 1.705 (1.121–2.593) | 0.013 |
| Monocyte (per 0.1 × 109/L increase) | 1.108 (1.029–1.193) | 0.006 | 1.085 (1.003–1.174) | 0.041 a |
| Neutrophil (per 1.0 × 109/L increase) | 0.959 (0.901–1.021) | 0.191 | ||
| Hemoglobin (per 1.0 g/L increase) | 0.988 (0.979–0.998) | 0.014 | 0.998 (0.987–1.010) | 0.754 |
| Platelet (per 1.0 × 109/L increase) | 1.004 (1.001–1.007) | 0.010 | 1.002 (0.998–1.005) | 0.294 |
| Albumin (per 1.0 g/L increase) | 0.926 (0.886–0.967) | 0.001 | 0.953 (0.905–1.002) | 0.060 |
| Fibrinogen (per 1.0 g/L increase) | 1.325 (1.122–1.564) | 0.001 | 1.236 (1.029–1.484) | 0.023 |
| D-dimer (per 1.0 mg/L increase) | 1.000 (0.999–1.000) | 0.463 | ||
DVT deep venous thrombosis, OR Odds ratio, CI confidence interval, BMI body mass index.
aAdjusted for age, time from injury to admission, hip fracture type, hemoglobin, platelet, albumin, and fibrinogen.
Discussion
Blood cell count is a simple, inexpensive, and widely available test in most hospitals. In this laboratory result, monocyte is a common indicator that constitutes nearly 10% of leucocytes in human. In the present study, we aimed to identify the association between monocyte count and preoperative DVT in older patients with hip fracture. We firstly found that the incidence of preoperative DVT after hip fracture was 19.0%, which was consistent with other studies.22,23 Most importantly, a high monocyte count was independently associated with an increased risk of DVT even after adjustment for potential confounders.
In this study, patients with a high monocyte count (> 0.6 × 109/L) had a 1.705-fold increased risk of DVT (95% CI: 1.121–2.593). When analyzing as a continuous variable, every 0.1 × 109/L increase in monocyte count was associated with a 8.5% higher risk of DVT (OR = 1.085, 95% CI: 1.003–1.174). In a large case-control study, Rezende et al 13 found that patients with a high monocyte count (> 0.77 × 109/L) had a 2.75-fold higher risk of VTE, while a low monocyte count (< 0.12 × 109/L) had a 0.56-fold lower risk of VTE. Similarly, Maldonado-Peña et al 11 reported that patients with monocytosis were more likely to develop DVT (OR = 9.35, 95% CI: 3.20–27.3), with an area under the curve (AUC) of 0.742, a sensitivity of 67.3%, a specificity of 80.9%, a positive predictive value (PPV) of 79.49% and a negative predictive value (NPV) of 69.39%. Moreover, some indexes based on the monocyte count, such as lymphocyte to monocyte ratio (LMR) and monocyte to high-density lipoprotein ratio (MHR), were also confirmed to be associated with DVT after total joint arthroplasty. 28 More clinical evidences have recently been summarized in a review article, and highlighted the relationship between monocyte and DVT, although the predictive ability was suboptimal. 29
Currently, the underlying mechanism of monocyte causing thrombosis is relatively well understood. 30 Monocytes are a major source of tissue factor (TF), which is a central initiator of DVT formation. 9 Recently, Amadio et al 8 showed that monocytes could directly trigger DVT formation through PTGI2/ANXA2/TF pathway. Also, monocytes are the main producers of inflammatory cytokines, such as interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α). 30 The crucial role of inflammation in thrombus formation has been well demonstrated, and the thromboembolic risk can be attenuated by their neutralizing antibody or blockade. 30 Interestingly, hip fracture itself could trigger a switch in monocyte function from phagocytic to inflammatory TNFα-producing cells, implying a possible mechanism for promoting thrombus formation in hip fracture patients. 31
Despite the above-mentioned studies, Basavaraj et al 12 designed a prospective cohort study to explore the relationship between monocyte count and future risk of VTE in a general population. After a median follow-up time of 12.5 years, they identified 429 VTE events in 25127 subjects, and subjects in the upper tertile of monocyte (≥ 0.7 × 109/L) were associated with an increased risk of VTE than those in the lower tertile (≤ 0.4 × 109/L) during the first year (HR = 2.51, 95% CI: 0.69–9.12). However, this association was gradually attenuated over time, and lost its statistical significance at the end of follow-up. Likewise, Liu et al 15 examined the association between inflammation / immune-based indexes and DVT in 1179 patients with tibial plateau fracture. The results showed that a high monocyte count (> 0.78 × 109/L) was associated with the risk of DVT in the univariate analysis, but failed to reach statistical significance in the multivariate analysis. In fact, monocytes have 3 different subsets: classical, intermediate and non-classical. Wypasek et al 6 showed that VTE was strongly associated with an increased number of non-classical and intermediate monocyte, while total monocyte count did not reach statistical significance. This may be one possible reason for the inconsistent results. Unfortunately, monocyte subset is not routinely tested in clinical practice, but a previous study found that intermediate monocyte increased immediately after acute hip fracture. 31 In addition, patients included in our study were significantly older than the above studies, with a mean age of 79.0 years. Since the incidence of VTE increases with age, this may be another important factor influencing the results.
Given the clear association between monocyte count and VTE risk, targeting monocyte was considered as an important novel therapeutic approach for the prevention and treatment of thrombosis.5,7–9 Shahneh et al 5 attempted to selectively reduce inflammatory monocytes by enforcing monocyte conversion using the nuclear receptor group 4 family A member 1 (Nur77) agonist, and this treatment decreased venous thrombus formation in wild-type mice. This effect was also observed upon the deletion of inflammatory monocytes using anti-C-C chemokine receptor type 2 (CCR2) antibody. 7 Similarly, total monocyte depletion by intravenous administration of clodronate or gadolinium chloride markedly reduced thrombus size.7,8 Notably, monocytes rapidly accumulate in skin wounds, and play an important role in wound repair. 32 Therefore, it is necessary to consider the side effect of monocyte depletion on wound healing in patients undergoing hip fracture surgery.
Furthermore, other risk factors associated with DVT in the multivariate analysis included intertrochanteric fracture, and elevated fibrinogen level. These findings have been reported by other studies.22,33 A recent meta-analysis conducted by Wang et al 34 found that advanced age, prolonged time from injury to admission, low albumin and hemoglobin levels, and high platelet level were associated with preoperative DVT in hip fracture patients. However, in our study, these factors reached statistical significance in the univariate analyses, but lost significance in the multivariate analysis. This may be due to the small number of patients with DVT.
However, several limitations should be mentioned. First, this was a retrospective study, and a selection bias may exist. In this study, patients were included consecutively, thus selection bias was minimized. For the same reason, some factors associated with DVT formation were not obtained for further analysis, such as previous history of VTE, anticoagulation medication before admission, and malnutrition inflammation complex syndrome (MICS). Second, as it was a classical case-control study, it is not possible to establish causal relationship. Third, the sample size was relatively small, which may influence statistical power. To make the results stable, we analyzed monocyte count continuously and categorically using multivariate logistic regression models, and this relationship persisted. To overcome these limitations, prospective studies with large sample sizes are needed to confirm our findings.
Conclusion
Taken together, a high monocyte count is independently associated with an increased risk of DVT in older patients with hip fracture. As monocyte is a simple indicator that can be obtained from the blood routine test, future studies should evaluate the potential role of monocyte in the prevention and treatment of thrombosis.
Acknowledgements
We would like to thank several nurses from the Department of Orthopedics in Deyang People's Hospital, for the help with the data inspection
Footnotes
Author Contributions: Zhicong Wang: Methodology, Formal analysis, Software, Visualization, Funding acquisition, Writing-original draft. Qing Zhou: Methodology, Formal analysis, Writing-review & editing, Project administration. Hailong Liu: Methodology, Formal analysis, Writing-review & editing, Project administration. Jianjun Zhang: Investigation, Supervision, Validation. Zhonglun Zhu: Investigation, Supervision, Validation. Jijun Wu: Investigation, Supervision, Validation. Xue Chen: Investigation, Supervision, Validation. Yuehong Liu: Conceptualization, Writing-review & editing, Supervision, Validation.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Sichuan Science and Technology Program, Deyang Science and Technology Bureau Project (grant number No. 2021JDR0337, No. 2019SZ118, No. 2019SZ125).
Data Sharing Statement: The data used during the current study are available from the corresponding author on reasonable request.
Consent to Participate: As this was a retrospective study, and data were analyzed anonymously, informed consent was therefore waived by the committee.
Ethical Approval: This study was approved by the Institutional Ethics Committee of Deyang People's Hospital (approval number: 2021-04-019-K01), and performed in accordance with the Declaration of Helsinki.
ORCID iD: Yuehong Liu https://orcid.org/0000-0001-8606-5371
References
- 1.Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398(10294):64‐77. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Z, Lei J, Shao X, et al. Trends in hospitalization and in-hospital mortality from VTE, 2007 to 2016, in China. Chest. 2019;155(2):342‐353. [DOI] [PubMed] [Google Scholar]
- 3.Rattan R, Parreco J, Eidelson Set al. Hidden burden of venous thromboembolism after trauma: a national analysis. J Trauma Acute Care Surg. 2018;85(5):899‐906. [DOI] [PubMed] [Google Scholar]
- 4.Nilius H, Mertins T, Boss Ret al. Long-term survival after venous thromboembolism: a prospective cohort study. Front Cardiovasc Med. 2021;8:749342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shahneh F, Christian Probst H, Wiesmann Set al. et al. Inflammatory monocyte counts determine venous blood clot formation and resolution. Arterioscler Thromb Vasc Biol. 2022;42(2):145-155. [DOI] [PubMed] [Google Scholar]
- 6.Wypasek E, Padjas A, Szymańska M, Plens K, Siedlar M, Undas A. Non-classical and intermediate monocytes in patients following venous thromboembolism: links with inflammation. Advances in Clinical and Experimental Medicine : Official Organ Wroclaw Medical University. 2019;28(1):51‐58. [DOI] [PubMed] [Google Scholar]
- 7.Laurance S, Bertin F, Ebrahimian Tet al. et al. Gas6 promotes inflammatory (CCR2CX3CR1) monocyte recruitment in venous thrombosis. Arterioscler, Thromb, Vasc Biol. 2017;37(7):1315‐1322. [DOI] [PubMed] [Google Scholar]
- 8.Amadio P, Tarantino E, Sandrini L, Tremoli E, Barbieri S. Prostaglandin-endoperoxide synthase-2 deletion affects the natural trafficking of Annexin A2 in monocytes and favours venous thrombosis in mice. Thromb Haemostasis. 2017;117(8):1486‐1497. [DOI] [PubMed] [Google Scholar]
- 9.von Brühl M, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rojnuckarin P, Uaprasert N, Sriuranpong V. Monocyte count associated with subsequent symptomatic venous thromboembolism (VTE) in hospitalized patients with solid tumors. Thromb Res. 2012;130(6):e279‐e282. [DOI] [PubMed] [Google Scholar]
- 11.Maldonado-Peña J, Rivera K, Ortega C, Betancourt M, Lugo J, Camargo E. Can monocytosis act as an independent variable for predicting deep vein thrombosis? Int J Cardiol. 2016;219:282‐284. [DOI] [PubMed] [Google Scholar]
- 12.Basavaraj M, Brækkan S, Brodin E, Østerud B, Hansen J. Monocyte count and procoagulant functions are associated with risk of venous thromboembolism: the Tromsø study. J Thrombosis and Haemostasis: JTH. 2011;9(8):1673‐1676. [DOI] [PubMed] [Google Scholar]
- 13.Rezende S, Lijfering W, Rosendaal F, Cannegieter S. Hematologic variables and venous thrombosis: red cell distribution width and blood monocyte count are associated with an increased risk. Haematologica. 2014;99(1):194‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao K, Zhang J, Li J, Meng H, Hou Z, Zhang Y. Incidence of and risk factors for new-onset deep venous thrombosis after intertrochanteric fracture surgery. Sci Rep. 2021;11(1):17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Zhu Y, Chen Wet al. et al. Relationship between the inflammation/immune indexes and deep venous thrombosis (DVT) incidence rate following tibial plateau fractures. J Orthop Surg Res. 2020;15(1):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao K, Wang Z, Tian S, Hou Z, Chen W, Zhang Y. Incidence of and risk factors for pre-operative deep venous thrombosis in geriatric intertrochanteric fracture patients. Int Orthop. 2022; 46(2):351-359. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Feng X, Zhang D, et al. Deep vein thrombosis in hospitalized patients with COVID-19 in Wuhan, China: prevalence, risk factors, and outcome. Circulation. 2020;142(2):114‐128. [DOI] [PubMed] [Google Scholar]
- 18.Tan Z, Hu H, Wang Z, Wang Y, Zhang Y. Prevalence and risk factors of preoperative deep venous thrombosis in closed patella fracture: a prospective cohort study. J Orthop Surg Res. 2021;16(1):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kendler DL, Bauer DC, Davison KS, et al. Vertebral fractures: clinical importance and management. Am J Med. 2016;129(2):221. e221–210. [DOI] [PubMed] [Google Scholar]
- 20.Zhang C, Feng J, Wang S, et al. Incidence of and trends in hip fracture among adults in urban China: a nationwide retrospective cohort study. PLoS Med. 2020;17(8):e1003180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Wei X, Huang H, et al. Deep vein thrombosis in bilateral lower extremities after hip fracture: a retrospective study of 463 patients. Clin Interv Aging. 2018;13:681‐689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He S, Zhang P, Qin H, Jiang N, Yu B. Incidence and risk factors of preoperative deep venous thrombosis following hip fracture: a retrospective analysis of 293 consecutive patients. Eur J Trauma Emergency Surg: Off Publ Eur Trauma Soc. 2022.Online ahead of print, doi: 10.1007/s00068-021-01861-3. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Zhou F, Xu Xet al. et al. Clinical predictors for deep vein thrombosis on admission in patients with intertrochanteric fractures: a retrospective study. BMC Musculoskelet Disord. 2021;22(1):328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Jiang W, Chen X, Yang L, Wang H, Liu Y. Systemic immune-inflammation index independently predicts poor survival of older adults with hip fracture: a prospective cohort study. BMC Geriatr. 2021;21(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Chen X, Yang L, Wang H, Jiang W, Liu Y. A new preoperative risk score for predicting mortality of elderly hip fracture patients: an external validation study. Aging Clin Exp Res. 2021;33(9):2519-2527. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Wang H, Yang L, Jiang W, Chen X, Liu Y. High platelet-to-lymphocyte ratio predicts poor survival of elderly patients with hip fracture. Int Orthop. 2021;45(1):13‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rabinov K, Paulin S. Roentgen diagnosis of venous thrombosis in the leg. Arch Surg. 1972;104(2):134‐144. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Yao Y, Yao C, Jiang Q. Predictive value of lymphocyte to monocyte ratio and monocyte to high-density lipoprotein ratio for acute deep vein thrombosis after total joint arthroplasty: a retrospective study. J Orthop Surg Res. 2018;13(1):211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue J, Ma D, Jiang J, Liu Y. Diagnostic and prognostic value of immune/inflammation biomarkers for venous thromboembolism: is it reliable for clinical practice? J Inflamm Res. 2021;14:5059‐5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Colling M, Tourdot B, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. 2021;128(12):2017‐2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baëhl S, Garneau H, Lorrain Det al. Alterations in monocyte phenotypes and functions after a hip fracture in elderly individuals: a 6-month longitudinal study. Gerontology. 2016; 62(5):477‐490. [DOI] [PubMed] [Google Scholar]
- 32.Min D, Nube V, Tao Aet al. Monocyte phenotype as a predictive marker for wound healing in diabetes-related foot ulcers. J Diabetes Complications. 2021;35(5):107889. [DOI] [PubMed] [Google Scholar]
- 33.Xing F, Li L, Long Y, Xiang Z. Admission prevalence of deep vein thrombosis in elderly Chinese patients with hip fracture and a new predictor based on risk factors for thrombosis screening. BMC Musculoskelet Disord. 2018;19(1):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang T, Guo J, Long Y, Yin Y, Hou Z. Risk factors for preoperative deep venous thrombosis in hip fracture patients: a meta-analysis. J Orthop Traumatol. 2022;23(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]