Abstract
Clinical manifestations of Covid-19 vary widely among patients. Recent studies suggest that up to 15% of patients with severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) infections develop gastrointestinal symptoms. The location of virus–host cell receptors angiotensin-converting enzyme 2 and transmembrane serine protease 2 has an important role in the pathophysiology and presentation of disease. They are expressed in the respiratory tract, as well as other organs and tissues including exocrine and endocrine pancreatic cells. These cells are therefore a possible target for the virus, which could explain the relationship between SARS-CoV-2 infection and pancreatic injury. We report a disastrous collateral effect of the Covid-19 pandemic on a 33-year-old man with chronic renal insufficiency and asymptomatic SARS-CoV-2 infection, who developed acute pancreatitis. Inflammation progressed rapidly toward necrosis and the development of a peripancreatic pseudoaneurysm which subsequently ruptured, causing death.
Keywords: Pancreatitis, Covid-19, pancreatic pseudoaneurysm, bleeding pseudoaneurysm, peripancreatic necrosis, severe acute respiratory syndrome coronavirus type 2
Introduction
Clinical manifestations of Covid-19 vary widely among patients, from mild disease to severe dyspnea or even death. Most (80.9%) patients with mild or moderate disease present with fever, cough, tiredness, and loss of taste or smell.1,2 However, recent studies show that up to 15% of patients with Covid-19 develop gastrointestinal symptoms such as loss of appetite, nausea, vomiting, diarrhea, and abdominal pain; gastrointestinal complaints as the only symptoms of disease are estimated to be present in about 10% of patients. 3
Besides the respiratory tract, severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) virus–host cell receptors angiotensin-converting enzyme 2 (ACE2) and trans-membrane serine protease 2 (TMPRSS2) are also expressed in other organs and tissues, which affects the pathophysiology and presentation of disease.4,5 In the pancreas, the receptors are expressed in both exocrine and endocrine pancreatic cells, making them a potential target for the virus, which explains the potential relationship between SARS-CoV-2 infection and pancreatic injury.4,6 Covid-19 is best known for causing thrombotic complications; 7 however, an increasing number of studies indicate that blood coagulation can be altered in terms of increased propensity to bleeding, regardless of the use of thromboprophylaxis.8,9
While most cases of acute pancreatitis in patients with Covid-19 are mild and self-limiting, complications in the form of progression to necrosis have been reported.10–12 However, to our knowledge, little is known about pancreatic complications, and there are no previous reports about the severe forms of acute pancreatitis complicated with pseudoaneurysm formation even though the management of patients affected with this can be problematic. We report a patient who developed acute necrotic pancreatitis during Covid-19, which was further complicated by pseudoaneurysm development and its rupture manifesting as acute gastrointestinal bleeding.
Case report
A 33-year-old man presented to the emergency department with epigastric pain radiating to the back, nausea, and vomiting. He was afebrile, with normal blood pressure (130/85 mmHg), heart rate (75 bpm), SpO2 98%, and a painful abdomen. On initial laboratory analysis, his white blood cell count, platelet count, and hemoglobin levels were within normal ranges, although lipase (1082 U/L), amylase (1426 U/L), urea (11 mmol/L), creatinine (235 mmol/L), and C-reactive protein levels (35.1 mg/L) were all elevated. Abdominal ultrasound showed an empty gallbladder, and an enlarged and edematous pancreas with no peripancreatic collections. The patient denied alcohol and tobacco consumption, and having taken any medications known to cause pancreatitis. His medical history included hypertension and mild chronic renal insufficiency. As all patients are required to have a valid and negative Covid-19 test report before admission, the Panbio™ Covid-19 Ag rapid test device (Abbott, Chicago, IL, USA) was used and the result was positive. His chest X-ray findings were normal. The patient was referred to a multi-specialized hospital, dedicated to patients with Covid-19.
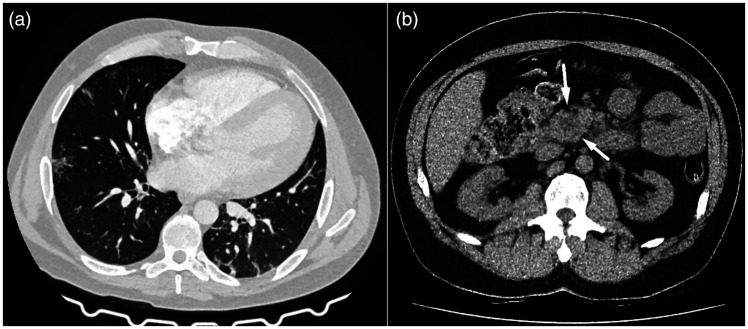
On the initial non-contrast chest computed tomography (CT) examination, discrete focal ground-glass opacities and interlobular septal thickening among consolidation areas of lung parenchyma were detected. The total severity CT score was 6/25, CORADS 6 (Figure 1a). Although the examination was performed without intravenous contrast application, fat stranding of peripancreatic adipose tissue and a slightly enlarged pancreatic head with an intraparenchymal hypodense zone were observed in the upper abdomen (Figure 1b).
Figure 1.
Non-contrast chest and abdominal CT examination. (a) Axial scan showing discrete peripheral ground-glass opacities typical of pulmonary manifestation of Covid-19 and (b) Axial view showing hypodense area inside the pancreatic head, suggestive of intraparenchymal necrosis. Discrete peripancreatic fat stranding can also be seen, without acute necrotic or liquid collection.
CT, computed tomography.
The patient was treated conservatively with antibiotic prophylaxis (imipenem, 0.5 g; 8 hourly), high volume crystalloid infusion with Ringer’s lactate, analgesics (tramadol, 100–200 mg daily), proton pump inhibitor (pantoprazole, 40 mg), and anticoagulant (fraxiparine, 0.3 mL) due to the D-dimer level of 1944 µg/L. On the fifth day of admission, he was stable with no complaints but routine laboratory analysis revealed a marked elevation of C-reactive protein (210.2 mg/L) and leukocytosis (22 × 109 IU/L).
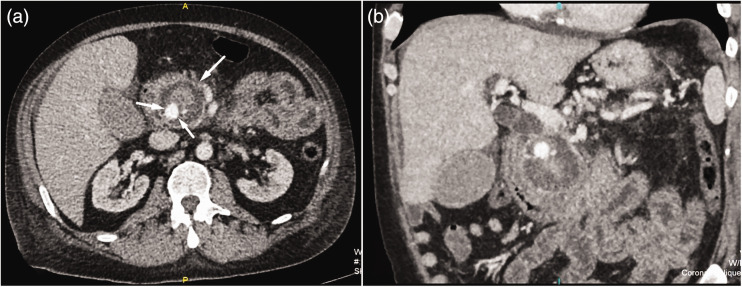
On the following day, his condition was complicated by the progression of renal failure (urea, 18 mmol/L; creatinine, 730 mmol/L), a discrete drop in hemoglobin (106 g/L), and tachycardia (120 bpm). A control ultrasound examination showed extensive pancreatic fluid collections in the head and tail. Surgical consultation was immediately obtained and progression towards pancreatic necrosis was suspected. Abdominal CT examination with intravenous contrast administration revealed acute necrotic collections, similar to walled-off pancreatic necrosis (WOPN), involving the pancreatic head and pancreaticoduodenal groove. These had a compressive effect on the distal common bile duct with consequent incipient biliary dilatation. Inside this 5-cm diameter hemorrhagic-necrotic formation was a 16-mm central enhancing component showing contrast extravasation, which correlated with pseudoaneurysm of the pancreaticoduodenal artery (Figure 2a, b).
Figure 2.
(a) Axial scan and (b) coronal reconstruction of contrast-enhanced abdominal CT showing central enhancing component inside walled-off pancreatic necrosis in the head of the pancreas. This correlates with the pseudoaneurysm originating from the pancreaticoduodenal artery.
Considering the level of patient deterioration and the potentially life-threatening pancreatic pseudoaneurysm, endovascular embolization was proposed as the best therapeutic option. Anticoagulant therapy was immediately excluded because the coagulation profile (anti-factor Xa assay, international normalized ratio, and thrombocytes) was within normal limits. Although the specialized Covid-19 hospital was equipped with adequate radiological diagnostics, angioembolization was not feasible because of the lack of human resources. Moreover, transfer of the patient to another center was refused in accordance with current Covid-19 safety protocols. Because the patient no longer had respiratory symptoms, he was referred to another hospital where angioembolization was technically feasible as soon as his PCR test was negative for SARS-CoV-2 infection. Nevertheless, massive rectal bleeding suddenly occurred.
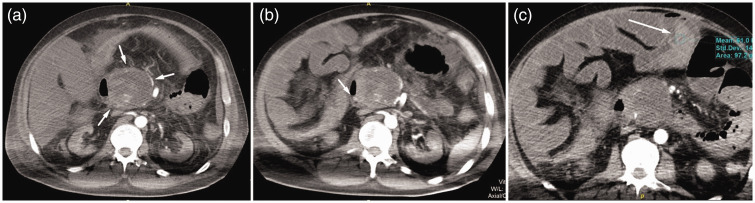
Despite all efforts to stabilize the patient with multiple blood transfusions, he remained pale, tachycardic with a heart rate of 140 bpm, and hypotensive with a systolic blood pressure of 80 to 100 mmHg and a diastolic blood pressure of 40 to 60 mmHg. Urgent control multidetector CT examination of his abdomen revealed a disintegrated pseudoaneurysm caused by its complete rupture and active hemorrhage within, and a more extensive WOPN than seen previously (7 cm maximal diameter). Additionally, a defect on the medial wall of the D2 duodenum and penetration of the necrotic collection into the lumen were observed. Furthermore, the small intestine was paretic with a dilated lumen filled with high-density contents corresponding to hemorrhage and coagulated blood (Figure 3a–c).
Figure 3.
(a) Multidetector CT of the abdomen in the arterial phase showing enlarged WOPN with a ruptured pseudoaneurysm and active intralesional bleeding. (b) Edematous medial wall of the D2 duodenal segment with a discrete defect suggesting fistulation with necrotic collection and (c) Hyperdense, hemorrhagic content in the lumen of the small intestine showing a small amount of free fluid in the peritoneal space
CT, computed tomography.
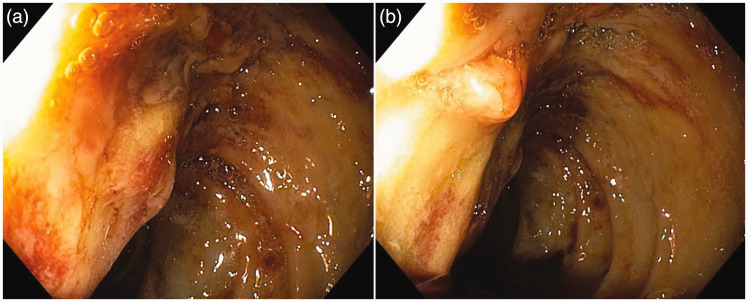
This finding was confirmed by gastro-duodenoscopy, which showed a pathologic communication between the necrotic collection and duodenum with penetration of the hemorrhagic content into the lumen (Figure 4a, b). Unfortunately, soon after, the patient developed cardiac arrest and died from hypovolemic shock caused by extensive gastrointestinal bleeding.
Figure 4.
(a) Duodenoscopy showing diffusely altered wall of the duodenum with extensive inflammation and exulceration and (b) Minor defect on the wall of the duodenum and penetration of the dense hemorrhagic content into the lumen.
This study conforms to CARE guidelines. 13 Written informed consent for publication was obtained from the patient’s sister.
Discussion
Acute pancreatitis is the most common gastrointestinal disease requiring immediate admission to hospital, with an annual incidence of 34 per 100,000 person-years in high-income countries. 14 It is unpredictable, potentially lethal, and characterized by a local and systemic inflammatory response. The prognosis mainly depends on the development of organ failure and secondary infection of the pancreas or peripancreatic necrosis. 15
Although alcohol abuse and gallstones are considered two main causes of acute pancreatitis, other etiologic agents have been identified including viral infections. This could reflect the fact that the pancreas, like many other organs, contains high numbers of ACE2 receptors in both islet cells and the exocrine gland, which is one receptor by which SARS-CoV-2 enters human cells. Indeed, Schepis et al. showed that SARS-CoV-2 could be detected and isolated from the pseudocyst content in patients with acute pancreatitis. 16 Besides ACE2 receptors and virus tropism for pancreatic cells, other factors such as treatment with non-steroidal anti-inflammatory drugs and glucocorticosteroids should be considered in the etiology of COVID-19-induced pancreatic injury. 17 Additionally, pancreatic injury might be caused by an inadequate immune reaction and the development of a cytokine storm, leading to endothelial cell damage with consecutive signs of multiorgan dysfunction. 18 All of these etiological factors are likely to have influenced the development of necrotic pancreatitis in our patient.
Necrotic pancreatitis is characterized by necrosis of the pancreatic parenchyma and/or peripancreatic fat with an acute necrotic collection which may form a WOPN during the course of disease. 15 As WOPN forms and expands, protein-lysing enzymes may erode the arterial wall, leading to pseudoaneurysm. 19 Although this is relatively uncommon, with an incidence of less than 10%, its overall mortality rate is 50%. 20 Risk factors for major vascular complications include necrotizing pancreatitis, infected WOPN, sepsis, previous pancreatic necrosectomy, long-term use of anticoagulant therapy, and underlying vasculitis. 21 In our case, encapsulated peripancreatic necrosis near the pancreaticoduodenal vascular structures was the main risk factor for pseudoaneurysm development.
The risk of rupture represents the most serious complication of pseudoaneurysms that have developed from necrotic pancreatitis. 22 To the best of our knowledge, there is no specified period of time in which a rupture could occur. In the case of rupture, a pseudoaneurysm typically manifests as melena, bleeding in the pancreatic or bile duct, or a massive hemorrhage in the peritoneal cavity with acute abdominal pain and shock. 19 Unfortunately, our patient’s pseudoaneurysm ruptured shortly after its detection on CT examination. Therefore, we recommend that patients with postinflammatory peripancreatic necrotic collections should be monitored intensively in the weeks following their detection. 23 The rapid progression of necrotic pancreatitis and pseudoaneurysm rupture in our patient followed by massive gastrointestinal bleeding might have been accelerated by damage to the intestinal mucosa associated with SARS-CoV-2 infection. Indeed, patients with Covid-19 were recently reported to be at a high risk of such bleeding,24,25 likely because of vasodilation and hyperemia of the intestinal mucosa which frequently occur in these patients. 26 Moreover, SARS-CoV-2 can have a direct cytotoxic effect on the gut, 27 while coagulopathy can occur following the prolonged use of anticoagulant drugs for thromboprophylaxis. 28
The management of pseudoaneurysms depends on their dimensions and the patient’s condition. Modern interventional radiology provides minimally invasive endovascular treatment, which is increasingly used because of improved technical capabilities and low morbidity rates. 29 Angioembolization offers exceptional diagnostic and therapeutic possibilities for the precise identification and localization of the pseudoaneurysm. Additionally, exclusion of the blood vessel responsible for pseudoaneurysm formation from circulation can be achieved by stenting or embolization.29,30
Conclusion
The association between Covid-19 and acute pancreatitis is not fully understood and further research into this area is needed. However, necrotic acute pancreatitis appears to be a rare but serious complication of SARS-CoV-2 infection, while pseudoaneurysm formation following WOPN can be life-threatening. Taking into account the unpredictability of rupture, as in our case, urgent endovascular treatment is required to prevent a fatal outcome.
Overcoming the challenges presented by Covid-19 has been more difficult because of disruption to health services. While the healthcare system tries to meet the demands of the majority, patients requiring specific and timely medical procedures may seem to be overlooked or to have to justify their health needs over others. In highlighting the gaps in healthcare, the Covid-19 pandemic may stir positive change and deliver improved care provision for all patients.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221098179 for Fatal gastrointestinal bleeding associated with acute pancreatitis as a complication of Covid-19: a case report by Milica Mitrovic, Boris Tadic, Aleksandra Jankovic, Ivan Rankovic and Jelena Djokic Kovac in Journal of International Medical Research
Footnotes
Author contributions: Conceptualization, J.D.K., M.M., and B.T.; methodology, J.D.K., and I.R.; resources, A.J., B.T., I.R., and J.D.K.; writing—original draft preparation, M.M., B.T, and J.D.K.; writing—review and editing, M.M, B.T., and A.J.; supervision, J.D.K., B.T., and M.M.
Conflicts of interest
The authors declare no conflict of interest.
Funding
This research received no external funding.
ORCID iDs
Milica Mitrovic https://orcid.org/0000-0002-7525-7615
Boris Tadic https://orcid.org/0000-0001-5400-1015
References
- 1.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–1242. 2020/02/25. DOI: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 2.Epidemiology Working Group for Ncip Epidemic Response CCfDC and Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41: 145–151. 2020/02/18. DOI: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Mao R, Qiu Y, He JS, et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2020; 5: 667–678. 2020/05/15. DOI: 10.1016/S2468-1253(20)30126-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaharuddin SH, Wang V, Santos RS, et al. Deleterious effects of SARS-CoV-2 infection on human pancreatic cells. Front Cell Infect Microbiol 2021; 11: 678482. 2021/07/21. DOI: 10.3389/fcimb.2021.678482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu F, Long X, Zhang B, et al. ACE2 expression in pancreas may cause pancreatic damage after SARS-CoV-2 infection. Clin Gastroenterol Hepatol 2020; 18: 2128–2130 e2122. 2020/04/26. DOI: 10.1016/j.cgh.2020.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alves AM, Yvamoto EY, Marzinotto MAN, et al. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz J Infect Dis 2020; 24: 561–564. 2020/09/23. DOI: 10.1016/j.bjid.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Chen R, Liu C, et al. Attention should be paid to venous thromboembolism prophylaxis in the management of COVID-19. Lancet Haematol 2020; 7: e362–e363. 2020/04/13. DOI: 10.1016/S2352-3026(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sposato B, Croci L, Di Tomassi M, et al. Spontaneous abdominal bleeding associated with SARS-CoV-2 infection: causality or coincidence? Acta Biomed 2021; 92: e2021199. 2021/05/15. DOI: 10.23750/abm.v92i2.10142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Samkari H, Karp Leaf RS, Dzik WH, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood 2020; 136: 489–500. 2020/06/04. DOI: 10.1182/blood.2020006520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maalouf RG, Kozhaya K, El Zakhemb A. Pancreatitis necrotizante inducida por SARS-CoV-2. Medicina Clinica 2021; 156: 629–630. DOI: 10.1016/j.medcli.2021.01.0050025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotar O, Khomiak I, Polanskyy O, et al . Case report of fatal acute necrotizing pancreatitis in patient with COVID-19: we should be aware of hemorrhagic complications. JOP 2020; 21: 167–171. [Google Scholar]
- 12.Kumaran NK, Karmakar BK, Taylor OM. Coronavirus disease-19 (COVID-19) associated with acute necrotising pancreatitis (ANP). BMJ Case Rep 2020; 13: e237903. 2020/09/10. DOI: 10.1136/bcr-2020-237903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gagnier JJ, Kienle G, Altman DG, et al. The CARE Guidelines: consensus-based clinical case reporting guideline development. Glob Adv Health Med 2013; 2: 38–43. 2014/01/15. DOI: 10.7453/gahmj.2013.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gungabissoon U, Delgado M, Cooper S, et al. The incidence of acute pancreatitis in the United States: identification of cases in an electronic healthcare database with supportive laboratory evidence. Pancreas 2021; 50: e70–e72. 2021/10/30. DOI: 10.1097/MPA.0000000000001887. [DOI] [PubMed] [Google Scholar]
- 15.Boxhoorn L, Voermans RP, Bouwense SA, et al. Acute pancreatitis. Lancet 2020; 396: 726–734. 2020/09/07. DOI: 10.1016/S0140-6736(20)31310-6. [DOI] [PubMed] [Google Scholar]
- 16.Schepis T, Larghi A, Papa A, et al. SARS-CoV2 RNA detection in a pancreatic pseudocyst sample. Pancreatology 2020; 20: 1011–1012. 2020/06/06. DOI: 10.1016/j.pan.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung WY, Abreu Lanfranco O. Contemporary review of drug-induced pancreatitis: A different perspective. World J Gastrointest Pathophysiol 2014; 5: 405–415. 2014/11/18. DOI: 10.4291/wjgp.v5.i4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegyi P, Szakacs Z, Sahin-Toth M. Lipotoxicity and cytokine storm in severe acute pancreatitis and COVID-19. Gastroenterology 2020; 159: 824–827. 2020/07/20. DOI: 10.1053/j.gastro.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoilat GJ, Mathew G, Ahmad H. Pancreatic pseudoaneurysm. StatPearls. Treasure Island (FL), 2021. [PubMed]
- 20.Bergert H, Hinterseher I, Kersting S, et al. Management and outcome of hemorrhage due to arterial pseudoaneurysms in pancreatitis. Surgery 2005; 137: 323–328. 2005/03/05. DOI: 10.1016/j.surg.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Barge JU, Lopera JE. Vascular complications of pancreatitis: role of interventional therapy. Korean J Radiol 2012; 13 Suppl 1: S45–S55. 2012/05/09. DOI: 10.3348/kjr.2012.13.S1.S45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tessier DJ, Stone WM, Fowl RJ, et al. Clinical features and management of splenic artery pseudoaneurysm: case series and cumulative review of literature. J Vasc Surg 2003; 38: 969–974. 2003/11/07. DOI: 10.1016/s0741-5214(03)00710-9. [DOI] [PubMed] [Google Scholar]
- 23.Udd M, Leppaniemi AK, Bidel S, et al. Treatment of bleeding pseudoaneurysms in patients with chronic pancreatitis. World J Surg 2007; 31: 504–510. 2007/02/27. DOI: 10.1007/s00268-006-0209-z. [DOI] [PubMed] [Google Scholar]
- 24.Zellmer S, Hanses F, Muzalyova A, et al. Gastrointestinal bleeding and endoscopic findings in critically and non-critically ill patients with corona virus disease 2019 (COVID-19): Results from Lean European Open Survey on SARS-CoV-2 (LEOSS) and COKA registries. United European Gastroenterol J 2021; 9: 1081–1090. DOI: 10.1002/ueg2.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trindade AJ, Izard S, Coppa K, et al. Gastrointestinal bleeding in hospitalized COVID-19 patients: a propensity score matched cohort study. J Intern Med 2021; 289: 887–894. DOI: 10.1111/joim.13232. [DOI] [PubMed] [Google Scholar]
- 26.Zhao J, Zhou G, Sun Y. Pathological and pathogenic findings of a local SARS case. Jie Fang Jun Yi Xue Za Zhi 2003; 28: 379–382. [Google Scholar]
- 27.Zhao X, Tao MH, Chen CY, et al. Clinical features and factors associated with occult gastrointestinal bleeding in COVID-19 patients. Infect Drug Resist 2021; 14: 4217–4226. DOI: 10.2147/idr.S335868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 7). Chin Med J (Engl) 2020; 133: 1087–1095. 2020/05/03. DOI: 10.1097/CM9.0000000000000819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitrovic M, Dugalic V, Kovac J, et al. Successful embolization of posterior inferior pancreaticoduodenal artery pseudoaneurysm on the grounds of chronic pancreatitis-case report and literature review. Medicina (Kaunas) 2020; 56: 617. 2020/11/20. DOI: 10.3390/medicina56110617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu JT, Yeh CN, Hung CF, et al. Management and outcome of bleeding pseudoaneurysm associated with chronic pancreatitis. BMC Gastroenterol 2006; 6: 3. 2006/01/13. DOI: 10.1186/1471-230X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221098179 for Fatal gastrointestinal bleeding associated with acute pancreatitis as a complication of Covid-19: a case report by Milica Mitrovic, Boris Tadic, Aleksandra Jankovic, Ivan Rankovic and Jelena Djokic Kovac in Journal of International Medical Research