Abstract
Background
Patients with MS have an altered gut microbiota compared to healthy individuals, as well as elevated small intestinal permeability, which may be contributing to the development and progression of the disease.
Objective
We sought to investigate if fecal microbiota transplantation was safe and tolerable in MS patients and if it could improve abnormal intestinal permeability.
Methods
Nine patients with MS were recruited and provided monthly FMTs for up to six months. The primary outcome investigated was change in peripheral blood cytokine concentrations. The secondary outcomes were gut microbiota composition, intestinal permeability, and safety (assessed with EDSS and MRI).
Results
The study was terminated early and was subsequently underpowered to assess whether peripheral blood cytokines were altered following FMTs. FMTs were safe in this group of patients. Two of five patients had elevated small intestinal permeability at baseline that improved to normal values following FMTs. Significant, donor-specific, beneficial alterations to the MS patient gut microbiota were observed following FMT.
Conclusion
FMT was safe and tolerable in this cohort of RRMS patients, may improve elevated small intestinal permeability, and has the potential to enrich for an MS-protective microbiota. Further studies with longer follow-up and larger sample sizes are required to determine if FMT is a suitable therapy for MS.
Keywords: Multiple sclerosis, fecal microbiota transplantation, microbiome, intestinal permeability
Introduction
Multiple Sclerosis (MS) is an autoimmune, inflammatory, demyelinating disease of the central nervous system influenced by genetic susceptibility and environmental factors. 1 The gut microbiota is one such environmental factor that has been implicated in the development and progression of the disease. 2 Past studies have repeatedly demonstrated that MS patients have numerous taxonomic alterations in their gut microbiota composition, including (but not limited to) relative increases in Pseudomonas, Blautia, Streptococcus, and Akkermansia spp., and decreases in Prevotella, Bacteroides, Parabacteroides, and Clostridia spp. compared to healthy individuals. 2 These findings suggest the microbiota as a potential interventional therapeutic target for MS.
The gut microbiota regulates intestinal permeability in a multifactorial manner. 3 Increased intestinal permeability due in part to a perturbed microbiota may allow microbial and dietary antigens to pass through the intestinal epithelium unregulated, trigger autoimmune responses in the host, and exacerbate MS pathophysiology.4,5 Elevated intestinal permeability has been found in 20–73% of MS patients,3,6 so modulating the gut microbiota in MS patients has the potential to subsequently improve this aspect of the disease.
Fecal microbiota transplantation (FMT) is gaining attention for its efficacy in treating both intestinal and extra-intestinal microbiota related diseases.7–9 Indeed, it has been previously shown to improve elevated intestinal permeability in patients with non-alcoholic fatty liver disease 10 and may have the same beneficial effect in patients with MS. Due to the links between MS and the gut microbiota, this study aimed to investigate the potential therapeutic benefit of FMT for relapsing-remitting MS (RRMS) patients. It was hypothesized that FMTs from healthy donors without a personal or family history of autoimmune diseases would be safe and well tolerated, decrease elevated gut permeability, shift the balance of cytokines in the peripheral blood to an anti-inflammatory state, and beneficially alter the microbiota composition of RRMS patients. The findings from this study will provide insights into the future use of FMT in the clinical treatment strategy for MS.
Materials and methods
Patient recruitment and group randomization
The original study plan was to enroll 40 patients with RRMS with 20 patients randomized into the early intervention group and 20 patients randomized into the late intervention group. Early intervention patients received one FMT per month for six months in the first six months of the study and were to be monitored for six months post-FMTs. Late intervention patients were recruited to the study and were monitored for six months prior to intervention. They then were planned to receive one FMT per month for six months in the last six months of the study. The study was terminated early upon request of the Research Ethics Board following the unexpected death of the principal investigator (MK). Therefore, total recruitment was stopped early and the total number of FMTs performed per subject were reduced in the late intervention arm. Details of the number of FMTs performed are found in Figure 1 and detailed below. The duration of follow-up was decreased in some patients as detailed below.
Figure 1.
CONSORT diagram of progress through the phases of the pilot randomized controlled trial.
Ten patients (three males and seven females) with RRMS were recruited to the study between October 2017 to May 2018 at a neurology clinic at University Hospital in London, ON, Canada (Figure 1). [ClinicalTrials.gov (NCT03183869)] The study was approved by Health Canada as well as Western University's Research Ethics Board (REB: 109306). All patients provided written informed consent. A single patient progressed to secondary progressive MS (SPMS) prior to the first FMT and thus was not included in the analysis. This patient tolerated the FMT and had no change in magnetic resonance imaging (MRI) activity following FMT; this patient's data are reported separately (Supplementary Table 1 and Supplementary Figure 1).
Patients were randomly assigned to either the early (n = 4) or late intervention group (n = 5). Patients were assigned to receive FMTs from either Donor 1 (n = 5) or Donor 2 (n = 4) based on donor availability. Patients 1, 2, 3, 4, 7, and 8 received 6 FMTs; Patient 5 received 5 FMTs; Patients 6 and 9 received 2 FMTs.
Study primary and secondary outcomes
The primary outcome was changes in peripheral blood cytokine concentrations; due to premature study termination and accompanying low sample size, this endpoint was not conclusively addressed, but the most complete data set (baseline vs. 4 weeks post-FMT) is reported here. The secondary outcomes were gut microbiota composition, intestinal permeability, and safety (assessed with EDSS and MRI). A summary of when each patient had each assessment performed can be found in Table 1.
Table 1.
Summary of patient assessments.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | |
|---|---|---|---|---|---|---|---|---|---|
| Visit 1 (MRI, EDSS, Blood, Urine, Gut Permeability, Toilet Paper Sample) | X FMT | X | X | X FMT | X | X | X FMT | X FMT | X |
| Visit 2 (EDSS, Blood, Urine, Toilet Paper Sample) | X FMT | X | X | X FMT | X | X | X FMT | X FMT | X |
| Visit 3 (EDSS, Blood, Urine, Toilet Paper Sample) | X FMT | X | X | X FMT | X | X | X FMT | X FMT | X |
| Visit 4 (EDSS, Blood, Urine, Toilet Paper Sample) | X FMT | X | X | X FMT | X | X | X FMT | X FMT | X |
| Visit 5 (EDSS, Blood, Urine, Toilet Paper Sample) | X FMT | X | X | X FMT | X | X | X FMT | X FMT | X |
| Visit 6 (EDSS, Blood, Urine, Toilet Paper Sample) | X FMT | X | X | X FMT | X | X | X FMT | X FMT | X |
| Visit 7 (MRI, EDSS, Blood, Urine, Gut Permeability, Toilet Paper Sample) | X | X FMT | X FMT | X | X FMT | X FMT | X | X | X FMT |
| Visit 8 (EDSS, Blood, Urine, Toilet Paper Sample) | X | X FMT | X FMT | X | X FMT | X FMT | X | X FMT | |
| Visit 9 (EDSS, Blood, Urine, Toilet Paper Sample) | X | X FMT | X FMT | X | X FMT | X | X | ||
| Visit 10 (EDSS, Blood, Urine, Toilet Paper Sample) | X | X FMT | X FMT | X | X FMT | ||||
| Visit 11 (EDSS, Blood, Urine, Toilet Paper Sample) | X | X FMT | X FMT | X | X FMT | ||||
| Visit 12 (MRI (early group) EDSS, Blood, Urine, Gut Permeability (early group) Toilet Paper Sample) | X | X FMT | X FMT | X | |||||
| Visit 13 (MRI (late group), EDSS, Blood, Urine, Gut Permeability (late group), Toilet Paper Sample | X | X |
X: indicates patient attended appointment; FMT: indicates patient received an FMT at this appointment.
FMT donor selection
Potential donors were screened using our previously published protocol. 11 Briefly, potential donors had their medical histories and physical examinations undertaken by a physician. Blood, stool, and urine were screened for 31 viral, bacterial, fungal, and protozoan agents in addition to biochemical characteristics. Donors were excluded if they engaged in behavior that was high risk for infectious disease transmission. Donors were also excluded if they had a personal and/or family history of malignancy, gastrointestinal disease, autoimmune disease, psychiatric disorder, metabolic syndrome, diabetes, early onset coronary disease, or liver disease as these conditions have been reported to have altered gut microbiome compositions. Two donors were selected for this study. Donor 1 provided FMTs to five patients, and Donor 2 provided FMTs to four patients.
Fecal microbiota transplant
Stool samples of 50–70 g were collected from donors and stored as whole stool at −80 °C for up to three months. The stool samples were thawed in a 37 °C water bath for one hour prior to preparation of the rectal enema. Two hundred and twenty milliliters of saline and 50–70 g of donor stool were placed inside of a BA614/STR filter bag (Seward, Islandia, NY) and were mixed using the Stomacher® 400 Circulator (Seward, Islandia, NY) at 230 rpm for 30 s. The filtered material was then transferred into an AMSure® Enema Bag (Amsino, Pomona, CA). The enema was prepared thirty minutes before the scheduled FMT and was stored at room temperature until the procedure took place. Rectal enema was used because it was a feasible and safe delivery route for repeated FMTs. 12
Blood, urine, vitals
Routine blood work, urinalysis, and vitals were taken at each visit (once per month for up to twelve months). Blood and urine were collected, and vitals were taken prior to administering the FMT at each monthly appointment. A summary of tests performed can be found in Supplementary Tables 2–4.
Cytokine analysis
Peripheral blood was collected into Ethylenediaminetetraacetic acid coated Vacutainer tubes (BD Biosciences, San Jose, CA) and spun immediately, and the plasma was collected and stored at −80 °C for later analysis. 13 Cytokine levels in plasma samples were quantified using multiplexed immunoassays (HTH17MOG-14 K; Millipore, MA, USA) using a Bio-Plex 200 system (Bio-Rad, CA, USA). The cytokine panel included both pro- and anti-inflammatory cytokines: Interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17A, IL-17E/IL-25, IL-17F, IL-21, IL-22, IL-23, IL-27, IL-28A, IL-31, IL-33, Granulocyte-macrophage colony-stimulating factor (GM-CSF), Interferon (IFN) γ, macrophage inflammatory protein (MIP)-3α, tumor necrosis factor (TNF) α and TNFβ. Levels of cytokines in plasma samples were determined using a standard curve as per manufacturer's instructions (Millipore). As a comparator, 10 healthy control blood donors (2 males, 7 females, 1 sex not recorded; age 40.3 ± 11.7 years) were locally recruited for one blood draw at the initiation of the study and their plasma was analyzed alongside patient samples.
EDSS and MRI
Clinical and radiological signs of disease activity or progression were measured using the Expanded Disability Status Scale (EDSS) 14 once per month for up to twelve months and MRI at baseline, six months, and twelve months. Due to early study closure the actual number of MRI follow-ups was reduced.
Intestinal permeability
Patients were asked to drink a solution of 5 g of lactulose (Calbiochem®, EMD Millipore Corp., Billerica, MA), 2 g of mannitol powder (BDH®, VWR analytical, Mississauga, ON), 1.5 g of Kool Aid (Kraft Foods, Ingleside, ON), 100 g of sucrose, and 450 mL of tap water the evening before their baseline, six month, and twelve month follow-ups. The subjects were asked to collect all the urine that they passed throughout the night and morning of their appointment and store it in a urine collection bottle. This bottle was brought to the clinic, the total volume of urine was recorded and then aliquoted into 10 mL. Concentrations of lactulose, mannitol and sucrose were determined using high performance liquid chromatography. 15
Fecal sample collection
Fecal samples were collected from patients at each time point using a validated toilet paper sampling method. 16 Briefly, patients collected a visibly soiled piece of toilet paper after passing a stool 1–3 days before their scheduled appointment. The subjects brought the fecal sample in a Fisherbrand™ Opaque Sterile Sampling Bag (Fischer Scientific, ThermoFisher Scientific, Mississauga, ON) to their appointments. The samples were then frozen at - 80 °C until DNA extraction took place. Fecal samples were collected once per month for up to twelve months.
DNA extraction
DNA from the toilet paper samples was extracted using the DNeasy® Powersoil® HTP 96 Kit (Qiagen, Toronto, Ontario, Canada), as per the manufacturer's instructions. Extracted DNA was stored at −20 °C until polymerase chain reaction (PCR) amplification.
DNA amplification
PCR amplification was completed using the Earth Microbiome universal primers, 515F and 806R, which are specific for the V4 variable region of the 16S rRNA gene. Primers and barcode sequences are listed in Supplementary Table 5. PCR reagent setup and amplification was performed as previously described. 17
Sequencing and data analysis
Sequencing was carried out at the London Regional Genomics Center (http://www.lrgc.ca; London, ON, CAN). Amplicons were quantified using pico green and pooled at equimolar concentrations prior to cleanup using QIAquick (Qiagen, Germantown, Maryland, USA). Using the 600-cycle MiSeq Reagent Kit, paired-end sequencing was carried out as 2 × 260 cycles with the addition of 5% ɸX-174 at a cluster density of ∼1100. Data were exported as raw fastq files (uploaded to NCBI Sequence Read Archive, BioProject ID: PRJNA703364).
The sequencing run yielded a total of 7,756,560 reads, ranging from 5325 to 233,072 reads per sample. After demultiplexing, an average of 13.9% of reads were removed from each sample following quality filtering performed utilizing the DADA2 pipeline (version 1.21.0). 18 Amplicon sequence variants (SVs) were assigned taxonomy with the SILVA (version 138) training set. 19 Samples and SVs were further pruned such that the final dataset used in all downstream analyses retained samples with >1000 reads, SVs present at > 1% abundance in any sample, and did not assign taxonomy to Eukaryota, Mitochondria, or Chloroplast. The final dataset included 236 SVs from 102 samples.
Functional potential of the gut microbiota was determined by inferencing gene content from taxonomic abundances with PICRUST2 software as previously described. 20 All SVs from the donor and MS patient samples were below the nearest-sequenced taxon index (NSTI) <2.0 cutoff recommended for a high level of confidence in predictions. Unstratified metagenome predictions for KEGG Ortholog (KO) and enzyme commission (EC) numbers were normalized to 16S rRNA copies, regrouped to MetaCyc reactions using the default mapping file, and then metabolic pathway abundances were inferenced with the MetaCyc pathway database.
Statistical analysis
Friedman test was used to compare EDSS at baseline to all subsequent time points. Wilcoxon matched-pairs signed rank test was used to compare biochemical test results in MS patients before and after FMT. 95% Confidence intervals were generated for each cytokine analyzed with GraphPad Prism (version 9.2.0). Wilcoxon matched-pairs signed rank test was used to compare cytokine concentrations at baseline to subsequent time points.
Microbiota analyses were performed conservatively in agreement with standards in the field, using CoDaSeq, 21 ALDEx2, 22 MaAsLin2, 23 Vegan 24 and core R packages. 25 All appropriate false-discovery rate corrections were employed, and P values, sample numbers, and names of statistical tests are provided in the main text and figure legends for Figures 2–5. Determination of data stratification, statistical tests, and figure preparation were performed in both GraphPad Prism and R (version 4.0.4). All tests of statistical significance utilized a P value of ≤ 0.05 or effect size of ≥|1| as a cut-off. Custom scripts are available at (https://github.com/kait-al/MS_FMT).
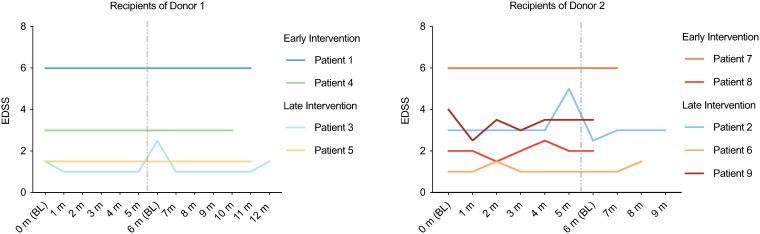
Figure 2.
EDSS is stable following multiple FMTs. Patients received one FMT per month for six months and EDSS was measured at each visit. Six patients received all six FMTs and nine patients received at least one FMT. EDSS did not significantly differ at any timepoint compared to baseline (Friedman test).
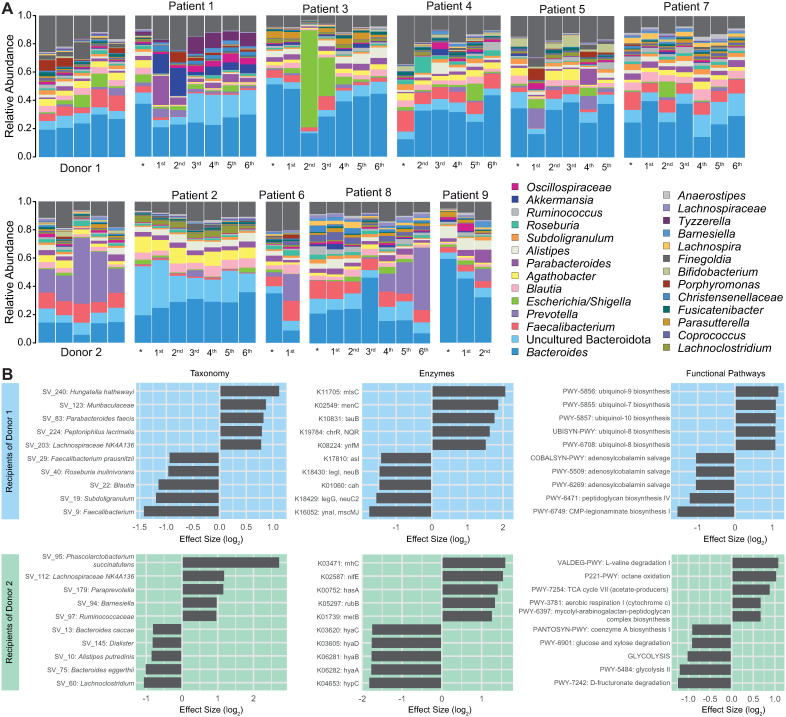
Figure 5.
Gut microbiota composition of RRMS patients following multiple FMTs. A) Each vertical bar represents the relative SV abundance within a single sample. Samples are grouped by participant, and in rows corresponding to their respective donor. * Represents the sample taken at baseline before any FMTs were administered, followed by samples taken following the patient's 1st to 6th FMTs. Relative abundance of SVs is colored by genera, with common genera shown in the legend. B) Effect sizes showing differential features were determined with ALDEx2. Comparisons were made for MS patients following their final FMT relative to their baseline, where positive values represent increased relative abundance, and negative values represent decreased relative abundance at the patient's final FMT compared to their baseline. Patients were grouped based on donor, and the ten most divergent taxonomic and functional features are shown for each comparison.
Results
FMT was safe and tolerable in MS patients
Nine patients (three males and six females) with RRMS were enrolled in this study. The patients had a mean age of 44 ± 8.2 years, the average age of diagnosis was 32.1 ± 8.5 years of age, and the average duration of MS was 14.6 ± 6.8 years. Patient demographics and medications can be found in Supplementary Table 6. Five of the nine patients recruited to this study were randomized to the late intervention group and observed for six months prior to receiving an FMT. Adverse events were documented and are summarized in Table 2. Only one adverse event was found to be related to FMT; a patient that developed hives after receiving an FMT. This resolved without treatment and did not recur after subsequent FMTs. Common adverse events included nausea, vomiting, and abdominal discomfort. Adverse events occurring during the observation period prior to FMT in the late intervention group are listed in Supplementary Table 7. No grade 3 or 4 adverse events occurred during the study.
Table 2.
Summary of adverse events.
| Adverse Event | Treatment Group (n = 9) | Related to Treatment | ||||||
|---|---|---|---|---|---|---|---|---|
| Severity | Severity | |||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| Nausea | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cramping | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Abdominal discomfort | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Difficulty swallowing | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yeast Infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Influenza virus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Common cold | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hypertension | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Kidney Stone | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ear infection | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Extreme fatigue | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hives | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Acne | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Routine blood work and urinalysis were performed, and vitals were taken at each appointment. There were no significant differences in routine laboratory parameters following the administration of six FMTs (Supplementary Tables 2–4). At baseline the mean EDSS was 3.0 ± 1.9 (n = 9). EDSS was measured at every visit and there was no significant change in EDSS following repeated FMTs (Figure 2). MRI was performed as a safety measure since patients may have MRI activity even in the absence of clinical relapse. No new MRI activity developed when MRIs from baseline were compared to MRIs performed following FMTs (data not shown).
Using a multiplex assay, the levels of cytokines, including pro-inflammatory (e.g. IL-6, IL-15, GM-CSF), regulatory (IL-10), Th17 (e.g. IL-17A, IL-17F, IL-21, IL-22) and Th1 (e.g. INFγ, IL-2, TNFβ) cytokines were measured in the plasma of patients at baseline and 4 weeks post the first FMT (Post-FMT). We did not observe a significant change in the levels of any of the cytokines measured post FMT (n = 9) or compared to healthy controls (n = 10), although the sample size was underpowered to accurately assess this endpoint (Table 3). The n of additional monthly FMTs decreased, further underpowering the evaluation of this endpoint, but again no change in the cytokine levels was detected in the corresponding plasma sample collected (12 weeks (n = 7), 24 weeks (n = 4), 28 weeks (n = 4) and 32 weeks (n = 3); data not shown).
Table 3.
Cytokine concentrations are unchanged following FMT.
| Cytokine | Concentration (pg/mL) | P value | Confidence interval of median paired difference | |
|---|---|---|---|---|
| Pre-FMT (n = 9) | Post-FMT (n = 9) | |||
| Mean ± SD | Mean ± SD | |||
| IL-17F | 90.0 ± 75.8 | 72.2 ± 80.28 | 0.52 | −115 −35 |
| GM-CSF | 177.8 ± 195.9 | 211.1 ± 222.8 | 0.63 | −137−137 |
| INFγ | 70.4 ± 45.1 | 53.1 ± 38.7 | 0.43 | −68−25 |
| IL-10 | 29.7 ± 24.2 | 20.1 ± 19.3 | 0.36 | −28−11 |
| MIP3a | 54.0 ± 35.1 | 29.6 ± 20.5 | 0.20 | −56−11 |
| IL-12p70 | 28.5 ± 20.1 | 24.3 ± 20.0 | 0.82 | −32−8 |
| IL-13 | 100.4 ± 195.7 | 99.1 ± 192.3 | >0.99 | −33−33 |
| IL-15 | 33.3 ± 39.2 | 26.7 ± 36.17 | 0.84 | −25−25 |
| IL-17A | 20.04 ± 19.5 | 19.0 ± 23.4 | 0.94 | −15−15 |
| IL-22 | 451.1 ± 452.1 | 470.0 ± 511.4 | 0.94 | −388−388 |
| IL-9 | 39.9 ± 39.9 | 28.5 ± 34.4 | 0.50 | −40−15 |
| IL-1β | 14.8 ± 11.9 | 10.4 ± 9.3 | 0.43 | −16−3 |
| IL-33 | 111.0 ± 59.2 | 73.9 ± 82.7 | 0.25 | −103−28 |
| IL-2 | 18.1 ± 18.1 | 14.5 ± 14.7 | 0.69 | −12 −12 |
| IL-21 | 46.2 ± 30.9 | 31.0 ± 21.9 | 0.30 | −47−10 |
| IL-4 | 474.4 ± 423.1 | 337.2 ± 379.4 | 0.74 | −466−466 |
| IL-23 | 9668 ± 8562 | 7787 ± 8744 | 0.57 | −12,732−4252 |
| IL-5 | 26.3 ± 21.5 | 21.1 ± 18.5 | 0.65 | −26−10 |
| IL-6 | 62.0 ± 123.1 | 66.3 ± 128.6 | 0.63 | −13−13 |
| IL-17E | 158.3 ± 159.3 | 174.4 ± 190.7 | 0.63 | −137−137 |
| IL-27 | 1672 ± 704.4 | 1359 ± 919.5 | 0.52 | −1177−597 |
| IL-31 | 170.0 ± 228.0 | 240.6 ± 329.5 | 0.95 | −323−303 |
| TNFα | 43.5 ± 35.9 | 51.5 ± 36.4 | 0.15 | −8−18 |
| TNFβ | 346.1 ± 610.2 | 360.0 ± 636.4 | 0.63 | −51−51 |
| IL28A | 2111 ± 2578 | 1668 ± 2569 | 0.43 | −1506−626 |
Elevated small intestinal permeability was normalized following FMT
Six patients had their small intestinal permeability assessed at both baseline and following completion of six FMTs. One patient's urine sample had undetectable levels of lactulose and mannitol at baseline and the samples from this patient were excluded from intestinal permeability analysis, thus only five patients were included in the analysis of intestinal permeability. Two patients had abnormal small intestinal permeability at baseline (>0.025 lactulose:mannitol, 26 ) which normalized following six FMTs (Figure 3). Both patients with elevated intestinal permeability were randomized to receive FMTs from donor 1.
Figure 3.
Abnormal small intestinal permeability normalized following six FMTs. Wilcoxon matched-pairs signed rank test was used to compare lactulose:mannitol at baseline and six months (p=0.375). Abnormal intestinal permeability is > 0.025 lactulose:mannitol, which is represented by the gray dotted line. Two patients had elevated small intestinal permeability at baseline and this improved in both post-FMT.
FMT was associated with significant alterations in the gut microbiota
The most abundant SVs present in the MS patients corresponded to the genera Bacteroides. At baseline, patients had significantly higher relative abundance of SVs 11, 30 and 15 (Bacteroides, Blautia faecis, and Bacteroides uniformis) than the healthy donors (effect size >1), while donors trended towards higher relative abundance of SVs 102 and 179 (Prevotella 9 and Paraprevotella, effect sizes of 0.96 and 0.92, respectively).
To determine whether patient and sample attributes (metadata) correlated with microbiota variation, CLR-transformed sample-wise Aitchison distances were evaluated using the envfit function from the vegan R package. With this approach, several metadata factors were determined to be microbiota confounders, including Patient, FMT donor and Early vs. Late randomization (Table 4). Adjusting for significant confounders with a general linear mixed model in MaAsLin2, several statistically significant multivariate associations were determined to occur over time (Table 5). These included an association with increased Muribaculaceae (family) in recipients of Donor 1, and increased peptidoglycan synthesis-associated ddl in recipients of Donor 2.
Table 4.
Significant covariates of microbiota taxonomic and functional variation.
| Microbiota Feature | Metadata | P value |
|---|---|---|
| Taxonomy | Patient | >0.001 |
| FMT Donor | >0.001 | |
| Time | 0.104 | |
| Early vs. Late | >0.001 | |
| Enzymes | Patient | >0.001 |
| FMT Donor | >0.001 | |
| Time | 0.429 | |
| Early vs. Late | 0.863 | |
| Functional Pathways | Patient | >0.001 |
| FMT Donor | >0.001 | |
| Time | 0.295 | |
| Early vs. Late | 0.282 |
Table 5.
Significant correlations in the microbiota over time after adjusting for confounders. Features are positively correlated with time (during FMT treatment period).
| Sample Cohort | Metadata | Feature | Coefficient | FDR |
|---|---|---|---|---|
| All samples | Time | SV 123: Muribaculaceae | 0.0005 | >0.001 |
| Donor 1 recipients | Time | SV 123: Muribaculaceae | 0.0006 | >0.001 |
| Donor 1 recipients | Time | SV 152: Muribaculaceae | 0.0005 | >0.001 |
| Donor 2 recipients | Time | K01921: ddl, D-alanine-D-alanine ligase | 4.29×10−6 | 0.029 |
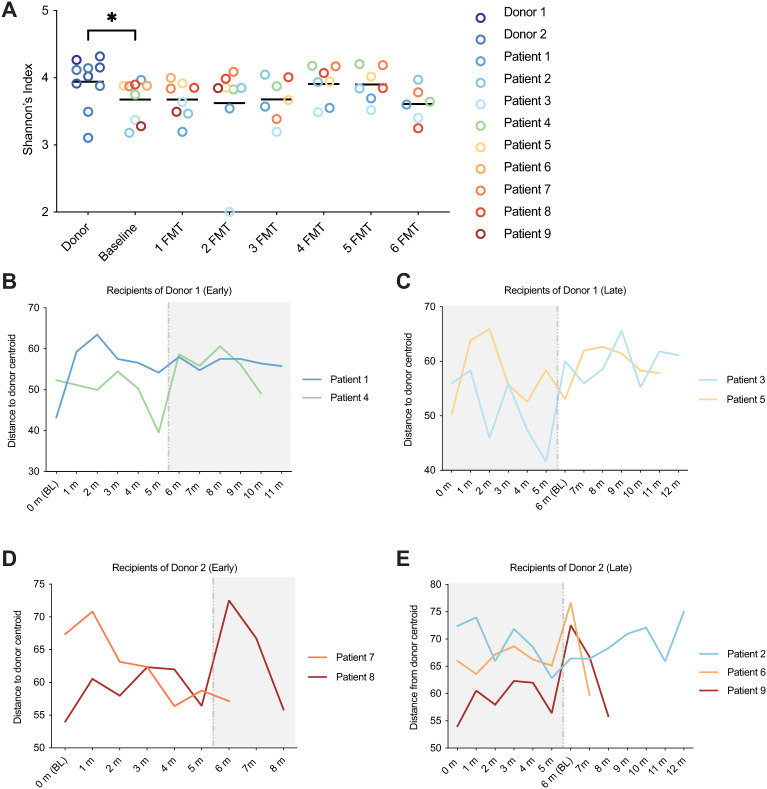
Alpha diversity as measured by Shannon's Index was calculated, and the donors were found to have a fecal microbiota diversity that was significantly higher than MS patients at baseline (P = 0.036, Mann-Whitney test), however, MS patients did not undergo a significant change in fecal microbiota diversity following repeated FMTs (Figure 4A). Microbiota beta diversity as measured by Aitchison distance (CLR-transformed Euclidian distance) was calculated and was compared between MS patients over time to the centroid value of their respective donor (Figure 4 B–E). Beta diversity between the MS patients and their donor did not significantly change over time, likely due to substantial intraindividual longitudinal variability (P = 0.399, simple linear regression).
Figure 4.
Microbiota diversity metrics of RRMS patients following multiple FMTs. A) Shannon's index of alpha diversity was higher in healthy donors than MS patients at baseline (p = 0.036, Mann-Whitney test), but did not significantly change in patients over time (P = 0.399, simple linear regression). B-E) Microbiota beta diversity was measured by Aitchison distance. Distance between MS patients and the centroid value of the Donor from which they received FMTs was not significantly different over time. White vs. grey portions of the plots represent FMT and non-FMT treatment periods, respectively.
Despite the substantial intraindividual longitudinal variability (Figure 5A), when comparing differentially abundant taxa and functional outputs between patients’ baseline and following their final FMT, the groups demonstrated numerous statistically significant alterations which were donor-specific (Figure 5B). Following the MS patient's final FMT, Donor 1 recipients were significantly enriched for SV 240 (Hungatella hathewayi), metal cation transporter mtsC, menaquinone biosynthesis-associated menC, taurine transporter tauB, and ubiquinol biosynthesis. Among other features, these patients were depleted in SVs 9, 19, and 22 (Faecalibacterium, Subdoligranulum, and Blautia, respectively), peptidoglycan biosynthesis and adenosylcobalamin salvage. Donor 2 recipients were dramatically enriched in SV 95 (Phascolarctobacterium succinatutens) and hyaluronan synthase hasA. Among other features, these patients were depleted in glycolysis and Hydrogenase-I (hyaABCD) (ALDEx2 effect sizes all >|1|).
Discussion
This study demonstrated that FMT was a safe and tolerable intervention in this group of MS patients, with the potential to normalize intestinal permeability and produce beneficial alterations to the gut microbiota. There were no significant changes observed in patients’ MRIs, EDSS, or plasma cytokine levels over the duration of the study, although the premature study termination and very low sample size due to the tragic death of the principal investigator is a significant limitation that may have hindered the detection of further beneficial outcomes of this intervention.
Previous studies identified an imbalance of cytokines in circulating blood in MS patients favoring a pro-inflammatory environment. This includes elevated levels of proinflammatory cytokines TNFα, 27 GM-CSF, 28 IL-1β, 29 and IL-6 30 compared to healthy controls, as well as a reduction in regulatory cytokines. 31 In the current study, the underpowered nature precluded accurate assessment of plasma cytokine levels, so although no significant changes were detected post-FMT or compared to healthy controls, this neither supports nor rejects the use of FMT in MS patients with respect to their cytokine profile. Recent data suggesting that FMT can induce a therapeutically useful pro-inflammatory state in patients with metastatic malignancy indicates that future studies in MS patients will need to continue to monitor for safety to assure that proinflammatory processes are not activated. 32
Current literature demonstrates that 20%-73% of MS patients have abnormal small intestinal permeability; 6 this is comparable to the 40% of patients in this study that had abnormal small intestinal permeability at baseline. Both patients with abnormal intestinal permeability improved to within the normal range (<0.025 lactulose:mannitol 26 ) following FMT. The microbiota is known to play a major role in maintaining intestinal barrier integrity, so a study with a larger sample size is needed to determine what changes in the microbiota may have caused this effect.
In this cohort, the MS patients had lower bacterial alpha diversity than the healthy donors at baseline; however, the diversity did not significantly change in the MS patients over time following multiple FMTs. This is in agreement with some, 33 but not other previous studies,34,35 which cite no difference in Shannon's Index between MS patients and healthy controls. It is therefore unlikely that bacterial alpha diversity is a significant indicator in MS, and the lack of change detected longitudinally in this study is unremarkable. Beta diversity as measured by Aitchison distance between the donor and the MS patients over time did not significantly decrease, meaning that the MS patients did not trend towards a more “donor like” microbiota. Thus, although significant changes were observed post-FMT, the donor microbiota engraftment did not increase following subsequent FMTs. However, longitudinal trends in patients 8 and 9 demonstrate persistent and cumulative effects of the repeated FMTs from Donor 2, indicating a patient- and donor-specific response to the intervention. Indeed, time was a significant covariate of microbiota variation from Donor 2, indicating that perhaps FMTs from this donor engrafted further with additional treatments.
The microbiota is a major regulator of both the innate and adaptive immune responses, 36 and bacterial species and their metabolites have been shown to both ameliorate and exacerbate MS.37,38 Here, potentially clinically relevant changes to the gut microbiota following FMT were conserved in MS patients in a donor-dependent manner. Specifically, post-FMT decreases in the MS-associated taxa Blautia and Subdoligranulum demonstrate that the intervention may exert beneficial taxonomic alterations, although a mechanistic relationship between these taxa and the disease is yet unknown. 2 Parabacteroides has previously been negatively associated with MS, and induced a protective, anti-inflammatory T cell response in mice;2,37 this genus increased following FMT in our cohort. A dramatic enrichment of Phascolarctobacterium succinatutens was observed post FMT. This species utilizes succinate to produce the multifaceted health promoting short chain fatty acid propionate.39,40 Propionate has repeatedly been shown to be depleted in both the plasma and stool of MS patients, and supplementation of propionate slows MS disease progression. 41 Health-associated Hungatella hathewayi was also enriched post-FMT. 42 Recent evidence suggests a causal link between H. hathewayi depletion in MS patients and decreased serum taurine levels. 42 Subsequent supplementation with H. hathewayi can increase circulating taurine and ameliorate MS in a mouse model.38,42 Importantly, the taurine-transporter tauB was also increased in our cohort post-FMT, suggesting a potential role of FMT in modulating the bioavailability of this noteworthy metabolite. 43
Further potentially advantageous, MS-ameliorating functional alterations occurred following FMT. Biosynthesis of the anti-inflammatory electron carrier ubiquinol was increased; oral ubiquinone supplementation has been previously shown to reduce oxidative stress, inflammation, fatigue and depression in MS patients.44–46 The menaquinone (Vitamin K)-related gene menC was increased; Vitamin K is depleted in MS patients and its oral supplementation has demonstrated protective effects in MS patients. 47 HasA, a gene encoding hyaluronan synthesis was also increased post-FMT. Although hyaluronic acid in the brain is associated with demyelinated lesions, 48 intestinal hyaluronic acid is known to decrease intestinal permeability by upregulating tight junction protein expression. 49 Hyaluronan has also been shown to greatly enrich the abundance of Akkermansia muciniphila, 49 a bacterium which is associated with MS but has only recently been linked to lower disability, and the potentially compensatory decreased immune response and amelioration of experimental autoimmune encephalomyelitis. 50 These factors all point to the ability for FMT to potentially exert clinically significant protective and preventative functional alterations to the MS microbiota.
Strengths of this study include that the microbiota of MS patients was followed for six months without any microbiome intervention, as well as for up to six months following FMT. To our knowledge, this is the first longitudinal study of the microbiome of MS patients and shows that the gut microbiota composition of MS patients can fluctuate over time without intervention, as has been previously reported in healthy individuals. 51 Repeated FMTs were administered in this study to ensure that changes in the gut microbiome persisted over time. Maintenance FMTs have proved to be beneficial at improving the efficacy of FMT for treating recurrent Clostridium difficile infections 52 and were used in a previous FMT study involving three MS patients. 53
The biggest limitation of this study was the small number of patients. While FMT did not cause any adverse events in these participants and two patients experienced an improvement in intestinal permeability, additional studies with larger more representative cohorts are required to determine the effect of FMT in MS patients. Another limitation was that patients did not receive a bowel prep or antibiotics prior to the initial FMT procedure, and this potentially limited the ability of the donor microbiota to colonize the MS patients. While antibiotics or bowel prep may have improved bacterial colonization after the first FMT, subsequent use of these methods could have had a deleterious effect on donor engraftment from previously administered FMTs. Dietary intake was not evaluated before or during this study and it is possible that changes in the gut microbiota composition could be a result of change in diet. Various MS treatments have been found to cause alterations in gut microbiota composition, and it is also possible that some of the changes observed in gut microbiota composition were caused by treatment instead of FMT. 54 However, we feel that treatment effects were very unlikely as patients did not have any MS treatment changes during the course of the study.
Overall, FMT was well tolerated in this group of MS patients, normalized elevated intestinal permeability, has the potential to beneficially alter the gut microbiota composition, and these alterations can persist with repeated FMT. Larger studies will be required to assess the efficacy of this intervention in the treatment of MS.
Supplemental Material
Supplemental material, sj-docx-1-mso-10.1177_20552173221086662 for Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial by Kait F. Al, Laura J Craven, Shaeley Gibbons, Seema Nair Parvathy, Ana Christina Wing and Chantelle Graf, Kate A Parham, Steven M Kerfoot, Hannah Wilcox, Jeremy P Burton, Marcelo Kremenchutzky, Sarah A Morrow, Courtney Casserly, Jon Meddings, Manas Sharma, Michael S. Silverman in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-doc-2-mso-10.1177_20552173221086662 for Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial by Kait F. Al, Laura J Craven, Shaeley Gibbons, Seema Nair Parvathy, Ana Christina Wing and Chantelle Graf, Kate A Parham, Steven M Kerfoot, Hannah Wilcox, Jeremy P Burton, Marcelo Kremenchutzky, Sarah A Morrow, Courtney Casserly, Jon Meddings, Manas Sharma, Michael S. Silverman in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Acknowledgements
We would like to acknowledge the London Health Sciences Foundation and Dave Postowoj for funding this study. We would like to thank Kim P Lee for performing the lactulose:mannitol small intestinal permeability analysis and Dr Susana Pearl for editing.
Footnotes
Author contributions: The study was designed by MK, MSS, JBP, and SK. KFA, LJC, and SG prepared the manuscript. KFA, LJC, and SG performed the microbiota analysis. HW extracted DNA from fecal samples. LJC and SNP prepared FMT material. CG administered FMTs and assessed patients at each visit. AW and MK recruited and assessed patients. SM and CC reviewed patients’ charts. JM performed the intestinal permeability analysis. MS analyzed MRI data. KP and SK performed cytokine analysis. All authors contributed to the revision of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the London Health Sciences Foundation,
ORCID iD: Michael S. Silverman https://orcid.org/0000-0002-0389-7656
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Shaeley Gibbons, Department of Microbiology and Immunology, Western University, London, ON, Canada.
Seema Nair Parvathy, Division of Infectious Diseases, Western University, London, ON, Canada.
Ana Christina Wing, Department of Neurology, London Health Sciences Centre, London, ON, Canada.
Chantelle Graf, Division of Infectious Diseases, Western University, London, ON, Canada.
Hannah Wilcox, Department of Microbiology and Immunology, Western University, London, ON, Canada.
Jeremy P Burton, Department of Microbiology and Immunology, Western University, London, ON, Canada; Lawson Health Research Institute, London, ON, Canada; Division of Urology, Department of Surgery, St Joseph’s Health Care, Western University, London, ON, Canada.
Courtney Casserly, Department of Neurology, London Health Sciences Centre, London, ON, Canada.
Jon Meddings, Division of Gastroenterology, University of Calgary, Calgary, AB, Canada.
Manas Sharma, Department of Radiology, Western University, London, ON, Canada.
Michael S. Silverman, Department of Microbiology and Immunology, Western University, London, ON, Canada Division of Infectious Diseases, Western University, London, ON, Canada; Lawson Health Research Institute, London, ON, Canada.
References
- 1.Gajofatto A, Benedetti MD. Treatment strategies for multiple sclerosis: when to start, when to change, when to stop? World J Clin Cases 2015; 3: 545–555. 2015/08/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schepici G, Silvestro S, Bramanti P, et al. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant 2019; 28: 1507–1527. 2019/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buscarinu MC, Fornasiero A, Romano S, et al. The contribution of gut barrier changes to multiple sclerosis pathophysiology. Front Immunol 2019; 10: 1916. 2019/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Torchinsky MB, Gobert M, et al. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature 2014; 510: 152–156. 2014/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haghikia A, Jorg S, Duscha A, et al. Dietary fatty acids directly impact central nervous system autoimmunity via the small intestine. Immunity 2015; 43: 817–829. 2015/10/22. [DOI] [PubMed] [Google Scholar]
- 6.Yacyshyn B, Meddings J, Sadowski D, et al. Multiple sclerosis patients have peripheral blood CD45RO + B cells and increased intestinal permeability. Dig Dis Sci 1996; 41: 2493–2498. 1996/12/01. [DOI] [PubMed] [Google Scholar]
- 7.Gupta S, Allen-Vercoe E, Petrof EO. Fecal microbiota transplantation: in perspective. Therap Adv Gastroenterol 2016; 9: 229–239. 2016/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilinski J, Winter K, Jasinski M, et al. Rapid resolution of COVID-19 after faecal microbiota transplantation. Gut 2021; 71: 230–232. 2021/07/08. DOI: 10.1136/gutjnl-2021-325010. [DOI] [PubMed] [Google Scholar]
- 9.Chen D, Wu J, Jin D, et al. Fecal microbiota transplantation in cancer management: current status and perspectives. Int J Cancer 2019; 145: 2021–2031. 2018/11/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craven L, Rahman A, Nair Parvathy S, et al. Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol 2020; 115: 1055–1065. 2020/07/04. [DOI] [PubMed] [Google Scholar]
- 11.Craven LJ, Nair Parvathy S, Tat-Ko J, et al. Extended screening costs associated with selecting donors for fecal microbiota transplantation for treatment of metabolic syndrome-associated diseases. Open Forum Infect Dis 2017; 4: ofx243. 2017/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohlke F, Stollman N. Fecal microbiota transplantation in relapsing clostridium difficile infection. Therap Adv Gastroenterol 2012; 5: 403–420. 2012/11/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mexhitaj I, Nyirenda MH, Li R, et al. Abnormal effector and regulatory T cell subsets in paediatric-onset multiple sclerosis. Brain 2019; 142: 617–632. 2019/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. 1983/11/01. [DOI] [PubMed] [Google Scholar]
- 15.Arrieta MC, Bistritz L, Meddings JB. Alterations in intestinal permeability. Gut 2006; 55: 1512–1520. 2006/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al KF, Bisanz JE, Gloor GB, et al. Evaluation of sampling and storage procedures on preserving the community structure of stool microbiota: a simple at-home toilet-paper collection method. J Microbiol Methods 2018; 144: 117–121. 2017/11/21. [DOI] [PubMed] [Google Scholar]
- 17.Al KF, Denstedt JD, Daisley BA, et al. Ureteral stent microbiota is associated with patient comorbidities but not antibiotic exposure. Cell Rep Med 2020; 1: 100094. 2020/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callahan BJ, McMurdie PJ, Rosen MJ, et al. DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 2016; 13: 581–583. 2016/05/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quast C, Pruesse E, Yilmaz P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 2013; 41: D590–D596. 2012/11/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douglas GM, Maffei VJ, Zaneveld JR, et al. PICRUSt2 for prediction of metagenome functions. Nat Biotechnol 2020; 38: 685–688. 2020/06/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gloor GB, Reid G. Compositional analysis: a valid approach to analyze microbiome high-throughput sequencing data. Can J Microbiol 2016; 62: 692–703. 2016/06/18. [DOI] [PubMed] [Google Scholar]
- 22.Fernandes AD, Macklaim JM, Linn TG, et al. ANOVA-like differential expression (ALDEx) analysis for mixed population RNA-seq. PLoS One 2013; 8: e67019. 2013/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mallick H, Rahnavard A, McIver LJ, et al. Multivariable association discovery in population-scale meta-omics studies. bioRxiv 2021. 2021.2001.2020.427420. DOI: 10.1101/2021.01.20.427420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oksanen JF, Blanchet G, Friendly M, et al. vegan: Community Ecology Package. R package version 2.5–6. https://CRAN.R-project.org/package=vegan (2019).
- 25.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria 2019.
- 26.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil 2010; 22: e15–e26. 2009/07/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol 2010; 67: 452–461. 2010/05/04. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Rezk A, Miyazaki Y, et al. Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med 2015; 7: 310ra166. 2015/10/23. [DOI] [PubMed] [Google Scholar]
- 29.Kallaur AP, Oliveira SR, Simao ANC, et al. Cytokine profile in patients with progressive multiple sclerosis and its association with disease progression and disability. Mol Neurobiol 2017; 54: 2950–2960. 2016/03/30. [DOI] [PubMed] [Google Scholar]
- 30.Barr TA, Shen P, Brown S, et al. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med 2012; 209: 1001–1010. 2012/05/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol 2007; 178: 6092–6099. 2007/05/04. [DOI] [PubMed] [Google Scholar]
- 32.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021; 371: 595–602. 2021/02/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen J, Chia N, Kalari KR, et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep 2016; 6: 28484. 2016/06/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun 2016; 7: 12015. 2016/06/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ventura RE, Iizumi T, Battaglia T, et al. Gut microbiome of treatment-naive MS patients of different ethnicities early in disease course. Sci Rep 2019; 9: 16396. 2019/11/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science 2010; 330: 1768–1773. 2011/01/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cekanaviciute E, Yoo BB, Runia TF, et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc Natl Acad Sci U S A 2017; 114: 10713–10718. 2017/09/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian J, Song M, Kaufman DL. Homotaurine limits the spreading of T cell autoreactivity within the CNS and ameliorates disease in a model of multiple sclerosis. Sci Rep 2021; 11: 5402. 2021/03/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosseini E, Grootaert C, Verstraete W, et al. Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 2011; 69: 245–258. 2011/04/28. [DOI] [PubMed] [Google Scholar]
- 40.Ikeyama N, Murakami T, Toyoda A, et al. Microbial interaction between the succinate-utilizing bacterium phascolarctobacterium faecium and the gut commensal bacteroides thetaiotaomicron. Microbiologyopen 2020; 9: e1111. 2020/08/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tobin D, Vige R, Calder PC. Review: the nutritional management of multiple sclerosis with propionate. Front Immunol 2021; 12: 676016. 2021/08/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li H, Xu H, Li Y, et al. Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat Commun 2020; 11: 3218. 2020/06/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eichhorn E, van der Ploeg JR, Leisinger T. Deletion analysis of the escherichia coli taurine and alkanesulfonate transport systems. J Bacteriol 2000; 182: 2687–2695. 2000/04/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moccia M, Capacchione A, Lanzillo R, et al. Coenzyme Q10 supplementation reduces peripheral oxidative stress and inflammation in interferon-beta1a-treated multiple sclerosis. Ther Adv Neurol Disord 2019; 12: 1756286418819074. 2019/03/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanoobar M, Dehghan P, Khalili M, et al. Coenzyme Q10 as a treatment for fatigue and depression in multiple sclerosis patients: a double blind randomized clinical trial. Nutr Neurosci 2016; 19: 138–143. 2015/01/21. [DOI] [PubMed] [Google Scholar]
- 46.Sanoobar M, Eghtesadi S, Azimi A, et al. Coenzyme Q10 supplementation ameliorates inflammatory markers in patients with multiple sclerosis: a double blind, placebo, controlled randomized clinical trial. Nutr Neurosci 2015; 18: 169–176. 2014/03/14. [DOI] [PubMed] [Google Scholar]
- 47.Popescu DC, Huang H, Singhal NK, et al. Vitamin K enhances the production of brain sulfatides during remyelination. PLoS One 2018; 13: e0203057. 2018/08/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Back SA, Tuohy TM, Chen H, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med 2005; 11: 966–972. 2005/08/09. [DOI] [PubMed] [Google Scholar]
- 49.Mao T, Su CW, Ji Q, et al. Hyaluronan-induced alterations of the gut microbiome protects mice against citrobacter rodentium infection and intestinal inflammation. Gut Microbes 2021; 13: 1972757. 2021/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox LM, Maghzi AH, Liu S, et al. Gut microbiome in progressive multiple sclerosis. Ann Neurol 2021; 89: 1195–1211. 2021/04/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Faith JJ, Guruge JL, Charbonneau M, et al. The long-term stability of the human gut microbiota. Science 2013; 341: 1237439. 2013/07/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kao D, Roach B, Silva M, et al. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA 2017; 318: 1985–1993. 2017/11/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Borody T, Leis S, Campbell J, et al. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am J Gastroenterol 2011; 106: S352. [Google Scholar]
- 54.Budhram A, Parvathy S, Kremenchutzky M, et al. Breaking down the gut microbiome composition in multiple sclerosis. Mult Scler 2017; 23: 628–636. 2016/12/14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mso-10.1177_20552173221086662 for Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial by Kait F. Al, Laura J Craven, Shaeley Gibbons, Seema Nair Parvathy, Ana Christina Wing and Chantelle Graf, Kate A Parham, Steven M Kerfoot, Hannah Wilcox, Jeremy P Burton, Marcelo Kremenchutzky, Sarah A Morrow, Courtney Casserly, Jon Meddings, Manas Sharma, Michael S. Silverman in Multiple Sclerosis Journal – Experimental, Translational and Clinical
Supplemental material, sj-doc-2-mso-10.1177_20552173221086662 for Fecal microbiota transplantation is safe and tolerable in patients with multiple sclerosis: A pilot randomized controlled trial by Kait F. Al, Laura J Craven, Shaeley Gibbons, Seema Nair Parvathy, Ana Christina Wing and Chantelle Graf, Kate A Parham, Steven M Kerfoot, Hannah Wilcox, Jeremy P Burton, Marcelo Kremenchutzky, Sarah A Morrow, Courtney Casserly, Jon Meddings, Manas Sharma, Michael S. Silverman in Multiple Sclerosis Journal – Experimental, Translational and Clinical