Abstract
The oxygenase component of toluene dioxygenase from Pseudomonas putida F1 is an iron-sulfur protein (ISPTOL) consisting of α (TodC1) and β (TodC2) subunits. Purified TodC1 gave absorbance and electron paramagnetic resonance spectra identical to those given by purified ISPTOL. TodC1 was reduced by NADH and catalytic amounts of ReductaseTOL and FerredoxinTOL. Reduced TodC1 did not oxidize toluene, and catalysis was strictly dependent on the presence of purified TodC2.
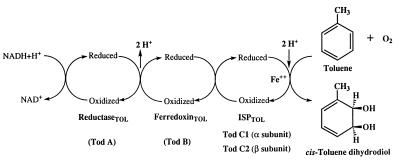
Toluene dioxygenase (TDO; EC 1.14.12) catalyzes the first reaction in the degradation of toluene by Pseudomonas putida F1 (33). TDO is a multicomponent enzyme system that oxidizes toluene to (+)-cis-(1S,2R)-dihydroxy-3-methylcyclohexa-3,5-diene (cis-toluene dihydrodiol) (10, 18, 33). The organization of the TDO system is shown in Fig. 1. Electrons are transferred from NADH through a flavoprotein reductase (ReductaseTOL [28]) to a Rieske [2Fe-2S] protein (FerredoxinTOL [29]). The latter reduces the oxygenase component, an iron-sulfur protein (ISPTOL [27]) which, in the presence of exogenous ferrous iron, catalyzes the stereospecific addition of dioxygen to the aromatic nucleus.
FIG. 1.
Electron flow in the TDO system.
ISPTOL has an α2β2 subunit composition, and the α and β subunits are encoded by the todC1 and todC2 genes, respectively (36). To fully understand the functions of the α and β subunits of ISPTOL in overall enzyme activity, including electron transfer, substrate binding, and oxygen activation, the individual subunits require further study. The purification of the β subunit from a clone expressing the todC2 gene has been recently reported (15). The α subunit encoded by the todC1 gene is the focus of this study. We report the construction of a high-expression clone for todC1, purification and properties of TodC1, electron transfer between FerredoxinTOL and TodC1, and the in vitro reconstitution of high levels of TDO activity from the purified TodC1 and TodC2 subunits. These studies provide essential preliminary information necessary for future detailed biophysical studies.
Construction of a todC1 expression clone.
In order to construct a todC1 high-expression clone, the todC2BA genes were deleted from pDTG601A (36) by digestion with PstI, followed by religation, to form plasmid pDTG612A. This clone contains the todC1 gene and the first 66 bp of the todC2 gene. To delete the remaining todC2 coding sequence, pDTG612A was digested with DraIII and PstI, treated with S1 nuclease to form blunt ends (25), and religated. The resulting plasmid, pDTG626, expresses high levels of todC1, and strain JM109(pDTG626) was used for the purification of TodC1.
Growth conditions for JM109(pDTG626) were first optimized by varying the temperature, medium, inducer concentration, and cell yield in order to produce maximal amounts of soluble TodC1. The best soluble preparation of TodC1 was obtained from cells grown in Luria broth (5) containing ampicillin (100 μg/ml) at 30°C and induced with 200 μM isopropyl-β-d-thiogalactopyranoside for 2 h when the turbidity at 600 nm reached 0.7. Cells were harvested by centrifugation and stored at −70°C.
Purification of TodC1.
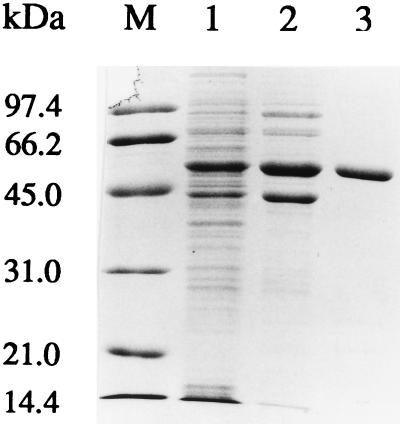
JM109(pDTG626) cell extract was prepared from frozen cells (51 g [wet weight]) as described previously (15), except that the frozen cells were suspended in buffer A (50 mM bis-Tris buffer, pH 6.8, containing 1 mM dithiothreitol and 5% glycerol). The cell extract (2.89 g of protein) was applied to a Q-Sepharose column (5 by 16 cm) (Pharmacia Biotech, Piscataway, N.J.) which had been previously equilibrated with buffer A at a flow rate of 2 ml/min. Unbound proteins were washed from the column with the same buffer at a flow rate of 4 ml/min. Bound proteins were eluted with a linear salt gradient of 0 to 600 mM KCl in buffer A at a flow rate of 2 ml/min. Fractions containing TodC1 were red-brown in color, and their absorption spectra were monitored from 300 to 700 nm. Fractions containing TodC1 were pooled and concentrated by ultrafiltration with a 30-kDa cutoff membrane filter (Amicon, Danvers, Mass.). The concentrated solution was exchanged into 5 mM potassium phosphate buffer (pH 6.8) by ultrafiltration as described above and applied to a hydroxyapatite column (1.0 by 15 cm; Bio-Rad Laboratories, Hercules, Calif.) that had been preequilibrated with 5 mM potassium phosphate buffer. Bound proteins were eluted with a 5 to 100 mM phosphate buffer (pH 6.8) gradient at a flow rate of 0.4 ml/min. Fractions containing TodC1 were concentrated, dialyzed against 50 mM 2-[N-morpholino]ethanesulfonic acid (MES) buffer (pH 6.8), and stored at −70°C. TodC1 was purified to approximately 95% homogeneity from the crude cell extract (Fig. 2, lane 3) by the two-step procedure. A 13-fold purification of the enzyme was achieved, with recovery of 56% of the activity present in the crude cell extract (Table 1). The increase in total activity after hydroxyapatite chromatography may be due to the removal of competing NADH oxidase activity. This aspect was not pursued further in the present study.
FIG. 2.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of samples taken during the purification of TodC1. Fractions obtained from different steps were analyzed on 12% polyacrylamide gels stained with Coomassie blue. Lanes: M, molecular mass standards; 1, cell extract (10 μg of protein); 2, Q-Sepharose column fraction containing TodC1 (7 μg of protein); 3, hydroxyapatite column fraction containing TodC1 (5 μg of protein).
TABLE 1.
Purification of TodC1 from JM109(pDTG626)a
| Purification step | Total protein (mg) | Activityb (U) | Sp act (U/mg of TodC1) | Recovery (%) | Purifi-cation (fold) |
|---|---|---|---|---|---|
| Crude cell extract | 2,893 | 554 | 0.19 | 100 | 1 |
| Q-Sepharose | 378 | 196 | 0.52 | 35 | 2.7 |
| Hydroxyapatite | 126 | 310 | 2.46 | 56 | 13 |
TDO activity was determined by measuring the formation of radioactive cis-toluene dihydrodiol (32). Purified TodC1 (20), TodB, and TodA (19) were used to assay pooled fractions from each column. Protein concentrations were determined by the method of Bradford (2) with bovine serum albumin as the standard.
One unit of activity was defined as the amount of protein required to form 1 μmol of cis-toluene dihydrodiol per min.
Reconstitution of TDO activity.
When purified TodC1 was incubated for 30 min at ambient temperature with saturating amounts of purified TodC2, TodB (FerredoxinTOL), and TodA (ReductaseTOL), the specific activity was 2.46 U/mg of TodC1 (Table 1). This activity is 66% of that obtained with purified native ISPTOL when specific activity is calculated per milligram of TodC1 (20) and over five times that given by TodC1 in crude cell extracts (15). The turnover number of reconstituted ISPTOL was 2.1 s−1, compared to a turnover number of 3.2 s−1 obtained with native ISPTOL (20). TDO activity was not observed when either TodC1 or TodC2 was omitted from the reconstitution assay. These results indicate that purified TodC1 and TodC2 can readily assemble in vitro to yield active ISPTOL and that both subunits are essential for TDO activity.
Properties of TodC1.
The properties of purified TodC1 and ISPTOL are shown in Table 2. The N-terminal amino acid sequence of TodC1 is identical to that predicted from its nucleotide sequence (35). The absorption spectrum of purified TodC1 showed a broad peak at 446 nm, with a shoulder around 558 nm and a peak at 325 nm, and was identical to the spectrum obtained with oxidized ISPTOL (20). Purified TodC2 shows no absorption in the 300 to 700-nm range (20).
TABLE 2.
Properties of purified TodC1 and ISPTOLa
| Protein | Absorption spectra, λmax (ɛ, mM−1, cm−1)b
|
Mean ± SD content (g-atoms mol−1) of:
|
EPR spectrae
|
|||||
|---|---|---|---|---|---|---|---|---|
| Oxidized | Reduced | Ironc | Acid-labile sulfided | Oxidized | Reduced
|
|||
| gx | gy | gz | ||||||
| TodC1 | 325 (10.33), 446 (6.67), 558 (3.67) | 376 (5.60), 526 (2.73) | 1.98 ± 0.14 | 1.91 ± 0.16 | Absent | 1.76 | 1.91 | 2.01 |
| ISPTOL | 325 (11.03), 446 (5.24), 558 (2.76) | 376 (4.93), 526 (2.50) | 2.02 ± 0.19 | 1.90 ± 0.08 | Absent | 1.76 | 1.91 | 2.01 |
ISPTOL was purified as described previously. The N-terminal sequence of purified TodC1 is MNQTDTSPIR.
For TodC1, based on α; for ISPTOL, based on αβ.
Determined by the method of Zabinski et al. (34).
Determined by the method of Beinert (1).
Recorded at 77 K with a Bruker ESP300 spectrometer with the following settings: 5.02 mW of microwave power, a 3,600-G centerfield, a 9.29-GHz microwave modulation frequency, a 42-s sweep time, and a receiver gain of 1.0 × 105.
TodC1 (α subunit) and ISPTOL (αβ heterodimer) each contained approximately 2 atoms each of iron and acid-labile sulfide (Table 2). TodC2 (β subunit) does not contain detectable amounts of iron or sulfur (20). These results are consistent with the presence of one Rieske [2Fe-2S] center in each TodC1 subunit.
The electron paramagnetic resonance (EPR) spectra of purified TodC1, native ISPTOL, and reconstituted ISPTOL were recorded at 77 K in both the oxidized and reduced states. Reduction was achieved by addition of excess sodium dithionite. The oxidized forms of TodC1, native ISPTOL, and reconstituted ISPTOL are EPR silent. The EPR spectrum of reduced TodC1 gave characteristic Rieske [2Fe-2S] signals at gx = 1.76, gy = 1.91, and gz = 2.01 (7). Identical reduced EPR spectra were given by native ISPTOL and reconstituted ISPTOL (Table 2).
Reconstituted and native ISPTOL behaved identically on native polyacrylamide gels (data not shown), indicating that the two preparations had the same subunit structure.
Electron transfer to purified TodC1.
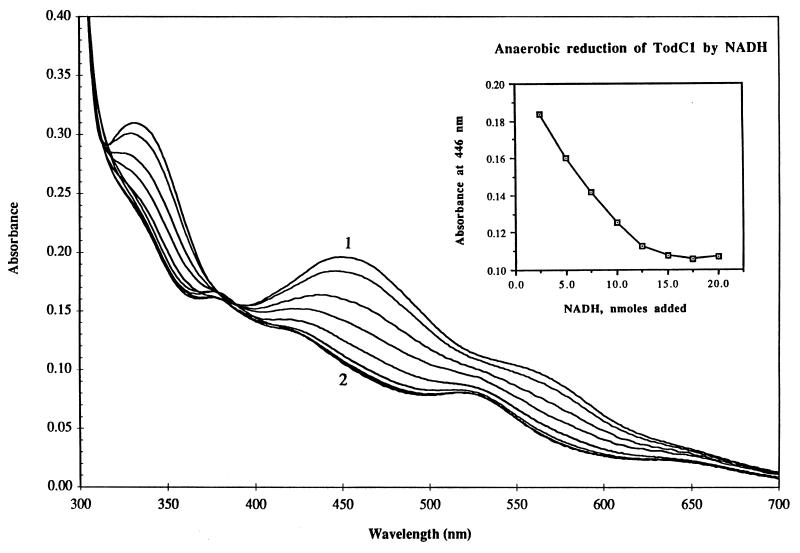
The reduction of TodC1 by NADH in the presence of catalytic quantities of TodA and TodB is shown in Fig. 3. When the absorbance at 446 nm was plotted against the amount of NADH added, a linear decrease in absorbance was observed (Fig. 3, inset). An endpoint was reached when 15 nmol of NADH was added to the solution containing 30 nmol of TodC1. These results show that TodC1 can accept electrons from reduced TodB (ferredoxinTOL) in the absence of TodC2 and also indicate that each TodC1 subunit can accept one electron. Upon exposure to air, reduced TodC1 was rapidly reoxidized to give its original oxidized spectrum.
FIG. 3.
Anaerobic reduction of TodC1. Absorption spectra were recorded on an Aminco DW2000 UV-visible spectrophotometer. Cuvettes were rendered anaerobic by alternately flushing and evacuating them with argon. Reaction mixtures contained TodC1 (30 nmol) in 1 ml of MES buffer (pH 6.8). Curve 1 shows the oxidized spectrum of TodC1. The curves between 1 and 2 are spectra of TodC1 obtained by anaerobic additions of NADH (2.5 nmol of each) in the presence of catalytic amounts of TodB (ferredoxinTOL, 90 μg of protein) and TodA (reductaseTOL, 45 μg of protein). The inset shows anaerobic reduction of TodC1 by NADH in the presence of TodB and TodA. The decrease in absorbance for TodC1 was monitored at 446 nm.
Discussion.
It is generally accepted that the α subunits of aromatic-ring-hydroxylating dioxygenases contain a Rieske [2Fe-2S] center and mononuclear iron with the latter being located at the active site of the enzyme (3). This generalization is based on rigorous biophysical studies conducted with phthalate (4, 11) and benzene (9, 21, 26) dioxygenases and the presence of the conserved motif C-X-H-X15–17-C-X2-H in the deduced amino acid sequences of all of the aromatic-ring-hydroxylating dioxygenases that have been reported to date (3, 23). These results have been confirmed by the recent report of the structure of the oxygenase component of naphthalene dioxygenase (16). In the current study, the purified α subunit (TodC1) of TDO, which contains the conserved Rieske center motif (36), was shown to have optical and EPR spectra that are characteristic of Rieske [2Fe-2S] proteins (Table 2). This preparation provided the opportunity to examine the role of TodC1 in electron transport and catalysis.
Figure 3 shows that TodC1 can accept electrons from reduced FerredoxinTOL in a reaction that is not dependent on the presence of the β subunit (TodC2). However, reduced TodC1, in the presence of air, was unable to oxidize toluene to cis-toluene dihydrodiol unless TodC2 was present. In a recent review, Butler and Mason suggested that the β subunit of the benzene dioxygenase oxygenase component might be involved in Ferredoxin docking and electron transfer (3). The results discussed above show that while the β subunit (TodC2) is essential for catalysis, it is not required for reduction of the Rieske [2Fe-2S] center in TodC1.
Active terminal oxygenase components from biphenyl dioxygenase (14), naphthalene dioxygenase (30), and benzene dioxygenase (21) have been reconstituted from separately produced α and β subunits in crude cell extracts with various degrees of success. Hurtubise et al. purified and characterized His-tagged α and β subunits of the oxygenase component from biphenyl dioxygenase (14). However, reconstitution experiments with purified His-tagged α and β subunits did not yield significant activity. Thus, the present work represents the first report of the reconstitution of a highly active form of a terminal oxygenase component (ISPTOL) from its purified α and β subunits.
The function of the β subunit of ISPTOL remains unclear. It contains no detectable prosthetic groups and is absolutely required for activity. A study of the isofunctional ISP β subunit from the toluate dioxygenase enzyme system suggested that it may play a role in substrate specificity (12). Work by Furukawa and coworkers with biphenyl and toluene dioxygenases has suggested that the β subunit may contribute to substrate specificity (8, 13), but accumulating evidence obtained with a variety of dioxygenase systems indicates that the α subunit is the major contributor to substrate specificity (6, 17, 22, 24, 31). All α subunits from aromatic ring-hydroxylating dioxygenases contain a conserved aspartate residue at position 205 (naphthalene dioxygenase numbering [16]). This amino acid may play a major role in the transfer of electrons from a Rieske [2Fe-2S] center in one α subunit to mononuclear iron near the active site in an adjacent α subunit. If this is the case, the β subunit may function mainly in a structural capacity to maintain contact between adjacent α subunits.
In conclusion, we report for the first time the purification and properties of the α subunit of ISPTOL, the detection of electron transport between purified TodC1 and FerredoxinTOL, the reconstitution of ISPTOL activity from the purified TodC1 and TodC2 subunits, and the absolute requirement of TodC2 for catalysis. These studies are an essential prerequisite for future investigations of subunit interactions and their role in substrate specificity, electron transfer, and oxygen activation.
Acknowledgments
This work was supported by U.S. Public Health Service grant GM29909 from the National Institute of General Medical Sciences.
We thank K. Lee for providing purified reductaseTOL, J. D. Haddock for helpful discussions, J. V. Parales for technical help in the N-terminal amino acid sequence determination, and G. Buettner for determining the EPR spectra at the University of Iowa ESR facility.
REFERENCES
- 1.Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990;4:2483–2491. doi: 10.1096/fasebj.4.8.2185975. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Butler C S, Mason J R. Structure-function analysis of the bacterial aromatic ring-hydroxylating dioxygenases. Adv Microb Physiol. 1997;38:47–84. doi: 10.1016/s0065-2911(08)60155-1. [DOI] [PubMed] [Google Scholar]
- 4.Cline J F, Hoffman B M, Mims W B, LaHaie E, Ballou D P, Fee J A. Evidence for N coordination to Fe in the [2Fe-2S] clusters of Thermus Rieske protein and phthalate dioxygenase from Pseudomonas. J Biol Chem. 1985;260:3251–3254. [PubMed] [Google Scholar]
- 5.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1980. [Google Scholar]
- 6.Erickson B D, Mondello F J. Enhanced biodegradation of polychlorinated biphenyls after site-directed mutagenesis of a biphenyl dioxygenase gene. Appl Environ Microbiol. 1993;59:3858–3862. doi: 10.1128/aem.59.11.3858-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fee J A, Findling K L, Yoshida T, Hille R, Tarr G E, Hearshen D O, Dunham W R, Day E P, Kent T A, Münck E. Purification and characterization of the Rieske iron-sulfur protein from Thermus thermophilus. J Biol Chem. 1984;259:124–133. [PubMed] [Google Scholar]
- 8.Furukawa K, Kimura N, Iwakiri R, Nishi A, Suyama A. Construction of hybrid operons conferring expanded capability for degrading aromatic hydrocarbons and chlorinated compounds. In: Nakazawa T, Furukawa K, Haas D, Silver S, editors. Molecular biology of pseudomonads. Washington, D.C: ASM Press; 1996. pp. 81–93. [Google Scholar]
- 9.Geary P J, Saboowalla F, Patil D, Cammack R. An investigation of the iron-sulphur proteins of benzene dioxygenase from Pseudomonas putida by electron-spin-resonance spectroscopy. Biochem J. 1984;217:667–673. doi: 10.1042/bj2170667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson D T, Hensley M, Yoshioka H, Mabry T J. Formation of (+)-cis-2,3-dihydroxy-1-methylcyclohexa-4,6-diene from toluene by Pseudomonas putida. Biochemistry. 1970;9:1626–1630. doi: 10.1021/bi00809a023. [DOI] [PubMed] [Google Scholar]
- 11.Gurbiel R J, Batie C J, Sivaraja M, True A E, Fee J A, Hoffman B M, Ballou D P. Electron-nuclear double resonance spectroscopy of 15N-enriched phthalate dioxygenase from Pseudomonas cepacia proves that two histidines are coordinated to the [2Fe-2S] Rieske-type clusters. Biochemistry. 1989;28:4861–4871. doi: 10.1021/bi00437a051. [DOI] [PubMed] [Google Scholar]
- 12.Harayama S, Rekik M, Timmis K N. Genetic analysis of a relaxed substrate specificity aromatic ring dioxygenase, toluate 1,2-dioxygenase, encoded by TOL plasmid pWWO of Pseudomonas putida. Mol Gen Genet. 1986;202:226–234. doi: 10.1007/BF00331641. [DOI] [PubMed] [Google Scholar]
- 13.Hirose J, Suyama A, Hayashida S, Furukawa K. Construction of hybrid biphenyl (bph) and toluene (tod) genes for functional analysis of aromatic ring dioxygenases. Gene. 1994;138:27–33. doi: 10.1016/0378-1119(94)90779-x. [DOI] [PubMed] [Google Scholar]
- 14.Hurtubise Y, Barriault D, Sylvestre M. Characterization of active recombinant His-tagged oxygenase component of Comamonas testosteroni B-356 biphenyl dioxygenase. J Biol Chem. 1996;271:8152–8156. doi: 10.1074/jbc.271.14.8152. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Parales R E, Lynch N A, Gibson D T. Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potential mononuclear non-heme iron coordination sites. J Bacteriol. 1996;178:3133–3139. doi: 10.1128/jb.178.11.3133-3139.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kauppi B, Lee K, Carredano E, Parales R E, Gibson D T, Eklund H, Ramaswamy S. Structure of an aromatic-ring-hydroxylating dioxygenase—naphthalene 1,2-dioxygenase. Structure. 1998;6:571–586. doi: 10.1016/s0969-2126(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 17.Kimura N, Nishi A, Goto M, Furukawa K. Functional analyses of a variety of chimeric dioxygenases constructed from two biphenyl dioxygenases that are similar structurally but different functionally. J Bacteriol. 1997;179:3936–3943. doi: 10.1128/jb.179.12.3936-3943.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobal V M, Gibson D T, Davis R E, Garza A. X-ray determination of the absolute stereochemistry of the initial oxidation product formed from toluene by Pseudomonas putida 39/D. J Am Chem Soc. 1973;95:4420–4421. doi: 10.1021/ja00794a048. [DOI] [PubMed] [Google Scholar]
- 19.Lee K. Biochemical studies on toluene and naphthalene dioxygenases. Ph.D. thesis. Iowa City: The University of Iowa; 1995. [Google Scholar]
- 20.Lynch N A, Jiang H, Gibson D T. Rapid purification of the oxygenase component of toluene dioxygenase from a polyol-responsive monoclonal antibody. Appl Environ Microbiol. 1996;62:2133–2137. doi: 10.1128/aem.62.6.2133-2137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mason J R, Butler C S, Cammack R, Shergill J K. Structural studies on the catalytic components of benzene dioxygenase from Pseudomonas putida. Biochem Soc Trans. 1997;25:90–95. doi: 10.1042/bst0250090. [DOI] [PubMed] [Google Scholar]
- 22.Mondello F J, Turcich M P, Lobos J H, Erickson B D. Identification and modification of biphenyl dioxygenase sequences that determine the specificity of polychlorinated biphenyl degradation. Appl Environ Microbiol. 1997;63:3096–3103. doi: 10.1128/aem.63.8.3096-3103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neidle E L, Hartnett C, Ornston L N, Bairock A, Rekik M, Harayama S. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J Bacteriol. 1991;173:5385–5395. doi: 10.1128/jb.173.17.5385-5395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parales R E, Emig M D, Lynch N A, Gibson D T. Substrate specificities of hybrid naphthalene and 2,4-dinitrotoluene dioxygenase enzyme systems. J Bacteriol. 1998;180:2337–2344. doi: 10.1128/jb.180.9.2337-2344.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 26.Shergill J K, Joannou C L, Mason J R, Cammack R. Coordination of the Rieske-type [2Fe-2S] cluster of the terminal iron-sulfur protein of Pseudomonas putida benzene 1,2-dioxygenase, studied by one- and two-dimensional electron spin-echo envelope modulation spectroscopy. Biochemistry. 1995;34:16533–16542. doi: 10.1021/bi00051a001. [DOI] [PubMed] [Google Scholar]
- 27.Subramanian V, Liu T-N, Yeh W-K, Gibson D T. Toluene dioxygenase: purification of an iron-sulfur protein by affinity chromatography. Biochem Biophys Res Commun. 1979;91:1131–1139. doi: 10.1016/0006-291x(79)91998-3. [DOI] [PubMed] [Google Scholar]
- 28.Subramanian V, Liu T-N, Yeh W-K, Narro M, Gibson D T. Purification and properties of NADH-ferredoxinTOL reductase: a component of toluene dioxygenase from Pseudomonas putida. J Biol Chem. 1981;256:2723–2730. [PubMed] [Google Scholar]
- 29.Subramanian V, Liu T-N, Yeh W-K, Serdar C M, Wackett L P, Gibson D T. Purification and properties of ferredoxinTOL: a component of toluene dioxygenase from Pseudomonas putida F1. J Biol Chem. 1985;260:2355–2363. [PubMed] [Google Scholar]
- 30.Suen W-C, Gibson D T. Recombinant Escherichia coli strains synthesize active forms of naphthalene dioxygenase and its individual α and β subunits. Gene. 1994;143:67–71. doi: 10.1016/0378-1119(94)90606-8. [DOI] [PubMed] [Google Scholar]
- 31.Tan H-M, Cheong C-M. Substitution of the ISPα subunit of biphenyl dioxygenase from Pseudomonas results in a modification of the enzyme activity. Biochem Biophys Res Commun. 1994;204:912–917. doi: 10.1006/bbrc.1994.2546. [DOI] [PubMed] [Google Scholar]
- 32.Wackett L P. Toluene dioxygenase from Pseudomonas putida F1. Methods Enzymol. 1990;188:39–45. doi: 10.1016/0076-6879(90)88010-8. [DOI] [PubMed] [Google Scholar]
- 33.Yeh W-K, Gibson D T, Liu T-N. Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun. 1977;78:401–410. doi: 10.1016/0006-291x(77)91268-2. [DOI] [PubMed] [Google Scholar]
- 34.Zabinski R, Münck E, Champion P M, Wood J M. Kinetic and Mössbauer studies on the mechanism of protocatechuic acid 4,5-oxygenase. Biochemistry. 1972;11:3212–3219. doi: 10.1021/bi00767a012. [DOI] [PubMed] [Google Scholar]
- 35.Zylstra G J, Gibson D T. Toluene degradation by Pseudomonas putida F1: nucleotide sequence of the todC1C2BADE genes and their expression in E. coli. J Biol Chem. 1989;264:14940–14946. [PubMed] [Google Scholar]
- 36.Zylstra G J, Gibson D T. Aromatic hydrocarbon degradation: a molecular approach. In: Setlow J K, editor. Genetic engineering: principles and methods. New York, N.Y: Plenum Press; 1991. pp. 183–203. [DOI] [PubMed] [Google Scholar]