Abstract
Fragility fracture is a worldwide problem and a main cause of disability and impaired quality of life. It is primarily caused by osteoporosis, characterized by impaired bone quantity and or quality. Proper diagnosis of osteoporosis is essential for prevention of fragility fractures. Osteoporosis can be primary in postmenopausal women because of estrogen deficiency. Secondary forms of osteoporosis are not uncommon in both men and women. Most systemic illnesses and organ dysfunction can lead to osteoporosis. The kidney plays a crucial role in maintaining physiological bone homeostasis by controlling minerals, electrolytes, acid-base, vitamin D and parathyroid function. Chronic kidney disease with its uremic milieu disturbs this balance, leading to renal osteodystrophy. Diabetes mellitus represents the most common secondary cause of osteoporosis. Thyroid and parathyroid disorders can dysregulate the osteoblast/osteoclast functions. Gastrointestinal disorders, malnutrition and malabsorption can result in mineral and vitamin D deficiencies and bone loss. Patients with chronic liver disease have a higher risk of fracture due to hepatic osteodystrophy. Proinflammatory cytokines in infectious, autoimmune, and hematological disorders can stimulate osteoclastogenesis, leading to osteoporosis. Moreover, drug-induced osteoporosis is not uncommon. In this review, we focus on causes, pathogenesis, and management of secondary osteoporosis.
Keywords: bone loss, fracture, bone mineral density, causes, management
1. Introduction
Osteoporosis is a condition characterized by bone fragility, secondary to either low bone mineral density (BMD) and/or microarchitectural deterioration that increases fracture risk. Postmenopausal estrogen deficiency is the primary cause of osteoporosis. In addition to postmenopausal women with primary osteoporosis (postmenopausal or age-related), more than half of perimenopausal and postmenopausal women referred to an osteoporosis center had one or more risk factors of secondary osteoporosis [1]. A fracture risk assessment tool (FRAX) helps to estimate the 10-year fracture risk by using clinical and radiological data. These clinical data include some, but not all, secondary causes of osteoporosis, such as smoking, excessive alcohol intake, type I diabetes mellitus, hyperthyroidism, chronic liver disease, and malnutrition [2]. Various secondary causes of osteoporosis are mentioned in Figure 1. Patients with newly diagnosed osteoporosis should be thoroughly evaluated including their history, a physical examination, and routine laboratory testing for detection of secondary causes. A systematic approach for detection of the underlying causes is illustrated in Figure 2. The management approach of patients with secondary osteoporosis is summarized in Figure 3. Proper recognition of the etiology of osteoporosis is an essential step in improving bone health, preventing further bone loss. Those patients can benefit from balanced nutrition, physical exercise, and avoiding long term glucocorticoid usage and other drugs that have negative impact on bone health. Using antiosteoporotic therapies in patients with high risk of fractures is recommended; the mechanism of action of the commonly used antiosteoporotic medications are illustrated in Figure 4. This article comprehensively discusses epidemiology, the various causes and pathogenesis of secondary osteoporosis. This topic not only covers the bone quantity problem but focuses on quality as well. Furthermore, the up-to-date management of secondary osteoporosis is thoroughly discussed.
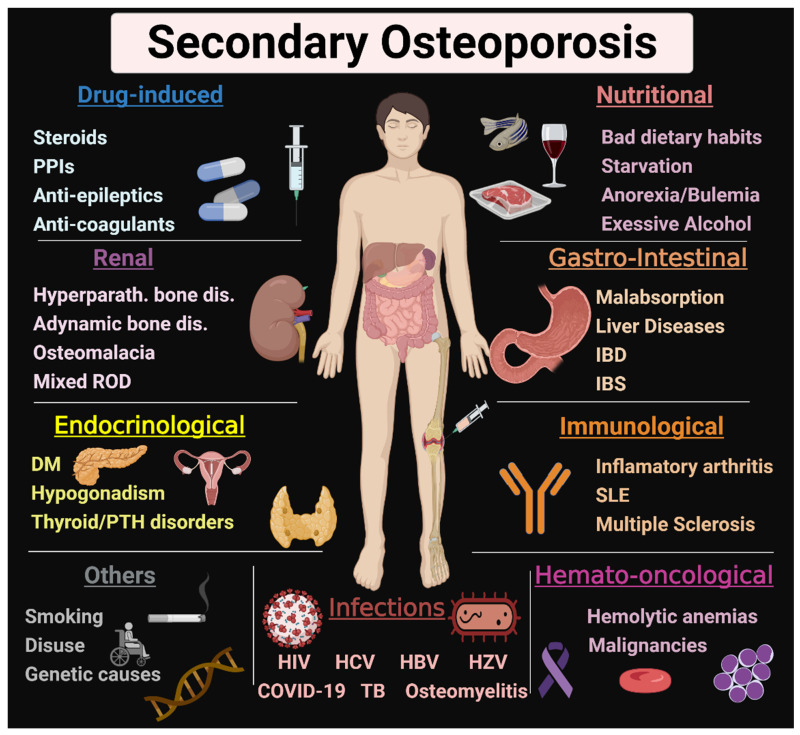
Figure 1.
Causes of secondary osteoporosis. Various causes of secondary osteoporosis are illustrated in this figure. They include ROD, DM, thyroid and parathyroid disorders, malabsorption, IBD, IBS, nutritional causes, drug-induced, infections, anemia, malignancies, inflammatory arthritis, SLE, smoking, and genetic causes. PPIs: proton pump inhibitors, ROD: renal osteodystrophy, DM: diabetes mellitus, PTH: parathyroid, IBD: inflammatory bowel disease, IBS: irritable bowel syndrome, SLE: systemic lupus erythematosus. HIV: human immunodeficiency virus, HCV: hepatitis C virus, HBV: hepatitis B virus, HZV: herpes zoster virus, TB: tuberculosis. This Figure was created with BioRender.com (accessed on 1 February 2022).
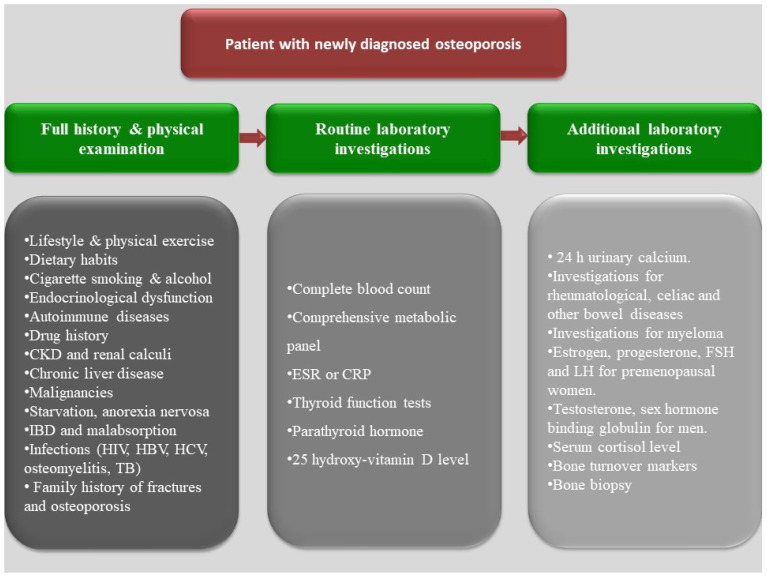
Figure 2.
Pragmatic diagnostic approach for newly diagnosed patients with osteoporosis. A systematic approach for the analysis and detection of a secondary cause of osteoporosis is recommended for all patients with a new diagnosis of osteoporosis. A full history and physical examination followed by a routine laboratory investigation for the most common and simple underlying causes of osteoporosis are required for most cases. Some additional investigation may be considered after routine lab for the suspected cases. CKD, chronic kidney disease, CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; IBD: inflammatory bowel diseases; HCV: hepatitis C virus; HBV: hepatitis B virus; HIV: human immunodeficiency virus; TB: tuberculosis; FSH: follicle stimulating hormone; LH: luteinizing hormone.
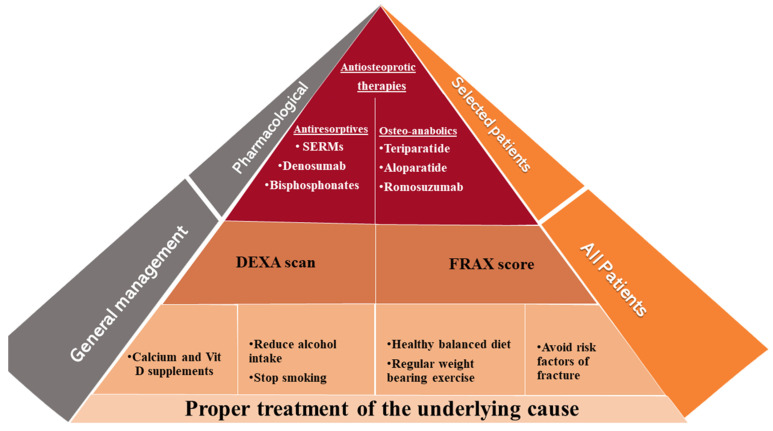
Figure 3.
Approach for prevention and management of secondary osteoporosis. Correction of the underlying causes of secondary osteoporosis is the cornerstone of prevention and treatment. All patients can benefit from non-pharmacological intervention, DEXA scan and assessment of fracture risk. Anti-osteoporotic medications (antiresorptives and osteoanabolics) can be used in selected cases with high fracture risk. DEXA: dual-energy X-ray absorptiometry, FRAX: fracture-risk algorithm, SERM: Selective estrogen receptor modulators.
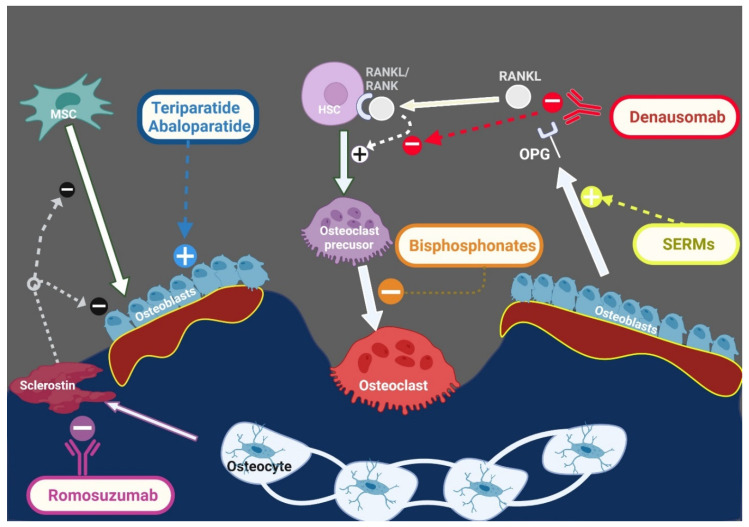
Figure 4.
Mechanism of action of common antiosteoporotic medications. Antiosteoporotic medications can be divided into two main categories: 1. Antiresorptives “on the right side” act mainly by inhibiting osteoclasts. Bisphosphonates act by inhibiting osteoclast differentiation from osteoclast precursors. The monoclonal antibody “denausumab” inhibits osteoclast differentiation by binding to RANKL, preventing its interaction with RANK. SERMs increase OPG production, thus inhibiting osteoclastogenesis. 2. Osteoanabolics “on the left side” stimulate bone formation via activation of PTH (teriparatide) or PTH-related peptide (abaloparatide) receptors. Romosuzumab is an anti-sclerostin monoclonal antibody. Thus, it stimulates osteoblast differentiation and function. MSC: mesenchymal stem cells, HSC: hematopoietic stem cells, SERMs: selective estrogen receptor modulators, OPG: osteoprotegerin, RANK: Receptor activator of nuclear factor κ B, RANKL: receptor activator of nuclear factor kappa-Β ligand. this figure was created with BioRender.com (accessed on 1 February 2022).
2. Renal Causes
Chronic kidney disease (CKD) is a well-established risk factor for bone loss [3]. The incidence of bone loss and fracture risk increases with decline in kidney function. Osteoporosis was reported in up to 32% of CKD patients, while osteopenia was found in about half [3,4,5,6]. However, the magnitude of the problem might be higher for various reasons. First, there is a high prevalence of vascular calcification in CKD patients, which results in a higher estimation of vertebral bone mass by DXA [7]. Second, CKD patients do not have a bone mass/quantity problem only, but a bone quality disorder as well [8]. Third, there is underutilization of osteoporosis diagnostic tools in CKD patients, despite the KDIGO recommendations. Up to 30–50% of fractured CKD patients had a T-score higher than −2.5 [9,10]. Advanced CKD patients have up to an 8-fold higher fracture risk when compared to the general population [11]. Osteoporotic fractures lead to a deleterious effect on the quality of life in CKD patients. One-year mortality after having a hip fracture is 17–27% in the general population [12,13], while it is up to 64% in patients with end-stage kidney disease (ESKD) [14,15].
Renal osteodystrophy (ROD), medication usage, hypogonadism, systemic inflammation, acidosis, and concurrent systemic illnesses contribute to bone loss in patients with CKD. Metabolic acidosis stimulates osteoclasts and induces robust bone resorption. ROD develops with early stages of CKD and progresses with further loss of kidney function [16]. There are many co-players in the pathogenesis of ROD. FGF-23, an osteocyte-secreted phosphaturic hormone, rises in early stages of CKD to prevent hyperphosphatemia [17,18]. Hyperphosphatemia occurs in late CKD stages despite increasing levels of FGF-23 due to klotho deficiency/resistance [19]. FGF-23 inhibits vitamin D activation and increases its catabolism [20,21]. Vitamin D deficiency/insufficiency, and hyperphosphatemia, contribute to secondary hyperparathyroidism in CKD patients [22,23,24,25]. Levels of sclerostin, DKK-1, and WNT pathway inhibitors increase with deterioration of kidney function [26]. They inhibit bone formation and promote low turnover bone disease [27]. On the other hand, the imbalance between osteoprotegerin (OPG) and receptor activator of nuclear factor kappa B ligand (RANKL) levels in CKD patients increases osteoclastogenesis and induces high turnover bone disease [28,29]. Moreover, disturbed gonadal hormones could be a major reason for osteoporosis. Many drugs commonly used in CKD patients such as heparin, warfarin, glucocorticoids, proton pump inhibitors, and diuretics can negatively affect bone health [30,31].
Many tools can be used in the diagnosis of osteoporosis in CKD patients, although there is no consensus on the optimal tool. DXA is the most widely used method. The Fracture Risk Assessment Tool (FRAX) helps to estimate the 10-year fracture risk; however, it does not include CKD as a secondary cause of osteoporosis [32]. Quantitative computed tomography (QCT) is not affected by vascular calcifications and could be a better tool, compared to DXA, especially for longitudinal follow-up and in obese patients [33]. However, its use is less common due to higher costs and radiation exposure. Both tools help to assess bone mass/quantity. On the other hand, TBS, high-resolution imaging techniques, finite element analysis, and Fourier transform infrared spectroscopy can be used in the assessment of bone quality. Bone turnover markers provide dynamic assessment of bone formation and resorption and facilitate ROD management [34]. Bone-specific alkaline phosphatase (BSAP) and intact procollagen-1 N-terminal peptide (P1NP) as bone formation markers, and tartrate-resistant acid phosphatase 5b (TRAP 5b) as a bone resorption marker are reliable in CKD patients [35]. Bone turnover markers and parathyroid hormone (PTH) do not only help to understand bone turnover status [36], but also to predict fracture risk [37,38]. Bone biopsy remains the gold standard to identify the mechanism and severity of bone loss [39]. It also helps to choose the appropriate medication, but it is limited by its invasive nature and lack of expertise. Assessment of bone histology in CKD patients should include three elements: turnover, mineralization, and volume [16,40]. Nowadays, the most common pathological findings in CKD patients are low turnover bone disease (LTBD), high turnover bone disease (HTBD), mixed ROD, while osteomalacia is less frequently seen in adults [41]. Recently published reviews have described the bone quality assessment and management in patients with CKD [7,42].
The primary step in osteoporosis management is to control the CKD metabolic derangements. Vitamin D deficiency, hyperphosphatemia, and hyperparathyroidism are common findings in these patients and have detrimental effects on bones. Patients should be instructed about fall risk prevention and non-pharmacological interventions to improve bone health. Smoking cessation, alcohol limitation, personalized exercise protocols, and well-balanced nutrition have a positive impact on bone, but are underutilized in CKD patients [42]. Optimizing calcium intake and the proper use of phosphate-lowering therapies, vitamin D, and calcimimetics can reduce fracture risks by improving ROD [43].
Determining the type of ROD and including high versus low turnover help to choose the appropriate treatment with higher efficacy and lower adverse events. Patients with HTBD are expected to benefit more from antiresorptives, e.g., bisphosphonates and denosumab, while patients with LTBD may benefit from osteoanabolics to improve bone formation.
Despite being excreted by the kidneys, bisphosphonates can be used in mild to moderate CKD patients without major safety concerns [44]. Their use in advanced CKD patients should be cautious with a concern for CKD progression [45]. Moreover, prolonged use of bisphosphonates in patients with advanced CKD might induce LTBD and increase the risk of atypical femur fracture [46]. Denosumab has been shown to improve BMD and reduce bone turnover in CKD patients in observational studies and small randomized control trials (RCTs) [47,48]. As opposed to bisphosphonates, it is not excreted through the kidneys, however close monitoring of serum calcium and vitamin D should be conducted for the risk of hypocalcemia.
On the other hand, osteoanabolics (teriparatide, abaloparatide, and romosozumab) have a promising role in mitigating bone loss in patients with LTBD. Teriparatide has been used in advanced CKD patients in several studies [49,50,51,52]. Abaloparatide was safe and effective in the early stages of CKD [53]. Romosozumab increased BMD in patients with mild to moderate CKD [54] and in dialysis patients [55].
3. Endocrinological Causes
3.1. Diabetes Mellitus
Diabetes is a chronic metabolic disease associated with an increased risk of fragility fracture. Adults with Type 1 diabetes mellitus (T1DM) have a greater risk of fracture, especially non-vertebral fracture, than those with type 2 diabetes (T2DM) [56,57]. Nevertheless, vertebral fractures are not uncommon and associated with increased mortality, but they are often underdiagnosed because they could be asymptomatic [58]. Diabetes can compromise bone metabolism, impair cell function or damage the extracellular matrix. This results in bone loss, alteration of bone microarchitecture, reduction of bone turnover and predisposition to low trauma fracture. The pathogenesis and risk factors of brittle bone in diabetes consist of obesity, increased insulin resistance, blood sugar disturbances, production of advanced glycation end products, muscle dysfunction, macro- and microvascular complications, and medications. Moreover, the associated comorbidities, such as thyroid disorders, gonadal dysfunction, and malabsorption may contribute to bone loss [59,60]. Notably, T1DM has been associated with reduced osteoblast activity, lower or similar BMD, and a higher risk of fracture [56,61,62,63]. Whereas T2DM is associated with an increased rate of bone loss and fracture, even with normal or high BMD [56,64]. A T-score threshold of −2.0 was suggested as a trigger for therapeutic intervention in T2DM [65]. However, the bone area at the total hip is a better surrogate for fragility fracture in elderly patients with T2DM compared to BMD [66].
Diabetes mainly affects bone quality, including disturbed bone material properties and increased cortical porosity, which are not measurable with BMD-DXA [59,67]. This emphasizes that bone density measurement by DXA underestimates the fracture risk in diabetic patients [68]. Trabecular bone score [69], peripheral quantitative computed tomography (pQCT), pQCT-based finite element analysis (pQCT-FEA) [70], and high resolution peripheral quantitative computed tomography (HR-pQCT) [71] are better tools to estimate fracture risk in diabetic patients. Invasive methods, such as microindentation and bone histomorphometry, are expensive and not widely available [68,72].
Diabetes causes skeletal fragility and applying strategies to reduce fracture is crucial. Furthermore, it seems there is a correlation between the degree of blood sugar control and the risk of fracture. [73,74]. In a large cohort study, there was a cubic relationship between HbA1c and risk of fracture [75]. Thiazolidinediones should be avoided in diabetic patients with increased bone fragility [76]. Moreover, there is growing evidence suggesting a negative outcome of sodium glucose cotransporter 2 (SGLT2) inhibitors on bone health. Alendronate use for 3 years resulted in an increase in BMD in diabetic patients with osteoporosis [77]. Anti-osteoporotic medications (mainly bisphosphonates) appear to prevent bone loss similarly in the spines of diabetic and non-diabetic individuals in a recent systematic review [78]. Use of daily subcutaneous injections of abaloparatide (80 mcg) was associated with improvement in BMD in diabetic patients [79].
3.2. Gonadal Disorders
Hypogonadism is a risk factor for osteoporosis. The peak bone mass and BMD are higher in men; however, if both a man and a woman have similar BMD, the man would have a higher risk of fracture. The incidence of osteoporosis in men under the age of 70 is significantly lower compared to women because the bone loss in women occurs earlier and at a higher rate [80,81]. Testosterone replacement therapy can improve BMD but results in hypogonadal older men were inconclusive. However, the volumetric BMD and bone strength significantly improved in hypogonadal older men who received testosterone treatment for one year [82,83].
3.3. Parathyroid Disorders (Hypoparathyroidism and Primary Hyperparathyroidism)
Hypoparathyroidism is a low bone turnover condition. The information regarding fracture risk is inconsistent [84,85,86], but patients with nonsurgical hypoparathyroidism seem to have a higher risk of vertebral fracture [86,87,88]. This could be potentially due to a longer period of bone changes in nonsurgical hypoparathyroidism compared to surgical hypoparathyroidism [86]. Therefore, we would speculate that the higher fracture risk is due to over-maturation and impaired quality of the bone. They have higher BMD by DXA at all skeletal sites, especially at the lumbar spine [89]. Furthermore, they typically have normal [89,90,91] or low [92] trabecular bone scores and are classified as degraded microarchitecture. Compared to the age and sex-matched controls, they often have higher volumetric BMD (trabecular and cortical), and higher cortical area and thickness by pQCT [89,93]. Nevertheless, HR-pQCT showed increased cortical volumetric BMD, but reduced cortical thickness and cortical porosity [89,94]. They also seem to have normal biomechanical strength determined by finite element modeling [94,95], but lower bone material strength index, measured by impact microindentation, than controls [86,96]. Calcium and vitamin D supplements are widely used. However, the long-term safety and efficacy of this practice are not very well studied. Donovan Tay et al. reported that long-term use of PTH (1-84) therapy reduced supplemental calcium and vitamin D requirements and increased lumbar spine and total hip BMD [97]. PTH (1-84) reduced urinary calcium and serum phosphorus levels and improved quality of life without increasing serious adverse events, compared to traditional management [98,99,100]. In a recent meta-analysis, compared to PTH, active vitamin D usage was associated with similar serum calcium levels but a trend toward lower urinary calcium levels [101]. Moreover, the long-term safety is not completely recognized, and dose-dependent increased risk of osteosarcoma is reported in rat studies [102,103]. This concern limited the long-term usage of PTH (1-84) as replacement therapy for hypoparathyroidism. Small studies reported heterogeneity regarding the efficacy of parathyroid tissue allotransplantation for treating hypoparathyroidism [104].
Primary hyperparathyroidism (PHPT) is associated with decreased BMD and increased fracture risk across various skeletal sites, especially at the lumbar spines [105,106]. BMD measurement by DXA is an acceptable predictor of fracture at hip and forearm but underdiagnoses vertebral fragility [107]. There are valuable tools, such as trabecular bone score, 3D-DXA [108], bone strain index (BSI) by finite element analysis of DXA [109], and HR-pQCT [110], to assess bone health and predict skeletal fragility [105]. HR-pQCT revealed altered microarchitecture of cortical and trabecular bone, including reduced cortical and trabecular volumetric densities, increased cortical porosity, and heterogeneity of trabecular distributions [110,111]. This is almost consistent with histomorphometric studies, except for preservation or even improvement of trabecular bone structure [112]. The assessment of bone material strength index at the tibia by using the impact microindentation technique showed impaired bone material properties in PHPT subjects, especially in those with fragility fracture [113]. Parathyroidectomy reduces calcium concentrations and increases BMD at different skeletal sites. It might reduce fracture risk better than active surveillance [114], but its advantages over medical therapy regarding risk of fracture, kidney stones and quality of life lack sufficient evidence [114,115]. Nevertheless, parathyroidectomy could improve bone strength assessed by HR-pQCT and finite element analysis [116]. In terms of medical therapy, optimization of calcium and vitamin D intake is suggested [117]. Calcium supplements can reduce PTH and increase femoral neck BMD in patients with asymptomatic PHPT [118]. There is no reason to restrict dietary calcium intake in the patients with mild PHPT, but close monitoring of calcium is necessary and calcium supplementation should be avoided in severe PHPT with elevated 1,25(OH)2D and higher serum PTH levels. Other medical therapies include bisphosphonates, cinacalcet, denosumab, and estrogen, which are appropriate for lowering calcium, increasing BMD or both [117].
3.4. Thyroid Disorders
Thyroid hormones play a pivotal role in bone metabolism. Hyperthyroidism, even subclinical, is a known risk factor for osteoporosis. It is associated with increased bone turnover, decreased bone mass, and increased fracture risk [119,120]. In addition, long-term TSH suppression in patients with differentiated thyroid cancer was associated with lower BMD in postmenopausal women [121]. Hyperthyroid women had impaired bone quality and quantity reported by HR-pQCT. Euthyroidism could improve volumetric BMD and cortical microarchitecture [119]. Overt hypothyroidism reduces bone formation. However, data on BMD and fracture risk are inconclusive [122].
3.5. Adrenal Disorders
Osteoporosis happens in 30–50% [123,124,125], and vertebral fractures in 30–70%, of patients with Cushing syndrome [126,127]. Cushing syndrome leads to excess glucocorticoid production, which negatively impacts bone metabolism through suppression of growth hormone and gonadal axis, besides altering the rhythmic production of parathyroid hormone [126]. Trabecular bone loss is more pronounced in patients with Cushing syndrome. Adrenal nodules with autonomous cortisol secretion [128], primary aldosteronism [129], pheochromocytoma [130,131], and congenital adrenal hyperplasia [132] are associated with deterioration of bone quality and quantity.
3.6. Growth Hormone
Despite acromegalic patients having a higher rate of bone formation, they have an increased risk of vertebral fractures because of increased bone turnover and poor bone quality. However, they may have increased, decreased, or similar BMD, compared to the general population [133,134]. They have higher cortical porosity and altered bone microarchitecture, which is attributed to altered bone remodeling and Wnt signaling.
Growth hormone deficiency is associated with low bone turnover osteoporosis, and loss of cortical greater than trabecular bone, which leads to increased fracture risks [135]. Growth hormone replacement initially increases bone turnover and reduces bone density. A maintenance treatment encourages improved bone mass, but its effects on fracture risk is not definite [136]. This could be due to increased DKK-1, a Wnt inhibitor, therefore increasing cortical porosity [137].
4. Gastrointestinal Causes
Malabsorption and chronic liver disease are well-known causes of osteoporosis, and they are included in the FRAX. Physiologic bone metabolism requires optimum amounts of nutrients, particularly minerals and vitamins. Vitamin D is a fat-soluble vitamin, so bone loss is remarkable in diseases associated with fat malabsorption [138,139,140,141,142,143]. Furthermore, in cases of steatorrhea, calcium absorption may be hindered by binding to excess fatty acids in the gastrointestinal (GI) lumen [144]. In this section, we will discuss the most common causes of GI-related osteoporosis.
4.1. Celiac Disease
Patients with celiac disease have a high prevalence of osteopenia and osteoporosis, 40% and 15%, respectively, even after excluding postmenopausal women [145]. It has been reported that 8% of patients with idiopathic low BMD have positive IgA anti-endomysial antibodies even though they are asymptomatic. Routine screening for celiac disease might be considered in idiopathic cases of osteoporosis [146,147]. A gluten-free diet can significantly improve BMD [148,149]. However, bone loss may persist due to the continuous inflammatory process leading to higher osteoclast activity and lower ability to generate bone matrix [150].
4.2. Chronic Pancreatitis
More than 50% of patients with chronic pancreatitis, particularly smokers and alcoholics, have low BMD. Pancreatic enzymes and vitamin D replacement significantly lowered the risk of fracture [151]. Cystic fibrosis can disturb bone health through mechanisms other than malabsorption. Cystic fibrosis transmembrane conductance regulator is expressed in bone cells, thus it might have a negative impact on bone metabolism. Additionally, bone resorption increases during pulmonary exacerbations as the proinflammatory cytokines stimulate osteoclast activity [152].
4.3. Short Bowel Syndrome
The prevalence of osteoporosis in patients with short bowel syndrome is 2-fold higher compared to matched controls [141]. Bone loss occurs because of micro and macronutrients’ malabsorption. Metabolic acidosis, either caused by chronic diarrhea or D-lactic acidosis by bacterial overgrowth, can also impair bone health [153].
4.4. Hepatic Osteodystrophy
Disturbed enterohepatic circulation of fat-soluble vitamins impairs bone metabolism. This is one of the main causes of bone loss in biliary disorders as primary biliary cholangitis (PBC) and sclerosing cholangitis. The prevalence of osteoporosis and fractures in PBC is up to 50% and 20%, respectively [154,155,156]. The etiology of chronic liver disease, alcohol, viral hepatitis, and autoimmune diseases, may contribute to the pathogenesis of hepatic osteodystrophy [154,157,158,159,160,161]. Cirrhosis complications such as malnutrition, impaired physical activity, and hypogonadism, along with disturbed vitamin D and K metabolism [162,163], can aggravate bone loss.
4.5. Peptic Ulcer Disease
Peptic ulcer disease is linked to osteoporosis, especially among males. Certain species of H-pylori infections may afflict bone metabolism by enhancing inflammatory status, reducing the circulatory ghrelin and estrogen levels, and increasing postprandial serotonin levels. Moreover, long-term use of PPIs can impair bone health [164,165].
4.6. Inflammatory Bowel Disease (IBD)
Patients with IBD have a higher risk of bone loss [166], poor bone quality [167,168], and fractures [169,170,171,172]. This can be explained by malnutrition, chronic inflammatory process, and immunosuppressive drugs [171,173,174]. Low turnover bone disease is the predominant underlying pathology in patients with osteoporosis and IBD [175,176]. The American College of Gastroenterology recommended using the conventional risk factors as indications for BMD screening in IBD patients using DXA scan [177]. The Cornerstone Health organization has expanded the indications for BMD screening to include maternal history of osteoporosis, malnourished or very thin patients, and amenorrheicor postmenopausal women [178]. Maldonado and colleagues highlighted the role of biomechanical CT to detect patients with an increased risk of fracture. 40% of those patients were not included in the Cornerstone checklist. Thus, IBD patients undergoing CT enterography may benefit from biomechanical CT screening for fracture risk [179]. Early suppression of the inflammatory process by anti-TNF is associated with better bone preservation [169,180]. In addition to calcium and vitamin D optimization, bisphosphonates are relatively safe and effective treatment options [181]. In an animal study, a natural compound (emodin) has been reported to inhibit osteoclast function and prevent IBD-related osteoporosis [182].
4.7. Irritable Bowel Syndrome
Patients with irritable bowel syndrome have a higher incidence of osteoporosis and fragility fractures [183]. This might be explained by chronic inflammation, overactivation of the hypothalamic-pituitary-adrenal axis, nutritional deficiency, and smoking. Further studies are needed to confirm the underlying mechanisms and to establish a treatment approach [184].
4.8. Dysbiosis
Microbiota is considered a hidden organ that has a bidirectional interaction with cellular responses. Certain species of microbiota are linked to osteoporosis and autoimmune diseases such as IBD, PBC, and sclerosing cholangitis [185,186]. The beneficial effects of probiotics were explained experimentally by manipulating the expression of OPG/RANKL, Wnt10b, and inflammatory cytokines [186,187].
Other GI disorders with increased risk of osteoporosis include post-gastrectomy [188], atrophic gastritis [189,190], and bariatric surgeries [191].
5. Nutritional Causes
Nutritional factors can potentially affect bone mass, metabolism, matrix, and microarchitecture. Insufficient nutrition leads to deficiency of protein, vitamins, and minerals, particularly calcium, phosphorus, and magnesium, which are essential for bone health [192]. The recommended daily calcium intake for adults is between 800–1200 mg daily [32,193], while it is 700 mg and 320–420 mg for phosphorus and magnesium, respectively [194]. The daily protein requirement is recommended to be 0.8 gm/kg for adults and 1–1.2 gm/kg for the elderly [195,196]. The vitamin D daily requirement ranges from 800 to 1000 IU [197].
Malnutrition can happen either because of poor nutrient intake, increased losses, and/or increased demand [198]. Bad dietary habits, anorexia nervosa, bulimia nervosa, prolonged total parental nutrition (TPN), bariatric interventions, and excess alcohol intake can cause secondary osteoporosis [199]. As osteoporosis and fractures are associated with many life-threatening events, their prevention is essential via balanced diet and physical exercise [200].
Starvation, the most severe form of malnutrition, can be caused by various socio-economic, environmental, and medical factors [201]. Starvation can negatively affect bone quantity and quality through minerals, vitamins, and collagen type I deficiency [201,202]. There is a positive relationship between malnutrition during early life, or even in utero, and early incidence of osteoporosis and fractures [203,204,205,206,207].
Vitamin D deficiency results in decreased calcium absorption and hypocalcemia leading to secondary hyperparathyroidism, consequently stimulating bone turnover and decreased BMD [208]. Treatment with vitamin D supplements has beneficial effects on bone health in patients with 25-hydroxy vitamin D levels less than 30 nmol/L [209,210]. On the other hand, prophylactic doses of vitamin D have a debatable role in the prevention of osteoporosis and fractures. [211,212,213,214,215,216].
Many observational studies reported a positive relationship between body mass index (BMI) and BMD [217]. Moreover, previous studies demonstrated that obesity could protect against fractures [218,219]. However, more recent studies did not show a positive impact of obesity on bone [220]. The Look AHEAD trial reported a modest increase in bone loss at the hip with intensive non-surgical weight loss interventions in obese type 2 diabetics [221,222]. Moreover, most bariatric surgeries were associated with bone loss and fragility [191,223]. This may be explained by mechanical unloading, secondary hyperparathyroidism due to malabsorption of calcium and vitamin D, decreased estrogen, leptin, and ghrelin, and increased adiponectin levels [191,224,225]. Therefore, it is recommended to receive adequate calcium and vitamin D and to monitor BMD after bariatric surgeries [226].
Patients with anorexia nervosa extremely limit their food intake because they are scared of weight gain [227]. This can lead to several medical complications including bone loss [228] with a 2–7-fold increased risk of fractures [229,230]. This is not only because of nutritional deficiencies but hormonal disturbances as well [231]. On the other hand, improving nutritional status corrects the endocrinological disorders and BMD in these patients [232]. Anti-osteoporotic medications may help to ameliorate bone loss in patients with persistently low BMI and amenorrhea [233]. Residronate use, either alone or combined with transdermal testosterone, resulted in improved spinal BMD [234,235]. Moreover, physiological doses of transdermal estrogen lead to increased spinal and hip BMD [236]. In a recent RCT, sequential therapy with recombinant human IGF-1 and risedronate was superior to risedronate alone in improving lumbar spine BMD in women with anorexia nervosa [237]. Furthermore, Fazeli et al. reported a significant increase in lumbar spine BMD after 6 months of teriparatide use [238].
Patients with prolonged TPN have an osteoporosis prevalence of 40 to 100% [239,240,241]. Despite TPN improving nutritional status, the prolonged need for TPN may induce dysbiosis [242], decreased gut calcium, and phosphorus absorption [239]. Moreover, it can induce hypercalciuria because of the hyperfiltration secondary to high amino acid infusion [243]. Routine vitamin D monitoring and management are necessary for patients with prolonged TPN because vitamin D deficiency is very prevalent among these patients [239]. Bisphosphonates improved BMD in patients with TPN-associated osteoporosis [244,245].
Bad dietary habits have been reported to be associated with osteoporosis. High dietary sugar may lead to osteoporosis [246] by glucose-induced hypercalciuria, hypermagnesuria [247,248], and decreasing vitamin D activation [249]. In addition, hyperglycemia can decrease osteoblast proliferation and increase osteoclast activation [250,251]. On the other hand, the effect of dietary salt on bone health is unclear [252].
Heavy alcohol intake has been associated with decreased BMD [253]. Mechanistically, it directly reduces osteoblast activity and increases osteoclastogenesis [254,255,256]. Indirectly, it can cause changes in body composition [257] and alterations in various hormones, including PTH, vitamin D, testosterone, and cortisol [258]. Alcohol abstinence may improve bone metabolism and increase BMD [259,260].
6. Drug-Induced
Drug-induced osteoporosis is the second most common cause of secondary osteoporosis. Despite their well-known adverse events, glucocorticoids are still one of the cornerstone immune-suppressive/modulator and anti-inflammatory therapies. Up to 40% of patients on long-term glucocorticoid therapy suffer from fractures during their lifetime [261,262].
Areas with high trabecular bone, such as lumbar spine and hip trochanter, are the classic sites for glucocorticoid-induced fractures [263]. Robust bone loss may reach up to 20% within the first year of therapy, and subsequently decline to 1 to 3% annually [264,265]. The fracture risk with glucocorticoid therapy is dose and time-dependent [262]. The impact of glucocorticoids on bone has been linked to their cumulative effect, which disturbs both bone quantity and quality. Glucocorticoids can induce bone loss irrespective of the route of administration. For instance, long-term inhaled glucocorticoids were associated with a 10% loss of BMD [266,267]. Even controlled-release budesonide and topical corticosteroid can negatively impact bone health [268,269].
Glucocorticoids initially decrease bone formation and increase RANKL/osteoprotegerin ratio, inducing high bone resorption [270,271]. The mechanism of bone loss with long-term usage is more attributed to suppressed bone formation rather than increased bone resorption. This could be due to the downregulation of the Wnt signaling pathway which impairs the osteoblast activity [272]. Additionally, glucocorticoids have an indirect impact on bone through their effects on calcium homeostasis, parathyroid gland activities, and vitamin D metabolism [273,274]. Furthermore, glucocorticoids lead to loss of muscle mass and strength which increases the risk of falls and fractures. They can also induce hypogonadism which decreases the anti-resorptive effect of testosterone and/or estrogen [275].
The use of a DXA scan and FRAX after 6 months of glucocorticoid therapy is recommended for those with a history of fragility fracture, patients of 40 years of age or older, and those with major osteoporotic risk factors [276].
For prevention of glucocorticoid-induced osteoporosis, daily intake of 1000–1200 mg calcium and 600–800 units of vitamin D, along with lifestyle modification, are highly recommended [275]. For adults with high risk of fracture, treatment with oral bisphosphonate is the preferred line of therapy [276]. Teriparatide is also effective in preventing and treating glucocorticoid-induced bone loss [277].
Antidepressants like selective serotonin reuptake inhibitors and monoamine oxidase inhibitors can induce low bone density and increase incidence of fracture [278,279,280,281]. It is not clear how these medications affect bone health, but it may be attributed to diminished osteoblast proliferation through the serotonin receptors and transporters [282].
Many studies showed significant bone loss with long-term use of antiepileptic drugs [283,284,285]. The pathogenesis is multifactorial, however accelerated vitamin D metabolism is a crucial co-player [286,287,288,289]. Bone loss occurs as a result of bone remodeling abnormalities rather than abnormal mineralization [290,291,292].
Aromatase inhibitors, adjuvant long-term therapies for breast cancer, lead to abrupt deprivation of estrogen and consequently, bone loss [293]. Moreover, concomitant use of gonadotropin-releasing hormone agonists induces up to 7% annual BMD loss [294]. The use of gonadotropin-releasing hormone agonists in prostate cancer patients is associated with increased fracture risk [295,296,297].
Antidiabetic medications can impact bone health either positively or negatively. Peroxisome proliferator-activated receptor gamma (PPARγ) plays an important role in the regulation of bone formation and energy metabolism, along with insulin sensitivity [298,299]. Its stimulation by thiazolidinediones induces bone resorption and inhibits bone formation [300]. Thiazolidinediones decreased the BMD and increased the risk of osteoporosis when compared to other anti-diabetic medications [301]. The effects of sodium-glucose cotransporter-2 (SGLT2) inhibitors on bone metabolism and fracture risk are receiving more attention because of their wide use. They may increase bone turnover, disturb bone microarchitecture, and reduce BMD [302]. In a recent study, Koshizaka and colleagues reported increased TRAP 5b with no change in BMD in a 24-week RCT [303].
In 2010, the FDA released a warning against long-term use of proton pump inhibitors (PPIs) as it may increase the incidence of osteoporosis and fracture risk [304]. The limited available evidence suggested that this might happen through histamine over-secretion [305], and affecting mineral homeostasis [306,307]. There is inconsistent data regarding the impact of PPIs on BMD.
Despite the negative effect of anticoagulants on bone metabolism having been studied for a long time, such effect is still debatable, and the underlying mechanisms are still poorly understood [308]. Unfractionated heparin was associated with significant bone loss compared to low molecular weight heparin [309,310,311]. Long-term use of warfarin was associated with decreased BMD and TBS [312]. In a recent study, this negative effect on bone was more pronounced in warfarin but was also found in direct oral anticoagulants [313].
7. Infection
Chronic active infections are not infrequent causes of bone loss, mainly due to cytokine release that stimulates osteoclastogenesis and suppresses osteoblast function. Human immunodeficiency virus (HIV)-infected patients have a three times higher prevalence of osteoporosis and up to four-fold increased risk of fractures compared to the general population [314,315]. This might be directly attributed to the HIV infection or secondary to the use of antiretroviral therapy (ART), concomitant alcohol use, smoking, associated hypogonadism, malnutrition, hepatitis B and/or C co-infection, and vitamin D insufficiency [316,317,318,319,320]. HIV infection promotes osteoblast apoptosis and osteoclast activation [321,322,323,324,325]. Furthermore, HIV infection induces a state of chronic inflammation in addition to immune system activation afflicting bone health [326,327,328]. Tenofovir disoproxil fumarate (TDF) is associated with osteoporosis and fractures more than the newer ART [329,330,331,332], as it induces multiple renal tubular defects and mineral losses [333,334]. The European AIDS Clinical Society (EACS) [335] guidelines recommend tenofovir alafenamide (TAF) as a first-line therapy instead of TDF in patients with progressive osteopenia or osteoporosis, as it is less toxic to the renal tubules [336,337]. Bisphosphonates are used effectively for the treatment of HIV-related bone disease [338]; however, bone anabolic drugs have not been adequately studied [315].
Hepatitis B and C viral (HBV; HCV) infections even without subsequent liver cirrhosis is associated with an increased risk of osteopenia and osteoporosis [158,339,340,341]. Furthermore, previous studies reported that the risk of osteoporosis was still higher in patients with HBV and HCV infections even after adjustment for other osteoporosis risk factors [158,342]. Of note, previous studies reported an increased risk of fractures in patients with HIV and HCV co-infection compared with HIV-infected patients [319]. Interestingly, HCV clearance led to a two-thirds reduction in fracture risk in postmenopausal women with osteoporosis [343].
Herpes zoster infection is associated with osteoporosis [344,345]. This negative effect on bone health may be due to the upregulation of inflammatory cytokines, especially in patients with post-herpetic neuralgia [346,347].
COVID-19 might predispose patients to osteoporosis [348]. This may be because of associated pro-inflammatory cytokine production and prolonged immobilization in severe cases [349]. Furthermore, there might be direct sequelae of infection on the skeleton [350]. The virus can decrease ACE2 expression in both osteoblasts and osteoclasts [351], leading to disordered bone formation and resorption. In addition, corticosteroids used in the treatment of COVID-19 have a negative impact on bone.
Osteomyelitis is commonly associated with significant bone loss and subsequent fragility fractures [352]. This is mainly attributed to the upregulation of inflammatory cytokines such as IL-1, IL-6, and TNFα with subsequent activation of RANKL and inhibition of osteoprotegerin [353,354].
Patients with active TB and TB survivors with pulmonary fibrosis have increased risk of osteoporosis [342,355]. Chronic systemic inflammation, concomitant malnutrition, and vitamin D deficiency are the main contributors to bone loss [354,356,357,358].
8. Hemato-Oncological Causes
Hematologic disorders are potentially able to damage bone through direct cellular effects or indirectly, mediated by several circulating factors [359]. The bone loss occurs mainly due to an imbalance between RANKL/RANK and WNT signaling pathways with subsequently increased bone resorption and decreased bone formation [360,361,362,363].
Anemia can lead to bone resorption and increases bone fragility [364,365]. Iron deficiency may negatively impact cytochromes’ P450 activity, which is essential for vitamin D metabolism and bone health [366]. β thalassemia causes ineffective erythropoiesis and bone marrow expansion that leads to medullary destruction and cortical thinning [367]. Moreover, pubertal delay, cytokine disturbances, growth hormone deficiency, iron bone deposition, deferoxamine-induced bone dysplasia, and vitamin D deficiency can further contribute to inadequate bone health in thalassemia patients [368,369,370,371,372,373]. Bisphosphonate may improve BMD [373,374], however its effect on fracture rate is uncertain in patients with thalassemia [375]. There is limited information but promising results observed by using denosumab or teriparatide to increase bone density in thalassemia patients [376,377].
The estimated prevalence of secondary osteoporosis in hemophilia patients is up to 58.7% [378]. The underlying mechanisms for low bone mass include vitamin D deficiency, limited physical activity secondary to hemophilic arthropathy, and the acquisition of osteoporosis-linked blood-born infections such as HIV [379,380,381]. In addition, Factor VIII deficiency is directly associated with increased bone resorption and decreased formation secondary to the imbalance in OPG/RANK/RANKL system [382,383]. Screening for osteoporosis should be implemented in the underweight, those with fragility fractures, HIV, and/or advanced hemophilic arthropathy [384]. Replacement of the deficient factor could minimize joint bleeding and hemoarthropathy and subsequently reduce the risk and progression of osteoporosis [385].
Both monoclonal gammopathy of undetermined significance and multiple myeloma patients are at increased hazard for osteoporosis and fragility fractures [386,387]. Myeloma cells stimulate the release of cytokines, IL-6, and IL-7, leading to activation of the RANKL/RANK pathway and enhanced bone resorption [361]. On the other hand, the expression of WNT inhibitors, Dkk-1, and secreted frizzled protein-2 is enhanced, leading to reduced bone formation [388,389]. Several guidelines recommend myeloma screening in elderly patients with osteoporosis and/or fragility fractures [390,391]. Bisphosphonate is recommended in myeloma patients as they have antineoplastic, immunomodulatory, and anticatabolic effects [392,393]. Nevertheless, renal impairment which is frequent in those patients is still an important hindrance [394] and may obligate the use of other safer drugs such as denosumab [395]. In addition, treatment of multiple myeloma, such as bortezomib, and monoclonal antibodies targeting DKK1 or sclerostin can reduce bone loss [396,397,398].
Osteoporosis is the most common skeletal pathology that occurs in 18 to 40% of systemic mastocytosis patients [399,400,401]. Bone involvement occurs due to bone marrow infiltration by mast cells, besides the release of circulating factors, such as histamine, prostaglandins, and interleukins (IL-1, IL-3, IL-6), which enhance osteoclast activity [402]. The manifestations consist of a wide clinical spectrum from asymptomatic condition to varying degrees of bone damage, such as osteopenia, osteoporosis, osteolytic lesions, and osteosclerosis [403]. Besides antiresorptive medications such as bisphosphonate and denosumab [404,405], interferon may improve bone pathology by controlling the disease activity [406]. Contrarily, safety concerns exist with the use of teriparatide as it may enhance the proliferation of malignant cells [407].
In patients with solid tumors, bone damage usually occurs either as a side effect of anticancer treatment or secondary to osteolytic metastasis, most commonly from breast cancer [408]. Moreover, cytotoxic chemotherapy and hormone deprivation therapies have detrimental effects on both bone quantity and quality [409,410,411]. Bone loss in patients receiving aromatase inhibitors or androgen deprivation therapy is up to ten times that in age-matched healthy controls [412,413]. Therefore, baseline and follow-up DXA scans are serially recommended based on the underlying diseases [414,415]. Bisphosphonate is advised in aromatase inhibitor receivers with higher fracture risk [416].
9. Rheumatological-Immunological Causes
The immune system plays an important role in bone homeostasis. Activated T cells affect bone health through the secretion of various cytokines [417]. Some experimental studies detected that Th17 cells are responsible for stimulating bone resorption, while T reg cells are peculiarly associated with inhibition of bone resorption [418]. Moreover, CD8+ T cells might have a protective function through the secretion of various factors, such as osteoprotegerin and interferon-γ which have an anti-osteoclastogenesis effect [419].
9.1. Inflammatory Arthritis
Inflammatory arthritis, including rheumatoid arthritis (RA), psoriatic arthritis, and spondyloarthropathy, is frequently associated with systemic skeletal complications, such as osteoporosis and fragility fractures [420].
Osteoporosis prevalence in patients with RA is about 30% and increases up to 50% in post-menopausal women [421,422]. Furthermore, a large meta-analysis revealed that patients with RA have a higher risk of fracture [423].
RA-related osteoporosis is described by two main features: local and systemic bone loss [424]. Several mechanisms are involved in the pathogenesis of bone loss including sustained inflammation, glucocorticoid use, decreased physical activity and increased secretion of proinflammatory cytokines such as IL-6, IL-1, and TNF-α [422,425]. Moreover, overexpression of RANKL promotes osteoclastogenesis [426]. There is enough evidence to support the role of autoantibodies in the pathogenesis of both local and systemic bone loss through osteoclast activation [427,428,429].
Disease-modifying anti-rheumatic drugs (DMARDs) don’t only control the inflammatory status but also help to avoid the long-term negative effects of corticosteroids on bone health [430]. The use of leflunomide was associated with a significant increase in lumbar spine BMD [431]. Moreover, TNF-inhibitors improved BMD and reduced the rate of fracture [432]. Other biological agents such as tocilizumab, rituximab, and abatacept significantly reduced bone resorption markers and RANKL expression [433,434]. On the other hand, the impact of methotrexate on bone loss is controversial [435].
Despite several studies demonstrating a significant association between psoriatic arthritis and bone loss/fragility fracture [436,437], others did not find such association [438]. Pro-inflammatory cytokines are involved in the mechanism of local bone loss [439].
On the other hand, patients with ankylosing spondylitis (AS) have lower BMD, even in the early stage of the disease [440]. The prevalence of osteopenia and osteoporosis is about 54% and 16%, respectively, within 10 years of the disease onset [440]. A large database showed a higher risk of vertebral and non-vertebral fractures among patients with AS [441]. The use of non-steroidal anti-inflammatory drugs was associated with decreased fracture risk [441]. TNF-inhibitors increased lumbar spine and total hip BMD, however they did not decrease the rate of vertebral fractures [442].
9.2. Systemic Lupus Erythematosus (SLE)
Osteoporosis and fragility fractures are well-known comorbidities in patients with SLE [443]. The incidence of fracture is up to 35% in this patient population [444]. Furthermore, asymptomatic vertebral, and non-vertebral fractures were associated with decreased quality of life and increased risk of mortality [445,446]. Pro-inflammatory cytokines directly affect bone mass [447]. Organ damage can indirectly cause bone mass loss. The disease duration and severity, besides the long-term glucocorticoid usage, are the main determinants of bone loss [448,449]. Lower levels of P1NP are predictive of bone loss and decrease BMD over 12 months in premenopausal SLE patients [450].
9.3. Multiple Sclerosis (MS)
Several studies showed that people with MS have lower BMD, higher rates of osteoporosis, and increased fracture risk compared to healthy controls [451,452,453]. Various risk factors contribute to bone loss in patients with MS, including disease duration and severity, vitamin D insufficiency, cumulative steroid dose, decreased ambulation, and inflammatory processes [453,454]. The pro-inflammatory osteopontin levels increase in patients with MS and correlate with femur neck BMD [455].
10. Others
10.1. Smoking
Smoking is incorporated within the FRAX score as a risk factor for osteoporosis [456]. It has direct harmful effects on osteogenesis and bone blood flow [457]. Indirectly, it afflicts serum levels of vitamin D, PTH [458,459], and sex hormones, particularly in females [460]. The effect of smoking cessation on BMD is unclear; however, it has been shown that it could increase BMD at femur and total hip [461] and reduce vertebral fractures [462].
10.2. Disuse Osteoporosis
Osteocytes have certain mechano-receptors that use weightbearing-induced signals to orchestrate bone turnover. Immobility leads to osteocyte dysfunction and subsequent inhibition of bone formation via downregulation of Wnt/β-catenin pathway [463]. This can be a systemic disorder with prolonged immobilization or a local disease among patients with hemiparesis, spinal cord injuries, or neuromuscular diseases. Physical exercise and rehabilitation programs are essential in preventing and treating this type of bone loss. The use of antiosteoporotic medications such as bisphosphonates, denosumab, teriparatide, and romosozumab might be indicated in refractory cases [463,464,465].
10.3. Genetic Causes of Osteoporosis
Genetics plays a crucial role in bone microarchitectural properties, skeletal strength, and the risk of osteoporosis. Rare, monogenic forms of osteoporosis start in childhood or young adulthood [466]. The most common one is osteogenesis imperfecta (OI), also known as ‘brittle bone disease’ [467]. Osteogenesis imperfecta is a genetic connective tissue disorder caused by defective bone formation, mainly due to impaired production and/or processing of type 1 collagen [468]. It is characterized by an abnormally high bone matrix mineralization. This is related to a larger number of crystals with the same volume of matrix [469,470]. Cortical porosity, thin trabeculae, abnormal bone quality, and low bone density with associated increased risk of fracture are common findings in OI [471,472,473]. There is limited evidence that bisphosphonates increase BMD and decrease the risk of fracture in patients with OI [474]. Moreover, denosumab had poor and inconclusive results [475]. Notably, romosuzumab increased BMD and improved turnover biomarkers in those patients [419]. Apart from OI, whole-genome sequencing studies were able to unmask new genetic variants that are associated with osteoporosis. The expression of these genetic variants is involved in different bone-protecting functions, such as vitamin D metabolism, mesenchymal stem cell osteogenic differentiation, and bone morphogenetic proteins. Some of these variants are population-specific and others are shared between patients with low BMD from different races [476,477].
Osteoporotic fractures are increasing exponentially [478] and are considered one of the major health care problems [479]. Osteoporosis is associated with a negative effect on fracture healing, especially in unstable and comminuted fractures, which indicate internal fixation [480,481]. The power of screw holding is decreased in the osteoporotic bone which, in turn, causes implant loosening, loss of fixation, and impaired healing. Antiosteoporotic medication should be considered to improve bone formation and the success rate of bone implants in osteoporotic fractures [482].
The current review is limited by the quantity and quality of the clinical studies in this field. Few RCTs demonstrated the impact of different anti-osteoporotic medications on bone.
11. Conclusions
Secondary osteoporosis is diagnosed when bone fragility is caused by a disease, drug, or nutritional deficiencies. It is an evolving, devastating health problem. Proper diagnosis and prevention are the cornerstones of preventing further bone loss and fragility fractures. Although causal treatment is essential, antiosteoporotic medications can further decrease the risk of fractures, as well as improve fracture healing. More RCTs are required to explore the safety and efficacy of antiosteoporotic drugs in various clinical settings.
Author Contributions
Conceptualization, A.E.-H. Writing—original draft preparation, M.M.S., S.E., E.N. and N.E. Writing—review and editing, M.A. (Mohamed Abdalbary), M.A. (Mostafa Abdelsalam) and K.A. Supervision, A.E.-H. and K.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
All the authors declared no conflict of interest in this work.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tannenbaum C., Clark J., Schwartzman K., Wallenstein S., Lapinski R., Meier D., Luckey M. Yield of Laboratory Testing to Identify Secondary Contributors to Osteoporosis in Otherwise Healthy Women. J. Clin. Endocrinol. Metab. 2002;87:4431–4437. doi: 10.1210/jc.2002-020275. [DOI] [PubMed] [Google Scholar]
- 2.Kanis J.A., Hans D., Cooper C., Baim S., Bilezikian J.P., Binkley N., Cauley J.A., Compston J.E., Dawson-Hughes B., El-Hajj Fuleihan G., et al. Interpretation and use of FRAX in clinical practice. Osteoporos. Int. 2011;22:2395. doi: 10.1007/s00198-011-1713-z. [DOI] [PubMed] [Google Scholar]
- 3.Najar M.S., Mir M.M., Muzamil M. Prevalence of osteoporosis in patients with chronic kidney disease (stages 3–5) in comparison with age-and sex-matched controls: A study from Kashmir Valley Tertiary Care Center. Saudi J. Kidney Dis. Transpl. 2017;28:538. doi: 10.4103/1319-2442.206439. [DOI] [PubMed] [Google Scholar]
- 4.Aggarwal H., Jain D., Yadav S., Kaverappa V. Bone mineral density in patients with predialysis chronic kidney disease. Ren. Fail. 2013;35:1105–1111. doi: 10.3109/0886022X.2013.815102. [DOI] [PubMed] [Google Scholar]
- 5.Fidan N., Inci A., Coban M., Ulman C., Kursat S. Bone mineral density and biochemical markers of bone metabolism in predialysis patients with chronic kidney disease. J. Investig. Med. 2016;64:861–866. doi: 10.1136/jim-2015-000043. [DOI] [PubMed] [Google Scholar]
- 6.Mazariegos C., Loaiza J., Sánchez-Polo V. Pos-275 osteoporosis in ckd: Prevalence and associated factors in a guatemalan center. Kidney Int. Rep. 2021;6:S116–S117. doi: 10.1016/j.ekir.2021.03.290. [DOI] [Google Scholar]
- 7.Asadipooya K., Abdalbary M., Ahmad Y., Kakani E., Monier-Faugere M.-C., El-Husseini A. Bone Quality in CKD Patients: Current Concepts and Future Directions–Part I. Kidney Dis. 2021;7:268–277. doi: 10.1159/000515534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malluche H.H., Porter D.S., Monier-Faugere M.C., Mawad H., Pienkowski D. Differences in bone quality in low- and high-turnover renal osteodystrophy. J. Am. Soc. Nephrol. 2012;23:525–532. doi: 10.1681/ASN.2010121253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qaseem A., Forciea M.A., McLean R.M., Denberg T.D. Treatment of Low Bone Density or Osteoporosis to Prevent Fractures in Men and Women: A Clinical Practice Guideline Update From the American College of Physicians. Ann. Intern. Med. 2017;166:818–839. doi: 10.7326/M15-1361. [DOI] [PubMed] [Google Scholar]
- 10.Camacho P.M., Petak S.M., Binkley N., Diab D.L., Eldeiry L.S., Farooki A., Harris S.T., Hurley D.L., Kelly J., Lewiecki E.M., et al. American association of clinical endocrinologists/american college of endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis- 2020 update executive summary. Endocr. Pract. 2020;26:564–570. doi: 10.4158/GL-2020-0524. [DOI] [PubMed] [Google Scholar]
- 11.Jadoul M., Albert J., Akiba T.y.c., Akizawa T., Arab L., Bragg-Gresham J., Mason N., Prutz K.-G., Young E., Pisoni R. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006;70:1358–1366. doi: 10.1038/sj.ki.5001754. [DOI] [PubMed] [Google Scholar]
- 12.Cenzer I.S., Tang V., Boscardin W.J., Smith A.K., Ritchie C., Wallhagen M.I., Espaldon R., Covinsky K.E. One-Year Mortality After Hip Fracture: Development and Validation of a Prognostic Index. J. Am. Geriatr. Soc. 2016;64:1863–1868. doi: 10.1111/jgs.14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morri M., Ambrosi E., Chiari P., Orlandi Magli A., Gazineo D., D’Alessandro F., Forni C. One-year mortality after hip fracture surgery and prognostic factors: A prospective cohort study. Sci. Rep. 2019;9:18718. doi: 10.1038/s41598-019-55196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coco M., Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am. J. Kidney Dis. 2000;36:1115–1121. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 15.Mittalhenkle A., Gillen D.L., Stehman-Breen C.O. Increased risk of mortality associated with hip fracture in the dialysis population. Am. J. Kidney Dis. 2004;44:672–679. doi: 10.1016/S0272-6386(04)00958-8. [DOI] [PubMed] [Google Scholar]
- 16.Moe S., Drüeke T., Cunningham J., Goodman W., Martin K., Olgaard K., Ott S., Sprague S., Lameire N., Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 17.Farrow E.G., Davis S.I., Summers L.J., White K.E. Initial FGF23-mediated signaling occurs in the distal convoluted tubule. J. Am. Soc. Nephrol. 2009;20:955–960. doi: 10.1681/ASN.2008070783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolek O.I., Hines E.R., Jones M.D., LeSueur L.K., Lipko M.A., Kiela P.R., Collins J.F., Haussler M.R., Ghishan F.K. 1α, 25-Dihydroxyvitamin D3 upregulates FGF23 gene expression in bone: The final link in a renal-gastrointestinal-skeletal axis that controls phosphate transport. Am. J. Physiol.-Gastrointest. Liver Physiol. 2005;289:G1036–G1042. doi: 10.1152/ajpgi.00243.2005. [DOI] [PubMed] [Google Scholar]
- 19.Koh N., Fujimori T., Nishiguchi S., Tamori A., Shiomi S., Nakatani T., Sugimura K., Kishimoto T., Kinoshita S., Kuroki T. Severely reduced production of klotho in human chronic renal failure kidney. Biochem. Biophys. Res. Commun. 2001;280:1015–1020. doi: 10.1006/bbrc.2000.4226. [DOI] [PubMed] [Google Scholar]
- 20.Shimada T., Hasegawa H., Yamazaki Y., Muto T., Hino R., Takeuchi Y., Fujita T., Nakahara K., Fukumoto S., Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J. Bone Miner. Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 21.Perwad F., Zhang M.Y., Tenenhouse H.S., Portale A.A. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am. J. Physiol.-Ren. Physiol. 2007;293:F1577–F1583. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 22.Tomasello S. Secondary hyperparathyroidism and chronic kidney disease. Diabetes Spectr. 2008;21:19–25. doi: 10.2337/diaspect.21.1.19. [DOI] [Google Scholar]
- 23.Almaden Y., Canalejo A., Hernandez A., Ballesteros E., Garcia-Navarro S., Torres A., Rodriguez M., Rodriguez M. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J. Bone Miner. Res. 1996;11:970–976. doi: 10.1002/jbmr.5650110714. [DOI] [PubMed] [Google Scholar]
- 24.Ritter C.S., Finch J.L., Slatopolsky E.A., Brown A.J. Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int. 2001;60:1737–1744. doi: 10.1046/j.1523-1755.2001.00027.x. [DOI] [PubMed] [Google Scholar]
- 25.Fukuda N., Tanaka H., Tominaga Y., Fukagawa M., Kurokawa K., Seino Y. Decreased 1, 25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J. Clin. Investig. 1993;92:1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamada-Ode K., Taniguchi Y., Shimamura Y., Fujimoto S., Terada Y. Serum dickkopf-related protein 1 and sclerostin may predict the progression of chronic kidney disease in Japanese patients. Nephrol. Dial. Transplant. 2019;34:1426–1427. doi: 10.1093/ndt/gfz078. [DOI] [PubMed] [Google Scholar]
- 27.Neto R., Pereira L., Magalhães J., Quelhas-Santos J., Martins S., Carvalho C., Frazão J.M. Sclerostin and DKK1 circulating levels associate with low bone turnover in patients with chronic kidney disease Stages 3 and 4. Clin. Kidney J. 2021;14:2401–2408. doi: 10.1093/ckj/sfab081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofbauer L.C., Heufelder A.E. Role of receptor activator of nuclear factor-κB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 2001;79:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 29.Gal-Moscovici A., Sprague S.M. Bone Health in Chronic Kidney Disease–Mineral and Bone Disease. Adv. Chronic Kidney Dis. 2007;14:27–36. doi: 10.1053/j.ackd.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 30.Taal M.W., Masud T., Green D., Cassidy M.J. Risk factors for reduced bone density in haemodialysis patients. Nephrol. Dial. Transplant. 1999;14:1922–1928. doi: 10.1093/ndt/14.8.1922. [DOI] [PubMed] [Google Scholar]
- 31.Evenepoel P., Claes K., Meijers B., Laurent M., Bammens B., Naesens M., Sprangers B., Pottel H., Cavalier E., Kuypers D. Poor Vitamin K Status Is Associated with Low Bone Mineral Density and Increased Fracture Risk in End-Stage Renal Disease. J. Bone Miner. Res. 2019;34:262–269. doi: 10.1002/jbmr.3608. [DOI] [PubMed] [Google Scholar]
- 32.Kanis J.A., Cooper C., Rizzoli R., Reginster J.Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019;30:3–44. doi: 10.1007/s00198-018-4704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nickolas T.L., Stein E., Cohen A., Thomas V., Staron R.B., McMahon D.J., Leonard M.B., Shane E. Bone mass and microarchitecture in CKD patients with fracture. J. Am. Soc. Nephrol. 2010;21:1371–1380. doi: 10.1681/ASN.2009121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delmas P.D., Confavreux E., Garnero P., Fardellone P., de Vernejoul M.C., Cormier C., Arce J.C. A combination of low doses of 17 beta-estradiol and norethisterone acetate prevents bone loss and normalizes bone turnover in postmenopausal women. Osteoporos. Int. 2000;11:177–187. doi: 10.1007/PL00004180. [DOI] [PubMed] [Google Scholar]
- 35.Jørgensen H.S., Behets G., Viaene L., Bammens B., Claes K., Meijers B., Naesens M., Sprangers B., Kuypers D., Cavalier E., et al. Diagnostic Accuracy of Noninvasive Bone Turnover Markers in Renal Osteodystrophy. Am. J. Kidney Dis. 2022;79:667–676.e1. doi: 10.1053/j.ajkd.2021.07.027. [DOI] [PubMed] [Google Scholar]
- 36.Sprague S.M., Bellorin-Font E., Jorgetti V., Carvalho A.B., Malluche H.H., Ferreira A., D’Haese P.C., Drueke T.B., Du H., Manley T., et al. Diagnostic Accuracy of Bone Turnover Markers and Bone Histology in Patients with CKD Treated by Dialysis. Am. J. Kidney Dis. 2016;67:559–566. doi: 10.1053/j.ajkd.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Nickolas T.L., Cremers S., Zhang A., Thomas V., Stein E., Cohen A., Chauncey R., Nikkel L., Yin M.T., Liu X.S., et al. Discriminants of prevalent fractures in chronic kidney disease. J. Am. Soc. Nephrol. 2011;22:1560–1572. doi: 10.1681/ASN.2010121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iimori S., Mori Y., Akita W., Kuyama T., Takada S., Asai T., Kuwahara M., Sasaki S., Tsukamoto Y. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients--a single-center cohort study. Nephrol. Dial. Transplant. 2012;27:345–351. doi: 10.1093/ndt/gfr317. [DOI] [PubMed] [Google Scholar]
- 39.Malluche H.H., Faugere M.C. Atlas of Mineralized Bone Histology. Karger; New York, NY, USA: 1986. [Google Scholar]
- 40.Malluche H.H., Monier-Faugere M.C. Renal osteodystrophy: What’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin. Nephrol. 2006;65:235–242. doi: 10.5414/CNP65235. [DOI] [PubMed] [Google Scholar]
- 41.Drüeke T.B., Massy Z.A. Changing bone patterns with progression of chronic kidney disease. Kidney Int. 2016;89:289–302. doi: 10.1016/j.kint.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Asadipooya K., Abdalbary M., Ahmad Y., Kakani E., Monier-Faugere M.C., El-Husseini A. Bone Quality in Chronic Kidney Disease Patients: Current Concepts and Future Directions—Part II. Kidney Dis. 2021;7:359–371. doi: 10.1159/000515542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wheeler D.C., Winkelmayer W.C. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Kidney Int. Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sugimoto T., Inoue D., Maehara M., Oikawa I., Shigematsu T., Nishizawa Y. Efficacy and safety of once-monthly risedronate in osteoporosis subjects with mild-to-moderate chronic kidney disease: A post hoc subgroup analysis of a phase III trial in Japan. J. Bone Miner. Metab. 2019;37:730–740. doi: 10.1007/s00774-018-0977-1. [DOI] [PubMed] [Google Scholar]
- 45.Robinson D.E., Ali M.S., Pallares N., Tebé C., Elhussein L., Abrahamsen B., Arden N.K., Ben-Shlomo Y., Caskey F.J., Cooper C., et al. Safety of Oral Bisphosphonates in Moderate-to-Severe Chronic Kidney Disease: A Binational Cohort Analysis. J. Bone Miner. Res. 2021;36:820–832. doi: 10.1002/jbmr.4235. [DOI] [PubMed] [Google Scholar]
- 46.Muschitz C., Thaler H.W., Dimai H.P., Resch H., Kocijan R., Kostic M., Geiger C., Kaider A., Muschitz G.K., Szivak M., et al. Atypical Femoral Fractures-Ongoing and History of Bone-Specific Therapy, Concomitant Diseases, Medications, and Survival. J. Clin. Densitom. 2016;19:359–367. doi: 10.1016/j.jocd.2015.05.070. [DOI] [PubMed] [Google Scholar]
- 47.Gopaul A., Kanagalingam T., Thain J., Khan T., Cowan A., Sultan N., Clemens K.K. Denosumab in chronic kidney disease: A narrative review of treatment efficacy and safety. Arch. Osteoporos. 2021;16:116. doi: 10.1007/s11657-021-00971-0. [DOI] [PubMed] [Google Scholar]
- 48.Iseri K., Watanabe M., Yoshikawa H., Mitsui H., Endo T., Yamamoto Y., Iyoda M., Ryu K., Inaba T., Shibata T. Effects of Denosumab and Alendronate on Bone Health and Vascular Function in Hemodialysis Patients: A Randomized, Controlled Trial. J. Bone Miner. Res. 2019;34:1014–1024. doi: 10.1002/jbmr.3676. [DOI] [PubMed] [Google Scholar]
- 49.Cejka D., Kodras K., Bader T., Haas M. Treatment of Hemodialysis-Associated Adynamic Bone Disease with Teriparatide (PTH1-34): A Pilot Study. Kidney Blood Press. Res. 2010;33:221–226. doi: 10.1159/000316708. [DOI] [PubMed] [Google Scholar]
- 50.Mitsopoulos E., Ginikopoulou E., Economidou D., Zanos S., Pateinakis P., Minasidis E., Memmos D., Thodis E., Vargemezis V., Tsakiris D. Impact of long-term cinacalcet, ibandronate or teriparatide therapy on bone mineral density of hemodialysis patients: A pilot study. Am. J. Nephrol. 2012;36:238–244. doi: 10.1159/000341864. [DOI] [PubMed] [Google Scholar]
- 51.Sumida K., Ubara Y., Hoshino J., Mise K., Hayami N., Suwabe T., Kawada M., Imafuku A., Hiramatsu R., Hasegawa E., et al. Once-weekly teriparatide in hemodialysis patients with hypoparathyroidism and low bone mass: A prospective study. Osteoporos. Int. 2016;27:1441–1450. doi: 10.1007/s00198-015-3377-6. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto J., Nakazawa D., Nishio S., Ishikawa Y., Makita M., Kusunoki Y., Nagai S., Fujieda Y., Takahata M., Yamada K., et al. Impact of Weekly Teriparatide on the Bone and Mineral Metabolism in Hemodialysis Patients With Relatively Low Serum Parathyroid Hormone: A Pilot Study. Ther. Apher. Dial. 2020;24:146–153. doi: 10.1111/1744-9987.12867. [DOI] [PubMed] [Google Scholar]
- 53.Bilezikian J.P., Hattersley G., Mitlak B.H., Hu M.Y., Fitzpatrick L.A., Dabrowski C., Miller P.D., Papapoulos S.E. Abaloparatide in patients with mild or moderate renal impairment: Results from the ACTIVE phase 3 trial. Curr. Med. Res. Opin. 2019;35:2097–2102. doi: 10.1080/03007995.2019.1656955. [DOI] [PubMed] [Google Scholar]
- 54.Miller P., Adachi J., Albergaria B.H., Cheung A.M., Chines A., Gielen E., Langdahl B., Miyauchi A., Oates M., Reid I., et al. OP0297|Efficacy and safety of romosozumab among postmenopausal women with osteoporosis and mild-to-moderate chronic kidney disease. Ann. Rheum. Dis. 2020;79:185. doi: 10.1136/annrheumdis-2020-eular.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato M., Inaba M., Yamada S., Emoto M., Ohno Y., Tsujimoto Y. Efficacy of romosozumab in patients with osteoporosis on maintenance hemodialysis in Japan; An observational study. J. Bone Miner. Metab. 2021;39:1082–1090. doi: 10.1007/s00774-021-01253-y. [DOI] [PubMed] [Google Scholar]
- 56.Vilaca T., Schini M., Harnan S., Sutton A., Poku E., Allen I.E., Cummings S.R., Eastell R. The risk of hip and non-vertebral fractures in type 1 and type 2 diabetes: A systematic review and meta-analysis update. Bone. 2020;137:115457. doi: 10.1016/j.bone.2020.115457. [DOI] [PubMed] [Google Scholar]
- 57.Ha J., Jeong C., Han K.D., Lim Y., Kim M.K., Kwon H.S., Song K.H., Kang M.I., Baek K.H. Comparison of fracture risk between type 1 and type 2 diabetes: A comprehensive real-world data. Osteoporos. Int. 2021;32:2543–2553. doi: 10.1007/s00198-021-06032-z. [DOI] [PubMed] [Google Scholar]
- 58.Koromani F., Oei L., Shevroja E., Trajanoska K., Schoufour J., Muka T., Franco O.H., Ikram M.A., Zillikens M.C., Uitterlinden A.G., et al. Vertebral Fractures in Individuals with Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care. 2020;43:137–144. doi: 10.2337/dc19-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shanbhogue V.V., Mitchell D.M., Rosen C.J., Bouxsein M.L. Type 2 diabetes and the skeleton: New insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4:159–173. doi: 10.1016/S2213-8587(15)00283-1. [DOI] [PubMed] [Google Scholar]
- 60.Asadipooya K., Uy E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019;3:1799–1818. doi: 10.1210/js.2019-00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stumpf U., Hadji P., van den Boom L., Böcker W., Kostev K. Incidence of fractures in patients with type 1 diabetes mellitus-a retrospective study with 4420 patients. Osteoporos. Int. 2020;31:1315–1322. doi: 10.1007/s00198-020-05344-w. [DOI] [PubMed] [Google Scholar]
- 62.Halper-Stromberg E., Gallo T., Champakanath A., Taki I., Rewers M., Snell-Bergeon J., Frohnert B.I., Shah V.N. Bone Mineral Density across the Lifespan in Patients with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2020;105:746–753. doi: 10.1210/clinem/dgz153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shah V.N., Harrall K.K., Shah C.S., Gallo T.L., Joshee P., Snell-Bergeon J.K., Kohrt W.M. Bone mineral density at femoral neck and lumbar spine in adults with type 1 diabetes: A meta-analysis and review of the literature. Osteoporos. Int. 2017;28:2601–2610. doi: 10.1007/s00198-017-4097-x. [DOI] [PubMed] [Google Scholar]
- 64.Ma L., Oei L., Jiang L., Estrada K., Chen H., Wang Z., Yu Q., Zillikens M.C., Gao X., Rivadeneira F. Association between bone mineral density and type 2 diabetes mellitus: A meta-analysis of observational studies. Eur. J. Epidemiol. 2012;27:319–332. doi: 10.1007/s10654-012-9674-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferrari S.L., Abrahamsen B., Napoli N., Akesson K., Chandran M., Eastell R., El-Hajj Fuleihan G., Josse R., Kendler D.L., Kraenzlin M., et al. Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos. Int. 2018;29:2585–2596. doi: 10.1007/s00198-018-4650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell A., Fall T., Melhus H., Lind L., Michaëlsson K., Byberg L. Type 2 diabetes and Change in Total Hip Bone Area and Bone Mineral Density in Elderly Swedish Men and Women. J. Clin. Endocrinol. Metab. 2021;106:2840–2854. doi: 10.1210/clinem/dgab490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khosla S., Samakkarnthai P., Monroe D.G., Farr J.N. Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021;17:685–697. doi: 10.1038/s41574-021-00555-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shah V.N., Snell-Bergeon J.K. Fracture risk in type 1 diabetes: Think beyond bone mineral density. J. Diabetes Complicat. 2019;33:107411. doi: 10.1016/j.jdiacomp.2019.107411. [DOI] [PubMed] [Google Scholar]
- 69.Leslie W.D., Aubry-Rozier B., Lamy O., Hans D. TBS (trabecular bone score) and diabetes-related fracture risk. J. Clin. Endocrinol. Metab. 2013;98:602–609. doi: 10.1210/jc.2012-3118. [DOI] [PubMed] [Google Scholar]
- 70.Jiang H., Robinson D.L., Nankervis A., Garland S.M., Callegari E.T., Price S., Lee P.V.S., Wark J.D. Bone Measures by Dual-Energy X-ray Absorptiometry and Peripheral Quantitative Computed Tomography in Young Women with Type 1 Diabetes Mellitus. J. Clin. Densitom. 2021;24:259–267. doi: 10.1016/j.jocd.2020.05.009. [DOI] [PubMed] [Google Scholar]
- 71.Heilmeier U., Joseph G.B., Pasco C., Dinh N., Torabi S., Darakananda K., Youm J., Carballido-Gamio J., Burghardt A.J., Link T.M., et al. Longitudinal Evolution of Bone Microarchitecture and Bone Strength in Type 2 Diabetic Postmenopausal Women With and Without History of Fragility Fractures-A 5-Year Follow-Up Study Using High Resolution Peripheral Quantitative Computed Tomography. Front. Endocrinol. 2021;12:599316. doi: 10.3389/fendo.2021.599316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jiang N., Xia W. Assessment of bone quality in patients with diabetes mellitus. Osteoporos. Int. 2018;29:1721–1736. doi: 10.1007/s00198-018-4532-7. [DOI] [PubMed] [Google Scholar]
- 73.Vestergaard P., Rejnmark L., Mosekilde L.J.D. Relative fracture risk in patients with diabetes mellitus, and the impact of insulin and oral antidiabetic medication on relative fracture risk. Diabetologia. 2005;48:1292–1299. doi: 10.1007/s00125-005-1786-3. [DOI] [PubMed] [Google Scholar]
- 74.Oei L., Zillikens M.C., Dehghan A., Buitendijk G.H., Castaño-Betancourt M.C., Estrada K., Stolk L., Oei E.H., van Meurs J.B., Janssen J.A.M.J.L. High bone mineral density and fracture risk in type 2 diabetes as skeletal complications of inadequate glucose control: The Rotterdam Study. Diabetes Care. 2013;36:1619–1628. doi: 10.2337/dc12-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Conway B.N., Long D.M., Figaro M.K., May M.E. Glycemic control and fracture risk in elderly patients with diabetes. Diabetes Res. Clin. Pract. 2016;115:47–53. doi: 10.1016/j.diabres.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Z.-N., Jiang Y.-F., Ding T.J.B. Risk of fracture with thiazolidinediones: An updated meta-analysis of randomized clinical trials. Bone. 2014;68:115–123. doi: 10.1016/j.bone.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 77.Keegan T.H., Schwartz A.V., Bauer D.C., Sellmeyer D.E., Kelsey J.L. Effect of alendronate on bone mineral density and biochemical markers of bone turnover in type 2 diabetic women: The fracture intervention trial. Diabetes Care. 2004;27:1547–1553. doi: 10.2337/diacare.27.7.1547. [DOI] [PubMed] [Google Scholar]
- 78.Anagnostis P., Paschou S.A., Gkekas N.N., Artzouchaltzi A.-M., Christou K., Stogiannou D., Vryonidou A., Potoupnis M., Goulis D.G.J.E. Efficacy of anti-osteoporotic medications in patients with type 1 and 2 diabetes mellitus: A systematic review. Endocrine. 2018;60:373–383. doi: 10.1007/s12020-018-1548-x. [DOI] [PubMed] [Google Scholar]
- 79.Dhaliwal R., Hans D., Hattersley G., Mitlak B., Fitzpatrick L.A., Wang Y., Schwartz A.V., Miller P.D., Josse R.G. Abaloparatide in postmenopausal women with osteoporosis and type 2 diabetes: A post hoc analysis of the ACTIVE study. JBMR Plus. 2020;4:e10346. doi: 10.1002/jbm4.10346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Golds G., Houdek D., Arnason T. Male Hypogonadism and Osteoporosis: The Effects, Clinical Consequences, and Treatment of Testosterone Deficiency in Bone Health. Int. J. Endocrinol. 2017;2017:4602129. doi: 10.1155/2017/4602129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rochira V., Balestrieri A., Madeo B., Zirilli L., Granata A.R., Carani C. Osteoporosis and male age-related hypogonadism: Role of sex steroids on bone (patho)physiology. Eur. J. Endocrinol. 2006;154:175–185. doi: 10.1530/eje.1.02088. [DOI] [PubMed] [Google Scholar]
- 82.Amory J.K., Watts N.B., Easley K.A., Sutton P.R., Anawalt B.D., Matsumoto A.M., Bremner W.J., Tenover J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004;89:503–510. doi: 10.1210/jc.2003-031110. [DOI] [PubMed] [Google Scholar]
- 83.Snyder P.J., Kopperdahl D.L., Stephens-Shields A.J., Ellenberg S.S., Cauley J.A., Ensrud K.E., Lewis C.E., Barrett-Connor E., Schwartz A.V., Lee D.C., et al. Effect of Testosterone Treatment on Volumetric Bone Density and Strength in Older Men With Low Testosterone: A Controlled Clinical Trial. JAMA Intern. Med. 2017;177:471–479. doi: 10.1001/jamainternmed.2016.9539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Underbjerg L., Sikjaer T., Mosekilde L., Rejnmark L. Postsurgical hypoparathyroidism--risk of fractures, psychiatric diseases, cancer, cataract, and infections. J. Bone Miner. Res. 2014;29:2504–2510. doi: 10.1002/jbmr.2273. [DOI] [PubMed] [Google Scholar]
- 85.Mendonça M.L., Pereira F.A., Nogueira-Barbosa M.H., Monsignore L.M., Teixeira S.R., Watanabe P.C., Maciel L.M., de Paula F.J. Increased vertebral morphometric fracture in patients with postsurgical hypoparathyroidism despite normal bone mineral density. BMC Endocr. Disord. 2013;13:1. doi: 10.1186/1472-6823-13-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pal R., Bhadada S.K., Mukherjee S., Banerjee M., Kumar A. Fracture risk in hypoparathyroidism: A systematic review and meta-analysis. Osteoporos. Int. 2021;32:2145–2153. doi: 10.1007/s00198-021-05966-8. [DOI] [PubMed] [Google Scholar]
- 87.Chawla H., Saha S., Kandasamy D., Sharma R., Sreenivas V., Goswami R. Vertebral Fractures and Bone Mineral Density in Patients With Idiopathic Hypoparathyroidism on Long-Term Follow-Up. J. Clin. Endocrinol. Metab. 2017;102:251–258. doi: 10.1210/jc.2016-3292. [DOI] [PubMed] [Google Scholar]
- 88.Underbjerg L., Sikjaer T., Mosekilde L., Rejnmark L. The Epidemiology of Nonsurgical Hypoparathyroidism in Denmark: A Nationwide Case Finding Study. J. Bone Miner. Res. 2015;30:1738–1744. doi: 10.1002/jbmr.2501. [DOI] [PubMed] [Google Scholar]
- 89.Silva B.C., Rubin M.R., Cusano N.E., Bilezikian J.P. Bone imaging in hypoparathyroidism. Osteoporos. Int. 2017;28:463–471. doi: 10.1007/s00198-016-3750-0. [DOI] [PubMed] [Google Scholar]
- 90.Cipriani C., Abraham A., Silva B.C., Cusano N.E., Rubin M.R., McMahon D.J., Zhang C., Hans D., Silverberg S.J., Bilezikian J.P. Skeletal changes after restoration of the euparathyroid state in patients with hypoparathyroidism and primary hyperparathyroidism. Endocrine. 2017;55:591–598. doi: 10.1007/s12020-016-1101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cipriani C., Minisola S., Bilezikian J.P., Diacinti D., Colangelo L., Piazzolla V., Angelozzi M., Nieddu L., Pepe J., Diacinti D. Vertebral Fracture Assessment in Postmenopausal Women with Postsurgical Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2021;106:1303–1311. doi: 10.1210/clinem/dgab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sakane E.N., Vieira M.C.C., Lazaretti-Castro M., Maeda S.S. Predictors of Poor Bone Microarchitecture Assessed by Trabecular Bone Score in Postsurgical Hypoparathyroidism. J. Clin. Endocrinol. Metab. 2019;104:5795–5803. doi: 10.1210/jc.2019-00698. [DOI] [PubMed] [Google Scholar]
- 93.Chen Q., Kaji H., Iu M.F., Nomura R., Sowa H., Yamauchi M., Tsukamoto T., Sugimoto T., Chihara K. Effects of an excess and a deficiency of endogenous parathyroid hormone on volumetric bone mineral density and bone geometry determined by peripheral quantitative computed tomography in female subjects. J. Clin. Endocrinol. Metab. 2003;88:4655–4658. doi: 10.1210/jc.2003-030470. [DOI] [PubMed] [Google Scholar]
- 94.Cusano N.E., Nishiyama K.K., Zhang C., Rubin M.R., Boutroy S., McMahon D.J., Guo X.E., Bilezikian J.P. Noninvasive Assessment of Skeletal Microstructure and Estimated Bone Strength in Hypoparathyroidism. J. Bone Miner. Res. 2016;31:308–316. doi: 10.1002/jbmr.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cohen A., Dempster D.W., Müller R., Guo X.E., Nickolas T.L., Liu X.S., Zhang X.H., Wirth A.J., van Lenthe G.H., Kohler T., et al. Assessment of trabecular and cortical architecture and mechanical competence of bone by high-resolution peripheral computed tomography: Comparison with transiliac bone biopsy. Osteoporos. Int. 2010;21:263–273. doi: 10.1007/s00198-009-0945-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Starr J.R., Tabacco G., Majeed R., Omeragic B., Bandeira L., Rubin M.R. PTH and bone material strength in hypoparathyroidism as measured by impact microindentation. Osteoporos. Int. 2020;31:327–333. doi: 10.1007/s00198-019-05177-2. [DOI] [PubMed] [Google Scholar]
- 97.Tay Y.D., Tabacco G., Cusano N.E., Williams J., Omeragic B., Majeed R., Gomez Almonte M., Bilezikian J.P., Rubin M.R. Therapy of Hypoparathyroidism With rhPTH(1-84): A Prospective, 8-Year Investigation of Efficacy and Safety. J. Clin. Endocrinol. Metab. 2019;104:5601–5610. doi: 10.1210/jc.2019-00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mannstadt M., Clarke B.L., Vokes T., Brandi M.L., Ranganath L., Fraser W.D., Lakatos P., Bajnok L., Garceau R., Mosekilde L., et al. Efficacy and safety of recombinant human parathyroid hormone (1-84) in hypoparathyroidism (REPLACE): A double-blind, placebo-controlled, randomised, phase 3 study. Lancet Diabetes Endocrinol. 2013;1:275–283. doi: 10.1016/S2213-8587(13)70106-2. [DOI] [PubMed] [Google Scholar]
- 99.Cusano N.E., Rubin M.R., McMahon D.J., Irani D., Tulley A., Sliney J., Jr., Bilezikian J.P. The effect of PTH(1-84) on quality of life in hypoparathyroidism. J. Clin. Endocrinol. Metab. 2013;98:2356–2361. doi: 10.1210/jc.2013-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Orloff L.A., Wiseman S.M., Bernet V.J., Fahey T.J., 3rd, Shaha A.R., Shindo M.L., Snyder S.K., Stack B.C., Jr., Sunwoo J.B., Wang M.B. American Thyroid Association Statement on Postoperative Hypoparathyroidism: Diagnosis, Prevention, and Management in Adults. Thyroid. 2018;28:830–841. doi: 10.1089/thy.2017.0309. [DOI] [PubMed] [Google Scholar]
- 101.Palui R., Das R.R., Roy A., Kamalanathan S., Kar S.S., Sahoo J., Selvarajan S., Satapathy A.K. Parathyroid Hormone Replacement versus Oral Calcium and Active Vitamin D Supplementation in Hypoparathyroidism: A Meta-analysis. Indian J. Endocrinol. Metab. 2020;24:206–214. doi: 10.4103/ijem.IJEM_579_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jolette J., Wilker C.E., Smith S.Y., Doyle N., Hardisty J.F., Metcalfe A.J., Marriott T.B., Fox J., Wells D.S. Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1-84 in a 2-year study in Fischer 344 rats. Toxicol. Pathol. 2006;34:929–940. doi: 10.1080/01926230601072301. [DOI] [PubMed] [Google Scholar]
- 103.Cipriani C., Irani D., Bilezikian J.P. Safety of osteoanabolic therapy: A decade of experience. J. Bone Miner. Res. 2012;27:2419–2428. doi: 10.1002/jbmr.1800. [DOI] [PubMed] [Google Scholar]
- 104.Parameswaran R., Samuel M., Satish R.L., Kripesh A., Moorthy V., Vajjhala R., Ng X.L., Yip G.W., Voon F.C.T., Chandran M. Parathyroid allotransplantation to treat post-thyroidectomy hypoparathyroidism: A review of case studies. Surgeon. 2021;19:183–192. doi: 10.1016/j.surge.2020.06.008. [DOI] [PubMed] [Google Scholar]
- 105.Ejlsmark-Svensson H., Rolighed L., Harsløf T., Rejnmark L. Risk of fractures in primary hyperparathyroidism: A systematic review and meta-analysis. Osteoporos. Int. 2021;32:1053–1060. doi: 10.1007/s00198-021-05822-9. [DOI] [PubMed] [Google Scholar]
- 106.Narayanan N., Palui R., Merugu C., Kar S.S., Kamalanathan S., Sahoo J., Selvarajan S., Naik D. The Risk of Fractures in Primary Hyperparathyroidism: A Meta-Analysis. JBMR Plus. 2021;5:e10482. doi: 10.1002/jbm4.10482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ejlsmark-Svensson H., Bislev L.S., Lajlev S., Harsløf T., Rolighed L., Sikjaer T., Rejnmark L. Prevalence and Risk of Vertebral Fractures in Primary Hyperparathyroidism: A Nested Case-Control Study. J. Bone Miner. Res. 2018;33:1657–1664. doi: 10.1002/jbmr.3461. [DOI] [PubMed] [Google Scholar]
- 108.Arboiro-Pinel R., Mahillo-Fernández I., Díaz-Curiel M. Bone Analysis by the Trabecular Bone Score and 3D-DXA in Postmenopausal Women with Primary Hyperparathyroidism. Endocr. Pract. 2021;28:83–89. doi: 10.1016/j.eprac.2021.08.006. [DOI] [PubMed] [Google Scholar]
- 109.Tabacco G., Naciu A.M., Messina C., Sanson G., Rinaudo L., Cesareo R., Falcone S., Manfrini S., Napoli N., Bilezikian J.P., et al. DXA-Based Bone Strain Index: A New Tool to Evaluate Bone Quality in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2021;106:2304–2312. doi: 10.1210/clinem/dgab317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein E.M., Silva B.C., Boutroy S., Zhou B., Wang J., Udesky J., Zhang C., McMahon D.J., Romano M., Dworakowski E., et al. Primary hyperparathyroidism is associated with abnormal cortical and trabecular microstructure and reduced bone stiffness in postmenopausal women. J. Bone Miner. Res. 2013;28:1029–1040. doi: 10.1002/jbmr.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hansen S., Beck Jensen J.E., Rasmussen L., Hauge E.M., Brixen K. Effects on bone geometry, density, and microarchitecture in the distal radius but not the tibia in women with primary hyperparathyroidism: A case-control study using HR-pQCT. J. Bone Miner. Res. 2010;25:1941–1947. doi: 10.1002/jbmr.98. [DOI] [PubMed] [Google Scholar]
- 112.Eriksen E.F. Primary hyperparathyroidism: Lessons from bone histomorphometry. J. Bone Miner. Res. 2002;17((Suppl. 2)):N95–N97. [PubMed] [Google Scholar]
- 113.Schoeb M., Winter E.M., Sleddering M.A., Lips M.A., Schepers A., Snel M., Appelman-Dijkstra N.M. Bone Material Strength Index as Measured by Impact Microindentation is Low in Patients with Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2021;106:e2527–e2534. doi: 10.1210/clinem/dgab207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang L., Liu X., Li H. Long-Term Skeletal Outcomes of Primary Hyperparathyroidism Patients After Treatment with Parathyroidectomy: A Systematic Review and Meta-Analysis. Horm. Metab. Res. 2018;50:242–249. doi: 10.1055/s-0043-125334. [DOI] [PubMed] [Google Scholar]
- 115.Anagnostis P., Vaitsi K., Veneti S., Potoupni V., Kenanidis E., Tsiridis E., Papavramidis T.S., Goulis D.G. Efficacy of parathyroidectomy compared with active surveillance in patients with mild asymptomatic primary hyperparathyroidism: A systematic review and meta-analysis of randomized-controlled studies. J. Endocrinol. Investig. 2021;44:1127–1137. doi: 10.1007/s40618-020-01447-7. [DOI] [PubMed] [Google Scholar]
- 116.Cusano N.E., Rubin M.R., Silva B.C., Tay Y.D., Williams J.M., Agarwal S., Omeragic B., Guo X.E., Bilezikian J.P. Skeletal Microstructure and Estimated Bone Strength Improve Following Parathyroidectomy in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2018;103:196–205. doi: 10.1210/jc.2017-01932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marcocci C., Bollerslev J., Khan A.A., Shoback D.M. Medical management of primary hyperparathyroidism: Proceedings of the fourth International Workshop on the Management of Asymptomatic Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2014;99:3607–3618. doi: 10.1210/jc.2014-1417. [DOI] [PubMed] [Google Scholar]
- 118.Jorde R., Szumlas K., Haug E., Sundsfjord J. The effects of calcium supplementation to patients with primary hyperparathyroidism and a low calcium intake. Eur. J. Nutr. 2002;41:258–263. doi: 10.1007/s00394-002-0383-1. [DOI] [PubMed] [Google Scholar]
- 119.Nicolaisen P., Obling M.L., Winther K.H., Hansen S., Hermann A.P., Hegedüs L., Bonnema S.J., Brix T.H. Consequences of Hyperthyroidism and Its Treatment for Bone Microarchitecture Assessed by High-Resolution Peripheral Quantitative Computed Tomography. Thyroid. 2021;31:208–216. doi: 10.1089/thy.2020.0084. [DOI] [PubMed] [Google Scholar]
- 120.Zhu H., Zhang J., Wang J., Zhao X., Gu M. Association of subclinical thyroid dysfunction with bone mineral density and fracture: A meta-analysis of prospective cohort studies. Endocrine. 2020;67:685–698. doi: 10.1007/s12020-019-02110-9. [DOI] [PubMed] [Google Scholar]
- 121.Ku E.J., Yoo W.S., Lee E.K., Ahn H.Y., Woo S.H., Hong J.H., Chung H.K., Park J.W. Effect of TSH Suppression Therapy on Bone Mineral Density in Differentiated Thyroid Cancer: A Systematic Review and Meta-analysis. J. Clin. Endocrinol. Metab. 2021;106:3655–3667. doi: 10.1210/clinem/dgab539. [DOI] [PubMed] [Google Scholar]
- 122.Delitala A.P., Scuteri A., Doria C. Thyroid Hormone Diseases and Osteoporosis. J. Clin. Med. 2020;9:1034. doi: 10.3390/jcm9041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ohmori N., Nomura K., Ohmori K., Kato Y., Itoh T., Takano K. Osteoporosis is more prevalent in adrenal than in pituitary Cushing’s syndrome. Endocr. J. 2003;50:1–7. doi: 10.1507/endocrj.50.1. [DOI] [PubMed] [Google Scholar]
- 124.Pecori Giraldi F., Moro M., Cavagnini F. Gender-related differences in the presentation and course of Cushing’s disease. J. Clin. Endocrinol. Metab. 2003;88:1554–1558. doi: 10.1210/jc.2002-021518. [DOI] [PubMed] [Google Scholar]
- 125.dos Santos C.V., Vieira Neto L., Madeira M., Alves Coelho M.C., de Mendonça L.M., Paranhos-Neto Fde P., Lima I.C., Gadelha M.R., Farias M.L. Bone density and microarchitecture in endogenous hypercortisolism. Clin. Endocrinol. 2015;83:468–474. doi: 10.1111/cen.12812. [DOI] [PubMed] [Google Scholar]
- 126.Fleseriu M., Auchus R., Bancos I., Ben-Shlomo A., Bertherat J., Biermasz N.R., Boguszewski C.L., Bronstein M.D., Buchfelder M., Carmichael J.D., et al. Consensus on diagnosis and management of Cushing’s disease: A guideline update. Lancet Diabetes Endocrinol. 2021;9:847–875. doi: 10.1016/S2213-8587(21)00235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tauchmanovà L., Pivonello R., Di Somma C., Rossi R., De Martino M.C., Camera L., Klain M., Salvatore M., Lombardi G., Colao A. Bone demineralization and vertebral fractures in endogenous cortisol excess: Role of disease etiology and gonadal status. J. Clin. Endocrinol. Metab. 2006;91:1779–1784. doi: 10.1210/jc.2005-0582. [DOI] [PubMed] [Google Scholar]
- 128.Moraes A.B., de Paula M.P., de Paula Paranhos-Neto F., Cavalari E.M.R., de Morais F.F.C., Curi D.S.C., Lima L.F.C., de Mendonça L.M.C., Farias M.L.F., Madeira M., et al. Bone Evaluation by High-Resolution Peripheral Quantitative Computed Tomography in Patients with Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2020;105:e2726–e2737. doi: 10.1210/clinem/dgaa263. [DOI] [PubMed] [Google Scholar]
- 129.Kim B.J., Kwak M.K., Ahn S.H., Kim H., Lee S.H., Koh J.M. Lower Trabecular Bone Score in Patients with Primary Aldosteronism: Human Skeletal Deterioration by Aldosterone Excess. J. Clin. Endocrinol. Metab. 2018;103:615–621. doi: 10.1210/jc.2017-02043. [DOI] [PubMed] [Google Scholar]
- 130.Kim B.J., Kwak M.K., Ahn S.H., Kim H., Lee S.H., Song K.H., Suh S., Kim J.H., Koh J.M. Lower Bone Mass and Higher Bone Resorption in Pheochromocytoma: Importance of Sympathetic Activity on Human Bone. J. Clin. Endocrinol. Metab. 2017;102:2711–2718. doi: 10.1210/jc.2017-00169. [DOI] [PubMed] [Google Scholar]
- 131.Yokomoto-Umakoshi M., Umakoshi H., Sakamoto R., Fukumoto T., Ogata M., Nakano Y., Iwahashi N., Kaneko H., Mizoguchi N., Hattori A., et al. Role of deteriorated bone quality in the development of osteoporosis in pheochromocytoma and paraganglioma. Bone. 2021;142:115607. doi: 10.1016/j.bone.2020.115607. [DOI] [PubMed] [Google Scholar]
- 132.Falhammar H., Frisén L., Hirschberg A.L., Nordenskjöld A., Almqvist C., Nordenström A. Increased Prevalence of Fractures in Congenital Adrenal Hyperplasia: A Swedish Population-Based National Cohort Study. J. Clin. Endocrinol. Metab. 2021;107:e475–e486. doi: 10.1210/clinem/dgab712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazziotti G., Biagioli E., Maffezzoni F., Spinello M., Serra V., Maroldi R., Floriani I., Giustina A. Bone turnover, bone mineral density, and fracture risk in acromegaly: A meta-analysis. J. Clin. Endocrinol. Metab. 2015;100:384–394. doi: 10.1210/jc.2014-2937. [DOI] [PubMed] [Google Scholar]
- 134.Silva P.P.B., Pereira R.M.R., Takayama L., Borba C.G., Duarte F.H., Trarbach E.B., Martin R.M., Bronstein M.D., Tritos N.A., Jallad R.S. Impaired Bone Microarchitecture in Premenopausal Women with Acromegaly: The Possible Role of Wnt Signaling. J. Clin. Endocrinol. Metab. 2021;106:2690–2706. doi: 10.1210/clinem/dgab260. [DOI] [PubMed] [Google Scholar]
- 135.Giustina A., Mazziotti G., Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr. Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jørgensen J.O.L., Johannsson G., Barkan A. Should patients with adult GH deficiency receive GH replacement? Eur. J. Endocrinol. 2021;186:D1–D15. doi: 10.1530/EJE-21-0534. [DOI] [PubMed] [Google Scholar]
- 137.Ueland T., Olarescu N.C., Jørgensen A.P., Otterdal K., Aukrust P., Godang K., Lekva T., Bollerslev J. Increased serum and bone matrix levels of the secreted Wnt antagonist DKK-1 in patients with growth hormone deficiency in response to growth hormone treatment. J. Clin. Endocrinol. Metab. 2015;100:736–743. doi: 10.1210/jc.2014-2912. [DOI] [PubMed] [Google Scholar]
- 138.Fedewa M.V., Bentley J.L., Higgins S., Kindler J.M., Esco M.R., MacDonald H.V. Celiac Disease and Bone Health in Children and Adolescents: A Systematic Review and Meta-Analysis. J. Clin. Densitom. 2020;23:200–211. doi: 10.1016/j.jocd.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 139.Duggan S.N., Smyth N.D., Murphy A., MacNaughton D., O’Keefe S.J.D., Conlon K.C. High prevalence of osteoporosis in patients with chronic pancreatitis: A systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2014;12:219–228. doi: 10.1016/j.cgh.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 140.Fan J., Wang Q., Sun L. Association between primary biliary cholangitis and osteoporosis: Meta-analysis. Clin. Rheumatol. 2017;36:2565–2571. doi: 10.1007/s10067-017-3844-x. [DOI] [PubMed] [Google Scholar]
- 141.Nygaard L., Skallerup A., Olesen S.S., Kohler M., Vinter-Jensen L., Kruse C., Vestergaard P., Rasmussen H.H. Osteoporosis in patients with intestinal insufficiency and intestinal failure: Prevalence and clinical risk factors. Clin. Nutr. 2018;37:1654–1660. doi: 10.1016/j.clnu.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 142.Yamanashi Y., Takada T., Kurauchi R., Tanaka Y., Komine T., Suzuki H. Transporters for the intestinal absorption of cholesterol, vitamin E, and vitamin K. J. Atheroscler. Thromb. 2017;24:347–359. doi: 10.5551/jat.RV16007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Maurya V.K., Aggarwal M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol. 2017;54:3753–3765. doi: 10.1007/s13197-017-2840-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ferraz R.R., Tiselius H.G., Heilberg I.P. Fat malabsorption induced by gastrointestinal lipase inhibitor leads to an increase in urinary oxalate excretion. Kidney Int. 2004;66:676–682. doi: 10.1111/j.1523-1755.2004.00790.x. [DOI] [PubMed] [Google Scholar]
- 145.Ganji R., Moghbeli M., Sadeghi R., Bayat G., Ganji A. Prevalence of osteoporosis and osteopenia in men and premenopausal women with celiac disease: A systematic review. Nutr. J. 2019;18:9. doi: 10.1186/s12937-019-0434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mustalahti K., Collin P., Sievanen H., Salmi J., Maki M. Osteopenia in patients with clinically silent coeliac disease warrants screening. Lancet. 1999;354:744–745. doi: 10.1016/S0140-6736(99)01990-X. [DOI] [PubMed] [Google Scholar]
- 147.Karakan T., Ozyemisci-Taskiran O., Gunendi Z., Atalay F., Tuncer C. Prevalence of IgA-antiendomysial antibody in a patient cohort with idiopathic low bone mineral density. World J. Gastroenterol. 2007;13:2978–2982. doi: 10.3748/wjg.v13.i21.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Siddh L., Sengar G., Nagraj N., Shyam R., Garg P. Body mass index in celiac disease and effect of a gluten-free diet on body mass index. Int. J. Adv. Med. 2016;3:813–815. doi: 10.18203/2349-3933.ijam20162611. [DOI] [Google Scholar]
- 149.Kemppainen T., Kroger H., Janatuinen E., Arnala I., Lamberg-Allardt C., Karkkainen M., Kosma V.M., Julkunen R., Jurvelin J., Alhava E., et al. Bone recovery after a gluten-free diet: A 5-year follow-up study. Bone. 1999;25:355–360. doi: 10.1016/S8756-3282(99)00171-4. [DOI] [PubMed] [Google Scholar]
- 150.Di Stefano M., Bergonzi M., Benedetti I., De Amici M., Torre C., Brondino N., Miceli E., Pagani E., Marseglia G.L., Corazza G.R., et al. Alterations of Inflammatory and Matrix Production Indices in Celiac Disease With Low Bone Mass on Long-term Gluten-free Diet. J. Clin. Gastroenterol. 2019;53:e221–e226. doi: 10.1097/MCG.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 151.Vujasinovic M., Nezirevic Dobrijevic L., Asplund E., Rutkowski W., Dugic A., Kahn M., Dahlman I., Saaf M., Hagstrom H., Lohr J.M. Low Bone Mineral Density and Risk for Osteoporotic Fractures in Patients with Chronic Pancreatitis. Nutrients. 2021;13:2386. doi: 10.3390/nu13072386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Haworth C.S. Impact of cystic fibrosis on bone health. Curr. Opin. Pulm. Med. 2010;16:616–622. doi: 10.1097/MCP.0b013e32833e2e94. [DOI] [PubMed] [Google Scholar]
- 153.Johnson E., Vu L., Matarese L.E. Bacteria, Bones, and Stones: Managing Complications of Short Bowel Syndrome. Nutr. Clin. Pract. 2018;33:454–466. doi: 10.1002/ncp.10113. [DOI] [PubMed] [Google Scholar]
- 154.Yadav A., Carey E.J. Osteoporosis in chronic liver disease. Nutr. Clin. Pract. 2013;28:52–64. doi: 10.1177/0884533612470145. [DOI] [PubMed] [Google Scholar]
- 155.Wariaghli G., Allali F., Maghraoui A.E., Hajjaj-Hassouni N. Osteoporosis in patients with primary biliary cirrhosis. Eur. J. Gastroenterol. Hepatol. 2010;22:1397–1401. doi: 10.1097/MEG.0b013e3283405939. [DOI] [PubMed] [Google Scholar]
- 156.Guanabens N., Pares A., Ros I., Caballeria L., Pons F., Vidal S., Monegal A., Peris P., Rodes J. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J. Hepatol. 2005;42:573–577. doi: 10.1016/j.jhep.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 157.Chappard D., Plantard B., Fraisse H., Palle S., Alexandre C., Riffat G. Bone changes in alcoholic cirrhosis of the liver. A histomorphometric study. Pathol. Res. Pract. 1989;184:480–485. doi: 10.1016/S0344-0338(89)80138-4. [DOI] [PubMed] [Google Scholar]
- 158.Chen C.H., Lin C.L., Kao C.H. Association Between Chronic Hepatitis B Virus Infection and Risk of Osteoporosis: A Nationwide Population-Based Study. Medicine (Baltimore) 2015;94:e2276. doi: 10.1097/MD.0000000000002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chen C.H., Lin C.L., Kao C.H. Relation Between Hepatitis C Virus Exposure and Risk of Osteoporosis: A Nationwide Population-Based Study. Medicine (Baltimore) 2015;94:e2086. doi: 10.1097/MD.0000000000002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schmidt T., Schmidt C., Strahl A., Mussawy H., Rolvien T., Jandl N.M., Casar C., Oheim R., Schinke T., Lohse A.W., et al. A System to Determine Risk of Osteoporosis in Patients with Autoimmune Hepatitis. Clin. Gastroenterol. Hepatol. 2020;18:226–233.e3. doi: 10.1016/j.cgh.2019.05.043. [DOI] [PubMed] [Google Scholar]
- 161.Valenti L., Varenna M., Fracanzani A.L., Rossi V., Fargion S., Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos. Int. 2009;20:549–555. doi: 10.1007/s00198-008-0701-4. [DOI] [PubMed] [Google Scholar]
- 162.Ehnert S., Aspera-Werz R.H., Ruoß M., Dooley S., Hengstler J.G., Nadalin S., Relja B., Badke A., Nussler A.K. Hepatic Osteodystrophy-Molecular Mechanisms Proposed to Favor Its Development. Int. J. Mol. Sci. 2019;20:2555. doi: 10.3390/ijms20102555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Barbu E.C., Chitu-Tisu C.E., Lazar M., Olariu C., Bojinca M., Ionescu R.A., Ion D.A., Badarau I.A. Hepatic Osteodystrophy: A Global (Re)View of the Problem. Acta Clin. Croat. 2017;56:512–525. doi: 10.20471/acc.2017.56.03.19. [DOI] [PubMed] [Google Scholar]
- 164.Zhao S., Ding L., Xie Q., Zhang J., Yang S., Xu W., Yang J., Xu Y., Zheng C. Is there an association between peptic ulcer disease and osteoporosis: A systematic review and cumulative analysis. Eur. J. Gastroenterol. Hepatol. 2021;33:9–16. doi: 10.1097/MEG.0000000000001981. [DOI] [PubMed] [Google Scholar]
- 165.Gennari L., Merlotti D., Figura N., Mingiano C., Franci M.B., Lucani B., Picchioni T., Alessandri M., Campagna M.S., Gonnelli S., et al. Infection by CagA-Positive Helicobacter pylori Strains and Bone Fragility: A Prospective Cohort Study. J. Bone Miner. Res. 2021;36:80–89. doi: 10.1002/jbmr.4162. [DOI] [PubMed] [Google Scholar]
- 166.Miranda-Bautista J., Verdejo C., Diaz-Redondo A., Breton I., Bellon J.M., Perez-Valderas M.D., Caballero-Marcos A., de Dios-Lascuevas M., Gonzalez-Rio E., Garcia-Sanchez C., et al. Metabolic bone disease in patients diagnosed with inflammatory bowel disease from Spain. Therap. Adv. Gastroenterol. 2019;12:1756284819862152. doi: 10.1177/1756284819862152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Soare I., Sirbu A., Martin S., Diculescu M., Mateescu B., Tieranu C., Fica S. Assessment of bone quality with trabecular bone score in patients with inflammatory bowel disease. Sci. Rep. 2021;11:20345. doi: 10.1038/s41598-021-99669-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Haschka J., Kraus D.A., Behanova M., Huber S., Bartko J., Schanda J.E., Meier P., Bahrami A., Zandieh S., Zwerina J., et al. Fractal-Based Analysis of Bone Microstructure in Crohn’s Disease: A Pilot Study. J. Clin. Med. 2020;9:4116. doi: 10.3390/jcm9124116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maldonado-Perez M.B., Castro-Laria L., Caunedo-Alvarez A., Montoya-Garcia M.J., Giner-Garcia M., Arguelles-Arias F., Romero-Gomez M., Vazquez-Gamez M.A. Does the Antitumor Necrosis Factor-alpha Therapy Decrease the Vertebral Fractures Occurrence in Inflammatory Bowel Disease? J. Clin. Densitom. 2019;22:195–202. doi: 10.1016/j.jocd.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 170.Bernstein C.N., Blanchard J.F., Leslie W., Wajda A., Yu B.N. The incidence of fracture among patients with inflammatory bowel disease. A population-based cohort study. Ann. Intern. Med. 2000;133:795–799. doi: 10.7326/0003-4819-133-10-200011210-00012. [DOI] [PubMed] [Google Scholar]
- 171.Komaki Y., Komaki F., Micic D., Ido A., Sakuraba A. Risk of Fractures in Inflammatory Bowel Diseases: A Systematic Review and Meta-Analysis. J. Clin. Gastroenterol. 2019;53:441–448. doi: 10.1097/MCG.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 172.Hidalgo D.F., Boonpheng B., Phemister J., Hidalgo J., Young M. Inflammatory Bowel Disease and Risk of Osteoporotic Fractures: A Meta-Analysis. Cureus. 2019;11:e5810. doi: 10.7759/cureus.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bravenboer N., Oostlander A.E., Van Bodegraven A.A. Bone loss in patients with inflammatory bowel disease: Cause, detection and treatment. Curr. Opin. Gastroenterol. 2021;37:128–134. doi: 10.1097/MOG.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 174.Morgan S., Hooper K.M., Milne E.M., Farquharson C., Stevens C., Staines K.A. Azathioprine Has a Deleterious Effect on the Bone Health of Mice with DSS-Induced Inflammatory Bowel Disease. Int. J. Mol. Sci. 2019;20:6085. doi: 10.3390/ijms20236085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Misof B.M., Roschger P., Klaushofer K., Rauch F., Ma J., Mack D.R., Ward L.M. Increased bone matrix mineralization in treatment-naive children with inflammatory bowel disease. Bone. 2017;105:50–56. doi: 10.1016/j.bone.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 176.Oostlander A.E., Bravenboer N., Sohl E., Holzmann P.J., van der Woude C.J., Dijkstra G., Stokkers P.C., Oldenburg B., Netelenbos J.C., Hommes D.W., et al. Histomorphometric analysis reveals reduced bone mass and bone formation in patients with quiescent Crohn’s disease. Gastroenterology. 2011;140:116–123. doi: 10.1053/j.gastro.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 177.Farraye F.A., Melmed G.Y., Lichtenstein G.R., Kane S.V. ACG Clinical Guideline: Preventive Care in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2017;112:241–258. doi: 10.1038/ajg.2016.537. [DOI] [PubMed] [Google Scholar]
- 178.IBD Checklists—Cornerstone Health. [(accessed on 1 December 2021)]. Available online: https://www.cornerstoneshealth.org/ibd-checklists.
- 179.Maldonado F.J., Al Bawardy B.F., Nehra A.K., Lee Y.S., Bruining D.H., Adkins M.C., Keaveny T.M., Johnson M.P., Fidler J.L., McCollough C.H., et al. Findings of CT-Derived Bone Strength Assessment in Inflammatory Bowel Disease Patients Undergoing CT Enterography in Clinical Practice. Inflamm. Bowel Dis. 2019;25:1072–1079. doi: 10.1093/ibd/izy341. [DOI] [PubMed] [Google Scholar]
- 180.Frei R., Fournier N., Zeitz J., Scharl M., Morell B., Greuter T., Schreiner P., Misselwitz B., Safroneeva E., Schoepfer A.M., et al. Early Initiation of Anti-TNF is Associated with Favourable Long-term Outcome in Crohn’s Disease: 10-Year-Follow-up Data from the Swiss IBD Cohort Study. J. Crohns Colitis. 2019;13:1292–1301. doi: 10.1093/ecco-jcc/jjz057. [DOI] [PubMed] [Google Scholar]
- 181.Yao L., Wang H., Dong W., Liu Z., Mao H. Efficacy and safety of bisphosphonates in management of low bone density in inflammatory bowel disease: A meta-analysis. Medicine. 2017;96:e5861. doi: 10.1097/MD.0000000000005861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Luo J.S., Zhao X., Yang Y. Effects of emodin on inflammatory bowel disease-related osteoporosis. Biosci. Rep. 2020;40:BSR20192317. doi: 10.1042/BSR20192317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lee S.Y., Hwang H.R., Yi Y.H., Kim J.M., Kim Y.J., Lee J.G., Cho Y.H., Tak Y.J., Lee S.H., Park E.J., et al. Association between Irritable Bowel Syndrome and Risk of Osteoporosis in Korean Premenopausal Women. Med. Princ. Pract. 2021;30:527–534. doi: 10.1159/000517909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wongtrakul W., Charoenngam N., Ungprasert P. The association between irritable bowel syndrome and osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2020;31:1049–1057. doi: 10.1007/s00198-020-05318-y. [DOI] [PubMed] [Google Scholar]
- 185.Cao R.R., He P., Lei S.F. Novel microbiota-related gene set enrichment analysis identified osteoporosis associated gut microbiota from autoimmune diseases. J. Bone Miner. Metab. 2021;39:984–996. doi: 10.1007/s00774-021-01247-w. [DOI] [PubMed] [Google Scholar]
- 186.Tu Y., Yang R., Xu X., Zhou X. The microbiota-gut-bone axis and bone health. J. Leukoc. Biol. 2021;110:525–537. doi: 10.1002/JLB.3MR0321-755R. [DOI] [PubMed] [Google Scholar]
- 187.Takimoto T., Hatanaka M., Hoshino T., Takara T., Tanaka K., Shimizu A., Morita H., Nakamura T. Effect of Bacillus subtilis C-3102 on bone mineral density in healthy postmenopausal Japanese women: A randomized, placebo-controlled, double-blind clinical trial. Biosci. Microbiota Food Health. 2018;37:87–96. doi: 10.12938/bmfh.18-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yoo S.H., Lee J.A., Kang S.Y., Kim Y.S., Sunwoo S., Kim B.S., Yook J.H. Risk of osteoporosis after gastrectomy in long-term gastric cancer survivors. Gastric Cancer. 2018;21:720–727. doi: 10.1007/s10120-017-0777-7. [DOI] [PubMed] [Google Scholar]
- 189.Kim H.W., Kim Y.H., Han K., Nam G.E., Kim G.S., Han B.D., Lee A., Ahn J.Y., Ko B.J. Atrophic gastritis: A related factor for osteoporosis in elderly women. PLoS ONE. 2014;9:e101852. doi: 10.1371/journal.pone.0101852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Aasarod K.M., Mosti M.P., Stunes A.K., Reseland J.E., Basso T., Syversen U., Fossmark R. Impaired skeletal health in patients with chronic atrophic gastritis. Scand. J. Gastroenterol. 2016;51:774–781. doi: 10.3109/00365521.2016.1141317. [DOI] [PubMed] [Google Scholar]
- 191.Paccou J., Caiazzo R., Lespessailles E., Cortet B. Bariatric Surgery and Osteoporosis. Calcif. Tissue Res. 2021;110:576–591. doi: 10.1007/s00223-020-00798-w. [DOI] [PubMed] [Google Scholar]
- 192.Hejazi J., Davoodi A., Khosravi M., Sedaghat M., Abedi V., Hosseinverdi S., Ehrampoush E., Homayounfar R., Shojaie L. Nutrition and osteoporosis prevention and treatment. Biomed. Res. Ther. 2020;7:3709–3720. doi: 10.15419/bmrat.v7i4.598. [DOI] [Google Scholar]
- 193.Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference IntakesIntakes . Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. National Academies Press (US); Washington, DC, USA: 1997. Dietary reference intakes. [PubMed] [Google Scholar]
- 195.Bauer J., Biolo G., Cederholm T., Cesari M., Cruz-Jentoft A.J., Morley J.E., Phillips S., Sieber C., Stehle P., Teta D. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013;14:542–559. doi: 10.1016/j.jamda.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 196.Rizzoli R., Stevenson J.C., Bauer J.M., van Loon L.J., Walrand S., Kanis J.A., Cooper C., Brandi M.-L., Diez-Perez A., Reginster J.-Y. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: A consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Maturitas. 2014;79:122–132. doi: 10.1016/j.maturitas.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 197.Rizzoli R. Vitamin D supplementation: Upper limit for safety revisited? Aging Clin. Exp. Res. 2021;33:19–24. doi: 10.1007/s40520-020-01678-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saunders J., Smith T. Malnutrition: Causes and consequences. Clin. Med. 2010;10:624. doi: 10.7861/clinmedicine.10-6-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Colangelo L., Biamonte F., Pepe J., Cipriani C., Minisola S. Understanding and managing secondary osteoporosis. Expert Rev. Endocrinol. Metab. 2019;14:111–122. doi: 10.1080/17446651.2019.1575727. [DOI] [PubMed] [Google Scholar]
- 200.Rizzoli R., Biver E., Brennan-Speranza T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021;9:606–621. doi: 10.1016/S2213-8587(21)00119-4. [DOI] [PubMed] [Google Scholar]
- 201.Kueper J., Beyth S., Liebergall M., Kaplan L., Schroeder J.E. Evidence for the adverse effect of starvation on bone quality: A review of the literature. Int. J. Endocrinol. 2015;2015:628740. doi: 10.1155/2015/628740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dulloo A.G., Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: A role for feedback signals from fat stores. Am. J. Clin. Nutr. 1998;68:599–606. doi: 10.1093/ajcn/68.3.599. [DOI] [PubMed] [Google Scholar]
- 203.Zong L., Cai L., Liang J., Lin W., Yao J., Huang H., Tang K., Chen L., Li L., Lin L. Exposure to famine in early life and the risk of osteoporosis in adulthood: A prospective study. Endocr. Pract. 2019;25:299–305. doi: 10.4158/EP-2018-0419. [DOI] [PubMed] [Google Scholar]
- 204.Hercshlag-Elkayam O., Even L., Shasha S.M. Clinical manifestations of “Hunger Disease” among children in the ghettos during the Holocaust. Harefuah. 2003;142:345–349. [PubMed] [Google Scholar]
- 205.Weisz G.M., Albury W.R. Hunger whilst “in utero” programming adult osteoporosis. Rambam Maimonides Med. J. 2014;5:e0004. doi: 10.5041/RMMJ.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Marcus E.-L., Menczel J. Higher prevalence of osteoporosis among female Holocaust survivors. Osteoporos. Int. 2007;18:1501–1506. doi: 10.1007/s00198-007-0389-x. [DOI] [PubMed] [Google Scholar]
- 207.Kin C.F.W., Shan W.S.Y., Shun L.J.C., Chung L.P., Jean W. Experience of famine and bone health in post-menopausal women. Int. J. Epidemiol. 2007;36:1143–1150. doi: 10.1093/ije/dym149. [DOI] [PubMed] [Google Scholar]
- 208.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocr. Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 209.Macdonald H.M., Reid I.R., Gamble G.D., Fraser W.D., Tang J.C., Wood A.D. 25-hydroxyvitamin D threshold for the effects of vitamin D supplements on bone density: Secondary analysis of a randomized controlled trial. J. Bone Miner. Res. 2018;33:1464–1469. doi: 10.1002/jbmr.3442. [DOI] [PubMed] [Google Scholar]
- 210.Reid I., Horne A., Mihov B., Gamble G., Al-Abuwsi F., Singh M., Taylor L., Fenwick S., Camargo C., Stewart A. Effect of monthly high-dose vitamin D on bone density in community-dwelling older adults substudy of a randomized controlled trial. J. Intern. Med. 2017;282:452–460. doi: 10.1111/joim.12651. [DOI] [PubMed] [Google Scholar]
- 211.Bolland M.J., Grey A., Avenell A. Effects of vitamin D supplementation on musculoskeletal health: A systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6:847–858. doi: 10.1016/S2213-8587(18)30265-1. [DOI] [PubMed] [Google Scholar]
- 212.Reid I.R., Bolland M.J., Grey A. Effects of vitamin D supplements on bone mineral density: A systematic review and meta-analysis. Lancet. 2014;383:146–155. doi: 10.1016/S0140-6736(13)61647-5. [DOI] [PubMed] [Google Scholar]
- 213.Zhao J.-G., Zeng X.-T., Wang J., Liu L. Association between calcium or vitamin D supplementation and fracture incidence in community-dwelling older adults: A systematic review and meta-analysis. JAMA. 2017;318:2466–2482. doi: 10.1001/jama.2017.19344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Burt L.A., Billington E.O., Rose M.S., Raymond D.A., Hanley D.A., Boyd S.K. Effect of high-dose vitamin D supplementation on volumetric bone density and bone strength: A randomized clinical trial. JAMA. 2019;322:736–745. doi: 10.1001/jama.2019.11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Liu C., Kuang X., Li K., Guo X., Deng Q., Li D. Effects of combined calcium and vitamin D supplementation on osteoporosis in postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2020;11:10817–10827. doi: 10.1039/D0FO00787K. [DOI] [PubMed] [Google Scholar]
- 216.Weaver C., Alexander D., Boushey C., Dawson-Hughes B., Lappe J., LeBoff M., Liu S., Looker A., Wallace T., Wang D. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos. Int. 2016;27:367–376. doi: 10.1007/s00198-015-3386-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Premaor M.O., Pilbrow L., Tonkin C., Parker R.A., Compston J. Obesity and fractures in postmenopausal women. J. Bone Miner. Res. 2010;25:292–297. doi: 10.1359/jbmr.091004. [DOI] [PubMed] [Google Scholar]
- 218.Schafer A.L. Decline in bone mass during weight loss: A cause for concern? J. Bone Miner. Res. 2016;31:36–39. doi: 10.1002/jbmr.2754. [DOI] [PubMed] [Google Scholar]
- 219.Jiang B.C., Villareal D.T. Weight loss-induced reduction of bone mineral density in older adults with obesity. J. Nutr. Gerontol. Geriatr. 2019;38:100–114. doi: 10.1080/21551197.2018.1564721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Compston J.E., Watts N.B., Chapurlat R., Cooper C., Boonen S., Greenspan S., Pfeilschifter J., Silverman S., Díez-Pérez A., Lindsay R. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011;124:1043–1050. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Schwartz A.V., Johnson K.C., Kahn S.E., Shepherd J.A., Nevitt M.C., Peters A.L., Walkup M.P., Hodges A., Williams C.C., Bray G.A. Effect of 1 year of an intentional weight loss intervention on bone mineral density in type 2 diabetes: Results from the Look AHEAD randomized trial. J. Bone Miner. Res. 2012;27:619–627. doi: 10.1002/jbmr.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lipkin E.W., Schwartz A.V., Anderson A.M., Davis C., Johnson K.C., Gregg E.W., Bray G.A., Berkowitz R., Peters A.L., Hodges A. The Look AHEAD Trial: Bone loss at 4-year follow-up in type 2 diabetes. Diabetes Care. 2014;37:2822–2829. doi: 10.2337/dc14-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Viégas M., Vasconcelos R.S.d., Neves A.P., Diniz E.T., Bandeira F. Bariatric surgery and bone metabolism: A systematic review. Arq. Bras. Endocrinol. Metabol. 2010;54:158–163. doi: 10.1590/S0004-27302010000200011. [DOI] [PubMed] [Google Scholar]
- 224.Carrasco F., Ruz M., Rojas P., Csendes A., Rebolledo A., Codoceo J., Inostroza J., Basfi-Fer K., Papapietro K., Rojas J. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes. Surg. 2009;19:41–46. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 225.Shaker J., Norton A., Woods M., Fallon M., Findling J. Secondary hyperparathyroidism and osteopenia in women following gastric exclusion surgery for obesity. Osteoporos. Int. 1991;1:177–181. doi: 10.1007/BF01625450. [DOI] [PubMed] [Google Scholar]
- 226.Gagnon C., Schafer A.L. Bone health after bariatric surgery. JBMR Plus. 2018;2:121–133. doi: 10.1002/jbm4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.American Psychiatric Association A. Diagnostic and Statistical Manual of Mental Disorders. Volume 3 American Psychiatric Association; Washington, DC, USA: 1980. [Google Scholar]
- 228.Westmoreland P., Krantz M.J., Mehler P.S. Medical complications of anorexia nervosa and bulimia. Am. J. Med. 2016;129:30–37. doi: 10.1016/j.amjmed.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 229.Vestergaard P., Emborg C., Støving R.K., Hagen C., Mosekilde L., Brixen K. Fractures in patients with anorexia nervosa, bulimia nervosa, and other eating disorders—a nationwide register study. Int. J. Eat. Disord. 2002;32:301–308. doi: 10.1002/eat.10101. [DOI] [PubMed] [Google Scholar]
- 230.Rigotti N.A., Neer R.M., Skates S.J., Herzog D.B., Nussbaum S.R. The clinical course of osteoporosis in anorexia nervosa: A longitudinal study of cortical bone mass. JAMA. 1991;265:1133–1138. doi: 10.1001/jama.1991.03460090081037. [DOI] [PubMed] [Google Scholar]
- 231.Steinman J., Shibli-Rahhal A. Anorexia nervosa and osteoporosis: Pathophysiology and treatment. J. Bone Metab. 2019;26:133–143. doi: 10.11005/jbm.2019.26.3.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Garnero P. Bone markers in osteoporosis. Curr. Osteoporos. Rep. 2009;7:84–90. doi: 10.1007/s11914-009-0014-3. [DOI] [PubMed] [Google Scholar]
- 233.Robinson L., Aldridge V., Clark E.M., Misra M., Micali N. Pharmacological treatment options for low bone mineral density and secondary osteoporosis in anorexia nervosa: A systematic review of the literature. J. Psychosom. Res. 2017;98:87–97. doi: 10.1016/j.jpsychores.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Miller K.K., Meenaghan E., Lawson E.A., Misra M., Gleysteen S., Schoenfeld D., Herzog D., Klibanski A. Effects of risedronate and low-dose transdermal testosterone on bone mineral density in women with anorexia nervosa: A randomized, placebo-controlled study. J. Clin. Endocrinol. Metab. 2011;96:2081–2088. doi: 10.1210/jc.2011-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Miller K.K., Grieco K.A., Mulder J., Grinspoon S., Mickley D., Yehezkel R., Herzog D.B., Klibanski A. Effects of risedronate on bone density in anorexia nervosa. J. Clin. Endocrinol. Metab. 2004;89:3903–3906. doi: 10.1210/jc.2003-031885. [DOI] [PubMed] [Google Scholar]
- 236.Misra M., Katzman D., Miller K.K., Mendes N., Snelgrove D., Russell M., Goldstein M.A., Ebrahimi S., Clauss L., Weigel T. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J. Bone Miner. Res. 2011;26:2430–2438. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Haines M.S., Kimball A., Meenaghan E., Bachmann K.N., Santoso K., Eddy K.T., Singhal V., Ebrahimi S., Dechant E., Weigel T., et al. Sequential Therapy with Recombinant Human IGF-1 Followed by Risedronate Increases Spine Bone Mineral Density in Women With Anorexia Nervosa: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2021;36:2116–2126. doi: 10.1002/jbmr.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fazeli P.K., Wang I.S., Miller K.K., Herzog D.B., Misra M., Lee H., Finkelstein J.S., Bouxsein M.L., Klibanski A. Teriparatide increases bone formation and bone mineral density in adult women with anorexia nervosa. J. Clin. Endocrinol. Metab. 2014;99:1322–1329. doi: 10.1210/jc.2013-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Napartivaumnuay N., Gramlich L. The prevalence of vitamin D insufficiency and deficiency and their relationship with bone mineral density and fracture risk in adults receiving long-term home parenteral nutrition. Nutrients. 2017;9:481. doi: 10.3390/nu9050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Saitta J.C., Ott S.M., Sherrard D.J., Walden C.E., Lipkin E.W. Metabolic bone disease in adults receiving long-term parenteral nutrition: Longitudinal study with regional densitometry and bone biopsy. J. Parenter. Enter. Nutr. 1993;17:214–219. doi: 10.1177/0148607193017003214. [DOI] [PubMed] [Google Scholar]
- 241.Verhage A.H., Cheong W.K., Allard J.P., Jeejeebhoy K.N. Increase in lumbar spine bone mineral content in patients on long-term parenteral nutrition without vitamin D supplementation. J. Parenter. Enter. Nutr. 1995;19:431–436. doi: 10.1177/0148607195019006431. [DOI] [PubMed] [Google Scholar]
- 242.Demehri F.R., Barrett M., Teitelbaum D.H. Changes to the intestinal microbiome with parenteral nutrition: Review of a murine model and potential clinical implications. Nutr. Clin. Pract. 2015;30:798–806. doi: 10.1177/0884533615609904. [DOI] [PubMed] [Google Scholar]
- 243.Bengoa J.M., Sitrin M.D., Wood R.J., Rosenberg I.H. Amino acid-induced hypercalciuria in patients on total parenteral nutrition. Am. J. Clin. Nutr. 1983;38:264–269. doi: 10.1093/ajcn/38.2.264. [DOI] [PubMed] [Google Scholar]
- 244.Nishikawa R., Siepler S., Siepler J., Diamantidis T., Okamoto R. Intravenous pamidronate improves bone mineral density in home parenteral nutrition patients. Clin. Nutr. 2003;22:S88. doi: 10.1016/S0261-5614(03)80330-8. [DOI] [Google Scholar]
- 245.Haderslev K.V., Tjellesen L., Sorensen H.A., Staun M. Effect of cyclical intravenous clodronate therapy on bone mineral density and markers of bone turnover in patients receiving home parenteral nutrition. Am. J. Clin. Nutr. 2002;76:482–488. doi: 10.1093/ajcn/76.2.482. [DOI] [PubMed] [Google Scholar]
- 246.Wyshak G., Frisch R.E. Carbonated beverages, dietary calcium, the dietary calcium/phosphorus ratio, and bone fractures in girls and boys. J. Adolesc. Health. 1994;15:210–215. doi: 10.1016/1054-139X(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 247.Lindeman R.D. Influence of various nutrients and hormones on urinary divalent cation excretion. Ann. N. Y. Acad. Sci. 1969;162:802–809. doi: 10.1111/j.1749-6632.1969.tb13011.x. [DOI] [PubMed] [Google Scholar]
- 248.Lemann J., Lennon E., Piering W., Prien E., Ricanati E. Evidence that glucose ingestion inhibits net renal tubular reabsorption of calcium and magnesium in man. J. Lab. Clin. Med. 1970;75:578–585. [PubMed] [Google Scholar]
- 249.Douard V., Sabbagh Y., Lee J., Patel C., Kemp F.W., Bogden J.D., Lin S., Ferraris R.P. Excessive fructose intake causes 1, 25-(OH) 2D3-dependent inhibition of intestinal and renal calcium transport in growing rats. Am. J. Physiol.-Endocrinol. Metab. 2013;304:E1303–E1313. doi: 10.1152/ajpendo.00582.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Baron R., Neff L., Louvard D., Courtoy P.J. Cell-mediated extracellular acidification and bone resorption: Evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 1985;101:2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Reginster J.-Y., Burlet N. Osteoporosis: A still increasing prevalence. Bone. 2006;38:4–9. doi: 10.1016/j.bone.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 252.DiNicolantonio J.J., Mehta V., Zaman S.B., O’Keefe J.H. Not salt but sugar as aetiological in osteoporosis: A review. Mo. Med. 2018;115:247. [PMC free article] [PubMed] [Google Scholar]
- 253.Ganry O., Baudoin C., Fardellone P. Effect of alcohol intake on bone mineral density in elderly women: The EPIDOS study. Am. J. Epidemiol. 2000;151:773–780. doi: 10.1093/oxfordjournals.aje.a010277. [DOI] [PubMed] [Google Scholar]
- 254.Maurel D., Boisseau N., Benhamou C., Jaffre C. Alcohol and bone: Review of dose effects and mechanisms. Osteoporos. Int. 2012;23:1–16. doi: 10.1007/s00198-011-1787-7. [DOI] [PubMed] [Google Scholar]
- 255.Maurel D., Jaffré C., Rochefort G.Y., Aveline P., Boisseau N., Uzbekov R., Gosset D., Pichon C., Fazzalari N., Pallu S. Low bone accrual is associated with osteocyte apoptosis in alcohol-induced osteopenia. Bone. 2011;49:543–552. doi: 10.1016/j.bone.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 256.Maurel D.B., Boisseau N., Benhamou C.-L., Jaffré C. Cortical bone is more sensitive to alcohol dose effects than trabecular bone in the rat. Jt. Bone Spine. 2012;79:492–499. doi: 10.1016/j.jbspin.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 257.Reid I. Relationships between fat and bone. Osteoporos. Int. 2008;19:595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- 258.Gaddini G.W., Turner R.T., Grant K.A., Iwaniec U.T. Alcohol: A simple nutrient with complex actions on bone in the adult skeleton. Alcohol. Clin. Exp. Res. 2016;40:657–671. doi: 10.1111/acer.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Song T.-H., Shim J.-C., Jung D.-U., Moon J.-J., Jeon D.-W., Kim S.-J., Oh M.-K. Increased Bone Mineral Density after Abstinence in Male Patients with Alcohol Dependence. Clin. Psychopharmacol. Neurosci. 2018;16:282. doi: 10.9758/cpn.2018.16.3.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Malik P., von Gleissenthall G., Gasser R.W., Moncayo R., Giesinger J.M., Mechtcheriakov S. Osteoprotegerin Levels Decrease in Abstinent Alcohol-Dependent Patients. Alcohol. Clin. Exp. Res. 2016;40:1235–1240. doi: 10.1111/acer.13063. [DOI] [PubMed] [Google Scholar]
- 261.Overman R.A., Yeh J.Y., Deal C.L. Prevalence of oral glucocorticoid usage in the United States: A general population perspective. Arthritis Care Res. (Hoboken) 2013;65:294–298. doi: 10.1002/acr.21796. [DOI] [PubMed] [Google Scholar]
- 262.Van Staa T.P., Leufkens H.G., Abenhaim L., Zhang B., Cooper C. Use of oral corticosteroids and risk of fractures. J. Bone Miner. Res. 2000;15:993–1000. doi: 10.1359/jbmr.2000.15.6.993. [DOI] [PubMed] [Google Scholar]
- 263.Van Staa T.P., Laan R.F., Barton I.P., Cohen S., Reid D.M., Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum. 2003;48:3224–3229. doi: 10.1002/art.11283. [DOI] [PubMed] [Google Scholar]
- 264.Kanis J.A., Johnell O., Oden A., Dawson A., De Laet C., Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos. Int. 2001;12:989–995. doi: 10.1007/s001980170006. [DOI] [PubMed] [Google Scholar]
- 265.De Vries F., Bracke M., Leufkens H.G., Lammers J.W., Cooper C., Van Staa T.P. Fracture risk with intermittent high-dose oral glucocorticoid therapy. Arthritis Rheum. 2007;56:208–214. doi: 10.1002/art.22294. [DOI] [PubMed] [Google Scholar]
- 266.Sutter S.A., Stein E.M. The Skeletal Effects of Inhaled Glucocorticoids. Curr. Osteoporos. Rep. 2016;14:106–113. doi: 10.1007/s11914-016-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Israel E., Banerjee T.R., Fitzmaurice G.M., Kotlov T.V., LaHive K., LeBoff M.S. Effects of inhaled glucocorticoids on bone density in premenopausal women. N. Engl. J. Med. 2001;345:941–947. doi: 10.1056/NEJMoa002304. [DOI] [PubMed] [Google Scholar]
- 268.Schoon E.J., Bollani S., Mills P.R., Israeli E., Felsenberg D., Ljunghall S., Persson T., Hapten-White L., Graffner H., Bianchi Porro G., et al. Bone mineral density in relation to efficacy and side effects of budesonide and prednisolone in Crohn’s disease. Clin. Gastroenterol. Hepatol. 2005;3:113–121. doi: 10.1016/S1542-3565(04)00662-7. [DOI] [PubMed] [Google Scholar]
- 269.Egeberg A., Schwarz P., Harslof T., Andersen Y.M.F., Pottegard A., Hallas J., Thyssen J.P. Association of Potent and Very Potent Topical Corticosteroids and the Risk of Osteoporosis and Major Osteoporotic Fractures. JAMA Derm. 2021;157:275–282. doi: 10.1001/jamadermatol.2020.4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Kondo T., Kitazawa R., Yamaguchi A., Kitazawa S. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J. Cell. Biochem. 2008;103:335–345. doi: 10.1002/jcb.21414. [DOI] [PubMed] [Google Scholar]
- 271.Gennari C. Differential effect of glucocorticoids on calcium absorption and bone mass. Br. J. Rheumatol. 1993;32((Suppl. 2)):11–14. doi: 10.1093/rheumatology/32.suppl_2.11. [DOI] [PubMed] [Google Scholar]
- 272.Ton F.N., Gunawardene S.C., Lee H., Neer R.M. Effects of low-dose prednisone on bone metabolism. J. Bone Miner. Res. 2005;20:464–470. doi: 10.1359/JBMR.041125. [DOI] [PubMed] [Google Scholar]
- 273.Davidson Z.E., Walker K.Z., Truby H. Clinical review: Do glucocorticosteroids alter vitamin D status? A systematic review with meta-analyses of observational studies. J. Clin. Endocrinol. Metab. 2012;97:738–744. doi: 10.1210/jc.2011-2757. [DOI] [PubMed] [Google Scholar]
- 274.Weinstein R.S. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinol. Metab. Clin. N. Am. 2012;41:595–611. doi: 10.1016/j.ecl.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Buckley L., Guyatt G., Fink H.A., Cannon M., Grossman J., Hansen K.E., Humphrey M.B., Lane N.E., Magrey M., Miller M., et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Rheumatol. 2017;69:1521–1537. doi: 10.1002/art.40137. [DOI] [PubMed] [Google Scholar]
- 276.Ak E., Bulut S.D., Bulut S., Akdag H.A., Oter G.B., Kaya H., Kaya O.B., Sengul C.B., Kisa C. Evaluation of the effect of selective serotonin reuptake inhibitors on bone mineral density: An observational cross-sectional study. Osteoporos. Int. 2015;26:273–279. doi: 10.1007/s00198-014-2859-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Saag K.G., Zanchetta J.R., Devogelaer J.P., Adler R.A., Eastell R., See K., Krege J.H., Krohn K., Warner M.R. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: Thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–3355. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]
- 278.Hung S.C., Lin C.H., Hung H.C., Lin C.L., Lai S.W. Use of Selective Serotonin Reuptake Inhibitors and Risk of Hip Fracture in the Elderly: A Case-Control Study in Taiwan. J. Am. Med. Dir. Assoc. 2017;18:350–354. doi: 10.1016/j.jamda.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 279.Bolton J.M., Morin S.N., Majumdar S.R., Sareen J., Lix L.M., Johansson H., Oden A., McCloskey E.V., Kanis J.A., Leslie W.D. Association of Mental Disorders and Related Medication Use With Risk for Major Osteoporotic Fractures. JAMA Psychiatry. 2017;74:641–648. doi: 10.1001/jamapsychiatry.2017.0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kang S., Han M., Park C.I., Jung I., Kim E.H., Boo Y.J., Kang J.I., Kim S.J. Use of serotonin reuptake inhibitors and risk of subsequent bone loss in a nationwide population-based cohort study. Sci. Rep. 2021;11:13461. doi: 10.1038/s41598-021-92821-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Ortuno M.J., Robinson S.T., Subramanyam P., Paone R., Huang Y.Y., Guo X.E., Colecraft H.M., Mann J.J., Ducy P. Serotonin-reuptake inhibitors act centrally to cause bone loss in mice by counteracting a local anti-resorptive effect. Nat. Med. 2016;22:1170–1179. doi: 10.1038/nm.4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Kruse R. Osteopathies in antiepileptic long-term therapy (preliminary report) Monatsschr. Kinderheilkd. 1968;116:378–381. [PubMed] [Google Scholar]
- 283.Beerhorst K., van der Kruijs S.J., Verschuure P., Tan I.Y., Aldenkamp A.P. Bone disease during chronic antiepileptic drug therapy: General versus specific risk factors. J. Neurol. Sci. 2013;331:19–25. doi: 10.1016/j.jns.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 284.Pack A.M., Olarte L.S., Morrell M.J., Flaster E., Resor S.R., Shane E. Bone mineral density in an outpatient population receiving enzyme-inducing antiepileptic drugs. Epilepsy Behav. 2003;4:169–174. doi: 10.1016/S1525-5050(03)00036-2. [DOI] [PubMed] [Google Scholar]
- 285.Verrotti A., Greco R., Morgese G., Chiarelli F. Increased bone turnover in epileptic patients treated with carbamazepine. Ann. Neurol. 2000;47:385–388. doi: 10.1002/1531-8249(200003)47:3<385::AID-ANA18>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 286.Verrotti A., Greco R., Latini G., Morgese G., Chiarelli F. Increased bone turnover in prepubertal, pubertal, and postpubertal patients receiving carbamazepine. Epilepsia. 2002;43:1488–1492. doi: 10.1046/j.1528-1157.2002.13002.x. [DOI] [PubMed] [Google Scholar]
- 287.Pascussi J.M., Robert A., Nguyen M., Walrant-Debray O., Garabedian M., Martin P., Pineau T., Saric J., Navarro F., Maurel P., et al. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J. Clin. Investig. 2005;115:177–186. doi: 10.1172/JCI21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Pack A.M., Morrell M.J., Randall A., McMahon D.J., Shane E. Bone health in young women with epilepsy after one year of antiepileptic drug monotherapy. Neurology. 2008;70:1586–1593. doi: 10.1212/01.wnl.0000310981.44676.de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Richens A., Rowe D.J. Disturbance of calcium metabolism by anticonvulsant drugs. Br. Med. J. 1970;4:73–76. doi: 10.1136/bmj.4.5727.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Mosekilde L., Melsen F. Dynamic differences in trabecular bone remodeling between patients after jejuno-ileal bypass for obesity and epileptic patients receiving anticonvulsant therapy. Metab. Bone Dis. Relat. Res. 1980;2:77–82. doi: 10.1016/0221-8747(80)90001-6. [DOI] [Google Scholar]
- 291.Weinstein R.S., Bryce G.F., Sappington L.J., King D.W., Gallagher B.B. Decreased serum ionized calcium and normal vitamin D metabolite levels with anticonvulsant drug treatment. J. Clin. Endocrinol. Metab. 1984;58:1003–1009. doi: 10.1210/jcem-58-6-1003. [DOI] [PubMed] [Google Scholar]
- 292.Coleman R. Effect of anastrozole on bone mineral density: 5-year results from the ‘Arimidex’, Tamoxifen, Alone or in Combination (ATAC) trial. J. Clin. Oncol. 2006;24:511. doi: 10.1200/jco.2006.24.18_suppl.511. [DOI] [PubMed] [Google Scholar]
- 293.Perez E.A., Josse R.G., Pritchard K.I., Ingle J.N., Martino S., Findlay B.P., Shenkier T.N., Tozer R.G., Palmer M.J., Shepherd L.E., et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: A companion study to NCIC CTG MA.17. J. Clin. Oncol. 2006;24:3629–3635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 294.Walsh P.C. Immediate versus deferred treatment for advanced prostatic cancer: Initial results of the Medical Research Council trial. The Medical Research Council Prostate Cancer Working Party Investigators Group. J. Urol. 1997;158:1623–1624. [PubMed] [Google Scholar]
- 295.Smith M.R., Lee W.C., Brandman J., Wang Q., Botteman M., Pashos C.L. Gonadotropin-releasing hormone agonists and fracture risk: A claims-based cohort study of men with nonmetastatic prostate cancer. J. Clin. Oncol. 2005;23:7897–7903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 296.Kokorovic A., So A.I., Serag H., French C., Hamilton R.J., Izard J.P., Nayak J.G., Pouliot F., Saad F., Shayegan B., et al. Canadian Urological Association guideline on androgen deprivation therapy: Adverse events and management strategies. Can Urol. Assoc. J. 2021;15:E307–E322. doi: 10.5489/cuaj.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Napoli N., Strollo R., Paladini A., Briganti S.I., Pozzilli P., Epstein S. The alliance of mesenchymal stem cells, bone, and diabetes. Int. J. Endocrinol. 2014;2014:690783. doi: 10.1155/2014/690783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Napoli N., Conte C., Pedone C., Strotmeyer E.S., Barbour K.E., Black D.M., Samelson E.J., Schwartz A.V. Effect of Insulin Resistance on BMD and Fracture Risk in Older Adults. J. Clin. Endocrinol. Metab. 2019;104:3303–3310. doi: 10.1210/jc.2018-02539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Napoli N., Chandran M., Pierroz D.D., Abrahamsen B., Schwartz A.V., Ferrari S.L., Bone I.O.F., Diabetes Working G. Mechanisms of diabetes mellitus-induced bone fragility. Nat. Rev. Endocrinol. 2017;13:208–219. doi: 10.1038/nrendo.2016.153. [DOI] [PubMed] [Google Scholar]
- 300.Yaturu S., Bryant B., Jain S.K. Thiazolidinedione treatment decreases bone mineral density in type 2 diabetic men. Diabetes Care. 2007;30:1574–1576. doi: 10.2337/dc06-2606. [DOI] [PubMed] [Google Scholar]
- 301.Yang B.R., Cha S.H., Lee K.E., Kim J.W., Lee J., Shin K.H. Effect of dipeptidyl peptidase IV inhibitors, thiazolidinedione, and sulfonylurea on osteoporosis in patients with type 2 diabetes: Population-based cohort study. Osteoporos. Int. 2021;32:1705–1712. doi: 10.1007/s00198-020-05801-6. [DOI] [PubMed] [Google Scholar]
- 302.Bilezikian J.P., Watts N.B., Usiskin K., Polidori D., Fung A., Sullivan D., Rosenthal N. Evaluation of Bone Mineral Density and Bone Biomarkers in Patients With Type 2 Diabetes Treated With Canagliflozin. J. Clin. Endocrinol. Metab. 2016;101:44–51. doi: 10.1210/jc.2015-1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Koshizaka M., Ishikawa K., Ishibashi R., Maezawa Y., Sakamoto K., Uchida D., Nakamura S., Yamaga M., Yokoh H., Kobayashi A., et al. Effects of ipragliflozin versus metformin in combination with sitagliptin on bone and muscle in Japanese patients with type 2 diabetes mellitus: Subanalysis of a prospective, randomized, controlled study (PRIME-V study) J. Diabetes Investig. 2021;12:200–206. doi: 10.1111/jdi.13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.U.S. Food & Drug Administration FDA Drug Safety Communication: Possible Increased Risk of Fractures of the Hip, Wrist, and Spine with the Use of Proton Pump Inhibitors. [(accessed on 15 November 2021)]; Available online: https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/fda-drug-safety-communication-possible-increased-risk-fractures-hip-wrist-and-spine-use-proton-pump#TableofEpidemiologicalStudiesevaluatingfractureriskwithprotonpumpinhibitors.
- 305.Yokota T., Matsui H., Matsuura B., Maeyama K., Onji M. Direct effects of proton pump inhibitors on histamine release from rat enterochromaffin-like cells. Eur. J. Pharmacol. 2003;481:233–240. doi: 10.1016/j.ejphar.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 306.Dammann H.G., Burkhardt F., Wolf N. The effects of oral rabeprazole on endocrine and gastric secretory function in healthy volunteers. Aliment. Pharmacol. Ther. 1999;13:1195–1203. doi: 10.1046/j.1365-2036.1999.00545.x. [DOI] [PubMed] [Google Scholar]
- 307.Marcuard S.P., Albernaz L., Khazanie P.G. Omeprazole therapy causes malabsorption of cyanocobalamin (vitamin B12) Ann. Intern. Med. 1994;120:211–215. doi: 10.7326/0003-4819-120-3-199402010-00006. [DOI] [PubMed] [Google Scholar]
- 308.Signorelli S.S., Scuto S., Marino E., Giusti M., Xourafa A., Gaudio A. Anticoagulants and Osteoporosis. Int. J. Mol. Sci. 2019;20:5275. doi: 10.3390/ijms20215275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Monov S.V., Monova D., Shumnalieva R. THU0500|Effects of long-term use of unfractionated heparin (UFH) or low-molecular-weight heparin (LMWH) on bone mineral density (BMD) in patients with nephrotic syndrome. Ann. Rheum. Dis. 2018;77:456. doi: 10.1136/annrheumdis-2018-eular.4255. [DOI] [Google Scholar]
- 310.Yang S., Niu Q., Gan L., Zhang X., Tu L., Zuo L. Effect of long-term use of unfractionated or low-molecular-weight heparin on bone mineral density in maintenance hemodialysis patients. Hemodial. Int. 2020;24:374–382. doi: 10.1111/hdi.12854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Yokoyama S., Ieda S., Nagano M., Nakagawa C., Iwase M., Hosomi K., Takada M. Association between oral anticoagulants and osteoporosis: Real-world data mining using a multi-methodological approach. Int. J. Med. Sci. 2020;17:471–479. doi: 10.7150/ijms.39523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Marques J.V.O., Nalevaiko J.Z., Oliveira M.F., Raetsch A.W.P., Marques G.L., Petterle R.R., Moreira C.A., Borba V.Z.C. Trabecular bone score (TBS) and bone mineral density in patients with long-term therapy with warfarin. Arch. Osteoporos. 2020;15:102. doi: 10.1007/s11657-020-00770-z. [DOI] [PubMed] [Google Scholar]
- 313.Nalevaiko J.Z., Marques J.V.O., Oliveira M.F., Raetsch A.W.P., Marques G.L., Petterle R.R., Moreira C.A., Borba V.Z.C. Bone density and quality in patients treated with direct-acting oral anticoagulants versus warfarin. Bone. 2021;150:116000. doi: 10.1016/j.bone.2021.116000. [DOI] [PubMed] [Google Scholar]
- 314.Brown T.T., Qaqish R.B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: A meta-analytic review. AIDS. 2006;20:2165–2174. doi: 10.1097/QAD.0b013e32801022eb. [DOI] [PubMed] [Google Scholar]
- 315.Starup-Linde J., Rosendahl S.B., Storgaard M., Langdahl B. Management of osteoporosis in patients living with HIV—A systematic review and meta-analysis. J. Acquir. Immune Defic. Syndr. 2020;83:1–8. doi: 10.1097/QAI.0000000000002207. [DOI] [PubMed] [Google Scholar]
- 316.McComsey G.A., Tebas P., Shane E., Yin M.T., Overton E.T., Huang J.S., Aldrovandi G.M., Cardoso S.W., Santana J.L., Brown T.T. Bone disease in HIV infection: A practical review and recommendations for HIV care providers. Clin. Infect. Dis. 2010;51:937–946. doi: 10.1086/656412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Grant P.M., Kitch D., McComsey G.A., Dube M.P., Haubrich R., Huang J., Riddler S., Tebas P., Zolopa A.R., Collier A.C. Low baseline CD4+ count is associated with greater bone mineral density loss after antiretroviral therapy initiation. Clin. Infect. Dis. 2013;57:1483–1488. doi: 10.1093/cid/cit538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Rochira V., Zirilli L., Orlando G., Santi D., Brigante G., Diazzi C., Carli F., Carani C., Guaraldi G. Premature decline of serum total testosterone in HIV-infected men in the HAART-era. PLoS ONE. 2011;6:e28512. doi: 10.1371/journal.pone.0028512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Maalouf N.M., Zhang S., Drechsler H., Brown G.R., Tebas P., Bedimo R. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. J. Bone Miner. Res. 2013;28:2577–2583. doi: 10.1002/jbmr.1988. [DOI] [PubMed] [Google Scholar]
- 320.Eckard A.R., McComsey G.A. Vitamin D deficiency and altered bone mineral metabolism in HIV-infected individuals. Curr. HIV/AIDS Rep. 2014;11:263–270. doi: 10.1007/s11904-014-0218-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Gibellini D., De Crignis E., Ponti C., Cimatti L., Borderi M., Tschon M., Giardino R., Re M.C. HIV-1 triggers apoptosis in primary osteoblasts and HOBIT cells through TNFα activation. J. Med. Virol. 2008;80:1507–1514. doi: 10.1002/jmv.21266. [DOI] [PubMed] [Google Scholar]
- 322.Butler J.S., Dunning E.C., Murray D.W., Doran P.P., O’Byrne J.M. HIV-1 protein induced modulation of primary human osteoblast differentiation and function via a Wnt/β-catenin-dependent mechanism. J. Orthop. Res. 2013;31:218–226. doi: 10.1002/jor.22196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Fakruddin J.M., Laurence J. HIV envelope gp120-mediated regulation of osteoclastogenesis via receptor activator of nuclear factor κB ligand (RANKL) secretion and its modulation by certain HIV protease inhibitors through interferon-γ/RANKL cross-talk. J. Biol. Chem. 2003;278:48251–48258. doi: 10.1074/jbc.M304676200. [DOI] [PubMed] [Google Scholar]
- 324.Cotter E.J., Malizia A.P., Chew N., Powderly W.G., Doran P.P. HIV proteins regulate bone marker secretion and transcription factor activity in cultured human osteoblasts with consequent potential implications for osteoblast function and development. AIDS Res. Hum. Retrovir. 2007;23:1521–1530. doi: 10.1089/aid.2007.0112. [DOI] [PubMed] [Google Scholar]
- 325.Gohda J., Ma Y., Huang Y., Zhang Y., Gu L., Han Y., Li T., Gao B., Gao G.F., Inoue J.-i. HIV-1 replicates in human osteoclasts and enhances their differentiation in vitro. Retrovirology. 2015;12:1–10. doi: 10.1186/s12977-015-0139-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Hileman C.O., Labbato D.E., Storer N.J., Tangpricha V., McComsey G.A. Is Bone Loss Linked to Chronic Inflammation in Antiretroviral-Naïve HIV-Infected Adults? A 48 Week Matched Cohort Study. AIDS. 2014;28:1759. doi: 10.1097/QAD.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.de Menezes E.G.M., Machado A.A., Barbosa F., de Paula F.J.A., Navarro A.M. Bone metabolism dysfunction mediated by the increase of proinflammatory cytokines in chronic HIV infection. J. Bone Miner. Metab. 2017;35:234–242. doi: 10.1007/s00774-016-0749-8. [DOI] [PubMed] [Google Scholar]
- 328.Vlot M.C., Grijsen M.L., Prins J.M., de Jongh R.T., de Jonge R., den Heijer M., Heijboer A.C. Effect of antiretroviral therapy on bone turnover and bone mineral density in men with primary HIV-1 infection. PLoS ONE. 2018;13:e0193679. doi: 10.1371/journal.pone.0193679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Stellbrink H.-J., Orkin C., Arribas J.R., Compston J., Gerstoft J., Van Wijngaerden E., Lazzarin A., Rizzardini G., Sprenger H.G., Lambert J. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin. Infect. Dis. 2010;51:963–972. doi: 10.1086/656417. [DOI] [PubMed] [Google Scholar]
- 330.McComsey G.A., Lupo S., Parks D., Poggio M.C., De Wet J., Kahl L.P., Angelis K., Wynne B., Vandermeulen K., Gartland M. Switch from tenofovir disoproxil fumarate combination to dolutegravir with rilpivirine improves parameters of bone health. AIDS. 2018;32:477. doi: 10.1097/QAD.0000000000001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Negredo E., Domingo P., Pérez-Álvarez N., Gutiérrez M., Mateo G., Puig J., Escrig R., Echeverría P., Bonjoch A., Clotet B. Improvement in bone mineral density after switching from tenofovir to abacavir in HIV-1-infected patients with low bone mineral density: Two-centre randomized pilot study (OsteoTDF study) J. Antimicrob. Chemother. 2014;69:3368–3371. doi: 10.1093/jac/dku300. [DOI] [PubMed] [Google Scholar]
- 332.Maggiolo F., Rizzardini G., Raffi F., Pulido F., Mateo-Garcia M.G., Molina J.-M., Ong E., Shao Y., Piontkowsky D., Das M. Bone mineral density in virologically suppressed people aged 60 years or older with HIV-1 switching from a regimen containing tenofovir disoproxil fumarate to an elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide single-tablet regimen: A multicentre, open-label, phase 3b, randomised trial. Lancet HIV. 2019;6:e655–e666. doi: 10.1016/S2352-3018(19)30195-X. [DOI] [PubMed] [Google Scholar]
- 333.Mateo L., Holgado S., Mariñoso M.L., Pérez-Andrés R., Bonjoch A., Romeu J., Olivé A. Hypophosphatemic osteomalacia induced by tenofovir in HIV-infected patients. Clin. Rheumatol. 2016;35:1271–1279. doi: 10.1007/s10067-014-2627-x. [DOI] [PubMed] [Google Scholar]
- 334.Carr A., Miller J., Eisman J.A., Cooper D.A. Osteopenia in HIV-infected men: Association with asymptomatic lactic acidemia and lower weight pre-antiretroviral therapy. AIDS. 2001;15:703–709. doi: 10.1097/00002030-200104130-00005. [DOI] [PubMed] [Google Scholar]
- 335.EACS (European AIDS Clinical Society) Version 9.0. 2017. [(accessed on 1 November 2021)]. Available online: http://www.eacsociety.org/files/guidelines_9.0-english.pdf.
- 336.Wang H., Lu X., Yang X., Xu N. The efficacy and safety of tenofovir alafenamide versus tenofovir disoproxil fumarate in antiretroviral regimens for HIV-1 therapy: Meta-analysis. Medicine. 2016;95:e5146. doi: 10.1097/MD.0000000000005146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Hill A., Hughes S.L., Gotham D., Pozniak A.L. Tenofovir alafenamide versus tenofovir disoproxil fumarate: Is there a true difference in efficacy and safety? J. Virus Erad. 2018;4:72–79. doi: 10.1016/S2055-6640(20)30248-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Brown T.T., Hoy J., Borderi M., Guaraldi G., Renjifo B., Vescini F., Yin M.T., Powderly W.G. Recommendations for evaluation and management of bone disease in HIV. Clin. Infect. Dis. 2015;60:1242–1251. doi: 10.1093/cid/civ010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Lai J.C., Shoback D.M., Zipperstein J., Lizaola B., Tseng S., Terrault N.A. Bone mineral density, bone turnover, and systemic inflammation in non-cirrhotics with chronic hepatitis C. Dig. Dis. Sci. 2015;60:1813–1819. doi: 10.1007/s10620-014-3507-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Orsini L.G., Pinheiro M.M., Castro C.H., Silva A.E., Szejnfeld V.L. Bone mineral density measurements, bone markers and serum vitamin D concentrations in men with chronic non-cirrhotic untreated hepatitis C. PLoS ONE. 2013;8:e81652. doi: 10.1371/journal.pone.0081652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Schiefke I., Fach A., Wiedmann M., Aretin A.V., Schenker E., Borte G., Wiese M., Moessner J. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J. Gastroenterol. 2005;11:1843. doi: 10.3748/wjg.v11.i12.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Lin M.-S., Chen P.-H., Wang P.-C., Lin H.-S., Huang T.-J., Chang S.-T., Chiu W.-N., Chen M.-Y. Association between hepatitis C virus infection and osteoporotic fracture risk among postmenopausal women: A cross-sectional investigation in Taiwan. BMJ Open. 2019;9:e021990. doi: 10.1136/bmjopen-2018-021990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Arase Y., Suzuki F., Suzuki Y., Akuta N., Kobayashi M., Sezaki H., Hosaka T., Kawamura Y., Yatsuji H., Hirakawa M., et al. Virus clearance reduces bone fracture in postmenopausal women with osteoporosis and chronic liver disease caused by hepatitis C virus. J. Med. Virol. 2010;82:390–395. doi: 10.1002/jmv.21691. [DOI] [PubMed] [Google Scholar]
- 344.Wu C.-H., Chai C.-Y., Tung Y.-C., Lu Y.-Y., Su Y.-F., Tsai T.-H., Tzou R.-D., Lin C.-L. Herpes zoster as a risk factor for osteoporosis: A 15-year nationwide population-based study. Medicine. 2016;95:e3943. doi: 10.1097/MD.0000000000003943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Min C., Bang W.J., Oh D.J., Sim S., Choi H.G. Association between Herpes Zoster and Osteoporosis: A Nested Case-Control Study Using a National Sample Cohort. BioMed Res. Int. 2019;2019:4789679. doi: 10.1155/2019/4789679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Lin S.-M., Wang C.-Y., Chen Y.-Y., Wang J.-H., Liang C.-C., Huang H.-K. Herpes zoster and the risks of osteoporosis and fracture: A nationwide cohort study. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:365–372. doi: 10.1007/s10096-018-3436-y. [DOI] [PubMed] [Google Scholar]
- 347.Shi H.-J., Cui Z.-Q. Correlation of serum inflammatory cytokine and immunoglobulin content with post-herpetic neuralgia in patients with acute herpes zoster. J. Hainan Med. Univ. 2017;23:97–100. [Google Scholar]
- 348.Hampson G., Stone M., Lindsay J., Crowley R., Ralston S. Diagnosis and Management of Osteoporosis during COVID-19: Systematic Review and Practical Guidance. Calcif. Tissue Int. 2021;109:351–362. doi: 10.1007/s00223-021-00858-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Napoli N., Elderkin A.L., Kiel D.P., Khosla S. Managing fragility fractures during the COVID-19 pandemic. Nat. Rev. Endocrinol. 2020;16:467–468. doi: 10.1038/s41574-020-0379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Queiroz-Junior C.M., Santos A.C.P.M., Galvão I., Souto G.R., Mesquita R.A., Sá M.A., Ferreira A.J. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone. 2019;128:115041. doi: 10.1016/j.bone.2019.115041. [DOI] [PubMed] [Google Scholar]
- 352.Hsieh E., Shiau S., Nolan O., Gibert C.L., Bedimo R.J., Rodriguez-Barradas M.C., Justice A.C., Womack J.A., Yin M.T. Increased fragility fracture rates in older men with osteomyelitis. Clin. Infect. Dis. 2019;69:1239–1242. doi: 10.1093/cid/ciz077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Jones D., Glimcher L.H., Aliprantis A.O. Osteoimmunology at the nexus of arthritis, osteoporosis, cancer, and infection. J. Clin. Investig. 2011;121:2534–2542. doi: 10.1172/JCI46262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Claro T., Widaa A., O’Seaghdha M., Miajlovic H., Foster T.J., O’Brien F.J., Kerrigan S.W. Staphylococcus aureus protein A binds to osteoblasts and triggers signals that weaken bone in osteomyelitis. PLoS ONE. 2011;6:e18748. doi: 10.1371/journal.pone.0018748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Choi C.-J., Choi W.-S., Kim C.-M., Lee S.-Y., Kim K.-S. Risk of sarcopenia and osteoporosis in male tuberculosis survivors: Korea National Health and Nutrition Examination Survey. Sci. Rep. 2017;7:13127. doi: 10.1038/s41598-017-12419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Nnoaham K.E., Clarke A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int. J. Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 357.Kaufmann S.H., Dorhoi A. Inflammation in tuberculosis: Interactions, imbalances and interventions. Curr. Opin. Immunol. 2013;25:441–449. doi: 10.1016/j.coi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 358.Sutherland J.S., Adetifa I.M., Hill P.C., Adegbola R.A., Ota M.O. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 359.Valderrábano R.J., Wu J.Y. Bone and blood interactions in human health and disease. Bone. 2019;119:65–70. doi: 10.1016/j.bone.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Morabito N., Gaudio A., Lasco A., Atteritano M., Pizzoleo M.A., Cincotta M., La Rosa M., Guarino R., Meo A., Frisina N. Osteoprotegerin and RANKL in the pathogenesis of thalassemia-induced osteoporosis: New pieces of the puzzle. J. Bone Miner. Res. 2004;19:722–727. doi: 10.1359/jbmr.040113. [DOI] [PubMed] [Google Scholar]
- 361.Giuliani N., Colla S., Sala R., Moroni M., Lazzaretti M., La Monica S., Bonomini S., Hojden M., Sammarelli G., Barille S. Human myeloma cells stimulate the receptor activator of nuclear factor-κB ligand (RANKL) in T lymphocytes: A potential role in multiple myeloma bone disease. Blood J. Am. Soc. Hematol. 2002;100:4615–4621. doi: 10.1182/blood-2002-04-1121. [DOI] [PubMed] [Google Scholar]
- 362.Kleber M., Ntanasis-Stathopoulos I., Dimopoulos M.A., Terpos E. Monoclonal antibodies against RANKL and sclerostin for myeloma-related bone disease: Can they change the standard of care? Expert Rev. Hematol. 2019;12:651–663. doi: 10.1080/17474086.2019.1640115. [DOI] [PubMed] [Google Scholar]
- 363.Rossini M., Viapiana O., Zanotti R., Tripi G., Perbellini O., Idolazzi L., Bonifacio M., Adami S., Gatti D. Dickkopf-1 and sclerostin serum levels in patients with systemic mastocytosis. Calcif. Tissue Int. 2015;96:410–416. doi: 10.1007/s00223-015-9969-5. [DOI] [PubMed] [Google Scholar]
- 364.Korkmaz U., Korkmaz N., Yazici S., Erkan M., Baki A.E., Yazici M., Ozhan H., Ataoğlu S. Anemia as a risk factor for low bone mineral density in postmenopausal Turkish women. Eur. J. Intern. Med. 2012;23:154–158. doi: 10.1016/j.ejim.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 365.Valderrábano R.J., Lee J., Lui L.-Y., Hoffman A.R., Cummings S.R., Orwoll E.S., Wu J.Y. Older men with anemia have increased fracture risk independent of bone mineral density. J. Clin. Endocrinol. Metab. 2017;102:2199–2206. doi: 10.1210/jc.2017-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Toxqui L., Vaquero M.P. Chronic iron deficiency as an emerging risk factor for osteoporosis: A hypothesis. Nutrients. 2015;7:2324–2344. doi: 10.3390/nu7042324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Tunacı M., Tunacı A., Engin G., Özkorkmaz B., Dinçol G., Acunaş G., Acunaş B. Imaging features of thalassemia. Eur. Radiol. 1999;9:1804–1809. doi: 10.1007/s003300050926. [DOI] [PubMed] [Google Scholar]
- 368.Scacchi M., Danesi L., Cattaneo A., Valassi E., Giraldi F.P., Argento C., D’Angelo E., Mirra N., Carnelli V., Zanaboni L. Bone demineralization in adult thalassaemic patients: Contribution of GH and IGF-I at different skeletal sites. Clin. Endocrinol. 2008;69:202–207. doi: 10.1111/j.1365-2265.2008.03191.x. [DOI] [PubMed] [Google Scholar]
- 369.Soliman A.T., El Banna N., Fattah M.A., ElZalabani M.M., Ansari B. Bone mineral density in prepubertal children with β-thalassemia: Correlation with growth and hormonal data. Metabolism. 1998;47:541–548. doi: 10.1016/S0026-0495(98)90237-2. [DOI] [PubMed] [Google Scholar]
- 370.Bordat C., Constans A., Bouet O., Blanc I., Trubert C.-L., Girot R., Cournot G. Iron distribution in thalassemic bone by energy-loss spectroscopy and electron spectroscopic imaging. Calcif. Tissue Int. 1993;53:29–37. doi: 10.1007/BF01352012. [DOI] [PubMed] [Google Scholar]
- 371.Dandona P., Menon R., Houlder S., Thomas M., Hoffbrand A., Flynn D. Serum 1, 25 dihydroxyvitamin D and osteocalcin concentrations in thalassaemia major. Arch. Dis. Child. 1987;62:474–477. doi: 10.1136/adc.62.5.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Chan Y.-L., Pang L.-M., Chik K.-W., Cheng J.C., Li C.-K. Patterns of bone diseases in transfusion-dependent homozygous thalassaemia major: Predominance of osteoporosis and desferrioxamine-induced bone dysplasia. Pediatr. Radiol. 2002;32:492–497. doi: 10.1007/s00247-002-0664-0. [DOI] [PubMed] [Google Scholar]
- 373.Morabito N., Russo G.T., Gaudio A., Lasco A., Catalano A., Morini E., Franchina F., Maisano D., La Rosa M., Plota M. The “lively” cytokines network in β-thalassemia major-related osteoporosis. Bone. 2007;40:1588–1594. doi: 10.1016/j.bone.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 374.Voskaridou E., Anagnostopoulos A., Konstantopoulos K., Stoupa E., Spyropoulou E., Kiamouris C., Terpos E. Zoledronic acid for the treatment of osteoporosis in patients with beta-thalassemia: Results from a single-center, randomized, placebo-controlled trial. Haematologica. 2006;91:1193–1202. [PubMed] [Google Scholar]
- 375.Gaudio A., Xourafa A., Rapisarda R., Zanoli L., Signorelli S.S., Castellino P. Hematological diseases and osteoporosis. Int. J. Mol. Sci. 2020;21:3538. doi: 10.3390/ijms21103538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Tournis S., Dede A.D., Savvidis C., Triantafyllopoulos I.K., Kattamis A., Papaioannou N. Effects of teriparatide retreatment in a patient with β-thalassemia major. Transfusion. 2015;55:2905–2910. doi: 10.1111/trf.13237. [DOI] [PubMed] [Google Scholar]
- 377.Voskaridou E., Ntanasis-Stathopoulos I., Papaefstathiou A., Christoulas D., Dimopoulou M., Repa K., Papatheodorou A., Peppa M., Terpos E. Denosumab in transfusion-dependent thalassemia osteoporosis: A randomized, placebo-controlled, double-blind phase 2b clinical trial. Blood Adv. 2018;2:2837–2847. doi: 10.1182/bloodadvances.2018023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Anagnostis P., Vakalopoulou S., Slavakis A., Charizopoulou M., Kazantzidou E., Chrysopoulou T., Vyzantiadis T.-A., Moka E., Agapidou A., Garipidou V. Reduced bone mineral density in patients with haemophilia A and B in Northern Greece. Thromb. Haemost. 2012;107:545–551. doi: 10.1160/TH11-08-05563. [DOI] [PubMed] [Google Scholar]
- 379.Forsyth A., Quon D., Konkle B. Role of exercise and physical activity on haemophilic arthropathy, fall prevention and osteoporosis. Haemophilia. 2011;17:e870–e876. doi: 10.1111/j.1365-2516.2011.02514.x. [DOI] [PubMed] [Google Scholar]
- 380.Wallny T., Scholz D., Oldenburg J., Nicolay C., Ezziddin S., Pennekamp P., Stoffel-Wagner B., Kraft C. Osteoporosis in haemophilia–an underestimated comorbidity? Haemophilia. 2007;13:79–84. doi: 10.1111/j.1365-2516.2006.01405.x. [DOI] [PubMed] [Google Scholar]
- 381.Simpson M.L., Valentino L.A. Vitamin D deficiency and Osteoporosis in Hemophilia: An Underappreciated Risk. American Society of Hematology; Washington, DC, USA: 2013. [Google Scholar]
- 382.Anagnostis P., Vakalopoulou S., Christoulas D., Paschou S., Papatheodorou A., Garipidou V., Kokkoris P., Terpos E. The role of sclerostin/dickkopf-1 and receptor activator of nuclear factor kB ligand/osteoprotegerin signalling pathways in the development of osteoporosis in patients with haemophilia A and B: A cross-sectional study. Haemophilia. 2018;24:316–322. doi: 10.1111/hae.13384. [DOI] [PubMed] [Google Scholar]
- 383.Baud’Huin M., Duplomb L., Téletchéa S., Charrier C., Maillasson M., Fouassier M., Heymann D. Factor VIII-von Willebrand factor complex inhibits osteoclastogenesis and controls cell survival. J. Biol. Chem. 2009;284:31704–31713. doi: 10.1074/jbc.M109.030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Kempton C.L., Antun A., Antoniucci D.M., Carpenter W., Ribeiro M., Stein S., Slovensky L., Elon L. Bone density in haemophilia: A single institutional cross-sectional study. Haemophilia. 2014;20:121–128. doi: 10.1111/hae.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Khawaji M., Åkesson K., Berntorp E. Long-term prophylaxis in severe haemophilia seems to preserve bone mineral density. Haemophilia. 2009;15:261–266. doi: 10.1111/j.1365-2516.2008.01912.x. [DOI] [PubMed] [Google Scholar]
- 386.Melton L.J., III, Rajkumar S.V., Khosla S., Achenbach S.J., Oberg A.L., Kyle R.A. Fracture risk in monoclonal gammopathy of undetermined significance. J. Bone Miner. Res. 2004;19:25–30. doi: 10.1359/jbmr.0301212. [DOI] [PubMed] [Google Scholar]
- 387.Melton L.J., III, Kyle R.A., Achenbach S.J., Oberg A.L., Rajkumar S.V. Fracture risk with multiple myeloma: A population-based study. J. Bone Miner. Res. 2005;20:487–493. doi: 10.1359/JBMR.041131. [DOI] [PubMed] [Google Scholar]
- 388.Kaiser M., Mieth M., Liebisch P., Oberländer R., Rademacher J., Jakob C., Kleeberg L., Fleissner C., Braendle E., Peters M. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur. J. Haematol. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 389.Oshima T., Abe M., Asano J., Hara T., Kitazoe K., Sekimoto E., Tanaka Y., Shibata H., Hashimoto T., Ozaki S. Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood. 2005;106:3160–3165. doi: 10.1182/blood-2004-12-4940. [DOI] [PubMed] [Google Scholar]
- 390.Berenson J.R., Anderson K.C., Audell R.A., Boccia R.V., Coleman M., Dimopoulos M.A., Drake M.T., Fonseca R., Harousseau J.L., Joshua D. Monoclonal gammopathy of undetermined significance: A consensus statement. Br. J. Haematol. 2010;150:28–38. doi: 10.1111/j.1365-2141.2010.08207.x. [DOI] [PubMed] [Google Scholar]
- 391.van de Donk N.W., Palumbo A., Johnsen H.E., Engelhardt M., Gay F., Gregersen H., Hajek R., Kleber M., Ludwig H., Morgan G. The clinical relevance and management of monoclonal gammopathy of undetermined significance and related disorders: Recommendations from the European Myeloma Network. Haematologica. 2014;99:984. doi: 10.3324/haematol.2013.100552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392.Derenne S., Amiot M., Barillé S., Collette M., Robillard N., Berthaud P., Harousseau J.L., Bataille R. Zoledronate is a potent inhibitor of myeloma cell growth and secretion of IL-6 and MMP-1 by the tumoral environment. J. Bone Miner. Res. 1999;14:2048–2056. doi: 10.1359/jbmr.1999.14.12.2048. [DOI] [PubMed] [Google Scholar]
- 393.Bellido T., Plotkin L.I. Novel actions of bisphosphonates in bone: Preservation of osteoblast and osteocyte viability. Bone. 2011;49:50–55. doi: 10.1016/j.bone.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Body J.J., Pfister T., Bauss F. Preclinical perspectives on bisphosphonate renal safety. Oncologist. 2005;10:3–7. doi: 10.1634/theoncologist.10-90001-3. [DOI] [PubMed] [Google Scholar]
- 395.Block G.A., Bone H.G., Fang L., Lee E., Padhi D. A single-dose study of denosumab in patients with various degrees of renal impairment. J. Bone Miner. Res. 2012;27:1471–1479. doi: 10.1002/jbmr.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Von Metzler I., Krebbel H., Hecht M., Manz R., Fleissner C., Mieth M., Kaiser M., Jakob C., Sterz J., Kleeberg L. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 397.Fulciniti M., Tassone P., Hideshima T., Vallet S., Nanjappa P., Ettenberg S.A., Shen Z., Patel N., Tai Y.-t., Chauhan D. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood J. Am. Soc. Hematol. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Delgado-Calle J., Anderson J., Cregor M.D., Condon K.W., Kuhstoss S.A., Plotkin L.I., Bellido T., Roodman G.D. Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia. 2017;31:2686–2694. doi: 10.1038/leu.2017.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Van der Veer E., Van Der Goot W., De Monchy J., Kluin-Nelemans H., Van Doormaal J. High prevalence of fractures and osteoporosis in patients with indolent systemic mastocytosis. Allergy. 2012;67:431–438. doi: 10.1111/j.1398-9995.2011.02780.x. [DOI] [PubMed] [Google Scholar]
- 400.Degboé Y., Eischen M., Nigon D., Apoil P.-A., Mailhol C., Tournier E., Laurent C., Hanssens K., Hermine O., Paul C. Prevalence and risk factors for fragility fracture in systemic mastocytosis. Bone. 2017;105:219–225. doi: 10.1016/j.bone.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 401.Escribano L., Álvarez-Twose I., Sánchez-Muñoz L., Garcia-Montero A., Núñez R., Almeida J., Jara-Acevedo M., Teodósio C., García-Cosío M., Bellas C. Prognosis in adult indolent systemic mastocytosis: A long-term study of the Spanish Network on Mastocytosis in a series of 145 patients. J. Allergy Clin. Immunol. 2009;124:514–521. doi: 10.1016/j.jaci.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 402.Rabenhorst A., Christopeit B., Leja S., Gerbaulet A., Kleiner S., Förster A., Raap U., Wickenhauser C., Hartmann K. Serum levels of bone cytokines are increased in indolent systemic mastocytosis associated with osteopenia or osteoporosis. J. Allergy Clin. Immunol. 2013;132:1234–1237.e7. doi: 10.1016/j.jaci.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 403.Rossini M., Zanotti R., Viapiana O., Tripi G., Orsolini G., Idolazzi L., Bonadonna P., Schena D., Escribano L., Adami S., et al. Bone involvement and osteoporosis in mastocytosis. Immunol. Allergy Clin. N. Am. 2014;34:383–396. doi: 10.1016/j.iac.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 404.Barete S., Assous N., De Gennes C., Grandpeix C., Feger F., Palmerini F., Dubreuil P., Arock M., Roux C., Launay J. Systemic mastocytosis and bone involvement in a cohort of 75 patients. Ann. Rheum. Dis. 2010;69:1838–1841. doi: 10.1136/ard.2009.124511. [DOI] [PubMed] [Google Scholar]
- 405.Orsolini G., Gavioli I., Tripi G., Viapiana O., Gatti D., Idolazzi L., Zanotti R., Rossini M. Denosumab for the treatment of mastocytosis-related osteoporosis: A case series. Calcif. Tissue Int. 2017;100:595. doi: 10.1007/s00223-017-0241-z. [DOI] [PubMed] [Google Scholar]
- 406.Laroche M., Livideanu C., Paul C., Cantagrel A. Interferon alpha and pamidronate in osteoporosis with fracture secondary to mastocytosis. Am. J. Med. 2011;124:776–778. doi: 10.1016/j.amjmed.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 407.Ogawa T., Ohshika S., Yanagisawa M., Kurose A., Ishibashi Y. Teriparatide may accelerate the growth of a pre-existing malignant tumor in an elderly patient with osteoporosis: A case report. Mol. Clin. Oncol. 2020;12:144–147. doi: 10.3892/mco.2019.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Infante M., Fabi A., Cognetti F., Gorini S., Caprio M., Fabbri A. RANKL/RANK/OPG system beyond bone remodeling: Involvement in breast cancer and clinical perspectives. J. Exp. Clin. Cancer Res. 2019;38:12. doi: 10.1186/s13046-018-1001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Cabanillas M.E., Lu H., Fang S., Du X.L. Elderly patients with non-Hodgkin lymphoma who receive chemotherapy are at higher risk for osteoporosis and fractures. Leuk. Lymphoma. 2007;48:1514–1521. doi: 10.1080/10428190701471973. [DOI] [PubMed] [Google Scholar]
- 410.Hamood R., Hamood H., Merhasin I., Keinan-Boker L. Hormone therapy and osteoporosis in breast cancer survivors: Assessment of risk and adherence to screening recommendations. Osteoporos. Int. 2019;30:187–200. doi: 10.1007/s00198-018-4758-4. [DOI] [PubMed] [Google Scholar]
- 411.Bruning P., Pit M., de Jong-Bakker M., Van den Ende A., Hart A., Van Enk A. Bone mineral density after adjuvant chemotherapy for premenopausal breast cancer. Br. J. Cancer. 1990;61:308–310. doi: 10.1038/bjc.1990.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Eastell R., Hannon R.A., Cuzick J., Dowsett M., Clack G., Adams J.E. Effect of an aromatase inhibitor on BMD and bone turnover markers: 2-year results of the Anastrozole, Tamoxifen, Alone or in Combination (ATAC) trial (18233230) J. Bone Miner. Res. 2006;21:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 413.Smith M.R., McGovern F.J., Zietman A.L., Fallon M.A., Hayden D.L., Schoenfeld D.A., Kantoff P.W., Finkelstein J.S. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N. Engl. J. Med. 2001;345:948–955. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 414.Gibson K., O’Bryant C.L. Screening and management of osteoporosis in breast cancer patients on aromatase inhibitors. J. Oncol. Pharm. Pract. 2008;14:139–145. doi: 10.1177/1078155208091866. [DOI] [PubMed] [Google Scholar]
- 415.Van Tongeren L.S., Duncan G.G., Kendler D.L., Pai H. Implementation of osteoporosis screening guidelines in prostate cancer patients on androgen ablation. J. Clin. Densitom. 2009;12:287–291. doi: 10.1016/j.jocd.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 416.Hadji P., Body J.J., Aapro M.S., Brufsky A., Coleman R.E., Guise T., Lipton A., Tubiana-Hulin M. Practical guidance for the management of aromatase inhibitor-associated bone loss. Ann. Oncol. 2008;19:1407–1416. doi: 10.1093/annonc/mdn164. [DOI] [PubMed] [Google Scholar]
- 417.Srivastava R.K., Dar H.Y., Mishra P.K. Immunoporosis: Immunology of Osteoporosis-Role of T Cells. Front. Immunol. 2018;9:657. doi: 10.3389/fimmu.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., Tanaka S., Kodama T., Akira S., Iwakura Y., et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Glorieux F.H., Devogelaer J.P., Durigova M., Goemaere S., Hemsley S., Jakob F., Junker U., Ruckle J., Seefried L., Winkle P.J. BPS804 Anti-Sclerostin Antibody in Adults With Moderate Osteogenesis Imperfecta: Results of a Randomized Phase 2a Trial. J. Bone Miner. Res. 2017;32:1496–1504. doi: 10.1002/jbmr.3143. [DOI] [PubMed] [Google Scholar]
- 420.Rotta D., Fassio A., Rossini M., Giollo A., Viapiana O., Orsolini G., Bertoldo E., Gatti D., Adami G. Osteoporosis in Inflammatory Arthritides: New Perspective on Pathogenesis and Treatment. Front. Med. 2020;7:613720. doi: 10.3389/fmed.2020.613720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Haugeberg G., Uhlig T., Falch J.A., Halse J.I., Kvien T.K. Bone mineral density and frequency of osteoporosis in female patients with rheumatoid arthritis: Results from 394 patients in the Oslo County Rheumatoid Arthritis register. Arthritis Rheum. 2000;43:522–530. doi: 10.1002/1529-0131(200003)43:3<522::AID-ANR7>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 422.Hauser B., Riches P.L., Wilson J.F., Horne A.E., Ralston S.H. Prevalence and clinical prediction of osteoporosis in a contemporary cohort of patients with rheumatoid arthritis. Rheumatology. 2014;53:1759–1766. doi: 10.1093/rheumatology/keu162. [DOI] [PubMed] [Google Scholar]
- 423.Jin S., Hsieh E., Peng L., Yu C., Wang Y., Wu C., Wang Q., Li M., Zeng X. Incidence of fractures among patients with rheumatoid arthritis: A systematic review and meta-analysis. Osteoporos. Int. 2018;29:1263–1275. doi: 10.1007/s00198-018-4473-1. [DOI] [PubMed] [Google Scholar]
- 424.Adami G., Saag K.G. Osteoporosis Pathophysiology, Epidemiology, and Screening in Rheumatoid Arthritis. Curr. Rheumatol. Rep. 2019;21:34. doi: 10.1007/s11926-019-0836-7. [DOI] [PubMed] [Google Scholar]
- 425.Stach C.M., Bäuerle M., Englbrecht M., Kronke G., Engelke K., Manger B., Schett G. Periarticular bone structure in rheumatoid arthritis patients and healthy individuals assessed by high-resolution computed tomography. Arthritis Rheum. 2010;62:330–339. doi: 10.1002/art.27252. [DOI] [PubMed] [Google Scholar]
- 426.Fessler J., Husic R., Schwetz V., Lerchbaum E., Aberer F., Fasching P., Ficjan A., Obermayer-Pietsch B., Duftner C., Graninger W., et al. Senescent T-Cells Promote Bone Loss in Rheumatoid Arthritis. Front. Immunol. 2018;9:95. doi: 10.3389/fimmu.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Castañeda S., Llorente I., García-Vicuña R., González-Álvaro I. Anti-citrullinated protein antibodies and bone loss in patients with early arthritis: Comment on the article “Anti-citrullinated protein antibodies and high levels of rheumatoid factor are associated with systemic bone loss in patients with early untreated rheumatoid arthritis” by Bugatti et al. Arthritis Res. Ther. 2017;19:152. doi: 10.1186/s13075-017-1362-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 428.Llorente I., Merino L., Ortiz A.M., Escolano E., González-Ortega S., García-Vicuña R., García-Vadillo J.A., Castañeda S., González-Álvaro I. Anti-citrullinated protein antibodies are associated with decreased bone mineral density: Baseline data from a register of early arthritis patients. Rheumatol. Int. 2017;37:799–806. doi: 10.1007/s00296-017-3674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Regueiro C., Ortiz A.M., Boveda M.D., Castañeda S., Gonzalez-Alvaro I., Gonzalez A. Association of high titers of anti-carbamylated protein antibodies with decreased bone mineral density in early arthritis patients. PLoS ONE. 2018;13:e0202583. doi: 10.1371/journal.pone.0202583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Orsolini G., Fassio A., Rossini M., Adami G., Giollo A., Caimmi C., Idolazzi L., Viapiana O., Gatti D. Effects of biological and targeted synthetic DMARDs on bone loss in rheumatoid arthritis. Pharmacol. Res. 2019;147:104354. doi: 10.1016/j.phrs.2019.104354. [DOI] [PubMed] [Google Scholar]
- 431.Kwon O.C., Oh J.S., Hong S., Lee C.K., Yoo B., Kim Y.G. Conventional synthetic disease-modifying antirheumatic drugs and bone mineral density in rheumatoid arthritis patients with osteoporosis: Possible beneficial effect of leflunomide. Clin. Exp. Rheumatol. 2019;37:813–819. [PubMed] [Google Scholar]
- 432.Fassio A., Adami G., Gatti D., Orsolini G., Giollo A., Idolazzi L., Benini C., Vantaggiato E., Rossini M., Viapiana O. Inhibition of tumor necrosis factor-alpha (TNF-alpha) in patients with early rheumatoid arthritis results in acute changes of bone modulators. Int. Immunopharmacol. 2019;67:487–489. doi: 10.1016/j.intimp.2018.12.050. [DOI] [PubMed] [Google Scholar]
- 433.Chen Y.M., Chen H.H., Huang W.N., Liao T.L., Chen J.P., Chao W.C., Lin C.T., Hung W.T., Hsieh C.W., Hsieh T.Y., et al. Tocilizumab potentially prevents bone loss in patients with anticitrullinated protein antibody-positive rheumatoid arthritis. PLoS ONE. 2017;12:e0188454. doi: 10.1371/journal.pone.0188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Tada M., Inui K., Sugioka Y., Mamoto K., Okano T., Koike T. Abatacept might increase bone mineral density at femoral neck for patients with rheumatoid arthritis in clinical practice: AIRTIGHT study. Rheumatol. Int. 2018;38:777–784. doi: 10.1007/s00296-017-3922-z. [DOI] [PubMed] [Google Scholar]
- 435.Tascioglu F., Oner C., Armagan O. The effect of low-dose methotrexate on bone mineral density in patients with early rheumatoid arthritis. Rheumatol. Int. 2003;23:231–235. doi: 10.1007/s00296-003-0298-z. [DOI] [PubMed] [Google Scholar]
- 436.Xia J., Xie S.Y., Liu K.Q., Xu L., Zhao P.P., Gai S.R., Guan P.L., Zhao J.Q., Zhu Y.P., Tsoi L.C., et al. Systemic evaluation of the relationship between psoriasis, psoriatic arthritis and osteoporosis: Observational and Mendelian randomisation study. Ann. Rheum. Dis. 2020;79:1460–1467. doi: 10.1136/annrheumdis-2020-217892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Kathuria P., Gordon K.B., Silverberg J.I. Association of psoriasis and psoriatic arthritis with osteoporosis and pathological fractures. J. Am. Acad. Dermatol. 2017;76:1045–1053.e3. doi: 10.1016/j.jaad.2016.11.046. [DOI] [PubMed] [Google Scholar]
- 438.Gulati A.M., Michelsen B., Diamantopoulos A., Grandaunet B., Salvesen Ø., Kavanaugh A., Hoff M., Haugeberg G. Osteoporosis in psoriatic arthritis: A cross-sectional study of an outpatient clinic population. RMD Open. 2018;4:e000631. doi: 10.1136/rmdopen-2017-000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Paine A., Ritchlin C. Altered Bone Remodeling in Psoriatic Disease: New Insights and Future Directions. Calcif. Tissue Int. 2018;102:559–574. doi: 10.1007/s00223-017-0380-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.van der Weijden M.A., Claushuis T.A., Nazari T., Lems W.F., Dijkmans B.A., van der Horst-Bruinsma I.E. High prevalence of low bone mineral density in patients within 10 years of onset of ankylosing spondylitis: A systematic review. Clin. Rheumatol. 2012;31:1529–1535. doi: 10.1007/s10067-012-2018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Muñoz-Ortego J., Vestergaard P., Rubio J.B., Wordsworth P., Judge A., Javaid M.K., Arden N.K., Cooper C., Díez-Pérez A., Prieto-Alhambra D. Ankylosing spondylitis is associated with an increased risk of vertebral and nonvertebral clinical fractures: A population-based cohort study. J. Bone Miner. Res. 2014;29:1770–1776. doi: 10.1002/jbmr.2217. [DOI] [PubMed] [Google Scholar]
- 442.Beek K.J., Rusman T., van der Weijden M.A.C., Lems W.F., van Denderen J.C., Konsta M., Visman I., Nurmohamed M.T., van der Horst-Bruinsma I.E. Long-Term Treatment With TNF-Alpha Inhibitors Improves Bone Mineral Density But Not Vertebral Fracture Progression in Ankylosing Spondylitis. J. Bone Miner. Res. 2019;34:1041–1048. doi: 10.1002/jbmr.3684. [DOI] [PubMed] [Google Scholar]
- 443.Gu C., Zhao R., Zhang X., Gu Z., Zhou W., Wang Y., Guo J., Bao Y., Sun C., Dong C., et al. A meta-analysis of secondary osteoporosis in systemic lupus erythematosus: Prevalence and risk factors. Arch. Osteoporos. 2019;15:1. doi: 10.1007/s11657-019-0667-1. [DOI] [PubMed] [Google Scholar]
- 444.Dey M., Bukhari M. Predictors of fracture risk in patients with systemic lupus erythematosus. Lupus. 2018;27:1547–1551. doi: 10.1177/0961203318768886. [DOI] [PubMed] [Google Scholar]
- 445.Le B., Waller J.L., Radhakrishnan R., Oh S.J., Kheda M.F., Nahman N.S., Jr., Carbone L. Osteoporotic fractures in patients with systemic lupus erythematosus and end stage renal disease. Lupus. 2018;27:17–24. doi: 10.1177/0961203317709953. [DOI] [PubMed] [Google Scholar]
- 446.Bultink I.E., Lems W.F. Lupus and fractures. Curr. Opin. Rheumatol. 2016;28:426–432. doi: 10.1097/BOR.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 447.Sarkissian A., Sivaraman V., Bout-Tabaku S., Ardoin S.P., Moore-Clingenpeel M., Mruk V., Steigelman H., Morris K., Bowden S.A. Bone turnover markers in relation to vitamin D status and disease activity in adults with systemic lupus erythematosus. Lupus. 2019;28:156–162. doi: 10.1177/0961203318815593. [DOI] [PubMed] [Google Scholar]
- 448.Briot K., Geusens P., Em Bultink I., Lems W.F., Roux C. Inflammatory diseases and bone fragility. Osteoporos. Int. 2017;28:3301–3314. doi: 10.1007/s00198-017-4189-7. [DOI] [PubMed] [Google Scholar]
- 449.Balasubramanian A., Wade S.W., Adler R.A., Saag K., Pannacciulli N., Curtis J.R. Glucocorticoid Exposure and Fracture Risk in a Cohort of US Patients With Selected Conditions. J. Bone Miner. Res. 2018;33:1881–1888. doi: 10.1002/jbmr.3523. [DOI] [PubMed] [Google Scholar]
- 450.Seguro L.P., Casella C.B., Caparbo V.F., Oliveira R.M., Bonfa A., Bonfa E., Pereira R.M. Lower P1NP serum levels: A predictive marker of bone loss after 1 year follow-up in premenopausal systemic lupus erythematosus patients. Osteoporos. Int. 2015;26:459–467. doi: 10.1007/s00198-014-2860-9. [DOI] [PubMed] [Google Scholar]
- 451.Coskun Benlidayi I., Basaran S., Evlice A., Erdem M., Demirkiran M. Prevalence and risk factors of low bone mineral density in patients with multiple sclerosis. Acta Clin. Belg. 2015;70:188–192. doi: 10.1179/2295333715Y.0000000002. [DOI] [PubMed] [Google Scholar]
- 452.Bisson E.J., Finlayson M.L., Ekuma O., Leslie W.D., Marrie R.A. Multiple sclerosis is associated with low bone mineral density and osteoporosis. Neurol. Clin. Pract. 2019;9:391–399. doi: 10.1212/CPJ.0000000000000669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Huang Z., Qi Y., Du S., Chen G., Yan W. BMI levels with MS Bone mineral density levels in adults with multiple sclerosis: A meta-analysis. Int. J. Neurosci. 2015;125:904–912. doi: 10.3109/00207454.2014.988332. [DOI] [PubMed] [Google Scholar]
- 454.Murphy O., Zandi M.S., Lindenberg N., Murphy E., Chataway J. Bone health in patients with multiple sclerosis relapses. Mult. Scler. Relat. Disord. 2016;6:75–80. doi: 10.1016/j.msard.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 455.Wen S.R., Liu G.J., Feng R.N., Gong F.C., Zhong H., Duan S.R., Bi S. Increased levels of IL-23 and osteopontin in serum and cerebrospinal fluid of multiple sclerosis patients. J. Neuroimmunol. 2012;244:94–96. doi: 10.1016/j.jneuroim.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 456.Kanis J.A., Harvey N.C., Johansson H., Odén A., Leslie W.D., McCloskey E.V. FRAX update. J. Clin. Densitom. 2017;20:360–367. doi: 10.1016/j.jocd.2017.06.022. [DOI] [PubMed] [Google Scholar]
- 457.Yoon V., Maalouf N., Sakhaee K.J. The effects of smoking on bone metabolism. Osteoporos. Int. 2012;23:2081–2092. doi: 10.1007/s00198-012-1940-y. [DOI] [PubMed] [Google Scholar]
- 458.Need A., Kemp A., Giles N., Morris H., Horowitz M., Nordin B. Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos. Int. 2002;13:83–88. doi: 10.1007/s198-002-8342-9. [DOI] [PubMed] [Google Scholar]
- 459.Jorde R., Saleh F., Figenschau Y., Kamycheva E., Haug E., Sundsfjord J. Serum parathyroid hormone (PTH) levels in smokers and non-smokers. The fifth Tromsø study. Eur. J. Endocrinol. 2005;152:39–45. doi: 10.1530/eje.1.01816. [DOI] [PubMed] [Google Scholar]
- 460.Allen A.M., Oncken C., Hatsukami D.J.C.A.R. Women and smoking: The effect of gender on the epidemiology, health effects, and cessation of smoking. Curr. Addict. Rep. 2014;1:53–60. doi: 10.1007/s40429-013-0003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Oncken C., Prestwood K., Kleppinger A., Wang Y., Cooney J., Raisz L. Impact of smoking cessation on bone mineral density in postmenopausal women. J. Women’s Health. 2006;15:1141–1150. doi: 10.1089/jwh.2006.15.1141. [DOI] [PubMed] [Google Scholar]
- 462.Thorin M.H., Wihlborg A., Åkesson K., Gerdhem P.J. Smoking, smoking cessation, and fracture risk in elderly women followed for 10 years. Osteoporos. Int. 2016;27:249–255. doi: 10.1007/s00198-015-3290-z. [DOI] [PubMed] [Google Scholar]
- 463.Rolvien T., Amling M. Disuse Osteoporosis: Clinical and Mechanistic Insights. Calcif. Tissue Int. 2021;110:592–604. doi: 10.1007/s00223-021-00836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.Cirnigliaro C.M., La Fountaine M.F., Parrott J.S., Kirshblum S.C., McKenna C., Sauer S.J., Shapses S.A., Hao L., McClure I.A., Hobson J.C., et al. Administration of Denosumab Preserves Bone Mineral Density at the Knee in Persons With Subacute Spinal Cord Injury: Findings From a Randomized Clinical Trial. JBMR Plus. 2020;4:e10375. doi: 10.1002/jbm4.10375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Qin W., Li X., Peng Y., Harlow L.M., Ren Y., Wu Y., Li J., Qin Y., Sun J., Zheng S., et al. Sclerostin Antibody Preserves the Morphology and Structure of Osteocytes and Blocks the Severe Skeletal Deterioration After Motor-Complete Spinal Cord Injury in Rats. J. Bone Miner. Res. 2015;30:1994–2004. doi: 10.1002/jbmr.2549. [DOI] [PubMed] [Google Scholar]
- 466.Hannan F.M., Newey P.J., Whyte M.P., Thakker R.V. Genetic approaches to metabolic bone diseases. Br. J. Clin. Pharmacol. 2019;85:1147–1160. doi: 10.1111/bcp.13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Forlino A., Marini J.C. Osteogenesis imperfecta. Lancet. 2016;387:1657–1671. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Styrkarsdottir U., Thorleifsson G., Eiriksdottir B., Gudjonsson S.A., Ingvarsson T., Center J.R., Nguyen T.V., Eisman J.A., Christiansen C., Thorsteinsdottir U., et al. Two Rare Mutations in the COL1A2 Gene Associate With Low Bone Mineral Density and Fractures in Iceland. J. Bone Miner. Res. 2016;31:173–179. doi: 10.1002/jbmr.2604. [DOI] [PubMed] [Google Scholar]
- 469.Nijhuis W.H., Eastwood D.M., Allgrove J., Hvid I., Weinans H.H., Bank R.A., Sakkers R.J. Current concepts in osteogenesis imperfecta: Bone structure, biomechanics and medical management. J. Child. Orthop. 2019;13:1–11. doi: 10.1302/1863-2548.13.180190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Fratzl-Zelman N., Schmidt I., Roschger P., Glorieux F.H., Klaushofer K., Fratzl P., Rauch F., Wagermaier W. Mineral particle size in children with osteogenesis imperfecta type I is not increased independently of specific collagen mutations. Bone. 2014;60:122–128. doi: 10.1016/j.bone.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 471.Kocijan R., Muschitz C., Haschka J., Hans D., Nia A., Geroldinger A., Ardelt M., Wakolbinger R., Resch H. Bone structure assessed by HR-pQCT, TBS and DXL in adult patients with different types of osteogenesis imperfecta. Osteoporos. Int. 2015;26:2431–2440. doi: 10.1007/s00198-015-3156-4. [DOI] [PubMed] [Google Scholar]
- 472.Vardakastani V., Saletti D., Skalli W., Marry P., Allain J.M., Adam C. Increased intra-cortical porosity reduces bone stiffness and strength in pediatric patients with osteogenesis imperfecta. Bone. 2014;69:61–67. doi: 10.1016/j.bone.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 473.Albert C., Jameson J., Smith P., Harris G. Reduced diaphyseal strength associated with high intracortical vascular porosity within long bones of children with osteogenesis imperfecta. Bone. 2014;66:121–130. doi: 10.1016/j.bone.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Dwan K., Phillipi C.A., Steiner R.D., Basel D. Bisphosphonate therapy for osteogenesis imperfecta. Cochrane Database Syst. Rev. 2016;10:Cd005088. doi: 10.1002/14651858.CD005088.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Li G., Jin Y., Levine M.A.H., Hoyer-Kuhn H., Ward L., Adachi J.D. Systematic review of the effect of denosumab on children with osteogenesis imperfecta showed inconsistent findings. Acta Paediatr. 2018;107:534–537. doi: 10.1111/apa.14154. [DOI] [PubMed] [Google Scholar]
- 476.Zheng H.F., Forgetta V., Hsu Y.H., Estrada K., Rosello-Diez A., Leo P.J., Dahia C.L., Park-Min K.H., Tobias J.H., Kooperberg C., et al. Whole-genome sequencing identifies EN1 as a determinant of bone density and fracture. Nature. 2015;526:112–117. doi: 10.1038/nature14878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Younes N., Syed N., Yadav S.K., Haris M., Abdallah A.M., Abu-Madi M. A Whole-Genome Sequencing Association Study of Low Bone Mineral Density Identifies New Susceptibility Loci in the Phase I Qatar Biobank Cohort. J. Pers. Med. 2021;11:34. doi: 10.3390/jpm11010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 478.Kanis J.A., McCloskey E.V., Johansson H., Cooper C., Rizzoli R., Reginster J.-Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 479.Yaacobi E., Sanchez D., Maniar H., Horwitz D.S.J.I. Surgical treatment of osteoporotic fractures: An update on the principles of management. Injury. 2017;48:S34–S40. doi: 10.1016/j.injury.2017.08.036. [DOI] [PubMed] [Google Scholar]
- 480.Ammarullah M.I., Afif I.Y., Maula M.I., Winarni T.I., Tauviqirrahman M., Akbar I., Basri H., van der Heide E., Jamari J.J.M. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials. 2021;14:7554. doi: 10.3390/ma14247554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 481.Jamari J., Ammarullah M.I., Saad A.P.M., Syahrom A., Uddin M., van der Heide E., Basri H. The effect of bottom profile dimples on the femoral head on wear in metal-on-metal total hip arthroplasty. J. Funct. Biomater. 2021;12:38. doi: 10.3390/jfb12020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Gorter E., Reinders C., Krijnen P., Appelman-Dijkstra N., Schipper I.J.B.R. The effect of osteoporosis and its treatment on fracture healing a systematic review of animal and clinical studies. Bone Rep. 2021;15:101117. doi: 10.1016/j.bonr.2021.101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.