Abstract
The acid-insoluble metal sulfides FeS2, MoS2, and WS2 are chemically attacked by iron(III) hexahydrate ions, generating thiosulfate, which is oxidized to sulfuric acid. Other metal sulfides are attacked by iron(III) ions and by protons, resulting in the formation of elemental sulfur via intermediary polysulfides. Sulfur is biooxidized to sulfuric acid. This explains leaching of metal sulfides by Thiobacillus thiooxidans.
Bacterial leaching, the biooxidation of metal sulfides to soluble metal sulfates and sulfuric acid, is effected by specialized bacteria. Three species of mesoacidophilic, chemolithotrophic bacteria are mainly involved: Thiobacillus ferrooxidans, Thiobacillus thiooxidans, and Leptospirillum ferrooxidans. T. ferrooxidans oxidizes reduced sulfur compounds to sulfate and iron(II) to iron(III) ions. T. thiooxidans is able to oxidize only reduced sulfur compounds, whereas L. ferrooxidans can oxidize only iron(II) ions (1, 4, 13, 15).
Recently, we described for pyrite (FeS2) degradation an iron(III) ion-mediated leaching mechanism via thiosulfate and polythionates (18). In this thiosulfate mechanism, the Fe-S2 bond is cleaved, after the S2 group has been oxidized by iron(III) hexahydrate ions to a thiosulfate group. Hydrolysis yields thiosulfate and an iron(II) ion. Thiosulfate is consecutively oxidized via tetrathionate, disulfane-monosulfonic acid, and trithionate to sulfate. Elemental sulfur occurs as a by-product only. The function of T. ferrooxidans and L. ferrooxidans is to supply the oxidizing iron(III) ions.
Although FeS2 is the most abundant metal sulfide on earth, it is not the most valuable one. Consequently, experiments were performed in order to evaluate whether the thiosulfate mechanism is also valid for other metal sulfides. A survey of mineralogical data on metal sulfides indicated that the structure of pyrite is almost unique (2, 28). For further tests, the differently structured metal sulfides sphalerite (ZnS), chalcopyrite (CuFeS2), galena (PbS), hauerite (MnS2), orpiment (As2S3), realgar (As4S4), and molybdenite (MoS2) were selected. For these, the formation of sulfur compounds by iron(III) ion-mediated chemical oxidation was analyzed. For the experiments, the metal sulfides were crushed, pulverized, and heat sterilized under N2. Fifty milliliters of a sterile 10 mM FeCl3 solution at pH 1.9 was added to 1 g of each metal sulfide powder in shake flasks. The suspension was sampled for analysis of reduced sulfur compounds (high-pressure liquid chromatography–diode array detection), sulfate (ion chromatography), metal ions (atomic absorption spectroscopy), iron(III)-iron(II) ions (photometry), and pH (electrode) as previously described (18). Experiments were done in triplicate. The results are shown in Table 1.
TABLE 1.
Formation of sulfur compounds by chemical metal sulfide oxidation with 10 mM Fe(III) chloride (3) at pH 1.9 and 28°C
| Metal sulfide | Formula | Structure (28) | Purity (%)a | S8 (%)b | SO42− (%)b | S4O62− (%)b | S5O62− (%)b |
|---|---|---|---|---|---|---|---|
| Pyrite | FeS2 | Disulfide | >99 | 16.1 | 81.7 | 1.3 | 0.9 |
| Molybdenite | MoS2 | Layer | 93 | 8.4 | 90.4 | 0.6 | 0.6 |
| Hauerite | MnS2 | Disulfide | >99 | 93.6 | 3.7 | 1.2 | 1.5 |
| Sphalerite | ZnS | Sphalerite | 95 | 94.9 | 4.8 | 0.1 | 0.2 |
| Chalcopyrite | CuFeS2 | Sphalerite | >99 | 92.2 | 7.3 | 0.3 | 0.2 |
| Galena | PbS | Halite | >99 | 99.9 | 0.1 | 0.0 | 0.0 |
| Orpiment | As2S3 | Layer | >99 | 94.8 | 5.2 | 0.0 | 0.0 |
| Realgar | As4S4 | Ring | >99 | 92.5 | 7.5 | 0.0 | 0.0 |
Purity calculations are based on ICP measurements of the elemental composition. No impurities were detected by X-ray diffraction except for some geerite (Cu8S5) in the case of chalcopyrite.
Percentages were calculated after 24 h of incubation except for galena (1 h), hauerite (5 h), and realgar (168 h), due to the different reaction rates. In the case of hauerite, traces of hexathionate were formed. The data are means of three parallel experiments. Standard deviations were less than 15%.
The oxidation products in the case of FeS2 and MoS2 consisted of up to 90% sulfate and about 1 to 2% polythionates. Because the valence bands of FeS2 and MoS2 are derived only from the metal orbitals, the valence bands do not contribute to the chemical bond between the metal and the sulfur moiety in the crystal (2, 25). Consequently, these metal sulfides are degradable only by an oxidizing attack, e.g., by iron(III) ions. None of these compounds is soluble in acid (proton attack) (21, 25, 26). Furthermore, both metal sulfides consist of pairs of sulfur atoms. These properties hold for WS2 as well (25, 26). Consequently, FeS2, MoS2, and WS2 are oxidized by the same, indirect thiosulfate mechanism. In leaching experiments with L. ferrooxidans and FeS2 the same sulfur compounds resulted (18). Thus, the same dissolution mechanism is active in bioleaching.
In contrast, all other metal sulfides mentioned above have valence bands, to which metal and sulfur orbitals contribute (2, 25, 26). Consequently, they are acid soluble. The experiments with these metal sulfides yielded elemental sulfur in amounts of more than 90%. Even MnS2, a disulfide like FeS2, but easily acid soluble, yielded mainly elemental sulfur. To explain the differences in end products, another mechanism for dissolution was sought.
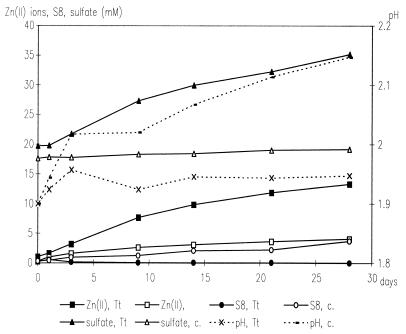
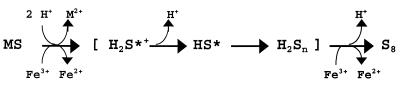
Experiments with T. thiooxidans on the bioleaching of ZnS demonstrated, in agreement with published data (5, 11), that a pure culture is able to dissolve ZnS and produce zinc(II) ions and sulfate at an almost constant pH (Fig. 1). It is known that ZnS is soluble in acid. Chemical experiments demonstrated the dissolution of this compound, too; however, elemental sulfur was formed (because sulfur-oxidizing bacteria were not present). Consequently, the pH started to increase and the dissolution of ZnS concomitantly decreased. To explain the mechanism of dissolution, the formation of intermediary polysulfides according to the work of Steudel (22) has to be considered. The mechanism becomes obvious from the following equations. The dissolution of a metal sulfide (MS) is started by proton attack (equation 1) and a consecutive oxidation of H2S by Fe(III) ions (equation 2).
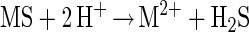 |
1 |
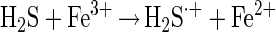 |
2 |
Due to the capability of Fe(III) ions to break metal sulfide bonds more effectively than protons (25, 26), the H2S·+ radical may preferentially be formed in one reaction without intermediately occurring H2S (equation 3).
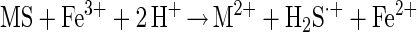 |
3 |
Polysulfide formation starts with dissociation of the strong acid H2S·+ to a HS· radical (equation 4).
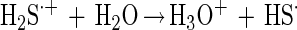 |
4 |
Two of the HS· radicals may react to a disulfide (equation 5).
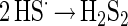 |
5 |
The disulfide may react again with Fe(III) ions (equation 6) or with another HS· radical (equation 7).
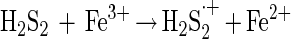 |
6 |
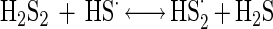 |
7 |
Tetrasulfide occurs by dimerization of two HS2· radicals (analogous to equation 5), or trisulfide occurs by reaction of a HS2· with a HS· radical. Further chain elongation to polysulfides proceeds by analogous reactions.
FIG. 1.
Leaching of ZnS by T. thiooxidans R20 (16). The strain had been adapted to grow on ZnS, before the experiment was started by addition of 109 cells to 1 g of ZnS (pulverized) in 50 ml of salt solution in shake flasks at 28°C in the dark. Tt, assays with T. thiooxidans; c., sterile control assays. Data are means of three parallel assays. Standard deviations were less than 15%. The experiment was reproduced twice.
In acidic solution, polysulfides decompose to rings of elemental sulfur, mainly S8 rings (>99%) (equation 8).
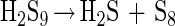 |
8 |
Formation of thiosulfate (polythionates and sulfate) occurs by side reactions (equations 9 and 10).
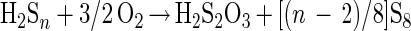 |
9 |
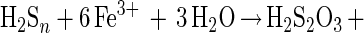 |
10 |
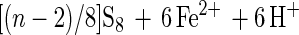 |
This oxidation mechanism does not necessarily require the presence of Fe(III) ions. An electron transfer from a semiconductive metal sulfide to an O2 molecule is also possible. The O2 molecule is reduced via a superoxide radical and a peroxide molecule to water (27). However, Fe(III) ions, usually present in acidic leach biotopes, are much more efficient in extracting electrons from a metal sulfide lattice than is O2 (25, 26).
The series of reactions 1 to 8 inherently explains the formation of elemental sulfur as the main sulfur compound oxidation product of acid-hydrolyzable metal sulfides. Thiosulfate, and consequently polythionates and sulfate, may also arise by side reactions (equations 9 and 10) (22).
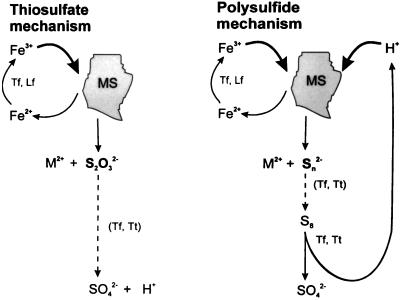
According to this polysulfide mechanism, elemental sulfur is formed. Since sulfur is reasonably stable under experimental and usually also environmental conditions, only in the case of the presence of sulfur-oxidizing bacteria can degradation occur (yielding the protons needed for a further dissolution of ZnS). This mechanism allows for the first time the unequivocal explanation of the ability of T. thiooxidans to leach some metal sulfides, i.e., the ones which are susceptible to hydrolysis by proton attack. Polysulfides were detected on the surface of oxidized chalcopyrite by Hackl et al. (8), confirming this mechanism. The degradation of metal sulfides via polysulfides is summarized in Fig. 2. As a consequence, two indirect oxidation mechanisms for metal sulfides exist.
FIG. 2.
Scheme for metal sulfide (MS) oxidation via polysulfides.
One mechanism is exclusively based on the oxidative attack of iron(III) ions on the acid-insoluble metal sulfides FeS2, MoS2, and WS2. Here, the main sulfur intermediate is thiosulfate. The second mechanism allows for a dissolution by an attack of iron(III) ions and/or by protons. In this case, the main sulfur intermediate is polysulfide (and consequently elemental sulfur). The two mechanisms may be simplified by the following equations:
Thiosulfate mechanism (FeS2, MoS2, and WS2)
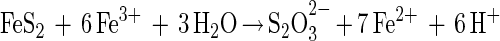 |
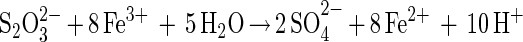 |
Polysulfide mechanism (e.g., ZnS, CuFeS2, or PbS)
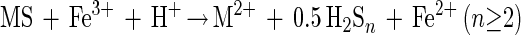 |
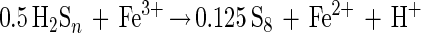 |
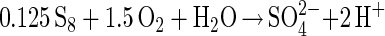 |
Consequently, bioleaching of metal sulfides means that the bacterial function is to generate sulfuric acid biologically to supply protons for hydrolysis attack and/or to keep the iron ions in an oxidized state [as iron(III) ions] for an oxidative attack (Fig. 3).
FIG. 3.
Bioleaching proceeds by two different indirect mechanisms via thiosulfate or via polysulfides and sulfur and is based on the properties of metal sulfides (MS). Dashed lines indicate occurrence of intermediate sulfur compounds.
Our conclusions are in agreement with data from the literature. In the absence of iron ions, T. ferrooxidans can solubilize acid-hydrolyzable, synthetic, iron-free sulfides like ZnS, CdS, NiS, CoS, CuS, and Cu2S (14, 24–26) but not the insoluble sulfides FeS2 (6), MoS2, and WS2 (25, 26). In the case of the former compounds, the leaching rates correlate with their solubility products (24, 26). This finding proves that the solubility product, besides pH, is decisive for the leachability of a metal sulfide in the case of a proton attack. We consequently conclude that in the absence of iron ions T. ferrooxidans acts like T. thiooxidans by acid production (sulfur oxidation). This conclusion is in agreement with the recent finding that the solubilization of Cu2+ from a copper ore is determined by the sulfur-oxidizing activity of T. ferrooxidans (23). The addition of iron ions to cultures of T. ferrooxidans growing with hydrolyzable, synthetic, iron-free sulfides generally enhanced leaching rates (25, 26). Because of the additional oxidative iron(III) ion attack, dissolution rates are much higher than those with T. ferrooxidans lacking iron ions or T. thiooxidans alone (5).
Thus, in summary bioleaching is effected by two indirect leaching mechanisms: via thiosulfate or via polysulfides and sulfur. Both mechanisms combine characteristics of the previously differentiated direct and indirect leaching mechanisms. Direct leaching means an attack on the crystal lattice of a metal sulfide through enzymatic oxidation by attached cells (4). This work shows that the mineralogy is also a contributing factor to the degradation pathway. The knowledge of these two mechanisms has implications for biotechnology and environmental problems connected to bioleaching. Sulfur compound-metabolizing enzymes are involved in metal sulfide oxidation. Their regulation or inhibition might influence the balance of sulfur compounds (9). For example, changing the balance from sulfur to sulfate would increase dissolution rates in bioleaching plants for gold recovery (12). Furthermore, cyanide consumption would be reduced (7). On the other hand, the formation of environmentally harmful acid rock drainage (17, 19, 20) might be reduced, if the oxidation could be stopped at the stage of elemental sulfur.
Acknowledgments
We thank M. Reiß and E. Gock for X-ray diffraction and ICP measurements.
This work was supported by grants to W.S. from BMBF via UBA (1490954) and DBU (05333).
REFERENCES
- 1.Bosecker K. Bioleaching: metal solubilization by microorganisms. FEMS Microbiol Rev. 1997;20:591–604. [Google Scholar]
- 2.Crundwell F K. The influence of the electronic structure of solids on the anodic dissolution and leaching of semiconducting sulphide minerals. Hydrometallurgy. 1988;21:155–190. [Google Scholar]
- 3.Dutrizac J E, MacDonald R J C. Ferric ion as a leaching medium. Miner Sci Eng. 1974;6:59–100. [Google Scholar]
- 4.Ehrlich H L. Geomicrobiology. New York, N.Y: Marcel Dekker, Inc.; 1996. [Google Scholar]
- 5.Garcia O, Bigham J M, Tuovinen O H. Sphalerite oxidation by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Can J Microbiol. 1995;41:578–584. [Google Scholar]
- 6.Gehrke T, Telegdi J, Thierry D, Sand W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl Environ Microbiol. 1998;64:2743–2747. doi: 10.1128/aem.64.7.2743-2747.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hackl R P, Jones L. Proceedings of the International Biohydrometallurgy Symposium—IBS ’97—Biomine ’97. Glenside, South Australia, Australia: Australian Mineral Foundation; 1997. Bacterial sulfur oxidation pathways and their effect on the cyanidation characteristics of biooxidized refractory gold concentrates; pp. M14.2.1–M142.10. [Google Scholar]
- 8.Hackl R P, Dreisinger D B, Peters E, King J A. Passivation of chalcopyrite during oxidative leaching in sulfate media. Hydrometallurgy. 1995;39:25–48. [Google Scholar]
- 9.Hazeu W, Batenburg-van der Vegte W H, Bos P, van der Pas R K, Kuenen J G. The production and utilization of intermediary elemental sulfur during the oxidation of reduced sulfur compounds by Thiobacillus ferrooxidans. Arch Microbiol. 1988;150:574–579. [Google Scholar]
- 10.Hutchins S R, Davidson M S, Brierley J A, Brierley C L. Microorganisms in reclamation of metals. Annu Rev Microbiol. 1986;40:311–336. doi: 10.1146/annurev.mi.40.100186.001523. [DOI] [PubMed] [Google Scholar]
- 11.Lizama H M, Suzuki I. Interaction of chalcopyrite and sphalerite with pyrite during leaching by Thiobacillus ferrooxidans and Thiobacillus thiooxidans. Can J Microbiol. 1991;37:304–311. [Google Scholar]
- 12.Rawlings D E, Silver S. Mining with microbes. Bio/Technology. 1995;13:773–778. [Google Scholar]
- 13.Rossi G. Biohydrometallurgy. Hamburg, Germany: McGraw-Hill; 1990. [Google Scholar]
- 14.Sakaguchi H, Torma A E, Silver M. Microbiological oxidation of synthetic chalcocite and covellite by Thiobacillus ferrooxidans. Appl Environ Microbiol. 1976;31:7–10. doi: 10.1128/aem.31.1.7-10.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sand W, Gehrke T, Hallmann R, Schippers A. Sulfur chemistry, biofilm, and the (in)direct attack mechanism—a critical evaluation of bacterial leaching. Appl Microbiol Biotechnol. 1995;43:961–966. [Google Scholar]
- 16.Sand W, Hallmann R, Rohde K, Sobotke B, Wentzien S. Controlled microbiological in-situ stope leaching of a sulphidic ore. Appl Microbiol Biotechnol. 1993;40:421–426. [Google Scholar]
- 17.Schippers A, Hallmann R, Wentzien S, Sand W. Microbial diversity in uranium mine waste heaps. Appl Environ Microbiol. 1995;61:2930–2935. doi: 10.1128/aem.61.8.2930-2935.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schippers A, Jozsa P-G, Sand W. Sulfur chemistry in bacterial leaching of pyrite. Appl Environ Microbiol. 1996;62:3424–3431. doi: 10.1128/aem.62.9.3424-3431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schippers A, Jozsa P-G, Sand W. Evaluation of the efficiency of measures for sulphidic mine waste mitigation. Appl Microbiol Biotechnol. 1998;49:698–701. [Google Scholar]
- 20.Schrenk M O, Edwards K J, Goodman R M, Hamers R J, Banfield J F. Distribution of Thiobacillus ferrooxidans and Leptospirillum ferrooxidans for generation of acid mine drainage. Science. 1998;279:1519–1522. doi: 10.1126/science.279.5356.1519. [DOI] [PubMed] [Google Scholar]
- 21.Singer P C, Stumm W. Acidic mine drainage: the rate-determining step. Science. 1970;167:1121–1123. doi: 10.1126/science.167.3921.1121. [DOI] [PubMed] [Google Scholar]
- 22.Steudel R. Mechanism for the formation of elemental sulfur from aqueous sulfide in chemical and microbiological desulfurization processes. Ind Eng Chem Res. 1996;35:1417–1423. [Google Scholar]
- 23.Sugio T, Akhter F. Solubilization of Cu2+ from copper ore by iron-oxidizing bacteria isolated from the natural environment and identification of the enzyme that determines Cu2+ solubilization activity. J Ferment Bioeng. 1996;82:346–350. [Google Scholar]
- 24.Torma A E, Sakaguchi H. Relation between the solubility product and the rate of metal sulfide oxidation by Thiobacillus ferrooxidans. J Ferment Technol. 1978;56:173–178. [Google Scholar]
- 25.Tributsch H, Bennett J C. Semiconductor-electrochemical aspects of bacterial leaching. 1. Oxidation of metal sulphides with large energy gaps. J Chem Technol Biotechnol. 1981;31:565–577. [Google Scholar]
- 26.Tributsch H, Bennett J C. Semiconductor-electrochemical aspects of bacterial leaching. Part 2. Survey of rate-controlling sulphide properties. J Chem Technol Biotechnol. 1981;31:627–635. [Google Scholar]
- 27.Tributsch H, Gerischer H. The oxidation and self-heating of metal sulphides as an electrochemical corrosion phenomenon. J Appl Chem Biotechnol. 1976;26:747–761. [Google Scholar]
- 28.Vaughan D J, Craig J R. Mineral chemistry of metal sulfides. Cambridge, United Kingdom: Cambridge University Press; 1978. [Google Scholar]