Abstract
To quantify hepatitis A virus (HAV) in experimentally contaminated mussels, we developed an internal standard RNA with a 7-nucleotide deletion for competitive reverse transcription (RT)-PCR. Deposited directly into the sample, this standard was used both as extraction control and as quantification tool. After coextraction and competitive RT-PCR, standard and wild-type products were detected by differential hybridization with specific probes and a DNA enzyme immunoassay. The quantifiable range with this reproducible method was 104 to 107 copies of HAV/gram or 400 to 106 50% tissue culture infective doses/ml.
Hepatitis A epidemics occur throughout the world and are generally due to fecal contamination of water or food (14, 26). In several outbreaks, the onset of clinical hepatitis has been linked to consumption of uncooked shellfish (8, 10, 21). Qualitative reverse transcription (RT)-PCR techniques are now in widespread use for the detection of hepatitis A virus (HAV) in shellfish (7, 13). We report a quantitative method using competitive RT-PCR in experimentally contaminated mussels in the presence of an internal standard RNA (IS-RNA) deposited directly into samples. PCR products were detected by differential microplate hybridization with specific probes for standard or wild-type fragments, followed by DNA enzyme immunoassay (DEIA). The IS-RNA not only makes quantification possible but also overcomes known difficulties, such as the influence of potential inhibitors and inefficiency in the RT reaction, and controls for potential disturbances in PCR (6, 9, 29, 33).
HAV (strain CF53; genotype Ib; titer, 107 50% tissue culture infective doses [TCID50]/ml) was supplied by J. M. Crance (CCRSA, Grenoble, France). Mussels (Mytilus edulis) grown on the French Atlantic coast were artificially contaminated by a 1-h immersion in reconstituted seawater containing 9 × 103 TCID50 of HAV per ml. After contamination, mussels were rinsed, shelled, and drained of excess fluid. Tissues were stored in 60-g aliquots at −20°C as described by Mignotte et al. (22). Virus was then recovered by two different extraction procedures using either borate (5) or glycine (30) buffers and was concentrated by organic flocculation (15) or precipitation with 10% polyethylene glycol 6000 (18). Concentrates were detoxified by filtration through a Sephadex LH20 gel (4) and frozen in 200-μl portions until analyzed. These extraction-concentration procedures have been optimized by Traoré et al. (31).
Total RNA was extracted from shellfish concentrates (200 μl) with acid guanidinium thiocyanate-phenol-chloroform using the RNAzol purification kit (Bioprobe Systems, Montreuil-sous-Bois, France). RNA pellets were resuspended in 40 μl of sterile water.
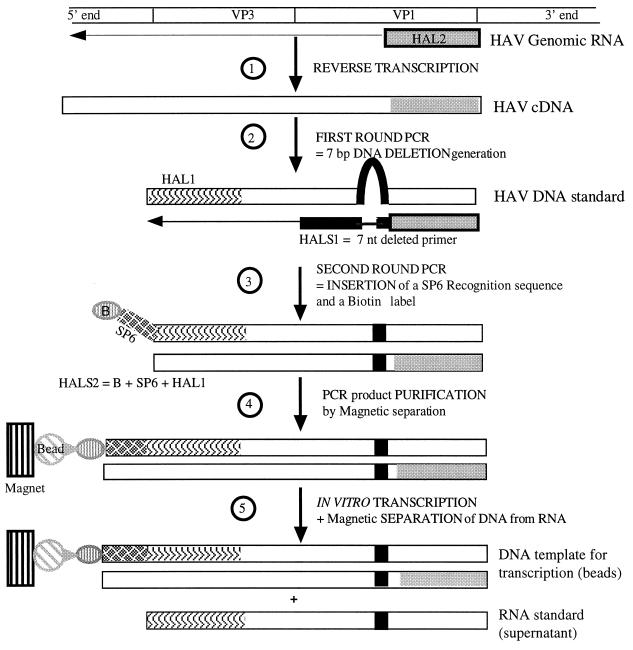
To generate the IS-RNA, two successive DNA intermediates were first constructed by PCR after cDNA synthesis by RT of genomic RNA from HAV strain CF53 (Fig. 1). The first intermediate was generated using a wild-type HAV PCR primer (HAL1) and a 10-nucleotide extended primer in which 7 internal nucleotides had been deleted (HALS1). The second DNA construction was derived from Repp et al. (28) and consisted of inserting a 34-nucleotide SP6 RNA polymerase recognition sequence and a biotin label subsequently used for RNA purification onto the 5′ end of the sense primer (HALS2) (Table 1). After purification on Biogel-P100 (Bio-Rad, Ivry-sur-Seine, France) to eliminate the remaining biotinylated primers, the resulting biotinylated 275-bp product was picked up with avidin-coated M-280 Dynabeads (Dynal, Oslo, Norway). In vitro transcription was performed using the Riboscribe RNA probe synthesis kit (TEBU, Le Perray-en-Yvelines, France) by adding the reaction mixture directly to the solid phase. After in vitro transcription, beads retaining DNA template were trapped in a magnetic field (MPCR-E magnet; Dynal), and the supernatant containing IS-RNA was recovered. Any remaining contaminant DNA was eliminated with RNase-free DNase I, which was itself subsequently eliminated (5 min, 95°C). A total of 85 μg of very pure IS-RNA was obtained, as determined by absorbance at 260 nm. IS-RNA dilutions were carried out in a Tris-EDTA 10:1 solution with 1 U of RNasin/μl and were conserved in 50-μl aliquots at −80°C until use. An Applied Biosystems model 373A DNA sequencing system was used to verify the sequences of all construction intermediates. No signal was detected after simple PCR on the same dilutions, proving that there was no contamination by the DNA template. For quantification, known quantities of IS-RNA were deposited either into the RT-PCR tube or directly into samples of the concentrate, which were undiluted or diluted (1:5, 1:25, 1:125).
FIG. 1.
Construction of IS-RNA for use in quantitative HAV RT-PCR. After cDNA synthesis using antisense primer (HAL2), a 7-bp shortened DNA fragment with a 10-nucleotide extended antisense primer in which 7 internal nucleotides had been deleted (HALS1) was generated by PCR. Deleted product was reamplified using a sense primer (HALS2) carrying a 34-nucleotide SP6 RNA polymerase recognition sequence (SP6) and a biotin label at its 5′ end (B). Avidin-coated magnetic beads (Bead) were used to purify the product of the second round of amplification. The supernatant was replaced, and the in vitro transcription mixture was added directly to the solid phase. After reaction, beads retaining DNA template were trapped in the magnetic field (Magnet), and the supernatant containing RNA molecules was recovered. The RNA standard obtained harbors a seven-nucleotide deletion and could be amplified with the same primers (HAL1 and HAL2) as wild-type HAV.
TABLE 1.
Sequence of primers used for RT-PCR detection or IS-RNA construction and probes used for selective detection of wild-type and IS-RNA sequences
| Primer or probe | Sequence | Position | Polarity |
|---|---|---|---|
| HAL1a | 5′-GTTTTGCTCCTCTTTATCATGCTATGGATGTTACTACAC-3′ | 2167–2205 | Sense |
| HAL2a | 5′-GGAAATGTCTCAGGTACTTTCTTTGCTAAAACTGGATCC-3′ | 2413–2375 | Antisense |
| HALS1b | 5′-GGAAATGTCTCAGGTACTTTCTTTGCTAAAACTGGATCCTCAADDDDDDDGATAGC-3′ | 2413–2358 | Antisense |
| HALS2b | 5′-Biotin-GCACATACGATTTAGGTGACACTATAGAATACAAGTTTTGCTCCTCTTTATCATGCT ATGGATGTTACTACAC-3′ | 2167–2205 | Sense |
| HA3c | 5′-Biotin-TCCTCAATTGTTGTGATAGC-3′ | 2377–2358 | Antisense |
| SSI3d | 5′-Biotin-GAGCTATCDDDDDDDTTGAGGATC-3′ | 2356–2379 | Sense |
HAV RT-PCR primer.
Primer specifically used for IS-RNA construction. HALS1 is an extended 10-nucleotide primer with 7 internal nucleotides deleted (D). HALS2 is a primer carrying SP6 recognition sequence (marked in italics and underlined) and a biotin at its 5′ end.
Probe with biotinylated 5′ end specific to the native HAV RNA sequence.
Probe with biotinylated 5′ end specific to the IS-RNA sequence.
Competitive RT-PCR consisted of coamplifying to saturation native HAV RNA and IS-RNA. The Titan one-step RT-PCR system (Boehringer, Mannheim, Germany) was run on an Omnigene Hybaid thermocycler in a total volume of 25 μl including 5 μl of RNA extract (previously denatured for 10 min at 65°C) with a 0.5 μM concentration of each of the primers HAL1 and HAL2 (1). Amplification conditions were provided by 30 min of reverse transcription at 42°C, 5 min of denaturation at 94°C, 10 initial amplification cycles (94°C, 30 s; 62°C, 30 s; 68°C, 45 s), 25 further amplification cycles (94°C, 30 s; 62°C, 30 s; and 68°C for 45 s in the first cycle, increasing in each subsequent cycle by 5-s increments), and finally a 7-min extension cycle at 68°C.
A DEIA was carried out with 6 μl of RT-PCR product using a commercial kit (GEN-ETIK-DEIA; Sorin Biomedica, Saluggia, Italy) with biotinylated probes specific to HA3 native RNA and SSI3 IS-RNA sequences (Table 1). DEIA conditions were the same for both probes and have already been described for the HA3 probe (1). The hybridization temperature was set at 52°C to increase signal-to-noise ratios. Positive and negative controls were included at each stage of the quantification procedure. Extraction controls consisted of HAV-negative shellfish concentrates with 2 × 105 copies of IS-RNA added (positive control) or without IS-RNA (negative control). Appropriate positive (103 copies of IS-RNA) and negative (sterile water) PCR controls were carried out. Negative and positive hybridization controls for IS-RNA and HAV-RNA products were coprocessed with tested samples.
The number of copies of IS-RNA synthesized was calculated from the end point in each dilution series after RT-PCRs in three independent experiments. The highest dilution (10−10) that yielded a reproducible DEIA signal (optical density at 450 nm [OD450] = 0.16 ± 0.05) was chosen as the optimal control, corresponding to about 10 equivalent genome copies, in view of the sensitivity of RT-PCR (1 to 10 TCID50). Native and IS-RNA–amplified products were differentiated by hybridization with specific probes overlapping the deleted sequence. The detection thresholds for each of the two specific probes were calculated as the mean plus 2 standard deviations of the OD450 of 20 samples negative for HAV but containing amplicons of the standard. This gave OD450 values of 0.10 for the probe SSI3 and an OD450 of 0.30 for the probe HA3. Our choice of this higher cutoff for quantification circumvents any potential influence from nonspecific hybridization. The cutoff calculated for qualitative use of this probe without a standard is 0.10 (1). The number of HAV genome copies per gram of shellfish tissue or per milliliter of concentrate was determined from the last dilution with the wild-type OD450 above 0.30 using the following formula: (HA3 OD450/SSI3 OD450) × IS-RNA copies in the RT-PCR tube × the dilution factor.
Although competitive RT-PCR methods run to saturation allow small numbers of copies of target RNA to be quantified, very few studies have been done on environmental samples, such as sediment and shellfish, or on complex clinical samples like stools. Methods developed for RT-PCR quantification of HAV (3, 12), enterovirus (2, 17, 19), and gastroenteritis RNA viruses (3) use a plasmid-generated deleted RNA standard coamplified with the wild-type RNA already extracted. IS-RNAs are distinguished from the target on the basis of size before or after restriction. For our IS-RNA, we chose a very simple PCR construction which is protected from contamination by DNA (28). The IS-RNA we obtained is very similar to the HAV sequence, differing from it only by one 7-nucleotide deletion. This is to ensure that both sequences are amplified equally under all circumstances, irrespective of the number of cycles or the efficiency of amplification (20, 27). Using a standard RNA during the amplification stages circumvents problems caused by inhibition of the RT and PCRs (32, 33) and controls for intertube variations in amplification conditions. By coamplifying 200 and 2,000 copies of IS-RNA with varying amounts of wild-type RNA (corresponding to viral concentrations of 2.5 × 102 to 5 × 104 TCID50/ml of RNA extract) we found that 2,000 copies of IS-RNA were necessary to achieve competition. Adding a standard at this point, however, cannot control for the viral losses associated with the extraction and purification of viral RNA from complex media. The results obtained also remain heavily dependent on the choice of extraction method (19).
Previous studies on sediment samples have demonstrated that adding a standard directly to the complex sample to be analyzed and then coextracting the RNAs gives more accurate and reproducible results (23). When the standard was introduced directly into the crushed shellfish preparation before extraction of total RNA, 2 × 105 copies of the IS-RNA were required. One hundred times more IS-RNA needs to be added before extraction of genome material than before amplification if a reproducible signal (OD450 = 1.81 ± 0.62; n = 8) is to be obtained. This concentration ratio between the two tests can be explained by the fact that only 2.5 × 104 copies of IS-RNA were amplified (5 μl for a total extract volume of 40 μl) and that the RNA extraction yield was less than 100%. Legeay et al. (16) also found a 10-fold difference between the results obtained when a standard RNA is added before RNA extraction or before amplification. Thus, despite the small size of the standard fragment (241 nucleotides), the efficiency with which this RNA was recovered by the chosen extraction procedure was satisfactory, probably because this small fragment is carried along by the considerable quantities of genome material that mussel tissue contains.
Quantification tests on crushed preparations of uncontaminated shellfish into which 2 × 105 copies of IS-RNA and known quantities of HAV varying between 0.1 and 105 TCID50/ml had been introduced were carried out. After total RNA extraction, amplification, and detection by immunoassay, we found that for concentrations of HAV below 100 TCID50/ml, the hybridization signal with probe HA3 was below the threshold of 0.30. The quantification threshold we obtained (400 TCID50/ml, or 104 copies) was comparable with the findings of Goswami et al. (11) of a detection limit of 2,000 particles per gram of shellfish. For concentrations of HAV above 104 TCID50/ml, OD450 values with probe HA3 showed no further increase above a certain ceiling. This saturation phenomenon could lead to underestimation of HAV quantities, and such samples must therefore be diluted to bring them back within the range in which values are proportional to quantity. By successively diluting each sample three times (by 1:5), the quantification range was extended to 104 to 107 copies per gram of mussel tissue or 400 to 106 TCID50/ml of homogenized mussel. We then compared the linearity of the signals obtained from these same shellfish samples with variable HAV content, for each of the quantification protocols in which the standard was added before extraction (Fig. 2B) or afterwards (Fig. 2A). Similar linearities of the signal were obtained for both methods (r2 = 0.9189 before extraction and r2 = 0.9364 after extraction). The value for the slope by adding the IS-RNA to the RT-PCR tube is approximately half the value reported in Fig. 2B. Adding the standard at an earlier stage seems to improve the discriminative power of the method. The potential range of HAV quantification is of the same amplitude as that of human immunodeficiency virus detection in plasma (25). Diluting samples several times also dilutes any inhibiting factors that may be present.
FIG. 2.
Estimation of linearity as a function of concentration of virus in the sample for the two quantification protocols. Results obtained with 2 × 103 copies of IS-RNA in the RT-PCR tube (A) or with 2 × 105 copies of IS-RNA added directly to the shellfish homogenate (B) are shown.
The reproducibility of our quantification method was tested by using two shellfish samples to which were added 104 or 106 TCID50/ml of titered HAV. Four successive dilutions (undiluted, 1:5, 1:25, and 1:125) of these samples were analyzed independently 10 times. The concentrations tested ranged from 80 to 106 TCID50/ml and were regularly distributed over the whole quantification range defined above (Table 2). The logarithm of the number of genome copies showed a linear increase from 4.67 to 6.08 log units as a function of the logarithm of concentration of virus in TCID50/ml (y = 0.36x + 4.56; r = 0.79); this was confirmed by a Snedecor’s test [F < Fs(5, 60) with α = 5%] (24). The standard deviations measured tended to widen at higher concentrations but nevertheless remained below 0.4 log. This quantification method is reproducible, therefore, despite the complexity of the samples tested and the considerable theoretical variability due to extraction techniques, amplification reactions, and immunoassays. These samples could be used as an external, interseries quality control.
TABLE 2.
Interassay reproducibility of the method for quantifying the HAV genome in crushed preparations of artificially contaminated shellfish
| Concn of HAV in crushed shellfish (TCID50/ml) | Genome copies
|
||
|---|---|---|---|
| Mean no./ga | Mean log no./ga | SD (log no./g) | |
| 80 | <104 | <4 | |
| 4 × 102 | 5.16 × 104 | 4.67 | 0.21 |
| 2 × 103 | 1.90 × 105 | 5.25 | 0.17 |
| 8 × 103 | 2.01 × 105 | 5.29 | 0.25 |
| 104 | 2.51 × 105 | 5.39 | 0.29 |
| 4 × 104 | 3.88 × 105 | 5.57 | 0.14 |
| 2 × 105 | 7.20 × 105 | 5.75 | 0.34 |
| 106 | 1.62 × 106 | 6.08 | 0.38 |
Mean number of copies and log number of copies of HAV genome per gram of shellfish tissue were determined from 10 separate quantification experiments.
The quantification technique with RNA coextraction was used on seven samples of mussels contaminated by immersion in seawater containing 9 × 103 TCID50 of HAV/ml, and subjected to different extraction-concentration procedures (31). All contaminated samples were positive with concentrations of virus between 5.07 × 104 and 106 copies per gram (Table 3). Standard deviations for three independent measurements on the same sample were less than 0.5 log, confirming the technique’s good reproducibility. Although viral extraction procedures involving organic flocculation and those using polyethylene glycol 6000 precipitation methods differed by a factor of 20, sample numbers were insufficient for valid interpretation.
TABLE 3.
Application of the method for quantifying the HAV genome to shellfish samples contaminated via natural routes (seven samples of mussels used)
| Virus extraction buffer (concentration process)a | Genome copies
|
||
|---|---|---|---|
| Mean no./gb | Mean log no./gb | SD (log no./g) | |
| Glycine (OF) | 9.38 × 105 | 5.97 | 0.45 |
| Glycine (OF, LH20) | 1.00 × 106 | 6.00 | 0.40 |
| Borate (OF) | 3.14 × 105 | 5.50 | 0.14 |
| Borate (PEG 6000) | 1.00 × 105 | 5.00 | 0.31 |
| Glycine (PEG 6000) | 5.48 × 104 | 4.74 | 0.22 |
| Borate (OF, LH20) | 5.38 × 104 | 4.73 | 0.09 |
| Glycine (PEG 6000, LH20) | 5.07 × 104 | 4.71 | 0.19 |
OF, organic flocculation; PEG 6000, viral precipitation using polyethylene glycol 6000; LH20, detoxification of samples by filtration on Sephadex LH20 gel.
Number of copies and log number of copies of HAV genome per gram of shellfish tissue were calculated with reference to last positive dilution of sample. Means of three independent quantification experiments on this last dilution are shown.
In conclusion, the RT-PCR quantitative assay provides an easy, reliable method for routine quantification of HAV genome copies in shellfish specimens. Using DEIA for detection allows the procedure to be automated, saving time and increasing reliability and accuracy. Moreover, adding IS-RNA standard during isolation of RNA makes it possible to control for the efficiency of both RNA isolation and RT and to detect samples rendered falsely negative by amplification inhibitors. This quantification method should allow numerous environmental shellfish to be screened for their level of contamination, the risk of viral infection associated with their consumption to be assessed, and methods of viral decontamination to be validated. Additional studies in order to demonstrate whether the addition of the IS-RNA one step earlier improves detection of viruses in naturally contaminated shellfish samples are in progress in our laboratory.
REFERENCES
- 1.Arnal C, Ferré-Aubineau V, Besse B, Billaudel S. Simplified RT-PCR procedure with detection by microplate hybridization for routine screening of hepatitis A virus. Can J Microbiol. 1998;44:298–302. [PubMed] [Google Scholar]
- 2.Arola A, Santti J, Ruuskanen O, Halonen P, Hyypia T. Identification of enteroviruses in clinical specimens with competitive PCR followed by genetic typing using sequence analysis. J Clin Microbiol. 1996;34:313–318. doi: 10.1128/jcm.34.2.313-318.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar R L, Neill F H, Romalde J L, Le Guyader F, Woodley C M, Metcalf T G, Estes M K. Detection of Norwalk virus and hepatitis A virus in shellfish tissues with the PCR. Appl Environ Microbiol. 1995;61:3014–3018. doi: 10.1128/aem.61.8.3014-3018.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beril C, Boher S, Schwartzbrod L. Detoxification by Sephadex LH20 of seafood concentrates for rotavirus assay. Water Sci Technol. 1991;24:417–421. [Google Scholar]
- 5.Boher S, Schwartzbrod L. Study of viral purification of oysters. Water Sci Technol. 1993;27:55–60. [Google Scholar]
- 6.Clementi M, Menzo S, Manzin A, Bagnarelli P. Quantitative molecular methods in virology. Arch Virol. 1995;140:1523–1539. doi: 10.1007/BF01322527. [DOI] [PubMed] [Google Scholar]
- 7.Cromeans T L, Nainan O V, Margolis H S. Detection of hepatitis A virus RNA in oyster meat. Appl Environ Microbiol. 1997;63:2460–2463. doi: 10.1128/aem.63.6.2460-2463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desenclos J C, Klontz K C, Wilder M H, Nainan O V, Margolis H S, Gunn R A. A multistate outbreak of hepatitis A caused by consumption of raw oysters. Am J Public Health. 1991;81:1268–1272. doi: 10.2105/ajph.81.10.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilliland G, Perrin S, Bunn H F. Competitive PCR for quantification of mRNA. In: Innis M A, Gelfand D H, Sninsky J J, White J J, editors. PCR protocols: a guide to methods and applications. New York, N.Y: Academic Press; 1990. pp. 60–69. [Google Scholar]
- 10.Goh K T, Chan L, Ding J L, Oon C J. An epidemic of cockles-associated hepatitis A in Singapore. Bull Acad Natl Med (Paris) 1984;62:893–897. [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami B B, Koch W H, Cebula T A. Detection of hepatitis A virus in Mercenaria mercenaria by coupled reverse transcription and polymerase chain reaction. Appl Environ Microbiol. 1993;59:2765–2770. doi: 10.1128/aem.59.9.2765-2770.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goswami B B, Koch W H. Competitor template RNA for detection and quantification of hepatitis A virus by PCR. BioTechniques. 1994;16:114–121. [PubMed] [Google Scholar]
- 13.Hafliger D, Gilgen M, Luthy J, Hubner P. Seminested RT-PCR systems for small round structured viruses and detection of enteric viruses in seafood. Int J Food Microbiol. 1997;37:27–36. doi: 10.1016/s0168-1605(97)00041-x. [DOI] [PubMed] [Google Scholar]
- 14.Halliday M L, Kang L Y, Zhou T K, Hu T K, Pan Q C, Fu T Y, Huang Y S, Hu S L. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shangai, China. J Infect Dis. 1991;164:852–859. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- 15.Katzenelson E, Fattal B, Hostovesky T. Organic flocculation: an efficient second-step concentration method for the detection of viruses in tap water. Appl Environ Microbiol. 1976;32:638–639. doi: 10.1128/aem.32.4.638-639.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legeay O, Bounaix S, Denis M, Arnauld C, Hutet E, Cariolet R, Albina E, Jestin A. Development of a RT-PCR test coupled with a microplate colorimetric assay for the detection of a swine Arterivirus (PRRSV) in boar semen. J Virol Methods. 1997;68:65–80. doi: 10.1016/s0166-0934(97)00110-9. [DOI] [PubMed] [Google Scholar]
- 17.Le Guyader F, Menard D, Dubois E, Haugarreau L, Kopecka H, Pommepuy M. Use of an RT-PCR control to evaluate viral removal. Water Sci Technol. 1997;35:461–465. [Google Scholar]
- 18.Lewis G D, Metcalf T G. Polyethylene-glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol. 1988;54:1983–1988. doi: 10.1128/aem.54.8.1983-1988.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martino T A, Sole M J, Penn L Z, Liew C C, Liu P. Quantification of enteroviral RNA by competitive polymerase chain reaction. J Clin Microbiol. 1993;31:2634–2640. doi: 10.1128/jcm.31.10.2634-2640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCulloch R K, Choong C S, Hurley D M. Evaluation of competitor type and size for use in the determination of mRNA by competitive PCR. PCR Methods Appl. 1995;4:219–226. doi: 10.1101/gr.4.4.219. [DOI] [PubMed] [Google Scholar]
- 21.Mele A, Rastelli M G, Gill O N. Recurrent epidemic hepatitis A associated with consumption of raw shellfish, probably controlled through public health measures. Am J Epidemiol. 1989;130:540–546. doi: 10.1093/oxfordjournals.aje.a115368. [DOI] [PubMed] [Google Scholar]
- 22.Mignotte B, Terver D, Schwartzbrod L. Comparative study of poliovirus recovery techniques from mussel tissues. Mar Pollut Bull. 1997;34:875–879. [Google Scholar]
- 23.Moller A, Jansson J K. Quantification of genetically tagged cyanobacteria in Baltic Sea sediment by competitive PCR. BioTechniques. 1997;22:512–518. doi: 10.2144/97223rr02. [DOI] [PubMed] [Google Scholar]
- 24.Morin J F. Régression linéaire corrélation linéaire. In: Besnard J C, Morin J F, editors. ImmunoStat outils statistiques en immuno-analyse. Paris, France: NucleoN; 1997. pp. 467–501. [Google Scholar]
- 25.Mulder J, McKinney N, Christopherson C C, Sninsky J, Greenfield L, Kwok S. Rapid and simple PCR assay for quantitation of human immunodeficiency virus type 1 in plasma: application to acute retroviral infection. J Clin Microbiol. 1994;33:292–300. doi: 10.1128/jcm.32.2.292-300.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu M J, Polish L B, Robertson B, Khanna B, Woodruff B A, Shapiro C N, Miller M A, Smith J D, Gedrose J K, Alter M J, Margolis H S. A multistate outbreak of hepatitis A associated with frozen strawberries. J Infect Dis. 1992;166:518–524. doi: 10.1093/infdis/166.3.518. [DOI] [PubMed] [Google Scholar]
- 27.Pannetier C, Delassus S, Darche S, Saucier C, Kourilsky P. Quantitative titration of nucleic acids by enzymatic amplification reactions run to saturation. Nucleic Acids Res. 1993;21:577–583. doi: 10.1093/nar/21.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Repp R, Borkhardt A, Gossen R, Kreuder J, Hammermann J, Lambert F. Construction of RNA standards for high-resolution automatic product analysis in quantitative competitive RT-PCR. BioTechniques. 1995;19:84–90. [PubMed] [Google Scholar]
- 29.Schwab K J, Estes M K, Neill F H, Atmar R J. Use of heat release and an internal standard control in reverse transcription PCR detection of Norwalk virus from stool samples. J Clin Microbiol. 1997;35:511–514. doi: 10.1128/jcm.35.2.511-514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobsey M D, Carrick R J, Jensen H R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978;36:121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Traoré O, Arnal C, Mignotte B, Maul A, Laveran H, Billaudel S, Schwartzbrod L. Reverse transcriptase-PCR detection of astroviruses, hepatitis A virus, and poliovirus in experimentally contaminated mussels: comparison of several extraction and concentration methods. Appl Environ Microbiol. 1998;64:3118–3122. doi: 10.1128/aem.64.8.3118-3122.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson I G. Inhibition and facilitation of nucleic acid amplification. Appl Environ Microbiol. 1997;63:3741–3751. doi: 10.1128/aem.63.10.3741-3751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmerman K, Mannhalter J K. Technical aspects of quantitative competitive PCR. BioTechniques. 1996;21:268–279. doi: 10.2144/96212rv01. [DOI] [PubMed] [Google Scholar]