Summary
Background
The iBreastExam electronically palpates the breast to identify possible abnormalities. We assessed the iBreastExam performance compared with clinical breast examination for breast lesion detection in high risk and symptomatic Nigerian women.
Methods
This prospective study was done at the Obafemi Awolowo University Teaching Hospital Complex (OAUTHC) in Nigeria. Participants were Nigerian women aged 40 years or older who were symptomatic and presented with breast cancer symptoms or those at high risk with a first-degree relative who had a history of breast cancer. Participants underwent four breast examinations: clinical breast examination (by an experienced surgeon), the iBreastExam (performed by recent nursing school graduates, who finished nursing school within the previous year), ultrasound, and mammography. Sensitivity, specificity, positive predictive values (PPV), and negative predictive values (NPV) of the iBreastExam and clinical breast examination for detecting any breast lesion and suspicious breast lesions were calculated, using mammography and ultrasound as the reference standard.
Findings
Between June 19 and Dec 5, 2019, 424 Nigerian women were enrolled (151 [36%] at high risk of breast cancer and 273 [64%] symptomatic women). The median age of participants was 46 years (IQR 42–52). 419 (99%) women had a breast imaging-reporting and data system (BI-RADS) assessment and were included in the analysis. For any breast finding, the iBreastExam showed significantly better sensitivity than clinical breast examination (63%, 95% CI 57–69 vs 31%, 25–37; p<0·0001), and clinical breast examination showed significantly better specificity (94%, 90–97 vs 59%, 52–66; p<0·0001). For suspicious breast findings, the iBreastExam showed similar sensitivity to clinical breast examination (86%, 95% CI 70–95 vs 83%, 67–94; p=0·65), and clinical breast examination showed significantly better specificity (50%, 45–55 vs 86%, 83–90; p<0·0001). The iBreastExam and clinical breast examination showed similar NPVs for any breast finding (56%, 49–63 vs 52%, 46–57; p=0·080) and suspicious findings (98%, 94–99 vs 98%, 96–99; p=0·42), whereas the PPV was significantly higher for clinical breast examination in any breast finding (87%, 77–93 vs 66%, 59–72; p<0·0001) and suspicious findings (37%, 26–48 vs 14%, 10–19; p=0·0020). Of 15 biopsy-confirmed cancers, clinical breast examination and the iBreastExam detected an ipsilateral breast abnormality in 13 (87%) women and missed the same two cancers (both <2 cm).
Interpretation
The iBreastExam by nurses showed a high sensitivity and NPV, but lower specificity than surgeon’s clinical breast examination for identifying suspicious breast lesions. In locations with few experienced practitioners, the iBreastExam might provide a high sensitivity breast evaluation tool. Further research into improved specificity with device updates and cost feasibility in low-resource settings is warranted.
Introduction
Breast cancer is the most common cancer affecting Nigerian women with rising incidence rates.1–3 Although breast cancer incidence is lower in west Africa than in North America, mortality rates are 50% higher, and 80% of Nigerian women with breast cancer present with stage 3 or 4 disease.1–5 Higher mortality rates are caused by multiple factors, including the scarce availability and access to symptom evaluation, screening, diagnosis, and treatment.4
Multiple studies have shown that screening mammography decreases advanced disease presentation and mortality.6–9 However, numerous barriers to mammography exist in Nigeria.4 In low-resource settings without access to mammography, ultrasound might be an appropriate alternative.10 However, ultrasound is operator dependent and requires equipment and image interpretation.11 Consequently, clinical breast examination by a health-care professional is the primary breast cancer detection method,12,13 and enables prompt evaluation of symptomatic women, can detect breast cancer at an earlier stage, and can reduce breast cancer mortality.14 However, clinical breast examination requires experienced examiners and quality can be inconsistent, with a wide sensitivity range (22–85%).15–17
Women in low-resource countries could benefit from a portable, low cost, easy to use alternative for breast cancer detection. The intelligent breast exam (iBreastExam; UE Life Sciences, Philadelphia, PA) is a 510(k) FDA-cleared device requiring minimal training.18 The iBreastExam uses sensors to electronically palpate the breast and evaluate tissue elasticity by making capacitive measurements and quantifying variations in tissue stiffness when pressed against the skin. The iBreastExam does not differentiate benign versus malignant findings, but identifies women who should undergo clinical evaluation and imaging for a possible abnormality, which could potentially be useful in low-resource regions.
Initial iBreastExam studies19–24 are promising, reporting 84–87% sensitivity, 80–94% specificity, and high negative predictive values (NPV; 94–98%). The iBreastExam can possibly provide a standardised breast examination with a report to facilitate physician communication.19 iBreastExam studies to date were done at academic centres in the USA and India. To our knowledge, there is no published literature on the iBreastExam in Africa. Importantly, the device has not undergone rigorous blinded comparison with clinical breast examination, mammography, and ultrasound using consistent imaging protocols. Our aim was to assess the iBreastExam performance compared with clinical breast examination for breast lesion detection in high risk and symptomatic Nigerian women, using mammography and ultrasound as the reference standard.
Methods
Study design and participants
This prospective study was done at the Obafemi Awolowo University Teaching Hospital Complex (OAUTHC) in Nigeria. Participants were Nigerian women aged 40 years or older who were symptomatic and presented with breast cancer symptoms or those at high risk with a first-degree relative who had a history of breast cancer. Patients were also recruited via community outreach programmes and media advertisements. Women with known breast cancer or non-intact skin were excluded. Written informed consent was obtained from all participants. This study is compliant with the Health Insurance Portability and Accountability Act and received institutional review board approval from the OAUTHC (protocol ERC/2017/6/30).
Procedures
Participants provided relevant history and underwent four breast examinations: clinical breast examination, the iBreastExam, ultrasound, and mammography. Imaging findings were considered twice; first, to assess the performance of the iBreastExam or clinical breast examination to detect any breast finding (benign or suspicious) and second, to assess the performance of each at detecting suspicious breast findings. Suspicious findings were defined as findings recommended for biopsy on the basis of breast imaging.
Each participant underwent systematic clinical breast examination by one of 14 surgeons (>5 years of experience following training) who were masked to other test results. Abnormal findings were recorded as positive and normal findings as negative.
The iBreastExam is a battery-powered, handheld probe with an array of dynamic pressure sensors (figure 1),18 which measure tissue elasticity by making capacitive measurements on the breast surface and quantifying tissue stiffness variations. The sensors are optimised to distinguish differences between hard or stiff areas compared with healthy breast tissue. These data are visualised on a 3D surface map providing a stiffness profile. Results are wirelessly transmitted to a tablet, which transfers results to a secure Microsoft Azure cloud. The iBreastExam produces a colour map that does not require physician interpretation, with red indicating an area warranting further evaluation (figure 2). During our study, the iBreastExam device was upgraded (from version W00009/W00010 to W00008) to simplify calibration, with subanalysis performed on data obtained before and after the upgrade.
Figure 1:
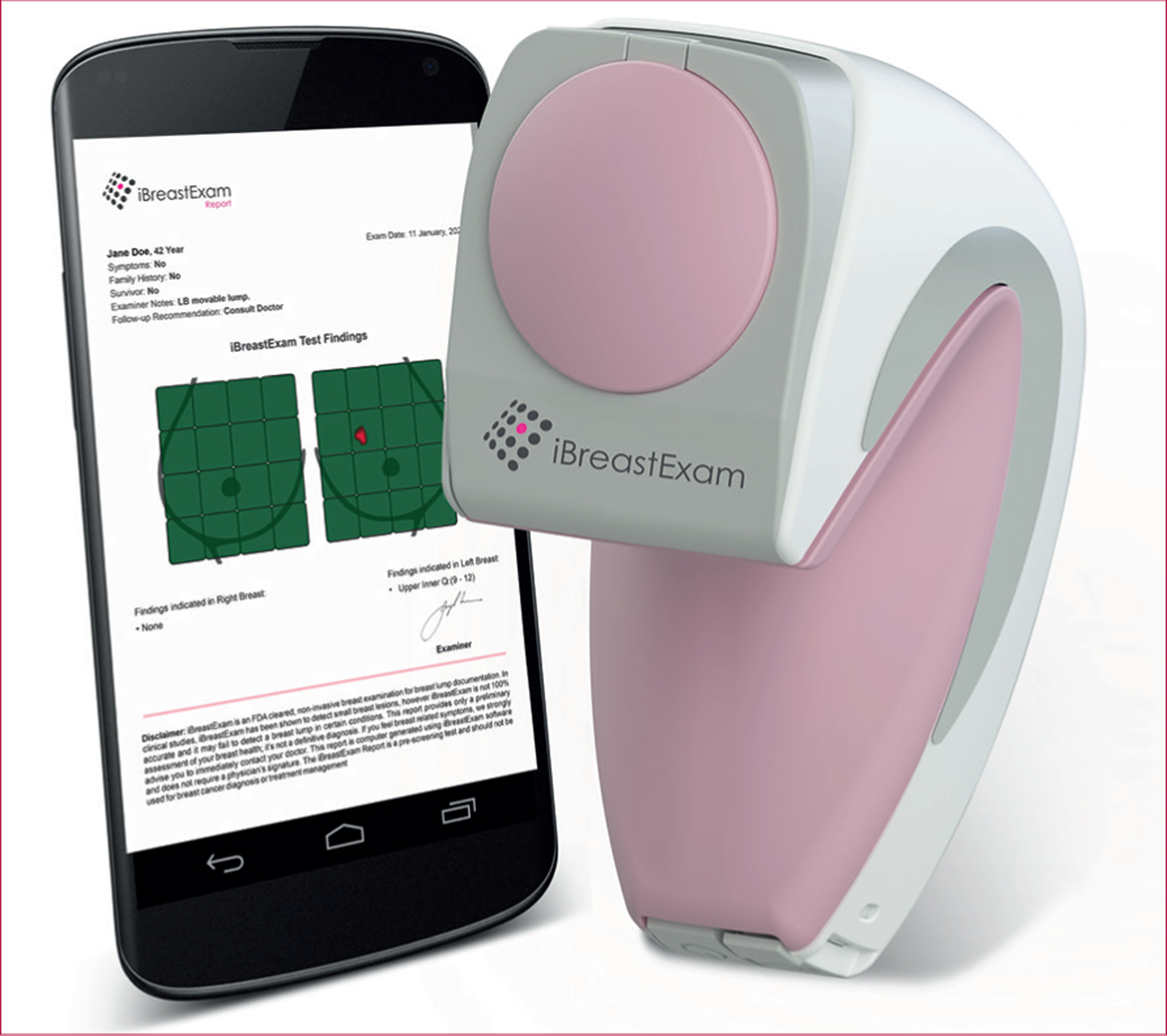
iBreastExam device and mobile tablet
Figure 2: iBreastExam colour map output.
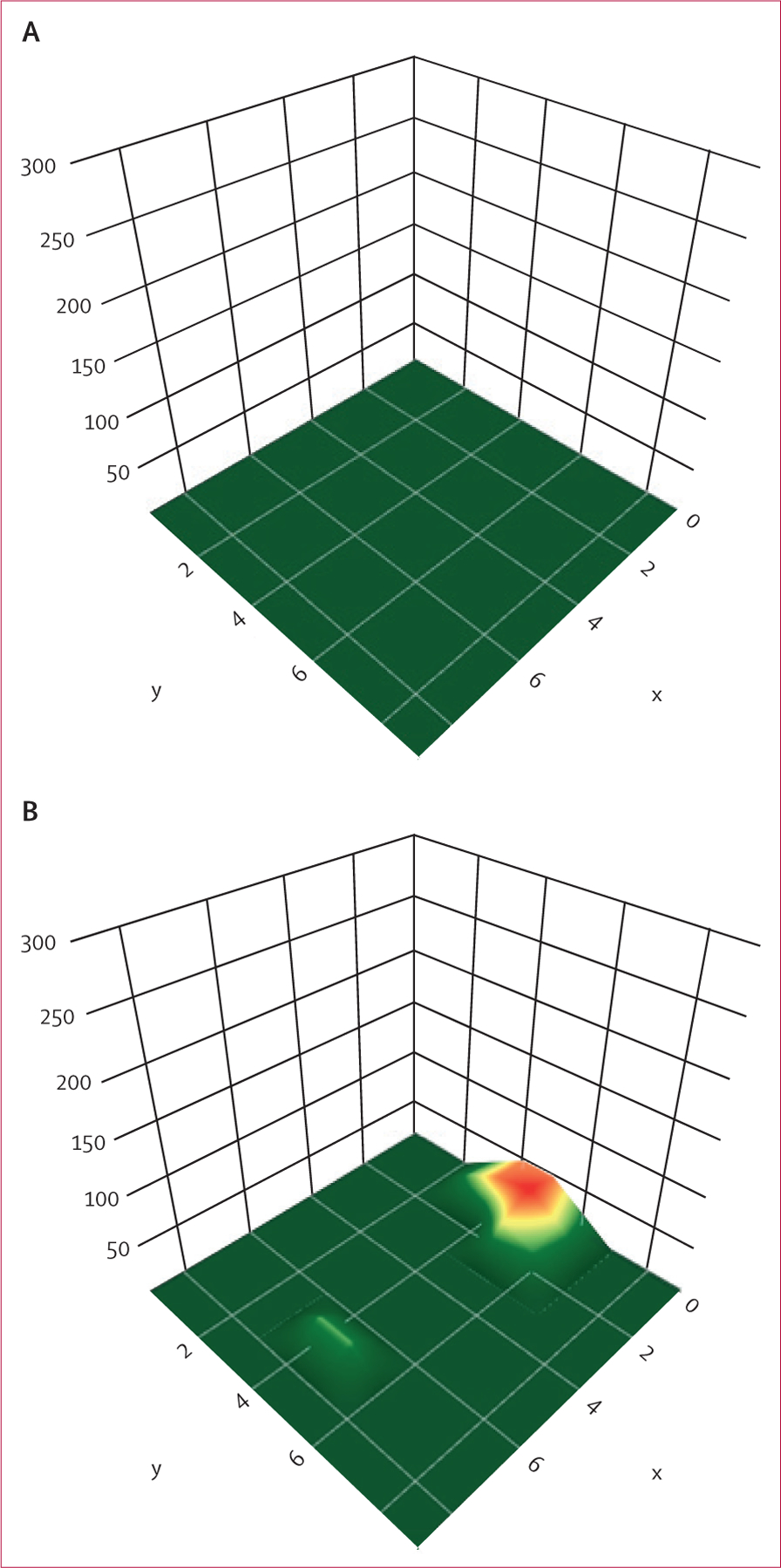
(A) Negative examination of breast tissue displaying all green. (B) An abnormal examination with a red area in the inner breast, indicating a possible abnormality warranting further evaluation.
After clinical breast examination, participants underwent the iBreastExam by one of four recent graduate nurses (ie, those who finished nursing school within the previous year), who were masked to other examination results. iBreastExam training was provided by one of the study’s principal investigators (OO) and the manufacturer. Before enrolment, each nurse performed 30 examinations with the iBreastExam which were reviewed by the principal investigator to establish technique proficiency (ie, correct device operation and inclusion of all breast tissue).
Patients were in the supine position and the iBreastExam systematically moved over the breasts. Red on the colour map was considered positive and green was negative. Women completed surveys regarding the experience. The time spent performing clinical breast examination and the iBreastExam was recorded.
Bilateral, whole breast, handheld ultrasound (Static Mindray DC-7; Mindray, Shenzhen, China or portable Landwind Medical P09; Landwind Medical, Shenzhen, China) was conducted by one radiologist from the OAUTHC with 12 years of experience, including 3 months of breast imaging training at the Memorial Sloan Kettering Cancer Center, with one of the study principal investigators (VLM). The radiologist was masked to clinical breast examination and the iBreastExam results. Lesion size and depth were measured on ultrasound.
Ultrasound data were used to quantify the iBreastExam and clinical breast examination performance at detecting any breast finding and suspicious findings, as classified by the breast imaging-reporting and data system (BI-RADS) categories (appendix p 1). The first analysis considered the breast ultrasound positive if any finding was identified (ie, BI-RADS 2–5), including benign and malignant masses, and negative if no finding was identified (BI-RADS 1). The second analysis considered the ultrasound positive for a suspicious finding if the imaging finding warranted a biopsy (ie, BI-RADS 3–5) and negative if no suspicious findings were present (BI-RADS 1–2). Biopsy was recommended for BI-RADS 3 findings, given patient inability to follow-up (because of numerous barriers such as financial resources, transportation limitations, inability to take time away from work or childcare obligations).
Digital 2D mammography was obtained at the OAUTHC (Full Field Digital GE Senographe 2000D; General Electric Healthcare, Chicago, IL, USA) and nearby commercial imagining centres (Full Field Digital Metaltronica spa Helianthus; Metaltronica, Pomezia, Italy, Siemens Mammomat 5000 Nova; Siemens Healthcare, Erlangan, Germany, and Philips Microdose L50; Philips Healthcare, Andover, MA, USA). The radiologist conducting ultrasounds also interpreted mammograms.
Similar to ultrasound, mammography was used to assess the iBreastExam and clinical breast examination performance to detect any breast finding and suspicious findings. The first analysis considered the mammogram positive for any finding if initial views showed a finding (BI-RADS 0 or 2–5), including benign and malignant findings, and negative if no finding was seen (BI-RADS 1). The second analysis considered the mammogram positive for a suspicious finding if biopsy was recommended (ie, BI-RADS 3–5) and negative if no suspicious findings were present (BI-RADS 1–2), meaning either healthy breast tissue or a benign finding. Similar to ultrasound, biopsy was recommended for BI-RADS 3 findings.
Breast density was assessed using mammography and considered dense if categorised as heterogeneously dense or extremely dense, and considered not dense if categorised as almost entirely fatty or with scattered fibroglandular density.25
Patients with findings that were BI-RADS 3–5 or suspicious on clinical breast examination underwent biopsy. Pathology results were recorded and correlated with clinical breast examination, the iBreastExam, ultrasound, and mammography results. For women diagnosed with cancer, treatment was initiated.
Statistical analysis
Sensitivity and specificity of the iBreastExam and clinical breast examination (in detecting an imaging finding) were determined using R (version 4.0.2). Generalised estimating equation models were built using the geepack package26 to account for the correlation between successive diagnostic measurements in the same individual, and determine the statistically significant covariates affecting sensitivity and specificity. The correlation structure, which minimised the quasi information criterion (compound symmetry), was used for the generalised estimating equation model with significant covariates selected using the backward elimination procedure. Size of the mass (≤2 cm vs >2 cm), depth of the mass, status of the iBreastExam upgrade, breast density, patient features (such as high risk vs symptomatic groups), and imaging features (such as breast density and lesion depth) were analysed as covariates in a generalised estimating equation logistic regression analysis with discordance as the binary outcome variable. Two different models were fit for the two different kinds of discordance (the iBreastExam was negative and clinical breast examination was positive and vice versa) with concordance used as a reference in both models.
Additionally, positive predictive value (PPV) and NPV were determined to assess the ability of the iBreastExam or clinical breast examination to predict a positive or negative imaging finding. The 95% CIs for sensitivity, specificity, PPV, and NPV were obtained using the Clopper-Pearson method for binomial proportions. Comparison between sensitivities and specificities at the patient level was performed using the McNemar test and comparison between PPVs and NPVs was performed using the Leisenring generalised score statistic test. Total agreement was calculated as a ratio of the number of findings that matched between two methods and the total number of findings. Type I error rate was set to 0·05. However, for multiple comparisons, such as between sensitivity and breast density, the Bonferroni correction was used and type I error rate was adjusted to 0·025.
This study is registered with ClinicalTrials.gov, NCT03473795
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Between June 19 and Dec 5, 2019, 424 Nigerian women were enrolled (151 [36%] at high risk of breast cancer and 273 (64%) symptomatic women; figure 3). The median age of participants was 46 years (IQR 42–52). Most women were married (360 [85%] of 424) and had children (390 [92%]). The median age at first pregnancy was 26 years (IQR 23–30) and 384 (91%) reported breastfeeding. Symptomatic women reported breast lumps (88 [32%] of 273), breast pain and discomfort (181 [66%]), nipple discharge (51 [19%]), or multiple symptoms. Some women reported additional symptoms (itching [n=8], tingling [n=1], axillary swelling [n=9], or skin changes [n=1]; appendix p 1).
Figure 3: Study profile.
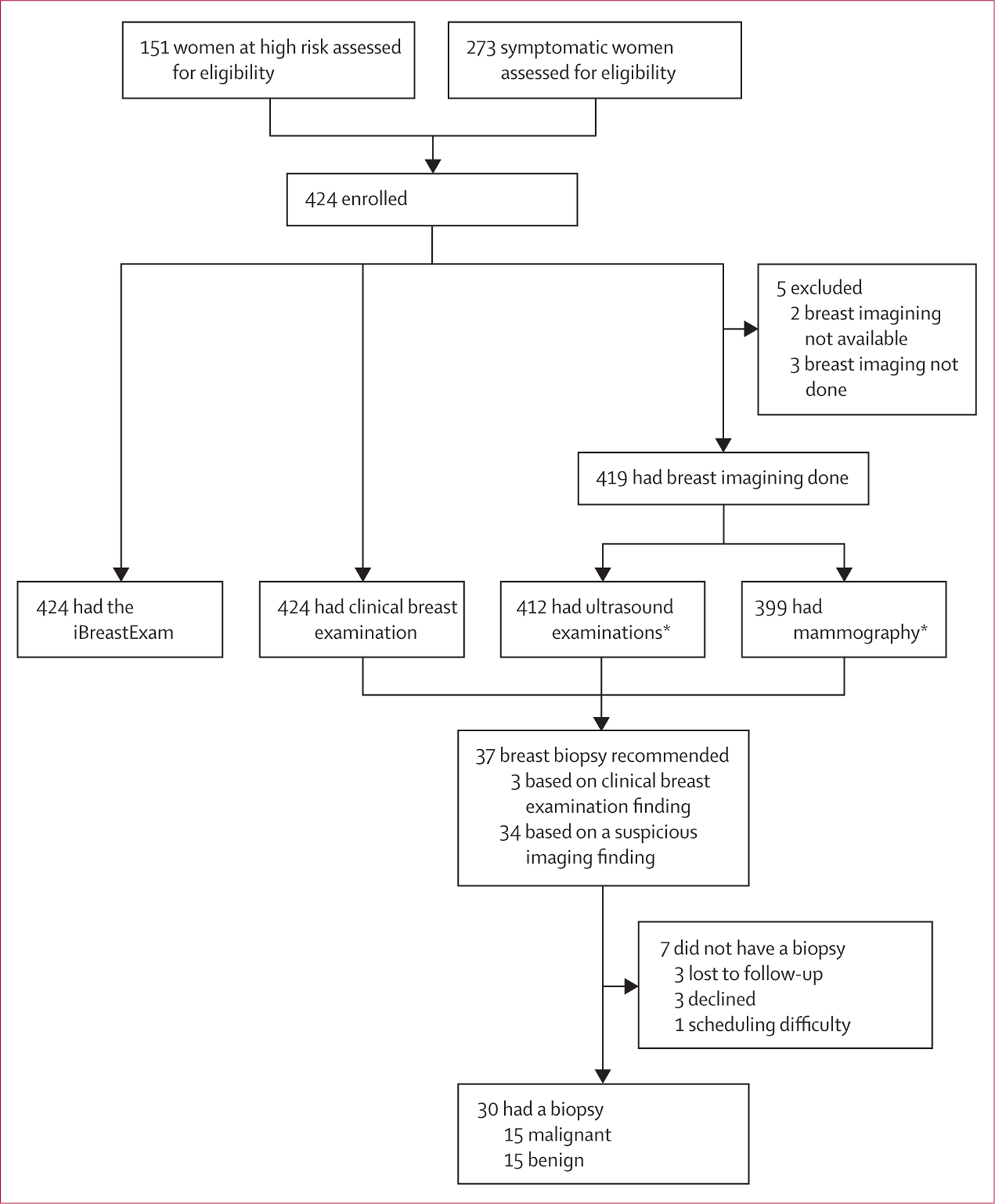
*Seven people excluded from ultrasound examinations and 20 from mammography because of logistical difficulties resulting in not being able to attend the imaging appointment.
All four examinations were performed in 392 (93%) of 424 participants. All 424 women had clinical breast examination and the iBreastExam, 412 (97%) had an ultrasound, and 401 (95%) had mammography. The average clinical breast examination took 2·7 min (range 1·0–10·0) and the iBreastExam took 6·2 min (3·0–20·0).
419 (99%) women had a BI-RADS assessment based on imaging (mammogram or ultrasound, or both). Five (1%) women without a BI-RADS category were excluded from the analysis because they did not have breast imaging (n=3) or mammography images were unavailable for radiologist review due to technical difficulty with transferring the images (n=2). The distribution of BI-RADS categories was as follows: three (1%) of 419 women were assigned BI-RADS 0, 381 (91%) assigned BI-RADS 1 or 2, three (1%) assigned BI-RADS 3, 15 (4%) assigned BI-RADS 4, and 17 (4%) assigned BI-RADS 5. Of three BI-RADS 0 women, one needed targeted ultrasound and two had additional mammography views, which were not performed because patients did not return for additional imaging. Thus, they were excluded from the analysis of suspicious findings, but considered positive in the assessment of any finding.
Biopsy was recommended for 37 (9%) of 424 women (five high risk and 32 symptomatic), but seven women did not receive biopsies despite recommendation (figure 3). 30 (81%) women underwent biopsy and half had a malignant finding; thus, 15 (4%) women had prevalent cancer (one [1%] of 151 at high risk and 14 [5%] of 273 symptomatic women). Of 14 symptomatic women, ten reported a lump, one had a lump and pain, two reported pain, and one had nipple discharge. Diagnoses included 11 invasive ductal carcinomas, one ductal carcinoma in situ, one papillary carcinoma, one metaplastic carcinoma, and one subtype unknown.
All diagnosed breast cancers had suspicious imaging findings. Average cancer size on imaging was 3·3 cm (range 1·3–7·0) larger than benign findings (2·2 cm, 0·4–4·2). Of 15 cancer biopsies, clinical breast examination and the iBreastExam detected an ipsilateral breast abnormality in 13 (87%) women (table 1) and missed the same two cancers (masses measuring 1·7 cm and 1·8 cm). Mammography identified 14 of 15 cancers and ultrasound identified 13 of 15.
Table 1:
Analysis of 15 diagnosed breast cancers by imaging features
| iBreastExam positive (n=13) | Clinical breast examination positive (n=13) | Mammogram positive (n=14) | Ultrasound positive (n=13) | |
|---|---|---|---|---|
| Mass alone (n=10 cancers) | 9/10 (90%) | 9/10 (90%) | 9/10 (90%) | 10/10 (100%) |
| Mass with calcifications (n=4 cancers) | 3/4 (75%) | 3/4 (75%) | 4/4 (100%) | 3/4 (75%) |
| Calcifications alone (n=1 cancer) | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | 0 |
Detected as a positive finding in the ipsilateral breast.
Clinical breast examination was positive in 84 (20%) of 424 women, whereas the iBreastExam was positive in 226 (53%). Clinical breast examination was positive in 75 (28%) of 273 symptomatic and nine (6%) of 151 high risk women. The iBreastExam was positive in 160 (59%) symptomatic women and 66 (44%) high risk women (appendix p 1). Sensitivities, specificities, NPV, and PPV for any breast finding and suspicious findings are shown in table 2.
Table 2:
The iBreastExam and clinical breast examination sensitivity, specificity, NPV, and PPV
| True positive | False negative | True negative | False positive | Sensitivity (95% CI) | Specificity (95% CI) | NPV (95% CI) | PPV (95%CI) | |
|---|---|---|---|---|---|---|---|---|
| Any breast finding | ||||||||
| The iBreastExam | 147 | 86 | 110 | 76 | 63% (57–69) | 59% (52–66) | 56% (49–63) | 66% (59–72) |
| Clinical breast examination | 71 | 162 | 175 | 11 | 31% (25–37) | 94% (90–97) | 52% (46–57) | 87% (77–93) |
| p value | ·· | ·· | ·· | ·· | <0·0001 | <0·0001 | 0·080 | <0·0001 |
| Suspicious breast finding | ||||||||
| The iBreastExam | 31 | 5 | 191 | 192 | 86% (70–95) | 50% (45–55) | 98% (94–99) | 14% (10–19) |
| Clinical breast examination | 30 | 6 | 331 | 52 | 83% (67–94) | 86% (83–90) | 98% (96–99) | 37% (26–48) |
| p value | ·· | ·· | ·· | ·· | 0·65 | <0·0001 | 0·42 | 0·0020 |
Findings were determined by breast imaging (n=419). NPV=negative predictive value. PPV=positive predictive value.
For any breast finding, the iBreastExam showed significantly better sensitivity than clinical breast examination (63%, 95% CI 57–69 vs 31%, 25–37; p<0·0001), and clinical breast examination showed significantly better specificity (94%, 90–97 vs 59%, 52–66; p<0·0001). For suspicious breast findings, the iBreastExam showed similar sensitivity to clinical breast examination (86%, 95% CI 70–95 vs 83%, 67–94; p=0·65), and clinical breast examination showed significantly better specificity (50%, 45–55 vs 86%, 83–90; p<0·0001). The iBreastExam and clinical breast examination showed similar NPVs for any breast finding (56%, 49–63 vs 52%, 46–57; p=0·080) and suspicious findings (98%, 94–99 vs 98%, 96–99; p=0·42), whereas the PPV was significantly higher for clinical breast examination in any breast finding (87%, 77–93 vs 66%, 59–72; p<0·0001) and suspicious findings (37%, 26–48 vs 14%, 10–19; p=0·0020).
Overall the iBreastExam accuracy was slightly higher than clinical breast examination for any breast finding (61%, 95% CI 57–66 vs 59%, 54–63), whereas for suspicious findings clinical breast examination was more accurate (86%, 83–89 vs 53%, 48–58). If the iBreastExam and clinical breast examination were used jointly and the finding was considered positive if either examination was positive, sensitivity for suspicious masses would improve to 92% (95% CI 77–98). However, specificity (49%, 44–54) and accuracy (53%, 48–58) of clinical breast examination and the iBreastExam combined is lower than clinical breast examination alone (appendix p 1). Results for high risk and symptomatic patient groups showed no significant difference in the iBreastExam (p=0·39) or clinical breast examination (p=0·27) performance when comparing to sensitivity and specificity.
After multiple comparison adjustment for the two kinds of discordance (the iBreastExam was negative and clinical breast examination was positive and vice versa) using the generalised estimating equation model, none of the covariates were significantly associated with the probability of discordance. Total agreement statistics were calculated at the quadrant level comparing the examinations for any finding and suspicious findings (table 3). Clinical breast examination showed better agreement with breast imaging (mammography and ultrasound) than the iBreastExam. The results from the generalised estimating equation indicated that none of the covariates considered in the model were significant at determining sensitivity and specificity for any breast finding. However, for suspicious findings, breast density was shown to have significant effect on specificity for the iBreastExam and clinical breast examination.
Table 3:
Total agreement statistics calculated by quadrant
| Any breast finding | Suspicious breast findings | |
|---|---|---|
| The iBreastExam versus clinical breast examination* | 88% (3748/4240) | NA |
| The iBreastExam versus mammography | 80% (3180/3990) | 88% (3515/3990) |
| The iBreastExam versus ultrasound | 87% (3584/4120) | 88% (3628/4120) |
| Clinical breast examination versus mammography | 87% (3451/3990) | 98% (3899/3990) |
| Clinical breast examination versus ultrasound | 96% (3941/4120) | 98% (4025/4120) |
NA=not applicable.
The iBreastExam and clinical breast examination did not differentiate between any and suspicious breast findings; thus, there is no agreement calculation comparing these two exams.
Considering that other clinical, pathological, and device-related factors such as age, lesion size, depth, and device upgrade could also play a role in determining sensitivity and specificity, we explored possible associations. Clinical breast examination and the iBreastExam sensitivities decreased for smaller lesions (≤2 cm) compared with larger lesions (>2 cm). For clinical breast examination, sensitivity decreased from 26% to 8% (p=0·021) and for the iBreastExam from 42% to 29% (p=0·093; appendix p 1). For clinical breast examination and the iBreastExam, specificities increased slightly when evaluating smaller lesions (from 95% to 96%; p=0·77 vs from 83% to 87%; p=0·59), although the differences were not statistically significant. Lesion depth did not significantly affect the iBreastExam or clinical breast examination performance (p=0·99 for both).
The iBreastExam and clinical breast examination showed significantly better specificity for suspicious masses in non-dense breasts than in dense breasts (appendix p 1). The effect of patient age (≤50 years or >50 years) had no significant difference in sensitivity and specificity in terms of performance for the detection of suspicious breast lesions with clinical breast examination (p=0·34) or the iBreastExam (p=0·79; appendix p 2).
The iBreastExam was upgraded during our study, with 192 studies conducted before the upgrade and 232 studies after; thus, the results were compared for these two groups. A decrease in false-positive results for suspicious findings was noted after the device upgrade (p<0·0001; appendix p 2).
All women completed an iBreastExam survey and 414 (98%) rated their experience from good to excellent, 414 (98%) reported a painless examination, and 406 (96%) reported a willingness to have an annual iBreastExam.
Discussion
In Nigeria, breast cancer evaluation in symptomatic women is scarce. Many women do not have access to imaging or high quality clinical breast examination. Our goal is to promote breast cancer detection in low-resource settings with highly mobile, scalable technology that requires minimal training. We designed a prospective study comparing a novel handheld breast device (iBreastExam) with clinical breast examination and used imaging as a reference standard. The iBreastExam was performed by recent nursing school graduates and showed significantly better sensitivity, but lower specificity, for detecting any breast finding compared with clinical breast examination performed by an experienced surgeon. For suspicious breast findings, higher iBreastExam sensitivity compared with clinical breast examination was not statistically significant and specificity was significantly lower. Although the non-inferiority of the iBreastExam to detect suspicious findings is an important result, the types of findings studied need to be distinguished. The detection of suspicious breast findings is crucial for a cancer diagnosis, whereas the detection of benign findings uses scarce recourses for diagnostic evaluation without benefiting patients.
The iBreastExam and clinical breast examination showed high NPV (98% for both) for suspicious findings confirming previous iBreastExam studies that have reported an NPV of 94–98%.19,22 These results are important in the context of the device’s intended use (ie, a low-resource setting) to enable community health workers to determine which patient should undergo additional evaluation. The ability to trust a negative result at the expense of lower specificity is important to avoid missing cancers; however, too low a specificity could generate excessive cost burden and strain under-resourced health-care systems.
We found that the iBreastExam positivity was greater than previously reported;23 however, given that 64% of our population was symptomatic, a higher positivity rate is not unexpected. For clinical use, higher iBreastExam positivity might be more acceptable in evaluating symptomatic women than screening asymptomatic women. The device upgrade during our study simplified calibration and decreased the number of false positive cases. After our study was finished, a newer version of the iBreastExam device became available, which auto-calibrates and might further improve specificity. Improved access to breast evaluation with the iBreastExam will need to outweigh the evaluation of false positives triggered by its use.
In low-income and middle-income countries, community health workers are an affordable and accessible health-care resource, often more readily available in the community than nurses and physicians. Low cost, user friendly technology, such as the iBreastExam, could possibly equip community health workers with minimal training to perform standardised breast examinations for the evaluation of symptomatic women. The iBreastExam could be combined with clinical history and clinical breast examination in a community setting to triage patients to determine who warrants further diagnostic evaluation. We found that if the iBreastExam and clinical breast examination were used jointly, sensitivity for suspicious masses would improve, but specificity would decrease. Further studies are warranted to determine whether the combination of these examinations with device modifications would improve specificity, particularly in asymptomatic women.
The iBreastExam and clinical breast examination showed better sensitivity and specificity for suspicious masses in non-dense breasts compared with dense breasts. Greater breast density increases breast cancer risk and lowers mammography sensitivity.27 Based on our study, the iBreastExam performs better in women with non-dense breasts. However, Xu and colleagues24 reported that the iBreastExam results were not influenced by breast density. Dense breasts are more common in young women. We found the iBreastExam and clinical breast examination showed no significant difference between women aged 50 years or younger and those older than 50 years; thus, further studies are warranted.
Given that 80% of breast cancers in Nigeria present as stage 3 or 4, and the mean breast tumour size at presentation to the OAUTHC is 10·5 cm,28 detection of masses smaller than 5 cm can result in cancer being diagnosed at an earlier and more treatable stage. For clinical breast examination and the iBreastExam, sensitivities decreased when evaluating smaller lesions (≤2 cm), which was not statistically significant for the iBreastExam. Similarly, previous studies19,21 reported that cancers missed by the iBreastExam were small (≤1·0 cm).
The iBreastExam took longer to perform than clinical breast examination, but examination length was reasonable. Nurses were iBreastExam trained after 30 cases, a practical number in comparison with clinical breast examination which might require more cases to establish competency. Patient acceptability of the iBreastExam was excellent, an important consideration for widespread applicability.
Limitations of our study include patient self-selection, as some patients were responding to media recruitment which could lead to a biased study population. Additionally, imaging is an imperfect reference standard for the true presence or absence of breast cancer and therefore, we cannot determine the sensitivity and specificity for cancer detection, we could only determine the sensitivity and specificity for detecting breast lesions as defined by imaging. Unfortunately, seven women had biopsy recommended but not performed, which limits the evaluation. In real-world clinical practice the iBreastExam cannot differentiate between women with any finding versus suspicious findings. Finally, our study did not examine use of the iBreastExam by community health workers or clinical breast examination and the iBreastExam by similarly trained workers. Additional investigation is needed to assess the cost placed on the health-care system at different performance variables.
In conclusion, the iBreastExam by recent nursing school graduates has shown a high NPV and sensitivity for identifying women with suspicious breast lesions, as defined by imaging, but lower specificity than a surgeon’s clinical breast examination. In geographical locations where clinical breast examination by experienced practitioners is unavailable, the iBreastExam might fill this gap by providing a high sensitivity breast evaluation tool in the community health setting. Whether low specificity can be further improved with device updates and whether the cost of evaluating positive iBreastExam findings is feasible in low-resource settings remains to be determined.
Supplementary Material
Research in context.
Evidence before this study
Breast cancer incidence is increasing worldwide and disproportionately affects women in low-income countries, such as Nigeria, with increasing late-stage disease presentation and higher mortality rates than in high-income countries. Nigeria has scarce availability and access to breast cancer screening, diagnostic services, and treatment. Clinical breast examination is the primary breast cancer detection method; however, it shows a wide sensitivity range (22–85%). Given the limitations of clinical breast examination, women in low-resource countries could benefit from a portable, low cost, easy to use alternative for early detection of breast cancer. The intelligent breast examination (iBreastExam; UE Life Sciences, Philadelphia, PA) is a 510(k) FDA-cleared device that uses sensors to electronically palpate the breast and evaluate tissue elasticity by making capacitive measurements and quantifying variations in tissue stiffness. Initial iBreastExam studies report a high sensitivity (84–87%), specificity (80–94%), and negative predictive value (94–98%). The iBreastExam studies to date were done at academic centres in the USA and India. We searched PubMed, Web of Science, and Scopus using the terms (“iBE” OR “iBreastExam”) AND (“Breast cancer”) AND (“LMIC” OR “Nigeria” OR “Africa” OR “West Africa”), with no date or language restrictions. We did not find any published trials of the iBreastExam in Africa or rigorous blinded trials comparing the iBreastExam with clinical breast examination, mammography, and ultrasound using clearly defined imaging protocols.
Added value of this study
Our study of the iBreastExam is the first in Africa, specifically Nigeria. We designed a rigorous prospective evaluation of the novel handheld iBreastExam device compared with clinical breast examination, using imaging as a reference standard, in high risk and symptomatic Nigerian women. The iBreastExam performed by recent nursing school graduates (ie, those who finished nursing school within the previous year) showed significantly better sensitivity, but lower specificity, for detecting any breast finding compared with clinical breast examination performed by experienced surgeons. For suspicious breast findings, higher sensitivity of the iBreastExam was not statistically significant and specificity remained significantly lower. Although, specificity improved with an upgrade of the iBreastExam device. We provide detailed and clear descriptions of the imaging parameters used, outcome definitions, and high quality evidence to guide future investigations.
Implications of all the available evidence
The high sensitivity and negative predictive value of the iBreastExam for any breast finding is promising; however, the low specificity needs to be addressed before making recommendations for clinical use. In geographical locations with few experienced practitioners to provide clinical breast examination, the iBreastExam might provide a high sensitivity breast evaluation tool in a community health setting. This study highlights the need for device adaptation and provides the foundation to guide future studies on the iBreastExam use by community health-care workers and cost analysis on the basis of performance in low-resource settings.
Acknowledgments
This study was funded by the Prevent Cancer Foundation Global Community Grant Award with additional support from the P30 Cancer Center Support Grant (P30 CA008748). UE LifeSciences provided the iBreastExam device and training and did not participate in the data analysis or manuscript writing. We thank Adedamola Nurat Adeyanju, Olamide Oluwadamilola Fayenuwo, Aanuoluwapo Olaitan, Katung Aba, Adeniyi Aderibigbe, Eze Okereke, Marguerite Samson, Elizabeth Morris, Murray Brennan, and Hedvig Hricak. We thank all resident doctors in the Department of Surgery at the Obafemi Awolowo University Teaching Hospitals Complex (Ife, Nigeria), Life Builders (Ibadan, Nigeria), and Lifefount Hospital (Ilorin, Nigeria). We also thank Alyssa Duck (medical editor; Memorial Sloan Kettering Cancer Center) for editorial assistance with final drafts of the manuscript. Opinions expressed by the authors in this Article are their own and should not be interpreted as representing the official viewpoint of the US Department of Health and Human Services, National Institutes of Health, or the National Cancer Institute.
Funding
Prevent Cancer Foundation Global Community Grant Award with additional support from the P30 Cancer Center Support Grant (P30 CA008748).
Footnotes
Declaration of interests
VLM reports consultant fees from Bayer Healthcare and Koios Medical, and a research grant from Pfizer, outside of the submitted work. OO and ADO report research grants from Pfizer, outside of the submitted work. All other authors declare no competing interests.
Data sharing
Anonymised participant data can be made available upon request to the corresponding author between 9 months and 36 months after publication. Proposals will be reviewed on the basis of scientific merit. After approval of a proposal, data can be shared via a secure online platform after signing a data access agreement.
For more on the iBreastExam see https://www.ibreastexam.com/
See Online for appendix
Contributor Information
Victoria L Mango, Breast Imaging Service, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Olalekan Olasehinde, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
Adeleye D Omisore, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
Funmilola O Wuraola, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
Olusola C Famurewa, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
Varadan Sevilimedu, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Gregory C Knapp, Department of Surgery, Dalhousie University, Halifax, NS, Canada.
Evan Steinberg, Department of Epidemiology and Biostatistics, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Promise R Akinmaye, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
Boluwatife D Adewoyin, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
Anya Romanoff, Department of Global Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Philip E Castle, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Bethesda, MD, USA.
Olusegun Alatise, Obafemi Awolowo University Teaching Hospital Complex, Ile-Ife, Nigeria.
T Peter Kingham, Department of Surgery, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
References
- 1.WHO International Agency for Research on Cancer. Population fact sheets Nigeria. 2020. https://gco.iarc.fr/today/data/factsheets/populations/566-nigeria-fact-sheets.pdf (accessed Nov 20, 2021). [Google Scholar]
- 2.Azubuike SO, Muirhead C, Hayes L, McNally R. Rising global burden of breast cancer: the case of sub-Saharan Africa (with emphasis on Nigeria) and implications for regional development: a review. World J Surg Oncol 2018; 16: 63–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akinde OR, Phillips AA, Oguntunde OA, Afolayan OM. Cancer mortality pattern in lagos university teaching hospital, lagos, Nigeria. J Cancer Epidemiol 2015; 2015: 842032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olasehinde O, Alatise OI, Arowolo OA, et al. Barriers to mammography screening in Nigeria: a survey of two communities with different access to screening facilities. Eur J Cancer Care (Engl) 2019; 28: e12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adesunkanmi ARK, Lawal OO, Adelusola KA, Durosimi MA. The severity, outcome and challenges of breast cancer in Nigeria. Breast 2006; 15: 399–409. [DOI] [PubMed] [Google Scholar]
- 6.Tabár L, Fagerberg CJ, Gad A, et al. Reduction in mortality from breast cancer after mass screening with mammography. Randomised trial from the Breast Cancer Screening Working Group of the Swedish National Board of Health and Welfare. Lancet 1985; 1: 829–32. [DOI] [PubMed] [Google Scholar]
- 7.Andersson I, Aspegren K, Janzon L, et al. Mammographic screening and mortality from breast cancer: the Malmö mammographic screening trial. BMJ 1988; 297: 943–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shapiro S, Strax P, Venet L. Periodic breast cancer screening in reducing mortality from breast cancer. JAMA 1971; 215: 1777–85. [PubMed] [Google Scholar]
- 9.Smith RA, Duffy SW, Gabe R, Tabar L, Yen AM, Chen TH. The randomized trials of breast cancer screening: what have we learned? Radiol Clin North Am 2004; 42: 793–806. [DOI] [PubMed] [Google Scholar]
- 10.Sood R, Rositch AF, Shakoor D, et al. Ultrasound for breast cancer detection globally: a systematic review and meta-analysis. J Glob Oncol 2019; 5: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans A, Trimboli RM, Athanasiou A, et al. Breast ultrasound: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging 2018; 9: 449–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alba LH, Díaz S, Gamboa O, et al. Accuracy of mammography and clinical breast examination in the implementation of breast cancer screening programs in Colombia. Prev Med 2018; 115: 19–25. [DOI] [PubMed] [Google Scholar]
- 13.Yip CH, Smith RA, Anderson BO, et al. Guideline implementation for breast healthcare in low- and middle-income countries: early detection resource allocation. Cancer 2008; 113 (suppl): 2244–56. [DOI] [PubMed] [Google Scholar]
- 14.Mittra I, Mishra GA, Dikshit RP, et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. BMJ 2021; 372: n256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fenton JJ, Barton MB, Geiger AM, et al. Screening clinical breast examination: how often does it miss lethal breast cancer? J Natl Cancer Inst Monogr 2005; 35: 67–71. [DOI] [PubMed] [Google Scholar]
- 16.Malmartel A, Tron A, Caulliez S. Accuracy of clinical breast examination’s abnormalities for breast cancer screening: cross-sectional study. Eur J Obstet Gynecol Reprod Biol 2019; 237: 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Veitch D, Goossens R, Owen H, Veitch J, Molenbroek J, Bochner M. Evaluation of conventional training in Clinical Breast Examination (CBE). Work 2019; 62: 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food & Drug Administration. 510(k) premarket notification 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K142926 (accessed March 5, 2021).
- 19.Clanahan JM, Reddy S, Broach RB, et al. Clinical utility of a hand-held scanner for breast cancer early detection and patient triage. JCO Glob Oncol 2020; 6: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu X, Gifford-Hollingsworth C, Sensenig R, Shih WH, Shih WY, Brooks AD. Breast tumor detection using piezoelectric fingers: first clinical report. J Am Coll Surg 2013; 216: 1168–73. [DOI] [PubMed] [Google Scholar]
- 21.Broach RB, Geha R, Englander BS, DeLaCruz L, Thrash H, Brooks AD. A cost-effective handheld breast scanner for use in low-resource environments: a validation study. World J Surg Oncol 2016; 14: 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Somashekhar S, Vijay R, Ananthasivan R, Prasanna G. Nonivasive and low-cost technique for early detection of clinically relevant breast lesions using a handheld point-of-care medical device (iBreastExam): prospective three-arm triple-blinded comparative study. Indian J Gynecol Oncol 2016; 14: 26. [Google Scholar]
- 23.Khandelwal R Health worker led breast examination in rural India using electro-mechanical hand-held breast palpation device. J Cancer Prev Curr Res 2018; 9: 138–41. [Google Scholar]
- 24.Xu X, Chung Y, Brooks AD, Shih WH, Shih WY. Development of array piezoelectric fingers towards in vivo breast tumor detection. Rev Sci Instrum 2016; 87: 124301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Radiology. ACR BI-RADS atlas, breast imaging reporting and data system 2013. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Bi-Rads (accessed Feb 1, 2022).
- 26.Højsgaard S, Halekoh U, Yan J. The R package geepack for generalized estimating equations. J Stat Softw 2006; 15: 1–11. [Google Scholar]
- 27.Nazari SS, Mukherjee P. An overview of mammographic density and its association with breast cancer. Breast Cancer 2018; 25: 259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olasehinde O, Alatise O, Omisore A, et al. Contemporary management of breast cancer in Nigeria: insights from an institutional database. Int J Cancer 2021; 148: 2906–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


