Abstract
Simple Summary
Ovarian cancer was the third most common gynecological cancer globally in 2020. Ovarian carcinoma is the most common type of ovarian cancer, comprising over 90% of all ovarian cancer cases. The risk of ovarian cancer increases in females with age, along with having a family history, having a family cancer syndrome, and breast cancer susceptibility gene (BRCA) mutations. Investigation of the latest disease burden, risk factors, and temporal trends of ovarian cancer is important for the reduction of its associated mortality globally. The global incidence and mortality rates of ovarian cancer for 185 countries in 2020 were retrieved from the Global Cancer Observatory (GLOBOCAN) database established by the International Agency for Research on Cancer (IARC, WHO, Lyon, France). The incidence of ovarian cancer has been increasing substantially among younger females, probably caused by the increasing prevalence of obesity, metabolic syndrome, estrogen exposure and nulliparity.
Abstract
This study aimed to investigate the most updated worldwide incidence and mortality, risk factors, and epidemiologic trend of ovarian cancer in different countries, regions, and age groups. The Global Cancer Observatory database was used for incidence and mortality rates of ovarian cancer in 2020. Data from Cancer Incidence in Five Continents and the WHO mortality database was accessed for trend analysis. Age-standardized rates (ASRs, per 100,000 persons) were calculated for incidence and mortality. The 10-year annual average percent change (AAPC) was estimated by Joinpoint regression analysis. There was an overall decreasing trend of ovarian cancer, yet its burden has been increasing in lower-income countries and among younger females in some countries. Intensive lifestyle modifications are warranted, especially for the populations at high risk for ovarian cancer, including smoking cessation, alcohol use reduction, physical activity, weight control, and treatment of metabolic diseases.
Keywords: incidence, mortality, risk factors, temporal trends, ovarian cancer
1. Introduction
Ovarian cancer was the third most common gynecological cancer globally in 2020 [1]. Ovarian carcinoma is the most common type of ovarian cancer, comprising over 90% of all ovarian cancer cases. There are five main histological types of ovarian carcinoma, of which high-grade serous carcinoma is the most common [2]. Ovarian cancer is often diagnosed at a late stage, making this malignancy the most lethal gynecological cancer. The prognosis of ovarian cancer is usually poor, with a 5-year survival rate of only 17% for a patient at an advanced stage [3]. Therefore, it is imperative to understand the etiology of ovarian cancer and identify its risk factors and populations at high risk in order to prevent this disease.
The risk of ovarian cancer increases in females with age, along with having a family history, having a family cancer syndrome, and breast cancer susceptibility gene (BRCA) mutations [4]. The reproductive-related risk factors for ovarian cancer include having children later or never having a full-term pregnancy, reaching menopause at an older age, and taking hormone therapy after menopause [5]. Other common modifiable lifestyle and metabolic risk factors include smoking, alcohol use, physical inactivity, unhealthy diet, obesity, and diabetes [6]. A comprehensive understanding of the disease burden attributable to the preventable risk factors for ovarian cancer is particularly important in the development of prevention and treatment strategies.
Investigation of the latest disease burden, risk factors, and temporal trends of ovarian cancer is important for the reduction of its associated mortality globally. However, previous studies have mainly focused on single countries, or have used relatively old data [7,8,9]. The associations between common modifiable risk factors and ovarian cancer burden have not been comprehensively examined at a country level. This study aimed to investigate the most updated worldwide incidence and mortality, risk factors, and epidemiologic trend of ovarian cancer in different countries, regions, and age groups.
2. Methods
2.1. Data Source
The global incidence and mortality rates of ovarian cancer for 185 countries in 2020 were retrieved from the Global Cancer Observatory (GLOBOCAN) database established by the International Agency for Research on Cancer (IARC, WHO) [1,10,11]. The gross domestic products (GDP) per capita and Human Development Index (HDI) were derived from the World Bank and United Nations, respectively. The Global Health Data Exchange (GHDx) [12] database was retrieved for the prevalence of smoking, alcohol use, unhealthy diet, physical inactivity, hypertension, diabetes, and lipid disorders in each country. Figures on the yearly incidence of ovarian cancer were collected from the Cancer Incidence in Five Continents I-X plus (CI5Plus) [13] for trend analysis. The CI5 is a global cancer database where the age and sex-specific cancer incidence are available for different countries to facilitate direct comparison between different countries [13]. Figures on mortality due to ovarian cancer were retrieved from the WHO Mortality Database [14]. The Nordic Cancer Registries (NORDCAN) [15,16] and the Surveillance, Epidemiology, and End Results (SEER) [17] were also accessed to obtain the most updated incidence and mortality of ovarian cancer for Northern European countries and the United States. A total of 48 countries/economies included for trend analysis were grouped into Asia, Oceania, North America, South America, Northern Europe, Western Europe, Southern Europe, Eastern Europe, and Africa. To confirm the data were correctly categorized, all extracted data of incidence and mortality rates for ovarian cancer in this study followed the International Classification of Disease and Related Health Problems’ 10th revision’s codes of ovarian cancer: “Malignant neoplasm of the ovary” (ICD-10, C56) [18]. To compare the data between different populations, all incidence and mortality rates were calculated using the age-standardized rates (ASRs) from the Segi–Doll standard population [19].
2.2. Statistical Analysis
Choropleth maps were plotted to present the global incidence and mortality of ovarian cancer. Associations between GDP per capita, HDI, the prevalence of smoking, alcohol use, unhealthy diet, physical inactivity, hypertension, diabetes, and lipid disorders and the burden of ovarian cancer were examined by univariable linear regression analysis. Beta coefficients (β) and the corresponding 95% confidence intervals (CI) were calculated from the regression. The β estimates refer to the degree of change in ASR of incidence or mortality of ovarian cancer per unit increase in the risk factors. For trend analysis of incidence and mortality rates for ovarian cancer, the data from the past 10 years were analyzed by joinpoint regression [20]. The ASRs and corresponding standard errors were transformed with the natural logarithm and calculated with binomial approximation. The annual percentage change (APC) was calculated by transforming ASRs and related SEs with joinpoint regression as percentage change rate by the assumption of a constant rate during the time interval [21]. For the calculation of the annual average percentage change (AAPC), the changepoint value was determined for the software to break the entire time interval. Each segment was weighted by the entire time interval proportion length. A maximum of one Joinpoint was used in the analysis. The temporal estimation outlined the incidence and mortality rates for ovarian cancer in the past decade. The AAPC was estimated to figure out the trend direction and degree by considering the slope and weighting of each segment. A positive value of AAPC indicated the upward inclination of the trend, and the significance of the trend was determined by its 95% C.I. (the trend was not significant if the 95% C.I. overlapped with zero) [21]. The epidemiological trends of ovarian cancer were analyzed in females aged 0–85+ years, and also in different age groups (≥50 years, <50 years, and <40 years). All p-values ≤ 0.05 were regarded as statistically significant.
3. Results
3.1. Global Incidence of Ovarian Cancer in 2020
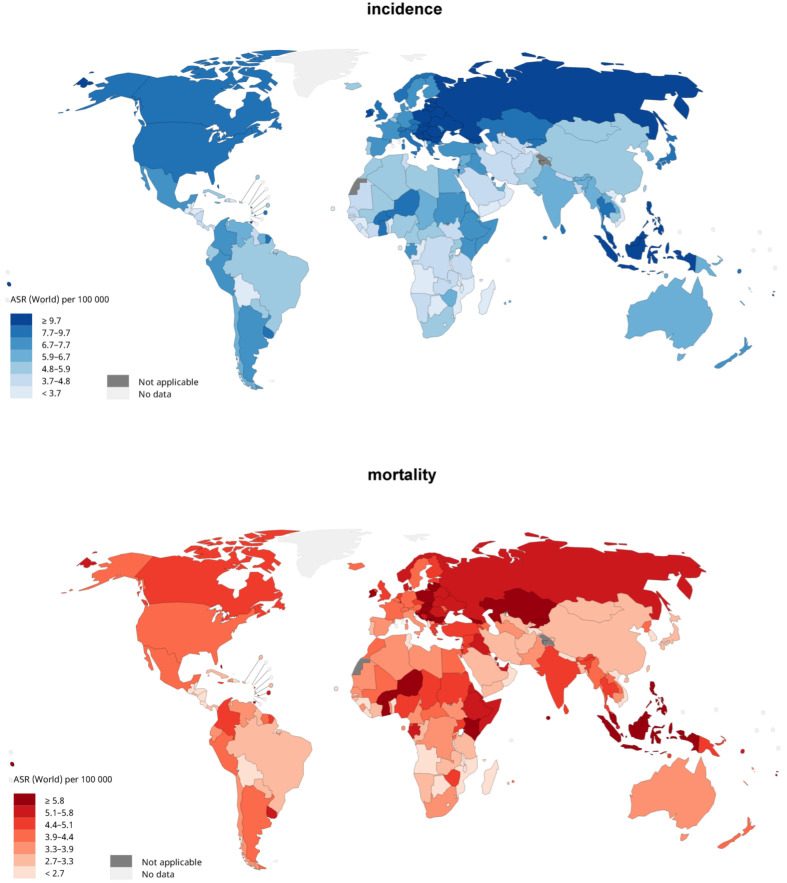
In 2020, a total of 313,959 new cases of ovarian cancer were recorded globally, with an ASR incidence of 6.6 per 100,000 (Figure 1). The highest incidence was found in Central and Eastern Europe (ASR = 10.7), followed by Northern Europe (ASR = 8.8), Polynesia (ASR = 8.8), North America (ASR = 8.1), and South East Asia (ASR = 8.1). The lowest incidence was observed in Central Africa (ASR = 4.4), the Caribbean (ASR = 4.6), and Southern Africa (ASR = 4.9). The highest incidence of ovarian cancer was observed in countries with a high-income level (ASR = 8.0), followed by countries with an upper–middle-income (ASR = 6.3), low–middle-income (ASR = 6.1), and low income (ASR = 5.3) levels.
Figure 1.
Global incidence and mortality of ovarian cancer, all ages, in 2020; Data source: https://gco.iarc.fr/today (accessed on 10 May 2021).
3.2. Global Mortality of Ovarian Cancer in 2020
In 2020, a total of 207,252 new deaths due to ovarian cancer were reported globally, with an ASR mortality of 4.2 per 100,000. The highest mortality was observed in Micronesia (ASR = 7.3), followed by Polynesia (ASR = 6.6), Central and Eastern Europe (ASR = 5.6), South East Asia (ASR = 5.2), and Melanesia (ASR = 5.2). The lowest mortality was observed in the Caribbean (ASR = 3.2), East Asia (ASR = 3.3), and Southern Africa (ASR = 3.3). The highest mortality was found in countries with a low–middle-income level (ASR = 4.3), followed by countries with high-income level (ASR = 4.1), low-income level (ASR = 4.1), and upper–middle-income level (ASR = 3.9).
3.3. Associations between Risk Factors and Incidence
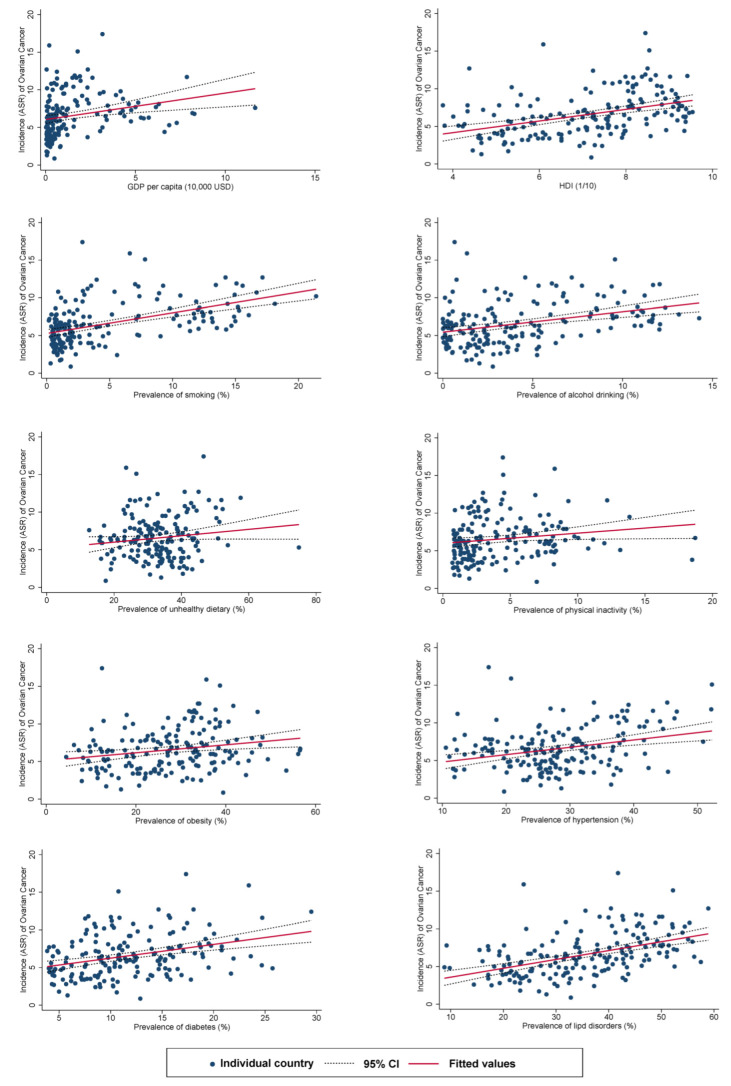
The associations between risk factors and the incidence of ovarian cancer are presented in Figure 2. Higher ASRs in the incidence of ovarian cancer was associated with a higher GDP per capita (β = 0.35, 95% C.I. 0.14 to 0.56, p = 0.001), HDI (β = 0.77, 95% C.I. 0.52 to 1.03, p < 0.001), and a higher prevalence of smoking (β = 0.27, 95% C.I. 0.20 to 0.35, p < 0.001), alcohol use (β = 0.27, 95% C.I. 0.16 to 0.38, p < 0.001), physical inactivity (β = 0.14, 95% C.I. 0.01 to 0.26, p = 0.036), obesity (β = 0.05, 95% C.I. 0.02 to 0.09, p = 0.005), hypertension (β = 0.10, 95% C.I. 0.05 to 0.14, p < 0.001), diabetes (β = 0.18, 95% C.I. 0.11 to 0.26, p < 0.001), and lipid disorders (β = 0.12, 95% C.I. 0.09 to 0.15, p < 0.001).
Figure 2.
Associations between risk factors and the incidence of ovarian cancer.
3.4. Associations between Risk Factors and Mortality
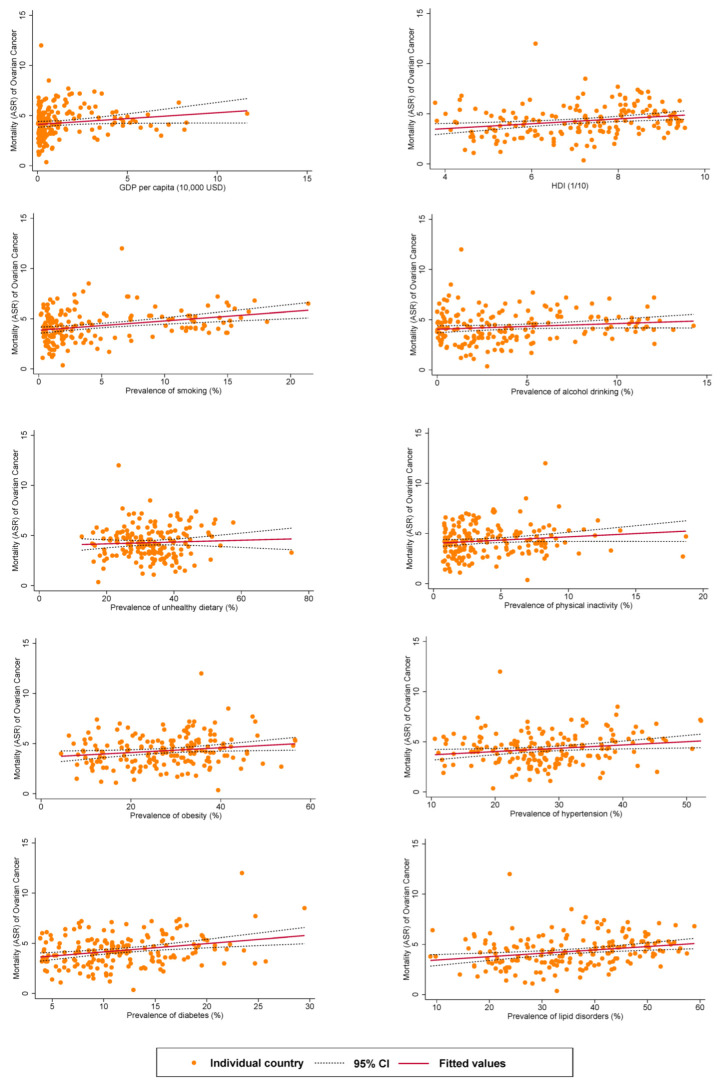
The associations between risk factors and mortality related to ovarian cancer are presented in Figure 3. Higher ASRs of mortality of ovarian cancer was associated with a higher HDI (β = 0.24, 95% C.I. 0.09 to 0.39, p < 0.001), and higher prevalence of smoking (β = 0.09, 95% C.I. 0.05 to 0.14, p < 0.001), obesity (β = 0.02, 95% C.I. 0.003 to 0.04, p = 0.023), hypertension (β = 0.03, 95% C.I. 0.01 to 0.06, p = 0.015), diabetes (β = 0.08, 95% C.I. 0.04 to 0.13, p < 0.001), and lipid disorders (β = 0.03, 95% C.I. 0.01 to 0.05, p = 0.001).
Figure 3.
Associations between risk factors and mortality of ovarian cancer.
3.5. Temporal Trends of Ovarian Cancer
The incidence and mortality trends of ovarian cancer between 1980 to 2018 are displayed in Figure S1. The joinpoint regression analysis results are displayed in Figure S2. Overall, there was a decreasing trend in the incidence and mortality rates of ovarian cancer globally. However, a substantial increase in incidence of ovarian cancer was observed in younger females.
3.6. Overall Incidence Trends of Ovarian Cancer
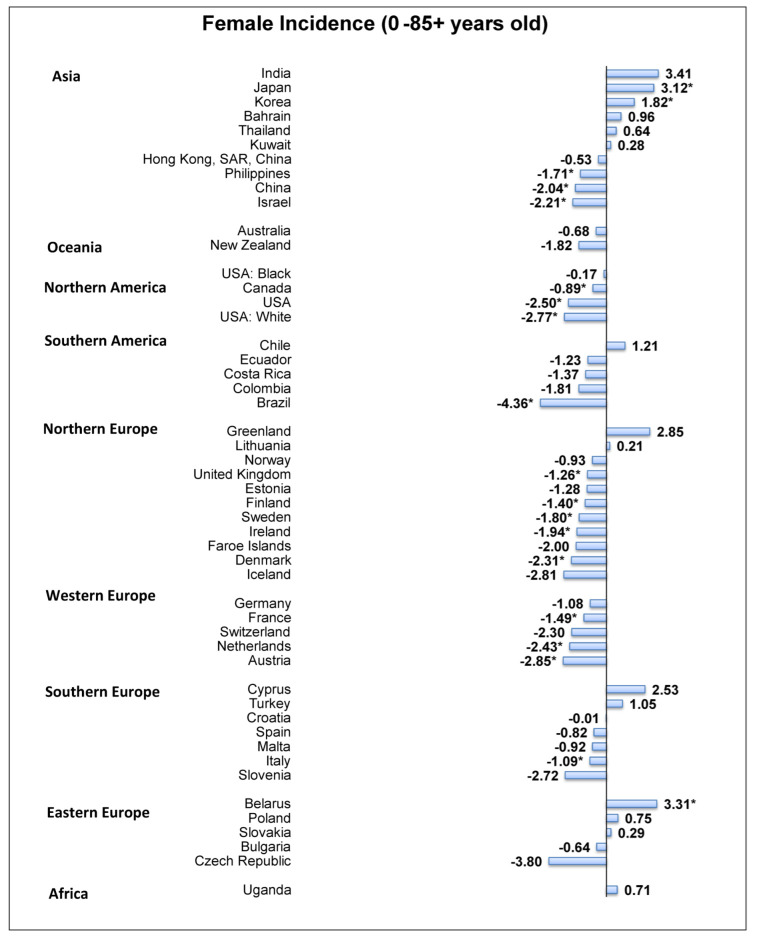
Of the 48 countries, 17 showed a decreased trend of ovarian cancer incidence from the past ten years up to 2018 (Figure 4). Among these countries, the lowest AAPC was from Brazil (AAPC = −4.36, 95% C.I. −6.37 to −2.20, p = 0.002) followed by the Czech Republic (AAPC = −3.80, 95% C.I. −5.86 to −1.70, p < 0.001), Austria (AAPC = −2.85, 95% C.I. −3.92 to −1.76, p < 0.001), the United States (AAPC = −2.50, 95% C.I. −3.45 to −1.55, p < 0.001), and the Netherlands (AAPC = −2.43, 95% C.I. −3.93 to −0.91, p = 0.006). By contrast, three countries had an increase in ovarian cancer incidence, including Belarus (AAPC = 3.31, 95% C.I. 2.38 to 4.26, p < 0.001), Japan (AAPC = 3.12, 95% C.I. 2.12 to 4.13, p < 0.001), and Korea (AAPC = 1.82, 95% C.I. 0.50 to 3.16, p = 0.013).
Figure 4.
AAPC of incidence of ovarian cancer aged 0–85+. * Statistically significant.
3.7. Overall Mortality Trends of Ovarian Cancer
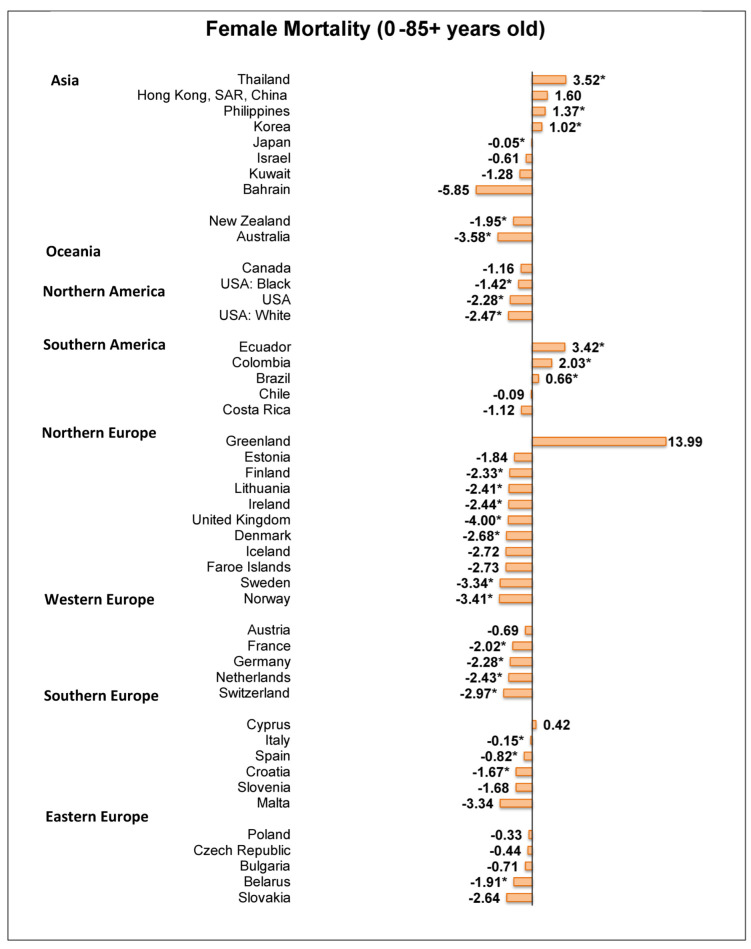
Of the 48 countries, 21 showed a decrease in the mortality of ovarian cancer (Figure 5). Among these countries, the lowest AAPC was from Australia (AAPC = −3.58, 95% C.I. −5.28 to −1.86, p < 0.001), followed by Norway (AAPC = −3.41, 95% C.I −4.42 to −0.24, p < 0.001), Sweden (AAPC = −3.34, 95% C.I −5.26 to −1.38, p = 0.005), Switzerland (AAPC = −2.97, 95% C.I. −4.52 to −1.39, p = 0.003), and Denmark (AAPC = −2.68, 95% C.I. −4.43 to −0.89, p = 0.009). By contrast, six countries had an increase in mortality due to ovarian cancer, including Thailand (AAPC = 3.52, 95% C.I. 2.77 to 4.27, p < 0.001), Ecuador (AAPC = 3.42, 95% C.I. 1.41 to 5.46, p = 0.004), Colombia (AAPC = 2.03, 95% C.I. 1.30 to 2.76, p < 0.001), the Philippines (AAPC = 1.37, 95% C.I. 0.71 to 2.03, p = 0.001), Korea (AAPC = 1.02, 95% C.I. 0.10 to 1.95, p = 0.033), and Brazil (AAPC = 0.66, 95% C.I. 0.19 to 1.13, p = 0.011).
Figure 5.
AAPC of mortality of ovarian cancer aged 085+. * Statistically significant.
3.8. Incidence Trends of Ovarian Cancer in Females Aged ≥ 50
In the 48 countries, 21 showed a decrease in incidence of ovarian cancer (Figure S3), with Brazil having highest decline in incidence (AAPC = −4.02, 95% C.I. −6.56 to −1.41, p = 0.008), followed by Slovenia (AAPC = −3.86, 95% C.I. −6.51 to −1.14, p = 0.012), the United States (AAPC = −3.01, 95% C.I. −4.23 to −2.22, p < 0.001), Switzerland (AAPC = −2.93, 95% C.I. −5.36 to −0.44, p = 0.027), and Austria (AAPC = −2.60, 95% C.I. −3.41 to −1.78, p < 0.001). On the contrary, only two countries had an increase in the incidence of ovarian cancer, including Japan (AAPC = 2.35, 95% C.I. 1.15 to 3.55, p = 0.002), and Belarus (AAPC = 1.88, 95% C.I. 1.21 to 2.54, p < 0.001).
3.9. Incidence Trends of Ovarian Cancer in Females Aged < 50
The decreasing trend was much less evident in females aged < 50 (Figure S4). Only four countries demonstrated a decrease in the incidence of ovarian cancer, including the Czech Republic (AAPC = −6.47, 95% C.I. −9.10 to −3.77, p < 0.001), Brazil (AAPC = −5.18, 95% C.I. −7.97 to −2.30, p = 0.003), Austria (AAPC = −3.43, 95% C.I. −6.27 to −0.50, 0.027), and the Netherlands (AAPC = −2.20, 95% C.I. −3.50 to −0.87, p = 0.005). By contrast, three countries showed an increase in the incidence of ovarian cancer including Belarus (AAPC = 5.61, 95% C.I. 2.90 to 8.39, p = 0.001), Japan (AAPC = 4.06, 95% C.I. 2.06 to 6.10, p = 0.001), and Korea (AAPC = 2.36, 95% C.I. 0.49 to 4.26, p = 0.019).
3.10. Incidence Trends of Ovarian Cancer in Females Aged < 40
Notably, no country showed a decreasing trend of ovarian cancer in females aged < 40 (Supplementary Figure S5). However, there were four countries showing an increasing trend of ovarian cancer, including India (AAPC = 6.89, 95% C.I. 0.95 to 13.18, p = 0.028), Belarus (AAPC = 6.69, 95% C.I. 2.48 to 11.08, p = 0.002), African Americans in the United States: (AAPC = 4.90, 95% C.I. 0.50 to 9.48, p = 0.033), and Japan (AAPC = 4.00, 95% C.I. 0.88 to 7.21, p = 0.018).
4. Discussion
4.1. Summary of Main Findings
There were several major findings: (1) the highest mortality rates due to ovarian cancer were observed in low–middle-income countries, and its incidence was highest in countries with high-income levels; (2) higher incidence of ovarian cancer was associated with a higher GDP per capita, HDI, prevalence of smoking, alcohol use, physical inactivity, obesity, hypertension, diabetes, and lipid disorders; (3) although there was an overall decreasing trend of incidence and mortality of ovarian cancer over the past decade, a substantial increase in incidence was observed in younger females.
4.2. Explanation of Findings and Relationship with Literature
The higher mortality rates of ovarian cancer in countries with lower-income levels found in this study are of concern. Although the highest incidence of ovarian cancer was observed in high-income countries, mortality was highest in countries with a lower-income level. In this analysis, incidence of ovarian cancer was associated with GDP per capita and HDI at the country level. The highest incidence rates in high-income countries may be driven by the higher prevalence of environmental, lifestyle, metabolic risk factors, nulliparity, menopausal hormone therapy use, and familial predisposition [22]. However, the largest mortality and mortality-to-incidence ratio were found in countries with lower-incomes. This could be due to the suboptimal resources available for early detection, treatment, and surveillance for cancer patients in these regions [23]. Based on this analysis, mortality rates due to ovarian cancer have been increasing for the past decade in some less developed countries, including Thailand, Ecuador, Colombia, the Philippines, and Brazil. These trends will likely continue in the future, suggesting that ovarian cancer is a significant health threat for these populations. More resources and targeted prevention strategies for ovarian cancer are needed in countries undergoing rapid economic development.
This study identified some preventable lifestyle and metabolic risk factors associated with the burden of ovarian cancer at a country level. These findings are generally consistent with previous observational studies using data from individuals. For example, a study using individual participant data for 28,114 women with ovarian cancer found that women who had smoked in the past had a 6% higher risk of ovarian cancer than those who had never smoked [24]. Consumption of at least one glass of wine per day, compared to no wine in the year before baseline, was associated with a 57% increased risk of developing ovarian cancer [25]. Women with the highest level of physical activity had an odds ratio of 0.73 (95% CI 0.56, 0.94) for ovarian cancer, compared with women with the lowest level of activity [26]. Overweight women had a 7% higher risk of ovarian cancer, and obese women had a 28% higher risk [27]. The presence of metabolic syndrome was also associated with ovarian cancer (relative risk (RR) = 3.42; 95% CI, 2.84 to 4.11) [28]
An overall decreasing trend of ovarian cancer was found, yet an increase in incidence was observed among younger females in some countries. Several factors may have contributed to this favorable trend, including the declining smoking prevalence, increasing use of oral contraceptives, and improvement in diagnosis and treatment. From 1980 to 2012, the prevalence of smoking among females decreased in North America, Australia, and New Zealand [29]. The use of oral contraceptives was usually higher in Western countries, which may have a protective effect for ovarian cancer [30]. The declining mortality rates for ovarian cancer might be attributed to the recent improvement in diagnosis and treatment. However, the increasing trend of incidence in younger females might be driven by the increasing prevalence of obesity, metabolic syndrome, estrogen exposure, and nulliparity among younger females. From 1985–2014, younger subjects (aged 15–40 years) showed a more drastic rise in prevalence (16.3 to 33.9%) than subjects aged >40 years (43.6 to 57.9%) [31]. The prevalence of metabolic syndrome also rose more rapidly in women and the younger population from 1991–2015 [32]. The increasing incidence may also be related to the rising presence of BRCA gene mutations in younger females. According to a recent study, rates of genetic testing for abnormal BRCA1 and BRCA2 genes have increased in females aged 40 or younger, which will increase their risk of ovarian cancer [33]
5. Limitation
Several limitations need to be mentioned in the current study. Firstly, there could be under-reporting of the incidence and mortality of ovarian cancer in the less developed countries with the underdevelopment in infrastructure and mechanism of cancer registries. Secondly, figures might have been overestimated for some countries as their incidence and mortality rates were represented by cancer registries in major cities. Thirdly, a direct comparison of the temporal trends of ovarian cancer between different countries may be problematic, as the cancer registries’ infrastructure may have changed over time. Nevertheless, this limitation is of less concern when comparing the trends of ovarian cancer between different age groups within the same country. Lastly, there was a lack of analysis on the incidence, mortality, risk factors, and epidemiological trends of ovarian cancer by different histological subtypes. This information is also important for the prevention of ovarian cancer as the epidemiological patterns could vary by different histological subtypes of ovarian cancer.
6. Conclusions
The incidence of ovarian cancer has been increasing substantially among younger females, probably caused by the increasing prevalence of obesity, metabolic syndrome, estrogen exposure and nulliparity. These trends are expected to continue due to economic growth and lifestyle transitions, particularly among countries with lower-income levels. Intensive lifestyle modifications are warranted, especially for the populations at high risk for ovarian cancer, including smoking cessation, alcohol use reduction, physical activity, weight control, and treatment of metabolic diseases. It is also important to improve early diagnosis, treatment, surveillance, and quality of life for patients with ovarian cancer. Longitudinal studies are needed to further confirm the drivers of these epidemiologic trends and provide more insight into the specific etiology and prognosis of ovarian cancer by histological subtypes.
Acknowledgments
The authors thank the staff at the World Health Organization and its collaborators who prepared these publicly available data. This study is based on publicly available data and solely reflects the opinion of its authors and not that of the World Health Organization.
Abbreviations
| AAPC | Average annual percent change |
| APC | Annual percentage change |
| ASR | Age-standardized rates |
| CI | Confidence interval |
| CI5 | Cancer Incidence in Five Continents |
| GDP | Gross domestic products |
| GHDx | Global Health Data Exchange |
| HDI | Human Development Index |
| IARC | International Agency for Research on Cancer |
| NORDCAN | Nordic Cancer Registries |
| RR | Relative risk |
| SEER | Surveillance, Epidemiology, and End Results |
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers14092230/s1, Figure S1: The plots of incidence and mortality trends for each country, Figure S2: The graphs of the joinpoint regression output, Figure S3: AAPC of incidence of ovarian cancer aged 50 years and older, Figure S4: AAPC of incidence of ovarian cancer aged <50 years old, Figure S5: AAPC of incidence of ovarian cancer aged <40 years old.
Author Contributions
J.H. and M.C.S.W., participated in the conception of the research ideas–study design–interpretation of the findings–writing of the first draft of the manuscript–provided intellectual input to the translational aspects of the study; W.C.C., C.H.N. and V.L., retrieved information from the relevant databases–performed the statistical analysis–presented the methodology and results; M.W., L.Z., D.E.L.-P.III, W.X., Z.-J.Z. and E.E., made critical revisions on the manuscripts–provided expert opinions on implications of the study findings. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Survey and Behavioural Research Ethics Committee, the Chinese University of Hong Kong (No. SBRE-20-332).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used for the analyses are publicly available from the World Health Organization websites (https://gco.iarc.fr/, https://apps.who.int/gho/data/node.main, accessed on 10 May 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Jayson G.C., Kohn E.C., Kitchener H.C., Ledermann J.A. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin L.A., Huang B., Miller R.W., Tucker T., Goodrich S.T., Podzielinski I., DeSimone C.P., Ueland F.R., van Nagell J.R., Seamon L.G. Ten-year relative survival for epithelial ovarian cancer. Obs. Gynecol. 2012;120:612–618. doi: 10.1097/AOG.0b013e318264f794. [DOI] [PubMed] [Google Scholar]
- 4.Momenimovahed Z., Tiznobaik A., Taheri S., Salehiniya H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health. 2019;11:287–299. doi: 10.2147/IJWH.S197604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salehi F., Dunfield L., Phillips K.P., Krewski D., Vanderhyden B.C. Risk factors for ovarian cancer: An overview with emphasis on hormonal factors. J. Toxicol. Environ. Health B Crit. Rev. 2008;11:301–321. doi: 10.1080/10937400701876095. [DOI] [PubMed] [Google Scholar]
- 6.Webb P.M., Jordan S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017;41:3–14. doi: 10.1016/j.bpobgyn.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Torre L.A., Trabert B., DeSantis C.E., Miller K.D., Samimi G., Runowicz C.D., Gaudet M.M., Jemal A., Siegel R.L. Ovarian cancer statistics, 2018. CA A Cancer J. Clin. 2018;68:284–296. doi: 10.3322/caac.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei K., Li Y., Zheng R., Zhang S., Liang Z., Cen H., Chen W. Ovary cancer incidence and mortality in China, 2011. Chin. J. Cancer Res. 2015;27:38–43. doi: 10.3978/j.issn.1000-9604.2015.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y., Luo G., Li M., Guo P., Xiao Y., Ji H., Hao Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer. 2019;19:984. doi: 10.1186/s12885-019-6139-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Agency for Research on Cancer. WHO Data Visualization Tools for Exploring the Global Cancer Burden in 2020. [(accessed on 10 November 2021)];2020 Available online: https://gco.iarc.fr/today/home.
- 11.Observatory G.C. Fact Sheet: Brain, Central Nervous System. 2021. [(accessed on 15 July 2021)]. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/31-Brain-central-nervous-system-fact-sheet.pdf.
- 12.Global Health Data Exchange. [(accessed on 15 July 2021)]. Available online: https://ghdx.healthdata.org/
- 13.Bray F., Colombet M., Mery L., Piñeros M., Znaor A., Zanetti R., Ferlay J. Cancer Incidence in Five Continents, Vol. XI (Electronic Version) Lyon: International Agency for Research on Cancer. 2020. [(accessed on 10 May 2020)]. Available online: https://ci5.iarc.fr.
- 14.World Health Organization WHO Mortality Database. [(accessed on 10 May 2021)];2021 Available online: https://www.who.int/healthinfo/mortality_data/en/
- 15.Danckert B.F.J., Engholm G., Hansen H.L., Johannesen T.B., Khan S., Køtlum J.E., Ólafsdóttir E., Schmidt L.K.H., Virtanen A., Storm H.H. NORDCAN: Cancer Incidence, Mortality, Prevalence and Survival in the Nordic Countries, Version 8.2. (26 March 2019) [(accessed on 10 May 2021)]. Available online: https://www-dep.iarc.fr/NORDCAN/english/frame.asp.
- 16.Engholm G., Ferlay J., Christensen N., Bray F., Gjerstorff M.L., Klint A., Kotlum J.E., Olafsdottir E., Pukkala E., Storm H.H. NORDCAN—A Nordic tool for cancer information, planning, quality control and research. Acta. Oncol. 2010;49:725–736. doi: 10.3109/02841861003782017. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services, N.I.o.H Surveillance, Epidemiology, and End Results (SEER) Program. [(accessed on 10 May 2021)];2020 Available online: https://seer.cancer.gov/about/
- 18.WHO International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2019 Edition. [(accessed on 21 July 2021)]. Available online: https://icd.who.int/browse10/2019/en#/C53.
- 19.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.J., Fay M.P., Feuer E.J., Midthune D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000;19:335–351. doi: 10.1002/(SICI)1097-0258(20000215)19:3<335::AID-SIM336>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 21.Clegg L.X., Hankey B.F., Tiwari R., Feuer E.J., Edwards B.K. Estimating average annual percent change in trend analysis. Stat. Med. 2009;28:3670–3682. doi: 10.1002/sim.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Vecchia C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017;26:55–62. doi: 10.1097/CEJ.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 23.Haier J., Sleeman J., Schäfers J. Editorial series: Cancer care in low- and middle-income countries. Clin. Exp. Metastasis. 2019;36:477–480. doi: 10.1007/s10585-019-10003-4. [DOI] [PubMed] [Google Scholar]
- 24.Beral V., Gaitskell K., Hermon C., Moser K., Reeves G., Peto R. Ovarian cancer and smoking: Individual participant meta-analysis including 28,114 women with ovarian cancer from 51 epidemiological studies. Lancet Oncol. 2012;13:946–956. doi: 10.1016/s1470-2045(12)70322-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang E.T., Canchola A.J., Lee V.S., Clarke C.A., Purdie D.M., Reynolds P., Bernstein L., Stram D.O., Anton-Culver H., Deapen D., et al. Wine and other alcohol consumption and risk of ovarian cancer in the California Teachers Study cohort. Cancer Causes Control. 2007;18:91–103. doi: 10.1007/s10552-006-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottreau C.M., Ness R.B., Kriska A.M. Physical activity and reduced risk of ovarian cancer. Obstet. Gynecol. 2000;96:609–614. doi: 10.1016/s0029-7844(00)00972-8. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z., Zhang T.T., Zhao J.J., Qi S.F., Du P., Liu D.W., Tian Q.B. The association between overweight, obesity and ovarian cancer: A meta-analysis. Jpn. J. Clin. Oncol. 2015;45:1107–1115. doi: 10.1093/jjco/hyv150. [DOI] [PubMed] [Google Scholar]
- 28.Huang J., Huang J.L.W., Wang J., Chung V.C.H., Wong M.C.S. Metabolic syndrome and risk of cancer in Chinese populations: A systematic review and meta-analysis in 57,260 individuals. Lancet. 2018;392:S15. doi: 10.1016/S0140-6736(18)32644-8. [DOI] [Google Scholar]
- 29.Ng M., Freeman M.K., Fleming T.D., Robinson M., Dwyer-Lindgren L., Thomson B., Wollum A., Sanman E., Wulf S., Lopez A.D., et al. Smoking Prevalence and Cigarette Consumption in 187 Countries, 1980–2012. JAMA. 2014;311:183–192. doi: 10.1001/jama.2013.284692. [DOI] [PubMed] [Google Scholar]
- 30.Bray F., Loos A.H., Tognazzo S., La Vecchia C. Ovarian cancer in Europe: Cross-sectional trends in incidence and mortality in 28 countries, 1953–2000. Int. J. Cancer. 2005;113:977–990. doi: 10.1002/ijc.20649. [DOI] [PubMed] [Google Scholar]
- 31.Wong M.C.S., Huang J., Wang J., Chan P.S.F., Lok V., Chen X., Leung C., Wang H.H.X., Lao X.Q., Zheng Z.-J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020;35:673–683. doi: 10.1007/s10654-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong M.C., Huang J., Pang T.W., Lok V., Chen X., Choi P., Wang H.H.-X., Zheng Z.-J. Tu1007 Worldwide Incidence and Prevalence of Metabolic Syndrome: A Systematic Review and Meta-Analysis of 14.6 Million Individuals. Gastroenterology. 2020;158:S-1003. doi: 10.1016/S0016-5085(20)33182-6. [DOI] [Google Scholar]
- 33.Rosenberg S.M., Ruddy K.J., Tamimi R.M., Gelber S., Schapira L., Come S., Borges V.F., Larsen B., Garber J.E., Partridge A.H. BRCA1 and BRCA2 Mutation Testing in Young Women with Breast Cancer. JAMA Oncol. 2016;2:730–736. doi: 10.1001/jamaoncol.2015.5941. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for the analyses are publicly available from the World Health Organization websites (https://gco.iarc.fr/, https://apps.who.int/gho/data/node.main, accessed on 10 May 2021).