Abstract
Objective
The aim of this study was to investigate the diagnostic value of peripheral blood neutrophil‐to‐lymphocyte ratio (NLR) combined with the thyroid imaging reporting and data system (TIRADS) for benign and malignant thyroid nodules.
Methods
A total of 585 adults were enrolled in the study. The receiver operating characteristic curves were used to determine the optimal cut‐off values for NLR and Kwak TIRADS (K‐TIRADS) grades, which were 1.87 and 4a, respectively. Thyroid nodules were scored as follows: NLR–K‐TIRADS score is 2 (both elevated K‐TIRADS grade and NLR), NLR–K‐TIRADS score is 1 (one of these was elevated) and NLR–k‐TIRADS score is 0 (neither were elevated).
Results
The proportions of malignant nodules with NLR‐K‐TIRADS scores of 2, 1 and 0 were 98.59%, 69.62% and 10.19%, and the difference was statistically significant (p < 0.001). In terms of the sensitivity of diagnosis of malignant nodules, NLR‐K‐TIRADS 1 tends to increase relative to K‐TIRADS grades ≥ 4a; in terms of specificity and positive predictive value for the diagnosis of malignant nodules, NLR–K‐TIRADS 2 was significantly higher than K‐TIRADS grades ≥ 4a (all p < 0.05).
Conclusions
NLR combined with K‐TIRADS grades may be a novel method for screening benign and malignant thyroid nodules.
Keywords: combined diagnosis, inflammation, neutrophil‐lymphocyte ratio, thyroid imaging reporting and data system, thyroid nodules
This study created a new preoperative screening method for benign and malignant thyroid nodules, the NLR–K‐TIRADS score, and NLR‐K‐TIRADS score method is better than K‐TIRADS classification alone in the diagnosis of malignant nodules.

1. INTRODUCTION
Thyroid nodules are a common disease of the endocrine system. Ultrasound is the preferred method for preoperative screening of thyroid nodules. The thyroid imaging reporting and data system (TIRADS) was first proposed in 2009 to optimize the management of thyroid nodules. 1 , 2 In 2011, Kwak et al 3 further subdivided the TIRADS classification into 1, 2, 3, 4a, 4b, 4c, 5 according to the number of suspicious signs of malignancy seen in the thyroid nodules. At present, the Kwak TIRADS (K‐TIRADS) classification has been widely used in clinical practice, but a recent meta‐analysis found that K‐TIRADS has high sensitivity and low specificity, 4 suggesting that the K‐TIRADS classification system is a good guide for diagnosing benign and malignant thyroid nodules, but it is still insufficient.
The neutrophil‐to‐lymphocyte ratio (NLR) is a novel marker of inflammation derived from routine blood count test. Its association with inflammation has been reported in inflammatory bowel disease, 5 diabetes mellitus type 2, 6 thyroiditis, 7 irritable bowel disease 8 and COVID‐19 infection. 9 Especially in thyroid cancer of inflammatory‐related diseases, studies have found that patients with thyroid cancer show higher NLR levels than patients with benign thyroid nodules. Further, patients with cervical lymph node metastasis have a higher NLR than those without 10 , 11 ; and patients with a higher risk of recurrence also have high NLR levels. 12
Neutrophil‐to‐lymphocyte ratio is a simple and cost‐effective routine test, and it would be surprising if NLR can make up for the deficiency of TIRADS grading in preoperative thyroid screening to a certain extent. No studies have been found that evaluate the combination of TIRADS grading with NLR in identifying the nature of thyroid nodules. Therefore, the present study aimed to analyse the combined NLR level and K‐TIRADS classification of patients with thyroid nodules to explore its diagnostic value for benign and malignant thyroid nodules.
2. METHODS
2.1. Study population
This was a retrospective study of the data of patients who were hospitalized for thyroid nodules for the first time in the General Surgery Department of the First Affiliated Hospital of Nanchang University between January 2017 and October 2019. Inclusion criteria included the following: thyroid nodules were detected on ultrasonography and graded using K‐TIRADS. 3 Exclusion criteria were as follows: a history of head and neck radiation, an infectious disease within the last 3 months, haematologic disease, other neoplastic disease, coronary heart disease, connective tissue disease, autoimmune disease (including Hashimoto's thyroiditis) and severe kidney or liver insufficiency. A total of 585 (98 male and 487 female) patients aged between 18 and 76 years were enrolled in the study. There were 326 malignant nodules (290 papillary carcinomas, 34 micro‐papillary carcinomas, and 2 follicular carcinomas) and 259 benign nodules. This study was approved by the Ethics Committee of the First Affiliated Hospital of Nanchang University and complied with the ethical guidelines of the Helsinki Declaration of 1975 (Ethic number: 2020‐04).
2.2. Data collection
Fasting (at least 8 h) venous blood was collected the morning before surgery. Routine blood samples were analysed using a Sysmex XE‐2100 haematology automated analyser (Sysmex, Kobe, Japan) with the supplied reagents, red blood cell controls and calibrators (Sysmex, Kobe, Japan). Chemiluminescence immunoassay (Roche Cobas e601) was used to determine serum free T3(FT3), free T4(FT4) and thyroid‐stimulating hormone(TSH). The normal ranges for serum concentrations of FT3, FT4 and TSH were 2.0–4.4 pg/ml, 0.93–1.70 ng/dl and 0.27–4.2 uIU/ml, respectively. The values of NLR were calculated using the formula: NLR = peripheral blood neutrophils/lymphocytes.
All ultrasonography was performed with Philips iU Elite colour Doppler ultrasound and performed by an experienced thyroid sonologist. The thyroid nodules were independently classified according to the K‐TIRADS grading. 3 One lesion was selected per patient. If the patient had multiple nodules, the one with the highest K‐TIRADS classification was selected, and the corresponding postoperative pathology report was recorded.
All postoperative thyroid specimens were evaluated by an experienced pathologist. The K‐TIRADS grading 3 is as follows: solid nodules, hypoechoic or very hypoechoic, irregular borders or tiny lobes, micro‐calcification and aspect ratio >1 are suspicious ultrasound features. The thyroid nodules are divided into five categories: Category 1, normal thyroid; Category 2, benign lesions; Category 3, possible benign nodules without the above suspicious ultrasound characteristics; Category 4, possible malignant nodules with the above 1, 2, 3 or 4 suspicious ultrasound features are 4a, 4b and 4c, respectively, and Category 5, nodules are highly likely to be malignant and have all five of the above suspicious ultrasound features.
2.3. Statistical analysis
SPSS software (version 26.0; SPSS, Chicago, IL, USA) was used for statistical analysis. The Kolmogorov‐Smirnov test was used to test for normal distribution. Continuous variables were not normally distributed and presented as medians with interquartile range (IQR) and compared using the Mann–Whitney U test. The count data were expressed as rate (%), and the chi‐square test was used to compare groups. The receiver operator characteristics (ROC) curve was used to determine the optimal cut‐off value for the NLR and K‐TIRADS grades. Multivariate logistic regression analyses were used to identify factors independently associated with malignant thyroid nodules. A p value < 0.05 was considered statistically significant.
3. RESULTS
3.1. Identification of independent predictors of malignant thyroid nodules
There were no significant differences between the two groups regarding gender, red blood cells, platelets, haemoglobin, FT3, FT4 and the incidence of hypertension and diabetes (all p > 0.05). However, there were statistically significant differences between the two groups regarding age, white blood cells, neutrophils, lymphocytes, NLR, TSH and the incidence of smoking (all p < 0.05; Table 1). After adjusting for age, sex, hypertension, diabetes, smoking and TSH, multivariate logistic regression analysis showed that high NLR and K‐TIRADS ≥ 4a were independent risk factor for malignant thyroid nodules (all p < 0.001; Table 2).
TABLE 1.
Comparison of demographic characteristics and biochemical indices of benign and malignant thyroid nodule patients
| Variables | Benign nodules (n = 259) | Malignant nodules (n = 326) | p Value |
|---|---|---|---|
| Age (years) | 50.00 (41.00–58.00) | 44.00 (34.00–51.00) | <0.001 |
| Sex | |||
| Male | 47 (18.15) | 51 (15.64) | 0.421 |
| Female | 212 (81.85) | 275 (84.36) | |
| Hypertension | 33 (12.74) | 40 (12.27) | 0.864 |
| Diabetes | 10 (3.86) | 5 ( 1.53) | 0.077 |
| Smoking | 17 (6.56) | 5 ( 1.53) | 0.001 |
| WBC (x109/L) | 5.13 (4.27–5.89) | 5.44 (4.67–6.54) | <0.001 |
| RBC (x1012/L) | 4.33 (4.04–4.61) | 4.36 (4.10–4.69) | 0.437 |
| PLT (x109/L) | 226.00 (193.00–265.00) | 230.00 (195.00–271.00) | 0.448 |
| HGB (g/L) | 128.50 (121.00–137.00) | 129.00 (121.00–137.00) | 0.860 |
| Neutrophil (x109/L) | 2.63 (2.13–3.17) | 3.05 (2.50–3.92) | <0.001 |
| Lymphocyte (x109/L) | 1.89 (1.62–2.28) | 1.76 (1.43–2.17) | 0.001 |
| NLR | 1.40 (1.17–1.64) | 1.77 (1.35–2.31) | <0.001 |
| FT3 (pg/ml) | 3.16 (2.93–3.42) | 3.13 (2.89–3.37) | 0.243 |
| FT4 (ng/dl) | 1.31 (1.20–1.42) | 1.30 (1.18–1.41) | 0.619 |
| TSH (uIU/ml) | 1.73 (1.19–2.52) | 2.02 (1.36–2.73) | 0.012 |
Values are expressed as a median (P25–P75) or N (%).
Bold values were considered statistically significant.
Abbreviations: FT3, free T3; FT4, free T4; HGB, haemoglobin; NLR, Neutrophil‐lymphocyte ratio; PLT, platelets; RBC, red blood cells; TSH, thyroid‐stimulating hormone; WBC, white blood cell.
TABLE 2.
Multivariate logistic analysis
| Variables | B | SE | Waldχ2 | OR(95%CI) | p Value |
|---|---|---|---|---|---|
| K‐TIRADS | |||||
| NLR | 1.58 | 0.31 | 26.02 | 4.84 (2.64–8.87) | <0.001 |
| 3 | 152.68 | 1 (reference) | – | ||
| 4a | 2.79 | 0.30 | 87.56 | 16.30 (9.09–29.26) | <0.001 |
| 4b | 3.90 | 0.39 | 99.38 | 49.42 (22.96–106.40) | <0.001 |
| 4c | 3.98 | 0.65 | 37.63 | 53.29 (14.96–189.80) | <0.001 |
| 5 | 4.13 | 0.81 | 25.95 | 62.13 (12.69–304.30) | <0.001 |
Bold values were considered statistically significant.
Abbreviation: NLR, Neutrophil‐lymphocyte ratio.
3.2. Relationship between K‐TIRADS grading and thyroid nodules
Among the 585 patients, the proportions of malignant nodules in K‐TIRADS3, K‐TIRADS4a, K‐TIRADS4b, K‐TIRADS4c and K‐TIRADS5 categories were 13.3%, 72.4%, 89.7%, 90.2% and 92.3%, respectively. With the higher the K‐TIRADS grade, the higher the proportion of malignant thyroid nodules (Figure 1).
FIGURE 1.
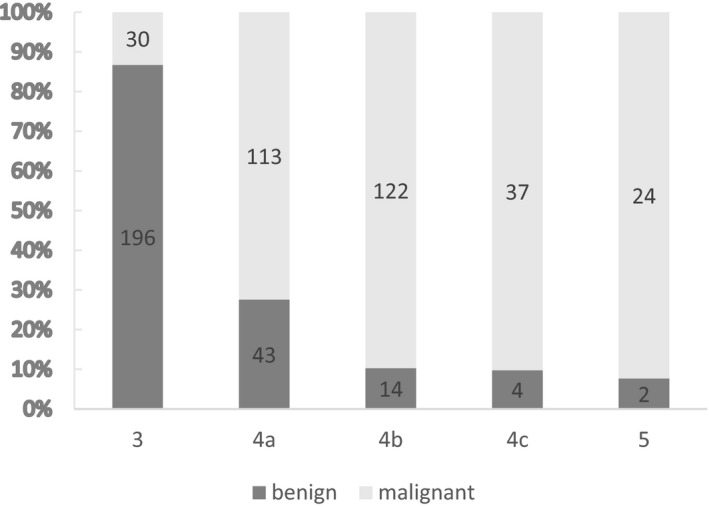
K‐TIRADS grades and thyroid nodule
3.3. ROC curve analysis and cut‐off value of the nlr and K‐TIRADS Grades
Receiver operator characteristics curve analysis indicated that the optimal cut‐off values for NLR and K‐TIRADS grading of thyroid malignant nodules were 1.87 and 4a, respectively, and their sensitivity and specificity were 45.7% and 95.0%, 90.8% and 75.7%, respectively. The area under the ROC curve for NLR and K‐TIRADS grades were 0.710 (95% confidence interval: 0.669–0.752) and 0.866 (95% confidence interval: 0.835–0.897), respectively (p < 0.001, p < 0.001; Figure 2).
FIGURE 2.
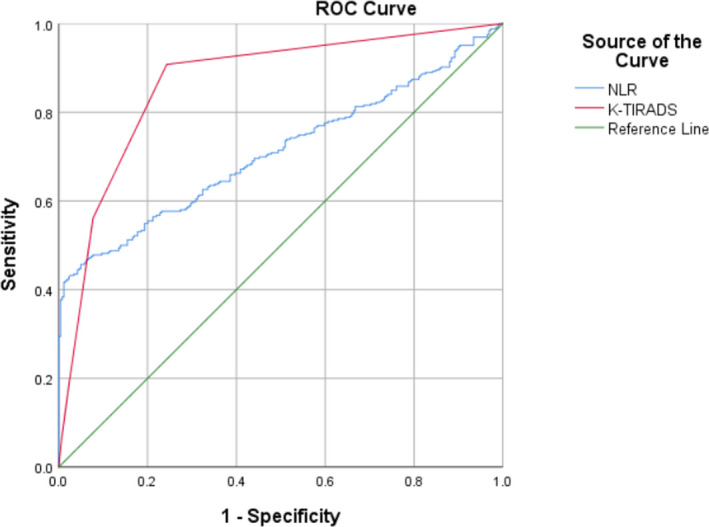
The ROC curve in predicting thyroid malignant nodules of NLR and KTIRADS grades
3.4. Relationship between NLR combined with K‐TIRADS grading and thyroid malignant nodules
Grouping NLR and K‐TIRADS by the best cut‐off value found that the proportion of malignant nodules with NLR ≥ 1.87 was significantly higher than that of NLR < 1.87 (p < 0.001), and the proportion of malignant nodules with K‐TIRADS grades ≥ 4a was significantly higher than that of K‐TIRADS grades < 4a (p < 0.001; Table 3).
TABLE 3.
The relationship between cut‐off values of NLR and K‐TIRADS grade and malignant nodules
| NLR ≥ 1.87 (n = 162) | NLR < 1.87 (n = 423) | p Value | K‐TIRADS grades ≥ 4a (n = 359) | K‐TIRADS grades < 4a (n = 226) | p Value | |
|---|---|---|---|---|---|---|
| Benign (n = 259) | 13 (8.02) | 246 (58.16) | <0.001 | 63 (17.55) | 196 (86.73) | <0.001 |
| Malignant (n = 326) | 149 (91.98) | 177 (41.84) | 296 (82.45) | 30 (13.27) |
Values are expressed as N (%).
Bold values were considered statistically significant.
Abbreviation: NLR, Neutrophil‐lymphocyte ratio.
According to the best cut‐off value of NLR and K‐TIRADS grading, patients were divided into the following three groups and assigned points: NLR–K‐TIRADS score is 2 (both elevated K‐TIRADS grade and NLR), NLR–K‐TIRADS score is 1 (one of these was elevated) and NLR–k‐TIRADS score is 0 (neither were elevated). The proportions of malignant thyroid nodules in the three groups were 98.59% (140/142), 69.62% (165/237) and 10.19% (21/206), and the difference was statistically significant (p < 0.001; Table 4). Subsequently, the diagnostic performance of K‐TIRADS and NLR–K‐TIRADS scores for malignant nodules was analysed, the results showed that in terms of the sensitivity of diagnosis of malignant nodules, NLR‐K‐TIRADS 1 tends to increase relative to K‐TIRADS grades ≥ 4a; in terms of specificity and positive predictive value for the diagnosis of malignant nodules, NLR–K‐TIRADS 2 was significantly higher than K‐TIRADS grades ≥ 4a (p < 0.05; Table 5).
TABLE 4.
The relationship between NLR–K‐TIRADS score and malignant thyroid nodules
| NLR–K‐TIRADS score [n (%)] | p Value | |||
|---|---|---|---|---|
| 0 (n = 206) | 1 (n = 237) | 2 (n = 142) | ||
| Benign (n = 259) | 185 (89.81) | 72 (30.38) | 2 (1.41) | <0.001 |
| Malignant (n = 326) | 21 (10.19) | 165 (69.62) | 140 (98.59) | |
Values are expressed as N (%).
Bold values were considered statistically significant.
Abbreviation: NLR, Neutrophil‐lymphocyte ratio.
TABLE 5.
Comparison of K‐TIRADS and NLR–K‐TIRADS for the diagnosis of nodule properties
| SEN (%) | SPE (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| NLR–K‐TIRADS ≥ 2 | 42.94%* (140/326) | 99.23%*,** (257/259) | 98.59%*,** (140/142) | 58.01%* (257/443) |
| NLR–K‐TIRADS ≥ 1 | 93.56%** (305/326) | 71.43%** (185/259) | 80.47%** (305/379) | 89.81%** (185/206) |
| K‐TIRADS grades ≥ 4a | 90.80% (296/326) | 75.68% (196/259) | 82.45% (296/359) | 86.73% (196/226) |
| NLR ≥ 1.87 | 45.71% (149/326) | 94.98% (246/259) | 91.98% (149/162) | 58.16% (246/423) |
Abbreviations: NLR, Neutrophil‐lymphocyte ratio; NPV, negative predictive value; PPV, positive predictive value; SEN, Sensitivity; SPE, specificity.
p < 0.05 compared with K‐TIRADS grades ≥ 4a.
p < 0.05 compared with NLR ≥ 1.87.
4. DISCUSSION
Thyroid cancer is the most common endocrine tumour. Early identification of benign and malignant thyroid nodules is very important for the treatment and prognosis of the disease. Most previous studies have independently investigated the clinical significance of NLR and TIRADS grades in malignant thyroid nodules. 10 , 11 , 13 However, we combined the NLR and K‐TIRADS grades to create the NLR–K‐TIRADS integration method to provide a new indicator for identifying the nature of thyroid nodules.
Reports in literature pointed out NLR's association with thyroid conditions 14 and thyroid nodules, 10 and our study has also proved this. We found that high levels of NLR are consistently an independent risk factor for thyroid cancer, and the best cut‐off value of NLR for predicting thyroid cancer was 1.87, with a sensitivity of 45.7% and a specificity of 95.0%. This finding was similar to the cut‐off value of NLR for predicting metastasis in medullary thyroid carcinoma presented by Xu et al. 15 Therefore, regular monitoring of NLR may be very helpful. This phenomenon is related to an increase in neutrophils, lymphopenia or both, which is also reflected in our research. Studies have found that in malignant conditions, the life span of neutrophils can be extended to more than twice the normal life span. Neutrophils reflect the host's inflammatory state. They can participate in different stages of the carcinogenic process by inducing angiogenesis, weakening the immune system, mediating tumour proliferation, inhibiting natural killer function and promoting tumour cell extravasation. 16 The cellular immunity mediated by lymphocytes is one of the body's responses to malignant tumours and plays an important anti‐tumour role. Anti‐tumour effector cells include T lymphocytes and natural killer cells, such as T regulatory cells, which can inhibit tumour‐promoting inflammation; CD8 + cytotoxic T lymphocytes can kill tumour cells directly. According to reports, lymphocytes are associated with improved prognosis in cancer patients. 17 , 18
Our study suggests that K‐TIRADS 4a is the optimal cut‐off value for the diagnosis of malignant nodules, and this has also been demonstrated in other studies. 19 , 20 , 21 The proportions of malignant nodules in K‐TIRADS 3, 4a, 4b, 4c, and 5 in our study were 13.3%, 72.4%, 89.7%, 90.2% and 92.3%, respectively. Some of these proportions differed from those reported by Kwak et al. 3 These differences may be related to our insufficient sample size, the study population and the differences between observers. However, our study is consistent with other studies 13 , 22 that the higher the TIRADS grade, the more likely the nodule is to be malignant.
Based on the complementary sensitivity and specificity of NLR ≥ 1.87 and K‐TIRADS ≥ 4a in the diagnosis of malignant nodules, we innovatively created the NLR–K‐TIRADS score. To the best of our knowledge, this is the first study to propose the NLR–K‐TIRADS scoring method to diagnose benign and malignant thyroid nodules. Our research found that the higher the NLR–K‐TIRADS score, the greater the possibility of malignant thyroid nodules. More importantly, in the diagnosis of malignant nodules, the diagnostic sensitivity of NLR‐K‐TIRADS 1 tends to increase relative to K‐TIRADS grades ≥ 4a alone. And the specificity and positive predictive value of NLR–K‐TIRADS 2 for the diagnosis of malignant nodules were significantly stronger than K‐TIRADS grades ≥ 4a alone. Sensitivity and positive predictive value are important determining factors for diagnostic tests, the above results suggest that peripheral blood NLR value combined with K‐TIRADS grading is superior to using K‐TIRADS grading alone for preoperative preliminary screening of benign and malignant thyroid nodules. Therefore, we suggest that for patients with NLR‐K‐TIRADS score of 1, clinicians should advise them to follow up regularly to closely observe the changes in their NLR values and K‐TIRADS grades, if it progresses to NLR‐K‐TIRADS score 2, pathological examination should be completed as soon as possible to clarify the nature of the nodule, which is conducive to the early treatment of thyroid cancer.
There are some limitations to this study. First, selection bias cannot be avoided because it is a retrospective study. Second, our sample size is limited, so these results only represent preliminary findings. Third, the ultrasound examinations of the patients in this study were performed by different sonographers, which may lead to differences in the classification of the thyroid nodules. This situation was inevitable due to the large number of patients undergoing surgery for thyroid nodules. However, as our ultrasound doctors are well‐trained, this should not impact our results. In addition, the single‐centre nature of this study may prevent globalization of the study outcomes to large communities, and a multi‐centre prospective, multi‐case study is needed for verification in the future.
5. CONCLUSIONS
We show that the NLR–K‐TIRADS scoring method is a promising diagnostic indicator that can be used to screen benign and malignant thyroid nodules and predict the risk of malignancy. We believe that the NLR–K‐TIRADS scoring system will soon play an important role in managing patients with thyroid nodules.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest regarding research, authorship and/or publication of this article.
ACKNOWLEDGEMENT
None.
Zhang J, Gong Z, Li S, et al. The value of neutrophil‐to‐lymphocyte ratio combined with the thyroid imaging reporting and data system in the diagnosis of the nature of thyroid nodules. J Clin Lab Anal. 2022;36:e24429. doi: 10.1002/jcla.24429
Funding information
This work was supported by the National Natural Science Foundation of China (grant number 81760168), the Key Research and Development Programs by Science and Technology Department of JiangXi Province (grant numbers 20201BBG71006) and Natural Science Program of Jiangxi Province (grant number 20192BAB205031).
DATA AVAILABILITY STATEMENT
All data in this study are available from the corresponding authors upon reasonable request.
REFERENCES
- 1. Park JY, Lee HJ, Jang HW, et al. A proposal for a thyroid imaging reporting and data system for ultrasound features of thyroid carcinoma. Thyroid. 2009;19:1257‐1264. [DOI] [PubMed] [Google Scholar]
- 2. Horvath E, Majlis S, Rossi R, et al. An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab. 2009;94:1748‐1751. [DOI] [PubMed] [Google Scholar]
- 3. Kwak JY, Han KH, Yoon JH, et al. Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology. 2011;260:892‐899. [DOI] [PubMed] [Google Scholar]
- 4. Migda B, Migda M, Migda MS, Slapa RZ. Use of the Kwak Thyroid Image Reporting and Data System (K‐TIRADS) in differential diagnosis of thyroid nodules: systematic review and meta‐analysis. Eur Radiol. 2018;28:2380‐2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Posul E, Yilmaz B, Aktas G, Kurt M. Does neutrophil‐to‐lymphocyte ratio predict active ulcerative colitis? Wien Klin Wochenschr. 2015;127:262‐265. [DOI] [PubMed] [Google Scholar]
- 6. Bilgin S, Aktas G, Zahid KM, et al. Association between novel inflammatory markers derived from hemogram indices and metabolic parameters in type 2 diabetic men. Aging Male. 2020;23:923‐927. [DOI] [PubMed] [Google Scholar]
- 7. Aktas G, Sit M, Dikbas O, et al. Elevated neutrophil‐to‐lymphocyte ratio in the diagnosis of Hashimoto's thyroiditis. Rev Assoc Med Bras. 1992;2017(63):1065‐1068. [DOI] [PubMed] [Google Scholar]
- 8. Gulali A, Tuba D, Burcin A, et al. Irritable bowel syndrome is associated with novel inflammatory markers derived from hemogram parameters. Family Med Primary Care Rev. 2020;22(2):107‐110. [Google Scholar]
- 9. Aktas G. Hematological predictors of novel Coronavirus infection. Rev Assoc Med Bras (1992). 2021;67(suppl 1):1‐2. [DOI] [PubMed] [Google Scholar]
- 10. Sit M, Aktas G, Erkol H, et al. Neutrophil to lymphocyte ratio is useful in differentiation of malign and benign thyroid nodules. P R Health Sci J. 2019;38:60‐63. [PubMed] [Google Scholar]
- 11. Cheong TY, Hong SD, Jung KW, So YK. The diagnostic predictive value of neutrophil‐to‐lymphocyte ratio in thyroid cancer adjusted for tumor size. PLoS One. 2021;16:e251446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu CL, Lee JJ, Liu TP, et al. Blood neutrophil‐to‐lymphocyte ratio correlates with tumor size in patients with differentiated thyroid cancer. J Surg Oncol. 2013;107:493‐497. [DOI] [PubMed] [Google Scholar]
- 13. Gao L, Xi X, Jiang Y, et al. Comparison among TIRADS (ACR TI‐RADS and KWAK‐ TI‐RADS) and 2015 ATA Guidelines in the diagnostic efficiency of thyroid nodules. Endocrine. 2019;64:90‐96. [DOI] [PubMed] [Google Scholar]
- 14. Afin H, Aktas G. Platelet to Lymphocyte and Neutrophil to Lymphocyte Ratios are useful in differentiation of thyroid conditions with normal and increased uptake. Ethiopian J Health Develop. 2021;35:1‐5. [Google Scholar]
- 15. Xu N, Jian Y, Wang Y, Tian W. Evaluation of neutrophil‐to‐lymphocyte ratio and calcitonin concentration for predicting lymph node metastasis and distant metastasis in patients with medullary thyroid cancer. Mol Clin Oncol. 2018;9:629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ocana A, Nieto‐Jimenez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour‐infiltrating lymphocytes in cancer: a systematic review with meta‐analysis. Br J Cancer. 2011;105:93‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yoon JH, Lee HS, Kim EK, Moon HJ, Kwak JY. Malignancy risk stratification of thyroid nodules: comparison between the thyroid imaging reporting and data system and the 2014 American thyroid association management guidelines. Radiology. 2016;278:917‐924. [DOI] [PubMed] [Google Scholar]
- 20. Zhang J, Liu BJ, Xu HX, et al. Prospective validation of an ultrasound‐based thyroid imaging reporting and data system (TI‐RADS) on 3980 thyroid nodules. Int J Clin Exp Med. 2015;8:5911‐5917. [PMC free article] [PubMed] [Google Scholar]
- 21. Srinivas MN, Amogh VN, Gautam MS, et al. A prospective study to evaluate the reliability of thyroid imaging reporting and data system in differentiation between benign and malignant thyroid lesions. J Clin Imaging Sci. 2016;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shen Y, Liu M, He J, et al. Comparison of different risk‐stratification systems for the diagnosis of benign and malignant thyroid nodules. Front Oncol. 2019;9:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data in this study are available from the corresponding authors upon reasonable request.


