Abstract
The type 2 diabetes mellitus (T2DM) is an urgent global health problem. T2DM patients are in a state of high oxidative stress and inflammation. Vitamin D and glutathione (GSH) play crucial roles in antioxidation and anti‐inflammation. However, T2DM patients have lower vitamin D and GSH levels than healthy persons. A randomized controlled trial was conducted to see the effect of the vitamin D supplementation on oxidative stress and inflammatory factors in T2DM patients. In this study, a total of 178 T2DM patients were randomly enrolled, 92 patients received regular treatment (T2DM group) and 86 patients in Vitamin D group received extra vitamin D 400 IU per day in addition to regular treatment. Serum vitamin D, GSH, GSH metabolic enzyme GCLC and GR, inflammatory factor MCP‐1, and IL‐8 levels were investigated. We found that the T2DM group has significantly higher concentrations of MCP‐1 and IL‐8 than those in the healthy donor group. After vitamin D supplementation for 90 days, T2DM patients had a 2‐fold increase of GSH levels, from 2.72 ± 0.84 to 5.76 ± 3.19 μmol/ml, the concentration of MCP‐1 decreased from 51.11 ± 20.86 to 25.42 ± 13.06 pg/ml, and IL‐8 also decreased from 38.21 ± 21.76 to 16.05 ± 8.99 pg/ml. In conclusion, our study demonstrated that vitamin D could regulate the production of GSH, thereby reducing the serum levels of MCP‐1 and IL‐8, alleviating oxidative stress and inflammation, providing evidence of the necessity and feasibility of adjuvant vitamin D treatment among patients with T2DM. On the other hand, vitamin D and GSH levels have important diagnostic and prognostic values in T2DM patients.
Keywords: glutathione, oxidative stress, serum inflammatory factors, type 2 diabetes, vitamin D
Diagram for randomized design of this study. In order to investigate of the correlation between vitamin D supplementation and serum glutathione levels of T2DM patients, a randomized controlled trial was conducted. A total of 178 T2DM patients were randomized to receive either vitamin D 400 IU per day or not. Healthy donors were also included in this study. Serum vitamin D, GSH, glutamate cysteine ligase (GCLC), glutathione reductase (GR), mononucleosis protein1 (MCP‐1), and leukocyte interleukin‐8 (IL‐8) levels of all three groups were investigated.

1. INTRODUCTION
With the development of society, the aging of the population and many other factors, the incidence of diabetes and prediabetes has increased over the years in China. The prevalence of diabetes was reported to be 10.9% in 2013, and the population with prediabetes is also growing, which has become a public health problem. Effective prevention and treatment strategies are needed to be carried out.1, 2 Diabetes is a chronic disease characterized by raised blood glucose levels. Type 2 diabetes (T2DM) is the most common type of diabetes. Patients with T2DM are also faced with complications like chronic functional impairment of eyes, renal disease, and cardiovascular disease in the progression of diabetes mellitus.
It is suggested that hyperglycemia increases oxidative stress; however, the antioxidant function in diabetic patients is diminished, resulting in the augment of oxidative stress.3, 4 High concentrations of glucose induce the secretion of inflammatory cytokine MCP‐1 and leukocyte interleukin‐8 (IL‐8) by activating the reactive oxygen species (ROS) and NF‐κB pathways.5, 6 High serum levels of MCP‐1 and IL‐8 accelerate the progression of diabetes and increase the incidence of complications.7, 8, 9 As a result, therapeutic agents against ROS have also been widely studied in the treatments of diabetes mellitus. 10
There is convincing evidence of the benefit of vitamin D on antioxidation.11, 12, 13 Vitamin D is a fat‐soluble vitamin responsible for building and maintaining bones by regulating calcium homeostasis. 14 The active hormone derived from vitamin D is 1,25(OH)2D3, which mediates numerous biological processes. The active vitamin D is associated with many other cellular functions like anti‐inflammatory and antioxidant properties to support physiological function and activity.15, 16, 17 It can be used to treat rickets, osteoporosis, hypothyroidism, psoriasis, and diseases caused by a lack of calcium or vitamin D. Vitamin D has also been reported to prevent the development of cancers. The deficiency of active vitamin D is related to the incidence of complications in T2DM patients. 18 Vitamin D possesses high antioxidant and anti‐inflammatory properties by increasing the formation of glutathione (GSH).19, 20 GSH helps to reduce the reactive oxygen level in the blood. 21 Meanwhile, long‐lasting hyperglycemia results in a low level of GSH. 22 In addition, GSH deficiency leads to the progression of diabetes and the development of complications.23, 24
Vitamin D is critical for maintaining homeostasis in T2DM patients. However, the effects of vitamin D in the treatment of T2DM in Southern Chinese people remain to be further investigated.
This study focuses on the effect of the supplementation of vitamin D on oxidative stress and inflammatory factors in patients with type 2 diabetes in a southern city of China. We conducted a randomized controlled trial (RCT) to follow up T2DM patients, to see in what manner GSH, MCP‐1, and IL‐8 levels would change after the extra supplementary of vitamin D. We aim to probe the diagnostic and prognostics values of the evaluation of vitamin D and GSH levels to monitor the progression of T2DM. This study also could provide evidence of the necessity and feasibility of adjuvant vitamin D treatment among patients with T2DM, providing evidence of the application of vitamin D in non‐bone diseases for further studies. We also follow up to study T2DM patients with vitamin D supplementation to see if they have a better prognosis and a lower incidence of complications.
2. MATERIALS AND METHODS
2.1. Patient enrollment
This study is a randomized controlled trial (RCT) and was approved by the Committee of Ethics and Research of the People's Hospital of Jiangmen (Approval No.:20200220‐8).
We have followed the CONSORT guideline 25 to perform the research.
A total of 200 adult patients diagnosed with type 2 diabetes were included and divided into two groups randomly. Patients with the following criteria were excluded: a history of cardiovascular disease, chronic liver or kidney disorders, endocrinology disorders, insulin treatment, cancer, and women in pregnancy or lactation. After excluding 22 patients who were lost to follow‐up, 86 patients in the Vitamin D supplement group (VitD group) and 92 patients in the standard treatment group (T2DM group) were finally enrolled in this study.
T2DM patients in the VitD group received 400 IU vitamin D daily for 90 days.
Volunteers of comparable age and gender were enrolled as healthy donor group (Healthy donor group, n = 102).
All the participants provided blood samples, and all the clinical characteristics were measured and collected. All the plasma of blood samples were separated by centrifugation and stored at −30℃ for further analysis.
2.2. Vitamin D, GSH, GCLC, MCP‐1, ROS and IL‐8 levels measurement
Plasma levels of vitamin D (Abbott 5P02, Control No. 08259UI00) were measured using the chemiluminescent microparticle immunoassay (CMIA) technique by Abbott i2000. In brief, vitamin D in the blood sample binds to anti‐vitamin D‐coated microparticles. After incubation, biotinylated vitamin D and anti‐Biotin acridinium‐labeled conjugate complexes were added to the reaction mixture and bound to the anti‐vitamin D‐coated particles. After washing, pre‐trigger solution and trigger solution were added to the reaction mixture. The resulting chemiluminescent reaction was measured as relative light units (RLUs) to calculate the amount of vitamin D in the sample.
The following ELISA kits were used to determine the levels of indicated components in the plasma: GSH (#BS‐E4238, Jiangsu Boshen Biotech company), GCL (#BS‐E7713, Jiangsu Boshen Biotech company), MCP‐1 (#BS‐E4072, Jiangsu Boshen Biotech company), and IL‐8 (#BS‐E3976, Jiangsu Boshen Biotech company), using the KHB ST‐960 Microplate Reader. ELISA is an immunoassay; briefly speaking, samples were incubated with the solidified antibody of indicated molecules and enzymatic labeling of antibody sequentially. Then, after adding the substrate of the enzyme, the substrate was catalyzed by the enzyme into colored products. The amount of the indicated molecules was positively linearly related to the amount of the colored products, so the concentrations of the tested molecules could be calculated.
The plasma concentrations of GR were tested using the U.V. spectrophotometric method (Zhongtuo biomedical Co., Ltd #ZF2110F). GR catalyzes the reduction of GSSG to GSH using NADPH, measuring the decrease in the rate of NADPH absorption peak at 340nm to calculate the activity of GR.
All appropriate tests, controls, and standards were measured according to the manufacturer's instructions.
2.3. Statistical analysis
The results are expressed as mean ± standard error. The statistical difference between groups was analyzed using Student's t‑test. Pearson's correlation analysis was performed to determine the relationships between variables. The results were considered significant at p < 0.05. The statistical calculations were done using GraphPad Prism 8.0.
3. RESULTS
3.1. T2DM patients have severer oxidative stress and lower vitamin D levels
Based on the promising correlation between vitamin D, GSH, and oxidative stress in T2DM patients, we investigated how the levels of those factors would change in T2DM patients compared with healthy persons. More importantly, we wanted to study how the serum levels of GSH and inflammatory factors would change in T2DM patients who accepted 400 U vitamin D3 supplementation for 90 days.
A total of 280 subjects were included in this study: 178 (63.6%) were diagnosed with T2DM and the others were healthy donors with normal glucose metabolism. Among the T2DM patients, they were randomly assigned to receive either the extra vitamin D supplementation (n = 86) or not (n = 92) in addition to standard medication treatments.
We first investigated oxidative stress in T2DM patients by testing the MCP‐1 and IL‐8 levels in the serum. The results showed that the serum levels of inflammatory biomarker MCP‐1 are much higher in the T2DM patients compared with healthy donors (53.64007 ± 35.91807 vs. 20.82569 ± 7.95332 pg/ml; p < 0.0001), as well as IL‐8 (37.76807 ± 22.57844 vs. 10.06893 ± 4.641069 pg/ml; p < 0.0001) (Figure 1A‐B), which indicates that the oxidative stress levels are much severer in T2DM patients.
FIGURE 1.
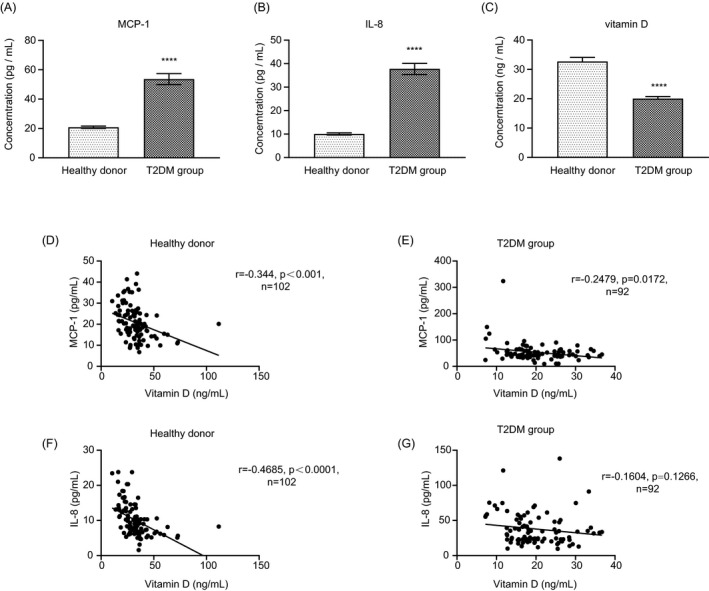
T2DM patients have high inflammatory marker levels and lower vitamin D levels. The serum MCP‐1 (A), IL‐8 (B), and vitamin D (C) levels of patients with T2DM and healthy donors. (D‐G) The correlations between blood vitamin D and MCP‐1 or IL‐8 levels of patients with T2DM and healthy donors. (ns: not significant, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p< 0.0001.)
On the other hand, we also tested the serum vitamin D levels of patients with T2DM and healthy donors. It is shown that serum vitamin D levels of T2DM patients (20.04 ± 6.85 ng/ml) are significantly lower than that of healthy donors (32.71 ± 13.83 ng/ml; Figure 1C).
These results suggested that T2DM patients generally lack vitamin D and are in a high oxidative and inflammatory condition.
We also found that the concentrations of blood inflammatory markers are significantly negatively correlated with the vitamin D levels (Figure 1D‐G), irrespective of healthy donor group or T2DM group, indicating that vitamin D may reduce the oxidative and inflammatory levels, which are consistent with previous studies.15, 16, 26, 27
In the vitamin D group, after the supplementation of vitamin D for 90 days, the serum vitamin D levels significantly increased and were back to normal levels (36.57654321 ± 12.66437866 vs. 17.21851852 ± 7.950170603 ng/ml; Figure 2A). These results showed that the supplementation of vitamin D besides regular treatment could recover the deficiency of vitamin D in the blood of these patients. In the meanwhile, the MCP‐1 and IL‐8 levels were significantly lower in the blood of T2DM patients after adjuvant treatment of vitamin D. MCP‐1 levels decreased from 51.11 ± 20.86 pg/ml to 25.42 ± 13.06 pg/ml, and the concentrations of IL‐8 also reduced from 38.21 ± 21.76 to 16.05 ± 8.99 pg/ml (Figure 2B, C).
FIGURE 2.
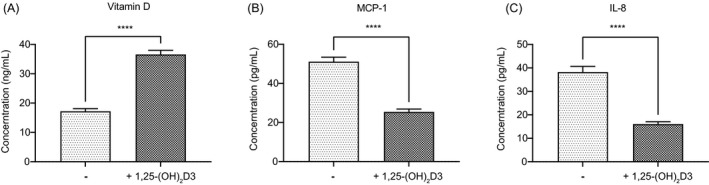
Vitamin D supplementation down‐regulates the oxidative stress. The serum vitamin D (A), MCP‐1 (B), and IL‐8 (C) levels in T2DM patients before and after the supplement of vitamin D. (ns: not significant, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.)
The result showed that active vitamin D3 could effectively lower the oxidative stress in T2DM patients.
3.2. Vitamin D lowers the oxidative stress by increasing the activity of GSH formation
With the evidence that vitamin D participates in the metabolism of GSH,28, 29 it functions as an effective antioxidant. It plays a vital role in alleviating the progression of diabetes and its complications. We also investigated the serum levels of GSH and its related metabolic enzymes GR and GCLC in T2DM patients. Concentrations of GSH in the healthy donor group and T2DM patients were 3.36 ± 0.52 and 2.75 ± 0.89 μmol/ml, respectively (Figure 3A). As expected, T2DM patients with lower vitamin D levels had lower levels of GSH in their blood. GR and GCLC are the two key enzymes that regulate the synthesis of GSH. Likewise, there were significantly lower GR and GCLC activities in T2DM patients than in healthy donors (Figure 3B, C). GR levels were 20.18 ± 17.42 vs. 65.11 ± 16.05 ng/L, and the GCLC activities were 42.74 ± 37.6 vs. 89.86 ± 90.72 U/mL.
FIGURE 3.
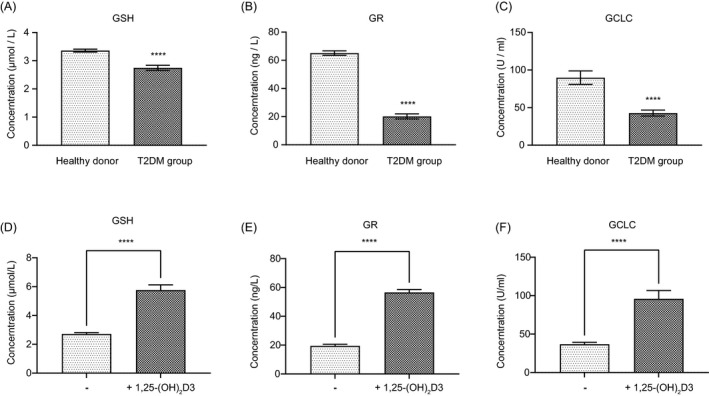
Vitamin D increases the formation of GSH. The serum GSH (A), GCLC (B), and GR (C) levels of patients with T2DM and healthy donors. The levels of GSH (A), GCLC (B), and GR (C) in T2DM patients before and after the supplement of vitamin D. (ns: not significant, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001.)
In the vitamin D group, after supplying vitamin D, GSH levels were increased from 2.72 ± 0.84 to 5.76 ± 3.19 μmol/mL, even slightly higher than the healthy persons (Figure 3D). At the same time, vitamin D increased GR levels from 19.50 ± 9.29 to 56.55 ± 17.76 ng/L and the activities of GCLC were also enhanced from 36.86 ± 22.47 vs. 95.93 ± 96.67 U/mL (Figure 3E, F). The results indicate that extra vitamin D supplements could significantly increase the formation and stabilization of GSH.
There was a positive correlation between blood vitamin D levels and GSH in healthy persons and T2DM patients, so were the two enzymes GR and GCLC (Figure 4A‐C). These results are consistent with the conclusions that vitamin D could upregulate the formation of GSH, 28 which also indicates that vitamin D could regulate the serum levels of GSH by affecting the concentrations and activities of GSH synthesis enzymes GR and GCLC.
FIGURE 4.
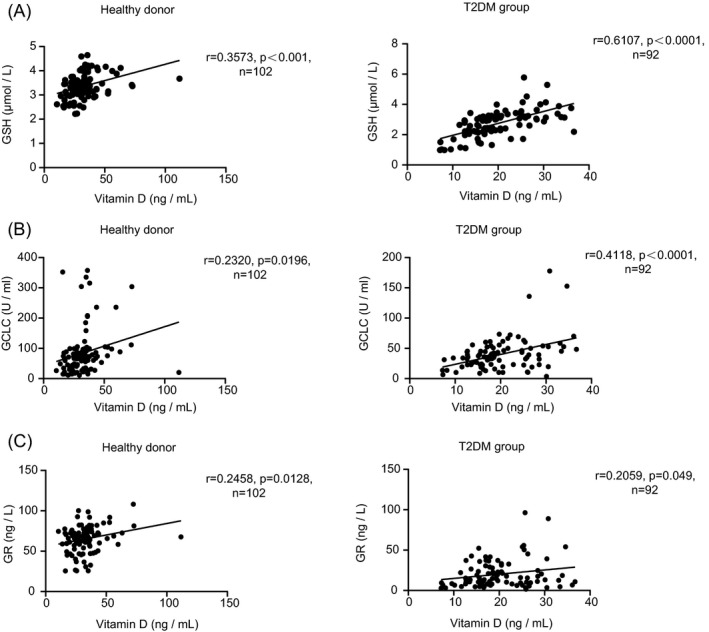
Correlations between blood vitamin D and GSH (A), GCLC (B), GR(C) levels of patients in healthy donors and the T2DM group
On the other hand, we found that the concentrations of MCP‐1 and IL‐8 are also significantly negatively correlated with the GSH levels (Figure 5A, B), irrespective of healthy donor group or T2DM group, indicating that GSH is upregulated by vitamin D and thereby alleviates the oxidative stress and inflammatory conditions.
FIGURE 5.
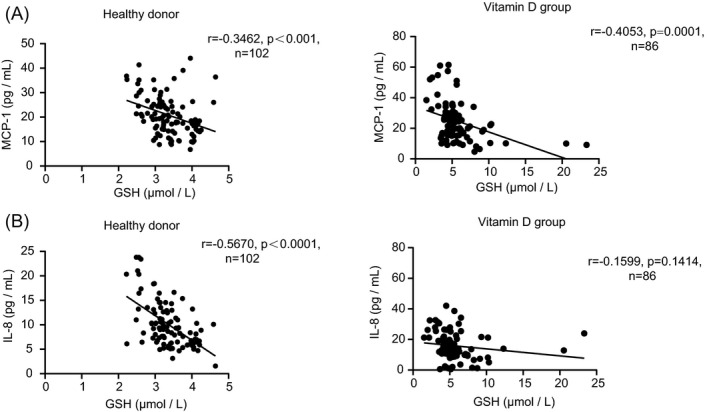
(A, B) Correlations between blood GSH and MCP‐1 or IL‐8 levels of patients in healthy donors and the T2DM group
In conclusion, the results above indicate that the supplementation of vitamin D in the treatment of diabetes is a benefit for T2DM patients. Vitamin D could significantly induce the formation of GSH by increasing and activating GCLC and GR, which could consequently reduce the oxidation and the secretion of inflammation cytokines like MCP‐1 and IL‐8 in T2DM patients, thereby slowing down the progressive deterioration of diabetes and reducing the risks of complications.
4. DISCUSSION
Under the induction of obesity, unhealthy lifestyle, aging, and other risk factors, the diabetic‐susceptible population will develop pancreatic β‐cell dysfunction and insulin resistance, leading to hyperglycemia.
Oxidative stress is involved in the occurrence and development of T2DM and its complications; therefore, related research on the prevention and control of T2DM is urgent. Previous studies have shown that the high glucose and free fatty acids level in plasma increases oxidative stress by enhancing the production of free radicals. 30 High glucose levels in the blood could boost the production of oxidative radicals, leading to insulin resistance, impaired insulin secretion, and diabetic vascular lesions,31, 32, 33 accelerating the deterioration of diabetes. On the other hand, high glucose levels may also cause oxidative stress30, 34, 35 and activate inflammatory responses in the body, causing the development and acceleration of a series of complications.23, 36, 37
High glucose concentrations induce the secretion of inflammatory cytokine MCP‐1 by activating the reactive oxygen species (ROS) and NF‐κB pathways.5, 6 MCP‐1 also has a potential effect on promoting renal complications in T2DM patients. 38 Hyperglycemia is also positively correlated to the high level of leukocyte interleukin‐8 (IL‐8), which results in a severe complication of diabetic encephalopathy. 5 In addition, ROS mediates the activation of NF‐κB pathways and induces the expression of IL‐8.39, 40
GSH is closely related to oxidative stress, which could scavenge oxygen free radicals effectively. Glutathione (GSH) is a tripeptide containing glutamate, cysteine, and glycine. GSH is an effective physiological antioxidant that helps to reduce the reactive oxygen level in the plasma. The deficiency of GSH positively correlates with aging and the prevalence of metabolic and cardiovascular diseases. 41 GSH exists in both reduced glutathione (G‐SH) and oxidized glutathione (GSSG) in blood. GSH is a convincing suppressor of radiative stress,42, 43 which contributes to the impairment of cells and tissues, leading to the progression of diabetes and the development of complications, as well as the dysregulation of other types of metabolic cycles. 44 GSH participates in many physiological and pathological processes, such as detoxification, regulates cell viability, and participates in the progression of chronic diseases like cardiovascular diseases, immune diseases, and diabetes. 23 Biosynthesis of GSH is tightly regulated by GCL activity, the catalytic component of which is GCLC. GR is responsible for maintaining the reduced form of GSH, which is responsible for eliminating radical oxidants.
Vitamin D possesses high antioxidant and anti‐inflammatory properties. It can effectively modulate the levels of GSH by affecting the activity and the expression levels of critical enzymes like GSH peroxidase superoxide dismutase (GPx1) and GR in GSH metabolism. 19 It is reported that the supplementation of vitamin D could significantly reduce the incidence of gestational diabetes and decrease the prevalence of kidney or cardiovascular complications in T2DM patients. 45 Some studies indicated that supplementation with vitamin D could modulate the inflammation symptoms and improve the defense against some chronic infections.26, 27, 46
However, the prevalence of vitamin D deficiency is surprisingly high and widespread among all ages and worldwide, and it may be associated with several chronic diseases,47, 48 including type 2 diabetes. Studies on the relationship between hyperglycemia, oxidative stress, and GSH implied that reductive glutathione could lower the oxidative stress, helping to control the circular glucose level and its complications. 49 Oxidative stress and diabetes, these two processes are likely to reinforce each other. However, the relationships between GSH, oxidative stress, and inflammatory cytokines remain to be investigated in T2DM patients.
According to our results, Vitamin D could induce the production of GSH by mediating the upregulation of GCLC and GR, thereby reducing the serum levels of MCP‐1 and IL‐8 to prevent the progression of diabetes and the incidence of complications.
This study found that vitamin D supplementation to increase GSH was beneficial in patients with T2DM, as demonstrated by reducing inflammatory factors MCP‐1 and IL‐8 in patients’ blood. Our results suggest that vitamin D could increase the stability of GR/GCLC by protecting them from degradation, and the mechanisms could be investigated in a further study.
We speculated that vitamin D might reduce the risk of complications of T2DM caused by the activated inflammatory processes, like the other anti‐inflammatory agents.50, 51, 52 Therefore, T2DM patients can benefit from vitamin D supplementation.
We conducted this study to investigate the predictive value of vitamin D and GSH level assessment in T2DM disease surveillance and patient prognosis. This study provides evidence for the necessity and feasibility of adjuvant vitamin D therapy for T2DM patients and further expands the application of vitamin D in disease treatment. On the other hand, the detections of vitamin D, GSH, GR, GCLC, MCP‐1, and IL‐8 are widely available in the department of laboratory medicine, and physicians can regularly monitor these indicators and formulate the treatments according to the health situation of the patient.
We also conducted a follow‐up study of T2DM patients supplemented with vitamin D to observe whether their prognosis would be better and the incidence of complications would be lower to evaluate the value of vitamin D in the adjuvant treatment of T2DM patients, providing evidence that vitamin D could be a promising biomarker of diabetes progression, as well as new guidance for the research on the development of diabetes.
This study is a single‐center study with several potential limitations, our study only includes patients in our hospital, and continuous measurements need to be further investigated. At the same time, racial and ethnic differences and biorhythm differences exist. But our study demonstrated the ability of vitamin D to improve circulating GSH levels and reduce inflammatory factors. These findings could provide evidence of the beneficial effects of vitamin D supplementation in patients with T2DM. In addition, the regular inspection of vitamin D and GSH concentrations in the blood of T2DM patients is a promising way to monitor the progression of this disease.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
JC.G, YG.W, and XL.F conceptually designed the study. JC.G, WG.H, XJ.F, XH.C, B.Z, and ZJ.L performed the experimental work. JC.G and WG.H analyzed the data. JC.G and XL.F wrote the manuscript. XJ.F, XH.C, B.Z, and ZJ.L discussed the hypothesis and reviewed the manuscript. All the authors read and approved the final manuscript.
PATIENT CONSENT FOR PUBLICATION
Not applicable.
ACKNOWLEDGMENT
Not applicable.
Gu J‐C, Wu Y‐G, Huang W‐G, et al. Effect of vitamin D on oxidative stress and serum inflammatory factors in the patients with type 2 diabetes. J Clin Lab Anal. 2022;36:e24430. doi: 10.1002/jcla.24430
Funding information
This work was supported by the Bureau of Science and Technology of Jiangmen Municipality under Grant (2020YLC030 to JC.G)
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article.
REFERENCES
- 1. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317(24):2515‐2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88‐98. [DOI] [PubMed] [Google Scholar]
- 3. Iacobini C, Vitale M, Pesce C, et al. Diabetic Complications and oxidative stress: a 20‐year voyage back in time and back to the future. Antioxidants (Basel). 2021;10(5):727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maria‐Luisa L‐D‐I‐V‐M, Cristina F‐M. Oxidative stress in diabetes mellitus and the role of vitamins with antioxidant actions. In: José AM‐G, ed. Oxidative stress and chronic degenerative diseases. IntechOpen; 2013. [Google Scholar]
- 5. Quan Y, Jiang CT, Xue B, et al. High glucose stimulates TNFalpha and MCP‐1 expression in rat microglia via ROS and NF‐kappaB pathways. Acta Pharmacol Sin. 2011;32(2):188‐193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wei M, Li Z, Xiao L, et al. Effects of ROS‐relative NF‐kappaB signaling on high glucose‐induced TLR4 and MCP‐1 expression in podocyte injury. Mol Immunol. 2015;68(2 Pt A):261‐271. [DOI] [PubMed] [Google Scholar]
- 7. Pan X, Kaminga AC, Wen SW, et al. Chemokines in prediabetes and type 2 diabetes: a meta‐analysis. Front Immunol. 2021;12: 622438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Scurt FG, Menne J, Brandt S, et al. Monocyte chemoattractant protein‐1 predicts the development of diabetic nephropathy. Diabetes Metab Res Rev. 2022;38(2):e3497. [DOI] [PubMed] [Google Scholar]
- 9. Loretelli C, Rocchio F, D'Addio F, et al. The IL‐8‐CXCR1/2 axis contributes to diabetic kidney disease. Metabolism. 2021;121: 154804. [DOI] [PubMed] [Google Scholar]
- 10. Zhang P, Li T, Wu X, et al. Oxidative stress and diabetes: antioxidative strategies. Front Med. 2020;14(5):583‐600. [DOI] [PubMed] [Google Scholar]
- 11. Cojic M, Kocic R, Klisic A, et al. The effects of vitamin D supplementation on metabolic and oxidative stress markers in patients with type 2 diabetes: a 6‐month follow up randomized controlled study. Front Endocrinol (Lausanne). 2021;12:610893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. El Askary A, Gharib AF, Almehmadi M, et al. The role of vitamin D deficiency and elevated inflammatory biomarkers as risk factors for the progression of diabetic nephropathy in patients with type 2 diabetes mellitus. Open Chemistry. 2021;19(1):1174‐1183. [Google Scholar]
- 13. Ao T, Kikuta J, Ishii M. The effects of vitamin D on Immune system and inflammatory diseases. Biomolecules. 2021;11(11):1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. DeLuca HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr. 2004;80(6 Suppl):1689S‐S1696. [DOI] [PubMed] [Google Scholar]
- 15. Pfeffer PE, Lu H, Mann EH, et al. Effects of vitamin D on inflammatory and oxidative stress responses of human bronchial epithelial cells exposed to particulate matter. PLoS One. 2018;13(8):e0200040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Almeida Moreira Leal LK, Lima LA, Alexandre de Aquino PE, et al. Vitamin D (VD3) antioxidative and anti‐inflammatory activities: Peripheral and central effects. Eur J Pharmacol. 2020;15(879):173099. [DOI] [PubMed] [Google Scholar]
- 17. Vojinovic J. Vitamin D receptor agonists’ anti‐inflammatory properties. Ann N Y Acad Sci. 2014;1317:47‐56. [DOI] [PubMed] [Google Scholar]
- 18. Ma L, Wang S, Chen H, et al. Diminished 25‐OH vitamin D3 levels and vitamin D receptor variants are associated with susceptibility to type 2 diabetes with coronary artery diseases. J Clin Lab Anal. 2020;34(4):e23137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ansari MGA, Sabico S, Clerici M, et al. Vitamin D supplementation is associated with increased glutathione peroxidase‐1 levels in Arab adults with prediabetes. Antioxidants (Basel). 2020;9(2):118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang C, Wang J, Xie X, et al. Low serum vitamin D concentration is correlated with anemia, microinflammation, and oxidative stress in patients with peritoneal dialysis. J Transl Med. 2021;19(1):411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsiao YF, Cheng SB, Lai CY, et al. The prognostic role of glutathione and its related antioxidant enzymes in the recurrence of hepatocellular carcinoma. Nutrients. 2021;13(11):4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kanikarla‐Marie P, Micinski D, Jain SK. Hyperglycemia (high‐glucose) decreases L‐cysteine and glutathione levels in cultured monocytes and blood of Zucker diabetic rats. Mol Cell Biochem. 2019;459(1–2):151‐156. [DOI] [PubMed] [Google Scholar]
- 23. Hakki Kalkan I, Suher M. The relationship between the level of glutathione, impairment of glucose metabolism and complications of diabetes mellitus. Pak J Med Sci. 2013;29(4):938‐942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matuz‐Mares D, Riveros‐Rosas H, Vilchis‐Landeros MM, et al. Glutathione participation in the prevention of cardiovascular diseases. Antioxidants. 2021;10(8):1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;24(8):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mangin M, Sinha R, Fincher K. Inflammation and vitamin D: the infection connection. Inflamm Res. 2014;63(10):803‐819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herscovitch K, Dauletbaev N, Lands LC. Vitamin D as an anti‐microbial and anti‐inflammatory therapy for Cystic Fibrosis. Paediatr Respir Rev. 2014;15(2):154‐162. [DOI] [PubMed] [Google Scholar]
- 28. Jain SK, Micinski D. Vitamin D upregulates glutamate cysteine ligase and glutathione reductase, and GSH formation, and decreases ROS and MCP‐1 and IL‐8 secretion in high‐glucose exposed U937 monocytes. Biochem Biophys Res Commun. 2013;437(1):7‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jain SK, Micinski D, Huning L, et al. Vitamin D and L‐cysteine levels correlate positively with GSH and negatively with insulin resistance levels in the blood of type 2 diabetic patients. Eur J Clin Nutr. 2014;68(10):1148‐1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49(2 Suppl 1):27‐29. [DOI] [PubMed] [Google Scholar]
- 31. Hurrle S, Hsu WH. The etiology of oxidative stress in insulin resistance. Biomed J. 2017;40(5):257‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6(3):456‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress‐A concise review. Saudi Pharm J. 2016;24(5):547‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yu T, Jhun BS, Yoon Y. High‐glucose stimulation increases reactive oxygen species production through the calcium and mitogen‐activated protein kinase‐mediated activation of mitochondrial fission. Antioxid Redox Signal. 2011;14(3):425‐437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luo J, Xiang Y, Xu X, et al. High glucose‐induced ROS production stimulates proliferation of pancreatic cancer via inactivating the JNK pathway. Oxid Med Cell Longev. 2018;2018:6917206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tsalamandris S, Antonopoulos AS, Oikonomou E, et al. The role of inflammation in diabetes: current concepts and future perspectives. Eur Cardiol. 2019;14(1):50‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tanifuji C, Suzuki Y, Geot WM, et al. Reactive oxygen species‐mediated signaling pathways in angiotensin II‐induced MCP‐1 expression of proximal tubular cells. Antioxid Redox Signal. 2005;7(9–10):1261‐1268. [DOI] [PubMed] [Google Scholar]
- 39. Ko JW, Lim SY, Chung KC, et al. Reactive oxygen species mediate IL‐8 expression in Down syndrome candidate region‐1‐overexpressed cells. Int J Biochem Cell Biol. 2014;55:164‐170. [DOI] [PubMed] [Google Scholar]
- 40. Choi SY, Lim JW, Shimizu T, et al. Reactive oxygen species mediate Jak2/Stat3 activation and IL‐8 expression in pulmonary epithelial cells stimulated with lipid‐associated membrane proteins from Mycoplasma pneumoniae. Inflamm Res. 2012;61(5):493‐501. [DOI] [PubMed] [Google Scholar]
- 41. Teskey G, Abrahem R, Cao R, et al. Glutathione as a marker for human disease. Adv Clin Chem. 2018;87:141‐159. [DOI] [PubMed] [Google Scholar]
- 42. Kwon DH, Lee H, Park C, et al. Glutathione induced immune‐stimulatory activity by promoting M1‐like macrophages polarization via potential ROS scavenging capacity. Antioxidants. 2019;8(9):413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fatehi‐Hassanabad Z, Chan CB, Furman BL. Reactive oxygen species and endothelial function in diabetes. Eur J Pharmacol. 2010;636(1–3):8‐17. [DOI] [PubMed] [Google Scholar]
- 44. Chen C, Mahar R, Merritt ME, et al. ROS and hypoxia signaling regulate periodic metabolic arousal during insect dormancy to coordinate glucose, amino acid, and lipid metabolism. Proc Natl Acad Sci U S A. 2021;118(1): 10.1073/pnas.2017603118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gunasegaran P, Tahmina S, Daniel M, et al. Role of vitamin D‐calcium supplementation on metabolic profile and oxidative stress in gestational diabetes mellitus: A randomized controlled trial. J Obstet Gynaecol Res. 2021;47(3):1016‐1022. [DOI] [PubMed] [Google Scholar]
- 46. Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID‐19 infections and deaths. Nutrients. 2020;12(4):988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kaur J, Ferguson SL, Freitas E, et al. Association of Vitamin D status with chronic disease risk factors and cognitive dysfunction in 50(‐)70 year old adults. Nutrients. 2019;11(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang H, Chen W, Li D, et al. Vitamin D and chronic diseases. Aging Dis. 2017;8(3):346‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sekhar RV, McKay SV, Patel SG, et al. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care. 2011;34(1):162‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pollack RM, Donath MY, LeRoith D, et al. Anti‐inflammatory agents in the treatment of diabetes and its vascular complications. Diabetes Care. 2016;39(Suppl 2):S244‐S252. [DOI] [PubMed] [Google Scholar]
- 51. Kong M, Xie K, Lv M, et al. Anti‐inflammatory phytochemicals for the treatment of diabetes and its complications: Lessons learned and future promise. Biomed Pharmacother. 2021;133: 110975. [DOI] [PubMed] [Google Scholar]
- 52. Liou GI. Diabetic retinopathy: role of inflammation and potential therapies for anti‐inflammation. World J Diabetes. 2010;1(1):12‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article.


