Abstract
Introduction
The intensification of coronavirus disease 2019 (COVID‐19) complications, severe symptoms, and high mortality rate has led researchers to focus on this significant issue. While respiratory and cardiac complications have been described as high‐risk manifestations in patients with COVID‐19, neurological complications can also enhance mortality. This study aimed to evaluate the prevalence of neurological complications arises from SARS‐CoV‐2 and assess the mortality rate from neurological complications.
Material and Methods
Literature review was conducted by searching in PubMed/Medline, Web of Sciences, and Embase. After performing search strategies with relevant terms, a number of articles were excluded, including review articles, systematic review or meta‐analysis, duplicate publication of same researchers, congress abstracts, animal studies, case reports, case series, and articles reporting a history of neurological features prior to COVID‐19 infection. After retrieving the data, statistical analysis was performed using the STATA Version 14 software.
Results
From 4455 retrieved publications, 20 articles were selected for further analysis. Among 18,258 included patients, 2791 showed neurological symptoms, which were classified into different groups. Headache, confusion, and fatigue were reported as the most non‐specific neurological features in confirmed COVID‐19 patients. Psychiatric symptoms, CNS disorders, cerebrovascular disorders, CNS inflammatory disorders, PNS disorders, neuromuscular disorders, etc., were defined as specific neurological manifestations. The pooled prevalence of neurological manifestations and mortality rate of COVID‐19 patients with neurological features were estimated to be 23.0% (95% CI: 17.8–29.2) and 29.1% (95% CI: 20.3–39.8), respectively.
Conclusion
Neurological manifestations may commonly happen in patients with COVID‐19. This study reported a high prevalence of neurological complications and mortality rates in COVID‐19 patients. Therefore, patients with COVID‐19 who indicated neurological symptoms should be taken seriously and should receive early treatment to prevent undesirable events.
Keywords: COVID‐19, nervous system, neurological manifestations, SARS‐CoV‐2
A total of 4455 studies were collected from PubMed/Medline, Embase, and Web of Science databases. After the removal of duplicates, articles were screened for title and abstract, and 119 papers met the criteria. Thereafter, the full‐text of these studies, which all reported the neurological symptoms of COVID‐19 infection, were evaluated. Following the exclusion of irrelevant studies, 20 articles were identified as qualified for final extraction and analysis.

1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19), an infection induced by severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2), originated in Wuhan, China in December 2019. This disease spread rapidly around the world and caused the deaths of millions of people. 1 In 2002 and 2012, the outbreaks of other members of this betacoronavirus, namely SARS‐CoV and MERS‐CoV, were, respectively, recorded. 2 According to the WHO report, more than 100 million cases were infected with SARS‐CoV‐2, and about four million deaths from COVID‐19 were reported.
Conforming to the Centers for Disease Control and Prevention (CDC), COVID‐19 is accompanied by three main symptoms, including common (fever, dry cough, headache, and fatigue), less common (pain, sore throat, diarrhea, and loss of taste and appetite), and severe (shortness of breath, chest pain, and dysfunction in speech). The disease is not limited to the respiratory system, but it encompasses a wide range of cardiovascular and neurological complications. 3 Search of genomes and coronavirus‐related sequences in the cerebrospinal fluid (CSF) verifies this assumption. 4 The intensified complications, severe symptoms, and high mortality rate of COVID‐19 have forced special attention to this disease. While respiratory and cardiac complications are considered high risks in patients with COVID‐19, neurological complications have also been demonstrated to enhance mortality rate. 5
SARS‐CoV‐2, the same as SARS‐CoV and MERS‐CoV viruses, has several routes of entry to central nervous system (CNS) and peripheral nervous system (PNS), including olfactory pathway and gut–brain axis as the neural pathways 6 and blood–brain barrier (BBB) as hematogenous pathway. 7 , 8 These accessibilities are provided by the expansion and expression of angiotensin‐converting enzyme 2 (ACE2) on nerve tissues and capillary endothelial tissues, 9 which are also present in brain endothelial cells and small intestine. 10 This notion corroborates earlier investigations that stated reduced RNA tracking in CSF may be the indication of direct SARS‐CoV‐2 invasion to nervous tissue in the cell‐to‐cell spread pattern. 11 , 12 However, inaccessibility to virus in the normal range of detection via the analysis of CSF samples and also the presence of hemoproteins product may relate to the lack of identifying SARS‐CoV‐2 in these samples. 13
The first step in brain damage caused by the SARS‐COV‐2 is triggering the inflammatory response of cytokines (e.g., IL‐6, IL‐12, and TNF‐α) after binding the virus to ACE2 and its proliferation in the CNS. 5 , 10 Neurological complications that affect CNS, PNS, and musculoskeletal system 8 , 13 entail impaired consciousness, stroke, anosmia, ataxia, and seizures, acute necrotizing encephalopathy, meningoencephalitis, and Guillain–Barre syndrome (GBS), recognized as PNS‐related symptoms, as well as Miller Fisher syndrome and hypogeusia that happen as a result of SARS‐CoV‐2 entry to peripheral neurons. 14 Collectively, the direct invasion of virus, immune‐induced complications, and cytokine storms are the main pathogenesis of the SARS‐CoV‐2. It is surmised that the immune system role is central to the development of neurological complications caused by this virus. 15 , 16 The present study aimed to evaluate the prevalence of neurological manifestations and mortality rate in patients with COVID‐19.
2. MATERIALS AND METHODS
The present systematic review was conducted by relying on Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statements. 17
2.1. Search strategy
Our systematic search was performed using databases viz PubMed/Medline, Web of Sciences, and Embase from January 1, 2019 to March 7, 2021. The terms employed in the search strategies were as follows: “Central nervous system” or “brain” or “neurologic” or “CNS “and “COVID‐19” or “severe acute respiratory syndrome coronavirus 2” or “novel coronavirus” or “SARS‐CoV‐2” or “SARS2” or”2019‐nCoV” or “nCoV disease” or “coronavirus disease‐19” or “coronavirus disease 2019” or “2019 novel coronavirus” or “Wuhan coronavirus” or “Wuhan seafood market pneumonia virus” or “Wuhan pneumonia”. There were no language restrictions; however, for non‐English articles, online translation systems were utilized. Besides, no limitations were considered for the location and type of articles.
2.2. Inclusion and exclusion criteria
All original studies presenting the neurological symptoms in COVID‐19 patients were regarded in our search. The results of final search were imported into EndNote X9 software (Thomson Reuters, San Francisco, CA), and duplicates were eliminated. We performed a three‐step screening to determine eligible results based on title, abstract, and full‐text. In all the studies included, patients developed neurological features and were positive for SARS‐CoV‐2. Reverse transcription‐polymerase chain reaction (RT‐PCR) was the main diagnosis method of confirming COVID‐19, but multiple studies added serological tests and computed tomography (CT) scan, as well. Regarding nervous system involvement, we took into consideration all reported neurological findings, comprising CNS and PNS symptoms, CNS inflammatory features, and cerebrovascular attributes, as well as neuromuscular, psychiatric, and non‐specific symptoms. All the methods used for the diagnosis of neurological symptoms were viewed as qualified. The exclusion of articles was based on any of the following papers: Review articles, systematic review or meta‐analysis, duplicate publication of same researchers, congress abstracts, and articles reporting a history of neurological features prior to COVID‐19 infection. Likewise, animal studies, case reports, and case series were excluded.
2.3. Data extraction and quality assessment
The data retrieved for each article were as follows: first author's name, location, publication time, sample size, number of COVID‐19 patients, gender, median age, COVID‐19 diagnosis method, neurological manifestations, diagnosis method of neurological findings, and number of deaths. Four authors extracted the information from the full‐text of 20 selected studies, independently. Inconsistencies between reviewers were resolved by consultation. Table 1 shows the extracted data.
TABLE 1.
Characteristics of the included studies
| References | Country | Published time | No. of patients with COVID‐19 | Male | Median age (years) | Covid‐19 diagnosis method | No. of patients with neurological manifestations | Diagnosis method for neurological manifestation | Neurological manifestations | Death |
|---|---|---|---|---|---|---|---|---|---|---|
| Helms 18 | France | Aug, 2020 | 140 | 100 | 62 | RT‐PCR, CT scan | 118 | CNE, RASS, CAM‐ICU, EEG, MRI, CSF analysis | NM | 21 |
| Karadaş 8 | Turkey | Jun, 2020 | 239 | 133 | 39 | RT‐PCR, CT scan | 83 | CNE, Brain CT, MRI, EEG | Headache 64, neuralgia 19, visual impairment 11, nizziness 16, auditory dysfunction 8, Numbness 12, Bifurcation in voice 3, Anosmia/hyposmia 18, Ageusia/dysgeusia 16, CVD 9, Impaired consciousness‐confusion 23, Sleep impairment 30, Orthostatic hypertension 8, Balance disorder 6, Myalgia 36, GBS 1, RLS 4 | NM |
| Frontera 19 | USA | Jan, 2021 | 4491 | 2607 | NM | RT‐PCR | 606 | CNE, Brain CT, MRI, EEG, CSF analysis | Encephalopathy 309, Stroke 84, Seizure 74, Hypoxic/ischemic brain injury 65, Movement disorder 41, Neuropathy 35, Myopathy 21, GBS 3 | 211 |
| Luigetti 20 | Italy | Jul, 2020 | 213 | 137 | 70.2 | RT‐PCR | 64 | CNE, CSF analysis | Headache 10, Dizziness 3, Balance disorder 3, Encephalopathy 86, Ageusia/dysgeusia 6, Anosmia/hyposmia 13, Seizure 6, Stroke 4, Encephalitis 1, Weakness 69, Myalgia 20, skeletal muscle injury 10 | 40 |
| Kandemirli 21 | Turkey | Oct, 2020 | 235 | NM | NM | RT‐PCR | 50 | CNE, MRI, CSF analysis | NM | NM |
| Sandoval 22 | Chile | Mar, 2021 | 90 | NM | NM | RT‐PCR, ELISA | 13 | CNE, EEG, Brain CT, MRI, CSF analysis | Seizure 3, Encephalopathy 6, myalgia 8, Anosmia/hyposmia 2, Other cranial nerves impairments 2, orthostatic intolerance 2 | 3 |
| Studart‐neto 53 | Brazil | Aug, 2020 | 1208 | NM | 57.4 | RT‐PCR, CT scan | 89 | CNE, Brain CT, MRI, CSF analysis | Encephalopathy 43, Stroke 11, Cerebral venous Thrombosis 2, Intracranial hemorrhage 2, Seizure 8, neuropathy 3, Rhabdomyolysis 2, headache 3, vertigo 2, movement disorder 6, impaired consciousness 35, psychomotor agitation 12, delayed awakening from sedation 5, focal neurological deficit 3, weakness 16 | 14 |
| Xiong 24 | China | Sep, 2020 | 917 | 504 | NM | RT‐PCR, CT scan | 39 | CNE, CSF analysis, Brain CT | Delirium 7, coma 14, Syncope 3, Stroke 1, Myalgia 2, headache 2, neuralgia 1 | 30 |
| Liguori 33 | Italy | Aug, 2020 | 103 | 59 | 55 | RT‐PCR | 94 | CNE | Sleep impairment 51, Ageusia/dysgeusia 48, headache 40, Anosmia/hyposmia 40, depression 39, Auditory Dysfunction 2, Confusion 23, Dizziness 27, Numbness/Paresthesia 6, Fatigue 33, daytime sleepiness 34, Myalgia 25, Anxiety 34 | NM |
| Scullen 25 | USA | Sep, 2020 | 76 | 40 | 59.8 | RT‐PCR, CT scan | 27 | CNE, EEG, Brain CT, MRI, SWI | Altered mental status 26, Encephalopathy 22, vasculopathy 5, Ageusia/dysgeusia 1, Weakness 1, Headache 2, visual impairment 14, Decerebrate posturing 1, Facial droop 1, Hemineglect 2, Hemiparesis or hemiplegia 4, Quadriplegia 1 | NM |
| Iltaf‐Sr 4 | Pakistan | Aug, 2020 | 350 | 245 | 49.5 | RT‐PCR | 68 | CNE | Headache 12, vertigo 12, numbness/paresthesia 11, consciousness 7, Anosmia/hyposmia 5, encephalitis 3, Stroke 2, GBS 1, Seizure 1 | NM |
| Khedr 26 | Egypt | Feb, 2021 | 439 | NM | 55.1 | RT‐PCR, CT scan | 117 | CNE, Brain CT, MRI, EMG | Headache 47, dizziness 50, myalgia 40, anxiety 10, suicidal trial 1, CVD 55, convulsions 5, encephalitis 6, Encephalopathy 4, attack of relapse of RR‐MS 2, transverse myelitis 2, meningoencephalitits 1, GBS 4, neuropathy 3, myasthenia gravis 2, myositis 2, isolated cranial nerve affection 31, Anosmia/hyposmia 31 | NM |
| Chougar 10 | France | Jul, 2020 | 1176 | NM | 61.2 | RT‐PCR, CT scan | 223 | CNE, MRI, EEG, CSF analysis | Focal neurological deficit 43, Seizure 13, altered mental status 21, headache 31, confusion 30, impaired consciousness 40, coma 3, delayed awakening from sedation 11, peripheral vestibular syndrome 1, Anosmia/hyposmia 22, visual impairment 6, GBS 1 | NM |
| Nersesjan 11 | Denmark | Jan, 2021 | 61 | 38 | 62.7 | RT‐PCR | 28 | CNE, Brain CT, MRI, EEG, CSF analysis, NCS | Weakness 21, Anosmia/hyposmia 18, headache 10, sensory symptoms 3, Seizure 4, hallucination 12, affect lability 2, paranoia 4, delirium 21, Stroke 4, Encephalopathy 19, peripheral facial palsy 2, myalgia 2, encephalitis 2, myelitis 1, rhabdomyolysis 1, neuropathy 1, altered mental status 18, dysexecutive function 20, delayed awakening from sedation 9, coma 2 | 12 |
| Abled‐Mannan 27 | UK | Oct, 2020 | 50 | NM | 11.7 | RT‐PCR, ELISA | 4 | CNE, Brain CT, MRI, CSF analysis, EMG | Encephalopathy 4, headache 3, dysarthria or dysphagia 2, meningism 1, ataxia 1, myalgia 4, reduced reflexes 2 | 0 |
| LaRovere 28 | USA | Feb, 2021 | 1695 | 909 | 9.1 | RT‐PCR, ELISA | 365 | CNE, Brain CT, MRI, SWI | Encephalopathy 15, Stroke 12, GBS 4, acute CNS infection/ADEM 8, acute fulminant cerebral edema 4 | 11 |
| Rifino 54 | Italy | Oct, 2020 | 1760 | 1162 | 64.9 | RT‐PCR, CT scan | 137 | CNE, EEG, EP, ENG‐EMG, Brain CT, MRI, CSF analysis | CVD 53, Stroke 48, Transient ischemic attacks 4, Cerebral venous thrombosis 1, neuropathy 45, GBS 17, Altered mental status 49, Encephalitis 5, Myelitis 2, Headache 3, Seizure 10, Syncope 3, Movement disorder 7 | 41 |
| Mao 30 | China | Apr, 2020 | 214 | 87 | 52.7 | RT‐PCR, CT scan | 78 | CNE, Brain CT | Dizziness 36, Headache 28, Impaired consciousness 16, CVD 6, Ataxia 1, Seizure 1, ageusia/dysageusia 12, Anosmia/hyposmia 11, visual impairment 3, Neuralgia 5, Myalgia 23 | NM |
| Eskandar 31 | Montefiore | Dec, 2020 | 4711 | NM | 63.4 | RT‐PCR | 581 | CNE, Brain CT, MRI | Altered mental status 258, Stroke 55, seizures 26 | 199 |
| Shekhar 32 | USA | Aug, 2020 | 90 | NM | 52.3 | RT‐PCR | 7 | CNE, MRI, EEG, Brain CT, DWI | Altered mental status 7, visual impirment 2, Seizure 4 | 2 |
Abbreviations: CNE, clinical neurological exams; CAM‐ICU, confusion assessment method for the ICU; CT, computerized tomography; CVD, cerebrovascular disease; EEG, electroencephalography; EMG, electromyography; ENG‐EMG, electroneurographic and electromyographic recordings; EP, evoked potentials; GBS: Guillain–Barre syndrome; MRI, magnetic resonance imaging; NCS, nerve conduction study; NM, not mentioned; RASS, Richmond Agitation‐Sedation Scale; RLS, restless leg syndrome; SWI, susceptibility weighted imaging.
2.4. Data synthesis and analysis
Analysis of data was performed by using STATA (version 14, IC; Stata Corporation, College Station, TX, USA), and the prevalence of neurological complications was estimated with confidence intervals (CIs) of 95%. The pooled frequency was calculated by a random effect model with 95% CI. Publication bias was also assessed by Begg's and Egger's tests, and p value <0.05 was considered an indication of statistically significant publication bias.
3. RESULTS
A total of 4455 studies were collected from three databases. After the removal of duplicates, articles were screened for title and abstract, and 119 papers met the criteria. Thereafter, the full‐text of these studies, which all reported the neurological symptoms of COVID‐19 infection, were evaluated. Following the exclusion of irrelevant studies, only 20 articles were identified as qualified for final extraction and analysis (Figure 1). These papers were appropriate for systematic review and entered into data extraction. Eighteen of these clinical studies used various methods, such as brain CT, MRI, EGG, and CSF for the diagnosis of neurological manifestations. 8 , 10 , 11 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 However, two other studies performed only clinical neurological exams. 4 , 33 In all 20 studies, RT‐PCR was examined using nasopharyngeal swab, but nine 8 , 10 , 18 , 23 , 24 , 25 , 26 , 29 , 30 and three 22 , 27 , 28 studies employed chest CT scan and ELISA, respectively, to confirm COVID‐19. Ten articles were originated from Europe, five from the USA, two from China, and others from Brazil, Pakistan, Egypt, and Chile. The information of these 20 studies is summarized in Table 1. A variety of neurological findings were reviewed and classified into different groups (Table 2). Among 20 articles, 18,258 cases were found by RT‐PCR test to be positive for SARS‐CoV‐2 (COVID‐19), and 2791 of patients had various neurological manifestations. The overall frequency of neurological symptoms in COVID‐19 patients was 15.28%. Additionally, 12 articles reported mortality. Among the 2791 patients, 582 succumbed in hospital; therefore, the total mortality rate was 20.85%.
FIGURE 1.

Flow diagram detailing review process and study selection
TABLE 2.
Categorized neurological findings of included studies
| Variables | No. of studies | n/N | Percentage (%) |
|---|---|---|---|
| Non‐specific symptoms | |||
| Headache | 13 | 255/1051 | 24.26 |
| Dizziness | |||
| Weakness | 5 | 132/436 | 30.27 |
| Fatigue | 3 | 38/144 | 26.39 |
| Impaired consciousness/confusion | 1 | 33/94 | 35.10 |
| Syncope | 6 | 174/635 | 27.40 |
| Neuropathy | 2 | 6/176 | 3.40 |
| Altered mental status | 5 | 87/977 | 8.81 |
| Neuralgia | 6 | 379/1003 | 37.78 |
| Sleep impairment | 3 | 25/200 | 12.50 |
| Daytime sleepiness | 2 | 81/177 | 45.76 |
| Bifurcation in voice | 1 | 34/94 | 36.17 |
| Balance disorder | 1 | 3/83 | 3.61 |
| Numbness/paresthesia | 2 | 10/177 | 5.65 |
| Vertigo | 3 | 29/245 | 11.83 |
| Reduced reflexes | 2 | 14/157 | 8.92 |
| Hemiparesis/hemiplegia | 1 | 2/4 | 50 |
| Quadriplegia | 1 | 4/27 | 14.81 |
| Peripheral facial palsy | 1 | 1/27 | 3.70 |
| Hemineglect | 1 | 2/28 | 7.14 |
| Focal neurological deficit | 1 | 2/27 | 7.40% |
| 2 | 46/312 | 14.74% | |
| Psychiatric symptoms | |||
| Depression | 1 | 39/94 | 41.50% |
| Anxiety | 2 | 44/211 | 20.85% |
| Delirium | 2 | 28/67 | 41.79% |
| Paranoia | 1 | 4/28 | 14.28% |
| Hallucination | 1 | 12/28 | 42.86% |
| Suicidal trial | 1 | 1/117 | 0.85% |
| Affect lability | 1 | 2/28 | 7.14% |
| Psychomotor agitation | 1 | 12/89 | 13.48% |
| CNS disorders | |||
| Seizure/convulsion | 12 | 155/2011 | 7.71 |
| Stroke | 9 | 221/1977 | 11.18 |
| Coma | 3 | 19/290 | 6.55 |
| Meningism | 1 | 1/4 | 25 |
| Isolated cranial nerve affection | 1 | 31/117 | 26.49 |
| Dysexecutive function | 1 | 20/28 | 71.43 |
| Orthostatic hypertension | 1 | 8/83 | 9.64 |
| Orthostatic intolerance | 1 | 2/13 | 15.38 |
| Ataxia | 2 | 2/82 | 2.44 |
| Acute fulminant cerebral edema | 1 | 4/365 | 1.09 |
| Other cranial nerve impairments | 1 | 2/13 | 15.38 |
| Cerebrovascular disorders | |||
| Cerebral venous thrombosis | 2 | 3/226 | 1.33 |
| Transient ischemic attacks | 1 | 4/137 | 2.92 |
| Intracranial hemorrhage | 1 | 2/89 | 2.25 |
| CVD | 4 | 123/415 | 29.64 |
| Vasculopathy | 1 | 5/27 | 18.52 |
| Hypoxic/ischemic brain injury | 1 | 65/606 | 10.72 |
| CNS inflammatory disorders | |||
| Encephalopathy | 8 | 422/1249 | 33.79 |
| Encephalitis | 5 | 17/414 | 4.10 |
| Transverse myelitis | 1 | 2/117 | 1.71 |
| Myelitis | 2 | 3/165 | 1.82 |
| Meningoencephalitis | 1 | 1/117 | 0.85 |
| GBS | 7 | 31/1599 | 1.94 |
| Acute CNS infection/ADEM | 1 | 8/365 | 2.19 |
| PNS disorders | |||
| Anosmia/hyposmia | 9 | 160/768 | 20.83 |
| Ageusia/dysageusia | 5 | 83/346 | 23.99 |
| Auditory dysfunction | 2 | 10/177 | 5.65 |
| Visual impairment | 5 | 36/418 | 8.61 |
| Peripheral vestibular syndrome | 1 | 1/223 | 0.45 |
| Sensory symptoms | 1 | 3/28 | 10.71 |
| Myasthenia gravis | 1 | 2/117 | 1.70 |
| Attack of relapse of RR‐MS | 1 | 2/117 | 1.70 |
| RLS | 1 | 4/83 | 4.82 |
| Neuromuscular disorders | |||
| Myalgia | 9 | 139/520 | 26.73 |
| Myopathy | 1 | 21/606 | 3.46 |
| Movement disorder | 3 | 54/832 | 6.49 |
| Myositis | 1 | 2/117 | 1.70 |
| Dysarthria/dysphagia | 1 | 2/4 | 50 |
| Rhabdomyolysis | 2 | 3/117 | 2.56 |
| Facial droop | 1 | 1/27 | 3.70 |
| Decerebrate posturing | 1 | 1/27 | 3.70 |
| Skeletal muscle injury | 1 | 10/64 | 15.62 |
| Others | |||
| Delayed awakening from sedation | 3 | 25/340 | 7.35 |
3.1. Non‐specific neurological features in confirmed COVID‐19 patients
Since the emerging COVID‐19 infection, a noticeable large number of studies have focused their attention on this outstanding issue; thus, various symptoms and signs have been reported for this disease. Based on the literature, headache, 34 confusion, 35 and fatigue 36 , 37 are the most common symptoms. In addition, altered mental status is realized as a common initial presentation in COVID‐19 patients. 38 Our results are in line with these findings. Among 20 included studies we reviewed, 13, 5, 3, 1, 6, and 6 studies reported headache, dizziness, weakness, fatigue, confusion, and altered mental status, respectively, as the most frequent symptoms. Five studies reported the observation of neuropathy features in 87 of 977 examined patients. 11 , 19 , 23 , 26 , 29 Two articles stated 45.76% (81/177) with sleep disorder, 8 , 33 and one of these studies mentioned daytime sleepiness in 36.17% (34/94). 33 The relative frequency of reduced reflex symptoms was 50% (2/4), standing as the most common symptoms. However, it could not be considered a notable manifestation due to the inadequate number of cases. 27 Hemiparesis and hemiplegia were detected in 14.81% (4/27), as reported by one study. 25
3.2. Characteristics of CNS disorders in confirmed COVID‐19 patients
Evidence has shown that coronavirus can target CNS and exert its neurotropic effects. 39 On the basis of this review, a broad spectrum of CNS manifestations was reported in coronavirus‐infected patients and classified into CNS disorders, CNS inflammatory disorders, and cerebrovascular disorders. The number of stroke (11.18%), coma (6.55%), and seizure/convulsion (7.71%) patients were much more than any other CNS disorders. Thirteen studies reported at least one of these disorders in COVID‐19 patients. 4 , 10 , 11 , 19 , 20 , 22 , 23 , 24 , 29 , 30 , 31 , 32 Remarkably, stroke, as a major concern in COVID‐19, was identified in an overall number of 211 out of 1977 patients. Neuroinvasion of coronavirus can disrupt immune system of CNS, leading to CNS inflammatory disorder. 40 , 41 Our results identified encephalopathy, 11 , 19 , 20 , 22 , 23 , 25 , 26 , 27 , 28 encephalitis, 4 , 11 , 20 , 26 , 29 transverse myelitis, 26 meningoencephalitis, 26 and GBS 4 , 8 , 10 , 19 , 26 , 28 , 29 as the inflammatory disorders of CNS. Among these disorders, encephalopathy had notable symptoms with a high frequency of 33.79% (422/1249). CNS and vascular system are coupled and inseparable systems influencing each other 42 ; therefore, alteration of CNS can affect vascular systems. This review is consistent with the clinical symptoms of these effects. Cerebral venous thrombosis, 23 , 29 vasculopathy, 25 and cardiovascular diseases 8 , 26 , 29 , 30 were prominent cerebrovascular manifestations of COVID‐19.
3.3. Characteristics of PNS and psychiatric symptoms in COVID‐19 patients
Nine studies reported 160 anosmia/hyposmia cases out of 768 COVID‐19 patients. 4 , 8 , 10 , 11 , 20 , 22 , 26 , 30 , 33 Six articles emphasized at least one of the following features: ageusia/dysgeusia (160 patients), auditory dysfunction (10 patients), and visual impairment (36 patients). Myopathy 19 and myalgia 8 , 11 , 20 , 22 , 24 , 26 , 27 , 30 , 33 had the overall frequencies of 3.46% and 26.73%, respectively. Furthermore, some rare cases of rhabdomyolysis, 11 , 23 myositis, 26 and dysarthria 27 were explored. Coronavirus can affect nervous system in a way that develops psychiatric symptoms. Depression (41.50%), anxiety (20.85%), delirium (41.79%), and hallucination (42.86%) were behavioral features observed as the most common psychiatric manifestations. 11 , 24 , 26 , 33 A few cases of paranoia, 11 suicidal trial, 26 and psychomotor agitation 23 were also reported by three articles.
3.4. Prevalence of neurological manifestations in COVID‐19 patients
From 20 studies examined, the pooled prevalence of neurological manifestations was estimated as 23% (95% CI 17.8–29.2) in COVID‐19 patients. Figure 2 depicts a forest plot for meta‐analysis of nervous system involvement in COVID‐19. There was no publication bias (p = 0.54 for Begg's rank correlation analysis and p = 0.06 for Egger's weighted regression analysis), as shown in Figure 3.
FIGURE 2.

Forest plot of meta‐analysis on neurological manifestations of COVID‐19
FIGURE 3.
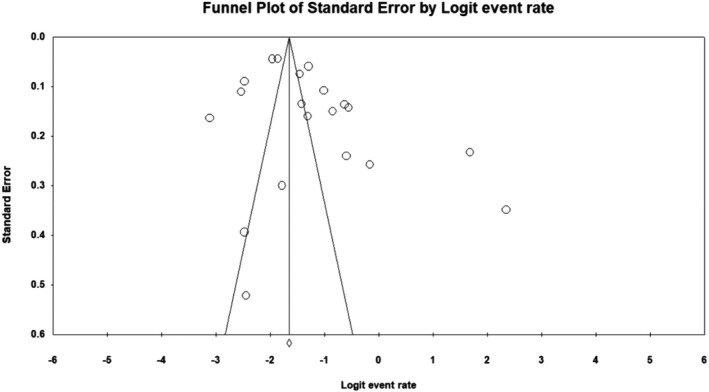
Funnel plot of meta‐analysis on neurological manifestations of COVID‐19
3.5. Mortality of COVID‐19 patients who developed nervous system symptoms
In total, 12 studies reported the number of deaths as the outcome. The pooled mortality of COVID‐19 patients with neurological features was estimated to be 29.1% (95% CI: 20.3–39.8). Figure 4 displays the meta‐analysis of the overall mortality rate. As indicated in Figure 5, no evidence of publication bias was found (p = 0.54 and p = 0.57 for Begg's rank correlation analysis and Egger's weighted regression analysis, respectively).
FIGURE 4.

Forest plot of meta‐analysis on mortality rate from neurological features of COVID‐19
FIGURE 5.

Funnel plot of meta‐analysis on the mortality rate from neurological features of COVID‐19
3.6. Risk of bias assessment
According to the quality assessment of the 20 included studies, all of them had a low risk of bias. We summarized the results of the critical appraisal (JBI checklist) of included studies in Table S1.
4. DISCUSSION
The present study was performed on 18,258 enrolled COVID‐19 patients, from which 2791 cases showed neurological complications. To the best of our knowledge, no survey has hitherto addressed the prevalence of neurological complications of COVID‐19, and this systematic review/meta‐analysis is the first study to investigate the mortality rate and prevalence of neurological manifestations, which is classified into several subgroups. In a study performed by Mao et al., the evaluation of neurological complications in COVID‐19 patients contributes to the early diagnosis of severe respiratory disease, prevention of death, and control of transmission cycle to other healthy individuals. 43 Therefore, through the evaluation of COVID‐19‐induced neurological damages and underlying mechanisms, more effective treatment strategies can be proposed to decline the severity and impact of SARS‐CoV‐2 on the nervous system. 23 The result of our study revealed that the overall prevalence of neurological complications of COVID‐19 is 23%. This high rate may have led to later recovery of patients with COVID‐19 and their longer hospital stay. Overexpression of ACE2 in nervous tissues and proinflammatory cytokines could be a major factor affecting vascular endothelium and elevating BBB permeability. 12 Neurological manifestations of COVID‐19 are defined in two general groups: non‐specific and specific symptoms. Psychiatric symptoms, CNS disorders, cerebrovascular disorders, CNS inflammatory disorders, PNS disorders, and neuromuscular disorders are recognized as non‐specific and other symptoms as specific neurological manifestations. Non‐specific neurological complications can arise from systematic behaviors, 44 whereas specific complications are associated with direct virus invasion to nervous tissue. However, Luigetti et al., 20 based on CSF results, ruled out this direct invasion of virus. Patients with severe respiratory distress of COVID‐19 showed more neurological complications, which supports the reports of intracranial arterial stroke in hospitalized patients, even those treated with intravenous immunoglobulin. 21 Emerging evidence has suggested a low frequency of COVID‐19 in children; inflammatory conditions in children with COVID‐19 receiving chimeric antigen receptor T‐cell therapy influence both CNS and PNS, giving rise to neurological manifestations. 27 Patients who experienced delirium and other serious neurological complications require long‐term stay in ICU with mechanical ventilation and higher doses of sedatives and neuroleptics than other asymptomatic patients, resulting in delayed discharge from the hospital. 18
Depression, anxiety, delirium, paranoia, hallucination, suicidal trial, affect lability, and psychomotor agitation were psychiatric symptoms observed in studied patients; depression (41.50%) and anxiety (20.85%) were the most frequently occurring symptoms. Delirium, a serious mental disability that diminish consciousness, showed an inflammatory origin following SARS‐CoV‐2 infection. 18 CNS disorders are characterized by seizure/convulsion, stroke, coma, meningism, isolated cranial nerve affection, dysexecutive function, orthostatic hypertension, orthostatic intolerance, ataxia, acute fulminant cerebral edema, and other cranial nerve impairments. Seizure/convulsion, stroke, and coma are the most frequently symptoms reported in studies; however, isolated cranial nerve affection (26.49%) and dysexecutive function (71.43%) were found separately by an article as the most examined symptoms. Increased blood coagulation and cytokine levels, as well as the employment of immune cell components have been exhibited to cause tissue destruction, ultimately leading to stroke in patients with SARS‐CoV‐2. Karadaş et al. 8 , 10 and Chougar et al. 8 , 10 believe that this rare complication is rooted in the virus invasion to blood vessels. Other prognosis for stroke includes the elevated levels of leukocytes, D‐dimer, CRP, lactate dehydrogenase, ferritin in serum, 4 , 45 and low count of lymphocyte. 43 Seizure is not a common neurological symptom of SARS‐CoV‐2, and the increased seizure reported in our investigation, according to Khedr et al.’s 26 study, may have a link to the neurology specialization admission of cases with neurological manifestations. However, the occurrence of seizures in patients with COVID‐19 has been denoted to be associated with metabolic changes, fever, 10 or the existence of tumors in CNS. 22 Cerebral venous thrombosis, transient ischemic attacks, intracranial hemorrhage, CVD, vasculopathy, and hypoxic/ischemic brain injury are the main symptoms appeared in cerebrovascular disorders, which CVD (29.64%), vasculopathy (18.52%), and hypoxic/ischemic brain injury (10.72%) were the major symptoms. Cerebrovascular endotheliitis and the simultaneous presence of several disorders such as hypertension and diabetes were associated with increased tissue neurotropism SARS‐CoV‐2 predisposition of patients with COVID‐19 to cerebrovascular disorder by ACE2 expression enhancement. SARS‐CoV‐2 disrupts blood pressure homeostasis by blocking ACE2, a factor involved in the reduction of blood pressure. 26 Encephalopathy, encephalitis, transverse myelitis, myelitis, meningoencephalitis, GBS and acute CNS infection/ADEM are inflammatory disorders of CNS. Encephalopathy, encephalitis, and GBS were extensively studied in articles, and encephalopathy reported as the most inflammatory disorders related to CNS following COVID‐19. Iltaf et al. 4 , 23 and Studart‐Neto et al. 4 , 23 speculated that encephalitis and encephalopathy are connected with the increased secretion of proinflammatory cytokines (e.g. IL‐2, ‐6, ‐7, TNF‐α, IFN‐γ) and antioxidant compounds (e.g., free radicals). GBS is a myelin‐destroying disorder induced by the immune system and causes muscular weakness, likely due to the similarity of the structure of SARS‐CoV‐2 epitopes with myelin proteins, which results in an autoimmune reaction. 10 , 26 Although being a rare complication, GBS is mostly caused by a wide range of viral and bacterial microorganisms through gastrointestinal infections such as Campylobacter and in rare cases, by respiratory infections such as SARS‐COV‐2. 4 , 12 In PNS disorders, the most frequent symptoms were anosmia/hyposmia, ageusia/dysageusia, and visual impairment. Other manifestations included auditory dysfunction, peripheral vestibular syndrome, sensory symptoms, myasthenia gravis, and relapse/attack of RR‐MS and RLS. Anosmia and ageusia disorders can be interpreted by the fact that the SARS‐CoV‐2 uses ACE2 receptors in the olfactory tissue to access the nerves. 22 , 26 Myalgia, myopathy, movement disorder, myositis, dysarthria/dysphagia, rhabdomyolysis, facial droop, decerebrate posturing, and skeletal muscle injury are known among neuromuscular disorders, which myalgia, as most the studied neuromuscular disorders, is considered to have inflammatory origin 46 and related to the high levels of lactate dehydrogenase and creatine kinase. 43 The latter may be relevant to the presence of ACE2 in muscles; however, this surmise needs further investigation.
Non‐specific neurological manifestation encompass headache, dizziness, weakness, fatigue, impaired consciousness/confusion, syncope, neuropathy, altered mental status, neuralgia, sleep impairment, daytime sleepiness, bifurcation in voice, balance disorder, numbness/paresthesia, vertigo, reduced reflexes, hemiparesis/hemiplegia, quadriplegia, peripheral facial palsy, hemineglect, and focal neurological deficit. Among these symptoms, headache, dizziness, impaired consciousness/confusion, and altered mental status were the most studied variables, while reduced reflexes, sleep impairment, daytime sleepiness, fatigue, dizziness, weakness, and headache showed high occurrence among patients with COVID‐19. Headache, as one of the frequent non‐specific manifestations, has reflected less association with mortality due to the fact that patients with headaches have less symptoms related to increased cytokine storm, such as D‐dimer, ferritin etc. Moreover, these patients had a history with lower blood pressure, as well as cardiovascular and nerve diseases. There is a direct relationship between headache and inflammation caused by COVID‐19 and high levels of IL‐6 as a marker of pain. 47
One of the significant findings of the present study was the mortality rate (29.1%) due to neurological complications of COVID‐19. Other complications of COVID‐19 have been described as respiratory and non‐respiratory complications, which there is no doubt that respiratory complications have more severe outcome, as stated by Vakili and co‐workers. 48 However, non‐respiratory complications are less prevalent but are more common in terms of mortality and can induce more risky situation for patients. According to LaRovere et al.’s study, neurological complications are not very serious; of 12% of patients who showed neurological complications, 66% developed death. 28 Obviously, the neurological complications of COVID‐19 are associated with increased in‐hospital mortality of patients, which this statement is in agreement with Chou et al.’s 49 cohort study. In‐hospital death of patients with COVID‐19 is also related to sex (i.e., males), diabetes mellitus, history of chronic pulmonary disease, increasing age, D‐dimer, body mass index, sequential organ failure assessment scores, and so forth. The number of neurological manifestations such as stroke and altered mentation (p < 0.05) in COVID‐19 patients may be a high‐risk factor for hospital‐related mortality. 31 , 50 Considering the reduced mortality rate of patients who were admitted in ICU, dexamethasone showed effective outcomes due to its inhibitory activity against immune response, which is essential for the inflammatory conditions. However, these corticosteroids induce neurological manifestations in long‐term administration. 51 The mortality rate was higher in patients with the in vitro symptoms of COVID‐19 who suffered from neurological complications. This finding agrees with the study conducted by Frontera et al. 19 who highlighted that patients with stroke are more prone to death than those who have stroke but not COVID‐19, which is attributed to prolonged hospitalization.
Mortality rate of neurological complications vary from country to country, owing to the age of patients and the level of access to treatment. 51 According to WHO recent report (14 July 2021), the case fatality rate of patients infected with COVID‐19 was estimated to be 2.15%. Since no study has compared the mortality rate of respiratory and neurological complications, the mortality rate of COVID‐19‐related neurological complications (29.1%) obtained by the present study were compared with that of severe respiratory complications of COVID‐19 (39%) reported in Hasan et al.’s 52 survey. They portrayed that the mortality rate of neurological complications in COVID‐19 patients was not higher than respiratory complications in these patients. Of note, serious neurological manifestations in patients with COVID‐19 cannot be ignored, and the mortality from respiratory complications of this disease varies in different countries, as reported to be 13% and 73% for Germany and Poland, respectively. 52
There were some limitations for the current study. First, in addition to the bias of publications, which is common among almost systematic reviews, there were few heterogeneities in the prevalence of neurological complications and the number of studies. Second, inclusion criteria and excluding case reports and non‐English studies may lead to dismiss specific neurological complications. Third, since the COVID‐19 pandemic has not yet over, the results and underlying mechanisms of this disease would certainly be much more impressive than we expect. Unlike some systematic reviews that reviewed the majority of studies conducted in China, this work attempted to avoid the risk of bias by reviewing articles from different countries. Moreover, as there are no reports of neurological mortality from COVID‐19 and no data on the overall prevalence of neurological complications, this review has notable superiority over other similar studies in providing comprehensive information during a wider period of time.
5. CONCLUSION
The results of this systematic review and meta‐analysis showed that neurological complications may commonly occur in patients with COVID‐19. Neurological complications in COVID‐19 patients can arise from virus invasion to nervous tissues and post reactions such as immune‐related damage. Our study reported the prevalence of neurological complications and mortality rate of 23% and 29.1%, respectively. It indicated that headache, confusion, and fatigue were the most common neurological complications caused by COVID‐19. Taken together, patients with COVID‐19 who indicated neurological symptoms should be taken seriously and should receive early treatment to prevent undesirable events.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
Marzie Mahdizade Ari, Mohamad Hosein Mohamadi, Negar Shadab Mehr, Sajjad Abbasimoghaddam, Amirhosein Shekartabar, Mohsen Heidary, and Saeed Khoshnood contributed to revising and final approval of the version to be published. All authors agreed and confirmed the manuscript for publication.
Supporting information
Table S1
Mahdizade Ari M, Mohamadi MH, Shadab Mehr N, et al. Neurological manifestations in patients with COVID‐19: A systematic review and meta‐analysis. J Clin Lab Anal. 2022;36:e24403. doi: 10.1002/jcla.24403
Marzie Mahdizade Ari, Mohamad Hosein Mohamadi, and Negar Shadab Mehr co‐first authorship who contributed equally to this work.
Sajjad Abbasimoghaddam and Amirhosein Shekartabar co‐second authorship who contributed equally to this work.
Contributor Information
Mohsen Heidary, Email: mohsenheidary40@gmail.com.
Saeed Khoshnood, Email: saeed.khoshnood22@gmail.com.
DATA AVAILABILITY STATEMENT
All the data in this review are included in the article.
REFERENCES
- 1. Pal M, Berhanu G, Desalegn C, Kandi V. Severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2): an update. Cureus. 2020;12:7423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Wit E, van Doremalen N, Falzarano D, Munster VJ. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14:523‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Trujillo Gittermann L, Valenzuela Feris S, von Oetinger GA. Relation between COVID‐19 and Guillain‐Barré syndrome in adults: a systematic review. Neurología (English Edition). 2020;35(9):646‐654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Iltaf S Sr, Fatima M, Salman S Sr, Salam J‐U, Abbas S. Frequency of neurological presentations of coronavirus disease in patients presenting to a tertiary care hospital during the 2019 coronavirus disease pandemic. Cureus. 2020;12:e9846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bridwell R, Long B, Gottlieb M. Neurologic complications of COVID‐19. Am J Emerg Med. 2020;38:1549.e3‐1549.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol. 2020;92:699‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karadaş Ö, Öztürk B, Sonkaya AR. A prospective clinical study of detailed neurological manifestations in patients with COVID‐19. Neurol Sci. 2020;41:1991‐1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gupta I, Reddy MK, Hussain MM, Murthy PM, Robert CA. Atypical neurological manifestations of COVID‐19. Cureus. 2020;12:e8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS‐CoV‐2 infection and neurological manifestations. Radiology. 2020;297(3):E313‐E323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nersesjan V, Amiri M, Lebech A‐M, et al. Central and peripheral nervous system complications of COVID‐19: a prospective tertiary center cohort with 3‐month follow‐up. J Neurol. 2021;268:3086‐3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Amanat M, Rezaei N, Roozbeh M, et al. Neurological manifestations as the predictors of severity and mortality in hospitalized individuals with COVID‐19: a multicenter prospective clinical study. BMC Neurol. 2021;21:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harapan BN, Yoo HJ. Neurological symptoms, manifestations, and complications associated with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and coronavirus disease 19 (COVID‐19). J Neurol. 2021;268:3059‐3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aslan C, Nikfarjam S, Asadzadeh M, Jafari R. Neurological manifestations of COVID‐19: with emphasis on Iranian patients. J Neurovirol. 2021;27:217‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chong Z‐Z, Souayah N. SARS‐CoV‐2 induced neurological manifestations entangles cytokine storm that implicates for therapeutic strategies. Curr Med Chem. 2021. [DOI] [PubMed] [Google Scholar]
- 16. Costela‐Ruiz VJ, Illescas‐Montes R, Puerta‐Puerta JM, Ruiz C, Melguizo‐Rodríguez L. SARS‐CoV‐2 infection: the role of cytokines in COVID‐19 disease. Cytokine Growth Factor Rev. 2020;54:62‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8:336‐341. [DOI] [PubMed] [Google Scholar]
- 18. Helms J, Kremer S, Merdji H, et al. Delirium and encephalopathy in severe COVID‐19: a cohort analysis of ICU patients. Crit Care. 2020;24:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frontera JA, Sabadia S, Lalchan R, et al. A prospective study of neurologic disorders in hospitalized patients with COVID‐19 in New York City. Neurology. 2021;96:e575‐e586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luigetti M, Iorio R, Bentivoglio AR, et al. Assessment of neurological manifestations in hospitalized patients with COVID‐19. Eur J Neurol. 2020;27:2322‐2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology. 2020;297:E232‐E235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandoval F, Julio K, Méndez G, et al. Neurologic features associated with SARS‐CoV‐2 infection in children: a case series report. J Child Neurol. 2021;36(10):853‐866. [DOI] [PubMed] [Google Scholar]
- 23. Studart‐neto A, Guedes BF, Tuma RDLE, et al. Neurological consultations and diagnoses in a large, dedicated COVID‐19 university hospital. Arq Neuropsiquiatr. 2020;78:494‐500. [DOI] [PubMed] [Google Scholar]
- 24. Xiong W, Mu J, Guo J, et al. New onset neurologic events in people with COVID‐19 in 3 regions in China. Neurology. 2020;95:e1479‐e1487. [DOI] [PubMed] [Google Scholar]
- 25. Scullen T, Keen J, Mathkour M, Dumont AS, Kahn L. Coronavirus 2019 (COVID‐19)—associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg. 2020;141:e437‐e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Khedr EM, Abo‐Elfetoh N, Deaf E, et al. Surveillance study of acute neurological manifestations among 439 Egyptian patients with COVID‐19 in Assiut and Aswan University Hospitals. Neuroepidemiology. 2021;55:109‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abdel‐Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID‐19 infection in children. JAMA Neurol. 2020;77:1440‐1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. LaRovere KL, Riggs BJ, Poussaint TY, et al. Neurologic involvement in children and adolescents hospitalized in the United States for COVID‐19 or multisystem inflammatory syndrome. JAMA Neurol. 2021;78:536‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID‐19 patients admitted to papa Giovanni XXIII hospital, Bergamo, Italy. J Neurol. 2021;268:2331‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eskandar EN, Altschul DJ, de la Garza Ramos R, et al. Neurologic syndromes predict higher in‐hospital mortality in COVID‐19. Neurology. 2021;96:e1527‐e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shekhar R, Sheikh AB, Suriya SS, Upadhyay S, Zafar A. Neurological complications among native Americans with COVID‐19: our experience at a tertiary care academic hospital in the US. J Stroke Cerebrovasc Dis. 2020;29:105260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Liguori C, Pierantozzi M, Spanetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS‐CoV2 infection. Brain Behav Immun. 2020;88:11‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Islam MA, Alam SS, Kundu S, et al. Prevalence of headache in patients with coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis of 14,275 patients. Front Neurol. 2020;11:562634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID‐19: a review article. Neurol Sci. 2020;41:1667‐1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Komaroff AL, Bateman L. Will COVID‐19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front Med. 2021;7:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O’Connor CM. COVID‐19 Fatigue: Not So Fast. American College of Cardiology Foundation; 2020. [Google Scholar]
- 38. Ward CF, Figiel GS, McDonald WM. Altered mental status as a novel initial clinical presentation for COVID‐19 infection in the elderly. Am J Geriatr Psychiatry. 2020;28:808‐811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tavčar P, Potokar M, Kolenc M, et al. Neurotropic viruses, astrocytes, and COVID‐19. Front Cell Neurosci. 2021;15:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kempuraj D, Selvakumar GP, Ahmed ME, et al. COVID‐19, mast cells, cytokine storm, psychological stress, and neuroinflammation. Neuroscientist. 2020;26:402‐414. [DOI] [PubMed] [Google Scholar]
- 41. Zayet S, Ben Abdallah Y, Royer P‐Y, Toko L, Gendrin V, Klopfenstein T. Encephalopathy in patients with COVID‐19: 'causality or coincidence?'. J Med Virol. 2021;93(2):1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tata M, Ruhrberg C, Fantin A. Vascularisation of the central nervous system. Mech Dev. 2015;138:26‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol. 2020;19(9):767‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eskandar EN, Altschul DJ, de la Garza RR, et al. neurologic syndromes predict higher in‐hospital mortality in COVID‐19. Neurology. 2021;96:e1527‐e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang H, Charmchi Z, Seidman RJ, Anziska Y, Velayudhan V, Perk J. COVID‐19 associated myositis with severe proximal and bulbar weakness. Muscle Nerve. 2020;62(3):57‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trigo J, García‐Azorín D, Planchuelo‐Gómez Á, et al. Factors associated with the presence of headache in hospitalized COVID‐19 patients and impact on prognosis: a retrospective cohort study. J Headache Pain. 2020;21:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vakili K, Fathi M, Pezeshgi A, et al. Critical complications of COVID‐19: a descriptive meta‐analysis study. Rev Cardiovasc Med. 2020;21:433‐442. [DOI] [PubMed] [Google Scholar]
- 49. Chou SH‐Y, Beghi E, Helbok R, et al. Global incidence of neurological manifestations among patients hospitalized with COVID‐19—a report for the GCS‐NeuroCOVID Consortium and the ENERGY Consortium. JAMA Netw Open. 2021;4:e2112131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhou F, Yu T, Du R, et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID‐19 in Wuhan, China: A Retrospective Cohort Study. The lancet. Vol 395, 10229 edn, 2020;1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Al‐Ramadan A, Rabab'h O, Shah J, Al‐Gharaibeh A. Acute and post‐acute neurological complications of COVID‐19. Neurol Int. 2021;13:102‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID‐19 patients with acute respiratory distress syndrome and corticosteroids use: a systematic review and meta‐analysis. Expert Rev Respir Med. 2020;14:1149‐1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Studart‐Neto A, Guedes BF, Camelo Filho AE, et al. Neurological consultations and diagnoses in a large, dedicated COVID‐19 university hospital. Arq Neuro‐Psiquiatr. 2020;78(8):494‐500. [DOI] [PubMed] [Google Scholar]
- 54. Rifino N, Censori B, Agazzi E, et al. Neurologic manifestations in 1760 COVID‐19 patients admitted to Papa Giovanni XXIII Hospital, Bergamo, Italy. J Neurol. 2021;268(7):2331‐2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All the data in this review are included in the article.


