Abstract
Introduction
Obesity is a major health problem that is associated with many physiological and mental disorders, such as diabetes, stroke, and depression. Gut microbiota has been affirmed to interact with various organs, including the brain. Intestinal microbiota and their metabolites might target the brain directly via vagal stimulation or indirectly through immune‐neuroendocrine mechanisms, and they can regulate metabolism, adiposity, homoeostasis and energy balance, and central appetite and food reward signaling, which together have crucial roles in obesity. Studies support the concept of bidirectional signaling within the gut–brain axis (GBA) in the pathophysiology of obesity, mediated by metabolic, endocrine, neural, and immune system mechanisms.
Materials and methods
Scopus, PubMed, Google Scholar, and Web of Science databases were searched to find relevant studies.
Results
The gut–brain axis (GBA), a bidirectional connection between the gut microbiota and brain, influences physiological function and behavior through three different pathways. Neural pathway mainly consists of the enteric nervous system (ENS) and vagus nerve. Endocrine pathway, however, affects the neuroendocrine system of the brain, particularly the hypothalamus–pituitary–adrenal (HPA) axis and immunological pathway. Several alterations in the gut microbiome can lead to obesity, by modulating metabolic pathways and eating behaviors of the host through GBA. Therefore, novel therapies targeting the gut microbiome, i.e., fecal microbiota transplantation and supplementation with probiotics and prebiotics, can be a potential treatment for obesity.
Conclusion
This study corroborates the effect of gut microbiome on physiological function and body weight. The results show that the gut microbiota is becoming a target for new antiobesity therapies.
Keywords: gut–brain axis, obesity, prebiotic, probiotic, review
Gut microbiota is a complex system of organisms, mainly different bacterial species, the gut microbiota interacts with the host, affects host systems and organs (e.g. the brain) and modulates the host physiological functions. The interaction between the gut microbiota and the brain, famously known as gut‐brain axis is a bidirectional connection via neural, immune, and endocrine pathways.
1. INTRODUCTION
Currently, obesity is a global concern, and its prevalence has dramatically grown since the past few decades. Based on the World Health Organization's 2016 report, about 1.9 billion of the world's adult population were overweight and 650 million of which were obese. Besides, by 2020, 39 million children aged 0–5 years old will be overweight or obese. 1 The prevalence of obesity in the world has tripled since 1975, with ~39% of the world's adult population having overweight and 13% having obesity in 2016. 2
Numerous studies have corroborated the association of obesity with many diseases, such as diabetes, stroke, metabolic disorders, and varied cancer types. It has also been affirmed that obesity can lower the quality of life and can give rise to mental health disorders such as depression and anxiety. 3 Considering these complications, the management and treatment of obesity are the major issues of concern. Obesity has a complex and multifactorial etiology, and the growing body of largely preclinical studies supports the concept of bidirectional signaling within the gut–brain axis (GBA) in the pathophysiology of obesity, mediated by metabolic, endocrine, neural, and immune system mechanisms. 4 Gut microbiota is a complex system of organisms, mainly different bacterial species. 5 The interaction between the gut microbiota and the brain, known as “the gut–brain axis (GBA)” is a bidirectional connection via neural, immune, and endocrine pathways. Signaling from the brain through the autonomic nervous system and the hypothalamic–pituitary–adrenal (HPA) axis influences many gastrointestinal processes, including transit and motility, mucus and fluid secretion, immune activation, intestinal permeability, and relative gut microbial abundance, and gene expression patterns in certain gut microorganisms. 6 Alterations in the gut luminal environment can affect gut microbial community composition and function. 7 Through these pathways, the gut microbiota interacts with the host, affects host systems and organs (e.g., the brain), and modulates the host physiological functions, including glucose metabolism and liver function. 8 Alteration in the gut microbiome composition can potentially associated with various chronic diseases, such as allergic asthma, inflammatory bowel disease, depression, and obesity. 9 , 10 , 11 , 12 The association of obesity with GBA has hitherto been investigated in numerous studies. A number of research works have attributed the gut microbiota to obesity, owing to the direct modulating action of GBA on the appetite‐related hormones, i.e., leptin (LEP), ghrelin, and glucagon‐like peptide 1 (GLP‐1). It has also been demonstrated that the neural connection of the GBA via the vagus nerve has a key role in changing eating behaviors and appetite. 13 , 14 Nonetheless, there is still much to investigate about the physiological mechanism of obesity and GBA and also its effect on appetite, eating behaviors, and other physiological mechanisms of the body. On the other hand, most of these surveys have mostly been performed on animal models. As the gut–microbiota–brain axis has an essential role in modulating obesity‐related behaviors and body function, targeting this pathway is a novel treatment method for the management of obesity. 15 GBA‐based treatments include probiotic, prebiotic, and synbiotic supplementations and fecal microbiota transplantation.
We review the gut microbiota as a key regulator of host metabolism, central appetite, and its role in metabolic disorders such as obesity, summarize the literature on potential mechanisms of gut–brain axis signaling, review how some bacterial strains might contribute to, or protect against, metabolic disease, and address how FMT and dietary interventions might be novel metabolic therapies in clinical practice in obesity management and discuss some of the recent clinical trials designed in this field.
2. OBESITY
Obesity is a medical condition in which the body weight is high above the desirable or acceptable weight, often due to excess accumulated fat in the body. According to body mass index (BMI), a BMI over 30 kg/m2 is considered obese, and a BMI greater than 40 kg/m2 is defined as morbidly obese. 16 , 17 Obesity, as a leading cause of death worldwide, affects a growing number of children and adults. In 2015, 100 million children and 600 million adults in over 195 countries were obese. 18 As reported before, obesity is correlated with various conditions and diseases, comprising type 2 diabetes mellitus, cardiovascular diseases, obstructive sleep apnea, and osteoarthritis, 3 suggesting the critical role of obesity in numerous disorders. 19 Obesity has also been considered a major feature of many syndromes, including Prader–Willi, Cohen, and Bardet–Biedl syndromes. 20 , 21 , 22 There are also several neuroendocrine factors but not related to growth hormone deficiency, pseudohypoparathyroidism, Cushing disease, hypothyroidism, and hypothalamic causes. Among genetic factors, single nucleotide polymorphism (SNP) can impact on obesity‐related genes controlling eating behavior and metabolism. A recent study on obesity has demonstrated the role of SNPs in fat mass, obesity‐associated LEP, and LEP receptor genes. 23 Besides genetics, a change in epigenetics, such as alteration in microRNA expression, noncoding microRNA, and DNA methylation, is correlated with obesity. 24 , 25 Epigenetics, unlike genetics, can be changed throughout the lifespan by lifestyle modification through physical activity, energy intake, and diet.
Obesity occurs in two main distinct processes: resetting the body weight set point and balancing prolonged positive energy. 26 The first process explains the reason why the studies of effective obesity treatments have faced multiple difficulties. 26 There are several pathophysiological mechanisms affecting appetite, thus potentially contributing to the development and establishment of obesity. More specifically, appetite is influenced by the interplay between the central nervous system (CNS) and the endocrine system through which signals from peripheral organs, particularly the digestion system, transport to CNS. In other words, LEP and ghrelin are produced peripherally but regulate eating behavior through their action on CNS, especially hypothalamus. 27 While much work have been conducted to give insight into the mechanisms of fat gain, there are still complex processes required to be clarified.
3. GUT–MICROBIOTA–BRAIN AXIS
The gut microbiota is a complex community containing trillions of microorganisms affecting normal physiology and host's susceptibility to disease. 5 The gut microbiota hosts more than 100 bacterial species, 150 times as many genes as human genome. 5 , 28 The gut flora is mainly dominated by bacteria, and by protozoa, viruses, archaea, and fungi. 29 Gut microbiota composition is a dynamic entity changing throughout the human life depending on the environmental (e.g., diet) and host (e.g., genetics and age) factors. 30 Gut microbiome possesses many functions; for instance, it maintains intestinal integrity, produces mucus, stimulates intestinal epithelium regeneration, and mediates the production of short‐chain fatty acid (SCFA). 31 Gut microbiota also mediates the maturation of innate immunity in the early stage of life and somehow performs sensing and modulating enormous amounts of signals from the environment, then behaves throughout the body. 32 The gut microbiota acts as an intermediate between the host and environment and may potentially influence human health. 33 An alteration in beneficial bacteria may exert notable effects on the individual's health; in particular, it induces some aspects of disease pathogenesis. Factors such as diet, illness, drug, and infection may also change microbiota. 34 , 35 , 36
The term “gut–microbiota–brain axis” is defined as a bidirectional communication between the brain and gut bacterial community through multiple systems forming a network (Figure 1). It has a significant role in maintaining the homeostasis of the CNS and gastrointestinal system. 37 , 38 The interaction pathways in this network include both indirect and direct signaling via the neuronal pathway, immune system, and chemical transmitter, as discussed below. 33 As different biological systems are engaged in these networks, their complexity needs to be explored.
FIGURE 1.
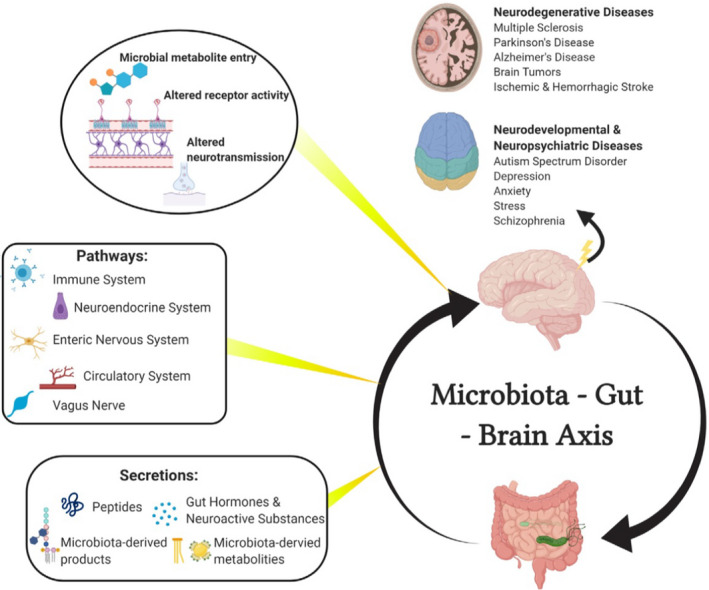
Structure of the gut–microbiota–brain axis (The figure was adopted and reproduced from Liu et al. with permission from the publisher) 116
3.1. Neural link between the gut and brain
The nerve fibers physically link the brain to the gut. The vagus nerve and autonomic nervous system (ANS) represent the major neuronal pathways (Figure 1). The former facilitates the bidirectional signals between the gut and brain stem, while the latter innervates the gut and enteric nervous system (ENS). The neuroanatomical routes controlling gut functions form a four‐level integrative organization. The first level is ENS comprising gut glial cells, submucosal ganglion, and myenteric ganglia. 39 , 40 The second level, prevertebral ganglia, is the key player in the peripheral visceral reflex response. 39 ANS, the third level in the spinal cord, brain stem dorsal motor nucleus of the vagus nerve, and nucleus tractus solitarius (NTS), projects and receives the signals from the efferent and afferent fiber of the vagus nerve, respectively. 41 The fourth level is the higher brain centers. Neuron signals from the cerebral cortex and subcortical area, including funnels and basal ganglia, pass downward to the medulla and pontine nuclei. Once passing through the afferent fiber of the vagus nerve, the NTS transfers to higher centers such as insular cortex, lobus limbicus, and thalamus. 42
In the afferent pathway, receptors are first involved in the gut tissue. Chemoreceptors are essential for identifying chemical stimuli (hormones and neurotransmitters), and mechanoreceptors are necessary for detecting alterations occurred in the intestinal volume. The vagus nerve, in turn, relays the signal from the gut receptors to CNS. 43 These gut receptors themselves are influenced by the gut microbiota. 44 Metabolites produced by the gut bacteria such as SCFA are sensed by a chemoreceptor, i.e., enteroendocrine cells (EECs), leading to calcium signaling that may be transmitted to specific fibers of the vagus nerve in gut epithelium. 45 The vagus nerve is responsible for downward signals from the CNS to visceral organs and tissues. It also has a role in the normal metabolism and immune system function. These downward pathways to the gut could influence the environment of the gut and eventually exert significant effects on the gut microbiota. 46 As described above, the vagus nerve provides a prominent channel for signaling both from and to the gut.
In many animal studies, the vagus nerve has been introduced as an important nerve that regulates the behavior of the gut‐brain communication. Lactobacillus rhamnosus JB‐1 has been shown to influence the expression of γ‐aminobutyric acid (GABA) receptors in the regions of the brain related to emotions and behavior (i.e., the hippocampus and amygdala nuclei) and also modulates the anxiety behavior. 47 Interestingly, in mice, vagotomy eliminates the influence of Lacticaseibacillus rhamnosus JB‐1 on the expression of GABA receptors. 47 The vagus nerve has been suggested as a mediator of gut effects on mood and behavior and is essential for the efficient impact of L. reuteri on social behaviors in autism spectrum disorder (ASD) models. 48 These findings point to the manipulation or activation of the vagus nerve as a possible approach for treating human disease. To support this assumption, the implantation of an electrical device stimulating the vagus nerve has been approved as an efficient treatment for resistant depression and epilepsy. 49
3.2. Immune system as a link between the gut and brain
Among the body organs, gut contains the highest number of immune cells; therefore, the immune system is a critical network between the brain and gastrointestinal tract (the gut) (Figure 1). 50 Investigations at both structural and cellular levels have signified that germ‐free (GF) animal models predispose to the development of immune deficiency. In GF models, secretory IgA T helper 17, CD4+, and CD8+ immune cells diminish. Also, a decrease is found in isolated lymphoid follicles, lamina propria, and Peyer's patches. On the other hand, re‐colonization of Bacteroides fragilis can maintain immune maturation of gut‐associated lymphoid tissue. 51 The immune system has also a major function in obesity. Previous surveys have unveiled that both high fat intake and the increased permeability of the gut barrier can lead to lipopolysaccharide (LPS) absorption, ultimately causing endotoxemia. 52 , 53 Notably, this endotoxemia could be hindered by some gut bacteria, which restore the gut barrier integrity. 54 Obesity is linked to neuroinflammatory disorders such as Alzheimer's disease and depression. Obese individuals are more predisposed to develop these types of pathologies; therefore, neuroinflammation may establish these associations. 55
3.3. Endocrine system as a link between the gut and the brain
The gut microbiota can affect many systems and organs of the body, such as lungs, liver, skin, adipose tissue, and brain through the endocrine system. The relationship between the gut microbiota and brain via the endocrine system is performed through modulating the neuroendocrine system of the brain and also through the production of hormone‐like metabolites by the gut microbiome, which circulates in the body and reaches other organs. 56 , 57
The gut microbiota is able to modify many behaviors, including sexual and social, stress‐related, learning, memory, eating, and obesity behaviors, by influencing the neuroendocrine system of the brain, particularly the hypothalamus–pituitary–adrenal (HPA) axis. 56 HPA axis, which chiefly reacts to stress‐related conditions, is a vital neuroendocrine pathway responsible for the physiological and developmental functions of the human body. 58
The connection between the gut–microbiota–brain axis and the neuroendocrine system has not fully been understood. However, it has been attributed to the modulation of the hormonal secretion or to the direct production of bacterial metabolites, namely SCFAs, neurotransmitters, and tryptophan (Figure 1). SCFAs, especially butyric acid and propionic acid, can enter the blood circulation and directly cause some alterations in the brain. These molecules can affect different metabolic pathways, i.e., glucose metabolism, catecholamine synthesis, and immunological pathways such as microglia maturation, thus giving rise to physiological and behavioral changes. 59 , 60 , 61 SCFAs also affect the hormonal secretion of EECs by the activation of some specific G protein‐coupled receptors, direct induction of the glucagon‐like peptide 1 (GLP‐1), release of peptide YY (PYY), and indirect release of ghrelin, which all are responsible for eating behaviors, including satiety, hunger, and appetite mechanisms. 14 , 62 , 63
The gut microbiota produces many neurotransmitters, such as catecholamines, GABA, and tryptophan, which can impact on the hypothalamus, thereby changing the neuroendocrine function. 56 GABA, which is mostly produced by Levilactobacillus brevis and Bifidobacterium dentium, can cross the blood‐brain barrier and induces its effects directly. 64 Many of these neurotransmitters are potentially associated with psychiatric and neurologic diseases, e.g., anxiety, depression, autism, and neurodegenerative disorders. 65 , 66
The immunological and endocrine pathways of the GBA are connected to each other in many ways, including the LPS of the Gram‐negative bacteria. These antigens cause immune and neuroendocrine activation following some external factors such as stress and diet. 56 , 67 It has been denoted that exposure to LPS in the early weeks of life can induce an increase in ACTH and corticosterone secretion in adulthood in response to stress‐related conditions. 68
Studies have exhibited a bidirectional relationship between stress‐related response and gut microbiota. It has also been displayed that alteration in the gut microbiome can cause changes in the body's response to stress, and experiencing stressful moments may modify the gut microbiome. The mechanism underlying this condition is the effect of the gut microbiota on the production of glucocorticoid hormones and some immune mediators. 56 , 69 , 70
4. ASSOCIATION OF OBESITY WITH GBA
Gut–brain axis has a significant impact on various aspects of physiology, including glucose homeostasis, feeding regulation, gut motility, and appetite. Using this system, therapeutics for many diseases, including T2DM and obesity, have been explored.
An extensive and complex networks of neurons and hormones act bilaterally between the gastrointestinal tract and the brain, and their receptors regulates appetite, food intake, and obesity. 71 The presence of nutrients in the gastrointestinal tract causes complex hormonal and neural signaling to the brain; the vagus nerve mediates this signaling. Information transmits from the gut to the NTS, and to the smooth muscles of the gut by effector fibers (Figure 2). 14 Thereafter, information from the NTS distributes to the hypothalamus, a region of the brain that regulates appetite, food intake, and energy balance in the neurons of the arcuate nucleus (ARC). The ARC consists of agouti‐related protein, orexigenic neuropeptide Y, cocaine‐ and amphetamine‐regulated transcript, anorexigenic peptides (LEP), and pro‐opiomelanocortin neurons. 14 Studies have shown that vagotomy in animal models increases food intake and weight gain by reducing anorexigenic hormone signaling. 72
FIGURE 2.
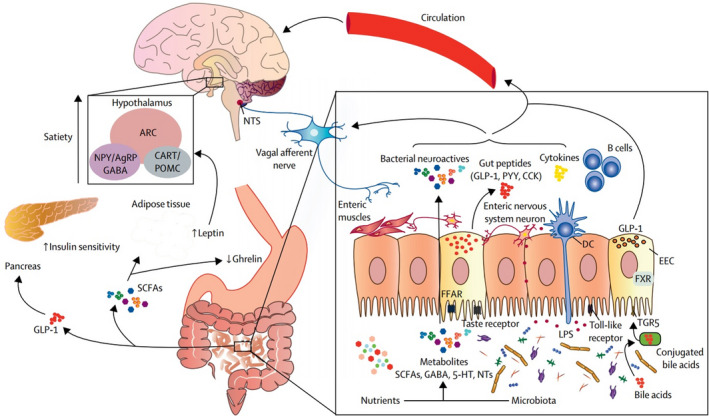
Association of obesity with gut–microbiota–brain axis (The figure was adopted and reproduced from Torres‐Fuentes et al. with permission from the publisher) 53
Some gut bacteria can modify the secretion of gut hormone, including GLP‐1, ghrelin, PYY, and LEP, thus hypothalamic neuroendocrine pathways affect appetite and satiety. 73 SCFAs derived from microbiota can bind to receptors on EECs and alter the release of enteric hormones into the systemic circulation. 74 Moreover, the activation of various taste (bitter, fat, umami, and sweet) receptors in EECs causes the secretion of ghrelin, GLP‐1, and cholecystokinin. 75 Acetate, the main SCFA secreted by intestinal bacteria, suppresses appetite via central hypothalamic mechanisms. 76
It is noteworthy that altered microbiota leads to an increase in acetate concentration, thus resulting in the upregulation of the parasympathetic nervous system and also the elevation of increased glucose‐stimulated insulin secretion, ghrelin secretion, and obesity. 77 Neuroactive metabolites (e.g., serotonin and GABA) affecting the central control of appetite are produced by the gut bacteria. 78 Common melanocortin neurons, which control bodyweight homoeostasis with serotonin, reduce appetite. 79 GABA, the main inhibitory neurotransmitter in the CNS is required for the normal regulation of energy balance. 78
Obesity‐associated microbiota increases the efficiency of calorie uptake from ingested foods. 80 Thus, an obesity‐associated microbiota in comparison to a lean‐associated gut microbiota provides more energy to the host from other indigestible carbohydrates and proteins by rising the production of different primary fermentation enzymes and nutrient transporters. 81
5. GBA‐BASED OBESITY TREATMENT
The results of surveys have revealed that the gut microbiota has a vital role in the regulation of metabolism, energy homoeostasis, and central appetite in the host. Today, the microbiota is utilized to treat metabolic disorders, like obesity. 82
5.1. Probiotics
Probiotics are “live microorganisms that confer a health benefit on the host when administered in adequate amounts.” Studies have demonstrated a link between probiotics and body weight reduction in animals and humans (Figure 3). 83 In obese individuals, inappropriate feeding causes a rise in the Firmicutes to Bacteroidetes ratio. This seems to facilitate energy extraction from the ingested food and increases energy storage in the host's adipose tissue. 84 Probiotics and synbiotics have been proposed to exert a decrease in body weight through different mechanisms. Probiotics help in the recovery of the tight junctions between epithelial cells, thus reducing intestinal permeability, preventing the translocation of bacteria, and reducing inflammation derived from lipopolysaccharides (LPS). The reduction in inflammation leads to an increase in insulin sensitivity in the hypothalamus, which improves satiety. Additionally, increased concentrations of leptin in adipose tissue, glucagon‐like peptide 1 (GLP‐1), and pancreatic polypeptide (PPY) in the intestine lead to a reduction in food intake due to an increase in satiety. 85
FIGURE 3.
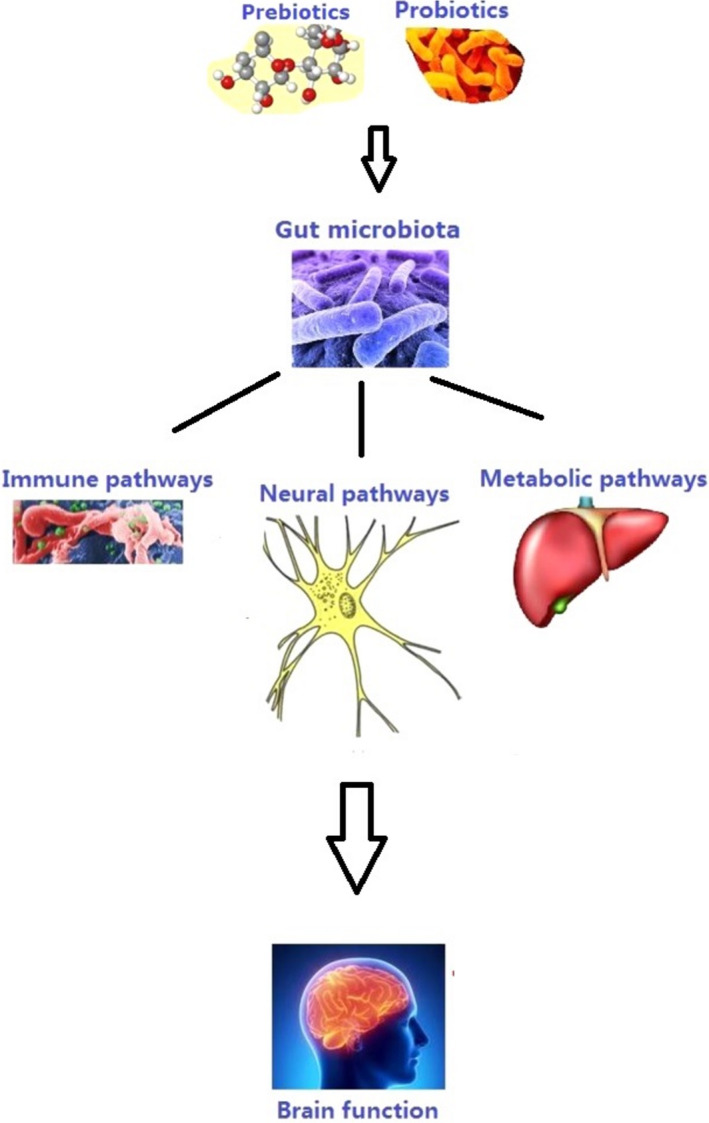
Association of probiotics and prebiotics with gut–microbiota–brain axis (The figure was adopted and reproduced from Liu et al. with permission from the publisher) 117
Thus, probiotics can modulate and maintain healthy microbiota. Several bacterial strains have displayed positive effects, such as the reduction of endotoxemia, adiposity, tissue inflammation, bodyweight, LEP levels, and energy intake. 73 Bifidobacterium and Lactobacillus spp. are the most common probiotic species that have shown these features; however, their impact on obesity seems to be strain and species‐specific. 86
5.2. Prebiotics
Prebiotics are “selectively fermented ingredients that result in benefits changes in the composition and/or activity of the gastrointestinal microbiota”. 86 Prebiotics promote the growth of beneficial bacteria in the gut. 87 These compounds may be considered a future target against obesity. Lactulose, inulin, fructooligosaccharides, and derivatives of galactose and β‐glucans are some typical examples. Oligosaccharides (e.g., insulin), fructooligosaccharides, galactooligosaccharides, and polyphenols are the most widespread prebiotics. These substances may serve as a medium for probiotics, which can stimulate their growth. 88
The consumption of prebiotics has a significant impact on gastrointestinal microbiota compounds and their metabolic activity. Prebiotics largely affect the modulation of lipid metabolism, the immunological system, enhanced absorbability of calcium, and modification of the bowel function. 89
Treatment with prebiotics increases the number of helpful bacteria such as Bifidobacterium and Lactobacillus spp. in the gastrointestinal tract. These bacteria exert effective impacts, comprising of antiobesity, reduced metabolic endotoxemia, decreased circulating proinflammatory cytokines, increased SCFA production, elevated expression of tight junction proteins, and enhanced intestinal barrier function. 73 The inulin‐type prebiotics promote the growth of beneficial lactobacilli and bifidobacteria, PYY response, and GLP‐1 and also decrease serum ghrelin concentration, which could influence the food intake. The number of beneficial bacteria in patients with obesity and type 2 diabetes is lower than that of healthy individuals. 73 More research is needed to elucidate the precise mechanisms between prebiotics and obesity, which are still unclear. 90
5.3. Synbiotics
Synbiotics are actually a combination of prebiotics and probiotics and possess synergistic effects. Synbiotics enhance the effectiveness of both prebiotics and probiotics and maximize their beneficial potential for the host's health. 91 Increasing the survival of probiotics in the gastrointestinal tract is the most important consequence of the production of synbiotics. 92 Synbiotics exert their positive effects by the provision of specific health impacts and the improved viability of probiotic microorganisms. 93 Synbiotics modulate the metabolic activity in the intestine through the development of microbiota, the maintenance of the intestinal biostructure, and the inhibition of potential pathogens present in the gastrointestinal tract. 94 Synbiotics reduce the number of undesirable metabolites such as the inactivation of nitrosamines and cancerogenic substances, in the gastrointestinal tract. Moreover, they elevate SCFAs, carbon disulfides, ketones, and methyl acetates, which potentially result in a positive effect on the host's health. 95
Synbiotics have several known efficient impacts on humans. They boost the number of beneficial microorganisms, such as Lactobacillus and Bifidobacterium, and balance the intestinal microbiota. 96 They also improve hepatic function in patients with cirrhosis. Synbiotics ameliorate the immune system function, inhibit bacterial translocation, and reduce the incidence of nosocomial infections in patients postsurgical procedures. 97 It appears that synbiotics are highly efficient in the reduction of blood sugar and fat levels, prevention of osteoporosis, and treatment of brain disorders associated with the abnormal hepatic function. 98
5.4. Akkermansia muciniphila
Akkermansia muciniphila (A. muciniphila) is a Gram‐negative, oval‐shaped, and nonmotile, chemoorganotroph and anaerobic microorganism. It is one of the most famous microorganisms in the gastrointestinal tract and has special effects, such as adipose tissue inflammation, metabolic endotoxemia, fat mass gain, and insulin resistance on the host. 99 A. muciniphila inoculation could cause the clearance of chylomicrons and triglycerides to avoid acute lipid overload in the circulation. These beneficial bacteria interact with different components of the diet, mainly insoluble fiber, release bioactive metabolites and send the signal to the host via the GBA. 100 A. muciniphila has the ability to produce enzymes degrading mucin and to utilize its mucin as a carbon and nitrogen source in the mucus layer of epithelium. This microorganism also modulates basal metabolism. Evidence has disclosed that there is a low number of A. muciniphila in patients with T2DM and obese people as gut microbial imbalance plays an essential role in upsetting the body's energy balance. Gut microbiota intervention is a potential therapeutic method for treating obesity‐related metabolic diseases, including hyperglycemia and hyperlipidemia. 101 The metabolic activity of A. muciniphila on the host leads to a change in the endotoxin level and SCFA production, and results in the elevation of fatty acid oxidation in the intestine and adipose tissue. A. muciniphila converts dietary fiber into butyrate, propionate, and acetate; these metabolites influence glucose and lipid homeostasis. Therefore, A. muciniphila can be considered a promising prebiotic from the improvement of metabolic syndromes, such as obesity. 102
5.5. Fecal microbiota transplantation
Direct microbiome therapies, including fecal microbiota transplantation (FMT), is a novel therapeutic option for metabolic syndrome and obesity. FMT is an extremely effective method in which the gut microbiota composition is transferred from a healthy donor to the patient's intestinal tract, typically by duodenal endoscopy or colonoscopy, normalizing the structure and function of the gut microbial community. 103 After performing FMT, donor microbial strains in human beings are involved in the colonization of the recipient gut microbiota and persist for at least three months. 104 However, donor‐recipient compatibilities are vital for the successful establishment of the donor's microbial strains in the recipient's gut. 104 The aforesaid approach has been very successful in treating Clostridium difficile infection, with more than 90% efficacy. 105 Given such a satisfactory effect, it can be expected that FMT can be useful in replacing effective gut microbiota and improving their performance for other diseases in which gut microbiota dysbiosis is involved, such as chronic constipation, irritable bowel syndrome, Crohn's disease, and ulcerative colitis. Moreover, there is increasing evidence that FMT might also have the potential to treat obesity and associated disorders such as type 2 diabetes. 103
When the composition of gut microbiota is transferred, often by duodenal endoscopy or colonoscopy, from healthy donors with a normal BMI to obese individuals diagnosed with type 2 diabetes, fecal microbiota diversity, and butyrate‐producing bacteria rise. 106 Of course, FMT can have some potential risks such as the spread of transmissible disease. Although there have been some mild effects such as diarrhea and fever, no side effects have been reported. 107
6. CLINICAL TRIALS
So far, various studies have focused on the effects of probiotics, prebiotics, and synbiotics on human health. There are several clinical trials in this area.
In a study conducted by Larsen et al., 108 50 obese patients were randomized to intake Ligilactobacillus salivarius Ls‐33 or placebo for 12 weeks. The fecal microbiota and concentrations of fecal SCFAs were assessed before and after the intervention. Ratios of Bacteroides‐Prevotella‐Porphyromonas group to Firmicutes bacteria and Roseburia intestinalis significantly increased after the administration of Ls‐33. In a double‐blind, placebo‐controlled trial study 87 subjects with high BMI were selected and randomized to intake L. gasseri SBT2055 or placebo daily for 12 weeks. The results represented that BMI, abdominal visceral fat area, and hip circumference decreased. 109 Another study of 40 obese adults who took Lactiplantibacillus plantarum for three weeks, a reduction was observed in BMI and arterial BP values. 110 Zarrati et al. 111 performed a survey on 75 healthy overweight and obese individuals randomized to intake L. acidophilus La5, Lacticaseibacillus casei DN001, B. lactis Bb12, or placebo for eight weeks. These strains changed the gene expression in the peripheral blood mononuclear cell, and altered BMI, fat percentage, and LEP levels. In Parnell and Reimer's double‐blind, placebo‐controlled trial, 48 healthy adults with a BMI (in kg/m2) >25 were given oligofructose or placebo daily for 12 weeks. Their results showed that body weight was reduced by 1.03 ± 0.43 kg with oligofructose supplementation, while the control group showed a weight gain of 0.45 ± 0.31 kg over 12 weeks (p = 0.01). In addition, glucose increased in the control group and decreased in the oligofructose group between the initial and final tests (p ≤ 0.05). Oligofructose supplementation did not affect plasma active glucagon‐like peptide 1 secretion. According to a visual analog scale designed to assess side effects, oligofructose was well tolerated. 112 An earlier study investigated the impact of a combination of L. rhamnosus CGMCC1.3724 (LPR) with oligofructose and inulin on 153 obese men and women for 36 weeks. The final outcome was weight loss and decrease in LEP and an increase in Lachnospiraceae. 113 Another investigation examined the impact of L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. longum, and L. bulgaricus combined with FOS on 70 children and adolescents with high BMI for eight weeks. Results suggested a reduction in BMI z‐score and waist circumference. 114 In an open‐label, randomized, controlled study on 77 obese children, Ipar et al. 115 implied some promising effects of L. acidophilus, L. rhamnosus, B. bifidum, B. longum, and E. faecium in combination with FOS. The supplement tested had an effective impact on anthropometric measurements and could decrease TC, LDL‐C, and total oxidative stress serum levels.
7. CONCLUSION
This study attempted to review the link between gut–microbiota–brain axis and obesity and discuss GBA‐based antiobesity treatments. According to BMI, a BMI over 30 kg/m2 is considered obese, and a BMI greater than 40 kg/m2 is defined as morbidly obese. Obesity is correlated with various conditions and diseases, comprising type 2 diabetes mellitus, cardiovascular diseases, obstructive sleep apnea, and osteoarthritis. Gut microbiota is a complex system of organisms, mainly different bacterial species, the gut microbiota interacts with the host, affects host systems and organs, and modulates the host physiological functions. GBA is a bidirectional connection via neural, immune, and endocrine pathways. The evidence base for interventional approaches, which have been shown to affect the composition and function of the intestinal microbiome, includes dietary strategies, oral probiotic/ prebiotic/synbiotic treatment, fecal microbiota transplantation, and bariatric surgery. Targeting the gut microbiota composition can be a potential treatment for obesity. However, further research is necessary to verify the effectiveness and efficiency of this therapeutic approach.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTION
Arezoo Asadi, Negar Shadab Mehr, Mohamad Hosein Mohamadi, Fazlollah Shokri, Mohsen Heidary, Nourkhoda Sadeghifard, and Saeed Khoshnood contributed to revising and final approval of the version to be published. All authors agreed and confirmed the manuscript for publication.
Asadi A, Shadab Mehr N, Mohamadi MH, et al. Obesity and gut–microbiota–brain axis: A narrative review. J Clin Lab Anal. 2022;36:e24420. doi: 10.1002/jcla.24420
Negar Shadab Mehr and Mohamad Hosein Mohamadi Co‐second authorship who contributed equally to this work.
Contributor Information
Mohsen Heidary, Email: mohsenheidary40@gmail.com.
Saeed Khoshnood, Email: saeed.khoshnood22@gmail.com.
DATA AVAILABILITY STATEMENT
All the data in this study are included in the manuscript.
REFERENCES
- 1. World Health Organization . https://www.who.int/news‐room/fact‐sheets/detail/obesity‐and‐overweight; 2021.
- 2. World Health Organization Obesity and Overweight 2016 . Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- 3. Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197‐1209. [DOI] [PubMed] [Google Scholar]
- 4. Osadchiy V, Martin CR, Mayer EA. The Gut‐Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol. 2019;17(2):322‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220‐230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moreira CG, Russell R, Mishra AA, et al. Bacterial adrenergic sensors regulate virulence of enteric pathogens in the gut. MBio. 2016;7(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sovran B, Hugenholtz F, Elderman M, et al. Age‐associated Impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep. 2019;9(1):1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mayer EA. Gut feelings: the emerging biology of gut‐brain communication. Nat Rev Neurosci. 2011;12(8):453‐466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular‐phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104(34):13780‐13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gonzalez A, Stombaugh J, Lozupone C, Turnbaugh PJ, Gordon JI, Knight R. The mind‐body‐microbial continuum. Dialogues Clin Neurosci. 2011;13(1):55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022‐1023. [DOI] [PubMed] [Google Scholar]
- 12. Russell SL, Gold MJ, Hartmann M, et al. Early life antibiotic‐driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012;13(5):440‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fetissov SO. Role of the gut microbiota in host appetite control: bacterial growth to animal feeding behaviour. Nat Rev Endocrinol. 2017;13(1):11‐25. [DOI] [PubMed] [Google Scholar]
- 14. Schellekens H, Dinan TG, Cryan JF. Lean mean fat reducing “ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology. 2010;58(1):2‐16. [DOI] [PubMed] [Google Scholar]
- 15. Aoun A, Darwish F, Hamod N. The Influence of the Gut Microbiome on Obesity in Adults and the Role of Probiotics, Prebiotics, and Synbiotics for Weight Loss. Prev Nutr Food Sci. 2020;25(2):113‐123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bleich SN, Cutler D, Murray C, Adams A. Why is the developed world obese? Annu Rev Public Health. 2008;29:273‐295. [DOI] [PubMed] [Google Scholar]
- 17. van Hout GC, van Oudheusden I, van Heck GL. Psychological profile of the morbidly obese. Obes Surg. 2004;14(5):579‐588. [DOI] [PubMed] [Google Scholar]
- 18. Collaborators GO. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017;377(1):13‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yazdi FT, Clee SM, Meyre D. Obesity genetics in mouse and human: back and forth, and back again. PeerJ. 2015;3:e856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DelParigi A, Tschöp M, Heiman ML, et al. High circulating ghrelin: a potential cause for hyperphagia and obesity in Prader‐Willi syndrome. J Clin Endocrinol Metab. 2002;87(12):5461‐5464. [DOI] [PubMed] [Google Scholar]
- 21. Goldstone AP, Beales PL. Genetic obesity syndromes. Obes Metab. 2008;36:37‐60. [DOI] [PubMed] [Google Scholar]
- 22. Guo D‐F, Rahmouni K. Molecular basis of the obesity associated with Bardet‐Biedl syndrome. Trends Endocrinol Metab. 2011;22(7):286‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dougkas A, Yaqoob P, Givens DI, Reynolds CK, Minihane AM. The impact of obesity‐related SNP on appetite and energy intake. Br J Nutr. 2013;110(6):1151‐1156. [DOI] [PubMed] [Google Scholar]
- 24. Rönn T, Volkov P, Davegårdh C, et al. A six months exercise intervention influences the genome‐wide DNA methylation pattern in human adipose tissue. PLoS Genet. 2013;9(6):e1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Widiker S, Karst S, Wagener A, Brockmann GA. High‐fat diet leads to a decreased methylation of the Mc4r gene in the obese BFMI and the lean B6 mouse lines. J Appl Genet. 2010;51(2):193‐197. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz MW, Seeley RJ, Zeltser LM, et al. Obesity pathogenesis: An endocrine society scientific statement. Endocr Rev. 2017;38(4):267‐296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flier JS. Obesity wars: molecular progress confronts an expanding epidemic. Cell. 2004;116(2):337‐350. [DOI] [PubMed] [Google Scholar]
- 28. de Vos WM, de Vos EA. Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev. 2012;70(Suppl 1):S45‐56. [DOI] [PubMed] [Google Scholar]
- 29. Dave M, Higgins PD, Middha S, Rioux KP. The human gut microbiome: current knowledge, challenges, and future directions. Transl Res. 2012;160(4):246‐257. [DOI] [PubMed] [Google Scholar]
- 30. de la Cuesta‐Zuluaga J, Kelley ST, Chen Y, et al. Age‐ and Sex‐Dependent Patterns of Gut Microbial Diversity in Human Adults. mSystems. 2019;4(4). doi: 10.1128/mSystems.00261-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burger‐van Paassen N, Vincent A, Puiman PJ, et al. The regulation of intestinal mucin MUC2 expression by short‐chain fatty acids: implications for epithelial protection. Biochemical Journal. 2009;420(2):211‐219. [DOI] [PubMed] [Google Scholar]
- 32. Nell S, Suerbaum S, Josenhans C. The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol. 2010;8(8):564‐577. [DOI] [PubMed] [Google Scholar]
- 33. Morais LH, Schreiber HL, Mazmanian SK. The gut microbiota‐brain axis in behaviour and brain disorders. Nat Rev Microbiol. 2021;19(4):241‐255. [DOI] [PubMed] [Google Scholar]
- 34. Cryan JF, O’Mahony S. The microbiome‐gut‐brain axis: from bowel to behavior. Neurogastroenterol Motil. 2011;23(3):187‐192. [DOI] [PubMed] [Google Scholar]
- 35. Davey KJ, O’Mahony SM, Schellekens H, et al. Gender‐dependent consequences of chronic olanzapine in the rat: effects on body weight, inflammatory, metabolic and microbiota parameters. Psychopharmacology. 2012;221(1):155‐169. [DOI] [PubMed] [Google Scholar]
- 36. Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14‐6ra. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cryan JF, O'Riordan KJ, Cowan CS, et al. The microbiota‐gut‐brain axis. Physiol Rev. 2019. [DOI] [PubMed] [Google Scholar]
- 38. Martin CR, Osadchiy V, Kalani A, Mayer EA. The brain‐gut‐microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anlauf M, Schäfer MKH, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459(1):90‐111. [DOI] [PubMed] [Google Scholar]
- 40. Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16:55‐59. [DOI] [PubMed] [Google Scholar]
- 41. Chang HY, Mashimo H, Goyal RK. Musings on the wanderer: what's new in our understanding of vago‐vagal reflex? IV. Current concepts of vagal efferent projections to the gut. Am J Physiol Gastrointest Liver Physiol. 2003;284(3):G357‐G366. [DOI] [PubMed] [Google Scholar]
- 42. Wang HX, Wang YP. Gut Microbiota‐brain Axis. Chin Med J (Engl). 2016;129(19):2373‐2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kaelberer MM, Buchanan KL, Klein ME, et al. A gut‐brain neural circuit for nutrient sensory transduction. Science. 2018;361(6408):eaat5236. doi: 10.1126/science.aat5236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Muller PA, Schneeberger M, Matheis F, et al. Microbiota modulate sympathetic neurons via a gut–brain circuit. Nature. 2020;583(7816):441‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell. 2017;170(1):185‐198.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bonaz B, Picq C, Sinniger V, Mayol J‐F, Clarençon D. Vagus nerve stimulation: from epilepsy to the cholinergic anti‐inflammatory pathway. Neurogastroenterol Motil. 2013;25(3):208‐221. [DOI] [PubMed] [Google Scholar]
- 47. Bravo JA, Forsythe P, Chew MV, et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci. 2011;108(38):16050‐16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sgritta M, Dooling SW, Buffington SA, et al. Mechanisms underlying microbial‐mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron. 2019;101(2):246‐259.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Milby AH, Halpern CH, Baltuch GH. Vagus nerve stimulation for epilepsy and depression. Neurotherapeutics. 2008;5(1):75‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Agustí A, García‐Pardo MP, López‐Almela I, et al. Interplay between the gut‐brain axis, obesity and cognitive function. Front Neurosci. 2018;12:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Delzenne NM, Neyrinck AM, Bäckhed F, Cani PD. Targeting gut microbiota in obesity: effects of prebiotics and probiotics. Nat Rev Endocrinol. 2011;7(11):639‐646. [DOI] [PubMed] [Google Scholar]
- 53. Torres‐Fuentes C, Schellekens H, Dinan TG, Cryan JF. The microbiota–gut–brain axis in obesity. Lancet Gastroenterol Hepatol. 2017;2(10):747‐756. [DOI] [PubMed] [Google Scholar]
- 54. Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16(6):341‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Guillemot‐Legris O, Muccioli GG. Obesity‐induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017;40(4):237‐253. [DOI] [PubMed] [Google Scholar]
- 56. Cussotto S, Sandhu KV, Dinan TG, Cryan JF. The Neuroendocrinology of the Microbiota‐Gut‐Brain Axis: A Behavioural Perspective. Front Neuroendocrinol. 2018;51:80‐101. [DOI] [PubMed] [Google Scholar]
- 57. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22(10):1079‐1089. [DOI] [PubMed] [Google Scholar]
- 58. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434‐445. [DOI] [PubMed] [Google Scholar]
- 59. De Vadder F, Kovatcheva‐Datchary P, Goncalves D, et al. Microbiota‐generated metabolites promote metabolic benefits via gut‐brain neural circuits. Cell. 2014;156(1–2):84‐96. [DOI] [PubMed] [Google Scholar]
- 60. Erny D, Hrabě de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965‐977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota‐gut‐brain axis? Neurochem Int. 2016;99:110‐132. [DOI] [PubMed] [Google Scholar]
- 62. Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short‐chain fatty‐acid binding G protein‐coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105(43):16767‐16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Tolhurst G, Heffron H, Lam YS, et al. Short‐chain fatty acids stimulate glucagon‐like peptide‐1 secretion via the G‐protein‐coupled receptor FFAR2. Diabetes. 2012;61(2):364‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Barrett E, Ross RP, O'Toole PW, Fitzgerald GF, Stanton C. γ‐Aminobutyric acid production by culturable bacteria from the human intestine. J Appl Microbiol. 2012;113(2):411‐417. [DOI] [PubMed] [Google Scholar]
- 65. Möhler H. The GABA system in anxiety and depression and its therapeutic potential. Neuropharmacology. 2012;62(1):42‐53. [DOI] [PubMed] [Google Scholar]
- 66. Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13(7):465‐477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Moreira AP, Texeira TF, Ferreira AB, Peluzio Mdo C, Alfenas RC. Influence of a high‐fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108(5):801‐809. [DOI] [PubMed] [Google Scholar]
- 68. Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic‐pituitary‐adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15(1 Pt 1):376‐384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bharwani A, Mian MF, Foster JA, Surette MG, Bienenstock J, Forsythe P. Structural & functional consequences of chronic psychosocial stress on the microbiome & host. Psychoneuroendocrinology. 2016;63:217‐227. [DOI] [PubMed] [Google Scholar]
- 70. Foster JA, Rinaman L, Cryan JF. Stress & the gut‐brain axis: Regulation by the microbiome. Neurobiol Stress. 2017;7:124‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sandhu KV, Sherwin E, Schellekens H, Stanton C, Dinan TG, Cryan JF. Feeding the microbiota‐gut‐brain axis: diet, microbiome, and neuropsychiatry. Transl Res. 2017;179:223‐244. [DOI] [PubMed] [Google Scholar]
- 72. Berthoud H‐R. The vagus nerve, food intake and obesity. Regul Pept. 2008;149(1–3):15‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Torres‐Fuentes C, Schellekens H, Dinan TG, Cryan JF. A natural solution for obesity: Bioactives for the prevention and treatment of weight gain. A Review. Nutr Neurosci. 2015;18(2):49‐65. [DOI] [PubMed] [Google Scholar]
- 74. Nøhr MK, Pedersen MH, Gille A, et al. GPR41/FFAR3 and GPR43/FFAR2 as cosensors for short‐chain fatty acids in enteroendocrine cells vs FFAR3 in enteric neurons and FFAR2 in enteric leukocytes. Endocrinology. 2013;154(10):3552‐3564. [DOI] [PubMed] [Google Scholar]
- 75. Calvo SS‐C, Egan JM. The endocrinology of taste receptors. Nat Rev Endocrinol. 2015;11(4):213‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Frost G, Sleeth ML, Sahuri‐Arisoylu M, et al. The short‐chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Perry RJ, Peng L, Barry NA, et al. Acetate mediates a microbiome–brain–β‐cell axis to promote metabolic syndrome. Nature. 2016;534(7606):213‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Delgado TC. Glutamate and GABA in appetite regulation. Front Endocrinol. 2013;4:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Xu Y, Jones JE, Kohno D, et al. 5‐HT2CRs expressed by pro‐opiomelanocortin neurons regulate energy homeostasis. Neuron. 2008;60(4):582‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity‐associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027‐1031. [DOI] [PubMed] [Google Scholar]
- 81. Krajmalnik‐Brown R, Ilhan ZE, Kang DW, DiBaise JK. Effects of gut microbes on nutrient absorption and energy regulation. Nutr Clin Pract. 2012;27(2):201‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. Diet‐induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3(4):213‐223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. So D, Whelan K, Rossi M, et al. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta‐analysis. Am J Clin Nutr. 2018;107(6):965‐983. [DOI] [PubMed] [Google Scholar]
- 84. Chassaing B, Koren O, Goodrich JK, et al. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature. 2015;519(7541):92‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Álvarez‐Arraño V, Martín‐Peláez S. Effects of probiotics and synbiotics on weight loss in subjects with overweight or obesity: A systematic review. Nutrients. 2021;13(10):3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Guarner F, Khan AG, Garisch J, et al. World gastroenterology organisation global guidelines: probiotics and prebiotics october 2011. J Clin Gastroenterol. 2012;46(6):468‐481. [DOI] [PubMed] [Google Scholar]
- 87. Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol. 2015;12(5):303‐310. [DOI] [PubMed] [Google Scholar]
- 88. Geurts L, Neyrinck AM, Delzenne NM, Knauf C, Cani PD. Gut microbiota controls adipose tissue expansion, gut barrier and glucose metabolism: novel insights into molecular targets and interventions using prebiotics. Beneficial Microbes. 2014;5(1):3‐17. [DOI] [PubMed] [Google Scholar]
- 89. Van Loo J, Clune Y, Bennett M, Collins JK. The SYNCAN project: goals, set‐up, first results and settings of the human intervention study. Br J Nutr. 2005;93(S1):S91‐S98. [DOI] [PubMed] [Google Scholar]
- 90. van der Beek CM, Canfora EE, Kip AM, et al. The prebiotic inulin improves substrate metabolism and promotes short‐chain fatty acid production in overweight to obese men. Metabolism. 2018;87:25‐35. [DOI] [PubMed] [Google Scholar]
- 91. Cencic A, Chingwaru W. The role of functional foods, nutraceuticals, and food supplements in intestinal health. Nutrients. 2010;2(6):611‐625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rioux KP, Madsen KL, Fedorak RN. The role of enteric microflora in inflammatory bowel disease: human and animal studies with probiotics and prebiotics. Gastroenterol Clin North Am. 2005;34(3):465‐482. [DOI] [PubMed] [Google Scholar]
- 93. Panesar PS, Kaur G, Panesar R, Bera MB. Synbiotics: potential dietary supplements in functional foods. Berkshire, UK; 2009. [Google Scholar]
- 94. De Vrese M, Schrezenmeir. Probiotics, prebiotics, and synbiotics. Food Biotechnol. 2008;1‐66. [DOI] [PubMed] [Google Scholar]
- 95. Manigandan T, Mangaiyarkarasi S, Hemalatha R, Hemalatha V, Murali N. Probiotics, prebiotics and synbiotics‐a review. Biomed Pharmacol J. 2012;5(2):295. [Google Scholar]
- 96. Sergeev IN, Aljutaily T, Walton G, Huarte E. Effects of Synbiotic Supplement on human gut microbiota, body composition and weight loss in obesity. Nutrients. 2020;12(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Zhang M‐M, Cheng J‐Q, Lu Y‐R, Yi Z‐H, Yang P, Wu X‐T. Use of pre‐, pro‐and synbiotics in patients with acute pancreatitis: a meta‐analysis. World J Gastroenterol. 2010;16(31):3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics‐a review. J Food Sci Technol. 2015;52(12):7577‐7587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Everard A, Belzer C, Geurts L, et al. Cross‐talk between Akkermansia muciniphila and intestinal epithelium controls diet‐induced obesity. Proc Natl Acad Sci. 2013;110(22):9066‐9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sonnenburg JL, Bäckhed F. Diet–microbiota interactions as moderators of human metabolism. Nature. 2016;535(7610):56‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Tailford LE, Crost EH, Kavanaugh D, Juge N. Mucin glycan foraging in the human gut microbiome. Front Genet. 2015;6:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xu Y, Wang N, Tan H‐Y, Li S, Zhang C, Feng Y. Function of Akkermansia muciniphila in obesity: interactions with lipid metabolism, immune response and gut systems. Front Microbiol. 2020;11:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Khoruts A, Sadowsky MJ. Understanding the mechanisms of faecal microbiota transplantation. Nat Rev Gastroenterol Hepatol. 2016;13(9):508‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li SS, Zhu A, Benes V, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science. 2016;352(6285):586‐589. [DOI] [PubMed] [Google Scholar]
- 105. Li YT, Cai HF, Wang ZH, Xu J, Fang JY. Systematic review with meta‐analysis: long‐term outcomes of faecal microbiota transplantation for Clostridium difficile infection. Aliment Pharmacol Ther. 2016;43(4):445‐457. [DOI] [PubMed] [Google Scholar]
- 106. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913‐916.e7. [DOI] [PubMed] [Google Scholar]
- 107. Marotz CA, Zarrinpar A. Focus: microbiome: treating obesity and metabolic syndrome with fecal microbiota transplantation. Yale J Biol Med. 2016;89(3):383. [PMC free article] [PubMed] [Google Scholar]
- 108. Larsen N, Vogensen FK, Gøbel RJ, et al. Effect of Lactobacillus salivarius Ls‐33 on fecal microbiota in obese adolescents. Clin Nutr. 2013;32(6):935‐940. [DOI] [PubMed] [Google Scholar]
- 109. Kadooka Y, Sato M, Imaizumi K, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64(6):636‐643. [DOI] [PubMed] [Google Scholar]
- 110. Sharafedtinov KK, Plotnikova OA, Alexeeva RI, et al. Hypocaloric diet supplemented with probiotic cheese improves body mass index and blood pressure indices of obese hypertensive patients‐a randomized double‐blind placebo‐controlled pilot study. Nutr J. 2013;12(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zarrati M, Salehi E, Nourijelyani K, et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight‐loss diet. J Am Coll Nutr. 2014;33(6):417‐425. [DOI] [PubMed] [Google Scholar]
- 112. Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89(6):1751‐1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Sanchez M, Darimont C, Drapeau V, et al. Effect of Lactobacillus rhamnosus CGMCC1. 3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr. 2014;111(8):1507‐1519. [DOI] [PubMed] [Google Scholar]
- 114. Safavi M, Farajian S, Kelishadi R, Mirlohi M, Hashemipour M. The effects of synbiotic supplementation on some cardio‐metabolic risk factors in overweight and obese children: a randomized triple‐masked controlled trial. Int J Food Sci Nutr. 2013;64(6):687‐693. [DOI] [PubMed] [Google Scholar]
- 115. Ipar N, Aydogdu SD, Yildirim GK, et al. Effects of synbiotic on anthropometry, lipid profile and oxidative stress in obese children. Beneficial Microbes. 2015;6(6):775‐781. [DOI] [PubMed] [Google Scholar]
- 116. Liu L, Huh JR, Shah K. Microbiota and the gut‐brain‐axis: Implications for new therapeutic design in the CNS. eBioMedicine. 2022;77:103908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu X, Cao S, Zhang X. Modulation of gut microbiota–brain axis by probiotics, prebiotics, and diet. J Agric Food Chem. 2015;63(36):7885‐7895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in this study are included in the manuscript.


