Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal chronic interstitial lung disease with no established treatment and is characterized by progressive scarring of the lung tissue and an irreversible decline in lung function. Chronic inflammation has been demonstrated to be the pathological basis of fibrosis. Emerging studies have revealed that most interleukin-17 (IL-17) isoforms are essential for the mediation of acute and chronic inflammation via innate and adaptive immunity. Overexpression or aberrant expression of IL-17 cytokines contributes to various pathological outcomes, including the initiation and exacerbation of IPF. Here, we aim to provide an overview of IL-17 family members in the pathogenesis of IPF.
Keywords: Interleukin-17 (IL-17) family, IL-17 receptor, Inflammation, Idiopathic pulmonary fibrosis
Background
Idiopathic pulmonary fibrosis (IPF) is a progressive and ultimately fatal disease characterized by irreversible scarring and progressive decline of lung function, with a median survival time of 2–3 years following diagnosis [1]. The pathogenesis underlying IPF is poorly understood. Increasing evidence suggests that it results from abnormal wound healing following repetitive alveolar injury, accompanied by chronic inflammation associated with various types of inflammatory cells [2, 3]. Additional critical mechanisms associated with IPF progression are damage and apoptosis of alveolar epithelial cells and proliferation and differentiation of fibroblasts to secrete the extracellular matrix [4].
The interleukin (IL)-17 family is composed of six members, IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, according to homology-based cloning analysis. To date, most IL-17 family members have been shown to be produced by numerous cell types; they participate in a wide range of inflammatory diseases, including asthma, pneumonitis, and pulmonary fibrosis [5]. These cytokines act on endothelial and epithelial mesenchymal lineages and immunocytes to induce the secretion of cytokines and chemokines, such as granulocyte macrophage-colony stimulating factor (GM-CSF), tumor necrosis factor-α (TNF-α), IL-1β, and IL-18 [6]; exert a key function in the migration and differentiation of neutrophils in the lungs [7]; and contribute to airway remodeling by promoting the production of the profibrotic mediators IL-6 and IL-11 by fibroblasts [8–12]. The prototypical IL-17 family member IL-17A plays a key role in various inflammatory conditions by promoting the expression of inflammatory factors, including cytokines, chemokines, matrix metalloproteinases, and acute phase proteins. Overexpression or abnormal activity of IL-17A under pathological conditions can drive pulmonary fibrosis [13]. IL-17B is a less-characterized member of the IL-17 family. A recent study demonstrated that IL-17B induced the production of proinflammatory cytokines by inducing downstream signaling molecules through IL-17 receptor A (IL-17RA) and IL-17 receptor B (IL-17RB), which promote Th17 cell differentiation or neutrophil recruitment and activation to drive bleomycin (BLM)-induced pulmonary fibrosis progression. IL-17E has been demonstrated to play an analogous and crucial role in accelerating BLM-induced pulmonary fibrosis. IL-17C and D have been reported to play key roles in inflammatory pathologies, including lung inflammation; however, there is little direct evidence for their role in IPF [14, 15]. In this review, we summarize the current knowledge regarding the biological functions of IL-17 family cytokines and their involvement in the pathogenesis and progression of IPF.
Biological characteristics of IL-17 family cytokines
IL-17 was first identified in 1993 [16, 17], and in the following years, five other IL-17 family members, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, were gradually discovered using sequence homology analysis. The first discovered IL-17 is termed IL-17A [18]. The sequences of IL-17A and IL-17F show approximately 55% homology, and they are often co-expressed. IL-17B, IL-17C, and IL-17D share 23–29% sequence homology, while IL-17E shares only 17% sequence identity with IL-17A, indicating that it is the most divergent subtype of the family [19, 20]. The IL-17 family members are secreted as disulfide-linked homodimers, with a molecular weight of 17–21 kDa.
The IL-17 cytokines exert their functions by activating their heterodimeric transmembrane receptors, which were first discovered in 1995 [21]. This discovery led to the successive identification of other homologous subunits, which are now termed as a new class of receptors: the IL-17R family, which consists of IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE, with IL-17RA as a common receptor. IL-17A and IL-17F bind to a dimeric IL17RA/RC complex, IL-17B and IL-17E bind to a dimeric 17RA/RB complex, and IL-17C binds to the IL-17RA/RE complex [22, 23]. However, the subunits of heterodimers specific to IL-17RD are yet to be identified [24–26].
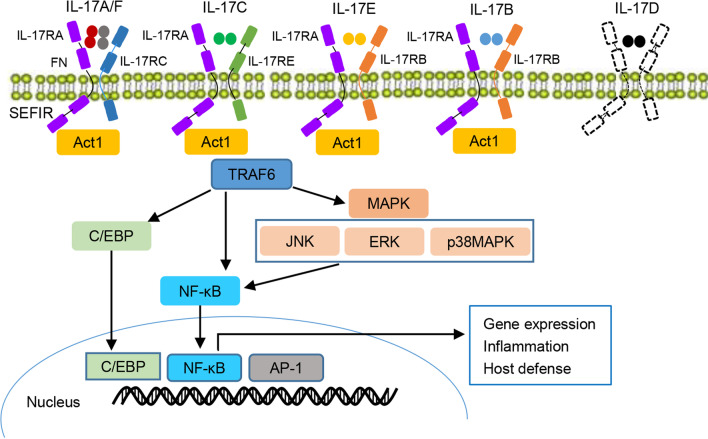
All receptor subunits are single transmembrane chains that contain an extracellular fibronectin (FN) III domain and share a cytoplasmic motif, similar expression to fibroblast growth factor (SEF)/IL-17R (SEFIR)Remove [27], which is conserved within the IL-17R family and is similar to the Toll/IL-1R homologous region (TIR domain) [28]. The SEFIR domain transduces the related signal to activate the adaptor protein nuclear factor-kappaB (NF-κB) activator 1 (Act1), followed by E3 ligase activation and the recruitment and ubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF6) [23]. TRAF6 subsequently activates multiple signaling pathways, such as members of the CCAAT/enhancer-binding protein (C/EBP) family and mitogen-activated protein kinase (MAPK) pathway, including JUN N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 MAPK, leading to the activation of NF-κB. In the nucleus, NF-κB induces the transcription of target genes directly or in combination with other transcription factors such as activator protein-1 (AP-1) (Fig. 1) [29–31].
Fig. 1.
IL-17 cytokines, receptors, and signaling. The IL-17 family is composed of six members, IL-17A–F, while the IL-17 receptor family is composed of five members, IL-17RA to IL-17RE (IL-17RD not shown). IL-17 signaling is activated through the binding of the IL-17 receptor complex to the adaptor protein Act1, which has been determined in IL-17C to recruit TRAF6 to drive the activation of downstream signaling pathways, MAPK, C/EBP, and NF-κB, contributing to the target gene expression as well as mediating the host defense and inflammatory response. IL-17R interleukin 17 receptor, TRAF6 tumor necrosis factor receptor-associated factor 6, C/EBP CCAAT/enhancer-binding protein, MAPK mitogen-activated protein kinase, NF-κB nuclear factor-kappaB, Act1 NF-κB activator 1, JNK JUN N-terminal kinase. ERK extracellular signal-regulated kinase, FN fibronectin, AP1 activator protein-1
Pro-inflammatory function of IL-17A and IL-17F in pulmonary fibrosis
IL-17A
IL-17A, the prototypic subtype of the IL-17 family, was first discovered in 1993 and named CTLA8 [32]. It was subsequently renamed IL-17 and, more recently, IL-17A.
IL-17A can be secreted by multiple cell types, including Th17, CD8+ T (Tc17), γδ T17, innate immune, and non-hematopoietic cells. It exerts its considerable influence via binding to the IL17RA/IL17RC heterodimer [22]. IL-17A production was reported to be significantly elevated in the bronchoalveolar lavage fluid of patients with IPF [33]. In IPF tissues, elevated IL-17 production can be observed in areas of active disease [34–36]. In these regions, IL-17 was originally reported to be mainly secreted by Th17 lymphocytes but was subsequently demonstrated to be produced by regenerating epithelium cells as well as immune cells, including γδ T cells, neutrophils, macrophages, and NK T cells [37]. IPF has been linked to repetitive epithelial cell injury, chronic inflammatory response, and activation of fibroblasts to secrete the extracellular matrix [38]. IL-17A is pivotal for multiple critical processes that promote fibrosis, including tissue repair, inflammatory response, and epithelial–mesenchymal transition (EMT) [39].
Multiple animal modeling studies have explored the role of IL-17A in driving early lung inflammation and fibrosis. During BLM injury, IL-17A expression is upregulated, contributing to the increased expression of other proinflammatory cytokines, including TNF-α, IL-1, IL-6, and TGF-β, as well as chemokines, such as IL-8, C-C motif chemokine (CCL) 2, C-X-C motif chemokine (CXCL) 1, and CXCL5, by endothelial cells and epithelial cells [40–42]. These molecules recruit certain inflammatory cells to the alveolar surface, and subsequent inflammation promotes pulmonary fibrosis [43]. Several studies have shown that BLM-stimulated IL-17 triggered significant neutrophilia and a marked increase in the levels of proinflammatory cytokines, such as IL-6 and IL-1β, and promoted pulmonary fibrosis [44]. Thus, IL-17A blockade by intraperitoneal anti-IL-17A alleviates the acute inflammatory and fibrotic features in mice [43]. Some studies have demonstrated that depletion of alveolar macrophages downregulates IL-17 responses to silica-induced early alveolitis and fibrosis by mediating the production of IL-23 and IL-1β [45].
IL-17A and its signaling cascade IL-17R subunits, as well as the post-translational modification of Act1, mediate tissue inflammation through various signaling pathways [44]. However, the receptor for IL-17A is also ubiquitously expressed in non-hematopoietic cells, including epithelial cells and fibroblasts, which play a vital role in the development of pulmonary fibrosis by EMT and differentiation into myofibroblasts, resulting in enhanced extracellular matrix deposition [46]. Additionally, IL-17 can play a role in lung fibrosis by suppressing autophagy in epithelial cells [47]. Consistent with this effect, IL-17A neutralization promoted autophagy, which presumably favored collagen resolution in the lungs [48]. In addition, in some studies, IL-17R production has been found to be elevated in fibroblasts following BLM challenge, and administration of exogenous IL-17 can accelerate fibroblast proliferation, leading to increased expression of α-smooth muscle actin (α-SMA) and collagen [49]. Mechanically, IL-17 stimulation of fibroblasts occurs via activation of the NF-κB/Act1 signaling pathway [50, 51].
IL-17F
IL-17F shares the highest sequence homology (55%) and overlapping biological function with IL-17A [52], and the cell sources of IL-17F are similar to IL-17A, including Th17 cells, γδ T cells, CD8+ T cells (Tc17), and innate lymphoid cells. Additionally, IL-17F signaling occurs through the same IL-17RA/RC receptor complex. Thus, IL-17F expression is upregulated in multiple inflamed human tissues, and the importance of IL-17F in autoimmune and inflammatory diseases is becoming increasingly apparent [52, 53]. By inducing various cytokines, such as IL-6, and CXC chemokines in human tracheal epithelial cells, vein endothelial cells, inflammatory cells, and fibroblasts, IL-17F plays vital roles in allergic and chronic inflammatory lung disease [54]. Previous research has shown that IL-17F induces similar pathological phenotypes, such as recruitment of neutrophils and the same downstream inflammatory genes via the signaling components of IL-17RA, Act1, and TRAF6, which have been identified as key players in the IL-17A-mediated inflammatory response [55]. In addition, specific overexpression of IL-17F in the lungs of mice leads to infiltration of macrophages and lymphocytes as well as mucus production [52]. Currently, there is no direct evidence for the contribution of IL-17F to the progression of IPF; however, these observations related to IL-17A and inflammatory responses suggest that IL-17F may be an effective target for the treatment of IPF.
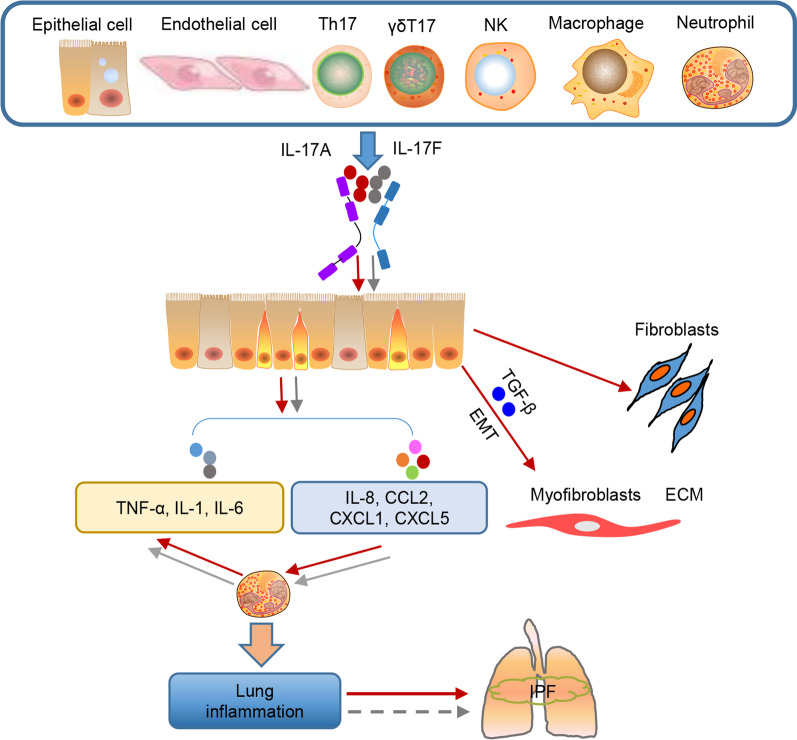
In summary, IL-17A derived from various cell types plays an important role in controlling the inflammatory response and fibrosis progression in IPF, and IL-17F may exert similar anti-inflammatory and anti-fibrotic effects, which need to be further explored (Fig. 2).
Fig. 2.
Main cellular sources and targets of IL-17A and IL-17F in IPF. IL-17A and F can be produced by Th17 cells and other immune cells such as γδ T cells, natural killer cells, macrophages, neutrophils, and non-hematopoietic cells such as epithelial and endothelial cells. IL-17A can contribute to pulmonary fibrosis by EMT, fibroblast proliferation, and differentiation to myofibroblasts. IL-17F stimulates lung inflammation by contributing to the infiltration of neutrophils, macrophages, and lymphocytes as well as promoting the expression of proinflammatory cytokines such as IL-6 and CXC chemokines; however, the role of IL-17F in IPF remains unclear. NK natural killer cells, Th17 T helper cell 17, TNF-α tumor necrosis factor-α, IL-1 interleukin-1, IL-6 interleukin-6, TGF-β transforming growth factor-β, IL-8 interleukin-8, CCL2 C-C motif chemokine 2, CXCL1 C-X-C motif chemokine ligand 1, CXCL5 C-X-C motif chemokine 5, EMT epithelial-mesenchymal transition, α-SMA α-smooth muscle actin, ECM extracellular matrix, IPF idiopathic pulmonary fibrosis
Pro-inflammatory function of IL-17B and IL-17D in pulmonary fibrosis
IL-17B
IL-17B was initially identified using a homology-based expressed sequence tag (EST) database [56, 57]. PCR and northern blot analysis showed that IL-17B expression was strong in the heart, testis tissues, and brain of mice, which have approximately 87.8% similarity to humans, and relatively low in other tissues, including in the liver, lungs, and skeletal muscle tissues [57]. IL-17B is functionally similar to IL-25 and elicits type 2 cytokine secretion from innate type 2 lymphocytes, NKT, and CD4+ CRTH2+ Th2 cells. Some reports revealed that this activity of IL-17B was dependent on the IL-17RA and IL-17RB receptor subunits [58]; however, it was shown to display a weak affinity of 7.6 nmol/L between IL-17B and IL-17RB [57].
Research on IL-17B has been limited; recent studies have shown that the epithelium weakly expresses IL-17B, whereas some connective tissue cells express abundant IL-17B, and neutrophils significantly express IL-17B [59]. Moreover, considerable amounts of IL-17B protein have been detected in naive and memory B cells, germinal center (GC) B cells, and neuronal cells.
The function of IL-17B has not been thoroughly investigated, but several studies have revealed that its function partially overlaps with that of IL-17A and that it exhibits proinflammatory effects under certain conditions [60]. For example, IL-17B increases the production of the inflammatory mediators IL-6, IL-23, and IL-1α in the peritoneal exudate cells and 3T3 cell line [61] as well as the production of TNF-α and IL-1β in THP-1 cells, a cell line derived from human monocyte/macrophage cells [56]. IL-17B promotes the recruitment of C-X-C chemokine receptor (CXCR)4+ or CXCR5+ GC B cells to CXCL12 and CXCL13, and intraperitoneal administration of recombinant human IL-17B causes the migration of neutrophils to the peritoneal cavity and triggers the secretion of chemoattractive factors from multiple cell types [57]. Additionally, IL-17B can enhance IL-33-driven type 2 immune responses [62]. These proinflammatory functions suggest that IL-17B may influence the progression of IPF, which is prompted by early inflammation of the lung. However, no direct study identified this until Yang et al. [63] reported in 2019 that the expression of IL-17B was induced by dysregulated microbiota and that it induced lung fibrosis in a BLM-induced mouse model by interacting with TNF-α to stimulate the secretion of Th17-cell-promoting genes and neutrophil-recruiting genes (Fig. 3). Further studies are required to determine the detailed mechanism of action of IL-17B in cellular and IPF models.
Fig. 3.
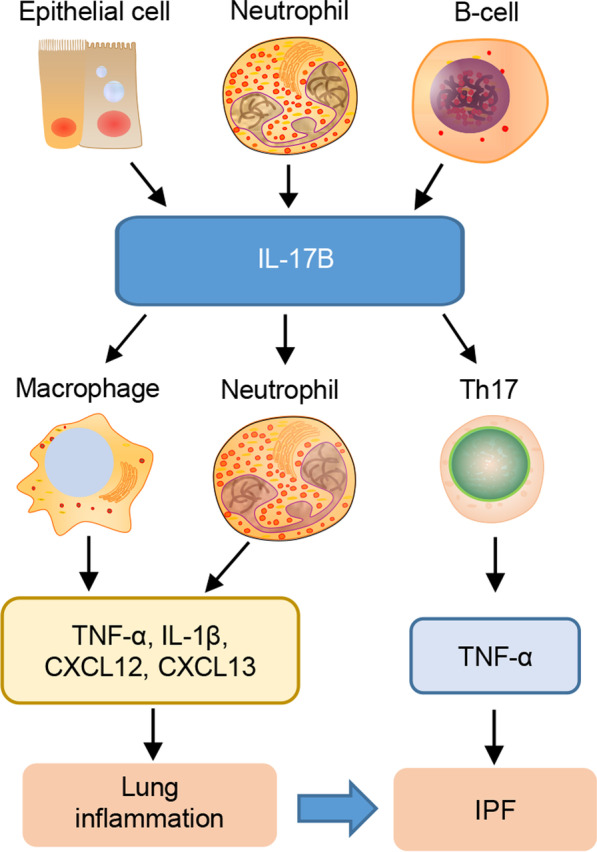
Main cellular sources and targets of IL-17B in IPF. IL-17B is mainly secreted from the epithelium, neutrophils, and B cells. It plays an important role in mediating lung inflammation by stimulating the expression of the inflammatory mediators IL-6, IL-23, TNF-α, IL-1β, and chemokines from macrophages, neutrophils, and Th17 cells. IL-17B induces lung fibrosis by cooperating with TNF-α to stimulate the secretion of Th17-cell-promoting genes and neutrophil-recruiting genes. Th17 T helper cell 17, TNF-α tumor necrosis factor-α, IL-1β interleukin-1β, CXCL12 C-X-C motif chemokine ligand 12, CXCL13 C-X-C motif chemokine ligand 13, IPF idiopathic pulmonary fibrosis
IL-17D
The IL-17D isoform of the IL-17 family, which maps to chromosome 13p11 in humans, discovered in 2002 displays 27% sequence identity with IL-17B [15]. The receptors and functions of IL-17D remain poorly understood. IL-17D mRNA is observed in a wide range of tissues, including the adipose tissue, lung, heart, pancreas, and brain. Intriguingly, IL-17D is detected only in B cells and resting CD4+ T cells among activated immune cells and rarely stimulates immune cells; it contributes to the secretion of proinflammatory factors through endothelial cells [64]. Knowledge of IL-17D in the regulation of pulmonary fibrosis remains limited, and further investigations are needed to understand the relationship between IL-17D-induced inflammatory response and pulmonary fibrosis development.
Effect of IL-17C in pulmonary fibrosis from its various inflammatory function
IL-17C was first discovered by Li et al. [56] and exhibited low sequence homology (23%) with IL-17A. IL-17C has been shown to be expressed in various cells, including epithelial cells, CD4+ T cells, DCs, and macrophages. IL-17C produced by epithelial cells has been reported to be associated with antimicrobial activity [65]. Stimulation of respiratory epithelial cells with whole bacteria in vitro and bacterial challenge in a mouse model rapidly induced IL-17C expression [66], leading to an enhanced lung inflammatory response [67].
Several studies have shown that IL-17C acts through an IL-17 receptor complex, consisting of the common IL-17RA subunit, which is shared with IL-17A, IL-17F, and IL-17B, and the specific IL-17RE subunit [23, 68]. IL-17RE has been shown to be located mainly on epithelial cells and Th17 cells. Th17 cells can produce elevated amounts of IL-17A, IL-17F, and IL-22 stimulated by IL-17C, indicating that IL-17C might promote cell differentiation or maintenance [68]. Additionally, IL-17RA and IL-17RE have been reported to be expressed on other types of cells; they enable IL-17C to play an important role in host defense, autoimmune, and inflammatory pathologies including lung inflammation [62, 63]. For example, adoptive transfer of IL-17C-transduced CD4+ T cells leads to significantly exacerbated collagen-induced arthritis [68]; IL-17C induces the release of TNF-α and IL-1β from THP-1, a monocytic cell line [61]; and adenoviral challenge of IL-17C in the lung triggers neutrophil recruitment [69].
A specific NF-κB binding site has been identified in the promoter region of IL-17C, and IL-17C production is dependent on NF-κB activation. Intriguingly, IL-17C binds to its receptor subunits IL-17RA and IL-17RE, activating the Act1 complex, which in turn activates the NF-kB and MAPK signaling molecules, contributing to the secretion of inflammatory factors and lung destruction [70].
The function of the IL-17C isoform in the progression of IPF has not been extensively investigated, and pulmonary fibrosis has been widely demonstrated to be driven by epithelial cell injury and the inflammatory response during wound healing, which is associated with the function of multiple immunocytes [71, 72]. Early lipopolysaccharide-induced lung injury can be established as a model of pulmonary fibrosis. Reports of related injury of epithelial cells, release of proinflammatory mediators, and IL-17C-induced neutrophil recruitment identified its important roles in lung inflammation. Boosted IL-17 production of IL-17C in IL-33-, NTHi- and cigarette smoke-induced lung inflammation has been recently reported [73, 74]. All of these findings suggest that IL-17C might be a key cytokine in the pathogenesis of pulmonary fibrosis (Fig. 4). Nevertheless, definitive evidence is yet to be provided, and the underlying mechanisms of action of IL-17C in regulating pulmonary fibrosis remain to be elucidated.
Fig. 4.
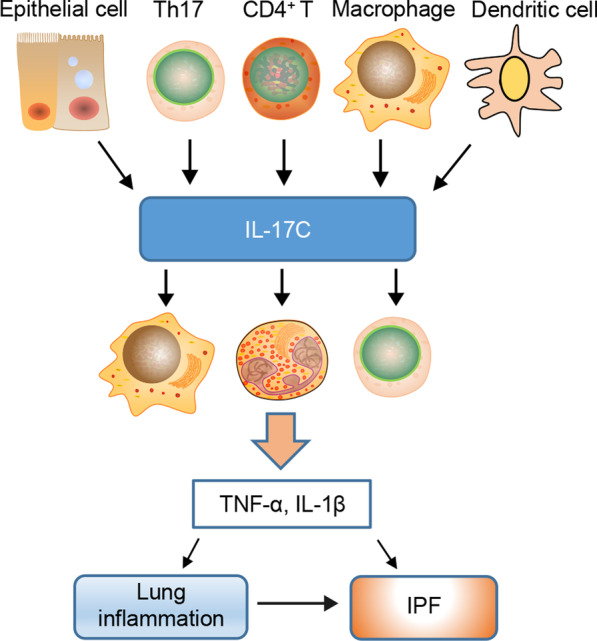
Main cellular sources and targets of IL-17C in lung inflammatory response. IL-17C is expressed in various cells, including epithelial cells, Th17 cells, CD4+ T cells, DCs, and macrophages. It acts on multiple types of cells, including CD4+ T cells, macrophages, and neutrophils, playing an important role in lung inflammation. Definitive evidence has not been reported for the role of IL-17C in regulating pulmonary fibrosis. Th17 T helper cell 17, TNF-α tumor necrosis factor-α, IL-1β interleukin-1β, IPF idiopathic pulmonary fibrosis
Protective and promotive roles of IL-17E in inflammatory response and fibrosis in lungs
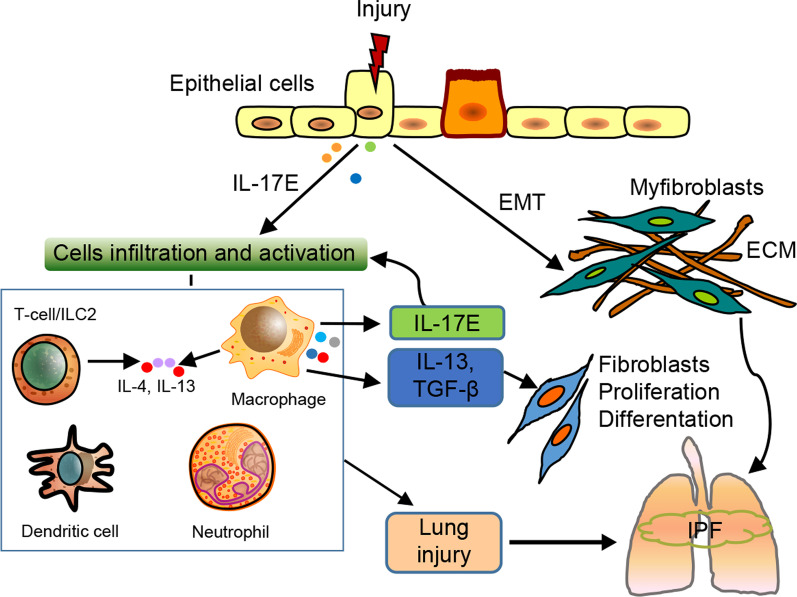
IL-17E, commonly known as IL-25, was originally discovered in Th2 cells in 2001 and later classified as an isoform of the IL-17 family. Later, scientists observed that IL-17E is secreted not only by Th2 cells but also by epithelial cells, endothelial cells, T cells, alveolar macrophages, ILC2s, DCs, eosinophils, and basophils, all of which are associated with inflammatory responses [75, 76]. IL-17E signals through a heterodimeric receptor composed of IL-17RA and IL-17RB, both of which are essential for cytokine expression in target cells during inflammatory disorders. IL-17E initiates allergic airway diseases by producing excessive cytokines, such as IL-4, IL-5, and IL-13 [77]. Remarkably, IL-17E plays protective role in some inflammatory responses, such as parasitic infection and dextran sulfate sodium-induced colitis [78, 79]. A few recent studies have revealed that IL-17 is involved in the development of lung fibrosis. Xu et al. [80] reported augmented levels of IL-17E (IL-25) and its receptor IL-17RB in the lung tissues of patients with IPF and showed that they drove lung fibrosis by mediating the EMT of alveolar epithelial cells as well as recruiting and activating lung fibroblasts. Hams et al. [81] observed a population of type 2 innate lymphoid cells (ILC2s) and increased production of IL-25 in the lungs of patients with IPF and reported that IL-25 promoted the release of IL-13 from ILC2s, which triggered collagen deposition during the IPF process. The relationship between IL-17E and IPF is summarized in Fig. 5. Further investigation is required for identifying other mechanisms by which IL-25 regulates IPF.
Fig. 5.
Involvement of IL-17E in IPF. IL-17E is produced from injured epithelial cells. It acts on T cells, ILC2s, alveolar macrophages, DC and neutrophils, which can stimulate the secretion of cytokines such as IL-17E, IL-13, and TGF-β, drive lung fibrosis by mediating EMT, as well as recruit and activate lung fibroblasts. IL-17E can additionally activate a series of cell types, leading to lung inflammation, which is critical in the development of IPF. ILC2s type 2 innate lymphoid cells, IL-4 interleukin-4, IL-13 interleukin-13, TGF-β transforming growth factor-β, EMT epithelial–mesenchymal transition, ECM extracellular matrix, IPF idiopathic pulmonary fibrosis
Conclusions
The potential role of IL-17 isoforms in pulmonary fibrosis has attracted increasing interest over the last few years. Several members of the IL-17 family have been identified to be promptly secreted by either immune cells or non-hematopoietic cells, playing vital roles via cascade signaling in key stages of IPF progression from the early inflammatory response to the late fibrotic process. Other undefined isoforms have been implicated in mediating protection against antimicrobial infections and inflammatory responses (Table 1). The current challenge is to precisely define the interplay among these IL-17 isoforms and determine their mechanisms of action in the pathogenesis of IPF.
Table 1.
Preclinical and clinical data of IL-17 family members in pulmonary inflammation and fibrosis
| IL-17 family member | Expression changes in inflammatory lung | Preclinical role in PF | Clinical role in PF |
|---|---|---|---|
| IL-17A | Elevated in BLM, IL-33 and LPS-induced lung inflammation [36, 44] | Contributing to fibrosis by promoting proinflammatory cytokines [39, 43]; triggering neutrophilia [44]; promoting EMT [46], accelerating fibroblasts proliferation, differentiation [46, 49] | Elevated in: lung of RA-ILD patients [35];airways of cystic fibrosis patients [34] |
| IL-17B | Expression of IL-17B was induced by dysregulated microbiota [63] | Elevated in BLM-induced PF mouse model by regulating Th17-cell-promoting genes and neutrophil-recruiting genes [63] | No data |
| IL-17C | IL-17C contributes to NTHi-induced inflammation and lung damage [74] | Remains limited | No data |
| IL-17D | Remains limited | Remains limited | No data |
| IL-17E | Protective roles in inflammatory response [75, 76] | Drove lung fibrosis by mediating EMT; recruiting and activating lung fibroblasts [80]; promotes IL-13 from ILC2s; triggering collagen deposition [81] | Elevated in lung of IPF patients [80] |
| IL-17F | Recruitment of neutrophils, macrophages, lymphocytes; promotes inflammatory cytokines [52, 55] | No direct evidence for the progression of IPF |
IL-17 interleukin 17, IPF idiopathic pulmonary fibrosis, BLM bleomycin, IL-33 interleukin-33, LPS Lipopolysaccharides, NTHi nontypeable Haemophilus influenzae, PF pulmonary fibrosis, EMT epithelial-mesenchymal transitions, Th17 cells T helper cell 17, IL-13 interleukin-13, ILC2s group II innate lymphoid cells, RA rheumatoid arthritis, ILD interstitial lung disease
Drugs that target IL-17 signaling are presently available in the market; brodalumab inhibits the human IL-17A receptor, and secukinumab and ixekizumab block IL-17A itself [82, 83]. These three FDA-approved IL-17 inhibitors have demonstrated marked efficacy in patients with inflammatory diseases, such as ankylosing spondylitis and psoriatic arthritis [83–85]. Through additional preclinical data and clinical trials that explore the efficacy of IL-17 neutralization for IPF, the usefulness of IL-17-related monoclonal antibodies in IPF should be considered. Furthermore, extensive information on the precise signaling mechanisms and biological functions of each IL-17 member would pave the way for therapeutic interventions that selectively target specific IL-17 members for the treatment of IPF in humans.
Acknowledgements
None.
Abbreviations
- AP-1
Activator protein-1
- BLM
Bleomycin
- C/EBP
CCAAT/enhancer-binding protein
- CXCR
C-X-C chemokine receptor
- EMT
Epithelial–mesenchymal transition
- ERK
Extracellular signal-regulated kinase
- EST
Expressed sequence tag
- FN
Fibronectin
- GC
Germinal center
- GM-CSF
Granulocyte macrophage-colony stimulating factor
- IL
Interleukin
- IL-17R
IL-17 receptor
- ILC2
Type 2 innate lymphoid cells
- IPF
Idiopathic pulmonary fibrosis
- JNK
JUN N-terminal kinase
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- NF-κB
Nuclear factor κB
- SEF
Similar expression to fibroblast growth factor
- SEFIR
SEF/IL-17R
- α-SMA
α-smooth muscle actin
- TNF-α
Tumor necrosis factor-α
- TRAF-6
Tumor necrosis factor receptor-associated factor 6
Author contributions
YJN and GY organized and executed the research projects. YJN, YHX, and SHW wrote and reviewed the manuscript. All of the above authors contributed to this article and approved the final submitted version. All authors read and approved the final manuscript.
Funding
This research was supported by Natural Science Foundation of Jiangsu Province (BK20180616), the Joint Funds for the Health and Education of Fujian Province (2019-WJ-31), and the Institute of Respiratory Diseases, Xiamen Medical College (HXJB-15).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Contributor Information
Yun-Juan Nie, Email: nieyunjuan@jiangnan.edu.cn.
Shuo-Hua Wu, Email: wushouhua0619@163.com.
Ying-Hua Xuan, Email: yhxuan@jiangnan.edu.cn.
Gen Yan, Email: gyan@stu.edu.cn.
References
- 1.Barratt SL, Creamer A, Hayton C, Chaudhuri N. Idiopathic pulmonary fibrosis (IPF): an overview. J Clin Med. 2018;7(8):201. doi: 10.3390/jcm7080201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez FJ, Collard HR, Pardo A, Raghu G, Richeldi L, Selman M, et al. Idiopathic pulmonary fibrosis. Nat Rev Dis Primers. 2017;3:17074. doi: 10.1038/nrdp.2017.74. [DOI] [PubMed] [Google Scholar]
- 3.Chanda D, Otoupalova E, Smith SR, Volckaert T, De Langhe SP, Thannickal VJ. Developmental pathways in the pathogenesis of lung fibrosis. Mol Aspects Med. 2019;65:56–69. doi: 10.1016/j.mam.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JM, Yoshida M, Kim MS, Lee JH, Baek AR, Jang AS, et al. Involvement of alveolar epithelial cell necroptosis in idiopathic pulmonary fibrosis pathogenesis. Am J Respir Cell Mol Biol. 2018;59(2):215–224. doi: 10.1165/rcmb.2017-0034OC. [DOI] [PubMed] [Google Scholar]
- 5.Song X, Qian Y. IL-17 family cytokines mediated signaling in the pathogenesis of inflammatory diseases. Cell Signal. 2013;25(12):2335–2347. doi: 10.1016/j.cellsig.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 6.Ito Y, Correll K, Zemans RL, Leslie CC, Murphy RC, Mason RJ. Influenza induces IL-8 and GM-CSF secretion by human alveolar epithelial cells through HGF/c-Met and TGF-alpha/EGFR signaling. Am J Physiol Lung Cell Mol Physiol. 2015;308(11):L1178–L1188. doi: 10.1152/ajplung.00290.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aulakh GK. Neutrophils in the lung: "the first responders". Cell Tissue Res. 2018;371(3):577–588. doi: 10.1007/s00441-017-2748-z. [DOI] [PubMed] [Google Scholar]
- 8.Pelaia G, Gallelli L, D'agostino B, Vatrella A, Cuda G, Fratto D, et al. Effects of TGF-beta and glucocorticoids on map kinase phosphorylation, IL-6/IL-11 secretion and cell proliferation in primary cultures of human lung fibroblasts. J Cell Physiol. 2007;210(2):489–497. doi: 10.1002/jcp.20884. [DOI] [PubMed] [Google Scholar]
- 9.Cook SA, Schafer S. Hiding in plain sight: interleukin-11 emerges as a master regulator of fibrosis, tissue integrity, and stromal inflammation. Annu Rev Med. 2020;71:263–276. doi: 10.1146/annurev-med-041818-011649. [DOI] [PubMed] [Google Scholar]
- 10.Montero P, Milara J, Roger I, Cortijo J. Role of JAK/STAT in interstitial lung diseases; molecular and cellular mechanisms. Int J Mol Sci. 2021;22(12):6211. doi: 10.3390/ijms22126211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prele CM, Yao E, O’donoghue RJ, Mutsaers SE, Knight DA. STAT3: a central mediator of pulmonary fibrosis? Proc Am Thorac Soc. 2012;9(3):177–82. doi: 10.1513/pats.201201-007AW. [DOI] [PubMed] [Google Scholar]
- 12.Jaffar J, Mcmillan L, Wilson N, Panousis C, Hardy C, Cho HJ, et al. Coagulation Factor-XII induces interleukin-6 by primary lung fibroblasts: a role in idiopathic pulmonary fibrosis? Am J Physiol Lung Cell Mol Physiol. 2021;322(2):L258–L272. doi: 10.1152/ajplung.00165.2021. [DOI] [PubMed] [Google Scholar]
- 13.Celada LJ, Kropski JA, Herazo-Maya JD, Luo W, Creecy A, Abad AT, et al. PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci Transl Med. 2018;10(460):eaar8356. doi: 10.1126/scitranslmed.aar8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaguchi S, Nambu A, Numata T, Yoshizaki T, Narushima S, Shimura E, et al. The roles of IL-17C in T cell-dependent and -independent inflammatory diseases. Sci Rep. 2018;8(1):15750. doi: 10.1038/s41598-018-34054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starnes T, Broxmeyer HE, Robertson MJ, Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J Immunol. 2002;169(2):642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 16.Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, et al. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155(12):5483–5486. [PubMed] [Google Scholar]
- 17.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183(6):2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y, Ao J, Ai C, Chen X. Molecular and functional identification of three interleukin-17A/F (IL-17A/F) homologues in large yellow croaker (Larimichthys crocea) Dev Comp Immunol. 2016;55:221–232. doi: 10.1016/j.dci.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 21.Yao Z, Fanslow WC, Seldin MF, Rousseau AM, Painter SL, Comeau MR, et al. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3(6):811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 22.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9(8):556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mcgeachy MJ, Cua DJ, Gaffen SL. The IL-17 family of cytokines in health and disease. Immunity. 2019;50(4):892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Carrozzi V, Sambandam A, Luis E, Lin Z, Jeet S, Lesch J, et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat Immunol. 2011;12(12):1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 25.Rickel EA, Siegel LA, Yoon BR, Rottman JB, Kugler DG, Swart DA, et al. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J Immunol. 2008;181(6):4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 26.Toy D, Kugler D, Wolfson M, Vanden Bos T, Gurgel J, Derry J, et al. Cutting edge: interleukin 17 signals through a heteromeric receptor complex. J Immunol. 2006;177(1):36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 27.Yang H, Zhu Y, Chen X, Li X, Ye S, Zhang R. Structure of a prokaryotic SEFIR domain reveals two novel SEFIR-SEFIR interaction modes. J Struct Biol. 2018;203(2):81–89. doi: 10.1016/j.jsb.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ely LK, Fischer S, Garcia KC. Structural basis of receptor sharing by interleukin 17 cytokines. Nat Immunol. 2009;10(12):1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadian Y, Bagood MD, Dahle SE, Sood A, Isseroff RR. Interleukin-17: potential target for chronic wounds. Mediators Inflamm. 2019;2019:1297675. doi: 10.1155/2019/1297675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8(3):247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 31.Schwandner R, Yamaguchi K, Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191(7):1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J Immunol. 1993;150(12):5445–5456. [PubMed] [Google Scholar]
- 33.Tiringer K, Treis A, Fucik P, Gona M, Gruber S, Renner S, et al. A Th17- and Th2-skewed cytokine profile in cystic fibrosis lungs represents a potential risk factor for Pseudomonas aeruginosa infection. Am J Respir Crit Care Med. 2013;187(6):621–629. doi: 10.1164/rccm.201206-1150OC. [DOI] [PubMed] [Google Scholar]
- 34.Brodlie M, Corris PA, Lordan J, Ward C. Interleukin-17 and cystic fibrosis lung disease. Am J Respir Crit Care Med. 2012;185(1):108–9; author reply 9–10. [DOI] [PubMed]
- 35.Zhang J, Wang D, Wang L, Wang S, Roden AC, Zhao H, et al. Profibrotic effect of IL-17A and elevated IL-17RA in idiopathic pulmonary fibrosis and rheumatoid arthritis-associated lung disease support a direct role for IL-17A/IL-17RA in human fibrotic interstitial lung disease. Am J Physiol Lung Cell Mol Physiol. 2019;316(3):L487–L497. doi: 10.1152/ajplung.00301.2018. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Guabiraba R, Besnard AG, Komai-Koma M, Jabir MS, Zhang L, et al. IL-33 promotes ST2-dependent lung fibrosis by the induction of alternatively activated macrophages and innate lymphoid cells in mice. J Allergy Clin Immunol. 2014;134(6):1422–32. e11. [DOI] [PMC free article] [PubMed]
- 37.Kono M, Miyashita K, Hirama R, Oshima Y, Takeda K, Mochizuka Y, et al. Prognostic significance of bronchoalveolar lavage cellular analysis in patients with acute exacerbation of interstitial lung disease. Respir Med. 2021;186:106534. doi: 10.1016/j.rmed.2021.106534. [DOI] [PubMed] [Google Scholar]
- 38.Chambers RC, Mercer PF. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann Am Thorac Soc. 2015;12(Suppl 1):S16–S20. doi: 10.1513/AnnalsATS.201410-448MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaikh SB, Prabhu A, Bhandary YP. Interleukin-17A: a potential therapeutic target in chronic lung diseases. Endocr Metab Immune Disord Drug Targets. 2019;19(7):921–928. doi: 10.2174/1871530319666190116115226. [DOI] [PubMed] [Google Scholar]
- 40.Albanesi C, Cavani A, Girolomoni G. IL-17 is produced by nickel-specific T lymphocytes and regulates ICAM-1 expression and chemokine production in human keratinocytes: synergistic or antagonist effects with IFN-gamma and TNF-alpha. J Immunol. 1999;162(1):494–502. [PubMed] [Google Scholar]
- 41.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34(2):149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 42.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, et al. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160(7):3513–3521. [PubMed] [Google Scholar]
- 43.Gouda MM, Bhandary YP. Acute lung injury: IL-17A-mediated inflammatory pathway and its regulation by curcumin. Inflammation. 2019;42(4):1160–1169. doi: 10.1007/s10753-019-01010-4. [DOI] [PubMed] [Google Scholar]
- 44.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207(3):535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golebski K, Ros XR, Nagasawa M, Van Tol S, Heesters BA, Aglmous H, et al. IL-1beta, IL-23, and TGF-beta drive plasticity of human ILC2s towards IL-17-producing ILCs in nasal inflammation. Nat Commun. 2019;10(1):2162. doi: 10.1038/s41467-019-09883-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang T, Liu Y, Zou JF, Cheng ZS. Interleukin-17 induces human alveolar epithelial to mesenchymal cell transition via the TGF-beta1 mediated Smad2/3 and ERK1/2 activation. PLoS ONE. 2017;12(9):e0183972. doi: 10.1371/journal.pone.0183972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipolla E, Fisher AJ, Gu H, Mickler EA, Agarwal M, Wilke CA, et al. IL-17A deficiency mitigates bleomycin-induced complement activation during lung fibrosis. FASEB J. 2017;31(12):5543–5556. doi: 10.1096/fj.201700289R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu H, Mi S, Li Z, Hua F, Hu ZW. Interleukin 17A inhibits autophagy through activation of PIK3CA to interrupt the GSK3B-mediated degradation of BCL2 in lung epithelial cells. Autophagy. 2013;9(5):730–742. doi: 10.4161/auto.24039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chrysanthopoulou A, Mitroulis I, Apostolidou E, Arelaki S, Mikroulis D, Konstantinidis T, et al. Neutrophil extracellular traps promote differentiation and function of fibroblasts. J Pathol. 2014;233(3):294–307. doi: 10.1002/path.4359. [DOI] [PubMed] [Google Scholar]
- 50.Heo YJ, Oh HJ, Jung YO, Cho ML, Lee SY, Yu JG, et al. The expression of the receptor for advanced glycation end-products (RAGE) in RA-FLS is induced by IL-17 via Act-1. Arthritis Res Ther. 2011;13(4):R113. doi: 10.1186/ar3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei L, Liu M, Xiong H, Peng B. Up-regulation of IL-23 expression in human dental pulp fibroblasts by IL-17 via activation of the NF-kappaB and MAPK pathways. Int Endod J. 2018;51(6):622–631. doi: 10.1111/iej.12871. [DOI] [PubMed] [Google Scholar]
- 52.Yang XO, Chang SH, Park H, Nurieva R, Shah B, Acero L, et al. Regulation of inflammatory responses by IL-17F. J Exp Med. 2008;205(5):1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hot A, Miossec P. Effects of interleukin (IL)-17A and IL-17F in human rheumatoid arthritis synoviocytes. Ann Rheum Dis. 2011;70(5):727–732. doi: 10.1136/ard.2010.143768. [DOI] [PubMed] [Google Scholar]
- 54.Glatt S, Baeten D, Baker T, Griffiths M, Ionescu L, Lawson ADG, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77(4):523–532. doi: 10.1136/annrheumdis-2017-212127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang SH, Dong C. IL-17F: regulation, signaling and function in inflammation. Cytokine. 2009;46(1):7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, et al. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc Natl Acad Sci USA. 2000;97(2):773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shi Y, Ullrich SJ, Zhang J, Connolly K, Grzegorzewski KJ, Barber MC, et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J Biol Chem. 2000;275(25):19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 58.Ramirez-Carrozzi V, Ota N, Sambandam A, Wong K, Hackney J, Martinez-Martin N, et al. Cutting edge: IL-17B uses IL-17RA and IL-17RB to induce Type 2 inflammation from human lymphocytes. J Immunol. 2019;202(7):1935–1941. doi: 10.4049/jimmunol.1800696. [DOI] [PubMed] [Google Scholar]
- 59.Kouri VP, Olkkonen J, Ainola M, Li TF, Bjorkman L, Konttinen YT, et al. Neutrophils produce interleukin-17B in rheumatoid synovial tissue. Rheumatology (Oxford) 2014;53(1):39–47. doi: 10.1093/rheumatology/ket309. [DOI] [PubMed] [Google Scholar]
- 60.Reynolds JM, Lee YH, Shi Y, Wang X, Angkasekwinai P, Nallaparaju KC, et al. Interleukin-17B antagonizes interleukin-25-mediated mucosal inflammation. Immunity. 2015;42(4):692–703. doi: 10.1016/j.immuni.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamaguchi Y, Fujio K, Shoda H, Okamoto A, Tsuno NH, Takahashi K, et al. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J Immunol. 2007;179(10):7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 62.Macgregor CH. Biosynthesis of membrane-bound nitrate reductase in Escherichia coli: evidence for a soluble precursor. J Bacteriol. 1976;126(1):122–131. doi: 10.1128/jb.126.1.122-131.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang D, Chen X, Wang J, Lou Q, Lou Y, Li L, et al. Dysregulated lung commensal bacteria drive interleukin-17B production to promote pulmonary fibrosis through their outer membrane vesicles. Immunity. 2019;50(3):692–706. e7. [DOI] [PubMed]
- 64.Shabgah AG, Fattahi E, Shahneh FZ. Interleukin-17 in human inflammatory diseases. Postepy Dermatol Alergol. 2014;31(4):256–261. doi: 10.5114/pdia.2014.40954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Larocque-De-Freitas IF, Rocha JDB, Nunes MP, Oliveira PV, Nascimento DO, Freire-De-Lima L, et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci Rep. 2018;8(1):16378. doi: 10.1038/s41598-018-34649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wonnenberg B, Jungnickel C, Honecker A, Wolf L, Voss M, Bischoff M, et al. IL-17A attracts inflammatory cells in murine lung infection with P. aeruginosa. Innate Immun. 2016;22(8):620–625. doi: 10.1177/1753425916668244. [DOI] [PubMed] [Google Scholar]
- 67.Wolf L, Sapich S, Honecker A, Jungnickel C, Seiler F, Bischoff M, et al. IL-17A-mediated expression of epithelial IL-17C promotes inflammation during acute Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol. 2016;311(5):L1015–L1022. doi: 10.1152/ajplung.00158.2016. [DOI] [PubMed] [Google Scholar]
- 68.Chang SH, Reynolds JM, Pappu BP, Chen G, Martinez GJ, Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011;35(4):611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, et al. New IL-17 family members promote Th1 or Th2 responses in the lung: in vivo function of the novel cytokine IL-25. J Immunol. 2002;169(1):443–453. doi: 10.4049/jimmunol.169.1.443. [DOI] [PubMed] [Google Scholar]
- 70.Conti HR, Whibley N, Coleman BM, Garg AV, Jaycox JR, Gaffen SL. Signaling through IL-17C/IL-17RE is dispensable for immunity to systemic, oral and cutaneous candidiasis. PLoS ONE. 2015;10(4):e0122807. doi: 10.1371/journal.pone.0122807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208(7):1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weiskirchen R, Weiskirchen S, Tacke F. Organ and tissue fibrosis: molecular signals, cellular mechanisms and translational implications. Mol Aspects Med. 2019;65:2–15. doi: 10.1016/j.mam.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 73.Cai T, Qiu J, Ji Y, Li W, Ding Z, Suo C, et al. IL-17-producing ST2(+) group 2 innate lymphoid cells play a pathogenic role in lung inflammation. J Allergy Clin Immunol. 2019;143(1):229–44. e9. [DOI] [PMC free article] [PubMed]
- 74.Vella G, Ritzmann F, Wolf L, Kamyschnikov A, Stodden H, Herr C, et al. IL-17C contributes to NTHi-induced inflammation and lung damage in experimental COPD and is present in sputum during acute exacerbations. PLoS ONE. 2021;16(1):e0243484. doi: 10.1371/journal.pone.0243484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med. 2007;204(7):1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu M, Dong C. IL-25 in allergic inflammation. Immunol Rev. 2017;278(1):185–191. doi: 10.1111/imr.12558. [DOI] [PubMed] [Google Scholar]
- 77.Tamachi T, Maezawa Y, Ikeda K, Kagami S, Hatano M, Seto Y, et al. IL-25 enhances allergic airway inflammation by amplifying a TH2 cell-dependent pathway in mice. J Allergy Clin Immunol. 2006;118(3):606–614. doi: 10.1016/j.jaci.2006.04.051. [DOI] [PubMed] [Google Scholar]
- 78.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464(7293):1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464(7293):1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xu X, Luo S, Li B, Dai H, Zhang J. Feature Article: IL-25 contributes to lung fibrosis by directly acting on alveolar epithelial cells and fibroblasts. Exp Biol Med (Maywood) 2019;244(9):770–780. doi: 10.1177/1535370219843827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hams E, Armstrong ME, Barlow JL, Saunders SP, Schwartz C, Cooke G, et al. IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc Natl Acad Sci U S A. 2014;111(1):367–372. doi: 10.1073/pnas.1315854111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Campa M, Mansouri B, Warren R, Menter A. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis. Dermatol Ther (Heidelb). 2016;6(1):1–12. doi: 10.1007/s13555-015-0092-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mease PJ, Genovese MC, Greenwald MW, Ritchlin CT, Beaulieu AD, Deodhar A, et al. Brodalumab, an anti-IL17RA monoclonal antibody, in psoriatic arthritis. N Engl J Med. 2014;370(24):2295–2306. doi: 10.1056/NEJMoa1315231. [DOI] [PubMed] [Google Scholar]
- 84.Chiricozzi A, Krueger JG. IL-17 targeted therapies for psoriasis. Expert Opin Investig Drugs. 2013;22(8):993–1005. doi: 10.1517/13543784.2013.806483. [DOI] [PubMed] [Google Scholar]
- 85.Marzo-Ortega H, Sieper J, Kivitz A, Blanco R, Cohen M, Delicha EM, et al. Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3-year results from the phase III trial, MEASURE 2. RMD Open. 2017;3(2):e000592. doi: 10.1136/rmdopen-2017-000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.