In the original article, there was a mistake in the caption for Figure 6C as published. A clerical error occurred when we prepared the paper. The correct caption appears below.
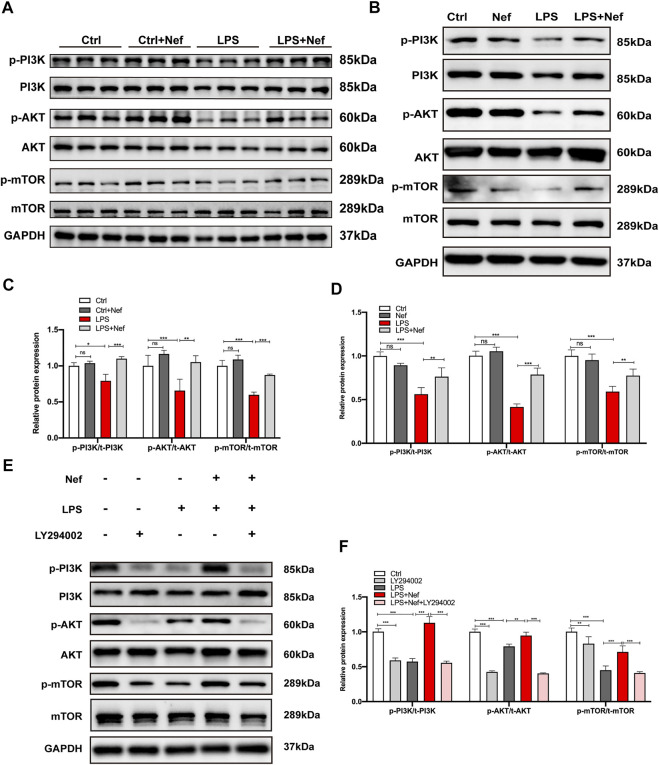
FIGURE 6.
Neferine reversed the LPS-induced downregulation of the PI3K/AKT/mTOR signaling pathway in vivo and in vitro. (A) Representative Western blot images of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR in mice. (B–E) Representative Western blot images of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR in H9c2 cells. (C) Densitometric quantification analysis of the protein expression levels of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR in mice. (D–F) Densitometric quantification analysis of the protein expression levels of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR in H9c2 cells. All data are expressed as mean ± standard deviation. All experiments were repeated at least three times. *p < 0.05, **p < 0.01, ***p < 0.001; ns: no significant difference.
“(C) Densitometric quantification analysis of the protein expression levels of p-PI3K, PI3K, p-AKT, AKT, p-mTOR, and mTOR in mice.”
In the original article, there was a mistake in Figures 2, 5, 6 as published. The image in the “LPS + Nef” group within Figure 2B, the image in the “LPS + Nef” group within Figure 5A, and the mTOR bands within Figure 6E, were uploaded with errors. The corrected Figures 2, 5, 6 appear below.
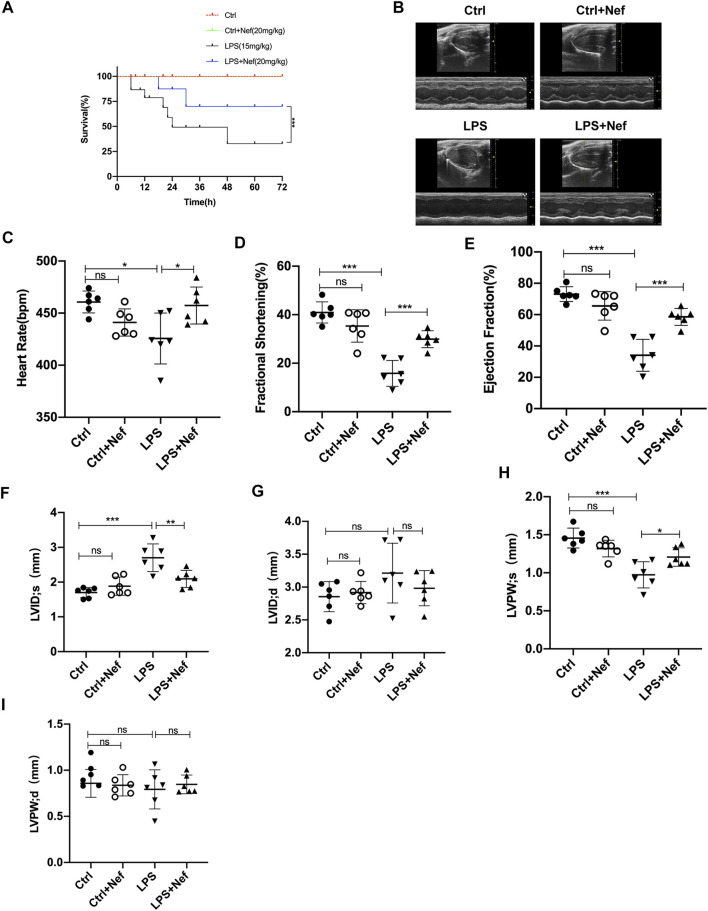
FIGURE 2.
Neferine preserved cardiac function and improved the survival rate in LPS-treated mice. (A) Neferine (20 mg/kg) was intraperitoneally administered 2 h before LPS injection (15 mg/kg) and then administered for three consecutive days. The mortality of mice within 72 h was recorded (n = 15 mice). (B–I) the mice were treated with neferine (20 mg/kg, intraperitoneally (i.p.) 2 h before LPS challenge (10 mg/kg, i.p.), and cardiac function was examined (n = 6). (B) Representative echocardiographic images. (C) Heart rate (HR). (D) Fractional shortening (FS). (E) Ejection fraction (EF). (F) Left ventricular internal systolic dimension (LVIDs). (G) Left ventricular internal diastolic dimension (LVIDd). (H) Left ventricular posterior wall systolic thickness (LVPWs). (I) Left ventricular posterior wall diastolic thickness (LVPWd). Data are expressed as mean ± standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001; ns: no significant difference.
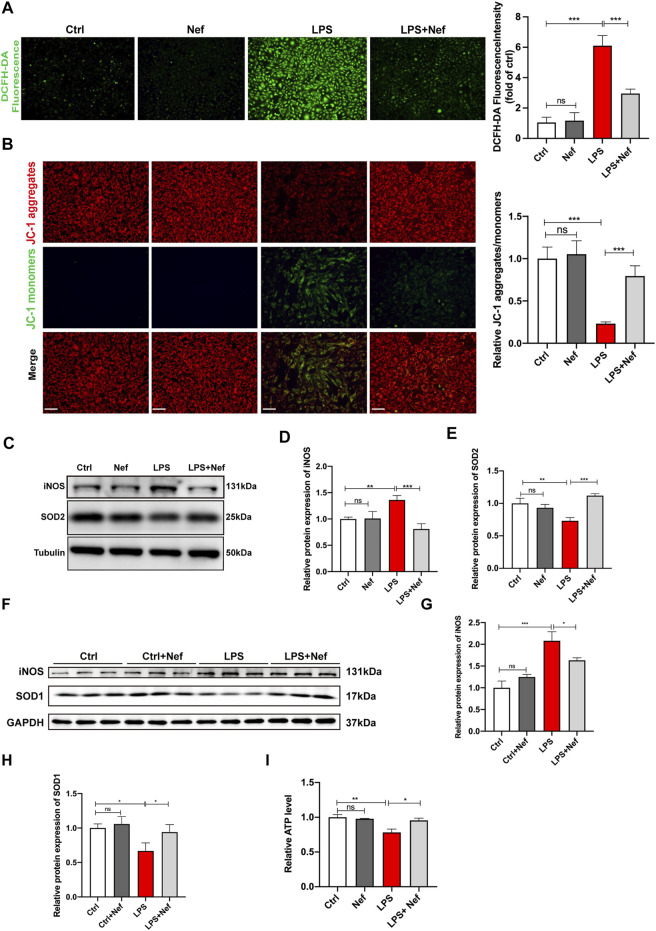
FIGURE 5.
Neferine reduced the production of reactive oxygen species (ROS) and prevented mitochondrial dysfunction. (A) DCFH-DA staining was used to evaluate the intracellular ROS level in H9c2 cells. Fluorescence intensity was measured. Scale bar, 50 μm. (B) Representative images of JC-1 staining in LPS-induced H9c2 cells. Fluorescence intensity was measured. Scale bar, 50 μm. (C–E) Western blot analysis and densitometric quantification of SOD2 and iNOS protein expression in H9c2 cells. (F) SOD1 and iNOS protein expression levels in septic mice were detected by Western blot (n = 6). (G,H) Densitometric quantification of SOD1 and iNOS protein expression levels. (I) ATP levels in H9c2 cells were analyzed. All data are expressed as mean ± SD. All experiments were repeated at least three times. *p < 0.05, **p < 0.01, ***p < 0.001; ns: no significant difference; DCFH-DA, 2′-7′dichlorofluorescein diacetate.
The authors apologize for this error and state that this does not change the scientific conclusions of the article in any way. The original article has been updated.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.