Abstract
Neonates, particularly those born preterm, have a high incidence of thrombocytopenia and bleeding, most commonly in the brain. Because of this, it has historically been accepted that neonates should be transfused at higher platelet counts than older children or adults, to decrease their bleeding risk. However, a number of observational studies and a recent large, randomized trial found a higher incidence of bleeding and mortality in neonates who received more platelet transfusions. The mechanisms underlying the deleterious effects of platelet transfusions in neonates are unknown, but it has been hypothesized that transfusing adult platelets into the very different physiological environment of a neonate may result in a “developmental mismatch” with potential negative consequences. Specifically, neonatal platelets are hyporeactive in response to multiple agonists and upon activation express less surface P‐selectin than adult platelets. However, this hyporeactivity is well balanced by factors in neonatal blood that promote clotting, such as the elevated hematocrit, elevated von Willebrand factor (VWF) levels, and a predominance of ultra‐long VWF polymers, with the net result of normal neonatal primary hemostasis. So far, most studies on the developmental differences between neonatal and adult platelets have focused on their hemostatic functions. However, it is now clear that platelets have important nonhemostatic functions, particularly in angiogenesis, immune responses, and inflammation. Whether equally important developmental differences exist with regard to those nonhemostatic platelet functions and how platelet transfusions perturb those processes in neonates remain unanswered questions.
Keywords: hemostasis, inflammation, neonate, platelet, platelet transfusion
Essentials.
Low platelet counts are common in premature neonates, who frequently receive platelet transfusions.
Studies have found an association between platelet transfusions and higher mortality in newborns.
Transfused platelets from adults are different from neonatal platelets in multiple ways.
Platelet transfusions might have negative effects on neonatal hemostasis and inflammation.
1. THROMBOCYTOPENIA AND PLATELET TRANSFUSIONS IN NEONATES
The incidence of thrombocytopenia is high among neonates admitted to the neonatal intensive care unit (NICU), ranging from 18% to 35% depending on the study. 1 , 2 The incidence is also inversely proportional to the gestational age of the infant, reaching ≈70% among the most premature neonates with a birth weight <1000 g. 3 Premature infants born with a weight <1500 g also have the highest incidence of intracranial bleeding of any patient population, with ≈20% of these infants developing an intracranial hemorrhage (usually intraventricular) during their hospital stay. When severe, intraventricular hemorrhages (IVHs) can cause ventricular dilatation or extend into the brain parenchyma, with the potential for significant long‐term neurodevelopmental consequences. 4 Due to the combined high incidence of thrombocytopenia and intracranial hemorrhage seen in this population, it has historically been accepted that preterm neonates should be transfused at higher platelet counts than older children or adults, in an attempt to prevent intracranial bleeding.
Prior surveys and observational studies have suggested that North American neonatologists administer platelet transfusions at higher platelet counts than their European counterparts. 5 A recent study of neonatal transfusion practices in seven US hospitals from 2013 to 2016 evaluated the platelet count before >1000 platelet transfusions administered to infants of different gestational ages. In this study, the median pretransfusion platelet count for the entire cohort was 71 × 109/L, and it was >45 × 109/L for all gestational and postnatal age groups examined. 6 In a multicenter, prospective observational study carried out in England in 2005 to 2006, in contrast, the median platelet count before transfusion was 27 × 109/L. 7
Until 2019, the evidence guiding platelet transfusion practices in this population was sparse, which likely contributed to the worldwide diversity in approaches. The first neonatal platelet transfusion threshold randomized trial was published in 1993 and included 152 preterm neonates who were followed during the first week of life, the period when nearly all IVHs occur. Neonates in the study were randomly assigned to receive a platelet transfusion either when the platelet count fell below 150 × 109/L (to maintain a normal platelet count) or when it fell below 50 × 109/L. Interestingly, the study found no difference in the incidence or severity of IVH between the two groups, suggesting that transfusing preterm neonates with platelet counts between 50 and 150 × 109/L did not reduce the bleeding risk. 8 However, this single study provided no information regarding the safety of tolerating platelet counts <50 × 109/L, or guidance for transfusion thresholds in preterm infants with late‐onset thrombocytopenia (after day of life 7).
In 2019, the results of the Platelets for Neonatal Transfusion–Study 2 (PlaNeT‐2), the largest randomized trial of platelet transfusion thresholds in neonates to date, were published. This study enrolled 660 neonates <34 weeks’ gestational age, and randomized them to receive platelet transfusions for platelet counts <50 × 109/L (liberal group) or <25 × 109/L (restrictive group). Overall, 90% of neonates randomly assigned to the liberal group received one or more platelet transfusions, compared to 53% of those randomly assigned to the restrictive group. Surprisingly, the liberal transfusion group exhibited a significantly higher incidence of the primary outcome of death or major bleeding in the 28 days following randomization, with both components of this composite outcome favoring the restrictive transfusion group. 9 Among secondary outcomes, infants randomly assigned to the liberal transfusion group also had a higher incidence of bronchopulmonary dysplasia (BPD), a disease of the lungs that affects preterm infants and is associated with inflammation and an arrest of pulmonary vascular and alveolar development.
A subsequent secondary analysis of the PlaNeT‐2 data used sophisticated mathematical modeling to examine whether the beneficial effects of restrictive transfusion thresholds varied depending on the neonate’s baseline risk of bleeding or mortality. 10 This analysis demonstrated that neonates with the highest baseline risk of bleeding/mortality (based on accepted clinical factors such as gestational age, underlying diagnosis, etc) benefitted just as much as low‐risk neonates from the restrictive transfusion thresholds. While these findings seemed surprising at first, they were in fact consistent with several observational studies that had found a poor correlation between platelet count and bleeding risk (suggesting that factors other than platelet count are better predictors of bleeding risk in neonates), 7 , 11 , 12 , 13 , 14 no effect of platelet transfusions in reducing the incidence or severity of IVH, 8 , 12 , 15 and an association between number of platelet transfusions and increased neonatal morbidity and mortality. 16 , 17 , 18 , 19 , 20 , 21 , 22
2. MECHANISMS UNDERLYING THE INCREASED BLEEDING RISK ASSOCIATED WITH PLATELET TRANSFUSIONS
The mechanisms through which platelet transfusions paradoxically increase bleeding risk are unknown. However, it is known that bleeding, particularly IVH, in preterm neonates is multifactorial, with vascular and hemodynamic factors likely playing a more important role in the pathogenesis than hemostatic factors. 23 Most intracranial hemorrhages in preterm neonates occur in the first few days of life and originate in the germinal matrix, a highly vascularized collection of neuronal and glial cells that lines the lateral ventricles in the developing brain. The vasculature of the germinal matrix is intrinsically fragile due to a developmental paucity of pericytes, an immature basal lamina, and a deficiency of glial fibrillary acidic protein. 24 When this fragile vasculature encounters disturbances in cerebral blood flow due to the impaired cerebral autoregulation of premature infants, blood vessels can rupture and bleed into the ventricles. Based on these facts, it is possible that the rapid volume expansion caused by a platelet transfusion could be a contributing factor to these hemorrhages. Platelet transfusions are usually administered to neonates at a dose of 10 to 15 mL/kg (15 mL/kg in the PlaNeT‐2 trial) given over ≈30–60 minutes. This is a significantly higher volume than that transfused to older children or adults, who usually receive ≈5 mL/kg of platelets over ≈30 minutes. Thus, it is conceivable that neonatal platelet transfusions administered at this dose and over a short period could result in a rapid expansion in neonatal blood volume that could at least contribute to the pathogenesis of IVH. Alternatively, it has been hypothesized that the transfusion of adult platelets into the very different physiological environment of a preterm neonate might result in a “developmental mismatch” between the transfused platelets and the recipient, with potential negative consequences.
3. HEMOSTATIC DEVELOPMENTAL DIFFERENCES BETWEEN NEONATAL AND ADULT PLATELETS
Morphologically, neonatal and adult platelets are indistinguishable from each other. Functionally, however, neonatal platelets are different from adult platelets in ways that meet the unique developmental needs of the fetus and neonate. One of the most striking characteristics of fetal/neonatal life is the rapid growth that occurs during this period of development, which includes a rapid expansion of the blood volume. At the same time that the blood volume expands, the platelet count also rises, which results in a 10‐fold expansion of the platelet mass during the first 2 weeks of postnatal life in the mouse 25 and an increase in platelet mass during the third trimester of gestation in humans. 26 Using mathematical modeling, it was demonstrated that this platelet mass expansion is facilitated by the longer life span of neonatal platelets, compared to adult platelets, which reduces the need to increase platelet production to meet the high platelet demands of the growing fetus/neonate. 25
In regards to their hemostatic functions, platelets isolated from human preterm and full‐term neonates are significantly hyporeactive compared to platelets from adults. This is demonstrated by lower levels of fibrinogen binding and surface P‐selectin expression in response to various agonists (including epinephrine, thrombin, thromboxane, ADP, collagen, and rhodocytin) in neonatal compared to adult platelets. The neonatal platelet hyporeactivity is the result of various developmental deficiencies in key platelet surface receptors and/or platelet signaling pathways. 27 Specifically, the decreased responsiveness of neonatal platelets to epinephrine and thrombin has been attributed to the decreased expression of α‐adrenergic receptors (for epinephrine) 28 , 29 and the thrombin receptors protease‐activated receptors 1 (PAR1) and 4 (PAR4). 30 , 31 In contrast, no developmental differences have been found in the expression levels of thromboxane receptors or of the ADP receptors P2Y1/P2Y12. 32 Instead, the decreased responsiveness of neonatal platelets to ADP and thromboxane is due to impaired intracellular signaling caused by the decreased guanosine triphosphatase activity of the α‐subunit of Gq. 33 , 34 With regard to collagen, one of the main collagen receptors on the platelet surface is glycoprotein VI (GPVI), which shares a common signal transduction pathway with the other hemi–immunoreceptor tyrosine–based activation motif receptor in platelets, C‐type lectin‐like receptor 2 (CLEC‐2). Two recent studies have shown a significant hyporesponsiveness of preterm and full‐term neonatal platelets to GPVI and CLEC‐2 ligands (collagen‐related peptide and rhodocytin, respectively), caused by a significant reduction in the protein levels of GPVI and CLEC‐2 coupled with an intracellular signaling defect. 31 , 35 In addition to these differences in specific receptors and signaling pathways, neonatal platelets also exhibit impaired calcium mobilization following agonist stimulation, reduced degranulation, and hypersensitivity to inhibition by prostaglandin, all of which might contribute to their relative hyporeactivity compared to adult platelets. 27
Importantly, despite the hyporeactivity of neonatal platelets, studies performed in the late 1980s found shorter bleeding times in healthy full term neonates compared to healthy adults. 36 Furthermore, several subsequent studies using a platelet function analyzer (PFA‐100), an objective and automated in vitro test of whole blood primary hemostasis, also found shorter closure times (CTs) in response to collagen/epinephrine (CT‐Epi) and collagen/ADP (CT‐ADP) in cord blood samples from full‐term neonates compared to blood samples form adults. 37 , 38 Taken together, these studies demonstrated that full‐term neonates have more robust primary hemostasis than healthy adults, despite the pronounced hyporeactivity of neonatal platelets. The explanation for this finding is that the platelet hyporeactivity is counterbalanced by several factors in neonatal blood that stimulate clotting, including a high hematocrit, high mean corpuscular volume, high levels of von Willebrand factor (VWF), and the predominance of ultra‐large VWF multimers. 39 , 40 , 41 , 42 The slightly shorter bleeding and closure times seen in neonates are the net result of this balance. Given these data, it is clear that the hyporeactivity of neonatal platelets is not a developmental deficiency, but rather an integral part of a different but well‐balanced neonatal primary hemostatic system (Table 1).
TABLE 1.
Key differences in neonatal platelet function, primary hemostasis, and compensatory factors in neonatal blood, compared to adults
|
Platelet function (in neonates vs adults) |
Primary hemostasis (in neonates vs adults) |
Compensatory factors (in neonates vs adults) |
|---|---|---|
| ↓↓ response to epinephrine | Shorter bleeding times | ↑ concentration of VWF |
| ↓ response to thrombin/TRAP | Shorter closure times (PFA‐100) b | ↑ proportion of VWF ultra‐large multimers |
| ↓ response to ADP | ↑ hematocrit | |
| ↓ response to thromboxane | ↑ mean corpuscular volume | |
| ↓↓ response to collagen | ||
| ↓↓ response to rhodocytin a | ||
| ↓ degranulation | ||
| ↑ sensitivity to inhibition by PGE1 |
Abbreviations: PFA‐100: Platelet Function Analyzer‐100; PGE1, prostaglandin E1; TRAP, thrombin receptor activating peptide; VWF, von Willebrand factor.
CLEC‐2 ligand.
Closure times in response to collagen/epinephrine and collagen/ADP.
4. NONHEMOSTATIC DEVELOPMENTAL DIFFERENCES BETWEEN NEONATAL AND ADULT PLATELETS
Over the past decade, there has been an exponential increase in the number of studies highlighting the many, previously unrecognized, functions of platelets outside of hemostasis. Platelets are now known to regulate biological processes that are critically important in fetal/neonatal life, including angiogenesis and vascular development, blood/lymphatic separation, immune responses, and inflammation. 43 , 44 , 45
Platelets have particularly extensive interactions with the immune system, and some would argue that they should be considered immune cells. Upon activation, platelets translocate P‐selectin from the alpha granules to their surface, where it is available to bind to its receptor, P‐selectin glycoprotein ligand‐1 (PSGL‐1), found on multiple cell types including neutrophils and monocytes. The P‐selectin–mediated interaction between platelets and neutrophils and platelets and monocytes can trigger immune cell activation and the release of cytokines/chemokines, facilitate cellular migration into tissues, and stimulate the formation and release of neutrophil extracellular traps (NETs) (Figure 1). 46 , 47 , 48 As previously mentioned, activated neonatal platelets exhibit significantly less surface P‐selectin compared to activated adult platelets, 49 although the mechanisms underlying this finding differ between species. In humans, the platelet content of P‐selectin is similar in neonates and adults, but neonatal platelets exhibit less surface P‐selectin upon agonist stimulation due to their overall hyporeactivity, coupled with a degranulation defect. 50 In mice, in contrast, platelet P‐selectin expression levels are developmentally regulated, and neonatal platelets contain significantly less P‐selectin than adult platelets. 51 P‐selectin surface expression levels are also lower in activated platelets from (human) preterm compared to term neonates. 49 Consistently, preterm neonates exhibit less platelet‐neutrophil aggregate formation than term neonates following platelet activation with thrombin receptor activating peptide or ADP, 52 a finding that might contribute to the preterm newborn’s susceptibility to infections. Neonates also have a markedly reduced ability to form NETs compared to adults, although this is due to a NET‐inhibitory factor produced by the placenta and present in neonatal blood for 3 to 14 days after birth.
FIGURE 1.
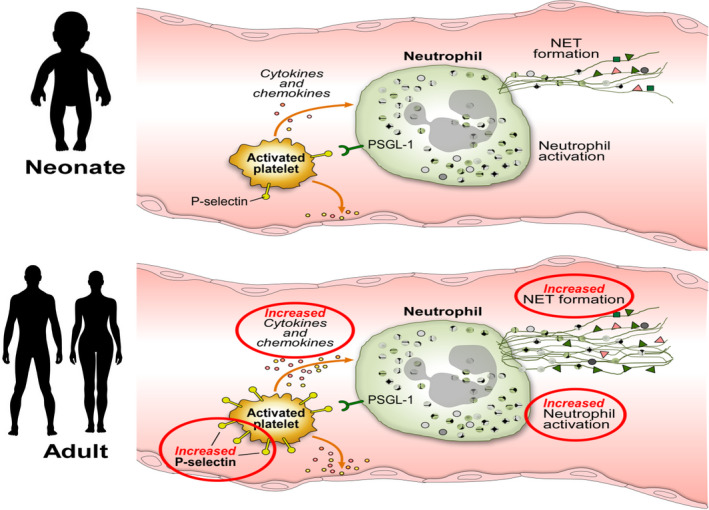
Schematic representation of key developmental differences between neonatal and adult platelets, and potential effects on immune cells. Upon activation, human neonatal platelets express less P‐selectin and release their alpha granule content (including cytokines and chemokines) less effectively than adult platelets. Decreased P‐selectin surface expression results in a reduced ability to interact with and activate immune cells, including neutrophils and monocytes. A neutrophil is shown as an example. The reduced NET formation in neonates is due to the presence of a placenta‐derived NET inhibitor. Abbreviations: NET, neutrophil extracellular trap; PSGL‐1, P‐selectin glycoprotein ligand‐1
In addition to interacting with immune cells, platelets express different toll‐like receptors and other pathogen sensors, 53 which enable the recognition of pathogen‐associated molecular patterns (PAMPs) and damage‐associated molecular patterns (DAMPs). In response to activation from these and other signals, platelets release their own cytokines and chemokines, which have complex regulatory functions on both innate and acquired immunity. How neonatal and adult platelets differ in regard to their immune (and other nonhemostatic) functions is unknown, but recent studies have compared the transcriptome and proteome of human neonatal (cord blood) and adult platelets. In regard to the transcriptome, there is an overall high correlation between neonatal and adult platelets. 29 Importantly, a majority of the mRNAs found in platelets at both developmental stages encoded proteins involved in the immune response, highlighting the importance of the platelets as immune cells. Among the 201 genes that were differentially expressed in neonatal versus adult platelets, transcripts related to protein synthesis, trafficking, and degradation were upregulated in neonatal platelets, while transcripts related to calcium transport or metabolism, actin cytoskeleton reorganization, and cell signaling were downregulated. 29 A comparison of the proteome of neonatal and adult platelets found 170 proteins to be differentially expressed. 54 Upregulated proteins included those involved in mitochondrial energy metabolism, long‐chain fatty acid metabolism, and iron binding, while proteins related to the inflammatory response, fibrinolysis, platelet activation, blood coagulation, and complement activation were downregulated in neonatal compared to adult platelets.
5. IMPLICATIONS OF THE DEVELOPMENTAL DIFFERENCES IN PLATELET FUNCTION TO NEONATAL TRANSFUSIONS
In the context of neonatal platelet transfusions, an important question is how the transfusion of comparatively hyperreactive adult platelets would affect the neonatal hemostatic and immunologic balance. To investigate the effects of platelet transfusion on neonatal hemostasis, Ferrer‐Marin et al 55 performed a series of in vitro studies mixing adult platelets with neonatal (cord) blood in which the platelet count had been reduced to ≈50 × 109/L, with the purpose of simulating miniaturized platelet transfusions into thrombocytopenic neonates. These experiments showed that the addition of adult platelets, but not endogenous neonatal platelets, to neonatal blood resulted in a significant shortening of the PFA‐100 closure time in response to collagen and epinephrine (CT‐Epi, a measure of primary hemostasis) to levels that have been associated with an increased cardiovascular risk. Although these effects have not been confirmed in vivo, this study provided the first experimental evidence in support of a potential “developmental mismatch” caused by the transfusion of adult platelets into neonatal blood and suggested that platelet transfusions could result in a prothrombotic neonatal phenotype. It is therefore conceivable that platelet transfusions could cause or exacerbate microvascular thrombosis in key organs such as the brain, lung, or intestine, which could contribute to the pathogenesis of serious neonatal morbidities like IVH, BPD, and necrotizing enterocolitis, respectively.
Recognition of the numerous nonhemostatic roles of platelets has also opened the door to other potential mechanisms through which the transfusion of adult platelets could positively or negatively affect neonates. A recent publication from the laboratory of Dr Lijun Xia raised the possibility of a novel mechanism through which platelets could contribute to brain bleeds at vulnerable periods of development. In this study, Hoover et al 56 found that the hemorrhages seen in the developing brain of mice lacking the platelet receptor CLEC‐2 or its ligand, podoplanin, were not due to a deficiency in platelet activation, as would have been expected, but rather were associated with the presence of hyperactivated embryonic megakaryocytes and platelets in the developing brain. Through a series of elegant experiments, the investigators demonstrated that podoplanin binding to CLEC‐2 normally restrains the collagen‐induced activation of embryonic megakaryocytes and platelets. In the absence of podoplanin or CLEC‐2, the unrestrained collagen I activation led to excessive angiopoietin secretion and endothelial activation, resulting in the formation of brain aneurysms that eventually ruptured and bled during midgestation. Interestingly, the bleeding phenotype was completely prevented by administration of aspirin and clopidogrel to the pregnant dam. 56 While the balance of podoplanin and collagen I in the developing human brain is unknown, human adult platelets are known to be hyperreactive compared to neonatal platelets in response to stimulation with collagen‐related peptide or rhodocytin, which bind to the platelet collagen receptor and CLEC‐2, respectively. 31 Thus, this study raised the intriguing possibility that hyperreactive platelets (ie, from adult donors) could contribute to neonatal bleeding by providing excessive angiogenic signals during a vulnerable period of brain vascular development.
From an immunologic perspective, the available data suggest that adult platelets, which express more P‐selectin upon activation and more effectively release their granule content, could be more proinflammatory than neonatal platelets (Figure 1). In support of a potential proinflammatory effect of platelet transfusions in neonates, we recently observed that transfusing healthy C57BL/6J (wild type [WT]) mice on postnatal day 10 (P10) with washed adult platelets resulted in significant increases in interleukin (IL)‐6 and granulocyte colony‐stimulating factor levels, among other cytokines, that were most pronounced 2 and 4 hours after transfusion, respectively. 57 Since platelet transfusions are frequently given to neonates with underlying inflammatory conditions, such as sepsis, we also examined the effects of transfusing adult WT platelets into WT P10 pups following a dose of lipopolysaccharide (LPS). In these experiments, 18 hours after LPS injection, the mice that had received a platelet transfusion had significantly higher IL‐6 levels than LPS‐injected, nontransfused littermates, which had nearly completely normalized their IL‐6. Interestingly, transfused mice also had higher levels of the anti‐inflammatory cytokine IL‐10, but when the correlation between the two was examined, at any level of IL‐10, IL‐6 levels were 2.3‐fold higher in transfused compared to nontransfused littermates, suggesting that platelet transfusions prolonged and amplified the LPS‐induced inflammation in newborn mice. 57 Recent work presented at the 2021 American Society of Hematology meeting by Maurya et al 58 also investigated the effect of adult versus neonatal platelets on monocyte inflammation and trafficking patterns. Using an in vitro coculture model of murine adult bone marrow monocytes and either neonatal or adult platelets, they found that monocyte inflammatory mRNAs (nitric oxide synthase 2, chemokine [C‐X‐C motif] ligand 1, chemokine [C‐C motif] ligand 2 [CCL2]) were increased in response to platelets regardless of developmental origin, but only coculture with adult platelets increased monocyte trafficking mRNA (C‐C chemokine receptor type 2 [CCR2]). This resulted in greater monocyte migration toward CCL2 (the CCR2 ligand) in a transwell chamber after treatment with adult, but not neonatal, platelets. Importantly, this was decreased upon blockage of P‐selectin. Taken together, these findings support the hypothesis that the transfusion of adult platelets into a neonate may alter the neonatal systemic immune response and immune cell migration, at least in part as a result of the developmental differences in P‐selectin expression.
In conclusion, it has become increasingly clear that neonatal platelets are different from adult platelets in ways that are designed to meet the unique needs of fetuses/neonates. Among other characteristics, they are hyporeactive in response to most agonists and express less P‐selectin upon activation than adult platelets. Because of these differences, transfusion of adult platelets into a sick neonate might cause a “developmental mismatch” with potential deleterious consequences for the recipient. The mechanisms through which platelet transfusions increase neonatal morbidity and mortality are not yet understood, but they might include rapid volume expansion leading to hemodynamic instability, providing inappropriate angiogenic stimulation during vulnerable periods of brain development, inducing a prothrombotic phenotype, and inducing or amplifying the neonatal inflammatory response.
6. ISTH 2021 PHILADELPHIA CONGRESS REPORT
Abstracts presented at the 2021 ISTH meeting provided new mechanistic and clinical insights in the field of neonatal bleeding and thrombosis. Murphy et al 59 reported interim results of a prospective observational study assessing thrombin generation (using a calibrated automated thrombography [CAT] assay) in platelet‐rich (PRP) and platelet‐poor (PPP) plasma from umbilical cord blood obtained from preterm (24‐31 weeks) and full‐term infants. Despite well‐known developmental differences in coagulation factor levels between preterm and term neonates, these investigators found no differences in any CAT parameter between PRPs obtained from preterm and full‐term neonates. Most surprisingly, in a subset of infants, no differences in thrombin generation were observed between PRP and PPP. This suggests that the phospholipid content of neonatal PPP, potentially from circulating extracellular vesicles, is sufficient to support thrombin generation in the absence of exogenous phospholipid (from platelets). 59 From a clinical perspective, these findings are consistent with the lack of correlation between platelet counts and bleeding reported in neonatal studies, 7 , 60 , 61 and with the lack of effectiveness of platelet transfusions to prevent bleeding. 9 , 60
IVH is a severe bleeding complication in extremely preterm neonates, and can be associated with serious neurodevelopmental consequences. At the ISTH meeting, Fejes et al 62 examined the proinflammatory effects of heme on human choroid plexus epithelial cells (HCPEpiCs) in vitro. Interestingly, they found that HCPEpiCs exposed to IVH cerebrospinal fluid or to heme had significantly upregulated IL‐8, IL‐1β and intercellular adhesion molecule 1 (ICAM‐1) mRNA expression levels compared to controls. IL‐8 and ICAM‐1 protein levels were also increased by heme. These findings indicate that bleeding products generated in the setting of IVH, such as heme, have proinflammatory effects on choroid plexus epithelial cells, which could contribute to the clinical manifestations and complications of IVH.
Other abstracts presented at the meeting examined the clinical management of thrombosis in neonates. Van Ommen et al 63 reported results from a multicenter prospective observational cohort study of preterm and term neonates with catheter‐related venous thrombosis, managed following the Neonatal Central‐Venous Line Observational Study on Thrombosis (NEOCLOT) protocol in 10 Dutch NICUs. A total of 116 neonates (92 preterms) were included, of which 1 died due to pulmonary embolism, 3 had recurrent thrombosis, and 9 (7.8%) had major bleeding. Two of those with major bleeding were on thrombolysis and 7 on low‐molecular‐weight‐heparin (LMWH). Five of the 7 infants on LMWH had subcutaneous catheter‐related major bleeding in the leg. The study concluded that the NEOCLOT protocol was effective and safe when not using subcutaneous catheters for LMWH administration. Another study by Cervio et al 64 investigated the effect of antithrombotic treatment in neonates and children with portal vein thrombosis, using a 10‐year prospective registry in a single tertiary care center in Argentina. The study included 47 patients, of which 20 were neonates. Portal vein thrombosis resolution was observed in 71% of patients treated with antithrombotic therapy versus 8.3% of those managed without anticoagulation. Cavernous transformation was diagnosed in 11 of 22 patients with nonrecanalized portal vein thrombosis. Thus, the study suggested that anticoagulation is associated with a high rate of recanalization of the portal vein, which might in turn decrease the risk of cavernous transformation.
7. FUTURE RESEARCH DIRECTIONS
Most of the studies characterizing developmental differences between neonatal and adult platelets so far have focused on their roles in hemostasis. However, it is likely that there are equally important developmental differences in regard to the platelets’ nonhemostatic functions, particularly in angiogenesis, vascular development, immune responses, and inflammation. Gaining a better understanding of the developmental differences in those platelet functions will be critical to elucidate the mechanisms underlying the increased morbidity and mortality associated with platelet transfusions in randomized trials and observational studies, and particularly the increased incidence of bronchopulmonary dysplasia. It is likely that the neonatal immune system, similar to the concept of developmental hemostasis, has unique developmentally regulated features aimed at meeting the needs of the newborn infant. Specifically, an attenuated inflammatory response is probably beneficial in early postnatal life to allow the normal colonization of the neonatal gastrointestinal tract and respiratory system by billions of microorganisms without mounting a damaging inflammatory response. The neonatal platelet hyporeactivity is likely an integral part of this balanced system, but the specific cellular interactions and pathways involved need to be elucidated. Furthermore, while some studies have shown significant improvement in platelet reactivity in newborn infants by 10 to 14 days of life, 49 , 65 more recent studies assessing platelet activation in different age groups (from neonates to adolescents) have found that age‐dependent improvements in platelet reactivity follow highly variable patterns, depending on the platelet agonist and the platelet activation marker studied. 66 , 67 Interestingly, some studies have identified platelet functional deficiencies extending into adolescence. 66 , 67 The mechanisms regulating the neonatal platelet hyporeactivity and the complex transition from a neonatal to an adult platelet are poorly understood, and should be the focus of future research. Similarly, it will be important to determine whether and how platelets contribute to the rapid angiogenesis needed to support the growth of a fetus and neonate, and how adult platelet transfusions affect these processes. 68
RELATIONSHIP DISCLOSURE
The authors have no relevant relationships or conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
Drs Davenport and Sola‐Visner reviewed the literature and wrote the manuscript.
Davenport P, Sola‐Visner M. Platelets in the neonate: Not just a small adult. Res Pract Thromb Haemost. 2022;6:e12719. doi: 10.1002/rth2.12719
Handling Editor: Prof. Yotis Senis
REFERENCES
- 1. Andrew M, Castle V, Saigal S, Carter C, Kelton JG. Clinical impact of neonatal thrombocytopenia. J Pediatr. 1987;110:457. [DOI] [PubMed] [Google Scholar]
- 2. Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C. Frequency and mechanism of neonatal thrombocytopenia. J Pediatr. 1986;108:749. [DOI] [PubMed] [Google Scholar]
- 3. Christensen RD, Henry E, Wiedmeier SE, et al. Thrombocytopenia among extremely low birth weight neonates: data from a multihospital healthcare system. J Perinatol. 2006;26:348. [DOI] [PubMed] [Google Scholar]
- 4. Bolisetty S, Dhawan A, Abdel‐Latif M, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 2014;133:55. [DOI] [PubMed] [Google Scholar]
- 5. Cremer M, Sola‐Visner M, Roll S, et al. Platelet transfusions in neonates: practices in the United States vary significantly from those in Austria, Germany, and Switzerland. Transfusion. 2011;51:2634. [DOI] [PubMed] [Google Scholar]
- 6. Patel R, Hendrickson J, Nellis M, et al. Variation in neonatal transfusion practice. J Pediatr. 2021;235:92–99.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stanworth SJ, Clarke P, Watts T, et al. Prospective, observational study of outcomes in neonates with severe thrombocytopenia. Pediatrics. 2009;124:e826. [DOI] [PubMed] [Google Scholar]
- 8. Andrew M, Vegh P, Caco C, et al. A randomized, controlled trial of platelet transfusions in thrombocytopenic premature infants. J Pediatr. 1993;123:285. [DOI] [PubMed] [Google Scholar]
- 9. Curley A, Stanworth SJ, Willoughby K, et al. Randomized trial of platelet‐transfusion thresholds in neonates. N Engl J Med. 2019;380:242. [DOI] [PubMed] [Google Scholar]
- 10. Fustolo‐Gunnink SF, Fijnvandraat K, van Klaveren D, et al. Preterm neonates benefit from low prophylactic platelet transfusion threshold despite varying risk of bleeding or death. Blood. 2019;134(26):2354–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baer VL, Lambert DK, Henry E, Christensen RD. Severe thrombocytopenia in the NICU. Pediatrics. 2009;124:e1095. [DOI] [PubMed] [Google Scholar]
- 12. Sparger KA, Assmann SF, Granger S, et al. Platelet transfusion practices among very‐low‐birth‐weight infants. JAMA Pediatr. 2016;170(7):687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deschmann E, Saxonhouse MA, Feldman HA, Norman M, Barbian M, Sola‐Visner M. Association of bleeding scores and platelet transfusions with platelet counts and closure times in response to adenosine diphosphate (CT‐ADPs) among preterm neonates with thrombocytopenia. JAMA Netw Open. 2020;3:e203394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Muthukumar P, Venkatesh V, Curley A, et al. Severe thrombocytopenia and patterns of bleeding in neonates: results from a prospective observational study and implications for use of platelet transfusions. Transfus Med. 2012;22:338–343. [DOI] [PubMed] [Google Scholar]
- 15. von Lindern JS, Hulzebos CV, Bos AF, Brand A, Walther FJ, Lopriore E. Thrombocytopaenia and intraventricular haemorrhage in very premature infants: a tale of two cities. Arch Dis Child Fetal Neonatal Ed. 2012;97:F348–F352. [DOI] [PubMed] [Google Scholar]
- 16. Del Vecchio A, Sola MC, Theriaque DW, et al. Platelet transfusions in the neonatal intensive care unit: factors predicting which patients will require multiple transfusions. Transfusion. 2001;41:803–808. [DOI] [PubMed] [Google Scholar]
- 17. Garcia MG, Duenas E, Sola MC, Hutson AD, Theriaque D, Christensen RD. Epidemiologic and outcome studies of patients who received platelet transfusions in the neonatal intensive care unit. J Perinatol. 2001;21:415–420. [DOI] [PubMed] [Google Scholar]
- 18. Bonifacio L, Petrova A, Nanjundaswamy S, Mehta R. Thrombocytopenia related neonatal outcome in preterms. Indian J Pediatr. 2007;74:269–274. [DOI] [PubMed] [Google Scholar]
- 19. Kenton AB, Hegemier S, Smith EO, et al. Platelet transfusions in infants with necrotizing enterocolitis do not lower mortality but may increase morbidity. J Perinatol. 2005;25:173–177. [DOI] [PubMed] [Google Scholar]
- 20. Baer VL, Lambert DK, Henry E, Snow GL, Sola‐Visner MC, Christensen RD. Do platelet transfusions in the NICU adversely affect survival? Analysis of 1600 thrombocytopenic neonates in a multihospital healthcare system. J Perinatol. 2007;27(12):790–796. [DOI] [PubMed] [Google Scholar]
- 21. Patel RM, Josephson CD, Shenvi N, et al. Platelet transfusions and mortality in necrotizing enterocolitis. Transfusion. 2019;59:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elgendy MM, Durgham R, Othman HF, et al. Platelet transfusion and outcomes of preterm infants: a cross‐sectional study. Neonatology. 2021;118:425–433. [DOI] [PubMed] [Google Scholar]
- 23. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ballabh P. Pathogenesis and prevention of intraventricular hemorrhage. Clin Perinatol. 2014;41(1):47–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu ZJ, Hoffmeister KM, Hu Z, et al. Expansion of the neonatal platelet mass is achieved via an extension of platelet lifespan. Blood. 2014;123:3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiedmeier SE, Henry E, Sola‐Visner MC, Christensen RD. Platelet reference ranges for neonates, defined using data from over 47,000 patients in a multihospital healthcare system. J Perinatol. 2009;29:130. [DOI] [PubMed] [Google Scholar]
- 27. Ferrer‐Marin F, Sola‐Visner M. Neonatal platelet physiology and implications for transfusion. Platelets. 2022;33(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Corby DG, O'Barr TP. Decreased alpha‐adrenergic receptors in newborn platelets: cause of abnormal response to epinephrine. Dev Pharmacol Ther. 1981;2:215. [PubMed] [Google Scholar]
- 29. Caparros‐Perez E, Teruel‐Montoya R, Lopez‐Andreo MJ, et al. Comprehensive comparison of neonate and adult human platelet transcriptomes. PLoS One. 2017;12:e0183042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schlagenhauf A, Schweintzger S, Birner‐Gruenberger R, Leschnik B, Muntean W. Newborn platelets: lower levels of protease‐activated receptors cause hypoaggregability to thrombin. Platelets. 2010;21:641–647. [DOI] [PubMed] [Google Scholar]
- 31. Hardy AT, Palma‐Barqueros V, Watson SK, et al. Significant hypo‐responsiveness to GPVI and CLEC‐2 agonists in pre‐term and full‐term neonatal platelets and following immune thrombocytopenia. Thromb Haemost. 2018;118:1009–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ngo ATP, Sheriff J, Rocheleau AD, et al. Assessment of neonatal, cord, and adult platelet granule trafficking and secretion. Platelets. 2020;31:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Israels SJ, Cheang T, Roberston C, McMillan‐Ward EM, McNicol A. Impaired signal transduction in neonatal platelets. Pediatr Res. 1999;45:687–691. [DOI] [PubMed] [Google Scholar]
- 34. Gelman B, Setty BN, Chen D, Amin‐Hanjani S, Stuart MJ. Impaired mobilization of intracellular calcium in neonatal platelets. Pediatr Res. 1996;39:692. [DOI] [PubMed] [Google Scholar]
- 35. Baker‐Groberg SM, Lattimore S, Recht M, McCarty OJ, Haley KM. Assessment of neonatal platelet adhesion, activation, and aggregation. J Thromb Haemost. 2016;14:815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrew M, Paes B, Bowker J, Vegh P. Evaluation of an automated bleeding time device in the newborn. Am J Hematol. 1990;35:275–277. [DOI] [PubMed] [Google Scholar]
- 37. Roschitz B, Sudi K, Kostenberger M, Muntean W. Shorter PFA‐100 closure times in neonates than in adults: role of red cells, white cells, platelets and von Willebrand factor. Acta Paediatr. 2001;90:664–670. [PubMed] [Google Scholar]
- 38. Saxonhouse MA, Garner R, Mammel L, et al. Closure times measured by the platelet function analyzer PFA‐100 are longer in neonatal blood compared to cord blood samples. Neonatology. 2010;97:242–249. [DOI] [PubMed] [Google Scholar]
- 39. Katz JA, Moake JL, McPherson PD, et al. Relationship between human development and disappearance of unusually large von Willebrand factor multimers from plasma. Blood. 1989;73:1851–1858. [PubMed] [Google Scholar]
- 40. Weinstein MJ, Blanchard R, Moake JL, Vosburgh E, Moise K. Fetal and neonatal von Willebrand factor (vWF) is unusually large and similar to the vWF in patients with thrombotic thrombocytopenic purpura. Br J Haematol. 1989;72:68–72. [DOI] [PubMed] [Google Scholar]
- 41. Saxonhouse MA, Sola MC. Platelet function in term and preterm neonates. Clin Perinatol. 2004;31:15–28. [DOI] [PubMed] [Google Scholar]
- 42. Schmugge M, Dunn MS, Amankwah KS, Blanchette VS, Freedman J, Rand ML. The activity of the von Willebrand factor cleaving protease ADAMTS‐13 in newborn infants. J Thromb Haemost. 2004;2:228–233. [DOI] [PubMed] [Google Scholar]
- 43. Franco AT, Corken A, Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126:582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weyrich AS. Platelets: more than a sack of glue. Hematology. 2014;2014:400–403. [DOI] [PubMed] [Google Scholar]
- 45. Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. [DOI] [PubMed] [Google Scholar]
- 46. Lisman T. Platelet‐neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018;371:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. [DOI] [PubMed] [Google Scholar]
- 48. Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nat Rev Immunol. 2013;13:34–45. [DOI] [PubMed] [Google Scholar]
- 49. Sitaru AG, Holzhauer S, Speer CP, et al. Neonatal platelets from cord blood and peripheral blood. Platelets. 2005;16:203–210. [DOI] [PubMed] [Google Scholar]
- 50. Caparros‐Perez E, Teruel‐Montoya R, Palma‐Barquero V, et al. Down regulation of the Munc18b‐syntaxin‐11 complex and beta1‐tubulin impairs secretion and spreading in neonatal platelets. Thromb Haemost. 2017;117:2079. [DOI] [PubMed] [Google Scholar]
- 51. Stolla MC, Catherman SC, Kingsley PD, et al. Lin28b regulates age‐dependent differences in murine platelet function. Blood Adv. 2019;3:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Esiaba I, Angeles DM, Milford TM, et al. Platelet‐neutrophil interactions are lower in cord blood of premature newborns. Neonatology. 2019;115:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ebermeyer T, Cognasse F, Berthelot P, Mismetti P, Garraud O, Hamzeh‐Cognasse H. Platelet innate immune receptors and TLRs: a double‐edged sword. Int J Mol Sci. 2021;22(15):7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stokhuijzen E, Koornneef JM, Nota B, et al. Differences between platelets derived from neonatal cord blood and adult peripheral blood assessed by mass spectrometry. J Proteome Res. 2017;16:3567–3575. [DOI] [PubMed] [Google Scholar]
- 55. Ferrer‐Marin F, Chavda C, Lampa M, Michelson AD, Frelinger AL 3rd, Sola‐Visner M. Effects of in vitro adult platelet transfusions on neonatal hemostasis. J Thromb Haemost. 2011;9:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoover CKY, Shao B, McDaniel MJ, et al. Heightened activation of embryonic megakaryocytes causes aneurysms in the developing brain of mice lacking podoplanin. Blood. 2021;137(20):2756–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Davenport P, Nolton E, Feldman H, Liu Z, Sola‐Visner M. Pro‐inflammatory effects of platelet transfusions in newborn mice with and without underlying inflammation. Blood. 2021;138(suppl 1):2146. [Google Scholar]
- 58. Maurya P, McGrath K, Ture SK, Palis J, Morrell C. Adult, but not noenatal platelet transfusions drive a monocyte trafficking phenotype in vitro and in vivo . Blood. 2021;138(suppl 1):2144. [Google Scholar]
- 59. Murphy C, Neary E, Kevane B, et al. The effect of platelets on thrombin generation in the premature infant: the EVENT study. Res Pract Thromb Haemost. 2021:123:OC 20.3. [Google Scholar]
- 60. Sparger K, Deschmann E, Sola‐Visner M. Platelet transfusions in the neonatal intensive care unit. Clin Perinatol. 2015;42(3):613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Deschmann E, Saxonhouse MA, Feldman HA, Norman M, Barbian M, Sola‐Visner M. Association between in vitro bleeding time and bleeding in preterm infants with thrombocytopenia. JAMA Pediatr. 2019;173:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fejes Z, Pocsi M, Balla A, et al. Investigation of preterm intraventricular hemorrhage induced inflammatory response and microRNA levels by heme and hypoxia in human choroid plexus epithelial cells. Res Pract Thromb Haemost. 2021:123:OC 20.2. [Google Scholar]
- 63. Van Ommen C, Bergman K, Boerma M, et al. NEOCLOT: management of catheter‐related venous thrombosis in preterm and term neonates. Res Pract Thromb Haemost. 2021:126:OC 20.1. [Google Scholar]
- 64. Cervio C, Hepner M, Bianco B, et al. Portal vein thrombosis (PVT) in neonates and children: a ten‐year‐prospective registry of a tertiary care single‐centre in Argentina. Res Pract Thromb Haemost. 2021;5:OC 20.4. [Google Scholar]
- 65. Bednarek FJ, Bean S, Barnard MR, Frelinger AL, Michelson AD. The platelet hyporeactivity of extremely low birth weight neonates is age‐dependent. Thromb Res. 2009;124(1):42–45. [DOI] [PubMed] [Google Scholar]
- 66. Herken K, Glauner M, Robert SC, et al. Age‐dependent control of collagen‐dependent platelet responses by thrombospondin‐1‐comparative analysis of platelets from neonates, children, adolescents, and adults. Int J Mol Sci. 2021;22(9):4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hezard N, Potron G, Schlegel N, Amory C, Leroux B, Nguyen P. Unexpected persistence of platelet hyporeactivity beyond the neonatal period: a flow cytometric study in neonates, infants and older children. Thromb Haemost. 2003;90:116. [PubMed] [Google Scholar]
- 68. Cakir B, Liegl R, Hellgren G, et al. Thrombocytopenia is associated with severe retinopathy of prematurity. JCI Insight. 2018;3:e99448. [DOI] [PMC free article] [PubMed] [Google Scholar]


