Abstract
Introduction
Interest revolving around coronavirus disease 2019 (COVID‐19) reinfection is escalating rapidly. By definition, reinfection denotes severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), PCR redetection, and COVID‐19 recurrence within three months of the initial symptoms. The main aim of the current systematic review was to evaluate the features of COVID‐19 relapse patients.
Materials and methods
For this study, we used a string of terms developed by a skilled librarian and through a systematical search in PubMed, Web of Science, and Embase for eligible studies. Clinical surveys of any type were included from January 2019 to March 2021. Eligible studies consisted of two positive assessments separated by a negative result via RT‐PCR.
Results
Fifty‐four studies included 207 cases of COVID‐19 reinfection. Children were less likely to have COVID‐19 relapse. However, the most patients were in the age group of 20–40 years. Asthenia (66.6%), headache (66.6%), and cough (54.7%) were prevalent symptoms in the first SARS‐CoV‐2 infection. Asthenia (62.9%), myalgia (62.9%), and headache (61.1%) were most frequent in the second one. The most common treatment options used in first COVID‐19 infection were lopinavir/ritonavir (80%), oxygen support (69.2%), and oseltamivir (66.6). However, for the treatment of second infection, mostly antibiotics (100%), dexamethasone (100%), and remdesivir (80%) were used. In addition, obesity (32.5%), kidney failure (30.7%), and hypertension (30.1%) were the most common comorbidities. Unfortunately, approximately 4.5% of patients died.
Conclusion
We found the potency of COVID‐19 recurrence as an outstanding issue. This feature should be regarded in the COVID‐19 management. Furthermore, the first and second COVID‐19 are similar in clinical features. For clinically practical comparison of the symptoms severity between two epochs of infection, uniform data of both are required. We suggest that future studies undertake a homogenous approach to establish the clinical patterns of the reinfection phenomena.
Keywords: COVID‐19, recurrence, reinfection, relapse, SARS‐CoV2
Totally, 1807 studies obtained from PubMed/Medline, Web of Science, and Embase databases. Following the removal of duplicates, the title and abstract of 998 studies were screened to select the studies which report the relapse of COVID‐19 after a negative RT‐PCR test. Finally, among the 152 full‐text articles, 54 studies were found to be eligible for data extraction.
1. INTRODUCTION
This is not the first time that coronavirus has caused problems in the world. Viruses such as severe acute respiratory syndrome coronavirus (SARS‐CoV) and Middle East respiratory syndrome coronavirus (MERS‐CoV) have also been prevalent in recent years. 1 The mortality rates of SARS‐CoV and MERS‐CoV epidemics have been estimated to be 10% and 35%, respectively. 2 The main problem of today's global health communities is conflict over the novel coronavirus (2019‐nCoV). The new virus was first identified in China, but is now found in many countries around the world. 1 There are currently several variants of coronavirus circulating among people, 3 , 4 and the virus is mostly transmitted through the respiratory tract. Various symptoms have been described for patients with COVID‐19, ranging from asymptomatic to severe. Kidney damage has also been reported in some cases. 5 According to the World Health Organization (WHO), COVID‐19‐infected patients can leave home quarantine after the improvement of their infectious symptoms and also the confirmation of two negative RT‐PCR tests (within 24 h). 6
Reinfection, relapse, recurrence, and reactivation are terms used for people infected with coronavirus and have become positive again after a period of negativity. 7 Based on a report from Guangdong Province in China, about 14% of patients who recover from COVID‐19 become reinfected with the virus. 8 In addition, there have been reports of reinfection in Korea and Japan. 9 Duration of immunization against coronavirus reinfection in recovering individuals is six months. 10 People who become infected with coronavirus for the second time often have milder symptoms and recover more quickly than those infected for the first time. 11 However, there are concerns about reinfection in people recovering from the coronavirus. The objective of this systematic review was to evaluate the prevalence and frequency of reinfection in people recovering from COVID‐19 and their clinical signs, as well as to assess the treatment methods.
2. MATERIALS AND METHODS
The present systematic review was conducted in accordance with Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statements. 12
2.1. Search strategy
We used PubMed/Medline, Web of Science, and Embase for a systematic search from January 1, 2019, to March 7, 2021. The search was based on the following string of terms: (“recurrence” OR “relapse” OR “reinfection” OR “reactivation”) AND (“COVID‐19” OR “severe acute respiratory syndrome coronavirus 2” OR “novel coronavirus” OR “SARS‐CoV‐2” OR “nCoV disease” OR “SARS2” OR “2019‐nCoV” OR “coronavirus disease‐19” OR “coronavirus disease 2019” OR “2019 novel coronavirus” OR “Wuhan coronavirus” OR “Wuhan seafood market pneumonia virus” OR “Wuhan pneumonia”). There was no limitation on language, location, and type of studies.
2.2. Inclusion and exclusion criteria
All the studies reported the reactivation or second infection of COVID‐19 were considered in the search. Total records were retrieved and entered into EndNote X9 software (Thomson Reuters). Following duplicate exclusion, a three‐stage screening was carried out to exploit the eligible studies based on title, abstract, and full text. The whole eligible studies reported the patients who were recovered from primary infection, but then developed a secondary COVID‐19 infection. RT‐PCR test was necessary inclusion criteria. Patients with a positive RT‐PCR for the first phase of COVID‐19, a negative RT‐PCR for recovery, and a second positive RT‐PCR for COVID‐19 recurrence were examined in the study. We excluded articles that reported only a serologic diagnosis test, without a nasopharyngeal swab RT‐PCR test, as well as duplicate publication of same studies, congress abstracts, reviews, systematic reviews and meta‐analysis, cellular and molecular studies, and animal studies. All types of manifestations and treatments were regarded without any restriction, and there was no limitation on comorbidities and underlying disorders.
2.3. Data extraction
The following data were acquired from each article: first author's name, location, publication time, type of study, number of relapsed patients, age, gender, interval between two infections, clinical manifestations, treatment, relative status, comorbidities, and outcome. Two investigators independently extracted the data from full text of 54 included studies. Inconsistencies between reviewers were resolved by consensus. The retrieved data are represented in Table 1.
TABLE 1.
Characteristics of the included studies
| First author | Country | Published time | Type of study | N. of relapsed patients | Median age at first infection | Male/female | Time between infections (days) | Clinical manifestations in first infection | Treatment in first infection | Clinical manifestations in second infection | Treatment in second infection | Status | Comorbidities | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lancman et al. 35 | USA | October 2020 | Case report | 1 | 55 | F | 42 | Fever, abdominal pain, cough, nausea, vomiting | HCQ, AZ, RDV | Fever, sore throat, abdominal pain, diarrhea, respiratory distress | Plasma, DEX, oxygen support | Worse | B cell ALL, diabetes mellitus, heart failure, asthma | Discharged |
| Chen et al. 36 | China | July 2020 | Case series | 4 | 32 |
M 2 F 2 |
12 | Fever 2, cough 3, fatigue 1 | ARB 1, IFN 1, CTM 1 | NR | NR | NR | NR | Discharged 4 |
| Nazir et al. 37 | India | October 2020 | Case report | 1 | 26 | M | 99 | None | HCQ, OTV, montair, RAN, vit B, vit C, zinc | None | HCQ, OTV, montair, RAN, vit B, vit C, zinc | ND | NR | Discharged |
| Selhorst et al. 38 | Belgium | November 2020 | Case report | 1 | 39 | F | 185 | Cough, dyspnea, headache, fever, malaise | NR | Dyspnea, rhinitis, sore throat | NR | Better | None | Discharged |
| Selvaraj et al. 33 | USA | December 2020 | Case report | 1 | 70–80 | M | 240 | Respiratory distress | ALB, Antitussives | Respiratory distress, fever, Myalgia, nausea, malaise | AZ, DEX, RDV, CRO, oxygen support | Worse | Obesity, neuropathy, asthma, sleep apnea, hypertension | Discharged |
| Bellesso et al. 39 | Brazil | December 2020 | Case report | 1 | 76 | F | 126 | Respiratory distress | CRO, VAN | Respiratory distress, hypoxemia, dyspnea | Meropenem, VAN, DEX, polymixin, linezolid, oxygen support | Worse | Hypercalcemia, anemia, Kidney failure, hypertension, under hemodialysis, MM, plasmacytoma, glucose intolerance | Died |
| Zhou et al. 40 | China | July 2020 | Case report | 1 | 40 | M | 5 | Fever, dyspnea, diarrhea | Oxygen support, ARB, mPDRL, Ig | Fever | Oxygen support, mPDRL | Better | Pneumonia | Discharged |
| Atici et al. 41 | Turkey | January 2021 | Case series | 2 | 46.5 |
M 1 F 1 |
102 | Fever 1, sore throat 1, headache 2, cough 1, asthenia 1, nausea 1, diarrhea 1, abdominal pain 1, myalgia 1 | HCQ 2, AZ 1, CRO 1 | Sore throat 2, fever 2, headache 2, myalgia 2, asthenia 1, nausea 1, cough 1, respiratory distress 1 | Favipiravir 2, AZ 1, CRO 1 | ND | None | Discharged 2 |
| Shoar et al. 42 | Iran | February 2021 | Case report | 1 | 31 | M | 79 | Fever, malaise, cough, respiratory distress, anosmia | Oxygen support, HCQ, DEX | Malaise, gingival aphthous ulcers, painful submandibular lymphadenopathy, fever, myalgia, skin desquamation during recovery | Naproxen | NR | NR | Discharged |
| Mulder et al. 43 | Netherland | October 2020 | Case report | 1 | 89 | F | 59 | Fever, fatigue, cough | NR | Fever, cough, dyspnea | NR | Worse | Waldenstrom macroglobulinemia | Died |
| Hanif et al. 44 | Pakistan | October 2020 | Case report | 1 | 58 | M | 49 | Fatigue, headache, sore throat | Oxygen support, AZ | Fever, headache, myalgia | NR | Better | Pneumonia | Discharged |
| Abdallah et al. 45 | USA | December 2020 | Case report | 1 | 30 | M | 30 | Chest pain, fever, and night sweat, progressive fatigue, anosmia | APAP | Chest pain, fatigue, dyspnea | AZ | ND | None | Discharged |
| Brito et al. 46 | Brazil | October 2020 | Case series | 2 | 42 |
M 1 F 1 |
21 | Fever 1, cough 2, sore throat 2, myalgia 2, fatigue 2, diarrhea 2, headache 2 | AZ 1, IVR 1 | Fever 2, cough 2, sore throat 2, myalgia 2, fatigue 2, diarrhea 2, headache 2, anosmia 2, dysgeusia 2, asthenia 1, nausea 1 | HCQ 2, AZ 2, IVR 2 | NR | None | Discharged 2 |
| Hussein et al. 47 | Iraq | December 2020 | Case report | 1 | 46 | M | 53 | Fever, cough | AZ, vit D, zinc | Fever, sore throat, cough, ageusia, anosmia | Favipiravir | NR | NR | Discharged |
| Kapoor et al. 48 | India | February 2021 | Case series | 3 | 33 | M 3 | 78.3 | Fever 1, cough 1 | RDV 1, oxygen support 1 | Fever 3, chills 1, respiratory distress 1, headache 1, vomiting 1 | RDV 1, plasma 1, IVIg 1, oxygen support 3 |
Worse 2 Better 1 |
MM 1, ALL 2, Pneumonia 1 | NR |
| Liu et al. 49 | China | August 2020 | Case report | 1 | 57 | F | 5 | Fever, cough | LPV, IFN, ARB hydrochloride | NR | LPV, IFN, ribavirin, budesonide | NR | NR | Discharged |
| Salcin et al. 50 | USA | December 2020 | Case report | 1 | 62 | F | 120 | Cough, respiratory distress | Antibiotics, HCQ, vit C, zinc | Tachycardia, tachypnea, hypoxia | Oxygen support, DEX, RDV, CRO, AZ, vit C, zinc, plasma, antibiotics, steroid | Worse | Hypertension, hypothyroidism, degenerative disk disease, previous L2‐L4 lumbar fusion, anxiety | Discharged |
| Santos et al. 51 | Brazil | February 2021 | Case control | 33 | 39.2 |
M 7 F 26 |
50.5 | Headache 29, asthenia 27, myalgia 16, arthralgia 10, sneeze 15, sore throat 19, dysgeusia 10, dyspnea 11, diarrhea 16, hyporexia 12, abdominal pain 10, nausea 10, vomiting 10, fever 7, cough 15, anosmia 8, skin lesions 8, dizziness 9, mental confusion 2 | AZ 20, corticosteroid 12, IVR 9, heparin 5, HCQ 5 | Headache 28, asthenia 29, myalgia 24, arthralgia 14, sneeze 22, sore throat 20, dysgeusia 17, dyspnea 19, diarrhea 16, hyporexia 15, abdominal pain 12, nausea 12, vomiting 12, fever 12, cough 21, anosmia 16, skin lesions 5, dizziness 12, mental confusion 5 | AZ 20, corticosteroid 26, IVR 21, heparin 12, HCQ 3, antibiotics 20, oxygen support 3 | NR | Obesity 10, diabetes mellitus 1, hypertension 5, asthma 1 |
Died 1 Discharged 32 |
| Goldman et al. 52 | USA | September 2020 | Case report | 1 | 60–69 | NR | 100 | Fever, chills, cough, dyspnea, chest pain | Oxygen support, steroids | Cough, asthenia, dyspnea | Oxygen support, RDV, DEX | Better | Emphysema, hypertension, pneumonia | NR |
| Duggan et al. 53 | USA | June 2020 | Case report | 1 | 82 | M | 10 | Fever, tachypnea, hypoxia, respiratory distress | Oxygen support | Fever, hypoxia, tachycardia, tachypnea, hypotension | Oxygen support | Worse | Parkinson's disease, diabetes, kidney failure, hypertension | Discharged |
| Coppola et al. 54 | Italy | August 2020 | Case report | 1 | 68 | M | 16 | Diarrhea, asthenia, fever, dyspnea, cough, myalgia | Tocilizumab, LPV/r, HCQ, oxygen support | Diarrhea, fever, myalgia, asthenia | NR | Better | Smoking, dyslipidemia, heart failure, carbohydrate intolerance | Discharged |
| Sicsic Jr et al. 55 | USA | February 2021 | Case report | 1 | 69 | F | 72 | Respiratory distress, cough, headache, fatigue, fever | AZ, OTV | Cough, fever, ageusia | RDV, antibiotics, DEX, oxygen support | Worse | Asthma, hypercholesterolemia, hypertension, sleep apnea | Discharged |
| Dou et al. 56 | China | July 2020 | Case report | 1 | 34 | M | 18 | Fever, chills, cough, sore throat, dizziness, fatigue | ARB, ribavirin, Ig, cefuroxime, LPV/r, IFN, cefoperazone sodium, sulbactam sodium, CTM | None | ARB, CQ phosphate, IFN | Better | Diabetes | Discharged |
| Novoa et al. 57 | Colombia | January 2021 | Case report | 1 | 44 | M | 89 | None | NR | Malaise, chills, headache, fever, sore throat | NR | Worse | None | Discharged |
| Tuan et al. 58 | USA | February 2021 | Case report | 1 | 43 | M | 7 | Respiratory distress, hypoxia, fever, myalgia, sore throat | Tocilizumab, HCQ, Ig, mPDRL, oxygen support | Respiratory distress | VAN, TZP, RDV, oxygen support | Worse | Diabetes, obesity, hypothyroidism | Discharged |
| Sharma et al. 59 | Qatar | December 2020 | Case report | 1 | 57 | M | 85 | None | CQ/HCQ, OTV | Fever, myalgia, headache, cough | AZ, OTV | Worse | Diabetes | Discharged |
| Bonifácio et al. 60 | Brazil | September 2020 | Case report | 1 | 24 | F | 36 | Headache, malaise, asthenia, fever, sore throat, nasal congestion | Naproxen, dipyrone | Malaise, myalgia, severe headache, fatigue, asthenia, fever, sore throat, anosmia, dysgeusia, diarrhea, cough, hyposmia | NR | Worse | Obesity | Discharged |
| Scaria et al. 61 | India | September 2020 | Case series | 2 | 26.5 |
M 1 F 1 |
100.5 | None | NR | None | NR | Worse 2 | NR | Discharged 2 |
| Ma et al. 62 | Hong Kong | September 2020 | Case report | 1 | 31 | F | 2 | Fever, dyspnea | NR | Myalgia, cough, fever | LPV/r | NR | Kidney failure, pneumocystis pneumonia, hypertension | Discharged |
| Zhang et al. 63 | China | Nov ember 2020 | Case series | 4 | 48.75 |
M 1 F 3 |
15.5 | Fever 4, cough 3, respiratory distress 1, sore throat 1, headache 1, myalgia 1 | LPV/r 4, HCQ 2, IFN 4, thymalfasin 1, CTM 4, ARB 2, thymopentin 1 | None | Thymalfasin 3, HCQ 4, CTM 4, IFN 4, ARB 1 | Better 4 | Hepatitis B | Discharged 4 |
| Fehdi et al. 64 | Morocco | May 2020 | Case report | 1 | 69 | M | 3 | Fever, cough, dyspnea, respiratory alkalosis, hypoxemia | HCQ, AZ, CRO, moxifloxacin, thromboprophylaxis | Respiratory distress | Oxygen support | Worse | Inflammatory syndrome | Died |
| Caralis 65 | USA | November 2020 | Case series | 7 | 60 |
M 5 F 2 |
NR | Cough 3, fever 4, fatigue 1, dyspnea 1, diarrhea 1, ageusia 1, anosmia 1, headache 1 | NR | Fever 1, headache 1, anosmia 1, ageusia 1, fatigue 2 | NR | Better 4, ND 3 | Arthritis 3, kidney failure 3, liver failure 3, HIV 3, sarcoidosis 3, diabetes 1, pneumonia 1 | Discharged 7 |
| Harrington et al. 66 | UK | 2021 | Case report | 1 | 78 | M | 223 | Fever | None | Respiratory distress, aphasia, hypoxia | Co‐amoxiclav, clarithromycin, DEX | Worse | Diabetes, diabetic nephropathy disease, COPD, sleep apnea, heart failure | NR |
| Ozaras et al. 67 | Turkey | October 2020 | Case report | 1 | 23 | F | 99 | Fever, chills, fatigue, cough, headache, sore throat, myalgia | APAP, AZ, HCQ | Fever, chills, fatigue, anorexia, ageusia, anosmia, myalgia | HCQ, APAP | NR | Smoking | Discharged |
| Prado‐Vivar et al. 68 | Ecuador | November 2020 | Case report | 1 | 46 | M | 47 | Headache, drowsiness | NR | Sore throat, nasal congestion, fever, back pain, cough, dyspnea | NR | Worse | NR | Discharged |
| Bellanti et al. 69 | Italy | October 2020 | Case report | 1 | 91 | F | 2 | Respiratory distress | DEX, TZP, daptomycin, enoxaparin, furosemide, amiodarone, bisoprolol, oxygen support | Fever, dyspnea, tachypnea, stranguria, respiratory distress | Paracetamol, cefepime, clarithromycin, oxygen support, caspofungin, furosemide, mPDRL | Worse | Diabetes, hypertension, atrial fibrillation, kidney failure, anxiety depressive disorder, UTI | Died |
| Yang et al. 70 | China | November 2020 | Cohort study | 93 | 34 |
M 36 F 57 |
8 | NR | Steroids 14 (mPDRL and/or DEX) | Cough 18, respiratory distress 3, fever 1 | NR | NR | NR | Discharged 93 |
| Du et al. 71 | China | August 2020 | Case series | 3 | 66 |
M 1 F 2 |
14 | Fever 2, dry cough 2, chest pain 1, diarrhea 1, respiratory distress 1, headache 1 | Antiviral 3, CTM 3, antibiotics 2 | None | CTM 3 | Better 3 | Hypertension 1, diabetes 1, COPD 1, digestive disease 1, renal impairment 1 | Discharged 3 |
| Tillett et al. 72 | USA | October 2020 | Case report | 1 | 25 | M | 10 | Sore throat, cough, headache, nausea, diarrhea | None | Fever, headache, dizziness, cough, nausea, diarrhea, hypoxia, respiratory distress, myalgia | Oxygen support | Worse | None | Discharge |
| Nonaka et al. 73 | Brazil | May 2021 | Case report | 1 | 45 | F | 142 | Diarrhea, myalgia, asthenia, sore throat | PRED | Headache, malaise, diarrhea, cough, sore throat, myalgia, ageusia, myalgia, insomnia, dyspnea, respiratory distress | NR | Worse | None | Discharged |
| Ravioli et al. 74 | Switzerland | May 2020 | Case series | 2 | 79 | F 2 | 20 | Fever 2, cough 2 | NR | Dyspnea 1, fever 1, confusion 1, cough 1 | HCQ 1, AZ 1, oxygen support 1 | Worse 1, ND 1 | Diabetes 1, heart failure 1, stroke 1 | Died 1, Discharged 1 |
| Jesus et al. 75 | Portugal | October 2020 | Case report | 1 | NR | M | 13 | None | PDRL | Fever, headache, myalgia, cough dyspnea, chest pain, tachypnea, respiratory distress | mPDRL, TZP, RDV | Worse | Pneumonia, cardiopulmonary arrest | Discharged |
| Zayet et al. 76 | France | February 2021 | Case series | 3 | 43 | F 3 | NR | Myalgia 2, fatigue 2, sore throat 1, cough 1, anosmia 1, dysgeusia 1, diarrhea 1, fever 1, chills 1 | NR | Myalgia 1, fatigue 1, dyspnea 2, chills 1, headache 2, cough 2, chest pain 1, anosmia 2, dysgeusia 2, vomiting 1, diarrhea 1 | NR | ND | Asthma 1 | NR |
| Ak et al. 77 | Turkey | December 2020 | Case report | 1 | 40 | M | 80 | Fever, cough | HCQ | Sore throat, cough, diarrhea, fever | HCQ, enoxaparin, moxifloxacin | Worse | None | Discharged |
| Chen et al. 78 | China | March 2020 | Case report | 1 | 46 | F | 3 | Fever, sore throat, cough, respiratory distress | OTV, ARB, LPV/r, moxifloxacin | NR | NR | Better | NR | Discharged |
| Wu et al. 79 | China | November 2020 | Case series | 2 | 27 |
M 1 F 1 |
14 | Fever 2 | IFN 2, LPV 2, silybin 1, CTM 1 | None | IFN | Better 2 | None | Discharged 2 |
| Lafaie et al. 80 | France | July 2020 | Case series | 3 | 86 | F 3 | 11 | Cough 2, fever 3, respiratory signs 2 asthenia 1, ageusia 1, tachypnea 1 | Levofloxacin 1, ofloxacin 1, mPDRL 1, CRO 1, anticoagulation 1, PDRL 1, rovamycine 1, corticosteroids 1 | Hyperthermia 1, respiratory distress 1, dehydration 1, hypernatremia 1, melena 1, dry cough 1, fever 1 | Levofloxacin 1, aztreonam 1, mPDRL 2, tocilizumab 1, furosemide 1, CRO 1, cotrimoxazole 1, plasma 1, oxygen support 1 | Worse 3 | Hypertension 3, beta‐lactam allergy, heart failure, diabetes, kidney failure, respiratory failure, hypothyroidism, Alzheimer's disease, arterial and rheumatoid arthritis | Died 3 |
| Mardani et al. 81 | Iran | July 2020 | Case report | 1 | 64 | F | 21 | Dyspnea, asthenia | CRO, clindamycin, LPV/r, HCQ | Respiratory distress | Meropenem, VAN, ampicillin, acyclovir, steroids, colistin | NR | Hypertension, heart failure, metastatic colorectal cancer, chemotherapy, bacterial meningitis, pneumonia | NR |
| Yadav et al. 82 | India | October 2020 | Case report | 1 | 3 | M | 42 | None | NR | None | NR | NR | Neuroblastoma, chemotherapy | Discharged |
| Mahallawi 83 | Saudi Arabia | September 2020 | Case report | 1 | 31 | M | NR | Myalgia, fever, headache, hyporexia, anosmia, ageusia | Paracetamol | NR | NR | NR | None | Discharged |
| West et al. 11 | UK | December 2020 | Case report | 1 | 25 | M | NR | Fever, headache, fatigue | NR | Fatigue, coryzal symptoms | NR | Better | None | Discharged |
| Varella et al. 84 | Brazil | August 2020 | Case report | 1 | 26 | M | 32 | Headache, asthenia | Home care | Fever, cough, headache, myalgia, arthralgia, anosmia, fatigue | AZ, analgesics and antipyretics | Worse | NR | Discharged |
| Mendoza et al. 85 | USA | August 2020 | Case report | 1 | 51 | M | NR | None | NR | Fever, severe dyspnea, severe respiratory distress | DEX, RDV, oxygen support | Worse | Hypertension and ESRD due to acute tubular necrosis, undergoing chronic hemodialysis thrice weekly | Discharged |
| Lee et al. 86 | South Korea | November 2020 | Major article | 6 | 29.5 |
M 2 F 4 |
12 | Fever 2, cough 1, sore throat 1, sputum 1, rhinorrhea 2, anosmia 2, chest pain 1, diarrhea 1, fatigue 1, anorexia 1 | Symptomatic care with oral antitussives and esomeprazole 1 | Fever 2, cough 1, sputum 1, chest pain 1, chill 1, dyspnea 1, rhinorrhea 1 | Symptomatic care 1 |
ND 1 Better 3 NR 2 |
Allergic rhinitis 1, dyslipidemia 1, Parkinson's disease 1, dementia 1, depression 1 | Discharged 6 |
Abbreviations: ALB, albuterol; APAP, acetaminophen; ARB, arbidol; AZ, azithromycin; COPD, chronic obstructive pulmonary disease; CQ, chloroquine; CRO, ceftriaxone; CTM, Chinese traditional medicine; DEX, dexamethasone; HCQ, hydroxychloroquine; IFN, interferon; Ig, immunoglobulin; IVIG, intravenous immunoglobulin; IVR, ivermectin; LPV, lopinavir; LPV/r, lopinavir/ritonavir; MM, multiple myeloma; mPDRL, methylprednisolone; ND, no difference; NR, not reported; OTV, oseltamivir; PDRL, prednisolone; PRED, prednisone; RAN, ranitidine; RDV, remdesivir; RPV, ritonavir; TZP, piperacillin/tazobactam; UTI, urinary tract infection; VAN, vancomycin.
2.4. Quality assessment
The critical appraisal checklist provided by the Joanna Briggs Institute (JBI) was used to perform a quality assessment of the studies. 13
3. RESULTS
3.1. Study characteristics
The search strategy yielded 1807 studies from three databases. Following the removal of duplicates, the title and abstract of 998 studies were examined. Among these studies, 152 were selected for full‐text assessment, and other 846 studies were eliminated due to irrelevancy. In all the selected studies, the relapse of coronavirus infection after a negative RT‐PCR test was reported. Among the 152 full‐text studies examined, only 54 studies were found to be eligible for data extraction (Figure 1). The included studies were original articles (5.5%, N = 3), case reports (72.2%, N = 39), and case series (22.2%, N = 12). Likewise, in the included studies, RT‐PCR tests were performed to detect both the first and the second infections. Thirteen studies were originated from Europe, 11 from the USA, 9 from China, and 6 from Brazil. These articles reported a total number of 207 patients who developed the second infection of coronavirus after a recovery, which was confirmed by a negative RT‐PCR test. Forty‐six studies reported the clinical features in the first infection; however, seven articles declared no symptoms. Only one study unrecorded the clinical features in the first infection. In addition, 42 investigations implied the medication and intervention.
FIGURE 1.
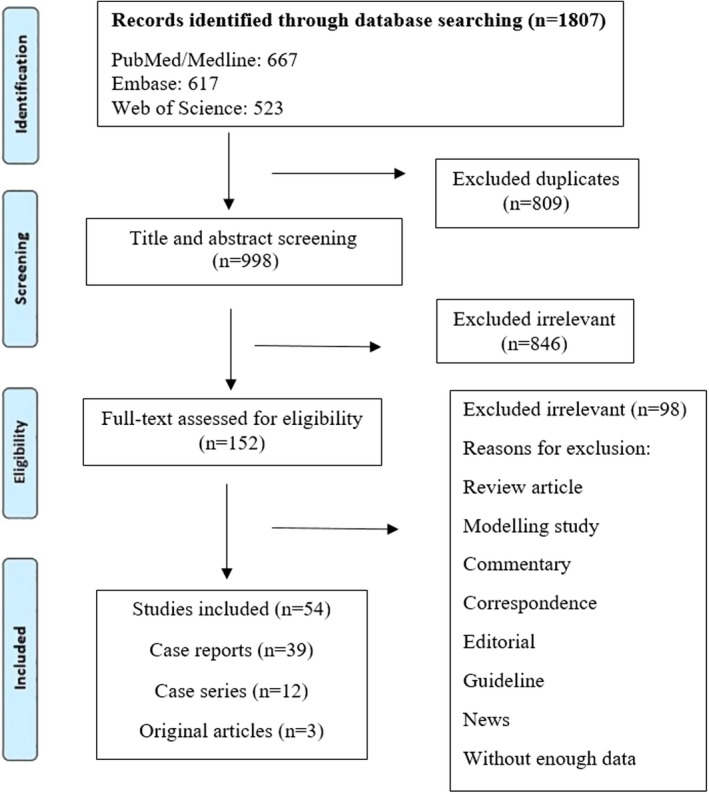
Flow diagram detailing review process and study selection
In the second phase of infection, 43 articles reported the clinical manifestations, seven articles stated no sign, and four articles did not list any symptoms. Also, 37 studies reported specifically the treatment of the secondary infection. To compare the severity of symptoms between two phases of infection, sufficient information of both is required. Only 41 investigations presented features for both periods of infection. From these 41 studies, information of 63 cases was identified as qualified for the comparison of manifestations. Moreover, the approximate interval between negative and second positive RT‐PCR was obtained from 49 studies. The length of this interval reflects the characteristics of COVID‐19 relapse.
During diagnosis, evaluation, and treatment, attention to comorbidities is necessary. Among included studies, 44 articles specified comorbidities. Of these observations, 11 studies did not found any notable underlying conditions or disorders. In this survey, we examined the outcome of the COVID‐19 relapse, which was categorized into discharge or death. The outcome was reported for a total number of 199 patients from 49 studies. The detailed information of is summarized in Table 1.
3.2. Demographic and general information
Considering the studies included, we reviewed 207 patients presented with secondary infection of COVID‐19 after a period of recovery. A negative RT‐PCR confirmed the recovery from the first phase of disease. Among the included articles, there were only three observational studies that reported 132 cases. Of all 207 cases, 122 (58.9%) patients were female, and 85 (41.1%) patients were male. As shown in Table 2, children were less likely to have a recurrence of COVID‐19. However, the most patients were in the age group of 20–40 years. The studies reported a wide range of 2–240 days between two coronavirus infections. We classified this duration into three groups: n ≤ 30, 30 < n < 90, and n ≥ 90. Thirty‐eight (77.5%) studies reported n ≤ 30 or 30 < n < 90 for the recovery duration, and only 11 (22.5%) investigations implied more than 90 days (Table 2).
TABLE 2.
Summary of the findings
| n/N (%) | No. of studies that mentioned | |
|---|---|---|
| Sex | ||
| Female | 122/207 (58.94) | 53 |
| Male | 85/207 (41.06) | |
| Age | ||
| <20 | 1/207 (0.48) | 1 |
| 20 ≤ n ≤ 40 | 157/207 (75.85) | 21 |
| 40 < n < 90 | 29/207 (14.01) | 16 |
| ≥90 | 20/207 (9.66) | 15 |
| Days between negative and second positive RT‐PCR | ||
| ≤30 | 23/49 (46.94) | 49 |
| 30 < n < 90 | 15/49 (30.61) | |
| ≥90 | 11/49 (22.45) | |
| Clinical manifestations in first infection | ||
| Asthenia | 34/51 (66.67) | 15 |
| Headache | 46/69 (66.67) | 22 |
| Cough | 52/95 (54.74) | 34 |
| Fever | 55/104 (52.88) | 43 |
| Sore throat | 33/66 (50) | 19 |
| Respiratory distress and signs | 13/27 (48.15) | 19 |
| Myalgia | 27/57 (47.37) | 16 |
| Diarrhea | 27/68 (39.71) | 14 |
| Fatigue | 14/37 (37.84) | 20 |
| Dyspnea | 20/54 (37.04) | 16 |
| Sneeze | 15/41 (36.59) | 8 |
| Chills | 4/12 (33.33) | 10 |
| Hyporexia | 13/42 (30.95) | 9 |
| Nausea | 13/45 (28.89) | 11 |
| Abdominal pain | 12/44 (27.27) | 10 |
| Malaise | 3/11 (27.27) | 10 |
| Vomiting | 11/42 (26.19) | 9 |
| Anosmia | 15/60 (25) | 14 |
| Dysgeusia | 11/44 (25) | 8 |
| Arthralgia | 10/41 (24.39) | 8 |
| Dizziness | 10/42 (23.81) | 9 |
| Tachypnea and respiratory alkalosis | 3/14 (21.43) | 10 |
| Chest pain | 4/19 (21.05) | 11 |
| Hypoxia | 2/10 (20) | 9 |
| Skin lesions | 8/41 (19.51) | 8 |
| Ageusia | 3/19 (15.79) | 10 |
| Rhinorrhea | 2/14 (14.29) | 8 |
| Drowsiness | 1/9 (11.11) | 8 |
| Hypoxemia | 1/9 (11.11) | 8 |
| Nasal congestion | 1/9 (11.11) | 8 |
| Night sweat | 1/9 (11.11) | 8 |
| Anorexia | 1/14 (7.14) | 8 |
| Sputum | 1/14 (7.14) | 8 |
| Mental confusion | 2/41 (4.88) | 8 |
| Treatment in first infection | ||
| Lopinavir/ritonavir | 8/10 (80) | 7 |
| Oxygen support | 9/13 (69.23) | 11 |
| Oseltamivir | 4/6 (66.66) | 6 |
| Interferon | 9/14 (64.29) | 7 |
| Chinese traditional medicine | 10/16 (62.5) | 6 |
| Azithromycin | 28/45 (62.22) | 11 |
| Antivirals | 3/5 (60) | 3 |
| Immunoglobulin | 3/5 (60) | 5 |
| Lopinavir | 3/5 (60) | 4 |
| Vitamins | 3/5 (60) | 5 |
| Zinc | 3/5 (60) | 5 |
| Acetaminophen | 2/4 (50) | 4 |
| Antibiotics | 3/6 (50) | 4 |
| Arbidol | 7/14 (50) | 7 |
| Ceftriaxone | 5/10 (50) | 5 |
| Dexamethasone | 2/4 (50) | 4 |
| Moxifloxacin | 2/4 (50) | 4 |
| Tocilizumab | 2/4 (50) | 4 |
| Methylprednisolone | 3/7 (42.86) | 5 |
| Hydroxychloroquine | 20/52 (38.46) | 15 |
| Albuterol | 1/3 (33.33) | 3 |
| Amiodarone | 1/3 (33.33) | 3 |
| Bisoprolol | 1/3 (33.33) | 3 |
| Cefoperazone sodium | 1/3 (33.33) | 3 |
| Cefuroxime | 1/3 (33.33) | 3 |
| Chloroquine | 1/3 (33.33) | 3 |
| Clindamycin | 1/3 (33.33) | 3 |
| Daptomycin | 1/3 (33.33) | 3 |
| Dipyrone | 1/3 (33.33) | 3 |
| Enoxaparin | 1/3 (33.33) | 3 |
| Furosemide | 1/3 (33.33) | 3 |
| Home care | 1/3 (33.33) | 3 |
| Montair | 1/3 (33.33) | 3 |
| Naproxen | 1/3 (33.33) | 3 |
| Paracetamol | 1/3 (33.33) | 3 |
| Piperacillin/tazobactam | 1/3 (33.33) | 3 |
| Prednisolone | 2/6 (33.33) | 4 |
| Prednisone | 1/3 (33.33) | 3 |
| Ranitidine | 1/3 (33.33) | 3 |
| Remdesivir | 2/6 (33.33) | 4 |
| Ribavirin | 1/3 (33.33) | 3 |
| Sulbactam sodium | 1/3 (33.33) | 3 |
| Thromboprophylaxis | 1/3 (33.33) | 3 |
| Vancomycin | 1/3 (33.33) | 3 |
| Ivermectin | 10/37 (27.03) | 4 |
| Silybin | 1/4 (25) | 3 |
| Antitussives | 2/9 (22.22) | 4 |
| Corticosteroids and steroids | 28/130 (21.54) | 4 |
| Anticoagulation | 1/5 (20) | 3 |
| Levofloxacin | 1/5 (20) | 3 |
| Ofloxacin | 1/5 (20) | 3 |
| Rovamycine | 1/5 (20) | 3 |
| Thymalfasin | 1/6 (16.67) | 3 |
| Thymopentin | 1/6 (16.67) | 3 |
| Heparin | 5/35 (14.29) | 3 |
| Esomeprazole | 1/8 (12.5) | 3 |
| Clinical manifestations in second infection | ||
| Asthenia | 34/54 (62.96) | 13 |
| Myalgia | 42/67 (62.69) | 23 |
| Headache | 44/72 (61.11) | 21 |
| Sore throat | 31/59 (52.54) | 18 |
| Dyspnea | 33/68 (48.53) | 21 |
| Sneeze | 22/47 (46.81) | 8 |
| Diarrhea | 25/58 (43.10) | 15 |
| Dysgeusia | 22/53 (41.51) | 11 |
| Anosmia | 24/62 (38.71) | 14 |
| Cough | 60/171 (35.09) | 28 |
| Fatigue | 10/31 (32.26) | 15 |
| Hyporexia | 15/47 (31.91) | 8 |
| Arthralgia | 15/48 (31.25) | 9 |
| Nausea | 16/53 (30.19) | 12 |
| Vomiting | 14/51 (27.45) | 10 |
| Abdominal pain | 13/48 (27.08) | 9 |
| Dizziness | 13/48 (27.08) | 9 |
| Malaise | 5/19 (26.32) | 12 |
| Fever | 46/186 (24.73) | 37 |
| Hypoxia | 4/18 (22.22) | 11 |
| Tachypnea | 4/18 (22.22) | 11 |
| Ageusia | 5/25 (20) | 12 |
| Chills | 5/28 (17.86) | 12 |
| Chest pain | 4/25 (16) | 11 |
| Respiratory distress | 18/127 (14.17) | 23 |
| Skin lesions and desquamation | 6/48 (12.5) | 9 |
| Tachycardia | 2/16 (12.5) | 9 |
| Mental confusion | 6/49 (12.24) | 9 |
| Anorexia | 1/15 (6.67) | 8 |
| Aphasia | 1/15 (6.67) | 8 |
| Back pain | 1/15 (6.67) | 8 |
| Coryzal symptoms | 1/15 (6.67) | 8 |
| Gingival aphthous ulcers | 1/15 (6.67) | 8 |
| Hypotension | 1/15 (6.67) | 8 |
| Hyposmia | 1/15 (6.67) | 8 |
| Hypoxemia | 1/15 (6.67) | 8 |
| Insomnia | 1/15 (6.67) | 8 |
| Lymphadenopathy | 1/15 (6.67) | 8 |
| Nasal congestion | 1/15 (6.67) | 8 |
| Rhinitis | 1/15 (6.67) | 8 |
| Stranguria | 1/15 (6.67) | 8 |
| Dehydration | 1/17 (5.88) | 8 |
| Hypernatremia | 1/17 (5.88) | 8 |
| Hyperthermia | 1/17 (5.88) | 8 |
| Melena | 1/17 (5.88) | 8 |
| Rhinorrhea | 1/20 (5) | 8 |
| Sputum | 1/20 (5) | 8 |
| Treatment in second infection | ||
| Acyclovir | 1/1 (100) | 1 |
| Ampicillin | 1/1 (100) | 1 |
| Analgesics | 1/1 (100) | 1 |
| Antipyretics | 1/1 (100) | 1 |
| Acetaminophen | 1/1 (100) | 1 |
| Arbidol | 1/1 (100) | 1 |
| Budesonide | 1/1 (100) | 1 |
| Caspofungin | 1/1 (100) | 1 |
| Cefepime | 1/1 (100) | 1 |
| Clarithromycin | 2/2 (100) | 2 |
| Co‐amoxiclav | 1/1 (100) | 1 |
| Colistin | 1/1 (100) | 1 |
| CQ phosphate | 1/1 (100) | 1 |
| Chinese traditional medicine | 7/7 (100) | 2 |
| Dexamethasone | 8/8 (100) | 8 |
| Enoxaparin | 1/1 (100) | 1 |
| Favipiravir | 2/2 (100) | 2 |
| Interferon | 7/7 (100) | 4 |
| Linezolid | 1/1 (100) | 1 |
| Lopinavir | 1/1 (100) | 1 |
| Lopinavir/ritonavir | 1/1 (100) | 1 |
| Meropenem | 2/2 (100) | 2 |
| Montair | 1/1 (100) | 1 |
| Moxifloxacin | 1/1 (100) | 1 |
| Naproxen | 1/1 (100) | 1 |
| Oseltamivir | 2/2 (100) | 2 |
| Paracetamol | 1/1 (100) | 1 |
| Polymixin | 1/1 (100) | 1 |
| Ranitidine | 1/1 (100) | 1 |
| Ribavirin | 1/1 (100) | 1 |
| Piperacillin/tazobactam | 2/2 (100) | 2 |
| Vancomycin | 3/3 (100) | 3 |
| Vitamins | 2/2 (100) | 2 |
| Zinc | 2/2 (100) | 2 |
| Methylprednisolone | 5/6 (83.33) | 4 |
| Corticosteroids and steroids | 28/35 (80) | 3 |
| Remdesivir | 8/10 (80) | 8 |
| Thymalfasin | 3/4 (75) | 1 |
| Azithromycin | 29/44 (65.91) | 9 |
| Ivermectin | 23/35 (65.71) | 2 |
| Antibiotics | 22/35 (62.86) | 3 |
| Ceftriaxone | 4/7 (57.14) | 4 |
| Furosemide | 2/4 (50) | 2 |
| Plasma | 4/8 (50) | 4 |
| Oxygen support | 21/54 (38.89) | 17 |
| Heparin | 12/33 (36.36) | 1 |
| Aztreonam | 1/3 (33.33) | 1 |
| Cotrimoxazole | 1/3 (33.33) | 1 |
| Immunoglobulin | 1/3 (33.33) | 1 |
| Levofloxacin | 1/3 (33.33) | 1 |
| Tocilizumab | 1/3 (33.33) | 1 |
| Hydroxychloroquine | 13/44 (29.55) | 7 |
| Arbidol | 1/4 (25) | 1 |
| Symptomatic care | 1/6 (16.67) | 1 |
| Status | ||
| Worse | 29/63 (46.03) | 41 |
| Better | 25/63 (39.68) | |
| ND | 9/63 (14.29) | |
| Comorbidities | ||
| Obesity | 13/40 (32.5) | 15 |
| Kidney failure | 8/26 (30.77) | 17 |
| Hypertension | 19/63 (30.16) | 24 |
| Pneumonia | 8/30 (26.67) | 19 |
| Heart failure | 6/23 (26.09) | 17 |
| Arthritis | 4/22 (18.18) | 13 |
| Hypothyroidism | 3/17 (17.65) | 14 |
| Sleep apnea | 3/17 (17.65) | 14 |
| Acute lymphoblastic leukemia | 3/18 (16.67) | 13 |
| Diabetes | 10/66 (15.15) | 22 |
| HIV | 3/21 (14.29) | 12 |
| Liver failure | 3/21 (14.29) | 12 |
| Sarcoidosis | 3/21 (14.29) | 12 |
| Anxiety | 2/16 (12.5) | 13 |
| Chemotherapy | 2/16 (12.5) | 13 |
| Glucose/carbohydrate intolerance | 2/16 (12.5) | 13 |
| Smoking | 2/16 (12.5) | 13 |
| Under hemodialysis | 2/16 (12.5) | 13 |
| Chronic obstructive pulmonary disease | 2/18 (11.11) | 12 |
| Multiple myeloma | 2/18 (11.11) | 13 |
| Alzheimer's disease and dementia | 2/21 (9.52) | 13 |
| Dyslipidemia | 2/21 (9.52) | 13 |
| Parkinson's disease | 2/21 (9.52) | 13 |
| Asthma | 5/53 (9.43) | 16 |
| Anemia | 1/15 (6.67) | 12 |
| Atrial fibrillation | 1/15 (6.67) | 12 |
| Bacterial meningitis | 1/15 (6.67) | 12 |
| Beta‐lactam allergy | 1/15 (6.67) | 12 |
| Cardiopulmonary arrest | 1/15 (6.67) | 12 |
| Degenerative disk disease | 1/15 (6.67) | 12 |
| End stage renal disease | 1/15 (6.67) | 12 |
| Emphysema | 1/15 (6.67) | 12 |
| Hepatitis B | 1/15 (6.67) | 12 |
| Hypercalcemia | 1/15 (6.67) | 12 |
| Hypercholesterolemia | 1/15 (6.67) | 12 |
| Inflammatory syndrome | 1/15 (6.67) | 12 |
| Lumbar fusion | 1/15 (6.67) | 12 |
| Metastatic colorectal cancer | 1/15 (6.67) | 12 |
| Nephropathy (diabetic) | 1/15 (6.67) | 12 |
| Neuroblastoma | 1/15 (6.67) | 12 |
| Neuropathy | 1/15 (6.67) | 12 |
| Plasmacytoma | 1/15 (6.67) | 12 |
| Respiratory failure | 1/15 (6.67) | 12 |
| Urinary tract infection | 1/15 (6.67) | 12 |
| Waldenstrom macroglobulinemia | 1/15 (6.67) | 12 |
| Stroke | 1/16 (6.25) | 12 |
| Digestive disease | 1/17 (5.88) | 12 |
| Renal impairment | 1/17 (5.88) | 12 |
| Allergic rhinitis | 1/21 (4.76) | 12 |
| Depression | 1/21 (4.76) | 12 |
| Outcome | ||
| Discharge | 190/199 (95.48) | 49 |
| Death | 9/199 (4.52) | |
Abbreviations: HIV, human immunodeficiency virus; n, number of patients with any variables; N, the total number of patients with COVID‐19; ND, no difference; No, number; RT‐PCR, reverse transcription‐polymerase chain reaction.
To compare the severity of the first infection with secondary, the reported features and symptoms were reviewed and extracted from the articles. Of 63 patients, 29 presented more severe manifestations, while 25 cases showed an ameliorated status, and 9 other cases indicated similar symptoms in both phases of the infection. Forty‐nine studies recorded the outcome of 199 patients, among whom 190 cases were discharged with an improved status, but 9 cases succumbed in hospital. Indeed, the survival rate is required to be taken into account when determining the potency of coronavirus reactivation.
3.3. Clinical manifestations
Some clinical signs were most frequent between the two infections, but prevalence was different. Moreover, the most prevalent clinical signs in the first infection were asthenia (66.6%), headache (66.6%), cough (54.7%), fever (52.8%), sore throat (50.0%), and respiratory distress (48.1%). However, in the second infection, asthenia (62.9%), myalgia (62.6%), headache (61.11%), sore throat (52.54%), and dyspnea (48.53%) were the common. Respiratory alkalosis (21.43%), drowsiness (11.11%), and night sweat (11.11%) occurred only in the first infection. The most frequent symptoms observed only in the second infection were as follows: asthenia (62.9%), myalgia (62.9%), and headache (61.1%). COVID‐19 recurrence was manifested with more mild signs in 39.6% of patients, while 46.0% presented with more severe signs, and other 14.3% did not show any prominent change between the two infections.
3.4. Treatment
Medications and treatments for the first COVID‐19 infection were reported in 40 studies. Among these treatments, hydroxychloroquine, azithromycin, and oxygen support were reported by 15, 11, and 11 articles, respectively. A total of 37 articles stated treatment for the second COVID‐19 infection. So that, oxygen support (17 studies), azithromycin (9 studies), dexamethasone (8 studies), and remdesivir (8 studies) were mostly reported. The most common treatment options used in first SARS‐CoV‐2 infection were lopinavir/ritonavir (80%), oxygen support (69.2%), and oseltamivir (66.6). However, for the treatment of second SARS‐CoV‐2 infection, mostly antibiotics (100%), dexamethasone (100%), and Remdesivir (80%) were used (Table 2).
3.5. Comorbidities
The evaluating comorbidities and underlying conditions can enlighten some aspects of COVID‐19. Based on the extracted data, a number of underlying diseases and conditions had a notable frequency. Obesity was highlighted as a condition in 32.5% of patients by 15 articles, whereas 22 studies stated diabetes with an overall prevalence of 15.15%. Hypertension and heart failure were reported to be 30.16% and 26.09% in 24 and 17 articles, respectively. Neurodegenerative disorders such as Alzheimer's (9.52%) and Parkinson's (9.52%) diseases were found as comorbidities (Table 2). However, evidence established an association between these types of comorbidities and COVID‐19; further investigations could clarify the detailed mechanisms of these relations.
4. DISCUSSION
To prevent reinfection or reactivation, four criteria can be considered for patients’ discharge. First, the patient should not have a fever for at least three days. Second, the patient's respiratory symptoms should considerably be ameliorated. Third, the radiological abnormalities shown in the CT scan and X‐ray images should substantially be improved. Four, as per WHO recommendation, patients should have two consecutive negative RT‐PCR results with a 24‐h interval. 14
Improved or discharged patients are connected to the members of the community, and they, therefore, are presented as a latent source of infection. 14 In this study, we assessed the prevalence and frequency of recurrence or reinfection in patients with COVID‐19 and performed investigations from various aspects, including factors related to host, virus, and environment. The emergence of new virus mutations is the main hypothesis on mechanisms of the COVID‐19 reinfection. The new variants of the SARS‐CoV‐2 can bind to human cells, and the produced antibodies in the first infection could not efficiently opsonize them. Actually, these variants can lead to evading the immune response. 15
Several factors influencing reinfection, including the initial load of the virus and the type of genome, are virus‐dependent. 16 The average duration of SARS‐CoV‐2 shedding is 20 days, which in some cases is 37 days. 17 A survey has suggested an average viral shedding of 53 days, with a maximum of 83 days. Patients in whom clinical symptoms had started earlier tented to have a longer duration of viral shedding and more severe disease. 18
The study by Elrashdy et al. found that the average time period between the previous discharge and the next positive test was 4–17 days. 14 In the present study, the highest incidence of reinfection was related to a period of less than 30 days (46.94%). In the periods of 30–90 days, the incidence of reinfection was 30.61%, and the lowest incidence (22.45%) was observed in the period of more than 90 days. Given the studies reviewed above, there is a discrepancy between the duration of subsequent coronavirus infection and the antibody‐induced immunity. Therefore, there is certainly other factors, such as the level of the individual's immune system or the accuracy of the tests, that affect this time period. Perhaps, the reason for the recurrence of the disease 7–14 days after discharge from the hospital is that the virus is still hidden in exosomes or extracellular vesicles and resumes activity after a period of "silences". 14
In this study, RT‐PCR was a necessary inclusion criterion. Thus, patients with only a serologic diagnosis test, without a nasopharyngeal swab RT‐PCR were excluded. RT‐PCR is the gold standard for diagnosing SARS‐CoV‐2; however, this test has low sensitivity due to test error or insufficient sample size. 19 The accuracy of RT‐PCR is 97%, 20 and the occurrence of false negatives in PCR of SARS‐CoV‐2 has been reported to be 30%, 21 which in some cases increases due to sampling error. 20 One of the reasons for the error in RT‐PCR is the prolonged conversion of nucleic acid, which causes recurrence or "turn positive". 22 In the early stages of infection, the SARS‐CoV‐2 is readily detected in the upper respiratory tract. As the disease progresses, the virus appears in the lower respiratory tract and other organs such as the intestines and blood. 23 Therefore, it is impossible to identify SARS‐CoV‐2 in the throat, and some patients may have positive CT scan, despite the negative RT‐PCR. 24
Incorrect sampling is another reason for recurrence in improved individuals, 14 although it is unlikely to happen due to the use of devices such as gloves, masks, and caps. 25
As the laboratory detection of virus nucleic acid can have false‐negative results, serological tests for specific IgG and IgM can be alternated. 19 Therefore, PCR alone is not adequate for discharging patients from hospital. Supplementary tests such as serological ones, together with the criteria recommended by the WHO and other specific health organizations, are needed to be performed in every country. There is a period of time between the apparent recovery in the clinic and the complete recovery from the SARS‐CoV‐2. Viral carriers with low symptoms pose a greater challenge to epidemic management and control. 26 Conducting two negative PCR at 24‐hour intervals is insufficient for detecting the virus; thus, repeating the test for a longer period of 48 h is recommended. In addition, immunological tests such as d‐dimer and absolute lymphocyte counts and even antibody testing should be performed. RT‐PCR results are negative on average 2.73 days after hospitalization. 26 Wolfel et al. in their study evaluated the hospitalized patients with COVID‐19. They demonstrated that after eight days of infection, the live virus is undetectable. 27
Gender, old age, and the type of disease are host‐dependent factors influencing the occurrence of reinfection and require immune system suppression. 16 In the present study, women became more infected than men (58.94% vs. 41.06). Children were less likely to have COVID‐19 relapse. However, the most patients were in the age group of 20–40 years. In addition, obesity (32.5%), kidney failure (30.7%), and hypertension (30.1%) were the most frequent underlying comorbidities observed among COVID‐19 relapse patients. Although studies have shown that underlying conditions cause the severity of COVID‐19 disease, but how each of these factors contribute to reinfection should be examined by designing new studies determining these effects separately or in combination. 28 , 29
The clinical features of patients with reinfection are similar to those of primary infection. The presence of asymptomatic patients among reactivated patients caused the recurrence of the asymptomatic contamination or infection with few symptoms. 14 , 16
In an earlier study, rhesus macaques became reinfected after recovery, without showing any symptoms. This finding highlights the need for strict protection from SARS‐CoV‐2 and its control, to hider the development of this severe disease. 30 The second time of infection severity is varied; some cases show mild, and some others indicate more severe symptoms. 10 In a former study, 46.03% of patients had a worse condition, and 39.68% had a better condition in the second than the first infection. One of the reasons for the deterioration condition of patients in the second infection, compared with first one, is the occurrence of an antibody‐dependent enhancement (ADE) that increases the infectivity of virus in the secondary infection. 31 However, a patient with strong immunity and more immune memory cells and T‐cell mediation could decrease the severity of the second infection. 10
Normally, people with a primary infection with mild symptoms and those with a suppressed immune system are more likely to get COVID‐19 for the second time because they do not produce an adequate immune response. It has also been demonstrated that 95.48% of the patients reinfected with the SARS‐CoV‐2 were discharged from hospital and 4.52% were died. The rate of death would have possibly been reduced if patients had received more care during their first‐time hospitalization. 10
SARS‐CoV‐2 reactivation may occur when using any antiviral drug. 16 In this survey, the most recurrence of the disease occurred after taking lopinavir/ritonavir, oseltamivir, interferon, and Chinese traditional medicine. These drugs may not have been able to fully eradicate viruses from the body, and some of them may remain in the body, causing reinfection. However, further investigation can evaluate the effectiveness of different drugs in complete eradication of the virus to eliminate the possibility of reinfection.
In a study performed by Okhuese, the proportion of infected population will continue to grow in the world if unvaccinated. At the same time, the rate of recovery will continue slowly. In other words, in this situation, the mortality rate can be determined based on the ratio of infection to recovery rate. The rate of reinfection with clinical clearance of the virus from the improved population decreases to zero over time. 32 Contrary to the results achieved in Okhuese's study, 33 despite the high prevalence of SARS‐CoV‐2, the rate of reinfection is still high. Therefore, more experimental and laboratory studies are needed to determine the cause of reinfection and its frequency. Of note, reinfection differs from reactivation. Reinfection is caused by different variants of SARS‐CoV‐2 virus, but reinfection occurs with the same strain. The only way to discriminate the reinfection and reactivation is by sequencing and molecular techniques. 34 Regrettably, the first two actions happen only in 5%–10%. 10 Designing studies to sequence the virus genome in the first and second infections is highly recommended. In this way, the cause of COVID‐19 recurrence is clarified, and its prevalence in the community is determined.
A number of limitations can be considered in this study. The first is the small number of original articles and short communication. The second is related to case series and case reports studies, which lack sufficient and accurate information on patients and are often reported descriptively. Therefore, accurate meta‐analysis calculations were impossible in this study.
5. CONCLUSION
The present study represents a large number of COVID‐19 reactivation over different countries. Overall, the recurrence of COVID‐19 in recovered patients may arises from various factors, including a false negative or positive in PCR, differences in tests, incorrect diagnosis by physicians to discharge a patient with COVID‐19, illness for reasons other than COVID‐19, the presence of various strains of SARS‐CoV‐2, and dysfunction of immune systems. Our results highlighted the potency of COVID‐19 recurrence as an outstanding issue. This feature needs to be regarded in the management of COVID‐19. The first and second COVID‐19 are the same in terms of clinical manifestations, but they are not distinguishable. So far, no acceptable marker has been found to predict the risk of reinfection. In addition, there is no validated test of whether a particular drug or treatment is associated with reinfection or reactivation. A careful follow‐up of discharged patients and accuracy in their discharge and removal from quarantine is of paramount importance to inhibit reinfection. Given the data discussed in this work, the first coronavirus infection can lead to the recurrence of COVID‐19. Regarding COVID‐19 infection, there are two hypothesis: (a) COVID‐19 infection reactivates following a period of dormancy, and (b) COVID‐19 increases the susceptibility to the second coronavirus invasion. Future experimental and clinical researches could examine these hypotheses and finally provide a clear view of COVID‐19 relapse.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTION
Maryam Koupaei, Mohamad Hosein Mohamadi, Ilya Yashmi, Amir Hossein Shahabi, Amir Hosein Shabani, Mohsen Heidary, and Saeed Khoshnood contributed in revising and final approval of the version to be published. All authors agreed and confirmed the study for publication.
INFORMED CONSENT
Not applicable.
CONSENT FOR PUBLICATION
Not applicable.
Koupaei M, Mohamadi MH, Yashmi I, et al. Clinical manifestations, treatment options, and comorbidities in COVID‐19 relapse patients: A systematic review. J Clin Lab Anal. 2022;36:e24402. doi: 10.1002/jcla.24402
Maryam Koupaei, Mohamad Hosein Mohamadi, and Ilya Yashmi shares co‐first authorship and contributed equally to this work.
Amir Hossein Shahabi and Amir Hosein Shabani shares co‐second authorship and contributed equally.
Contributor Information
Mohsen Heidary, Email: mohsenheidary40@gmail.com.
Saeed Khoshnood, Email: Saeed.khoshnood22@gmail.com.
DATA AVAILABILITY STATEMENT
All the data in this review are included in the study.
REFERENCES
- 1. Perlman S. Another decade, another coronavirus. N Engl J Med. 2020;382(8):760‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. He F, Deng Y, Li W. Coronavirus disease 2019: what we know? J Med Virol. 2020;92(7):719‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaidyanathan G. Coronavirus variants are spreading in India—what scientists know so far. Nature. 2021;593(7859):321‐322. [DOI] [PubMed] [Google Scholar]
- 4. Le Page M. The global threat of the coronavirus variants. New Sci. 2021;249(3318):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatr. 2020;87(4):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS‐CoV‐2 test results. Am J Emerg Med. 2021;39(256):e1‐e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Habadi MI, Balla Abdalla TH, Hamza N, Al‐Gedeei A. COVID‐19 reinfection. Cureus. 2021;13(1):e12730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liuqian L, Huang S, Wei H. 14% of Recovered Covid‐19 Patients in Guangdong Tested Positive Again. 2020.
- 9. Villamil JFP, Olivera MJ. COVID‐19: is reinfection a threat or not? Iran J Publ Health. 2020;49(Suppl 1):112‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stokel‐Walker C. What we know about covid‐19 reinfection so far. BMJ. 2021;372:n99. [DOI] [PubMed] [Google Scholar]
- 11. West J, Everden S, Nikitas N. A case of COVID‐19 reinfection in the UK. Clin Med. 2021;21(1):e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 13. Institute J . The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews Checklist for Analytical Cross Sectional Studies. The Joanna Briggs Institute North Adelaide, Australia; 2017.
- 14. Elrashdy F, Aljaddawi AA, Redwan EM, Uversky VN. On the potential role of exosomes in the COVID‐19 reinfection/reactivation opportunity. J Biomol Struct Dyn. 2021;39(15):5831‐5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sanaie S, Golipour E, Shamekh A, Sadaie MR, Mahmoodpoor A, Yousefi M. Immune response variables and viral mutations impact on COVID‐19 reinfection and relapse. Int Immunopharmacol. 2021;100:108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ye G, Pan Z, Pan Y, et al. Clinical characteristics of severe acute respiratory syndrome coronavirus 2 reactivation. J Infect. 2020;80(5):e14‐e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li N, Wang X, Lv T. Prolonged SARS‐CoV‐2 RNA shedding: not a rare phenomenon. J Med Virol. 2020;92(11):2286‐2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong X, Cao Y‐Y, Lu X‐X, et al. Eleven faces of coronavirus disease 2019. Allergy. 2020;75(7):1699‐1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xie X, Zhao ZZW, Zheng C, Wang F, Liu J. Chest CT for typical 2019‐nCoV pneumonia: relationship to negative RT‐PCR testing. Radiology. 2020;296:200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in different types of clinical specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xiao AT, Tong YX, Zhang S. False negative of RT‐PCR and prolonged nucleic acid conversion in COVID‐19: rather than recurrence. J Med Virol. 2020;92(10):1755‐1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang W, Du R‐H, Li B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID‐19: comparison to RT‐PCR. Radiology. 2020;296(2):E115‐E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chaturvedi R, Naidu R, Sheth S, Chakravarthy K. Efficacy of serology testing in predicting reinfection in patients with SARS‐CoV‐2. Disaster Med Publ Health Prep. 2021;15(2):e29‐e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan J, Kou S, Liang Y, Zeng J, Pan Y, Liu L. PCR assays turned positive in 25 discharged COVID‐19 patients. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. [DOI] [PubMed] [Google Scholar]
- 28. Heidary M, Asadi A, Noorbakhsh N, et al. COVID‐19 in HIV‐positive patients: a systematic review of case reports and case series. J Clin Lab Anal. 2022. 581:e24308. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koupaei M, Naimi A, Moafi N, et al. Clinical characteristics, diagnosis, treatment, and mortality rate of TB/COVID‐19 coinfectetd patients: a systematic review. Front Med. 2021;8:740593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bao L, Deng W, Gao H, et al. Reinfection could not occur in SARS‐CoV‐2 infected rhesus macaques. bioRxiv 2020.03. 13.990226. 2020.
- 31. Halstead SB. Dengue hemorrhagic fever: two infections and antibody dependent enhancement, a brief history and personal memoir. Rev Cubana Med Trop. 2002;54(3):171‐179. [PubMed] [Google Scholar]
- 32. Victor OA. Estimation of the probability of reinfection with COVID‐19 by the susceptible‐exposed‐infectious‐removed‐undetectable‐susceptible model. JMIR Public Health Surveillance. 2020;6(2):e19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Parry J. Covid‐19: Hong Kong Scientists Report First Confirmed Case of Reinfection. British Medical Journal Publishing Group; 2020. [DOI] [PubMed] [Google Scholar]
- 34. Lancman G, Mascarenhas J, Bar‐Natan M. Severe COVID‐19 virus reactivation following treatment for B cell acute lymphoblastic leukemia. J Hematol Oncol. 2020;13(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y, Bai W, Liu B, et al. Re‐evaluation of retested nucleic acid‐positive cases in recovered COVID‐19 patients: report from a designated transfer hospital in Chongqing, China. J Infect Public Health. 2020;13(7):932‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nazir N, Ahirwar A, Jain S. Reinfection in a healthcare worker with COVID‐19 in a hospital in North India. Anaesth Pain Intensive Care. 2020;24(5):572. [Google Scholar]
- 37. Selhorst P, Van Ierssel S, Michiels J, et al. Symptomatic SARS‐CoV‐2 re‐infection of a health care worker in a Belgian nosocomial outbreak despite primary neutralizing antibody response. medRxiv. 2020. [DOI] [PMC free article] [PubMed]
- 38. Selvaraj V, Herman K, Dapaah‐Afriyie K. Severe, symptomatic reinfection in a patient with COVID‐19. RI Med J. 2013;2020(103):24‐26. [PubMed] [Google Scholar]
- 39. Bellesso M, Bruniera FR, Trunkel AT, Nicodemo IP. Second COVID‐19 infection in a patient with multiple myeloma in Brazil‐reinfection or reactivation? Hematol Transfus Cell Ther. 2021;43:109‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhou X, Zhou J, Zhao J. Recurrent pneumonia in a patient with new coronavirus infection after discharge from hospital for insufficient antibody production: a case report. BMC Infect Dis. 2020;20(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Atici S, Ek ÖF, Yildiz MS, Şikgenç MM, Güzel E, Soysal A. Symptomatic recurrence of SARS‐CoV‐2 infection in healthcare workers recovered from COVID‐19. J Infect Dev Ctries. 2021;15(01):69‐72. [DOI] [PubMed] [Google Scholar]
- 42. Shoar S, Khavandi S, Tabibzadeh E, Khavandi S, Naderan M, Shoar N. Recurrent coronavirus diseases 19 (COVID‐19): a different presentation from the first episode. Clin Case Rep. 2021;9(4):2149‐2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mulder M, van der Vegt DS, Munnink BBO, et al. Reinfection of severe acute respiratory syndrome coronavirus 2 in an immunocompromised patient: a case report. Clin Infect Dis. 2021;73(9):e2841‐e2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hanif M, Haider MA, Ali MJ, Naz S, Sundas F. Reinfection of COVID‐19 in Pakistan. A first case report. Cureus. 2020;12(10):e11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abdallah H, Porterfield F, Fajgenbaum D. Symptomatic relapse and long‐term sequelae of COVID‐19 in a previously healthy 30‐year‐old man. BMJ Case Rep. 2020;13(12):e239825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Brito CAA, Lima PMA, de Brito MCM, de Oliveira DB. Second episode of COVID‐19 in health professionals: report of two cases. Int Med Case Rep J. 2020;13:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hussein NR, Musa DH, Naqid IA, Saleem SM, Ibrahim N. The first case of COVID‐19 reinfection in Duhok City, Kurdistan Region of Iraq. A case report. J Kermanshah Univ Med Sci. 2020;24(4):e111454. [Google Scholar]
- 48. Kapoor R, Nair RK, Nayan N, Bhalla S, Singh J. Reinfection or reactivation of coronavirus‐19 in patients with hematologic malignancies: case report series. SN Compr Clin Med. 2021;3(2):670‐674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu F, Cai Z‐B, Huang J‐S, et al. Repeated COVID‐19 relapse during post‐discharge surveillance with viral shedding lasting for 67 days in a recovered patient infected with SARS‐CoV‐2. J Microbiol Immunol Infect. 2021;54(1):101‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salcin S, Fontem F. Recurrent SARS‐CoV‐2 infection resulting in acute respiratory distress syndrome and development of pulmonary hypertension: a case report. Respir Med Case Rep. 2021;33:101314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Adrielle dos Santos L, Filho PGDG, Silva AMF, et al. Recurrent COVID‐19 including evidence of reinfection and enhanced severity in thirty Brazilian healthcare workers. J Infect. 2021;82(3):399‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Goldman J, Wang K, Röltgen K, et al. Reinfection with SARS‐CoV‐2 and Failure of Humoral Immunity: a case report. Medrxiv. 2020.
- 53. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. A case report of possible novel coronavirus 2019 reinfection. Am J Emerg Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coppola A, Annunziata A, Carannante N, Di Spirito V, Fiorentino G. Late reactivation of SARS‐CoV‐2: a case report. Front Med. 2020;7:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sicsic I Jr, Chacon AR, Zaw M, Ascher K, Abreu A, Chediak A. A case of SARS‐CoV‐2 reinfection in a patient with obstructive sleep apnea managed with telemedicine. BMJ Case Rep. 2021;14(2):e240496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dou C, Xie X, Peng Z, et al. A case presentation for positive SARS‐CoV‐2 RNA recurrence in a patient with a history of type 2 diabetes that had recovered from severe COVID‐19. Diabetes Res Clin Pract. 2020;166:108300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Novoa W, Miller H, Mattar S, Faccini‐Martínez ÁA, Rivero R, Serrano‐Coll H. A first probable case of SARS‐CoV‐2 reinfection in Colombia. Ann Clin Microbiol Antimicrob. 2021;20(1):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tuan J, Spichler‐Moffarah A, Ogbuagu O. A new positive SARS‐CoV‐2 test months after severe COVID‐19 illness: reinfection or intermittent viral shedding? BMJ Case Rep. 2021;14(2):e240531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sharma R, Sardar S, Arshad AM, Ata F, Zara S, Munir W. A patient with asymptomatic SARS‐CoV‐2 infection who presented 86 days later with COVID‐19 pneumonia possibly due to reinfection with SARS‐CoV‐2. Am J Case Rep. 2020;21:e927154‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bonifácio LP, Pereira APS, Araújo DCDAE, et al. Are SARS‐CoV‐2 reinfection and Covid‐19 recurrence possible? A case report from Brazil. Rev Soc Bras Med Trop. 2020;53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Scaria V. Asymptomatic reinfection in two healthcare workers from India with genetically distinct SARS‐CoV‐2. 2020.
- 62. Akya A, Bozorgomid A, Ghadiri K, et al. Usefulness of blood parameters for preliminary diagnosis of brucellosis. J Blood Med. 2020;11:107‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Zhang R‐Z, Deng W, He J, et al. Case report: recurrence of positive SARS‐CoV‐2 results in patients recovered from COVID‐19. Front Med. 2020;7:882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Fehdi MA, Erragh A, Zerhouni A, Aissaoui O, Nsiri A, Alharrar R. Case report: a COVID‐19 reactivation case. Pan Afr Med J. 2020;35(35). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Caralis P. Case reports of COVID 19 recurrence. J Prim Care Community Health. 2021;12:2150132720982752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Harrington D, Kele B, Pereira S, et al. Confirmed reinfection with SARS‐CoV‐2 variant VOC‐202012/01. Clin Infect Dis. 2021;73(10):1946‐1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ozaras R, Ozdogru I, Yilmaz A. Coronavirus disease 2019 re‐infection: first report from Turkey. New Microbes New Infect. 2020;38:100774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Prado‐Vivar B, Becerra‐Wong M, Guadalupe JJ, et al. A case of SARS‐CoV‐2 reinfection in Ecuador. Lancet Infect Dis. 2021;21(6):e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bellanti F, Lo Buglio A, Custodero G, et al. Fatal relapse of COVID‐19 after recovery? A case report of an older Italian patient. J Infect. 2021;82(1):e49‐e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yang C, Jiang M, Wang X, et al. Viral RNA level, serum antibody responses, and transmission risk in recovered COVID‐19 patients with recurrent positive SARS‐CoV‐2 RNA test results: a population‐based observational cohort study. Emerg Microbes Infect. 2020;9(1):2368‐2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Du H‐W, Chen J‐N, Pan X‐B, et al. Prevalence and outcomes of re‐positive nucleic acid tests in discharged COVID‐19 patients. Eur J Clin Microbiol Infect Dis. 2021;40(2):413‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tillett RL, Sevinsky JR, Hartley PD, et al. Genomic evidence for reinfection with SARS‐CoV‐2: a case study. Lancet Infect Dis. 2021;21(1):52‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nonaka CKV, Franco MM, Gräf T, Mendes AVA, de Aguiar RS, Giovanetti M, et al. Genomic Evidence of a Sars‐Cov‐2 Reinfection Case With E484K Spike Mutation in Brazil. 2021.
- 74. Ravioli S, Ochsner H, Lindner G. Reactivation of COVID‐19 pneumonia: a report of two cases. J Infect. 2020;81(2):e72‐e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de Jesus RP, Silva R, Aliyeva E, et al. Reactivation of SARS‐CoV‐2 after asymptomatic infection while on high‐dose corticosteroids. Case report. SN Compr Clin Med. 2020;2(11):2402‐2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zayet S, Royer P‐Y, Toko L, Pierron A, Gendrin V, Klopfenstein T. Recurrence of COVID‐19 after recovery? A case series in health care workers, France. Microbes Infect. 2021;23(4–5):104803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ak R, Yilmaz E, Seyhan AU, Doganay F. Recurrence of COVID‐19 Documented with RT‐PCR. J Coll Physicians Surg Pak. 2021;30(1):S26‐S28. [DOI] [PubMed] [Google Scholar]
- 78. Cao S, Wu A, Li J, Li Y, Xia M, Wu J. Recurrent recurrence of positive SARS‐CoV‐2 RNA in a COVID‐19 patient. 2020.
- 79. Wu J, Cheng J, Shi X, et al. Recurrence of SARS‐CoV‐2 nucleic acid positive test in patients with COVID‐19: a report of two cases. BMC Pulm Med. 2020;20(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lafaie L, Célarier T, Goethals L, et al. Recurrence or relapse of COVID‐19 in older patients: a description of three cases. J Am Geriatr Soc. 2020;68(10):2179‐2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mardani M, Nadji SA, Sarhangipor KA, Sharifi‐Razavi A, Baziboroun M. COVID‐19 infection recurrence presenting with meningoencephalitis. New Microbes New Infect. 2020;37:100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yadav SP, Thakkar D, Bhoyar RC, et al. Asymptomatic reactivation of SARS‐CoV‐2 in a child with neuroblastoma characterised by whole genome sequencing. IDCases. 2021;23:e01018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mahallawi W. Case report: a recovered SARS CoV‐2 patient protected from reinfection. Front Med. 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Alonso FOM, Sabino BD, Guimarães MAAM, Varella RB. Recurrence of SARS‐CoV‐2 infection with a more severe case after mild COVID‐19, reversion of RT‐qPCR for positive and late antibody response: case report. J Med Virol. 2021;93(2):655‐656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Munoz Mendoza J, Alcaide ML. COVID‐19 in a patient with end‐stage renal disease on chronic in‐center hemodialysis after evidence of SARS‐CoV‐2 IgG antibodies. Reinfection or inaccuracy of antibody testing. Idcases. 2020;22:e00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Lee J‐S, Kim SY, Kim TS, et al. Evidence of severe acute respiratory syndrome coronavirus 2 reinfection after recovery from mild coronavirus disease 2019. Clin Infect Dis. 2021;73(9):e3002‐e3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data in this review are included in the study.


