Abstract
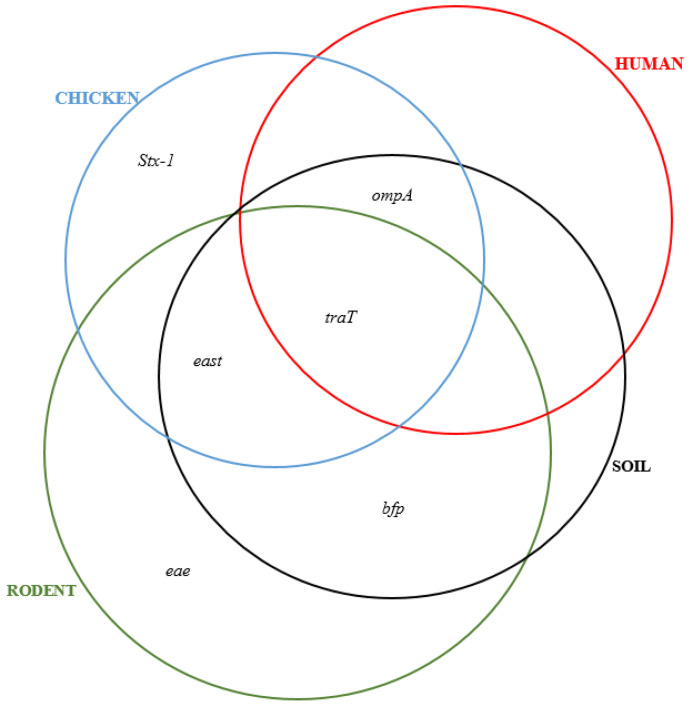
The interaction of rodents with humans and chicken in the household environment can facilitate transmission of multidrug-resistant (MDR) Escherichia coli (E. coli), causing infections that are difficult to treat. We investigated the presence of genes encoded for carbapenem, extended spectrum beta-lactamases (ESBL), tetracycline and quinolones resistance, and virulence among 50 MDR E. coli isolated from human (n = 14), chicken (n = 12), rodent (n = 10), and soil (n = 14) samples using multiplex polymerase chain reaction (PCR). Overall, the antimicrobial resistance genes (ARGs) detected were: blaTEM 23/50 (46%), blaCTX-M 13/50 (26%), tetA 23/50 (46%), tetB 7/50 (14%), qnrA 12/50 (24%), qnrB 4/50 (8%), blaOXA-48 6/50 (12%), and blaKPC 3/50 (6%), while blaIMP, blaVIM, and blaNDM-1 were not found. The virulence genes (VGs) found were: ompA 36/50 (72%), traT 13/50 (26%), east 9/50 (18%), bfp 5/50 (10%), eae 1/50 (2%), and stx-1 2/50 (4%), while hlyA and cnf genes were not detected. Resistance (blaTEM, blaCTX-M, blaSHV, tetA, tetB, and qnrA) and virulence (traT) genes were found in all sample sources while stx-1 and eae were only found in chicken and rodent isolates, respectively. Tetracycline resistance phenotypes correlated with genotypes tetA (r = 0.94), tetB (r = 0.90), blaKPC (r = 0.90; blaOXA-48 (r = 0.89), and qnrA (r = 0.96). ESBL resistance was correlated with genotypes blaKPC (r = 0.93), blaOXA-48 (r = 0.90), and qnrA (r = 0.96) resistance. Positive correlations were observed between resistance and virulence genes: qnrB and bfp (r = 0.63) also blaTEM, and traT (r = 0.51). Principal component analysis (PCA) indicated that tetA, tetB, blaTEM, blaCTX-M, qnrA, and qnrB genes contributed to tetracycline, cefotaxime, and quinolone resistance, respectively. While traT stx-1, bfp, ompA, east, and eae genes contributed to virulence of MDR E. coli isolates. The PCA ellipses show that isolates from rodents had more ARGs and virulence genes compared to those isolated from chicken, soil, and humans.
Keywords: multidrug-resistant, rodents, chicken, humans, soil, E. coli, PCR, genes
1. Introduction
Escherichia coli (E. coli) is a versatile bacterial pathogen that has the ability to cause various infections, most of which are difficult to treat [1,2]. In fact, this bacterium is listed by the World Health Organization (WHO) as one of the critical antimicrobial-resistant bacteria that can cause severe and often deadly infections such as bloodstream infections and pneumonia [3]. The pathogenicity of E. coli strains is enhanced by a variety of virulence and resistance genes [4,5]. E. coli strains producing extended-spectrum β-lactamases (ESBLs) and carbapenemases are potentially recognized pathogens that can resist most β-lactam antibiotics [6,7]. ESBLs are plasmid-mediated enzymes that hydrolyse β-lactam containing antimicrobial agents including penicillins, cephalosporins, and aztreonam. ESBLs are grouped into three main types: TEM, SHV, and CTX-M [8,9]. Carbapenemases are a major group of β-lactamases capable of hydrolysing penicillins, cephalosporins, monobactams, and carbapenems. They include β-lactamases of classes B (IMP and VIM), D (OXA-23 to -27), and A (IMI, KPC, NMC, and SME) [10,11]. Tetracycline resistance genes (tetA and tetB) coded for efflux pumps have been frequently detected in human and animal E. coli isolates [12]. The genes qnrA and qnrB are known to confer quinolone resistance in E. coli strains and spread horizontally through plasmids [13].
Important virulence factors of E. coli are encoded by several genes including: locus enterocyte effacement (LEE), intimin, bundle forming (bae, bfpA)) [14,15], Shiga toxins, adhesins (stx1, stx2, eaeA, ehxA, and bfpA) [15,16], heat-labile, heat stable, and colonization factors (elt, est) [14,16]. E. coli is a typical One Health pathogen, with the potential of resistomes spreading between humans, animals, and the environment, where such interactions exist [17]. In Tanzania, studies conducted in the Karatu ecosystem have revealed intense interactions between humans, rodents, and chicken, leading to frequent occurrence and recurrence of zoonotic infections [18,19,20]. Previous studies have suggested that the role of rodents in the transmission of multidrug-resistant (MDR) bacterial infections to humans and environmental contamination [21,22,23,24]. In a recent phenotypic study conducted in Karatu, we isolated E. coli strains from chickens, humans, rodents, and soils which showed high levels of resistance to cefotaxime (79.7%), imipenem (79.8%), and tetracycline (73.7%); 512 out of 650 (78.8%) were MDR [25].
We hypothesize that the intense interactions between chickens, humans, rodents, and soils may lead to the transfer of ARGs and VRGs among them. However, molecular characterization of ARGs and VGs was not conducted in the phenotypic study [25]. Knowledge of ARGs and VGs is important in understanding the pathogenicity and virulence of E. coli [26]. This study was conducted in Karatu, Northern Tanzania to provide insights of molecular epidemiology of ARGs and VGs occurring in E. coli isolated among chickens, humans, rodents, and soils in households. To our knowledge, this is the first study in Tanzania that has investigated the genotypic diversity of E. coli isolated among chicken, humans, rodents, and soils in households. Multiplex PCR [27] was used for detection of genes encoding for tetracycline resistance (tetA, tetB), ESBL (blaCTX-M, blaSHV, and blaTEM), metallo beta-lactamases (blaVIM, blaIMP, and blaNDM), and virulence genes bfp, east, hlyA, traT, eaeA, ompA, cnf, and stx-1. The working assumption is that MDR E. coli strains circulating in Karatu carry a variety of virulence genes capable of causing life-threatening infections that are difficult to treat.
2. Materials and Methods
2.1. Study Area
This study was conducted between June 2020 and March 2021 in the Karatu district in the northern zone of Tanzania, located between latitudes 3°10′ and 4°00′ S, and longitude 34°47′ and 35°56′ E. The district has a population of 230,166 people comprised of 117,769 men and 112,397 women with an average of five people per household [28]. Karatu has an altitude range from 1000 to 1900 m above sea level with two wet seasons annually (short rains between October and December and long rains from March to June).
2.2. Bacterial Isolates
A total of 50 MDR E. coli isolates from chicken cloaca swabs (12), human nasal swabs (14), rodents’ deep pharyngeal swabs (10), and household soil (14) samples, particularly those with higher phenotypic resistance to tetracycline, imipenem, and cefotaxime, were selected for genomic DNA extraction and further genomic analyses. All selected isolates were preserved in nutrient broth (TSB) with 50% glycerol (v/v) at −80 °C until DNA extraction. Isolates that were resistant to at least three different classes of antibiotics were considered as multidrug-resistant (MDR) [29].
2.3. DNA Extraction
The genomic DNA of all phenotypically MDR E. coli strains were extracted by using Zymo Research Fungal and Bacterial Genomic DNA MiniPrepTM kit (Zymo Research, Irvine, CA, USA), following the manufacturer’s instructions. The purity, quality, and quantity of DNA were determined using a nanodrop spectrophotometer (NanoDrop, Thermo Scientific, Ramsey, NJ, USA) and agarose gel electrophoresis. The extracted DNA samples were stored at −80 °C until when PCR analyses were performed.
2.4. Detection of Antimicrobial Resistance and Virulence Genes
Multiplex PCR [27] was used to detect the tetracycline (tetA and tetB), ESBL (blaCTX-M, blaSHV, and blaTEM), and Metallo beta-lactamases (blaVIM, blaIMP, and blaNDM) resistance and virulence (bfp, east, hlyA, traT, eae, ompA, cnf, and stx1) genes. Briefly, lyophilized primers (Macrogen, Amsterdam, The Netherlands) for target genes (in supplementary materials) were reconstituted using nuclease-free water to obtain 100 μM stock and 10 μM working solutions before storage at −20 °C. PCR was carried out in a total volume of 25 μL containing 12.5 μL of 1 X Taq PCR Master Mix (Bio Basic, Canada), 1 μL of the forward primer and 1 μL of the reverse primer, 3 μL of DNA template, and 7.5 μL nuclease-free water. Multiplex PCRs were conducted using amplification conditions indicated in Table 1. PCR products were separated by electrophoresis on 1.5% (w/v) agarose gel pre-stained with Gel Red (Merck, Darmstadt, Germany) at 120 Volts for 1 h, and visualized under UV light using a BioDoc-itTM imaging system (Ultra-Violet Products, Cambridge, UK). PCR product size was determined by conducting electrophoresis along with a GeneRuler 100 bp Plus DNA Ladder (Bioneer, Daedeok-gu, Republic of Korea). DNA from E. coli American Type Culture Collection (ATCC) 29522 strain was used for quality assurance.
Table 1.
Detection of virulence genes of MDR E. coli isolates from different sample sources.
| Genes | Different Sample Sources n (%) | ||||
|---|---|---|---|---|---|
| Humans (n = 14) |
Chickens (n = 12) | Rodents (n = 10) |
Soil (n = 14) |
Total (n = 50) |
|
| Bfp | 0 (0.0) | 0 (0.0) | 3 (30.0) | 2 (14.3) | 5 (10.0) |
| East | 0 (0.0) | 4 (33.3) | 3 (30.0) | 2 (14.3) | 9 (18.0) |
| hlyA | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| traT | 4 (28.6) | 4 (33.3) | 4 (40.0) | 1 (7.1) | 13 (26.0) |
| eae | 0 (0.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 1 (2.0) |
| ompA | 10 (71.4) | 12 (100.0) | 7 (70.0) | 7 (50.0) | 36 (72.0) |
| cnf | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| stx-1 | 0 (0.0) | 1 (8.3) | 1 (10.0) | 0 (0.0) | 2 (4.0) |
| Total | 2 (14.3) | 4 (33.3) | 6 (60.0) | 4 (33.3) | 16 (32.0) |
| χ2-square | 52.29 | 46.43 | 2.00 | 26.67 | |
| p-value | 0.001 | 0.001 | 0.0188 | 0.0004 | |
2.5. Statistical Analysis
The data obtained were entered into an Excel spreadsheet (Microsoft® Office Excel 2010) and analysed. The differences in occurrence of the genes (%) between categories were compared by chi-square test using R-software, version 4.0.2 (R Foundation for Statistical computing, Vienna, Austria) [30]. Principal component analysis (PCA) was used to investigate the distribution and relationships of antimicrobial resistance and virulence genes of MDR E. coli isolates with respect to their different sample sources. Any p-value less than 0.05 was considered statistically significant.
3. Results
3.1. Carbapenems, ESBL, Tetracycline, and Quinolones Resistance Genes in MDR E. coli Isolates from Different Sample Sources
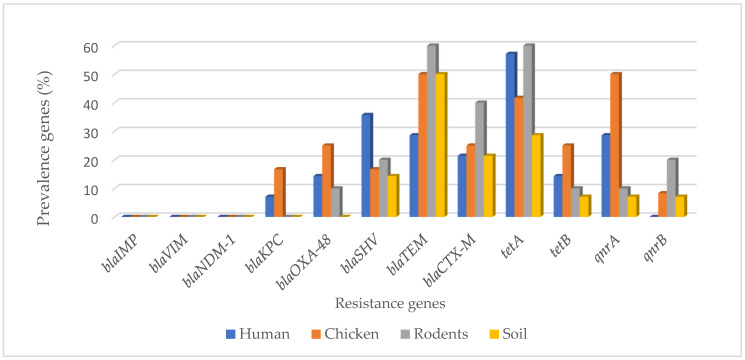
Overall, the resistance genes blaTEM (46%), blaCTX-M (26%), tetA (46%), tetB (14%), qnrA (24%), qnrB (8%), blaOXA-48 (12%), and blaKPC (6%) were detected (Figure 1) and distributed in isolates from human 8/14 (57.1%), chicken 9/12 (75.0%), rodent 8/10 (80.0%), and soil 7/14 (50.0%) samples, as shown in Table 2. For human isolates the most common ARGs were tetA 8/14 (57.1%) and blaSHV 5/14 (35.7%), while for chicken the most common ones were tetA 5/12 (41.7%) and qnrA 6/12 (50%), for rodents they were blaTEM 6/12 (50%), and tetA 6/12 (50%), and for soil they were blaTEM 7/14 (50%) and tetA 4/14 (28.6%).
Figure 1.
Occurrence of resistance genes in MDR E. coli isolates from different sample types.
Table 2.
Prevalence of antimicrobial resistance genes in MDR E. coli isolates from different sample types.
| Genes | Different Types of Sample Sources n (%) | ||||
|---|---|---|---|---|---|
| Human (n = 14) | Chicken (n = 12) | Rodents (n = 10) | Soil (n = 14) |
Total Isolates (n = 50) |
|
| blaIMP | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| blaVIM | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| blaNDM-1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| blaKPC | 1 (7.1) | 2 (16.7) | 0 (0.0) | 0 (0.0) | 3 (6.0) |
| blaOXA-48 | 2 (14.3) | 3 (25.0) | 1 (10.0) | 0 (0.0) | 6 (12.0) |
| blaSHV | 5 (35.7) | 2 (16.7) | 2 (20.0) | 2 (14.3) | 11 (22.0) |
| blaTEM | 4 (28.6) | 6 (50.0) | 6 (60.0) | 7 (50.0) | 23 (46.0) |
| blaCTX-M | 3 (21.4) | 3 (25.0) | 4 (40.0) | 3 (21.4) | 13 (26.0) |
| tetA | 8 (57.1) | 5 (41.7) | 6 (60.0) | 4 (28.6) | 23 (46.0) |
| tetB | 2 (14.3) | 3 (25.0) | 1 (10.0) | 1 (7.1) | 7 (14.0) |
| qnrA | 4 (28.6) | 6 (50.0) | 1 (10.0) | 1 (7.1) | 12 (24.0) |
| qnrB | 0 (0.0) | 1 (8.3) | 2 (20.0) | 1 (7.1) | 4 (8.0) |
| Total | 8 (57.1) | 9 (75.0) | 8 (80.0) | 7 (50.0%) | 32 (64.0) |
| χ2-square | 52.29 | 46.43 | 2.00 | 26.67 | |
| p-value | 0.001 | 0.001 | 0.0188 | 0.0004 | |
3.2. Detection of Virulence Genes in MDR E. coli Isolates from Different Sample Sources
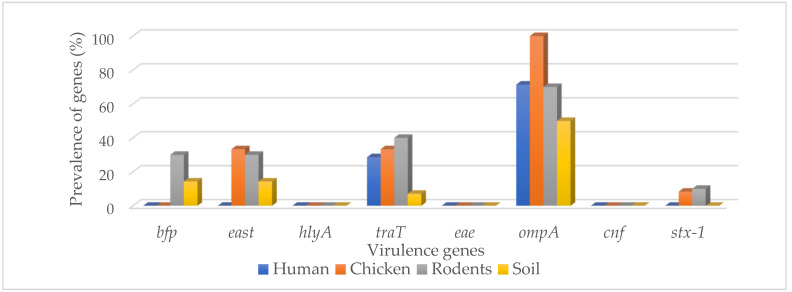
Overall, the virulence genes were: ompA (72%), traT (26%), east (18%), bfp (10%), eae (2%), and stx-1 (4%), while hlyA and cnf were not detected (Table 1). For humans the most common VRs were traT 4/14 (28.6%) and ompA 10/14 (71.4%), while for chicken they were traT 4/12 (33.3%) and ompA 12/12 (100%), for rodents they were traT 4/10 (40%) and ompA 7/10 (70%), and for soil isolates they were predominated by ompA 7/14 (50%) and east 2/14 (14.3) (Figure 2).
Figure 2.
Prevalence of virulence genes in different types of the sample source.
3.3. Comparison between Phenotypic and Genotypic Antibiotic Resistance
We found positive correlations between tetracycline resistance and tetA (0.94), tetB (=0.90), carbapenem resistance and blaKPC (0.90) and blaOXA-48 (0.89), and quinolone resistance and qnrA (0.96). We also found correlation between tetracycline resistance and genotypes for carbapenem (blaKPC = 0.90, blaOXA-48 = 0.91), cefotaxime and qnrA (0.96), and quinolone resistance and qnrA (0.94). Cefotaxime resistance was correlated with genotypes for carbapenem (blaKPC = 0.93, blaOXA-48 = 0.90) and quinolone (qnrA = 0.96) resistance (Table 3). However, we found weak and negative correlation between phenotypes and genotypes for ESBL resistance (CTX-M = 0.60, blaTEM = −0.63 and blaSHV = 0.33) (Table 3).
Table 3.
Correlation between phenotypes and genotypes of MDR E. coli isolates.
| Genotypes of Isolates | Phenotypic Resistance of Isolates | |||
|---|---|---|---|---|
| Correlation Coefficients (r) | ||||
| Tetracycline | Imipenem | Ciprofloxacin | Cefotaxime | |
| tetA | 0.53 | 0.51 | 0.62 | 0.43 |
| tetB | 0.90 | 0.90 | 0.86 | 0.93 |
| blaKPC | 0.90 | 0.90 | 0.86 | 0.93 |
| blaOXA-48 | 0.91 | 0.89 | 0.90 | 0.90 |
| qnrA | 0.94 | 0.94 | 0.90 | 0.96 |
| qnrB | −0.69 | −0.71 | −0.67 | −0.69 |
| blaCTX-M | −0.54 | −0.58 | −0.45 | −0.61 |
| blaTEM | −0.71 | −0.69 | −0.78 | −0.63 |
| blaSHV | 0.43 | 0.41 | 0.51 | 0.33 |
As shown in Table 4, we found correlations between qnrB and bfp genes (r = 0.63) and with blaTEM and traT genes (r = 0.51) and the remaining displayed weak and negative correlations.
Table 4.
Correlation between resistance and virulence genes of MDR E. coli isolates.
| ABR Genes | Virulence Genes | |||||
|---|---|---|---|---|---|---|
| Correlation Coefficients (r) | ||||||
| bfp | east | traT | eae | ompA | stx-1 | |
| blaKPC | −0.11 | 0.30 | 0.41 | −0.05 | 0.11 | −0.07 |
| blaOXA-48 | −0.16 | −0.06 | 0.15 | 0.38 | −0.05 | 0.22 |
| blaSHV | −0.24 | −0.34 | −0.44 | −0.10 | 0.24 | −0.15 |
| blaTEM | 0.39 | 0.44 | 0.51 | 0.17 | −0.39 | 0.01 |
| blaCTX-M | 0.05 | 0.39 | 0.31 | 0.23 | −0.22 | 0.08 |
| tetA | 0.36 | −0.10 | 0.11 | 0.15 | 0.26 | 0.22 |
| tetB | −0.18 | 0.06 | −0.05 | −0.08 | 0.18 | −0.11 |
| qnrA | −0.26 | 0.03 | 0.00 | −0.11 | 0.09 | 0.10 |
| qnrB | 0.63 | −0.19 | 0.12 | −0.05 | 0.13 | 0.30 |
3.4. Co-Occurrence between Resistance and Virulence Genes
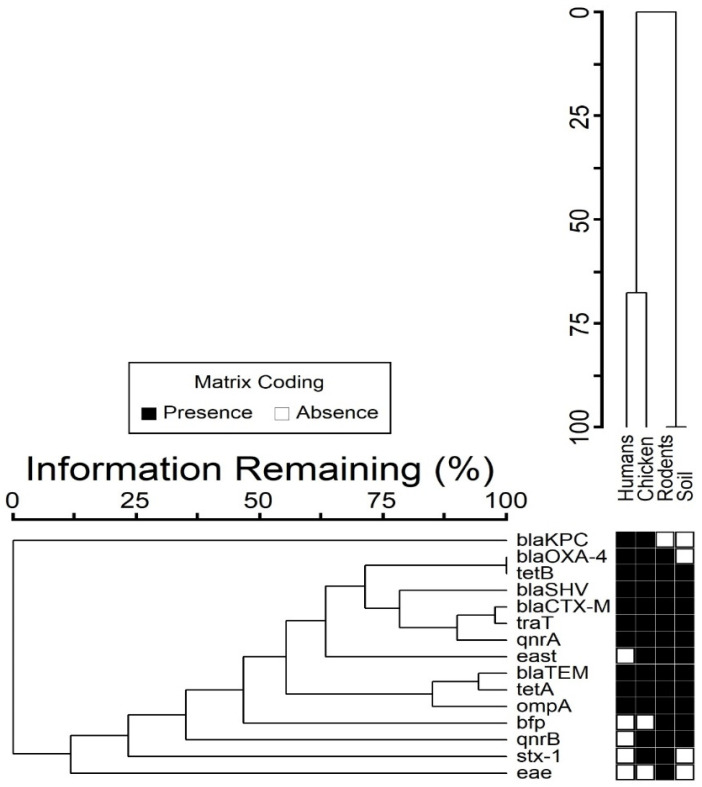
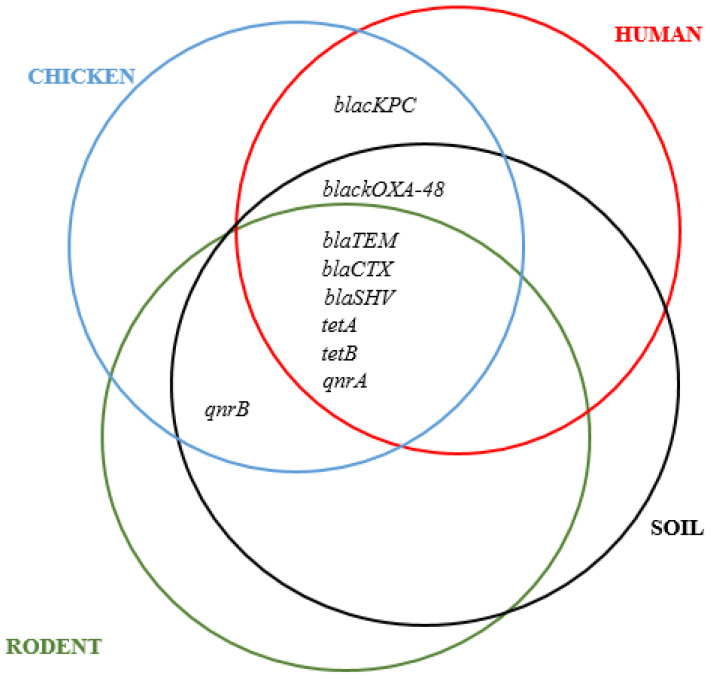
We observed that 38 out of 50 (76%) MDR E. coli isolates had at least one virulence gene. A co-existence of up to six resistance genes and at least one virulence gene was noted. In some cases, four resistance genes co-existed with four virulence genes (Figure 3). The combination consisting of blaTEM, blaCTX-M, tetA, ompA, and traT genes was common with 55% co-occurrence (Figure 4 and Figure 5).
Figure 3.
Co-occurrence of resistance and virulence genes in isolates from different sample sources.
Figure 4.
Distribution of resistance genes in various sample sources.
Figure 5.
Distribution of virulence genes in various sample sources.
3.5. Principal Component Analysis Results
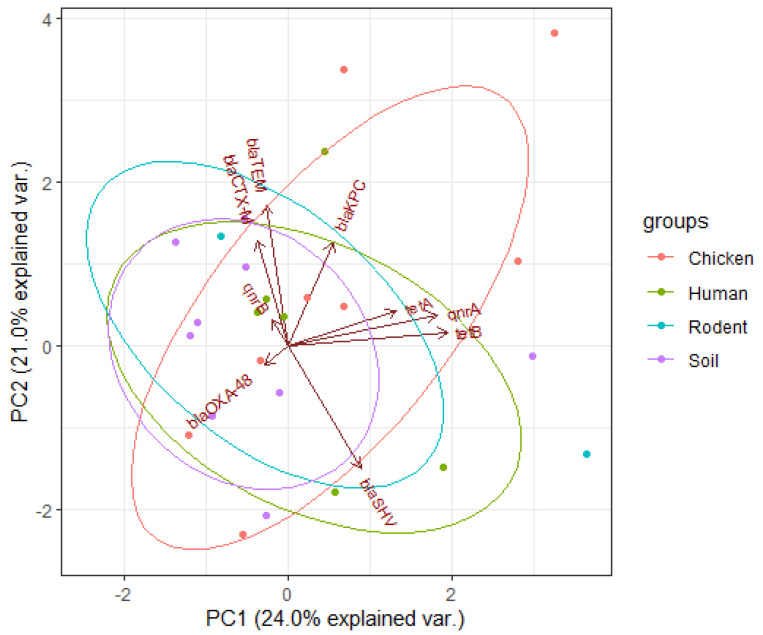
According to Figure 6 below, the arrows (vectors) for tetA, qnrA, and tetB genes aligned closer to each other in principal component 1 (PC1) indicating greater and positive correlations among them. The lengths of arrows show that tetB gene contributed more to the resistance of isolates followed by qnrB and tetA genes. The vectors for blaTEM, blaCTX-M, and qnrB genes are close to each other and to PC2 showing their influence on resistance. These genes had greater and positive correlations between them, but all were negatively correlated to the blaSHV gene. The lengths of the vectors indicate that the blaTEM gene had a higher influence on resistance of isolates (PC2), followed by the blaCTX-M while qnrB had the lowest. According to PCA plane, rodent and chicken ellipses are extended in the upper quadrants indicating higher proportions of ARGs, followed by those from human and soil.
Figure 6.
Principal component analysis of resistance genes of E. coli isolates. The dots represent isolates from different sources of samples, arrows indicate the original variables (resistance genes of the isolates), and ellipses indicate a region that contains 95% of all samples of a particular source.
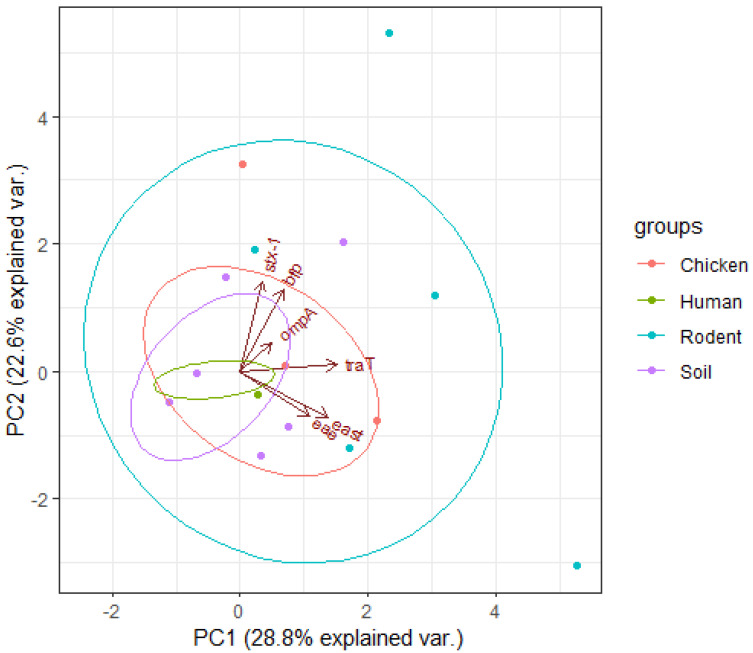
The smaller angle between traT gene vector and PC1 indicates a greater and positive correlation between them (Figure 7). The same behaviour was displayed by east and eae genes which show a greater and positive correlation between them. Along PC2, stx-1, bfp and ompA genes had greater and positive correlations with PC2 indicating higher influence on virulence of isolates. The different sizes of loadings indicated higher and positive correlations between them. Different sizes of ellipses indicate variation in the prevalence of virulence genes across different sources of isolates. Rodent isolates had more virulence genes followed by chicken and soil isolates, while those from humans had the lowest gene prevalence.
Figure 7.
Principal component analysis for virulence genes of E. coli isolates. The dots represent isolates from different sources of samples, arrows indicate the original variables (virulence genes of the isolates), and ellipses indicate a region that contains 95% of all samples of a particular source.
4. Discussion
The study found 32/50 (64%) of MDR E. coli isolates carrying at least one AMR gene, with 10/50 (20%) having more than three. At the same time, 38 out of 50 (76%) MDR E. coli isolates had at least one virulence gene and 8/50 (16%) had more than three. PCA results showed that most of the resistance and virulence genes were found in isolates from rodents and chicken samples compared with human and soil isolates (Figure 6 and Figure 7). The most detected AMR genes included: tetA (46%), blaTEM (46%), blaCTX-M (26%), qnrA (24%), blaSHV (22%), tetB (8%), and blaOXA-48 (12%). This finding is in agreement with the results of our previous study in Karatu that reported higher resistance of E. coli to tetracycline (73.7%), imipenem (79.8%), and cefotaxime (79.7%) where 512 out of 650 (78.8%) isolates were multidrug-resistant [25]. Interestingly, the highest prevalence of AMR genes was observed in isolates from rodent (80.0%) followed by those from chicken (75.0%), human (57.1%), and lastly soil (50.0%) samples. Our findings imply that rodents that invade households have a potential to spread MDR E. coli infections with ARGs to other hosts, as observed by others [31,32,33]. The increased prevalence of resistance genes in isolates from chicken can be associated with frequent use and misuse of antibiotics in the prevention and treatment of poultry diseases, which is a common practice in the area as reported by previous studies [26,34,35]. The high prevalence of ESBL genes; blaSHV (20%), blaCTX-M (40%), blaTEM (60%), tetracycline; tetA (60%), and quinolone; qnrB (20%) resistance genes indicate the widespread of MDR E. coli infections in the Karatu district. This keeps with findings of a study conducted in nearby Arusha that found blaTEM, blaCTX-M, tetA, tetB, and qnrs [34,35,36]. This pattern can be explained by the frequent use and misuse of these antibiotics in veterinary and human medicines in the area [35], rendering these groups of antibiotics to be less effective. We found strong and positive correlations between tetracycline resistance and tetA (r = 0.94) and tetB (r = 0.90), carbapenem resistance and blaKPC (r = 0.94), as well as blaOXA-48 (r = 0.89) and quinolone resistance with qnrA (r = 0.96), highlighting the dominant role of genes in causing resistance [37,38,39]. Similarly, we found strong and positive correlation between tetracycline resistance phenotypes and genotypes for carbapenem (blaKPC = 0.90, blaOXA-48 = 0.91), quinolone (qnrA = 0.94), as well as ESBL and carbapenem (blaKPC = 0.93, blaOXA-48 = 0.90) and quinolone (qnrA = 0.96) resistance genotypes. Such associations have been reported in previous studies [40,41] and can be explained by the fact that most of these genes are carried on similar transferrable plasmids [42,43].
Overall, the detected virulence genes were: bfp 5/50 (10%), east 9/50 (18%), traT 13/50 (26%), eae 1/50 (2%), ompA 36/50 (72%), and stx-1 2/50 (4%). For isolates obtained from human samples, the most common virulence genes were: traT (28.6%) and ompA (71.4%), for chickens ompA (100%), traT (33.3%), east (33.3%), and stx1 (8.3%) for rodents ompA (70%), eae (10%), traT (40%), east (30%), bfp (30%), and stx1 (10%). Isolates from soil samples contained bfp (14.3%), east (14.3%), traT (7.1%), and ompA (50%). The bundle forming pilus (bfp) gene codes for adherence of E. coli strains to intestinal epithelial cells of the host [44], while eae gene promotes secretion of intimin protein for bacterial adherence to enterocytes [45]. The gene stx-1 encodes production of the Shiga toxin (stx) protein in some E. coli strains responsible for haemolytic uremic syndrome (HUS) and bloody diarrhoea in humans [45,46]. The gene east codes for production of heat-stable enterotoxin 1 in Enteroaggregative E. coli (EAST1) which induces diarrhoea in humans and livestock [47]. The gene ompA codes for outer membrane protein A, which enables intracellular survival of E. coli strains and protects them against host defence mechanism [48]. Meanwhile the traT gene codes for outer membrane protein, an important factor during urethral tract infections in humans [49]. The presence of wide-ranging virulence factors indicates that the MDR E. coli isolates circulating in Karatu have the ability to cause life-threatening infections that can be difficult to treat, given the fact that they occur in antibiotic-resistant isolates. We noted some significant differences with other studies. In this study, the prevalence of ompA in rodent isolates (70%) was lower than 93.5% reported in China by Guan et al. [50]. The 50% occurrence of ompA in E. coli from soil samples was greater than 42% documented in Indiana, USA [51]. However, we did not detect stx1, eae, and hlyA genes contrary to Cooley et al. [52] who reported stx1 (100%), eae (100%), and hlyA (40%) in soil, livestock, wild birds, and water samples, respectively. Interestingly, we found a higher prevalence of virulence genes (60%) among E. coli isolates from rodent samples compared to previous studies in Berlin (0%) [21], in Hanoi (1.7%) [53], and in Vancouver (3.8%) [54]. These geographical related differences can be attributed to variations in levels of antibiotics use as well as environmental factors [55]. In this study, we found co-occurrence of resistance and virulence genes in 38/50 (76%) of the isolates. The most common combinations were: blaSHV, tetA, and ompA in humans; blaTEM, tetA, tetB, qnrA, and ompA in chicken; blaTEM, blaCTX-M, tetA, and ompA in rodents; and blaTEM, tetA, and ompA in soil isolates. Importantly, we found varying correlation between ARGs and VGs among the isolates. We found positive correlation between blaTEM and traT genes (r = 0.51) and qnrB and bfp genes (r = 0.63), while negative correlations were revealed between blaOXA-48 and ompA (r = −0.05), blaSHV and traT (r = −0.44), and tetA and east (r = −0.10). This finding is keeping with those of other studies, showing that acquisition of resistance to certain antimicrobial agents may be associated with an increase or decrease in the virulence levels of a microorganism. This result seems to indicate that acquisition of resistance to certain antibiotics may be associated with an increase or decrease in the virulence levels depending on location and mechanism of transfer of specific genes [27,56,57].
5. Conclusions
Our study revealed that MDR E. coli isolates from humans, chicken, rodents, and household soils harbour different ARGs (blaTEM, blaCTX-M, blaSHV, tetA, tetB, qnrA, and qnrB) and VGs (bfp, east, traT, ompA and stx-1). The PCA results show that traT, stx-1, bfp, ompA, east, and eae genes influenced the virulence of MDR E. coli isolates. Resistance (blaTEM, blaCTX-M, blaSHV, tetA, tetB, and qnrA) and virulence (traT) genes were detected in isolates from all sample sources, while stx-1 and eae genes were specific to chicken and rodent isolates only. Interestingly, rodents had the highest percentage of both ARGs and VGs, indicating their potential in carriage and transmission of infections to other hosts in the environment. This situation urgently calls for One Health-based interventions including improving hygiene and control of rodents in households.
Acknowledgments
We strongly appreciate the good cooperation from all participants, district medical office, and the livestock department during our study in the Karatu district. We also acknowledge for the field technical assistance from Dismas Joseph and Ramadani Kigunguli and laboratory technical assistance from Lawrence Mdimi and Mariam Makange.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph19095388/s1, Table S1. List of primers used for amplification of selected antimicrobial resistance and virulence genes of E. coli isolates. Table S2. Multiplex PCR conditions used during amplification of antibiotic resistance and virulence genes of MDR E. coli isolates.
Author Contributions
Collection of samples, laboratory processing, and data analysis V.S.S., supervision and verification of the analytical methods M.I.M. and G.M. Manuscript developed by V.S.S. with input from M.I.M., G.M. and A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The ethical clearance for the study was issued by the National Institute for Medical Research (NIMR) of Tanzania (NIMR/HQ/R.8a/Vol.IX/3386). NIMR is the national institutional review board that ensures all health research follows the national health research ethics requirements for research involving human subjects. Sokoine University of Agriculture Institutional Animal Care and Use Committee (IACUC) approved the use of animals in this study. The permission to work in the study area was sought from the Regional Administrative Office (Arusha).
Informed Consent Statement
Informed verbal consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was funded by the Government of Tanzania and the World bank through the Africa Centre of Excellence for Innovative Rodent Pest Management and Biosensor Technology Development (IRPM & BTD) at Sokoine University of Agriculture.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Song Y., Yu L., Zhang Y., Dai Y., Wang P., Feng C., Liu M., Sun S., Xie Z., Wang F. Prevalence and characteristics of multidrug-resistant mcr-1-positive Escherichia coli isolates from broiler chickens in Tai’an, China. Poult. Sci. 2020;99:1117–1123. doi: 10.1016/j.psj.2019.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim Y.B., Yoon M.Y., Ha J.S., Seo K.W., Noh E.B., Son S.H., Lee Y.J. Molecular characterization of avian pathogenic Escherichia coli from broiler chickens with colibacillosis. Poult. Sci. 2020;99:1088–1095. doi: 10.1016/j.psj.2019.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shrivastava S.R., Shrivastava P.S., Ramasamy J. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018;32:76–77. doi: 10.4103/jms.jms_25_17. [DOI] [Google Scholar]
- 4.Sarowska J., Futoma-Koloch B., Jama-Kmiecik A., Frej-Madrzak M., Ksiazczyk M., Bugla-Ploskonska G., Choroszy-Krol I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019;11:10. doi: 10.1186/s13099-019-0290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abd El-Baky R.M., Ibrahim R.A., Mohamed D.S., Ahmed E.F., Hashem Z.S. Prevalence of virulence genes and their association with antimicrobial resistance among pathogenic E. coli isolated from Egyptian patients with different clinical infections. Infect. Drug. Resist. 2020;13:1221–1236. doi: 10.2147/IDR.S241073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tooke C.L., Hinchliffe P., Bragginton E.C., Colenso C.K., Hirvonen V.H., Takebayashi Y., Spencer J. β-Lactamases and β-Lactamase Inhibitors in the 21st Century. J. Mol. Biol. 2019;431:3472–3500. doi: 10.1016/j.jmb.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masoud S.M., El-Baky A., Mahmoud R., Aly S.A., Ibrahem R.A. Co-Existence of Certain ESBLs, MBLs and Plasmid Mediated Quinolone Resistance Genes among MDR E. coli Isolated from Different Clinical Specimens in Egypt. Antibiotics. 2021;10:835. doi: 10.3390/antibiotics10070835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim M.E., Bilal N.E., Magzoub M.A., Hamid M.E. Prevalence of extended-spectrum β-lactamases-producing Escherichia coli from Hospitals in Khartoum State, Sudan. Oman Med. J. 2013;28:116–120. doi: 10.5001/omj.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yano H., Uemura M., Endo S., Kanamori H., Inomata S., Kakuta R., Ichimura S., Ogawa M., Shimojima M., Ishibashi N. Molecular characteristics of extended-spectrum β-lactamases in clinical isolates from Escherichia coli at a Japanese tertiary hospital. PLoS ONE. 2013;8:e64359. doi: 10.1371/journal.pone.0064359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Queenan A.M., Bush K. Carbapenemases: The versatile β-lactamases. Clin. Microbiol. Rev. 2007;20:440–458. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Livermore D.M. The impact of carbapenemases on antimicrobial development and therapy. Curr. Opin. Investig. Drugs (Lond. Engl. 2000) 2002;3:218–224. [PubMed] [Google Scholar]
- 12.Bryan A., Shapir N., Sadowsky M.J. Frequency and distribution of tetracycline resistance genes in genetically diverse, nonselected, and nonclinical Escherichia coli strains isolated from diverse human and animal sources. Appl. Environ. Microbiol. 2004;70:2503–2507. doi: 10.1128/AEM.70.4.2503-2507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poirel L., Cattoir V., Nordmann P. Plasmid-mediated quinolone resistance; interactions between human, animal, and environmental ecologies. Front. Microbiol. 2012;3:24. doi: 10.3389/fmicb.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allocati N., Masulli M., Alexeyev M.F., Di Ilio C. Escherichia coli in Europe: An overview. Int. J. Environ. Res. Public Health. 2013;10:6235–6254. doi: 10.3390/ijerph10126235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robins-Browne R.M., Holt K.E., Ingle D.J., Hocking D.M., Yang J., Tauschek M. Are Escherichia coli pathotypes still relevant in the era of whole-genome sequencing? Front. Cell. Infect. Microbiol. 2016;6:141. doi: 10.3389/fcimb.2016.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomes T.A., Elias W.P., Scaletsky I.C., Guth B.E., Rodrigues J.F., Piazza R.M., Ferreira L., Martinez M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016;47:3–30. doi: 10.1016/j.bjm.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White A., Hughes J.M. Critical importance of a one health approach to antimicrobial resistance. EcoHealth. 2019;16:404–409. doi: 10.1007/s10393-019-01415-5. [DOI] [PubMed] [Google Scholar]
- 18.Kilonzo B., Mbise T., Mwalimu D., Kindamba L. Observations on the endemicity of plague in Karatu and Ngorongoro, northern Tanzania. Tanzan. J. Health Res. 2006;8:1–6. doi: 10.4314/thrb.v8i1.14262. [DOI] [PubMed] [Google Scholar]
- 19.Makundi R.H., Massawe A.W., Mulungu L.S., Katakweba A., Mbise T.J., Mgode G. Potential mammalian reservoirs in a bubonic plague outbreak focus in Mbulu District, northern Tanzania, in 2007. [(accessed on 2 February 2022)]. Available online: https://www.degruyter.com/document/doi/10.1515/MAMM.2008.038/pdf.
- 20.Ziwa M.H., Matee M.I., Hangombe B.M., Lyamuya E.F., Kilonzo B.S. Plague in Tanzania: An overview. Tanzan. J. Health Res. 2013;15:4. doi: 10.4314/thrb.v15i4.7. [DOI] [PubMed] [Google Scholar]
- 21.Guenther S., Bethe A., Fruth A., Semmler T., Ulrich R.G., Wieler L.H., Ewers C. Frequent combination of antimicrobial multiresistance and extraintestinal pathogenicity in Escherichia coli isolates from urban rats (Rattus norvegicus) in Berlin, Germany. PLoS ONE. 2012;7:e50331. doi: 10.1371/journal.pone.0050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feng A.Y., Himsworth C.G. The secret life of the city rat: A review of the ecology of urban Norway and black rats (Rattus norvegicus and Rattus rattus) Urban Ecosyst. 2014;17:149–162. doi: 10.1007/s11252-013-0305-4. [DOI] [Google Scholar]
- 23.Hassell J.M., Ward M.J., Muloi D., Bettridge J.M., Robinson T.P., Kariuki S., Ogendo A., Kiiru J., Imboma T., Kangethe E.K. Clinically relevant antimicrobial resistance at the wildlife–livestock–human interface in Nairobi: An epidemiological study. Lancet Planet. Health. 2019;3:e259–e269. doi: 10.1016/S2542-5196(19)30083-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desvars-Larrive A., Ruppitsch W., Lepuschitz S., Szostak M.P., Spergser J., Feßler A.T., Schwarz S., Monecke S., Ehricht R., Walzer C. Urban brown rats (Rattus norvegicus) as possible source of multidrug-resistant Enterobacteriaceae and meticillin-resistant Staphylococcus spp., Vienna, Austria, 2016 and 2017. Eurosurveillance. 2019;24:1900149. doi: 10.2807/1560-7917.ES.2019.24.32.1900149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonola V.S., Katakweba A.S., Misinzo G., Matee M.I. Occurrence of Multi-Drug-Resistant Escherichia coli in Chickens, Humans, Rodents and Household Soil in Karatu, Northern Tanzania. Antibiotics. 2021;10:1137. doi: 10.3390/antibiotics10091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cepas V., Soto S.M. Relationship between Virulence and Resistance among Gram-Negative Bacteria. Antibiotics. 2020;9:719. doi: 10.3390/antibiotics9100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao M., Zhang X., Gao Y., Song S., Xu D., Yan L. Development and application of multiplex PCR method for simultaneous detection of seven viruses in ducks. BMC Vet. Res. 2019;15:103. doi: 10.1186/s12917-019-1820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.United Republic of Tanzania, 2012 Population and Housing Census-Population Distribution by Administrative Areas. [(accessed on 2 February 2022)]. Available online: http://tanzania.countrystat.org/fileadmin/user_upload/countrystat_fenix/congo/docs/Census%20General%20Report-2012PHC.pdf.
- 29.Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M., Giske C., Harbarth S., Hindler J., Kahlmeter G., Olsson-Liljequist B. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 30.R: A Language and Environment for Statistical Computing. [(accessed on 2 February 2022)]. Available online: https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006.
- 31.Gakuya F., Kyule M., Gathura P., Kariuki S. Antimicrobial susceptibility and plasmids from Escherichia coli isolated from rats. East Afr. Med. J. 2001;78:518–522. doi: 10.4314/eamj.v78i10.8960. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Sangwan N., Li H.Y., Su J.Q., Oyang W.Y., Zhang Z.J., Gilbert J.A., Zhu Y.G., Ping F., Zhang H.L. The antibiotic resistome of swine manure is significantly altered by association with the Musca domestica larvae gut microbiome. ISME J. 2017;11:100–111. doi: 10.1038/ismej.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poirel L., Madec J.-Y., Lupo A., Schink A.-K., Kieffer N., Nordmann P., Schwarz S. Antimicrobial resistance in Escherichia coli. Microbiol. Spectr. 2018;6:4. doi: 10.1128/microbiolspec.ARBA-0026-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comparison of Antibiotic Resistant Escherichia coli Obtained from Drinking Water Sources in Northern Tanzania: A Cross-Sectional Study. [(accessed on 2 February 2022)]. Available online: https://www.researchgate.net/publication/309765672_Comparison_of_antibiotic_resistant_Escherichia_coli_obtained_from_drinking_water_sources_in_northern_Tanzania_a_cross-sectional_study. [DOI] [PMC free article] [PubMed]
- 35.Antimicrobial Resistance Profiles of Escherichia coli Isolated from Broiler and Layer Chickens in Arusha and Mwanza, Tanzania. [(accessed on 2 February 2022)]. Available online: https://www.hindawi.com/journals/ijmicro/2021/6759046/ [DOI] [PMC free article] [PubMed]
- 36.Sale of Fluoroquinolones in Northern Tanzania: A Potential Threat for Fluoroquinolone Use in Tuberculosis Treatment. [(accessed on 2 February 2022)]. Available online: https://academic.oup.com/jac/article/65/1/145/727526?login=false#. [DOI] [PubMed]
- 37.Comparison of the Prevalence of Antibiotic-Resistant Escherichia coli Isolates from Commercial-Layer and Free-Range Chickens in Arusha District, Tanzania. [(accessed on 2 February 2022)]. Available online: https://www.researchgate.net/publication/308594993_Comparison_of_the_prevalence_of_antibiotic-resistant_Escherichia_coli_isolates_from_commercial-layer_and_free-range_chickens_in_Arusha_district_Tanzania.
- 38.Subbiah M., Caudell M.A., Mair C., Davis M.A., Matthews L., Quinlan R.J., Quinlan M.B., Lyimo B., Buza J., Keyyu J. Antimicrobial resistant enteric bacteria are widely distributed amongst people, animals and the environment in Tanzania. Nat. Commun. 2020;11:228. doi: 10.1038/s41467-019-13995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genetic Relatedness of Multidrug Resistant Escherichia coli Isolated from Humans, Chickens and Poultry Environments. [(accessed on 2 February 2022)]. Available online: https://aricjournal.biomedcentral.com/articles/10.1186/s13756-021-00930-x. [DOI] [PMC free article] [PubMed]
- 40.Mahmoud N.E., Altayb H.N., Gurashi R.M. Detection of Carbapenem-Resistant Genes in Escherichia coli Isolated from Drinking Water in Khartoum, Sudan. [(accessed on 2 February 2022)]; doi: 10.1155/2020/2571293. Available online: https://pubmed.ncbi.nlm.nih.gov/32612664/ [DOI] [PMC free article] [PubMed]
- 41.Schages L., Wichern F., Kalscheuer R., Bockmühl D. Winter is coming–Impact of temperature on the variation of beta-lactamase and mcr genes in a wastewater treatment plant. Sci. Total Environ. 2020;712:136499. doi: 10.1016/j.scitotenv.2020.136499. [DOI] [PubMed] [Google Scholar]
- 42.Gutkind G.O., Di Conza J., Power P., Radice M. β-Lactamase-mediated resistance: A biochemical, epidemiological and genetic overview. Curr. Pharm. Des. 2013;19:164–208. doi: 10.2174/138161213804070320. [DOI] [PubMed] [Google Scholar]
- 43.Evans B.A., Amyes S.G. OXA β-lactamases. Clin. Microbiol. Rev. 2014;27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamilton M.J., Hadi A.Z., Griffith J.F., Ishii S., Sadowsky M.J. Large scale analysis of virulence genes in Escherichia coli strains isolated from Avalon Bay, CA. Water Res. 2010;44:5463–5473. doi: 10.1016/j.watres.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel J., Yin H.B., Bauchan G., Mowery J. Inhibition of Escherichia coli O157: H7 and Salmonella enterica virulence factors by benzyl isothiocyanate. Food Microbiol. 2020;86:103303. doi: 10.1016/j.fm.2019.103303. [DOI] [PubMed] [Google Scholar]
- 46.Pan Z., Chen Y., McAllister T.A., Gänzle M., Plastow G., Guan L.L. Abundance and Expression of Shiga Toxin Genes in Escherichia coli at the Recto-Anal Junction Relates to Host Immune Genes. Front. Cell. Infect. Microbiol. 2021;11:148. doi: 10.3389/fcimb.2021.633573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ménard L.-P., Dubreuil J.D. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 (EAST1): A new toxin with an old twist. Crit. Rev. Microbiol. 2002;28:43–60. doi: 10.1080/1040-840291046687. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y., Kim K.S. Role of OmpA and IbeB in Escherichia coli K1 invasion of brain microvascular endothelial cells in vitro and in vivo. Pediatr. Res. 2002;51:559–563. doi: 10.1203/00006450-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 49.Firoozeh F., Saffari M., Neamati F., Zibaei M. Detection of virulence genes in Escherichia coli isolated from patients with cystitis and pyelonephritis. Int. J. Infect. Dis. 2014;29:219–222. doi: 10.1016/j.ijid.2014.03.1393. [DOI] [PubMed] [Google Scholar]
- 50.Guan J., Liu S., Lin Z., Li W., Liu X., Chen D. Severe sepsis facilitates intestinal colonization by extended-spectrum-β-lactamase-producing Klebsiella pneumoniae and transfer of the SHV-18 resistance gene to Escherichia coli during antimicrobial treatment. Antimicrob. Agents Chemother. 2014;58:1039–1046. doi: 10.1128/AAC.01632-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duffitt A.D., Reber R.T., Whipple A., Chauret C. Gene expression during survival of Escherichia coli O157: H7 in soil and water. Int. J. Microbiol. 2011;2011:340506. doi: 10.1155/2011/340506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cooley M.B., Jay-Russell M., Atwill E.R., Carychao D., Nguyen K., Quiñones B., Patel R., Walker S., Swimley M., Pierre-Jerome E. Development of a robust method for isolation of Shiga toxin-positive Escherichia coli (STEC) from fecal, plant, soil and water samples from a leafy greens production region in California. PLoS ONE. 2013;8:e65716. doi: 10.1371/journal.pone.0065716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Le Huy H., Koizumi N., Ung T.T.H., Le T.T., Nguyen H.L.K., Hoang P.V.M., Nguyen C.N., Khong T.M., Hasebe F., Haga T., et al. Antibiotic-resistant Escherichia coli isolated from urban rodents in Hanoi, Vietnam. J. Vet. Med. Sci. 2020;82:653–660. doi: 10.1292/jvms.19-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Himsworth C.G., Zabek E., Desruisseau A., Parmley E.J., Reid-Smith R., Jardine C.M., Tang P., Patrick D.M. Prevalence and characteristics of Escherichia coli and Salmonella spp. in the feces of wild urban Norway and black rats (Rattus norvegicus and Rattus rattus) from an inner-city neighborhood of Vancouver, Canada. J. Wildl. Dis. 2015;51:589–600. doi: 10.7589/2014-09-242. [DOI] [PubMed] [Google Scholar]
- 55.Bengtsson-Palme J., Hammaren R., Pal C., Östman M., Björlenius B., Flach C.-F., Fick J., Kristiansson E., Tysklind M., Larsson D.J. Elucidating selection processes for antibiotic resistance in sewage treatment plants using metagenomics. Sci. Total Environ. 2016;572:697–712. doi: 10.1016/j.scitotenv.2016.06.228. [DOI] [PubMed] [Google Scholar]
- 56.Soto S., Zuniga S., Ulleryd P., Vila J. Acquisition of a pathogenicity island in an Escherichia coli clinical isolate causing febrile urinary tract infection. Eur. J. Clin. Microbiol. Infect. Dis. 2011;30:1543–1550. doi: 10.1007/s10096-011-1258-2. [DOI] [PubMed] [Google Scholar]
- 57.Hacker J., Blum-Oehler G., Mühldorfer I., Tschäpe H. Pathogenicity islands of virulent bacteria: Structure, function and impact on microbial evolution. Mol. Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.