Abstract
Backgrounds
As a regulator of cell cycle, cell division cycle‐associated 5 (CDCA5) is involved in the progression of various malignant tumors. However, the potential relationship between CDCA5 and lung cancer has not been reported.
Methods
In our study, we analyzed the expression of CDCA5 in a variety of malignant tumors, performed Kaplan‐Meier survival analysis of lung adenocarcinoma (LUAD), explored the potential relationship between CDCA5 expression and clinicopathological characteristics, assessed the predictive capability of at different stages of clinicopathological characteristics, revealed the enriched functions and signaling pathways among LUAD paitents with high CDCA5 expression, and investigated the correlation between PD‐1, PD‐L1, and CDCA5 through bioinformatics analyses. Subsequently, we performed quantitative real‐time reverse transcription‐polymerase chain reaction (qRT‐PCR) and western blotting (WB) to demonstrate that CDCA5 mediates the p53‐p21 pathway and regulates the cell cycle.
Result
CDCA5 is probably involved in the occurrence and development of NSCLC, and function as a reliable biomarker for predicting the survival outcomes of patients with early stage of patients with LUAD. Furthermore, CDCA5 may be a promising indicator of immunotherapy efficacy. In addition, silencing the expression of CDCA5 significantly increased the proportion of apoptotic NSCLC cells, and caused NSCLC cells to be arrested in the G1 phase.
Conslusion
In conclusion, CDCA5 regulated the cell cycle of NSCLC cells by mediating the p53‐p21 signaling pathway, participating in the development and progression of NSCLC patients.
Keywords: apoptosis, bioinformatics analyses, CDCA5, G1 phase arrest, non‐small cell lung cancer
Schematic diagram of CDCA5 regulating G1 arrest in NSCLC cells by mediating the p53‐p21 signaling pathway.
1. INTRODUCTION
Lung cancer is known as the malignant tumor with the highest morbidity and mortality worldwide. 1 The American Cancer Society predicted that the morbidity and mortality of lung cancer among men and women in the United States ranked second. 2 Moreover, according to Chinese official forecasts, lung cancer had become the malignant tumor with the highest morbidity and mortality in 2015. 3 It is estimated that approximately 85% of lung cancers are clinically NSCLC and approximately 60% of lung cancers are pathologically LUAD. 4 The existing clinical breakthrough of molecular targeted therapy and immunotherapy have dramatically changed treatment patterns and prognosis for LUAD patients, which have improved their prognosis and quality of life significantly. 5 , 6 However, the 5‐year survival rate for advanced NSCLC patients with distant metastasis is only 7%. 7 In view of the above, it is of clinical importance to explore the complex molecular mechanisms for improving the prognosis of NSCLC patients.
According to the data from Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer‐pku.cn/), in normal humans, CDCA5 is highly expressed in blood and male testis, while lower in other organs and tissues. 8 The information from Genecards (https://www.genecards.org/) indicates that CDCA5 is an encoding gene encoding Sororin, and is closely related to Cornelia De Lange syndrome and Roberts‐Sc Phocomalia syndrome. 9 The cohesion of sister chromatids in mitosis ensures the precise separation of chromosomes in meiotic and mitotic cells, and researchers have revealed that Sororin functions as a stabilizer of cohesion and plays a critical role in cell division. 10 , 11 In addition, Sororin is involved in double‐strand break repair, mitotic cell cycle, and positive regulation of exit from mitosis. 10 , 12 In summary, the Sororin encoded by CDCA5 is a cell cycle regulator that regulates cell division, and participates in a variety of biological activities.
There is now growing evidence that CDCA5 is overexpressed as an oncogene in a variety of common malignancies and has been proven to play a crucial part in tumorigenesis and development, including NSCLC, 13 hepatocellular carcinoma, 14 , 15 prostate cancer, 16 , 17 gastric cancer, 18 , 19 ovarian cancer, 20 nasopharyngeal carcinoma, 21 bladder cancer, 22 esophageal squamous cell carcinoma, 23 colorectal cancer, 24 breast cancer, 25 oral squamous cell carcinoma. 26 So far, only Nguyen et al. have discussed the relationship between CDCA5 and NSCLC in detail, and they reported that CDCA5, recognized as an independent prognostic factor of NSCLC, played an essential role in the growth of lung cancer cells. 13 Based on the results of their research, we speculate that CDCA5 is probably participated in the occurrence and development of NSCLC by regulating the cell cycle of NSCLC patients. Recently, the important immune checkpoints PD‐1 and PD‐L1 have become significant targets for immunotherapy in patients with LUAD. However, the potential relationship between CDCA5 and PD‐1/PD‐L1 has not been reported.
In the present study, we performed bioinformatics analyses to exhibit the expression of CDCA5 in 18 common malignant tumors, calculated the correlation between the expression of CDCA5 and the survival, expression of common immune checkpoint inhibitors (ICIs) and clinicopathological characteristics of LUAD patients, and explored the enriched functions and signaling pathways in highly expressed CDCA5 patients with LUAD. Furthermore, we discussed the potential relationship between CDCA5 and PD‐1/PD‐L1 for the first time by bioinformatics analysis. Following the results of bioinformatics analyses, we subsequently designed experiments to explore the changes in downstream genes expression in p53‐p21 signaling pathway, apoptosis, and cell cycles in NSCLC cells after silencing CDCA5.
2. MATERIALS AND METHODS
2.1. Data acquisition
The transcriptome data and corresponding clinical data of LUAD patients were obtained from The Cancer Genome Atlas (TCGA, https://portal.gdc.cancer.gov/) 27 database. Subsequently, the duplicate data and data with a follow‐up time of 0 were excluded.
2.2. Pan‐cancer analysis of CDCA5
The Wilcoxon rank‐sum test was performed to exhibit the expression of CDCA5 in tumor tissues and adjacent normal tissues in 18 common malignant tumors, and a multiple box‐plot was plotted for visualization. Furthermore, Wilcoxon rank‐sum test was conducted to explore whether there is a statistically significant difference in the CDCA5 expression between tumor tissues and adjacent normal tissues of LUAD patients.
2.3. Survival analysis of CDCA5
To investigate the relationship between the expression of CDCA5 and the overall survival (OS) of LUAD patients, Kaplan–Meier survival analysis was conducted to detect whether there was a statistical difference in OS between patients with high‐expression and low‐expression of CDCA5, in which median expression of CDCA5 was recognized as the cut‐off point to divide LUAD patients into different groups.
2.4. Correlation analyses between the expression of CDCA5 and clinicopathological characteristics
To explore the potential relationship between the expression of CDCA5 and common clinicopathological characteristics (e.g., Stage, T, and N), several Wilcoxon signed‐rank tests were employed, and multiple box plots were generated for visualization. To further explore the predictive capability of CDCA5 for survival outcomes under different stages of clinicopathological characteristics (e.g., Age, Gender, Stage, T, N, and M), a series of survival curves were generated for visualization.
2.5. GSEA function enrichment analysis
The Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets by software GSEA 4.0.1 (https://www.gsea‐msigdb.org/gsea/index.jsp) were utilized to identify related signaling pathways in different expression groups, in which the threshold was set as followed: p < 0.05 and (false discovery rate) FDR < 0.25.
2.6. Expression analyses of common ICIs
The National Comprehensive Cancer Network (NCCN) Guideline proposed that anti‐PD‐1 and anti‐PD‐L1 therapy are the fundamental treatment of immunotherapy for NSCLC patients. To wonder whether CDCA5 could function as a biomarker for the efficacy of immunotherapy, Wilcoxon rank‐sum tests were conducted to compare the expression of common ICIs (such as PD‐1, PD‐L1) between LUAD patients with high and low CDCA5 expression.
2.7. Experimental materials
Human NSCLC lines (A549 and HCC827) were purchased from American Type Culture Collection (ATCC) cell bank; DMEM medium and pancreatin were purchased from American Gibco Company; fetal bovine serum (FBS) was purchased from Beijing Huamei Company; and Cysteinyl aspartate‐specific proteinase 3 (Caspase‐3) activity detection kits and apoptosis detection kits were purchased from China Beyotime Company; and methlthiazoletrazolium (MTT) was purchased from American Sigma Company; anti‐CDCA5 monoclonal antibody, anti‐p53 monoclonal antibody, anti‐p21 monoclonal antibody, anti‐β‐actin monoclonal antibody, and corresponding secondary antibody were purchased from American Cell Signaling Technology Company.
2.8. Cell transfection
The A549 and HCC827 cells were nurtured in 5% CO2 at 37˚C, subsequently seeded in 6‐well plates (2.5 × 105 cells/well), and were transfected with the mixture of Lipofectamine 2000 and CDCA5 small interfering RNA (CDCA5 siRNA, sc‐61599) or its negative control (NC). The successful transfection was verified by qRT‐PCR and WB.
2.9. qRT‐PCR
Isolated from A549 and HCC827 cells by Trizon (Invitrogen, USA), total RNAs were subsequently reverse‐transcribed into complementary DNAs (cDNAs) by Prime Script RT kit (TaKaRa, Dalian, China). Then, the cDNAs were stained by SYBR Green dyestuff (TaKaRa, Dalian, China), and were performed for qRT‐PCR by ABI 7500 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). The relative expression of CDCA5 was evaluated by 2−△△Ct method, and β‐actin was considered as a normalization control. The primer sequences were as follows: CDCA5: forward: 5′‐GAGGTCCCAGCGGAAATCAG‐3′, reverse: 5′‐TCTTTAAGACGATGGGCTTTCTG‐3′; β‐actin: forward: 5′‐TGGATCAGCAAGCAGGAGTA‐3′, reverse: 5′‐TCGGCCACATTGTGAACTTT‐3′.
2.10. Quantification of CDCA5, P53, P21 protein
After 48 h transfection, the two groups of cells were lysed with RIPA lysate, added with loading buffer based on their respective doses, and boiled for 10 min. Then, the 20‐gram protein of two groups was added into NuPAGE precast gels, incubated with CDCA5 (1:200), P53 (1:200), P21 (1:200), Glyceraldehyde‐3‐Phosphate Dehydrogenase (GAPDH, 1:200) primary antibody overnight at 4℃, incubated with the secondary antibody (1:5000) at room temperature for 30 min, and developed color by ECL solutions. Next, Quantity One 1‐D software was operated to quantify the optical density (OD) values of western blot bands. The relative expression of target proteins was calculated as the ratio of the OD value of the target protein to the OD value of GAPDH.
2.11. Detection of cell apoptosis
Resuspended by EDTA‐free trypsin, the A549 and HCC827 cells were transferred into the flow tubes after three washes of PBS, which were subsequently incubated with PI and Annexin V‐FITC for 15 min in the dark. The flow cytometry was operated to detect apoptosis of A549 and HCC827 cells.
2.12. Detection of cell cycle
After 48 h of transfection, A549 and HCC827 cells were fixed with 70% ethanol overnight, treated with RNase for 30 min, and strained with PI for 20 min. Subsequently, the cell cycles of two groups of cells were calculated by flow cytometer.
2.13. Statistical analysis
All the bioinformatics analysis above was run through R‐x64‐4.0.4 (https://www.r‐project.org/), and the results are marked as followed: ***<0.001, **<0.01, and *<0.05. Besides, the remaining analyses were conducted by SPSS 22.0 software, in which data were expressed as means ± standard deviation (SD), independent sample t‐tests were performed for comparison between two groups, one‐way analysis of variances (ANOVAs) were utilized for comparison between multiple groups, least significant difference t‐tests (LSD t tests) were performed for differences between groups. Moreover, p < 0.05 indicated that the differences were statistically significant.
3. RESULTS
3.1. CDCA5 is highly expressed in malignant tumors
We downloaded the transcriptome data and corresponding clinical data of 551 samples, including 54 normal samples and 497 LUAD samples. According to the results of pan‐cancer analysis, we discovered that among the 18 common malignant tumors, CDCA5 was highly expressed and statistically significant (Figure 1A), which indicated CDCA5 was probably an oncogene of promising research value that participated in the occurrence and development of a variety of malignant tumors.
FIGURE 1.
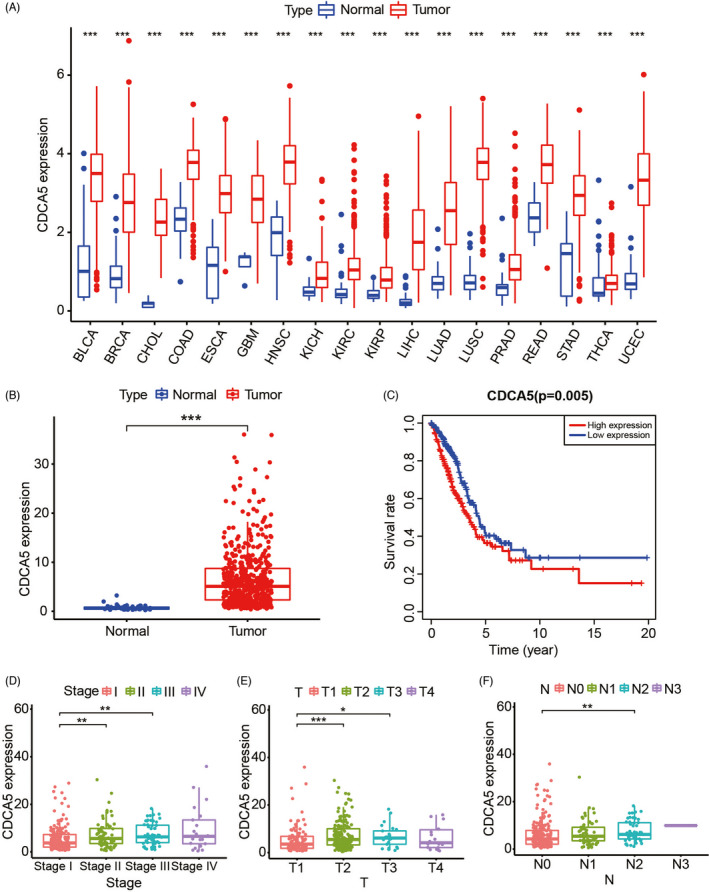
CDCA5 is an oncogene involved in the development and progression of LUAD. A, CDCA5 is overexpressed in 18 common malignant tumors. B, The expression of CDCA5 in tumor tissues of patients with LUAD is significantly higher than in adjacent tissues. C, LUAD with high expression of CDCA5 has a relatively poor prognosis. D–F, Advanced clinicopathological characteristics, including Stage (D), T (E), N (F), of patients with LUAD are closely associated with high expression of CDCA5
3.2. CDCA5, an oncogene, is involved in the occurrence and development of NSCLC
Analyses of expression and survival of CDCA5 in patients with LUAD showed that CDCA5 was highly expressed and statistically significant, and overexpression of CDCA5 always led to poor prognosis (Figure 1B,C). Moreover, the multi‐box plots indicated that compared with LUAD patients with early periods of Stage (Stage I, Figure 1D), T (T1, Figure 1E), N (N0, Figure 1F), the CDCA5 expression of LUAD patients with corresponding later periods was significantly higher. As shown in these survival curves, we concluded that compared with their later stage, the expression of CDCA5 exhibited excellent predictive capability in the early stage of Stage (Stage I‐II, Figure 2C), T (T1‐2, Figure 2D), N (N0, Figure 2E), M (M0, Figure 2F). Furthermore, patients >65 years of age (Figure 2A) with high CDCA5 expression, both male and female (Figure 2B), tended to exhibit a relatively better survival advantage. The results above indicated that overexpression of CDCA5 probably played a crucial part in the development and progression of LUAD, and CDCA5 could act as an early diagnostic indicator to predict the prognosis of patients with LUAD.
FIGURE 2.
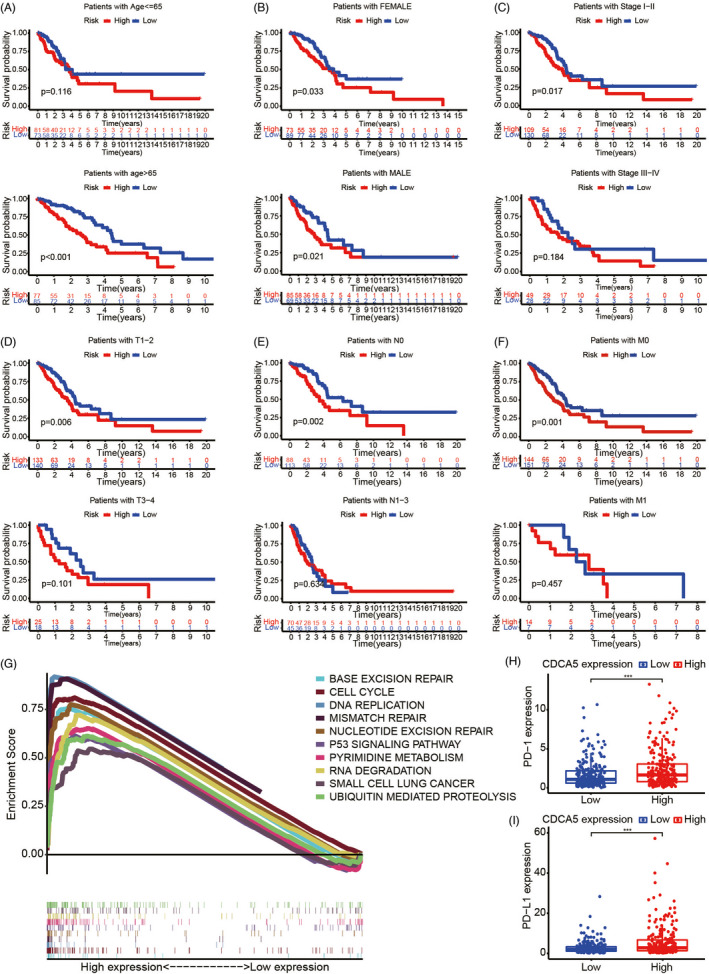
Exploration of the clinical significance of CDCA5. A–F, The Kaplan–Meier survival analyses suggest that the overexpression of CDCA5 exhibits excellent predictive capability in patients >65 years of age (A), both male and female (B), and early stage of Stage (C), T (D), N (E), and M (F), which always predict poor prognosis for patients with LUAD. G, GSEA reveals that several fundamental activities of cells, including cell cycle, base excision repair, DNA replication, mismatch repair, nucleotide excision repair, pyrimidine metabolism, RNA degradation, ubiquitin‐mediated proteolysis, and p53 signaling pathway were enriched in LUAD samples with higher expression of CDCA5. H–I, The expression of PD‐1 (H), and PD‐L1 (I) in LUAD samples with high CDCA5 expression are higher than LUAD samples with low CDCA5 expression
3.3. Bioinformatics analysis of clinical significance of CDCA5
The GESA function enrichment analysis revealed that several fundamental activities of cells, including cell cycle, base excision repair, DNA replication, mismatch repair, nucleotide excision repair, pyrimidine metabolism, RNA degradation, ubiquitin‐mediated proteolysis, and p53 signaling pathway were enriched in LUAD patients with high expression of CDCA5 (Figure 2G). Therefore, we speculated that overexpression of CDCA5 regulated the cell cycle of NSCLC via mediating p53 signaling pathway. Expression analyses suggested that the expression of PD‐1 (Figure 2H), and PD‐L1 (Figure 2I) in LUAD patients with high CDCA5 expression were relatively higher, which revealed that LUAD patients with high CDCA5 expression were probably more sensitive to anti‐PD‐1 and anti‐PD‐L1 therapies.
3.4. CDCA5 modulates cell cycle of NSCLC via p53‐p21 signaling pathway
The A549 and HCC827 cell lines were successfully transfected by si‐CDCA5, respectively, which were verified by qRT‐PCR (Figure 3A) and WB (Figure 3B). According to the Schematic diagram (Figure 4) on the KEGG website (https://www.kegg.jp), 28 we found that it was through p21 signaling pathway that p53 regulated cell cycle. Subsequently, we discovered that after knocking down the expression of CDCA5, the expression of p53 and p21 in A549 and HCC827 decreased correspondingly (Figure 3C), which indicated that overexpression of CDCA5 may participate in the regulation of the cell cycle of NSCLC via p53‐p21 signaling pathway.
FIGURE 3.
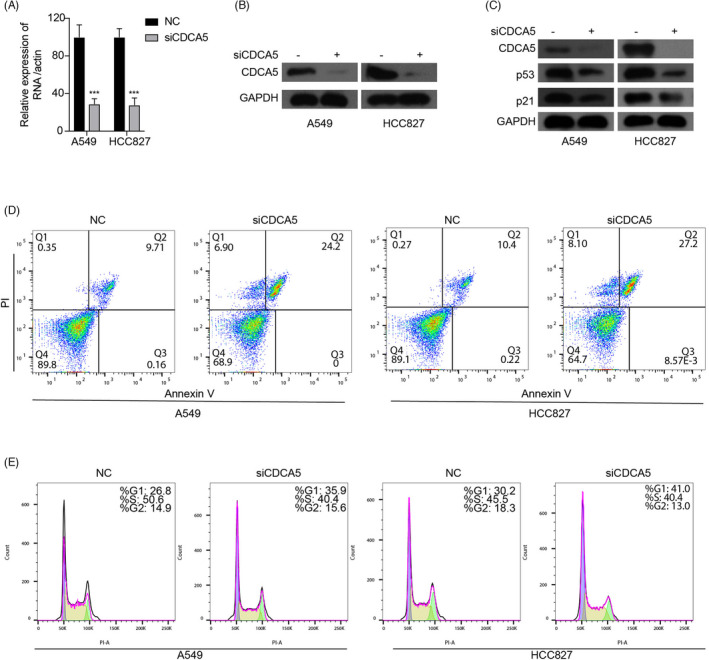
Silencing CDCA5 expression increases the proportion of apoptotic NSCLC cells and arrests NSCLC cells in G1 phase. A and B, qRT‐PCR (A) and WB (B) indicate that A549 and HCC827 cell lines are successfully transfected by si‐CDCA5. C, The expression of p53 and p21 in A549 and HCC827 decreas correspondingly after knocking down the expression of CDCA5. D, The proportion of A549 and HCC827 apoptotic cells increase correspondingly after knocking down the expression of CDCA5 by si‐CDCA5. E, Compared with the negative controls, the proportion of G1 phase arrest of A549 and HCC827 cells transfected with si‐CDCA5 is significantly increased
FIGURE 4.
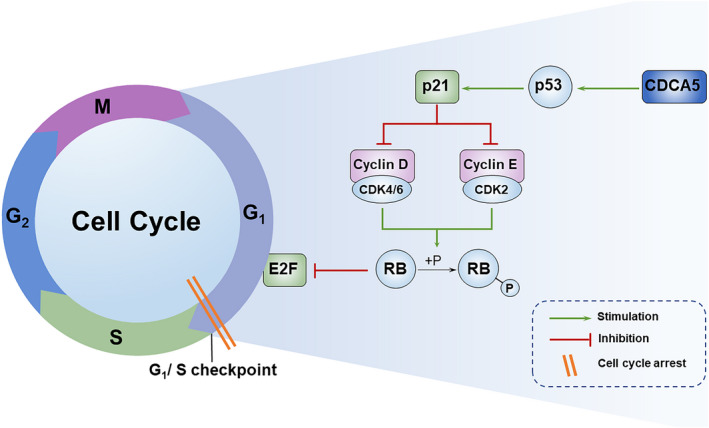
Schematic diagram of CDCA5 regulating G1 arrest in NSCLC cells by mediating the p53‐p21 signaling pathway
3.5. Silencing CDCA5 leads to apoptosis and G1 phase arrest cell cycle of NSCLC cells
The results of the flow cytometer revealed that after knocking down the expression of CDCA5 by si‐CDCA5, the proportion of A549 and HCC827 apoptotic cells increased correspondingly (Figure 3D), which indicated that the silence of CDCA5 effectively enhanced the apoptosis of NSCLC cells. In addition, cell cycle analyses suggested that compared with their negative controls, the proportion of G1 phase arrest of A549 and HCC827 cells transfected with si‐CDCA5 increased significantly (Figure 3E), which revealed that silencing CDCA5 led to G1 phase arrest of NSCLC cells.
4. DISCUSSION
In recent years, the emergence of bioinformatic analyses has functioned as an effective and reliable pattern to study the complex molecular pathogenesis of NSCLC. 29 Increasing scholars have screened effective and reliable targets through bioinformatics analyses to predict the prognosis, explore the tumor immunity, and even select suitable anti‐tumor drugs of patients with tumors, which benefited greatly the patients with malignant tumors. 30 , 31
CDCA5, acts as a cell cycle regulator, is combined with nuclear chromatin from S phase to metaphase and is subsequently released into the cytoplasm upon nuclear envelope breakdown. 9 Through bioinformatics analyses, we concluded that overexpression of CDCA5, an oncogene overexpressed in 18 common malignant tumors, was closely associated with advanced clinicopathological characteristics in patients with LUAD, probably leading to poor prognosis.
Increasing evidence have demonstrated that CDCA5 is involved in the regulation of the cell cycle of various malignant tumors, and is involved in the progression of a variety of malignant tumors by mediating p53 signaling pathway. For example, Xing et al. conducted the integrated analysis of CDCA protein family in patients with pancreatic cancer, and proposed that CDCA protein family affected cell cycle and DNA repair through the P53‐E2F pathway, in which E2F was a gene located in the downstream of the p53‐p21 signaling pathway. 32 Moreover, Zhang et al. reported that silencing CDCA5 induced G1 phase arrest via down‐regulating cyclin E1 to inhibit the proliferation of gastric cancer cells. 19 It is worth mentioning that cyclin E is located downstream of the p53‐p21 signaling pathway and regulates downstream cell cycle arrest (Figure 4). According to the results of GSEA, we found that the cell cycle and p53 signaling pathway were significantly enriched in LUAD patients with CDCA5 highly expressed. Therefore, we speculated that similar to pancreatic cancer and gastric cancer, CDCA5 played a crucial role in the regulation of cell cycle in patients with NSCLC through p53‐p21 signaling pathway. To verify the results of bioinformatics analyses, we subsequently conducted the WB for validation that the protein content of p53 and p21 decreased correspondingly after knocking down the expression of CDCA5. According to the results of flow cytometry, we discovered that after silencing the expression of CDCA5, the apoptosis of NSCLC cells enhanced and most of the cells were arrested in the G1 phase. Moreover, through bioinformatic analyses, we concluded that LUAD patients with high CDCA5 expression tended to be more suitable for the anti‐PD‐1 and anti‐PD‐L1 therapies, and CDCA5 had an excellent predictive ability for predicting the survival outcomes of LUAD patients in the early stage of common clinicopathological characteristics.
In conclusion, the silencing of CDCA5 mediates the p53‐p21 signaling pathway to regulate the cell cycle of NSCLC cells, participating in the progression of NSCLC. As a potential indicator for selecting suitable immunotherapy, CDCA5 may also act as a reliable biomarker for predicting the survival outcomes of patients with LUAD. Therefore, CDCA5 is probably an effective and reliable therapeutic target for patients with NSCLC with promising research value.
CONFLICT OF INTEREST
All authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Hang Chen and Guodong Xu contributed to the conception of the study. Wei Shen, Dimin Tong, and Jie Chen performed the R language. Hongxiang Li, Zeyang Hu, Shuguang Xu, and Sufang He contributed significantly to analysis and manuscript preparation. Wei Shen and Hang Chen performed the data analyses and wrote the manuscript. Zhen Ge, Jianan Zhang, and Qiqi Mao helped perform the analysis with constructive discussions.
PATIENT CONSENT STATEMENT
Not applicable.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
Not applicable.
CLINICAL TRIAL REGISTRATION
Not applicable.
CONSENT FOR PUBLICATION
Written informed consent for publication was obtained from all participants.
ACKNOWLEDGMENT
We thank the TCGA database for generously sharing a large amount of data.
Shen W, Tong D, Chen J, et al. Silencing oncogene cell division cycle associated 5 induces apoptosis and G1 phase arrest of non‐small cell lung cancer cells via p53‐p21 signaling pathway. J Clin Lab Anal. 2022;36:e24396. doi: 10.1002/jcla.24396
Wei Shen, Dimin Tong, Jie Chen, Hongxiang Li, Zeyang Hu, Shuguang Xu and Sufang He contributed equally to this work.
Funding information
The study was funded by the Basic Public Welfare Project of Ningbo (Grant No. 2019C50041), the Natural Science Foundation of Zhejiang (Grant No. LY22H160004), and the Natural Science Foundation of Ningbo (Grant No. 2021J278).
Contributor Information
Hang Chen, Email: 791097532@qq.com.
Guodong Xu, Email: xuguodong@nbu.edu.cn.
DATA AVAILABILITY STATEMENT
All data analyzed during the current study are accessible from the TCGA database (https://portal.gdc.cancer.gov/).
REFERENCES
- 1. Nasim F, Sabath B, Eapen G. Lung cancer. Med Clin N Am. 2019;103(3):463‐473. [DOI] [PubMed] [Google Scholar]
- 2. Siegel R, Miller K, Fuchs H, Jemal A. Cancer statistics, 2021. CA: Cancer J Clin. 2021;71(1):7‐33. [DOI] [PubMed] [Google Scholar]
- 3. Chen W, Zheng R, Baade P, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115‐132. [DOI] [PubMed] [Google Scholar]
- 4. Shi J, Wang L, Wu N, et al. Clinical characteristics and medical service utilization of lung cancer in China, 2005–2014: Overall design and results from a multicenter retrospective epidemiologic survey. Lung Cancer. 2019;128:91‐100. [DOI] [PubMed] [Google Scholar]
- 5. Patel S, Weiss J. Advances in the treatment of non‐small cell lung cancer: immunotherapy. Clin Chest Med. 2020;41(2):237‐247. [DOI] [PubMed] [Google Scholar]
- 6. Hirsch F, Scagliotti G, Mulshine J, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299‐311. [DOI] [PubMed] [Google Scholar]
- 7. American Cancer Society . Non‐small cell lung cancer survival rates by stage[OL]. www.cancer.org/cancer/non‐small‐cell‐lung‐cancer/detection‐diagnosis‐staging/survival‐rates.html Date last updated: 04/20/21
- 8. Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98‐W102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stelzer G, Rosen N, Plaschkes I, et al. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Prot Bioinf. 2016;54:1.30.31‐31.30.33. [DOI] [PubMed] [Google Scholar]
- 10. Rankin S, Ayad N, Kirschner M. Sororin, a substrate of the anaphase‐promoting complex, is required for sister chromatid cohesion in vertebrates. Mol Cell. 2005;18(2):185‐200. [DOI] [PubMed] [Google Scholar]
- 11. Nishiyama T, Ladurner R, Schmitz J, et al. Sororin mediates sister chromatid cohesion by antagonizing Wapl. Cell. 2010;143(5):737‐749. [DOI] [PubMed] [Google Scholar]
- 12. Schmitz J, Watrin E, Lénárt P, Mechtler K, Peters J‐M. Sororin is required for stable binding of cohesin to chromatin and for sister chromatid cohesion in interphase. Curr Biol. 2007;17(7):630‐636. [DOI] [PubMed] [Google Scholar]
- 13. Nguyen M, Koinuma J, Ueda K, et al. Phosphorylation and activation of cell division cycle associated 5 by mitogen‐activated protein kinase play a crucial role in human lung carcinogenesis. Can Res. 2010;70(13):5337‐5347. [DOI] [PubMed] [Google Scholar]
- 14. Zhang X, Yan Z, Wang L, Zhang S, Gao M. STAT1‐induced upregulation of lncRNA RHPN1‐AS1 predicts a poor prognosis of hepatocellular carcinoma and contributes to tumor progression via the miR‐485/CDCA5 axis. J Cell Biochem. 2020;121:4741‐4755. [DOI] [PubMed] [Google Scholar]
- 15. Chen H, Chen J, Zhao L, et al. CDCA5, transcribed by E2F1, promotes oncogenesis by enhancing cell proliferation and inhibiting apoptosis via the AKT pathway in hepatocellular carcinoma. J Cancer. 2019;10(8):1846‐1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chong Y, Xue L. Downregulation of CDCA5 can inhibit cell proliferation, migration, and invasion, and induce apoptosis of prostate cancer cells. Crit Rev Eukaryot Gene Expr. 2021;31(1):29‐40. [DOI] [PubMed] [Google Scholar]
- 17. Ji J, Shen T, Li Y, Liu Y, Shang Z, Niu Y. CDCA5 promotes the progression of prostate cancer by affecting the ERK signalling pathway. Oncol Rep. 2021;45(3):921‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang Z, Zhang S, Du J, et al. Cyclin‐dependent kinase 1 (CDK1) is co‐expressed with CDCA5: their functions in gastric cancer cell line MGC‐803. Med Sci Monit. 2020;26:e923664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Z, Shen M, Zhou G. Upregulation of CDCA5 promotes gastric cancer malignant progression via influencing cyclin E1. Biochem Biophys Res Comm. 2018;496(2):482‐489. [DOI] [PubMed] [Google Scholar]
- 20. Gao A, Hu Y, Zhu W. CDCA5 is negatively regulated by miR‐326 and boosts ovarian cancer progression. J BUON. 2021;26(2):544‐552. [PubMed] [Google Scholar]
- 21. Liu D, Gong H, Tao Z, Chen S, Kong Y, Xiao B. LINC01515 promotes nasopharyngeal carcinoma progression by serving as a sponge for miR‐325 to up‐regulate CDCA5. J Mol Histol. 2021;52(3):577‐587. [DOI] [PubMed] [Google Scholar]
- 22. Fu G, Xu Z, Chen X, Pan H, Wang Y, Jin B. CDCA5 functions as a tumor promoter in bladder cancer by dysregulating mitochondria‐mediated apoptosis, cell cycle regulation and PI3k/AKT/mTOR pathway activation. J. 2020;11(9):2408‐2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu J, Zhu C, Yu Y, et al. Systematic cancer‐testis gene expression analysis identified CDCA5 as a potential therapeutic target in esophageal squamous cell carcinoma. EBioMedicine. 2019;46:54‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shen A, Liu L, Chen H, et al. Cell division cycle associated 5 promotes colorectal cancer progression by activating the ERK signaling pathway. Oncogenesis. 2019;8(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Phan N, Wang C, Li K, et al. Distinct expression of CDCA3, CDCA5, and CDCA8 leads to shorter relapse free survival in breast cancer patient. Oncotarget. 2018;9(6):6977‐6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tokuzen N, Nakashiro K, Tanaka H, Iwamoto K, Hamakawa H. Therapeutic potential of targeting cell division cycle associated 5 for oral squamous cell carcinoma. Oncotarget. 2016;7(3):2343‐2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tomczak K, Czerwińska P, Wiznerowicz M. The Cancer Genome Atlas (TCGA): an immeasurable source of knowledge. Contemp Oncol. 2015;19:A68‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kanehisa M, Sato Y, Kawashima M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022;31(1):47‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zou J, Wang L, Tang H, Liu X, Peng F, Peng C. Ferroptosis in non‐small cell lung cancer: progression and therapeutic potential on it. J Mol Med. 2021;22(24):13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen H, Hu Z, Sang M, et al. Identification of an autophagy‐related lncRNA prognostic signature and related tumor immunity research in lung adenocarcinoma. Front Genet. 2021;12:767694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H, Shen W, Ni S, et al. Construction of an immune‐related lncRNA signature as a novel prognosis biomarker for LUAD. Aging (Albany NY). 2021;13:20684‐20697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xing C, Wang Z, Zhu Y, et al. Integrate analysis of the promote function of Cell division cycle‐associated protein family to pancreatic adenocarcinoma. Int J Med Sci. 2021;18(3):672‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data analyzed during the current study are accessible from the TCGA database (https://portal.gdc.cancer.gov/).


