Abstract
Introduction
Since COVID‐19 outbreak, various studies mentioned the occurrence of neurological disorders. Of these, encephalitis is known as a critical neurological complication in COVID‐19 patients. Numerous case reports and case series have found encephalitis in relation to COVID‐19, which have not been systematically reviewed. This study aims to evaluate the clinical symptoms, diagnosis, treatment, and outcome of COVID‐19‐associated encephalitis.
Methods
We used the Pubmed/Medline, Embase, and Web of Science databases to search for reports on COVID‐19‐associated encephalitis from January 1, 2019, to March 7, 2021. The irrelevant studies were excluded based on screening and further evaluation. Then, the information relating diagnosis, treatment, clinical manifestations, comorbidities, and outcome was extracted and evaluated.
Results
From 4455 initial studies, 45 articles met our criteria and were selected for further evaluation. Included publications reported an overall number of 53 COVID‐19‐related encephalitis cases. MRI showed hyperintensity of brain regions including white matter (44.68%), temporal lobe (17.02%), and thalamus (12.76%). Also, brain CT scan revealed the hypodensity of the white matter (17.14%) and cerebral hemorrhages/hemorrhagic foci (11.42%) as the most frequent findings. The IV methylprednisolone/oral prednisone (36.11%), IV immunoglobulin (27.77%), and acyclovir (16.66%) were more preferred for COVID‐19 patients with encephalitis. From the 46 patients, 13 (28.26%) patients were died in the hospital.
Conclusion
In this systematic review, characteristics of COVID‐19‐associated encephalitis including clinical symptoms, diagnosis, treatment, and outcome were described. COVID‐19‐associated encephalitis can accompany with other neurological symptoms and involve different brain. Although majority of encephalitis condition are reversible, but it can lead to life‐threatening status. Therefore, further investigation of COVID‐19‐associated encephalitis is required.
Keywords: COVID‐19, encephalitis, SARS‐CoV‐2, systematic review
At the first screening, 4455 papers were retrieved. In the second phase, after removing duplicates, 2171 papers remained. These papers were screened by title and abstract, and 119 were selected for detailed full‐text evaluation. Applying the criteria to the full‐text documents, 45 articles were eligible for inclusion in the systematic review.

1. INTRODUCTION
Humans have been struggling with the COVID‐19 epidemic for nearly 2 years. 1 As of early August 2020, more than 17.5 million cases of COVID‐19 were identified in 188 countries, including 680,000 deaths. 2 The disease that often has respiratory symptoms but sometimes also has extrapulmonary manifestations such as neurological symptoms. 1 On average, neurological symptoms appear three weeks after respiratory symptoms. 3 Less common clinical manifestations of COVID‐19 include headache, brain status alteration, chest pain, abdominal pain, diarrhea, and nausea. 2 Nervous manifestations can range from a mild nervous agitation to severe encephalitis. 4 The roll of central nervous system in SARS‐CoV‐2 epidemic has been determined. 3 Ischemic stroke, central nervous system (CNS) inflammation, encephalopathy, and myelitis are common clinical manifestations of the CNS in COVID‐19 patients. 5 Encephalitis means inflammation of the brain, 6 which is mainly caused by the autoimmune process and/or the viral infection. 5 Encephalitis is one of the main and devastating complications associated with CNS. 7 In previous epidemics, MERS‐CoV and SARS‐CoV viruses have caused brain complications such as polyneuropathy, ischemic stroke, encephalitis, and brain status change in patients with Middle East respiratory syndrome coronavirus and severe acute respiratory syndrome coronavirus. 8 , 9 Several case reports and case series have reported the patients with COVID‐19‐associated encephalitis, which in some cases have been fatal. 4 , 10 , 11 The pooled mortality rate from COVID‐19‐associated encephalitis is reported to be 13.4%. 12 In a multicenter study by Pilotto et al. in Italy, 25 out of 45 people with encephalitis tested positive for SARS‐CoV‐2. They found that there is a wide range of clinical manifestations in patients and the response to treatment depends on the specific CNS manifestations. 13 Due to the importance of encephalitis in COVID‐19 patients and the risk of death for them, it is necessary to conduct a detailed systematic review on this study. Therefore, the aim of this study was to evaluate the clinical symptoms, diagnosis, treatment, and outcome of COVID‐19‐associated encephalitis.
2. MATERIALS AND METHODS
This systematic review was performed according to “Preferred Reporting Items for Systematic Reviews and Meta‐Analyses” (PRISMA) statement. 14
2.1. Search strategy
We used Pubmed/Medline, Embase, and Web of Science databases for this literature. The articles included were only those published in English from January 1, 2019, to March 7, 2021. The search keywords used were “encephalitis,” “brain,” “neurologic,” “COVID‐19,” “severe acute respiratory syndrome coronavirus 2,” “SARS‐CoV‐2,” “2019‐nCoV,” “nCoV disease,” “coronavirus disease‐19,” “2019 novel coronavirus,” and “Wuhan pneumonia.”
2.2. Inclusion/exclusion criteria
Case reports and case series reporting encephalitis in patients with COVID‐19 were included. These studies met the following inclusion criteria: (i) COVID‐19 patients were confirmed and diagnosed with RT‐PCR as suggested by WHO; (ii) the raw data for clinical symptoms, diagnosis, treatment, and outcome of COVID‐19‐associated encephalitis were addressed. Studies without enough data, review article, modelling study, commentary, correspondence, editorial, guideline, and news were excluded. All potentially relevant articles were then screened for eligibility. Two reviewers independently screened the records by title, abstract, and full texts to exclude those not related to the current study.
2.3. Data collection
The extracted data included first author name; country where the study was conducted; year of publication; type of study; number of patients investigated; distribution of age and sex in the population; diagnosis methods; data for clinical, radiological, and laboratory findings; therapy, and the patient outcome.
2.4. Quality assessment
We used the case reports/case series appraisal checklist supplied by the Joanna Briggs Institute (JBI) to evaluate the quality of the studies. 15
3. RESULTS
3.1. Study selection and general characteristics
As shown in Figure 1, at the first screening, 4455 papers were retrieved. In the second phase, after removing duplicates, 2171 papers remained. These papers were screened by title and abstract, and 119 were selected for detailed full‐text evaluation. Applying the criteria to the full‐text documents, 45 articles were eligible for inclusion in the systematic review. The results of various studies including participants’ clinical manifestations, comorbidities, diagnosis, treatment, and outcome are reported in Tables 1 and 2. Moreover, a summary of the case report and case series findings are reported in Table 3.
FIGURE 1.
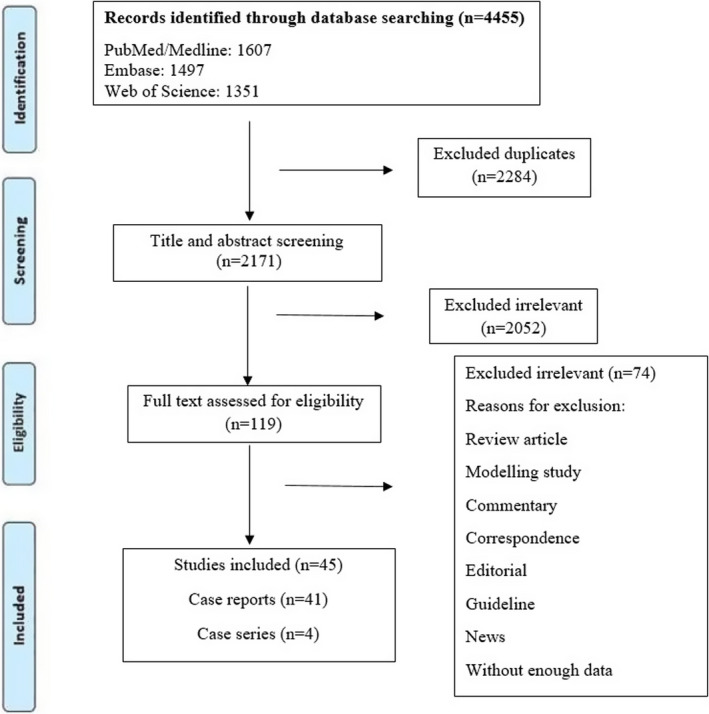
Flow diagram detailing review process and study selection
TABLE 1.
Characteristics of the case series studies
| First author | Country | Published time | No. of patients | Median age (years) | Male/female | Encephalitis diagnosis method | CT results | MRI results | Special encephalitis treatment | SARS‐CoV‐2 diagnosis method | COVID‐19 treatment | Clinical manifestations | SARS‐CoV‐2 diagnosis in CSF sample | Comorbidities | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lopes CCB 32 | Brazil | December 2020 | 2 | 50 | 1M, 1F | 2 brain CT scan, 2 brain MRI, 2 EEG | 2 bilateral lesions in the centrum semiovale/1 focal lesions in globus pallidus and internal capsule/1 signal abnormalities in the white matter, including corpus callosum | 2 multifocal hyperintensity in centrum semiovale, 1 lesions in the cerebellar white matter and globus pallidus | NM | 2 RT‐PCR | 1 hydroxychloroquine | 2 fever, 2 RS, 1 renal failure, 2 delayed awakening after sedation withdrawal, 2 DC, 1 coma, 1 four‐limb weakness | 2 negative | 2 hypertension, 1 diabetes, 1 obesity, 1 smoking | 1 death, 1 partial recovery |
| Kihira S 33 | USA | October 2020 | 5 | 48.6 | 3M, 2F | MRI, head CT, EEG | 5 unremarkable | 5 hyperintensity in the white matter, 3 confluent diffusion restriction in the cerebral white matter, 2 hyperintensity in the splenium of corpus callosum/1 scattered frontoparietal hyperintensity/1 microhemorrhages in corpus callosum/1 intraventricular hemorrhage | 1 plasma exchange | 5 RT‐PCR | NM | 1 myocardial infarction, 2 fever, 5 RS, 3 acute renal failure, 1 cardiogenic shock, 1 abdominal pain, 1 cardiac arrest, 1 nausea, 1 vomiting, 1 chills, 5 AMS, 1 focal seizures, 1 lower limbs paralysis | 3 negative, 1 NP, 1 NM | 1 Hypertension, 1 diabetes mellitus type 2, 1 pyelonephritis, 1 gestation, 1 obesity | NM |
| Barreto‐Acevedo E 34 | Peru | June, 2020 | 2 | 50.5 | 1M, 1F | MRI, CSF analysis, brain tomography | 2 unremarkable | 1unremarkable, 1 NP | Dexamethasone | RT‐PCR, serological test | NM | 2 fever, 2 chills, 1 malaise, 1 headache, 2 AMS, 2 seizures | NP | 1 obesity | 1death, 1 transferred to other hospital |
| Delorme C 35 | France | August 2020 | 4 | 66.75 | 2M, 2F |
Brain MRI, CSF analysis, brain FDG‐PET/CT imaging, EEG |
4 NP |
1 unremarkable/1 non‐specific Hyperintensity of the white matter/1 right T2 orbitofrontal Hyperintensity |
3 IV immunoglobulin, 3 IV corticosteroids | 4 RT‐PCR | NM | 4 cognitive impairment, 2 cerebellar syndrome, 1 myoclonus, 1 psychiatric symptoms, 4 fever, 3 RS, 2 anosmia, 1 ageusia, 1 diarrhea, 2 fatigue, 2 agitation, 1 psychomotor slowing, 1 convulsive status, 1 apraxia, 1 dysexecutive syndrome | 4 negative | 1 temporal lobe epilepsy (hippocampal sclerosis), 1 diabetes mellitus type2, 1 hypertension | 4 discharged |
TABLE 2.
Characteristics of case report studies
| First author | Country | Published time | Age (years) | Sex | Encephalitis diagnosis method | CT results | MRI results | Special encephalitis treatment | SARS‐CoV‐2 diagnosis method | COVID‐19 treatment | Clinical manifestations | SARS‐CoV‐2 diagnosis in CSF sample | Comorbidities | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kumar N 36 | India | October 2020 | 35 | M | Head CT | Hypodensities in both thalami and left caudate nucleus | NP | Propofol infusion, mannitol, IV methylprednisolone | RT‐PCR | Hydroxychloroquine, azithromycin, IV amoxicillin‐clavulanic acid | Fever, vomiting, DC | NP | Invasive meningioma | Death |
| Novi G 37 | Italy | September 2020 | 64 | F | Brain and spine MRI, CSF analysis | NP | Multiple enhancing lesions of the brain, bilateral optic nerve enhancement | IV methylprednisolone, prednisone | RT‐PCR, antibody testing | IV immunoglobulin | RS, anosmia, ageusia, visual impairment, behavioral changes, headache, hyperreflexia | Positive | Vitiligo, hypertension, monoclonal gammopathy | Discharged |
| Ayuso LL 38 | Spain | September 2020 | 72 | F | Brain MRI, immunoblot analysis | NP | Hyperintensity in cerebellum, contrast enhancement on the floor of the fourth ventricle | IV methylprednisolone, prednisone | RT‐PCR, chest CT | Hydroxychloroquine, azithromycin, ceftriaxone | Psychiatric symptoms, fever, AMS, dizziness, visual impairment, unsteadiness, cerebellar signs | NP | Hypertension, hyperlipidemia, smoking, depression | Discharged |
| Khan Z 39 | USA | November 2020 | 30 | M | Head CT | Opacifications of paranasal sinuses, hypodensity of the white matter | Hyperintensity in the white matter of cerebral hemispheres | Acyclovir | RT‐PCR, chest CTy | NM | Seizure, DC, behavioral changes, myoclonus, AMS, psychiatric symptoms | NP | Obesity | Still hospitalized |
| Westhoff TH 40 | Germany | July 2020 | 69 | M | Brain MRI, CSF analysis | NP | Linear meningeal enhancement/hyperintensity in the white matter | NM | RT‐PCR, chest CT | Hydroxychloroquine | Fever, RS, diarrhea, pancreas and kidney allograft dysfunction, seizure, hemi‐neglect, fatigue | Positive | Immunosuppression | Discharged |
| Kamal YM 41 | United Arab Emirate | September 2020 | 31 | M | CSF analysis, head CT, brain MRI | Bilateral hypodensities in the external capsules, the insular cortex and white matter of the frontal lobes | Bilateral diffusion restriction in the temporal and frontal lobes/bilateral hyperintensity in the temporal lobe cortex | IV acyclovir sodium | RT‐PCR | Chloroquine, lopinavir–ritonavir | Behavioral changes, AMS, agitation, drowsiness | Positive | None | Discharged |
| Rebeiz T 21 | USA | September 2020 | NM | M | CSF analysis, brain MRI | Subarachnoid hemorrhage within the mesial parietal region/nonspecific hypo‐attenuation in the splenium of the corpus callosum | Diffusion restriction and hyperintensity of the corpus callosum, left thalamus and frontal cortex | Acyclovir, ceftriaxone, vancomycin | RT‐PCR | NM | Behavioral changes, fever, AMS, psychiatric symptoms | NP | None | Death |
| Zoghi A 42 | Iran | June 2020 | 21 | M | CSF analysis, brain and cervical MRI | NP | Hyperintensity in the internal capsule, cerebral peduncles, pons and the corpus callosum | IV vancomycin, meropenem, acyclovir | Chest CT, antibody testing | Plasma exchange | Anorexia, vomiting, food intolerance, malaise, lower limbs paralysis, weakness, urinary retention, drowsiness | Negative | None | NM |
| Moriguchi T 9 | Japan | May 2020 | 24 | M | Brain MRI | NP | Hyperintensity along the wall of right lateral ventricle, right temporal lobe and hippocampus, slight hippocampus atrophy | IV ceftriaxone, vancomycin, acyclovir, steroids | Chest CT, RT‐PCR | Favipiravir | Fatigue, fever, headache, RS, seizure, unconsciousness | Positive | NM | Still hospitalized |
| Haqiqi A 43 | United kingdom | January 2021 | 56 | M | Head CT, brain MRI, CSF analysis | Diffuse hypodensity of the white matter/multiple bilateral white matter hemorrhagic foci involving the corpus callosum | Hyperintensity of the white matter/diffuse hemosiderin staining throughout the white matter and the corpus callosum/some cystic hemorrhagic areas within both cerebral hemispheres | None | RT‐PCR | Supportive care | RS, acute kidney injury, DC | Negative | Hypertension, chronic kidney disease, hypercholesterolemia, asthma, obesity | Discharged |
| Pizzanelli C 44 | Italy | January 2021 | 74 | F | Brain MRI, total body PET/TC | Unremarkable | Hyperintensity in the temporal lobes, mild hippocampal thickening | IV methylprednisolone, oral prednisolone | Chest CT, RT‐PCR | Remdesevir, dexamethasone | Fever, RS, seizure, AMS, oral automatism, weakness, ideo‐motor slowing | Negative | None | Discharged |
| Al Mazrouei SS 1 | United Arab Emirates | September 2020 | 43 | M | Head CT, brain MRI | Hypodensity of bilateral thalami | Hyperintensity in the frontal lobes, insula, thalamus and globus pallidus | NM | RT‐PCR, Chest CT | NM | Fever, RS, weakness, fatigue, DC | NP | Diabetes mellitus type2 | Death |
| Sirous R 2 | USA | August 2020 | 50 | M | MRI, magnetic resonance angiography, magnetic resonance venography | Mild cerebral generalized parenchymal volume loss with sulcal enlargement | Cerebral edema with mass effect, downward cerebellar tonsillar herniation/compression and displacement of the brainstem and 4th ventricle | NM | RT‐PCR | IV hydroxychloroquine | Fatigue, headache, nausea, vomiting, lethargy, AMS | NP | None | Death |
| Mardani M 45 | Iran | July 2020 | 64 | F | CSF analysis | Unremarkable | NP | NM | RT‐PCR, CSF analysis | IV ceftriaxone, clindamycin, hydroxychloroquine, lopinavir/ritonavir | RS, weakness, DC | Positive | Hypertension, ischemic heart disease, metastatic colorectal cancer | NM |
| Vandervorst F 46 | Belgium | July 2020 | 29 | M | Brain MRI | Unremarkable | Hyperintensity of the left temporal cortex/mild gyral expansion | NM | RT‐PCR, chest CT | IV acyclovir, hydroxychloroquine | Weakness, RS, anorexia, Anosmia, ageusia, AMS, short‐term memory deficits, psychiatric symptoms | Negative | None | Discharged |
| Freire‐Álvarez E 31 | Spain | October 2020 | 39 | M | Brain MRI | Unremarkable | Hyperintensity at the cortical and subcortical right frontal regions, right thalamus and mammalary body, temporal lobes and cerebral peduncles | IV immunoglobulin, tocilizumab | RT‐PCR, chest CT | Lopinavir/ritonavir, subcutaneous interferon beta‐1b | Fatigue, DC, malaise, fever, AMS, headache, drowsiness, minimal stiff neck, language disorder, paraphasia | Negative | NM | Clinical improvement, Still hospitalized |
| Parsons T 47 | Germany | May 2020 | 51 | F | Brain MRI, EEG | NP | Hyperintensities in the white matter | Methylprednisolone, IV Immunoglobulin | RT‐PCR, chest CT | NM | RS, fever, vomiting, unresponsiveness, flaccid muscles | Negative | NM | NM |
| Al‐olama M 48 | United Arab Emirates | May 2020 | 36 | M | Brain CT, CT angiography | Hematoma in the right frontal lobe with surrounding edema/extracerebral hemorrhage/cortical swelling/bilateral supratentorial leptomeningeal increased enhancement | NP | NM | PCR | NM | Fever, RS, headache, body pain, diarrhea, vomiting, drowsiness, AMS | NP | None | Still hospitalized |
| Goodloe TB 49 | Alabama | January, 2021 | 52 | M | Bead CT, EEG | Unremarkable | Unremarkable | Vancomycin, ceftriaxone, azithromycin, acyclovir | RT‐PCR | NM | AMS, agitation, fever | NP | Hypertension, diabetes mellitus type2, end‐stage renal disease, coronary artery disease | Discharged |
| Sattar SBA 23 | USA | September 2020 | 44 | M | Brain MRI, head CT, CSF analysis | Few scattered foci of white matter hypo‐attenuation | Abnormal medial cortical signals in the bilateral frontal lobes | NM | RT‐PCR, chest CT | Hydroxychloroquine, azithromycin | Fever, RS, seizure, AMS, unresponsiveness | Positive | None | Discharged |
| Haider A 29 | USA | March 2020 | 66 | M | EEG, brain MRI | Unremarkable | Small lacunar infarcts and a patchy area of bright signals in the cortical and lateral periventricular regions | Tocilizumab, IV immunoglobulin, rituximab | RT‐PCR | NM | Seizure, AMS, behavioral changes | NP | Benign prostatic hypertrophy, fatty liver disease, hypertension | Discharged |
| Cariddi LP 50 | Italy | June, 2020 | 64 | F | Head CT, brain MRI | Bilateral hypodensity of the white matter/a small left occipital parenchymal hemorrhage | Bilateral edema with bilateral occipital foci of subacute hemorrhage | NM | RT‐PCR | Hydroxychloroquine, darunavir/cobicistat | Fever, RS, visual impairment, AMS, drowsiness, reduced tendon reflexes | Negative | Hypertension, gastroesophageal reflux disease, hyperuricemia, dyslipidemia, obstructive sleep apnea, atrial fibrillation | Partial recovery |
| Sofijanova A 51 | Republic of Macedonia | November 2020 | 9 month | NM | Head CT, biochemical blood test | Enlargement of the lateral ventricles, with intraventricular masses, internal hydrocephalus | NP | Anti‐edematous therapy | NM | Cephalosporin, aminoglycoside, antiviral drug | RS, convulsive status, fever, DC, vomiting, seizure | NP | NM | Transferred to another hospital |
| Ghosh R 11 | India | August 2020 | 44 | F | Brain MRI, CSF analysis | NP | T2‐weighted hyperintensity in the parietal lobes with peri‐lesional edema | IV methylprednisolone | RT‐PCR | Ceftriaxone, vancomycin, acyclovir | Myalgia, RS, hypogeusia, hyposmia, AMS, seizure, unconsciousness, reduced tendon refluxes, loss of sphincter control | NP | None | Death |
| Pilotto A 52 | Italy | August, 2020 | 60 | M | EEG, brain MRI, CSF analysis | Unremarkable | Unremarkable | Methylprednisolone | RT‐PCR, chest CT | Ritonavir/lopinavir, hydroxychlroroquine | Fever, RS, cognitive fluctuations, DC, AMS, behavioral changes, asthenia | Negative | None | Discharged |
| Azab MA 3 | Egypt | February, 2021 | 89 | M | MRI, post‐mortem biopsy | NP | Hyperintensity near the basal ganglia and thalami | NM | Serological test | Acyclovir, acetaminophen | Rash, seizure, tremors, RS, cerebellar signs, fever, headache, dizziness, myalgia | NP | NM | Death |
| Abdi S 53 | Iran | June, 2020 | 58 | M | Brain MRI, CSF analysis | NP | Hyperintensity of the white matter/involvement of cortical and deep gray matter and midbrain | IV dexamethasone | RT‐PCR, chest CT | NM | Drowsiness, gait disturbance, DC | Negative | NM | Death |
| Dharsandiya M 54 | India | August, 2020 | 68 | M | Head CT, blood test, CSF analysis | Age‐related cortical atrophy (unremarkable) | NP | Methylprednisolone, tocilizumab | RT‐PCR, chest CT | Azithromycin, hydroxychloroquine, gamma globulin | Fever, RS, renal failure, viral sepsis, autonomic disturbance, AMS, seizure | NP | Diabetes, hypertension | Death |
| Babar A 55 | USA | October, 2020 | 20 | F | Brain MRI, CSF analysis, EEG | Unremarkable | Unremarkable | Methylprednisolone | RT‐PCR | Levofloxacin, acyclovir | RS, ageusia, insomnia, Fever, AMS, psychiatric symptoms | Negative | Obesity, anxiety | Discharged |
| Virhammar J 56 | Sweden | June, 2020 | 55 | F | Head CT, CSF analysis, EEG, brain MRI | Hypodensities in the thalami and midbrain | Hyperintensity in subinsular regions, thalami, and brainstem/involvement of temporal lobes, hippocampi, and cerebral peduncles | IV immunoglobulin | RT‐PCR, chest CT | Acyclovir, plasma exchange | Fever, myalgia, impaired brain stem reflexes, myoclonus, lethargy, DC | Positive | None | Discharged to rehabilitation |
| Farhadian S 57 | USA | June, 2020 | 78 | F | Brain MRI, EEG, CSF analysis | NP | Generalized atrophy/hyperintensity in white matter | NM | RT‐PCR, chest CT | Hydroxychloroquine | Seizure like activity, RS, fever, AMS | Negative | Immunosuppression due to kidney transplantation | Discharged |
| de Miranda Henriques‐Souza AM 58 | Brazil | October, 2020 | 12 | F | Brain and spine MRI, CSF analysis | NP | Bilateral restricted diffusion in the white matter/hyperintensity of the corpus callosum | Methylprednisolone | RT‐PCR | NM | Tetraplegia, fever, deep areflexia, skin rash, headache, RS, acute motor weakness, numbness | Negative | None | Discharged |
| Afshar H 59 | Iran | August 2020 | 39 | F | Brain MRI | NP | Hyperintensities in bilateral thalami, temporal lobes and pons | IV immunoglobulin, IV methylprednisolone | Chest CT | Meropene, levofloxacin, linezolide, hydroxychloroqine, atazanavir, IV immunoglobulin | Fever, myalgia, anorexia, drowsiness, RS, DC, headache, seizure | Negative | None | Discharged |
| Crosta F 60 | Italy | December 2020 | 79 | M | EEG, brain MRI | Unremarkable | Hyperintensity of the left temporal cortex, with mild gyral expansion | NM | RT‐PCR | Clarithromycin, dexamethasone | Fever, AMS, anosmia, RS, ageusia, DC, short‐term memory deficits, psychiatric symptoms | NP | Hypertension, diabetes, chronic heart failure | Discharged |
| Sangare A 61 | France | November 2020 | 56 | M | EEG, brain MRI | NP | Hemorrhagic lesions in the pontine tegmentum and subinsular regions, including corpus callosum | IV methylprednisolone, plasma exchange with albumin | RT‐PCR, chest CT | Cephalosporin linezolide, trimethoprime‐sulfamethoxazole, meropenem aminosid | Fever, RS, reversible acute kidney failure, visual impairment, unresponsiveness | Negative | Hypertension | Discharged |
| El‐Zein RS 62 | USA | September 2020 | 40 | M | EEG, blood tests, CSF analysis | Unremarkable | Unremarkable | IV immunoglobulin | Simplexa SARS‐CoV‐2 assay | Hydroxychloroquine | Fever, fatigue, AMS, RS, psychiatric symptoms, increased agitation | Negative | None | Discharged |
| Etemadifar M 4 | Iran | September 2020 | 51 | M | Head CT, brain MRI | Generalized brain edema/signs of brain herniation | Generalized brain edema, downward herniation of cerebellar tonsils and brainstem, hyperintensities in bilateral cerebral cortices and corpus striatum | NM | RT‐PCR | Hydroxychloroquine, lopinavir/ritonavir, IV acyclovir, IV dexamethasone | Headache, drowsiness, nausea, vomiting, RS, seizure, cardiac arrest, impaired brain stem reflexes | NP | Hypothyroidism migraine | Death |
| Peng LV 63 | China | February, 2021 | 90 | F | CSF analysis, physical and neurological examination | Unremarkable | NP | Mannitol and anti‑viral therapy (Ganciclovir) | RT‑PCR, Chest CT | NM | Fever, RS, fatigue, unconsciousness, unresponsiveness, increased muscle tension | Negative | Cerebral lacunar infarction with no neurological deficits‐ live in a healthcare unit | Death (irrelevant cause) |
| Hayashi M 10 | Japan | August 2020 | 75 | M | Neurological examination, brain MRI | NP | Hyperintensity in the splenium of corpus callosum | Corticosteroid pulse, meropenem | RT‐PCR | Favipiravir, corticosteroid pulse | Urinary incontinence, diarrhea, DC, cerebellar signs, fever, AMS, tremor, gait disturbance | NP |
Mild Alzheimer's disease |
Death |
| Kumar A 64 | USA | November 2020 | 35 | F | Brain MRI, EEG, CSF analysis | Unremarkable | Hyperintensity in the white matter involving bilateral cerebral peduncles | Methylprednisolone, IV immunoglobulin, plasma exchange | RT‐PCR, serological tests | NM | Anosmia, ageusia, gait disturbance, neuropathy, weakness, drowsiness, lethargy | Negative | Gastric bypass surgery, anemia | Discharged to a long‐term care facility |
| Muccioli L 65 | Italy | September 2020 | 47 | F | EEG, brain MRI | NP | Hyperintensity in the white matter | Tocilizumab | Chest CT, RT‐PCR | NM | Asthenia, RS, ageusia, hyposmia, language disturbance, pain in the extremities, fever, AMS, headache, agitation | Negative | None | Discharged |
TABLE 3.
Summary of the case reports and case series findings
| Variables | No. of studies | n/N | % |
|---|---|---|---|
| Gender | |||
| Male | 29 | 32/53 | 60.38 |
| Female | 19 | 21/53 | 39.62 |
| Age | |||
| <30 (years old) | 6 | 6/53 | 11.32 |
| 31–50 (years old) | 16 | 18/53 | 33.96 |
| >51 (years old) | 25 | 29/53 | 54.72 |
| Age/sex | |||
| <30 (years old) | |||
| Male | 4 | 4/6 | 66.67 |
| Female | 2 | 2/6 | 33.33 |
| 31–50 (years old) | |||
| Male | 11 | 12/18 | 66.67 |
| Female | 6 | 6/18 | 33.33 |
| >51 (years old) | |||
| Male | 15 | 16/29 | 55.17 |
| Female | 12 | 13/29 | 44.83 |
| Clinical manifestation | |||
| Neurological manifestations | |||
| Decreased consciousness/unconsciousness | 17 | 18/54 | 33.33 |
| Behavioral changes | 6 | 6/54 | 11.11 |
| Altered mental status | 24 | 29/54 | 53.70 |
| Cerebellar signs | 4 | 5/54 | 9.25 |
| Seizure | 15 | 16/54 | 29.62 |
| Agitation | 5 | 6/54 | 11.11 |
| Headache | 11 | 11/54 | 20.37 |
| Memory deficits | 2 | 2/54 | 3.70 |
| Unresponsiveness | 4 | 4/54 | 7.40 |
| Convulsive status | 2 | 2/54 | 3.70 |
| Cognitive impairment | 2 | 5/54 | 9.26 |
| Language disturbance | 2 | 2/54 | 3.70 |
| Paraphasia | 1 | 1/54 | 1.85 |
| Tremors | 2 | 2/54 | 3.70 |
| Lower limbs paralysis | 2 | 2/54 | 3.70 |
| Gait disturbance | 3 | 3/54 | 5.55 |
| Unsteadiness | 1 | 1/54 | 1.85 |
| Hemi‐neglect | 1 | 1/54 | 1.85 |
| Impaired brain stem reflexes | 2 | 2/54 | 3.70 |
| Pain | 3 | 3/54 | 5.55 |
| Coma | 1 | 1/54 | 1.85 |
| Apraxia | 1 | 1/54 | 1.85 |
| Dysexecutive syndrome | 1 | 1/54 | 1.85 |
| Psychomotor slowing | 1 | 1/54 | 1.85 |
| Ideo‐motor slowing | 1 | 1/54 | 1.85 |
| Oral automatism | 1 | 1/54 | 1.85 |
| Neuropathy | 1 | 1/54 | 1.85 |
| Reduced tendon reflexes | 2 | 2/54 | 3.70 |
| Loss of sphincter control | 1 | 1/54 | 1.85 |
| Deep areflexia | 1 | 1/54 | 1.85 |
| Psychiatric symptoms | |||
| Psychiatric symptoms | 8 | 8/54 | 14.81 |
| General symptoms | |||
| Fever | 32 | 38/54 | 70.37 |
| Vomiting | 8 | 8/54 | 14.81 |
| Nausea | 3 | 3/54 | 5.55 |
| Diarrhea | 4 | 4/54 | 7.40 |
| Anosmia/hyposmia | 7 | 8/54 | 14.81 |
| Ageusia/dysgeusia | 8 | 8/54 | 14.81 |
| Dizziness | 2 | 2/54 | 3.70 |
| Malaise | 3 | 3/54 | 5.55 |
| Fatigue | 8 | 9/54 | 16.66 |
| Drowsiness | 9 | 9/54 | 16.66 |
| Weakness/asthenia | 10 | 10/54 | 18.51 |
| Lethargy | 3 | 3/54 | 5.55 |
| Chills | 2 | 3/54 | 5.55 |
| Anorexia | 3 | 3/54 | 5.55 |
| Food intolerance | 1 | 1/54 | 1.85 |
| Insomnia | 1 | 1/54 | 1.85 |
| Numbness | 1 | 1/54 | 1.85 |
| Neuromuscular symptoms | |||
| Myalgia | 4 | 4/54 | 7.40 |
| Hyperreflexia | 1 | 1/54 | 1.85 |
| Myoclonus | 3 | 3/54 | 5.55 |
| Neck stiffness | 1 | 1/54 | 1.85 |
| Flaccid muscles | 1 | 1/54 | 1.85 |
| Tetraplegia | 1 | 1/54 | 1.85 |
| Increased muscle tension | 1 | 1/54 | 1.85 |
| Other | |||
| Respiratory symptoms | 30 | 37/54 | 68.51 |
| Visual impairment | 4 | 4/54 | 7.40 |
| Renal dysfunction | 8 | 10/54 | 18.51 |
| Cardiac dysfunction | 2 | 4/54 | 7.40 |
| Rash | 2 | 2/54 | 3.70 |
| Viral sepsis | 1 | 1/54 | 1.85 |
| Delayed awakening after sedation | 1 | 2/54 | 3.70 |
| Autonomic disturbances | 1 | 1/54 | 1.85 |
| Comorbidities | |||
| Hypertension | 13 | 14/48 | 29.16 |
| Diabetes mellitus | 7 | 7/48 | 14.58 |
| Obesity | 6 | 6/48 | 12.50 |
| Neurologic disorders | 5 | 5/48 | 10.41 |
| Cardiologic disorder | 4 | 4/48 | 8.33 |
| Dyslipidemia | 2 | 2/48 | 4.16 |
| Anemia | 1 | 1/48 | 2.08 |
| Psychiatric disorders | 2 | 2/48 | 4.16 |
| Renal dysfunction | 3 | 3/48 | 6.25 |
| Immunosuppressive state | 2 | 2/48 | 4.16 |
| Smoking | 2 | 2/48 | 4.16 |
| Hypercholesterolemia | 1 | 1/48 | 2.08 |
| Hypothyroidism | 1 | 1/48 | 2.08 |
| Vitiligo | 1 | 1/48 | 2.08 |
| Monoclonal gammopathy | 1 | 1/48 | 2.08 |
| Asthma | 1 | 1/48 | 2.08 |
| Colorectal cancer | 1 | 1/48 | 2.08 |
| Fatty liver disease | 1 | 1/48 | 2.08 |
| Gastroesophageal reflux disease | 1 | 1/48 | 2.08 |
| Hyperuricemia | 1 | 1/48 | 2.08 |
| Obstructive sleep apnea | 1 | 1/48 | 2.08 |
| Benign prostatic hypertrophy | 1 | 1/28 | 3.57 |
| Gestation | 1 | 1/20 | 5.00 |
| No comorbidities | 15 | 15/48 | 31.25 |
| Presence of SARS‐CoV‐2 RNA in the CSF sample | |||
| Positive | 7 | 7/34 | 20.58 |
| Negative | 21 | 27/34 | 79.41 |
| SARS‐CoV‐2 diagnosis method | |||
| RT‐PCR | 40 | 49/53 | 92.45 |
| Chest CT | 20 | 20/53 | 37.73 |
| Serological testing (anti‐SARS‐CoV‐2 antibody) | 5 | 6/53 | 11.32 |
| Simplexa SARS‐CoV‐2 assay | 1 | 1/53 | 1.88 |
| Encephalitis diagnosis method | |||
| Brain MRI | 36 | 44/54 | 81.48 |
| Head CT scan | 15 | 20/54 | 37.03 |
| CSF analysis | 21 | 25/54 | 46.29 |
| Electroencephalogram | 15 | 23/54 | 42.59 |
| Total body PET/TC | 1 | 1/54 | 1.85 |
| FDG‐PET/CT imaging | 1 | 4/54 | 7.40 |
| CT angiogram | 1 | 1/54 | 1.85 |
| Magnetic resonance angiography and venography | 1 | 1/54 | 1.85 |
| Biochemical blood tests | 3 | 3/54 | 5.55 |
| Post‐mortem biopsy | 1 | 1/54 | 1.85 |
| Physical and neurological examination | 2 | 2/54 | 3.70 |
| Immunoblot analysis | 1 | 1/54 | 1.85 |
| Brain tomography | 1 | 1/54 | 1.85 |
| Special encephalitis treatment | |||
| Dexamethasone | 2 | 3/36 | 8.33 |
| Plasma exchange | 3 | 3/36 | 8.33 |
| IV methylprednisolone/oral prednisone | 13 | 13/36 | 36.11 |
| IV immunoglobulin | 8 | 10/36 | 27.77 |
| Corticosteroids | 2 | 4/36 | 11.11 |
| Steroids | 1 | 1/36 | 2.77 |
| Propofol infusion | 1 | 1/36 | 2.77 |
| Mannitol | 2 | 2/36 | 5.55 |
| Acyclovir | 6 | 6/36 | 16.66 |
| Ceftriaxone | 3 | 3/36 | 8.33 |
| Vancomycin | 4 | 4/36 | 11.11 |
| Meropenem | 2 | 2/36 | 5.55 |
| Tocilizumab | 4 | 4/36 | 11.11 |
| Azithromycin | 1 | 1/36 | 2.77 |
| Rituximab | 1 | 1/36 | 2.77 |
| Anti‐edematous therapy | 1 | 1/36 | 2.77 |
| COVID‐19 treatment | |||
| Hydroxychloroquine | 15 | 15/30 | 50.00 |
| Chloroquine | 1 | 1/30 | 3.33 |
| Azithromycin | 4 | 4/30 | 13.33 |
| IV amoxicillin‐clavulanic acid | 1 | 1/30 | 3.33 |
| IV immunoglobulin | 2 | 2/30 | 6.66 |
| Ceftriaxone | 3 | 3/30 | 10.00 |
| Dexamethasone | 3 | 3/30 | 10.00 |
| Favipiravir | 2 | 2/30 | 6.66 |
| Ritonavir/lopinavir | 5 | 5/30 | 16.66 |
| Plasma exchange | 2 | 2/30 | 6.66 |
| Remdesevir | 1 | 1/30 | 3.33 |
| Clarithromycin | 1 | 1/30 | 3.33 |
| Corticosteroid pulse | 1 | 1/30 | 3.33 |
| Clindamycin | 1 | 1/30 | 3.33 |
| Interferon beta‐1b | 1 | 1/30 | 3.33 |
| Darunavir/cobicistat | 1 | 1/30 | 3.33 |
| Cephalosporin | 2 | 2/30 | 6.66 |
| Aminoglycoside | 1 | 1/30 | 3.33 |
| Vancomycin | 1 | 1/30 | 3.33 |
| Linezolide | 2 | 2/30 | 6.66 |
| Acyclovir | 6 | 6/30 | 20.00 |
| Acetaminophen | 1 | 1/30 | 3.33 |
| Gamma globulin | 1 | 1/30 | 3.33 |
| Levofloxacin | 2 | 2/30 | 6.66 |
| Meropene | 1 | 1/30 | 3.33 |
| Atazanavir | 1 | 1/30 | 3.33 |
| Trimethoprime‐sulfamethoxazole | 1 | 1/30 | 3.33 |
| Meropenem aminosid | 1 | 1/30 | 3.33 |
| Outcome | |||
| Death | 13 | 13/46 | 28.26 |
| Discharged | 20 | 23/46 | 50.00 |
| Discharged to rehabilitation/partial recovery | 4 | 4/46 | 8.69 |
| Still hospitalized | 4 | 4/46 | 8.69 |
| Transferred to another hospital | 2 | 2/46 | 4.34 |
| Brain MRI pattern | |||
| Unremarkable | 6 | 6/47 | 12.76 |
| Hyperintensity in the white matter | 15 | 21/47 | 44.68 |
| Hyperintensity in the corpus callosum | 5 | 6/47 | 12.76 |
| Hyperintensity in the cerebellum | 3 | 3/47 | 6.38 |
| Hyperintensity of the thalamus | 6 | 6/47 | 12.76 |
| Hyperintensity in the temporal lobe | 8 | 8/47 | 17.02 |
| Hyperintensity in the frontal lobe | 5 | 5/47 | 10.63 |
| Hyperintensity in the brainstem | 3 | 3/47 | 6.38 |
| Hyperintensity in the parietal lobe | 2 | 2/47 | 4.25 |
| Hyperintensity along the wall of lateral ventricle | 1 | 1/47 | 2.12 |
| Hemorrhagic/microhemrorrhagic areas | 4 | 5/47 | 10.63 |
| Signs of brain edema | 4 | 4/47 | 8.51 |
| Confluent diffusion restriction in the white matter | 2 | 4/47 | 8.51 |
| Compression and displacement of the brainstem and fourth ventricle | 1 | 1/47 | 2.12 |
| Downward cerebellar tonsilar herniation | 2 | 2/47 | 4.25 |
| Mild gyral expansion | 2 | 2/47 | 4.25 |
| Involvement of cortical and deep gray matter and midbrain | 1 | 1/47 | 2.12 |
| Diffuse hemosiderin staining throughout the white matter and corpus callosum | 1 | 1/47 | 2.12 |
| Linear meningeal enhancement | 1 | 1/47 | 2.12 |
| Contrast enhancement on the floor of the fourth ventricle | 1 | 1/47 | 2.12 |
| Bilateral optic nerve enhancement | 1 | 1/47 | 2.12 |
| Slight hippocampus atrophy | 1 | 1/47 | 2.12 |
| Mild hippocampal thickening | 1 | 1/47 | 2.12 |
| Generalized brain atrophy | 1 | 1/47 | 2.12 |
| Head CT scan pattern | |||
| Unremarkable | 15 | 20/35 | 57.14 |
| Hypodensity of the white matter | 6 | 6/35 | 17.14 |
| Hypodensity of the thalamus | 3 | 3/35 | 8.57 |
| Hypodensity of the corpus callosum | 2 | 2/35 | 5.71 |
| Hypodensity in the cerebellum | 2 | 3/35 | 8.57 |
| Cerebral hemorrhages/hemorrhagic foci | 4 | 4/35 | 11.42 |
| Brain swelling and edema | 2 | 2/35 | 5.71 |
| Brain herniation | 1 | 1/35 | 2.85 |
| Opacification of paranasal sinuses | 1 | 1/35 | 2.85 |
| Internal hydrocephalus | 1 | 1/35 | 2.85 |
| Parenchymal hematoma with surrounding edema | 1 | 1/35 | 2.85 |
| Cerebral parenchymal volume loss with sulcal enlargement | 1 | 1/35 | 2.85 |
| Enlargement of the lateral ventricles with intraventricular masses | 1 | 1/35 | 2.85 |
| Increased supratentorial leptomeningeal enhancement | 1 | 1/35 | 2.85 |
3.2. Study population
From a total of 45 studies, 53 patients with COVID‐19‐associated encephalitis were enrolled from 18 countries. Forty‐one (93.18%) studies were case reports and 4 (6.82%) were case series. The most significant number of studies was conducted in the USA (n = 10), followed by Italy (n = 6) and Iran (n = 5).
3.3. Demographic data
Demographic information of the individuals with COVID‐19‐associated encephalitis can be found in Tables 1 and 2. The patients were 21 female and 32 male with mean age of 52.12 years ranged between 9 months and 89 years. The highest incidence of COVID‐19‐associated encephalitis was observed in people over 50 years of age (54.72%).
3.4. Diagnostic methods
COVID‐19 was most often diagnosed by RT‐PCR (92.45%) and chest CT (37.73%). In addition, serological tests (11.32%) and simplexa assay (1.88%) were used to detect SARS‐CoV‐2 virus (Table 3). Brain MRI (81.48%), CSF analysis (46.29%), electroencephalography (42.59%), and head CT (37.03%) were the most frequently used methods to diagnose encephalitis (Table 3). The most common brain MRI patterns were hyperintensity in the white matter (44.68%), hyperintensity in the temporal lobe (17.02%), and hyperintensity of the thalamus (12.76%). In addition, hypodensity of the white matter (17.14%) and cerebral hemorrhages/hemorrhagic foci (11.42%) were the most common head CT scan patterns.
3.5. Clinical manifestations
Clinical manifestations were reported in five categories including (A) neurological manifestations such as altered mental status (53.70%), decreased consciousness/unconsciousness (33.33%), and seizure (29.62%); (B) psychiatric symptoms (14.81%); (C) general symptoms such as fever (70.37%), headache (20.37%), weakness/asthenia (18.51%), and drowsiness (16.66%); (D) neuromuscular symptoms such as myalgia (7.40%), myoclonus (5.55%); and (E) other clinical manifestation such as respiratory symptoms (68.51%), renal dysfunction (18.51%), and visual impairment (7.40%).
3.6. Comorbidities
The most common comorbidities were hypertension (29.16%), diabetes mellitus (14.58%), obesity (12.50%), and neurologic disorders (10.41%). The less common comorbidities were anemia (2.08%), hypercholesterolemia (2.08%), hypothyroidism (2.08%), vitiligo (2.08%), and asthma (2.08%).
3.7. Treatment options
A wide range of treatment options was used to treat COVID‐19. The most common of which were hydroxychloroquine (50%), acyclovir (20%), and ritonavir/lopinavir (16.66%), respectively. Common encephalitis treatment modalities included IV methylprednisolone/oral prednisone (36.11%), IV immunoglobulin (27.77%), acyclovir (16.66%). In Table 3, we summarize all of the drugs used.
3.8. Outcomes
In total, 58.69% of the patients with COVID‐19‐associated encephalitis discharged and 13.05% of them were still hospitalized. The pooled mortality rate of these patients was 28.26%.
3.9. Risk of bias assessment
The results of the critical appraisal (JBI checklist) of included studies are summarized in Table S1. Overall, 45 articles were identified as having a low risk of bias (quality assessment score >7).
4. DISCUSSION
Encephalitis is one of the specific neurological manifestations of COVID‐19 that can cause severe damage to the patient. 16 In this study, we reviewed case series and case reports to evaluate the clinical symptoms, diagnosis, treatment, and outcome of COVID‐19‐associated encephalitis.
The patients with COVID‐19‐associated encephalitis can show encephalitis weeks after the onset of symptoms of COVID‐19 or to have symptoms of COVID‐19 and encephalitis at the same time. 12 Our study indicated that the clinical manifestations in patients with COVID‐19‐associated encephalitis can be both central nervous system symptoms (i.e., headache, dizziness, and impaired consciousness) and peripheral nervous system symptoms (i.e., hypogeusia, hyposmia, etc.). The most common symptoms were related to altered mental status (53.7%), decreased consciousness/unconsciousness (33.3%), and seizure (29.6%).
These results were consistent with a systematic review performed by Siow et al. They also reported that decreased level of consciousness (77.1%), alter in mental state (72.3%), and seizures (38.2%) were the most common symptoms in patients with COVID‐19‐associated encephalitis. 12
Correia et al. 17 conducted a systematic review on the neurological manifestations of patients with COVID‐19. The rate of altered consciousness in their study was reported to be 11.2%. The difference in the results of their study with us could be due to differences in the time frame of each study and the number of patients admitted.
Furthermore, headache (20.37%) and weakness/asthenia (18.51%) were other clinical symptoms of COVID‐19‐associated encephalitis in the present study. Correia et al. 17 and Siow et al. 12 reported headache rates of 16.8% and 27.3%, respectively.
In this study, myalgia (7.4%) was the most frequent neuromuscular symptom. The prevalence of myalgia in a meta‐analysis done by Li et al. 18 was 35.8%.
Fever (70.37%) and respiratory failure (68.51%) were the most common symptoms of COVID‐19 in our evaluation. Heidary et al. 19 achieved the same results in their study. They reported that clinical symptoms of COVID‐19 included coughing (81.3%), fever (62.8%), and dyspnea (60%). Also, Koupaei et al. 20 demonstrated that the COVID‐19 patients mostly suffered from fever (78.8%), cough (63.7%), and respiratory distress (22.6%).
So far, several cases of COVID‐19‐associated encephalitis have been reported in people who did not have symptoms of COVID‐19. The presence of asymptomatic people with encephalitis recommends that performing the diagnostic tests is necessary to prevent the spread of the disease. 21 , 22 On the contrary, CNS involvement is similar in the SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV viruses. Thus, it is recommended that more sensitive and specific tests be performed. 23
In this study, the most common methods used to diagnose encephalitis were MRI (81.48%), CSF analysis (46.29%), electroencephalogram (42.59%), and head CT scan (37.03%). Among the analysis performed on CSF, only 79.41% were positive and showed the presence of viral RNA. This may be due to the mechanism of encephalitis that the virus has not entered CSF and cannot be detected. Moreover, in the early stages of the disease, CSF may have a normal level and cause a false‐negative result. 5
The most common MRI findings included hyperintensity in the white matter, hyperintensity in the temporal lobe, and hyperintensity in the corpus callosum, respectively. Although the CT findings of patients with COVID‐19‐associated encephalitis usually are not remarkable, 24 our study showed that the most findings are hypodensity of the white matter (17.14%) and cerebral hemorrhages/hemorrhagic foci (11.42%).
Probably, some of the signs in the imaging are related to the subcortical white matter hyperintensities and microbleeds in the deep gray nuclei caused by underlying diseases. 12
The association between underlying diseases such as hypertension, diabetes, chronic obstructive pulmonary disease (COPD), cardiovascular disease, and cerebrovascular disease has been identified with COVID‐19. People with the above underlying diseases are more likely than others to develop COVID‐19 and the severity of the disease. 25 In the present study, patients with COVID‐19‐associated encephalitis had a higher percentage of hypertension (29.16%) and diabetes mellitus (14.58%).
Angiotensin‐converting enzyme 2 (ACE2), the receptor for SARS‐CoV‐2, is abundant in various organs. 3 Diabetes can increase the serum ACE2. Thus, it is not surprising that diabetes is a common comorbidity in patients with COVID‐19‐associated encephalitis. 26
In this study, COVID‐19‐associated encephalitis was more common in people over 50 years of age (54.72%). It seems that elderly people with several underlying diseases are less able to physiological rearrangement, which makes them more prone to encephalitis. 27
Although various treatments have been used to treat COVID‐19‐associated encephalitis, none of them can be used with certainty. At the time of the COVID‐19 epidemic, physicians should suspect SARS‐CoV‐2 as a differentiating factor when certain diseases and neurological symptoms occur. 21 Our survey showed that IV methylprednisolone/oral prednisone (36.11%), IV immunoglobulin (27.77%), and acyclovir (16.66%) were the common treatment options to treat encephalitis. The healing role of IV immunoglobulin in severe cases of COVID‐19 has been confirmed in several studies. 28 , 29 , 30 , 31
There are some limitations in this study. First, only case reports and case series were enrolled in this systematic review. Thus, the existence of publication bias should be considered. Second, since our search was limited to articles published in English, some relevant articles in other languages have missed. Third, some studies lacked sufficient data.
5. CONCLUSION
In this systematic review, various aspects of COVID‐19‐associated encephalitis including clinical symptoms, diagnosis, treatment, and outcome were studied. COVID‐19‐associated encephalitis is one of the complications of SARS‐CoV‐2, which may accompany with other neurological symptoms and make the patient's condition worse. It usually occur in severe cases and can increase the mortality rate. Thus, it is recommended to pay special attention to neurological symptoms during the COVID‐19 epidemic. Lack of proper attention causes problems such as delay in COVID‐19 diagnosis, virus transmission, and increased mortality. Therefore, further studies on COVID‐19‐associated encephalitis are suggested.
CONFLICT OF INTEREST
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTION
Maryam Koupaei, Negar Shadabmehr, Mohamad Hosein Mohamadi, Arezoo Asadi, Sajjad Abasi Moghadam, Amirhosein Shekartabar, Mohsen Heidary, and Fazlollah Shokri contributed in revising and final approval of the version to be published. All the authors agreed and confirmed the study for publication.
Supporting information
Table S1
Koupaei M, Shadab Mehr N, Mohamadi MH, et al. Clinical symptoms, diagnosis, treatment, and outcome of COVID‐19‐associated encephalitis: A systematic review of case reports and case series. J Clin Lab Anal. 2022;36:e24426. doi: 10.1002/jcla.24426
Koupaei and Shadab Mehr contributed equally to this work.
Mohamadi and Asadi contributed equally to this work.
Abbasimoghaddam and Shekartabar contributed equally to this work.
Contributor Information
Mohsen Heidary, Email: mohsenheidary40@gmail.com.
Fazlollah Shokri, Email: f.shokri_sbmu@yahoo.com.
DATA AVAILABILITY STATEMENT
All the data in this review are included in the study.
REFERENCES
- 1. Al Mazrouei SS, Saeed GA, Al Helali AA, Ahmed M. COVID‐19‐associated encephalopathy: neurological manifestation of COVID‐19. Radiol Case Rep. 2020;15(9):1646‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sirous R, Taghvaei R, Hellinger JC, Krauthamer AV, Mirfendereski S. COVID‐19‐associated encephalopathy with fulminant cerebral vasoconstriction: CT and MRI findings. Radiol Case Rep. 2020;15(11):2208‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Azab MA, Azzam AY. SARS‐CoV‐2 associated viral encephalitis with mortality outcome. Interdiscip Neurosurg. 2021;25:101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Etemadifar M, Salari M, Murgai AA, Hajiahmadi S. Fulminant encephalitis as a sole manifestation of COVID‐19. Neurol Sci. 2020;41(11):3027‐3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhagat R, Kwiecinska B, Smith N, et al. New‐onset seizure with possible limbic encephalitis in a patient with COVID‐19 infection: a case report and review. J Investig Med High Impact Case Rep. 2021;9:2324709620986302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellul M, Solomon T. Acute encephalitis–diagnosis and management. Clin Med. 2018;18(2):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Garg RK, Paliwal VK, Gupta A. Encephalopathy in patients with COVID‐19: a review. J Med Virol. 2021;93(1):206‐222. [DOI] [PubMed] [Google Scholar]
- 8. Gu J, Gong E, Zhang BO, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005;202(3):415‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis. 2020;94:55‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hayashi M, Sahashi Y, Baba Y, Okura H, Shimohata T. COVID‐19‐associated mild encephalitis/encephalopathy with a reversible splenial lesion. J Neurol Sci. 2020;415:116941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh R, Dubey S, Finsterer J, Chatterjee S, Ray BK. SARS‐CoV‐2‐associated acute hemorrhagic, necrotizing encephalitis (AHNE) presenting with cognitive impairment in a 44‐year‐old woman without comorbidities: a case report. Am J Case Rep. 2020;21:e925641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siow I, Lee KS, Zhang JJY, Saffari SE, Ng A. Encephalitis as a neurological complication of COVID‐19: a systematic review and meta‐analysis of incidence, outcomes, and predictors. Eur J Neurol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pilotto A, Padovani A. Reply to the Letter “COVID‐19‐associated encephalopathy and cytokine‐mediated neuroinflammation”. Ann Neurol. 2020;88(4):861‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 15. Institute J . The Joanna Briggs Institute Critical Appraisal Tools for Use in JBI Systematic Reviews Checklist for Analytical Cross Sectional Studies. The Joanna Briggs Institute; 2017. [Google Scholar]
- 16. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID‐19. Brain Behav Immun. 2020;88:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Correia AO, Feitosa PWG, Moreira JLS, Nogueira SAR, Fonseca RB, Nobre MEP. Neurological manifestations of COVID‐19 and other coronaviruses: a systematic review. Neurol Psychiatry Brain Res. 2020;37:27‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li L‐Q, Huang T, Wang Y‐Q, et al. COVID‐19 patients’ clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol. 2020;92(6):577‐583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heidary M, Asadi A, Noorbakhsh N, et al. COVID‐19 in HIV‐positive patients: a systematic review of case reports and case series. J Clin Lab Anal. 2022;36(4):e24308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Koupaei M, Naimi A, Moafi N, et al. Clinical characteristics, diagnosis, treatment, and mortality rate of TB/COVID‐19 coinfectetd patients: a systematic review. Front Med. 2021;8:740593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rebeiz T, Lim‐Hing K, Khazanehdari S, Rebeiz K. Behavioral changes without respiratory symptoms as a presenting sign of COVID‐19 encephalitis. Cureus. 2020;12(9):e10469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Radmard S, Epstein SE, Roeder HJ, et al. Inpatient neurology consultations during the onset of the SARS‐CoV‐2 New York City pandemic: a single center case series. Front Neurol. 2020;11:805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sattar SBA, Haider MA, Zia Z, Niazi M, Iqbal QZ. Clinical, radiological, and molecular findings of acute encephalitis in a COVID‐19 patient: a rare case report. Cureus. 2020;12:e10650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kihira S, Delman BN, Belani P, et al. Imaging features of acute encephalopathy in patients with COVID‐19: a case series. Am J Neuroradiol. 2020;41(10):1804‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID‐19: evidence from meta‐analysis. Aging. 2020;12(7):6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elemam NM, Hannawi H, Al Salmi I, Naeem KB, Alokaily F, Hannawi S. Diabetes mellitus as a comorbidity in COVID‐19 infection in the United Arab Emirates. Saudi Med J. 2021;42(2):170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Esme M, Topeli A, Yavuz BB, Akova M. Infections in the elderly critically‐Ill patients. Front Med. 2019;6:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shao Z, Feng Y, Zhong LI, et al. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID‐19: a multicenter retrospective cohort study. Clin Transl Immunol. 2020;9(10):e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haider A, Siddiqa A, Ali N, Dhallu M. COVID‐19 and the brain: acute encephalitis as a clinical manifestation. Cureus. 2020;12(10):e10784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Afshar H, Yassin Z, Kalantari S, et al. Evolution and resolution of brain involvement associated with SARS‐CoV2 infection: a close clinical–paraclinical follow up study of a case. Mult Scler Relat Disord. 2020;43:102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freire‐Álvarez E, Guillén L, Lambert K, et al. COVID‐19‐associated encephalitis successfully treated with combination therapy. Clin Infect Pract. 2020;7:100053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lopes CCB, Brucki SMD, Passos Neto CEB, et al. Acute disseminated encephalomyelitis in COVID‐19: presentation of two cases and review of the literature. Arq Neuropsiquiatr. 2020;78(12):805‐810. [DOI] [PubMed] [Google Scholar]
- 33. Kihira S, Delman BN, Belani P, et al. Imaging features of acute encephalopathy in patients with COVID‐19: a case series. AJNR Am J Neuroradiol. 2020;41(10):1804‐1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barreto‐Acevedo E, Mariños E, Espino P, Troncoso J, Urbina L, Valer N. Acute encephalitis associated with SARS‐CoV‐2: first case report in Peru. Rev Neuropsiquiatr. 2020;116‐122. [Google Scholar]
- 35. Delorme C, Paccoud O, Kas A, et al. COVID‐19‐related encephalopathy: a case series with brain FDG‐positron‐emission tomography/computed tomography findings. Eur J Neurol. 2020;27(12):2651‐2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kumar N, Kumar S, Kumar A, et al. Acute necrotizing encephalitis as a probable association of COVID‐19. Indian J Crit Care Med. 2020;24(10):991‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Novi G, Rossi T, Pedemonte E, et al. Acute disseminated encephalomyelitis after SARS‐CoV‐2 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7(5):e797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Llorente Ayuso L, Torres Rubio P, Beijinho do Rosário RF, Giganto Arroyo ML, Sierra‐Hidalgo F. Bickerstaff encephalitis after COVID‐19. J Neurol. 2021;268(6):2035‐2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Khan Z, Singh S, Foster A, Mazo J, Graciano‐Mireles G, Kikkeri V. A 30‐year‐old male with COVID‐19 presenting with seizures and leukoencephalopathy. SAGE Open Med Case Rep. 2020;8:2050313X20977032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Westhoff TH, Seibert FS, Bauer F, et al. Allograft infiltration and meningoencephalitis by SARS‐CoV‐2 in a pancreas‐kidney transplant recipient. Am J Transplant. 2020;20(11):3216‐3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kamal YM, Abdelmajid Y, Al Madani AAR. Cerebrospinal fluid confirmed COVID‐19‐associated encephalitis treated successfully. BMJ Case Rep. 2020;13(9):e237378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. A case of possible atypical demyelinating event of the central nervous system following COVID‐19. Mult Scler Relat Disord. 2020;44:102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Haqiqi A, Samuels TL, Lamb FJ, Moharrum T, Myers AE. Acute haemorrhagic leukoencephalitis (Hurst disease) in severe COVID‐ 19 infection. Brain Behav Immun Health. 2021;12:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pizzanelli C, Milano C, Canovetti S, et al. Autoimmune limbic encephalitis related to SARS‐CoV‐2 infection: case‐report and review of the literature. Brain Behav Immun Health. 2021;12:100210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mardani M, Nadji SA, Sarhangipor KA, Sharifi‐Razavi A, Baziboroun M. COVID‐19 infection recurrence presenting with meningoencephalitis. New Microbes New Infect. 2020;37:100732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vandervorst F, Guldolf K, Peeters I, et al. Encephalitis associated with the SARS‐CoV‐2 virus: a case report. Interdiscip Neurosurg. 2020;22:100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Parsons T, Banks S, Bae C, Gelber J, Alahmadi H, Tichauer M. COVID‐19‐associated acute disseminated encephalomyelitis (ADEM). J Neurol. 2020;267(10):2799‐2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Al‐Olama M, Rashid A, Garozzo D. COVID‐19‐associated meningoencephalitis complicated with intracranial hemorrhage: a case report. Acta Neurochir. 2020;162(7):1495‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goodloe TB 3rd, Walter LA. COVID‐19 presenting as encephalopathy in the emergency department: a case report. Clin Pract Cases Emerg Med. 2021;5(1):26‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Princiotta Cariddi L, Tabaee Damavandi P, Carimati F, et al. Reversible encephalopathy syndrome (PRES) in a COVID‐19 patient. J Neurol. 2020;267(11):3157‐3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sofijanova A, Bojadzieva S, Duma F, Superlishka E, Murtezani A, Jordanova O. Severe encephalitis in infant with COVID‐19: a case report. Open Access Maced J Med Sci. 2020;8(T1):514‐517. [Google Scholar]
- 52. Pilotto A, Odolini S, Masciocchi S, et al. Steroid‐responsive encephalitis in coronavirus disease 2019. Ann Neurol. 2020;88(2):423‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Abdi S, Ghorbani A, Fatehi F. The association of SARS‐CoV‐2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J Neurol Sci. 2020;416:117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dharsandiya M, Shah K, Patel K, Patel T, Patel A, Patel A. SARS‐CoV‐2 viral sepsis with meningoencephalitis. Indian J Med Microbiol. 2020;38(2):219‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Babar A, Lewandowski U, Capin I, et al. SARS‐CoV‐2 encephalitis in a 20‐year old healthy female. Pediatr Infect Dis J. 2020;39(10):e320‐e321. [DOI] [PubMed] [Google Scholar]
- 56. Virhammar J, Kumlien E, Fällmar D, et al. Acute necrotizing encephalopathy with SARS‐CoV‐2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95(10):445‐449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Farhadian S, Glick LR, Vogels CBF, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID‐19. Res Sq. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. de Miranda Henriques‐Souza AM, de Melo ACMG, de Aguiar Coelho Silva Madeiro B, Freitas LF, Sampaio Rocha‐Filho PA, Gonçalves FG. Acute disseminated encephalomyelitis in a COVID‐19 pediatric patient. Neuroradiology. 2021;63(1):141‐145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Afshar H, Yassin Z, Kalantari S, et al. Evolution and resolution of brain involvement associated with SARS‐CoV2 infection: a close clinical – paraclinical follow up study of a case. Mult Scler Relat Disord. 2020;43:102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Crosta F, Simeone PG, Sanrocco C, et al. Neurological features of COVID‐19 infection: a case series of geriatric patients. J Gerontol Geriatr. 2020;68:235‐239. [Google Scholar]
- 61. Sangare A, Dong A, Valente M, et al. Neuroprognostication of consciousness recovery in a patient with COVID‐19 related encephalitis: preliminary findings from a multimodal approach. Brain Sci. 2020;10(11):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. El‐Zein RS, Cardinali S, Murphy C, Keeling T. COVID‐19‐associated meningoencephalitis treated with intravenous immunoglobulin. BMJ Case Rep. 2020;13(9):e237364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lv PU, Peng F, Zhang Y, et al. COVID‐19‐associated meningoencephalitis: a care report and literature review. Exp Ther Med. 2021;21(4):362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kumar A, Olivera A, Mueller N, Howard J, Lewis A. Delayed SARS‐COV‐2 leukoencephalopathy without severe hypoxia. J Neurol Sci. 2020;418:117146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Muccioli L, Pensato U, Cani I, et al. COVID‐19‐related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J Neuroimmunol. 2020;349:577400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All the data in this review are included in the study.


