Abstract
Background
The increase in rare opportunistic microbial infections caused by Morganella morganii is alarming across the globe. It has been reported that in cases of urinary tract infections (UTIs) caused by M. morganii, however, few studies investigated children. Our study aimed to analyze the risk factors, antimicrobial susceptibility, and clinical characteristics, so as to improve the clinical diagnosis and therapy of M. morganii infection.
Methods
Between April 1, 2017 and April 1, 2021, 11 cases of pediatric UTIs caused by M. morganii were included in this retrospective study. Medical records were reviewed and analyzed.
Results
The study population included 10 males and one female between 11 months and 13 years old (mean age: 4 years 9 months). The most common comorbidity was nephrotic syndrome (72.7%, 8/11). Six patients (54.5%) were in the immunosuppressed state due to chemotherapy or immunosuppressant therapy. Ten cases defined as lower UTIs with no specific clinical manifestations had normal or slightly elevated leukocyte counts and procalcitonin (PCT) levels, and normal C‐reactive protein (CRP) levels. One child diagnosed upper UTIs accompanied with fever, high level of leukocyte counts, CRP, and PCT. The M. morganii presented 100% susceptibility to aztreonam, ertapenem, meropenem, piperacillin/tazobactam, cefepime, ceftazidime, cefotetan, ticarcillin/clavulanic acid, and cefoperazone/sulbactam. Almost all patients had good responses to third‐generation cephalosporins antibiotic therapy.
Conclusion
Clinical vigilance for the possibility of M. morganii in pediatric UTIs in combination with underlying disease or immunosuppression is warranted. Treatment strategies should be proposed according to the clinical condition and the antibiotic susceptibility results.
Keywords: bacterial infections, children, community‐acquired, Morganella morganii, urinary tract infection
In this prospective study, urinary tract infections (UTIs) caused by M. morganii among children had good responses to third generation cephalosporins antibiotic therapy. Continuous monitoring of antimicrobial resistance trends of M. morganii are necessary to inform paediatricians aiming at reducing the associated morbidities and mortalities.
1. INTRODUCTION
Morganella morganii is a gram‐negative, rod‐shaped, and facultative anaerobic bacillus, which belongs to human gut commensal microbiota. 1 It is considered as a non‐negligent opportunistic pathogen that mainly causes various infections, such as sepsis, abscess, urinary tract infections (UTIs), chorioamnionitis, and cellulitis. 2 Furthermore, on rare occasions, it may cause potentially fatal systemic infection, especially in postoperative environment and nosocomial as well as in young children and patients with impaired immune system. 1 UTIs are among the most common bacterial infections in children and are associated with significant short‐ and long‐term morbidity. 3 , 4 Precise treatment decisions help to prevent chronification of UTIs in children, related the development of antibiotic resistance, voiding dysfunctions, renal scarring, and systemic abiosis. Uropathogens involved in UTIs should be identified with precision to allow targeted therapeutic decisions. 5 In recent years, more and more published clinical case reports have attempted to clarify the clinical manifestation and management strategies of UTIs caused by M. morganii. 6 , 7 M. morganii is recognized as a new clinical treatment challenge because of ongoing acquisition of antimicrobial resistance genes, which may bring more extensive and challenging multidrug resistance issue. 8 The first study investigating community‐acquired UTIs caused by M. morganii in children has been reported in Turkey. 9 Our study aims to explore the risk, antimicrobial susceptibility, and clinical characteristics, so as to improve the treatment and prognosis of children with UTI caused by M. morganii.
2. MATERIALS AND METHODS
2.1. Samples
From April 1, 2017 to April 1, 2021, a total of 31,705 children were admitted and treated in the pediatric department of the First Affiliated Hospital of Xiamen University. Among the 625 patients proven UTIs, 11 cases with M. morganii UTIs were retrospectively evaluated. The medical records of children diagnosed with UTIs caused by M. morganii were reviewed. Patient information, including demographics, medical history, laboratory findings, treatments, and outcomes were summarized and analyzed.
2.2. Instruments and reagents
Urine specimens may have originated from clean‐catch midstream urine or aseptic bladder catheterizations. Urine samples were inoculated onto eosin methylene blue agar (Autobio Diagnostics Co Ltd.) and Colombian blood agar medium (Autobio Diagnostics Co Ltd.). All inoculated plates were incubated in Thermo M3111 incubator (Thermo Inc.). Pathogen identifications were performed using the Vitek MS‐CHCA (BioMérieux Inc.) and Vitek‐MS automated microbial identification system (BioMérieux Inc.). The cut‐off used for significant bacterial presence was ≥1000–50,000 colony‐forming units (CFU)/ml for urine specimen from bladder catheterization (≥104 CFU/ml with symptoms or ≥105 CFU/ml without symptoms for urine specimen from midstream void). 10
BioMerieux mini Vidas automated immunoassay analyzer (BioMérieux Inc.) and procalcitonin (PCT) kit were used to detect serum PCT. C‐reactive protein (CRP) was detected by VITROS 5,1 FS analyzer (Ortho‐Clinical Diagnostics) using the manufacturer's reagents. The new UF‐1000i analyzer (Sysmex) was used for urinalysis. The urinalysis with positive leucocyte esterase and/or nitrite, bacteriuria, or pyuria is highly sensitive for UTI.
2.3. Antimicrobial susceptibility testing
Antimicrobial susceptibility testing of M. morganii isolates was determined by the automated VITEK 2 compact microbiology analyzer (BioMérieux Inc.) using antimicrobial susceptibility testing (AST)‐GN67 and XN04 cards (BioMérieux Inc.). Results were interpreted as sensitive, intermediate, and resistance based on the Clinical and Laboratory Standards Institute's (CLSI) criterias. 11 , 12 , 13 , 14 , 15 The following antimicrobial agents were used at the concentrations shown: amikacin (30 µg), ampicillin/sulbactam (10/10 µg), amtronam (30 µg), ertapenem (10 µg), trimethoprime/sulfamethoxazole (1.25/23.75 µg), ciprofloxacin (5 µg), fosfomycin (200 µg), chloramphenicol (30 µg), meropenem (10 µg), minocycline (30 µg), moxifloxacin (5 µg), naproxic acid (30 µg), norfloxacin (10 µg), piperacillin (100 µg), piperacillin/tazobactan (100/10 µg), gentamicin (10 µg), ticacillin (75 µg), ticacillin/clavulanic acid (75/10 µg), cefepime (30 µg), cefuroxime (30 µg), cefoperazone/sulbactam (75/30 µg), ceftriaxone (30 µg), cephalothifen (30 µg), cefotaxime (30 µg), ceftazidime (30 µg), cefotetan (30 µg), cefazolin (30 µg), cefazoxime (30 µg), tobramycin (10 µg), imipenem (10 µg), levofloxacin (5 µg), amoxicillin/potassium clavulanate (20/10 µg), ampicillin (10 µg), furantoin (300 µg), tetracycline (30 µg), and tigecycline (15 µg). For statistical analysis purpose, “intermediate” sensitivity results of bacterial isolates were grouped to “resistant” sensitivity results. Multi‐drug resistance was defined as resistance of an isolate to three or more antimicrobial classes tested. 16
2.4. Quality control
The quality control strains of pathogen identification were E. coli ATCC 8739 strains (Biomerieux Inc.). For quality control of susceptibility tests, E. coli ATCC25922 strains (National Center for Clinical Laboratories) were used.
2.5. Ethical clearance
The study was approved by Ethical Review Committee of the First Affiliated Hospital of Xiamen University with number [2022] ERSR‐012.
3. RESULTS
In the 625 patients proven UTI, only 11 cases were identified M. morganii positive in clean‐catch midstream urine culture. During the same period, M. morganii was not detected in the cultures of other body fluids (sputum, cerebrospinal fluid, broncho‐alveolar lavage fluid, pleural effusion, peritoneal effusion, secretion, or exudate, etc.) and catheter culture (central venous catheter, tracheal catheter, thoracic drainage tube, abdominal drainage tube, etc.). The study population included 10 males and one female between 11 months and 13 years old (mean age: 4 years 9 month). The most common comorbidity was nephrotic syndrome (72.7%, 8/11), though systemic lupus erythematosus (SLE) (1/11), acute lymphocytic leukemia (ALL) (1/11), and left ureteropelvic junction obstruction (1/11) also occurred. Six patients (54.5%) were in the immunosuppressed state due to chemotherapy or immunosuppressant therapy. Only one patient (case 6) had fever and none of the patients had urinary tract irritation symptoms. The M. morganii strains were isolated from the samples collected for the culture and urinalysis at the first day of hospitalization. At the time of presentation, all patients had no central venous catheter or urinary catheter. (The characteristics and the clinical manifestation of patients are shown in Table 1). Ten cases defined as lower UTIs with no specific clinical manifestations observed had normal or slightly elevated leukocyte counts and PCT levels, and normal CRP levels. No other bacterial coinfection was isolated from all the urine and blood cultures. Only one child (case 6) diagnosed with upper UTIs accompanied high level of white blood cell (WBC) count, CRP, and PCT. Urinalyses were positive for leukocytes in 11 (100%), hematuria in 3 (36.4%), nitrite in 1 (9.1%), and bacteriuria in 7 (63.6%) children. The laboratory findings were presented in Table 2.
TABLE 1.
Characteristics and the clinical manifestation of patients complicated with Morganella morganii
| Case | Gender | Age | Comorbidity | Use immunosuppressant/chemotherapy drugs | Medical history and cause of admission |
|---|---|---|---|---|---|
| 1 | Male | 7 years | Nephrotic syndrome | None | Edema for 10 days |
| 2 | Male | 1 year and 8 months | Nephrotic syndrome | None | Eyelid edema for 12 days and abnormal urine test for 7 days |
| 3 | Male | 5 years and 10 months | Nephrotic syndrome | Prednison | Nephrotic syndrome for 6 months, urine protein positive accompanied by cough for 10 days |
| 4 | Male | 2 years and 4 months | Nephrotic syndrome | None | Edema for 18 days and cough for 3 days |
| 5 | Male | 13 years | SLE and lupus nephritis | Prednisolone, Mycophenolate Mofetil | SLE for 1 year, with tawny urine for 1 month and abdominal pain for 3 days |
| 6 | Male | 11 months | Left ureteropelvic junction obstruction | None | Fever for 1 day with abnormal urine test |
| 7 | Male | 2 years and 8 months | Nephrotic syndrome | Prednisone | Systemic edema for 8 days and abnormal urine test for 5 days |
| 8 | Male | 2 years and 6 months | Nephrotic syndrome | Prednisone | Nephrotic syndrome for 1 months, urine protein positive for 1 day |
| 9 | Male | 3 years and 9 months | ALL | Chemotherapy | ALL for 9 months and hospitalized for chemotherapy |
| 10 | Female | 9 years | Nephrotic syndrome | Prednison | Nephrotic syndrome for 2 years, urine protein positive for 2 days |
| 11 | Male | 3 years and 7 months | Nephrotic syndrome | None | Cough for half a month, eyelid edema for 10 days |
Abbreviations: ALL, acute lymphocytic leukemia; SLE, systemic lupus erythematosus.
TABLE 2.
Laboratory findings of patients
| Case | WBC count (109/L) | Neutrophil (%) | CRP (mg/L) | PCT (ng/ml) | Blood culture and other bacterial infections | WBC count of urinalysis (/µl) | Bacteriua of urinalysis (/µl) | RBC count of urinalysis (/µl) | Nitrite of urinalysis | B ultrasonography of urinary system | Infection time |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10.93 | 59.2 | 7 | 0.464 | Negative | 38 | 2.8 | 5 | Negative | Bilateral renal enlargement | 2017/04/08 |
| 2 | 6.56 | 17.3 | <0.499 | 0.107 | Negative | 54.1 | 46.9 | 5.8 | Negative | Normal | 2018/01/04 |
| 3 | 11.6 | 80.2 | 0.89 | 0.049 | Negative | 77 | 483 | 1.5 | Negative | Normal | 2018/09/04 |
| 4 | 14.16 | 59.5 | <0.499 | 0.112 | Negative | 59.8 | 38.7 | 2.9 | Negative | Normal | 2019/10/23 |
| 5 | 7.5 | 61.27 | 4.6 | 0.074 | Negative | 64.5 | 34.1 | 31.9 | Negative | Normal | 2019/11/17 |
| 6 | 13.73 | 43.5 | 156.64 | 1.59 | Negative | 6454.7 | 45.5 | 14.4 | Negative | Left kidney hydronephrosis | 2019/02/08 |
| 7 | 11.03 | 25.4 | <0.499 | 0.266 | Negative | 70.4 | 11.3 | 0.7 | Negative | Normal | 2019/04/03 |
| 8 | 8.68 | 48.6 | <0.499 | 0.042 | Negative | 66.3 | 11.3 | 1.1 | Negative | Normal | 2020/01/14 |
| 9 | 3.18 | 37.5 | <0.499 | / | Negative | 242 | >99,999 | 2.9 | Positive | Normal | 2020/05/25 |
| 10 | 7.5 | 52.3 | 2 | 0.211 | Negative | 56 | 240 | 29.4 | Negative | Normal | 2020/09/03 |
| 11 | 6.34 | 56.9 | <0.499 | 0.12 | Negative | 43.7 | 4.6 | 29.6 | Negative | Normal | 2021/03/12 |
Reference intervals were the following: CRP (0–10 mg/L), PCT (<0.046 ng/ml, WBC count of urinalysis [0–18/µl]), RBC count of urinalysis (0–15/µl), bacteriua of urinalysis (0–11.4/µl).
Abbreviations: CRP, C‐reactive protein; PCT, procalcitonin.
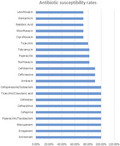
Susceptibility test results of M. morganii isolates against various antibiotics were shown in Table S1. The M. morganii presented 100% susceptibility to aztreonam (11/11), ertapenem (11/11), meropenem (11/11), piperacillin/tazobactam (11/11), cefepime (11/11), ceftazidime (11/11), cefotetan (10/10), ticarcillin/clavulanic acid (7/7), and cefoperazone/sulbactam (6/6). The M. morganii was almost sensitive to amikacin (90.9%, 10/11), ceftriaxone (10/11) and cefotaxime (10/11), and norfloxacin (81.8%, 9/11). The susceptibility testing demonstrated 72.7% (8/11) susceptibility to ciprofloxacin, moxifloxacin, nalidixic acid, gentamicin, ticarcillin, levofloxacin, but 100% resistance to cefuroxime axetil (11/11), cefalotin (11/11), cefazolin (3/3) and 90.9% to ampicillin/sulbactam (10/11).
Almost all patients (10/11) had good responses to third‐generation cephalosporins antibiotic therapy according to guideline. 4 Furthermore, one child (case 5) withdrew treatment because of economic problem. None of the children developed any complications including sepsis, shock, meningitis, etc. The clinical information of 11 patients with M. morganii infection was presented in Table 3.
TABLE 3.
Clinical information of patients
| Case | Antibiotics before urine culture results | Antibiotics after urine culture results | Duration of effective antibiotics therapy | Length of stay | Outcome of infection |
|---|---|---|---|---|---|
| 1 | Flucloxacillin (3 days) | Cefotaxime (3 days) | 3 days | 11 days | Cure |
| 2 | None | Cefixime (3 days) | 3 days | 12 days | Cure |
| 3 | Cefotaxime (5 days) | Ceftazidime (3 days) | 3 days | 11 days | Cure |
| 4 | Cefaclor (3 days) | Ceftriaxone (3 days) | 3 days | 27 days | Cure |
| 5 | None | Withdrawing treatment | Loss to follow‐up | 2 days | Loss to follow‐up |
| 6 | Cefixime (1 day) | Cefotaxime (10 days)and cefixime (3 days) | 14 days | 13 days | Cure |
| 7 | Amoxicillin/clavulanate potassium (5 days) | Ceftriaxone (7 days) | 7 days | 13 days | Cure |
| 8 | None | Cefixime (5 days) | 5 days | 9 days | Cure |
| 9 | Compound sulfamethoxazole (8 months) | Cefixime (3 days) | 3 days | 2 days | Cure |
| 10 | Metronidazole and amoxicillin/potassium clavulanate (2 days) | Cefixime (3 days) | 3 days | 13 days | Cure |
| 11 | Amoxicillin/clavulanate potassium (3 days) | Cefixime (3 days) | 3 days | 8 days | Cure |
4. DISCUSSION
Morganella morganii has been considered a clinical and community‐acquired pathogen with opportunistic infections detected in diverse organ infections across all age groups globally. 17 M. morganii has been reported causing various infections such as bacteremia, osteomyelitis, and UTIs. Claire et al. reported a 3‐year‐old girl with hematologic malignancy suffering from M. morganii bacteremia was successfully treated with ceftazidime‐avibactam and aztreonam combination. 18 M. morganii was cultured positive in blood sample and cephalohematoma aspirated from a neonate developing an infected right parietal cephalohematoma and underlying osteomyelitis. 6 The previous studies of UTIs caused by M. morganii were almost reported in adult. In Jan Hrbacek et al.’s 19 survey from Central European Urology Department between 2011–2019, M. morganii (n = 194, 26.2%) was positive in the pathogens surveyed in urine cultures. Leyla et al. (2019) 20 reported three M. morganii strains were isolated from the urine samples of patients with community‐acquired UTIs. In 2020, a study from Turkey presented 11cases of community‐acquired UTIs caused by M. morganii in children. 9 Similar studies have not been conducted in China.
Compared with the only other pediatric study in Turkish population, 9 we had observed some differences and similar results in these two samples. The clinical manifestations of UTIs in children are nonspecific and largely depend on the age. UTIs in newborn and young infants always manifest as diverse and non‐specific symptoms including temperature instability, hyperexcitability, jaundice, lethargy, abdominal pain, irritability, etc. 10 In older children, the manifestations are more specific including irritative voiding symptoms, fever, chillness, vomiting, abdominal pain, and costovertebral knocking pain, etc. 21 In the Turkish population, 9 irritability (n = 5, 45.5%) and dysuria (n = 5, 45.5%) were the most frequent symptoms of infants with UTIs. Nevertheless, almost all the children had no specific clinical symptoms except for one child with fever in our research. In both samples, none of the patients developed renal failure or electrolyte abnormalities and had clinical signs of bacteremia/sepsis. Of the 11 Turkish patients with urine studies, 81.8% had leukocyturia and 45.5% had hematuria. Similarly, the urinalysis which was positive for leukocytes and hematuria in our sample was 100% and 36.4%, respectively. Nitrite test has a low sensitivity (about 50%) but high specificity (98%) for pediatric UTIs. 21 However, children with positive nitrite was 9.1% (1/11) in our study compared to 54.5% (6/11) in the Turkish study. Atm B et al. 9 showed that 45.5% of children had normal CRP and leukocyte levels in UTI caused by M. morganii. In our study, 10 cases with lower UTIs had normal or slightly elevated leukocyte counts and PCT levels, and normal CRP levels. Only one child (case 6) diagnosed upper UTIs accompanying high level of WBC, CRP, and PCT had left kidney hydronephrosis. Our results were in accord with past reports indicating that CRP had a lower specificity for identifying children with renal parenchymal involvement, whereas a cut‐off value of 1.0 ng/ml of PCT had been shown to be predictive of acute pyelonephritis in young children. 22
The previous investigations had revealed risk factors for M. morganii infections including old age, repeat hospitalizations, nursing home residence, urethral catheter, and prematurity, etc. 23 , 24 None of the children enrolled in our study underwent invasive procedure including peripheral central vein catheterization, urethral catheterization or other operation, which was in accord with the study of Turkish population. 9 M. morganii infections were frequently associated with a history of immunocompromised condition or immunodeficiency diseases such as acquired immune deficiency syndrome, and X‐linked agammaglobulinemia. 17 , 25 In accordance with previous research features, six patients (54.5%) in our study were in the immunosuppressed state due to chemotherapy or immunosuppressant therapy. Unlike the Turkish research, 9 all of our patients have comorbidities, the most common of which is nephrotic syndrome (72.7%, 8/11). The causes of UTIs in nephrotic children include a low level of serum IgG, hypoproteinemia, decreased bactericidal activity of the leukocytes, declined perfusion of the spleen, corticosteroids treatment, and loss of properdin in the urine. 26 M. morganii should be placed as a clinically significant pathogen in the causative possibilities of UTIs in nephrotic children. One patient was detected left ureteropelvic junction obstruction which is an established risk factor for recurrent UTIs. However, there is no convincing evidence that malformation in the urinary system maybe a risk factor for M. morganii infection.
Various mechanisms including intrinsic, acquired, and adaptive resistances can lead to antibiotic resistance. M. morganii has intrinsic resistance to amoxicillin, oxacillin, most of the first‐ and second‐generation cephalosporins, ampicillin, macrolides, lincosamides, glycopeptides, fosfomycin, fusidic acid, and colistin. 2 Jan Hrbacek et al. 19 detected antibiotic susceptibility of 194 M. morganii strains from urine cultures and revealed the cumulative resistance rates of ampicillin (95.7%), amoxiillin/clavulanic acid (92.9%), cefuroxime (89.3%), and nitrofurantoin (95.9%). High resistance to cefuroxime axetil (100%), cefalotin (100%), cefazolin (100%), amoxicillin/clavulanate potassium (100%), nitrofurantoin (100%) and ampicillin/sulbactam (90.9%) was observed in our study. Multidrug‐resistant M. morganii strains carrying mobile genetic elements including plasmids integrons have been presented in the literatures. Fatimah et al. 27 identified that M. morganii (n = 13) counted for 3.7% of extended‐spectrum beta‐lactamase (ESBL) infections and modest‐to‐high resistant to tigecycline (6/13). In the study of N. Mbelle et al., 28 they detected that two multidrug‐resistant M. morganii strains from UTIs were only susceptible to carbapenems, amikacin, and tigecycline. Xiaobing Guo et al. 29 also confirmed that M. morganii L241 exhibited resistance to almost all of the β‐lactam antibiotics including piperacillin/tazobactam, cefotaxime, ceftazidime, cefepime, cefpirome, ertapenem, imipenem, and meropenem with the exception of aztreonam. Nevertheless, there is no multidrug‐resistant M. morganii strain observed in our research. More attention should be paid to the multi‐drug resistance and extensively drug‐resistant M. morganii strains which are increasing globally resulting in treatment failure. 17
M. morganii is normally susceptible to aztreonam, aminoglycosides, antipseudomonal penicillins, third‐ and fourth‐generation cephalosporins, carbapenems, quinolones, trimethoprim/sulfamethoxazole and chloramphenicol. 2 Atm et al., 9 who described antibiotic resistance of M. morganii in pediatric UTIs, reported 100% susceptibility to imipenem, meropenem, piperacillin‐tazobactam and 90.9% to amikacin. Compared to the data of Turkish study, 9 our study proved the same susceptibility rate of meropenem, piperacillin‐tazobactam and amikacin, but susceptibility rate of imipenem (44.4%, 4/9) was lower in our survey. Although the exact reasons for conflicting results of imipenem susceptibility are unclear, differences in age, race, geographic area, and methodology may explain the discrepancies. The antibiotic susceptibility rates of an adults’ UTIs investigation enrolling 194 strains of M. morganii were compared to our results including meropenem (98.9% vs. 100%), amikacin (95.7% vs. 90.9%), ertapenem (86.8% vs. 100%), piperacillin/tazobactam (86.4% vs. 100%), ceftazidime (86.3% vs. 100%), cefepime (85.7% vs. 100%), cefotaxime (83.7% vs. 90.9%), ofloxacin (73.9% vs. 72.7%), cotrimoxazole (72.5% vs. 63.6%), and ciprofloxacin (65.6% vs. 72.7%). 19 The differences may be due to the small sample size of our study group and different clinical features between children and adults. For the treatment of M. morganii infections, gentamicin was the most frequently used antibiotic, and ciprofloxacin, amikacin, and ceftriaxone were also used frequently in adult patients, but the use of the third‐generation cephalosporins is still recommended in the children in the consideration of the aminoglycoside‐induced nephrotoxicity or cochleotoxicity. 30 In Turkish study, hospitalized children received piperacillin‐tazobactam, whereas outpatients were given amikacin, with good clinical response. 9 In our study, almost all patients (10/11) had good responses to third‐generation cephalosporins antibiotic treatment. None of the children developed any complications including sepsis, shock, meningitis, etc.
To our knowledge, this study firstly described the clinical manifestations and resistance patterns of M. morganii in pediatric UTIs in China. However, our research has certain limitations. Firstly, this was a single‐center study with the limitation of sample size. Additionally, the manifestations related information was limited in our retrospective study.
In summary, our study described that lower UTIs caused by M. morganii with no specific clinical manifestations had normal or slightly elevated leukocyte counts and PCT levels, and normal CRP levels, but pyelonephriti may accompany with fever symptom, high level of WBC, CRP, and PCT. Immunocompromised condition is one of M. morganii infection risk factors. The M. morganii presented high susceptibility to aztreonam, ertapenem, meropenem, piperacillin/tazobactam, cefepime, ceftazidime, cefotetan, ticarcillin/clavulanic acid, and cefoperazone/sulbactam. Almost all patients had good responses to third‐generation cephalosporins antibiotic treatment. Most UTIs caused by M. morganii patients have a good prognosis with no further complications.
Morganella morganii is a conditionally pathogenic microorganism with relatively few cases of clinical infection among children. Clinical vigilance for the possibility of M. morganii in pediatric UTIs in combination with underlying disease or immunosuppression is warranted. Continuous monitoring of antimicrobial resistance trends of M. morganii is necessary to inform paediatricians aiming at reducing the associated morbidities and mortalities. Treatment strategies should be proposed according to clinical condition and the antibiotic susceptibility results.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Chen Xianrui and Shi Huixuan designed the study. Shi Huixuan analyzed and interpreted the data, and drafted the article. Yao Yonghua and Xu Jinping acquired the data. Chen Xianrui is the corresponding author who contributed to conception and design, critical revision of the article for important intellectual content. All authors read and approved the final manuscript.
Supporting information
Table S1
ACKNOWLEDGMENTS
We would like to acknowledge all staff members for participation in and contribution to this study. We also thank all patients included in this research.
Shi H, Chen X, Yao Y, Xu J. Morganella morganii: An unusual analysis of 11 cases of pediatric urinary tract infections. J Clin Lab Anal. 2022;36:e24399. doi: 10.1002/jcla.24399
DATA AVAILABILITY STATEMENT
The data that support the findings of the current study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Zaric RZ, Jankovic S, Zaric M, Milosavljevic M, Stojadinovic M, Pejcic A. Antimicrobial treatment of Morganella morganii invasive infections: systematic review. Indian J Med Microbiol. 2021;39(4):404‐412. [DOI] [PubMed] [Google Scholar]
- 2. Liu H, Zhu J, Hu Q, Rao X. Morganella morganii, a non‐negligent opportunistic pathogen. Int J Infect Dis. 2016;50:10‐17. [DOI] [PubMed] [Google Scholar]
- 3. Tullus K, Shaikh N. Urinary tract infections in children. Lancet. 2020;395(10237):1659‐1668. [DOI] [PubMed] [Google Scholar]
- 4. Veauthier B, Miller MV. Urinary tract infections in young children and infants: common questions and answers. Am Fam Physician. 2020;102(5):278‐285. [PubMed] [Google Scholar]
- 5. Lemberger U, Quhal F, Bruchbacher A, Shariat SF, Hiess M. The microbiome in urinary tract infections in children – an update. Curr Opin Urol. 2021;31(2):147‐154. [DOI] [PubMed] [Google Scholar]
- 6. Staudt MD, Etarsky D, Ranger A. Infected cephalohematomas and underlying osteomyelitis: a case‐based review. Childs Nerv Syst. 2016;32(8):1363‐1369. [DOI] [PubMed] [Google Scholar]
- 7. Paul SP, Newman LM, Mubashar Y, Turner PC. Morganella morganii: a rare cause of early onset neonatal sepsis and meningitis. Br J Hosp Med. 2020;81(10):1‐3. [DOI] [PubMed] [Google Scholar]
- 8. Xiang G, Lan K, Cai Y, et al. Clinical molecular and genomic epidemiology of Morganella morganii in China. Front Microbiol. 2021;12:744291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Atm B, Kara SS, Aslan MH. Community‐acquired pediatric urinary tract infections caused by Morganella Morganii. J Pediatr Res. 2020;7(2):121‐125. [Google Scholar]
- 10. Stein R, Dogan HS, Hoebeke P, et al. Urinary tract infections in children: EAU/ESPU guidelines. Eur Urol. 2015;67(3):546‐558. [DOI] [PubMed] [Google Scholar]
- 11. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing CLSI Supplement M100S, 26th ed. Clinical and Laboratory Standards Institute; 2016. [Google Scholar]
- 12. Clinical and Laboratory Standards Institute (CLSI) . Performance Standards for Antimicrobial Susceptibility Testing, 27th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- 13. Clinical and Laboratory Standards Institute (CLSI) . Performance Standards for Antimicrobial Susceptibility Testing. 28th informational supplement, MS100‐S28. CLSI; 2018. [Google Scholar]
- 14. Clinical and Laboratory Standards Institute (CLSI) . Performance Standards for Antimicrobial Susceptibility Testing. 29th informational supplement, MS100‐S29. CLSI; 2019. [Google Scholar]
- 15. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing, 30th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute; 2020. [Google Scholar]
- 16. Magiorakos AP, Srinivasan A, Carey R, et al. Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268‐281. [DOI] [PubMed] [Google Scholar]
- 17. Bandy A. Ringing bells: Morganella morganii fights for recognition. Public Health. 2020;182:45‐50. [DOI] [PubMed] [Google Scholar]
- 18. Hobson AC, Bonacorsi S, Fahd M, et al. Successful treatment of bacteremia due to NDM‐1‐producing Morganella morganii with aztreonam and ceftazidime‐avibactam combination in a pediatric patient with hematologic malignancy. Antimicrob Agents Chemother. 2018;63(2):e02463‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hrbacek J, Cermak P, Zachoval R. Current antibiotic resistance trends of uropathogens in central Europe: survey from a tertiary hospital urology department 2011–2019. Antibiotics. 2020;9(9):2011‐2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Minnullina L, Pudova D, Shagimardanova E, Shigapova L, Sharipova M, Mardanova A. Comparative genome analysis of uropathogenic Morganella morganii strains. Front Cell Infect Microbiol. 2019;9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang SS, Tsai JD, Kanematsu A, Han CH. Asian guidelines for urinary tract infection in children. J Infect Chemother. 2021;27(11):1543‐1554. [DOI] [PubMed] [Google Scholar]
- 22. t Hoen LA, Bogaert G, Radmayr C, et al. Update of the EAU/ESPU guidelines on urinary tract infections in children. J Pediatr Urol. 2021;17(2):200‐207. [DOI] [PubMed] [Google Scholar]
- 23. Erlanger D, Assous MV, Wiener‐Well Y, Yinnon AM, Ben‐Chetrit E. Clinical manifestations, risk factors and prognosis of patients with Morganella morganii sepsis. J Microbiol Immunol Infect. 2019;52(3):443‐448. [DOI] [PubMed] [Google Scholar]
- 24. Learman BS, Brauer AL, Eaton KA, Armbruster CE. A rare opportunist, Morganella morganii, decreases severity of polymicrobial catheter‐associated urinary tract infection. Infect Immun. 2019;88(1):e00691‐e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cho YK, Kook H, Woo YJ, Choi YY, Ma JS, Hwang TJ. Morganella morganii pericarditis in a child with X‐linked agammaglobulinemia. Pediatr Int. 2010;52(3):489‐491. [DOI] [PubMed] [Google Scholar]
- 26. Sorkhi H, Riahi SM, Ebrahimpour S, Shaikh N, Rostami A. Urinary tract infection in children with nephrotic syndrome: a systematic review and meta‐analysis. Microb Pathog. 2019;137:103718. [DOI] [PubMed] [Google Scholar]
- 27. Faa A, Aar B, Saa C, et al. ESBL expression and antibiotic resistance patterns in a hospital in Saudi Arabia: Do healthcare staff have the whole picture? J Infect Public Health. 2020;13(5):759‐766. [DOI] [PubMed] [Google Scholar]
- 28. Mbelle N, Osei Sekyere J, Feldman C, Maningi NE, Modipane L, Essack SY. Genomic analysis of two drug‐resistant clinical Morganella morganii strains isolated from UTI patients in Pretoria, South Africa. Lett Appl Microbiol. 2020;70(1):21‐28. [DOI] [PubMed] [Google Scholar]
- 29. Guo X, Rao Y, Guo L, et al. Detection and genomic characterization of a Morganella morganii isolate from China that produces NDM‐5. Front Microbiol. 2019;10:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mizrahi A, Delerue T, Morel H, et al. Infections caused by naturally AmpC‐producing Enterobacteriaceae: can we use third‐generation cephalosporins? A narrative review. Int J Antimicrob Agents. 2020;55(2):105834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
The data that support the findings of the current study are available from the corresponding author upon reasonable request.


