Abstract
The terminal oxidase complexes encoded by coxMNOP and coxWXYZ were studied by analysis of mutations in each of the two oxidases. Carbon monoxide difference spectra obtained from membranes of coxMNOP mutant bacteroids were like those obtained for the wild type, whereas bacteroid membranes of a coxWXYZ mutant were deficient in CO-reactive cytochrome b. Experiments involving cyanide inhibition of oxidase activity were consistent with the conclusion that the coxX mutant is deficient in a membrane-associated O2-binding component. The viable cell number (bacteria that could be recultured from crushed nodules) was 20 to 29% lower for the coxX mutant than for the wild-type or the CoxN− strain. In three separate greenhouse studies, nodules of a coxX mutant had significantly lower (28 to 34% less) acetylene reduction rates than the wild-type nodules did, and plants inoculated with a double mutant (coxMNOP coxWZYZ) had rates 30% lower than those of wild-type-inoculated plants.
Bradyrhizobium japonicum exists both as a free-living soil organism and as a symbiotic bacteroid that fixes N2 in the low-O2 environment of the legume root nodule (see references 1 and 2). In symbiosis, it is incumbent upon the vigorously respiring bacteroid to generate sufficient ATP and reductant to supply the energy-intensive nitrogen fixation process. Nevertheless, the O2 level in the bacteroid must be maintained at levels low enough so as not to inactivate nitrogenase (3). Although there is no doubt as to the multiplicity of O2-binding components in bacteroids, their characteristics and especially their O2 affinity properties relative to each other are largely unknown.
Spectral and inhibition studies on membranes isolated from free-living and bacteroid forms of B. japonicum have revealed the existence of a number of terminal oxidases. These include an aa3-type cytochrome c oxidase, a heme b-containing ubiquinol oxidase, a high-O2-affinity cytochrome c oxidase, and an unusual putative flavoprotein oxidase (see reference 18). A cytochrome c oxidase complex that contains seven to eight subunits and CO-reactive cytochrome c was purified from B. japonicum bacteroid membranes (12). This oxidase is capable of functioning at O2 concentrations of less than 1.0 μM. At least one of the subunits, a heme c-containing peptide, was unique to the symbiotic state. Most of the subunits of this complex appear to correspond to those encoded by the fixNOQP gene cluster (19), which encodes a cbb3-type oxidase that has an O2 affinity (Km) of approximately 7 nM (20) and is expressed microaerobically. Molecular approaches to identify genes encoding the many B. japonicum terminal oxidases have been successful; for example, genes for four terminal oxidases belonging to the heme-copper cytochrome family of terminal oxidases have been cloned. These are gene clusters fixNOQP (19), coxMNOP (5), coxBA (4, 7), and coxWXYZ (21–23)). coxBA encodes two subunits of the cytochrome aa3 oxidase complex and is expressed only under conditions of high aeration (8, 9). coxMNOP and coxWXYZ encode complexes with similarities to CuA-containing cytochrome c oxidases (5) and b-type ubiquinol oxidases (22, 23), respectively. Based on the predicted properties of CoxWXYZ and due to the lack of detectable heme O in B. japonicum (23), it was concluded that the CoxWXYZ complex is a bb3-type ubiquinol oxidase. The expression and key roles played by B. japonicum FixNOQP and CoxBA in symbiosis and in free-living culture, respectively, have been previously described (8, 20), and the unique roles of CoxWXYZ and CoxMNOP in microaerobic H2-dependent growth were recently reported (24).
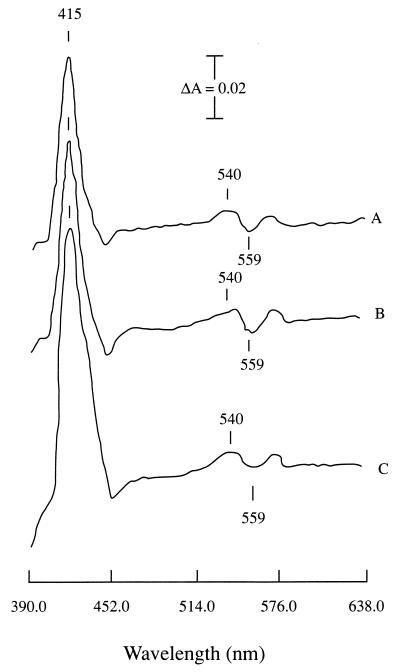
To determine the physiological roles of the terminal oxidases encoded by coxWXYZ and coxMNOP, bacterial strains that contain mutations in each of these two terminal oxidases were analyzed. The coxWXYZ mutant, strain JHK12, has a Kmr cassette inserted in the coxX open reading frame (23). Strain Bj3430 was described previously; it contains an omega insertion in the coxN open reading frame of the coxMNOP complex (5). A strain with mutations in both of these oxidases has also been recently described (24). Dithionite-reduced minus air-oxidized difference spectra for the wild-type and coxX mutant membranes obtained from bacteroids (13, 15) were similar to those obtained previously (13) for wild-type B. japonicum bacteroid membranes (data not shown). Therefore, we initially thought the CoxWXYZ oxidase was not expressed in the symbiotic state. However, we also reasoned that the abundance of net cytochrome b in bacteroid membranes could make mutants lacking any one of them indistinguishable from the wild type in simple difference spectral studies. Therefore, CO difference spectra (with dithionite as the reductant) were used to more precisely analyze membrane terminal oxidase content. Spectral analysis with CO (15, 25) was consistent with the conclusion that coxX mutant membranes are deficient in the symbiotic expression of a CO-reactive cytochrome b (see Fig. 1). A CO-reactive cytochrome b previously identified in wild-type bacteroid membranes (1, 15) exhibits a trough in the area of 558 nm and shoulders at 572 and 540 nm; these features are reduced by more than 30% in the membranes of the coxX mutant. Concomitantly, the 415-nm peak, attributed primarily to a cytochrome c-552 in bacteroids (15, 16, 18), is 32% larger in the mutant strain than in the wild type. These percent differences were based on equivalent membrane protein concentrations and were reproducible in three separate experiments. CO difference spectra of the coxN mutant strain membranes revealed no differences from those of the wild type (Fig. 1); the scan shows that the mutant had the same size trough at 559 nm (A540 minus A559) as the wild type.
FIG. 1.
Carbon monoxide difference spectra of dithionite-reduced bacteroid membranes of wild-type B. japonicum JH (A), coxN mutant strain Bj3430 (B), and coxX mutant strain JHK12 (C). The amount of CO-reactive cytochrome b was based on the A540 minus the A559. Protein concentrations in milligrams per milliliter were as follows: for JH, 3.1; for Bj3430, 3.3; and for JHK12, 3.0.
Previously, we titrated the oxidase activity of B. japonicum membranes by using different cyanide concentrations ranging from 2 × 10−8 to 5 × 10−4 M so that we could assess the complement of terminal respiratory components in microaerobically incubated cells from the wild type, JHK12, and Bj3430 strains (24). The inhibition of O2 uptake by CN− for wild-type membranes was triphasic, with Kis of approximately 0.1, 0.70, and 50 μM. Both of the terminal oxidase mutant strains exhibited a different inhibition pattern than was seen for the wild type in the 1.0 μM cyanide concentration region (24). Previous CN− titration inhibition patterns of O2-dependent H2 oxidation activity on B. japonicum bacteroid membranes (15) revealed three inhibition phases with Kis of 0.8, 9.4, and 90.9 μM. In the present study (with a different B. japonicum parent strain), three Kis were also observed by CN− inhibition of NADH-dependent O2 uptake. These were at 0.4 μM, between 1.0 and 2.0 μM, and around 100 μM cyanide. However, the data (not shown) for bacteroid membranes did not clearly differentiate between inhibitory phases of the mutants and the wild type.
To assess the possible roles of these oxidase complexes in symbiotic nitrogen fixation, soybean plants were inoculated with the wild-type and mutant terminal oxidase strains and tested for acetylene reduction activity. In three separate greenhouse experiments, the coxX mutant had significantly less activity than that of the parent strain. Among the three experiments, the coxX mutant strain had activities ranging from 66 to 72% of the parent strain, and all these differences were statistically significant due to the number of replicates used (see Table 1). Plants inoculated with the double mutant had symbiotic nitrogen fixation rates comparable to those of the coxX mutant. In contrast, strain Bj3430 (coxN mutant) exhibited no statistically significant differences in nitrogen fixation from the wild-type nodules (Table 1). The latter result is consistent with earlier studies on the coxN mutant (5).
TABLE 1.
Symbiotic nitrogen fixation abilities of strains
| Expt | n | C2H4 reduction (μmol/h/g of nodule wt) by straina:
|
|||
|---|---|---|---|---|---|
| JH | JHK12 | Bj3430 | JHKS4 | ||
| 1 | 24 | 18.2 ± 4.9 | 11.9 ± 3.7 | ||
| 2 | 35 | 16.1 ± 4.1 | 10.8 ± 3.2 | ||
| 3 | 29 | 19.5 ± 6.9 | 14.0 ± 3.6 | 17.5 ± 5.9 | 13.6 ± 3.8 |
All results are given as means ± standard deviations. According to Student’s t distribution test (14), t* is >2.5 for all comparisons of strain JH versus JHK12 and strain JH versus JHKS4, so the results for strain JHK12 (all experiments) and JHKS4 (experiment 3) are significantly less than those for strain JH at the 99% confidence level. The Bj3430 results are not significantly different from the strain JH results. Procedures for growth of soybeans and plant nutrient supplementation (12) and assays for acetylene (C2H2) reduction of root nodules have been described previously (10, 17). Nodule weights varied from 0.28 to 0.85 g per plant. n is the number of replicates, with each individual n being all the nodules from one plant.
A clue as to the nature of the deficiency affecting the symbiosis by the coxX mutant strain came from nodule bacteroid occupancy (viability) studies. On a per gram of nodule weight basis, the coxX mutant had significantly fewer bacteria than the parent strain that could be recultured in the free-living state when plated on agar medium. The procedures were performed as described previously (6), except that the nodules were initially rinsed with 70% ethanol rather than 95% ethanol and the GSY medium for plating contained 50 μg of cycloheximide per ml. The other antibiotics in the medium were as follows: for strain JH, 100 μg of rifampin per ml; for strain JHK12, 100 μg each of rifampin and kanamycin per ml; and for strain Bj3430, 100 μg of streptomycin per ml. In three separate experiments, the wild-type strain had 5.7 × 1011 ± 0.9 × 1011 (mean ± standard deviation), 7.5 × 1011 ± 1.1 × 1011, and 6.4 × 1011 ± 0.8 × 1011 viable bacteria per g of nodule weight. The JHK12 strain (coxX mutant) had approximately 80, 71, and 75%, respectively, of these wild-type viable cell numbers for the same amount of nodule fresh mass. The coxN mutant did not incur a significant loss in the viable number of bradyrhizobia reisolated. It is unclear whether the coxX mutation affects nodule physiology, which in turn moderately affects the number of viable bacteroids, or if the terminal oxidase mutation affects the ability of bacteroids per se to divide during nodule development. Yet a third possibility is that the reestablishment of the free-living organism from the bacteroid condition is influenced by the lack of CoxWXYZ. Nevertheless, these results, like the symbiotic nitrogen fixation results described above, indicate an important role for the CoxWXYZ terminal oxidase in symbiosis. Most likely, the FixNOQP functions in nanomolar levels of free O2 in mature bacteroids, whereas the CoxWXYZ oxidase functions in micromolar levels of O2 in the earlier stages of mature bacteroid or nodule development. CoxMNOP apparently does not function in or significantly affect the symbiosis.
REFERENCES
- 1.Appleby C A. Plant hemoglobin properties, function, and genetic origin. In: Ludden P W, Burris J E, editors. Nitrogen fixation and CO2 metabolism. New York, N.Y: Elsevier Science Publishing Co.; 1984. pp. 41–51. [Google Scholar]
- 2.Appleby C A. The origin and functions of haemoglobin in plants. Sci Progress Oxford. 1994;76:365–398. [Google Scholar]
- 3.Batut J, Boistart P. Oxygen control in Rhizobium. Antonie Leeuwenhoek. 1994;66:129–150. doi: 10.1007/BF00871636. [DOI] [PubMed] [Google Scholar]
- 4.Bott M, Bollinger M, Hennecke H. Genetic analysis of the cytochrome c–aa3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol. 1990;4:2147–2157. doi: 10.1111/j.1365-2958.1990.tb00576.x. [DOI] [PubMed] [Google Scholar]
- 5.Bott M, Preisig O, Hennecke H. Genes for a second terminal oxidase in Bradyrhizobium japonicum. Arch Microbiol. 1992;158:335–343. doi: 10.1007/BF00245362. [DOI] [PubMed] [Google Scholar]
- 6.Frustaci J M, O’Brian M R. Characterization of a Bradyrhizobium japonicum ferrochelatase mutant and isolation of the hemH gene. J Bacteriol. 1992;174:4223–4229. doi: 10.1128/jb.174.13.4223-4229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabel C, Maier R J. Nucleotide sequence of the coxA gene encoding subunit 1 of cytochrome aa3 of Bradyrhizobium japonicum. Nucleic Acids Res. 1990;18:6143. doi: 10.1093/nar/18.20.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabel C, Maier R J. Oxygen-dependent transcriptional regulation of cytochrome aa3 in Bradyrhizobium japonicum. J Bacteriol. 1993;175:128–132. doi: 10.1128/jb.175.1.128-132.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gabel C, Bittinger M A, Maier R J. Cytochrome aa3 gene regulation in members of the family Rhizobiaceae: comparison of copper and oxygen effects in Bradyrhizobium japonicum and Rhizobium tropici. Appl Environ Microbiol. 1994;60:141–148. doi: 10.1128/aem.60.1.141-148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia-Horsman J A, Barquera B, Rumbley J, Ma J, Gennis R B. The superfamily of heme-copper respiratory oxidases. J Bacteriol. 1994;176:5587–5600. doi: 10.1128/jb.176.18.5587-5600.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham L, Maier R J. Variability in molybdenum uptake activity in Bradyrhizobium japonicum strains. J Bacteriol. 1987;169:2555–2560. doi: 10.1128/jb.169.6.2555-2560.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keefe R G, Maier R J. Purification and characterization of an O2-utilizing cytochrome-c oxidase complex from Bradyrhizobium japonicum bacteroid membranes. Biochim Biophys Acta. 1993;1183:91–104. doi: 10.1016/0005-2728(93)90008-4. [DOI] [PubMed] [Google Scholar]
- 13.Mutaftschiev S, O’Brian M R, Maier R J. Hydrogen oxidation activity in membranes from Rhizobium japonicum. Biochim Biophys Acta. 1983;722:372–380. [Google Scholar]
- 14.Noether G. Introduction to statistics: a fresh approach. Boston, Mass: Houghton Mifflin Co.; 1971. [Google Scholar]
- 15.O’Brian M R, Maier R J. Involvement of cytochromes and a flavoprotein in hydrogen oxidation in Rhizobium japonicum bacteroids. J Bacteriol. 1983;155:481–487. doi: 10.1128/jb.155.2.481-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brian M R, Maier R J. Expression of cytochrome o in hydrogen uptake constitutive mutants of Rhizobium japonicum. J Bacteriol. 1985;161:507–514. doi: 10.1128/jb.161.2.507-514.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brian M R, Kirshbom P M, Maier R J. Tn5-induced cytochrome mutants of Bradyrhizobium japonicum: effects of the mutations on cells grown symbiotically and in culture. J Bacteriol. 1987;169:1089–1094. doi: 10.1128/jb.169.3.1089-1094.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brian M R, Maier R J. Molecular aspects of the energetics of nitrogen fixation in the Rhizobium-legume symbiosis. Biochim Biophys Acta. 1989;974:229–246. doi: 10.1016/s0005-2728(89)80239-7. [DOI] [PubMed] [Google Scholar]
- 19.Preisig O, Anthamatten D, Hennecke H. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci USA. 1993;90:3309–3313. doi: 10.1073/pnas.90.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Preisig O, Zufferey R, Thöny-Meyer L, Appleby C A, Hennecke H. A high-affinity cbb3-type cytochrome oxidase terminates the symbiosis-specific respiratory chain of Bradyrhizobium japonicum. J Bacteriol. 1996;178:1532–1538. doi: 10.1128/jb.178.6.1532-1538.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surpin M A, Moshiri F, Murphy A M, Maier R J. Genetic evidence for a fourth terminal oxidase from Bradyrhizobium japonicum. Gene. 1994;143:73–77. doi: 10.1016/0378-1119(94)90607-6. [DOI] [PubMed] [Google Scholar]
- 22.Surpin M A. The cloning and characterization of a cytochrome terminal oxidase complex from the symbiotic nitrogen fixing bacterium Bradyrhizobium japonicum. Ph.D. thesis. Baltimore, Md: The Johns Hopkins University; 1995. [Google Scholar]
- 23.Surpin M A, Lübben M, Maier R J. The Bradyrhizobium japonicum coxWXYZ gene cluster encodes a bb3-type ubiquinol oxidase. Gene. 1996;183:201–206. doi: 10.1016/s0378-1119(96)00559-8. [DOI] [PubMed] [Google Scholar]
- 24.Surpin M A, Maier R J. Roles of the Bradyrhizobium japonicum terminal oxidase complexes in microaerobic H2-dependent growth. Biochim Biophys Acta. 1998;1364:37–45. doi: 10.1016/s0005-2728(98)00003-6. [DOI] [PubMed] [Google Scholar]
- 25.Wong T-Y, Maier R J. Hydrogen-oxidizing electron transport components in nitrogen-fixing Azotobacter vinelandii. J Bacteriol. 1984;159:348–352. doi: 10.1128/jb.159.1.348-352.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]