Abstract
Objective:
To analyze the effect of economic and racial/ethnic residential segregation on breast cancer-specific survival (BCSS) in South Florida, a diverse metropolitan area that mirrors the projected demographics of many United States (US) regions.
Summary Background Data:
Despite advances in diagnosis and treatment, racial and economic disparities in BCSS. This study evaluates these disparities through the lens of racial and economic residential segregation, which approximate the impact of structural racism.
Methods:
Retrospective cohort study of stage I-IV BC patients treated at our institution from 2005–2017. Our exposures include index of concentration at the extremes (ICE), a measurement of economic and racial neighborhood segregation, which was computed at the census-tract level using American Community Survey data. The primary outcome was BCSS.
Results:
Random effects frailty models predicted that patients living in low-income neighborhoods had higher mortality compared to those living in high-income neighborhoods (HR:1.56, 95%CI:1.23–2.00). Patients living in low-income non-Hispanic Black (NHB) and Hispanic neighborhoods had higher mortality compared to those living in high-income non-Hispanic White (NHW) neighborhoods (HR: 2.43, 95%CI:1.72, 3.43) and (HR: 1.99, 95%CI: 1.39, 2.84), after controlling for patient characteristics, respectively. In adjusted race-stratified analysis, NHWs living in low-income NHB neighborhoods had higher mortality compared to NHWs living in high-income NHW neighborhoods (HR:4.09, 95%CI:2.34–7.06).
Conclusions:
Extreme racial/ethnic and economic segregation were associated with lower BCSS. We add novel insight regarding NHW and Hispanics to a growing body of literature that demonstrate how the ecological effects of structural racism—expressed through poverty and residential segregation—shape cancer survival.
Keywords: Structural Racism, Breast Cancer
Mini-Abstract:
Using random effects frailty models, we show that extreme racial/ethnic and economic segregation was associated with lower breast cancer-specific survival. This adds novel insight to a growing body of literature that demonstrate how the ecological effects of structural racism—expressed through poverty and residential segregation—shape cancer survival.
Introduction
Despite significant advances in breast cancer screening, diagnosis, and treatment, well-documented racial/ethnic and socioeconomic survival disparities persist. Non-Hispanic black (NHB) women continue to have the lowest breast cancer-specific survival compared to their non-Hispanic white (NHW) and Hispanic counterparts.1–3 The drivers of this survival gap are multifactorial, with increasing inquiry on residential segregation.4–6 Specifically, residential segregation refers to the geographic separation of marginalized economic and racial/ethnic groups and is a product of structural racism and classism expressed through discriminatory housing policies in the twentieth century.5 Though many of these policies have been removed, residential segregation remains a fundamental cause of health disparities in the United States (US).4–7
Residential segregation warrants consideration as a standard ecological risk factor for monitoring cancer inequities given its role as a population driver of cancer outcomes across the cancer continuum.4, 8–11 Residential segregation contributes to disadvantaged and under-resourced neighborhoods and to negative health outcomes as a result of exposure to substandard housing, access to health care, and social isolation.5 The literature has consistently shown that residential segregation is a driver of breast cancer survival.4, 8–11 However, many of these studies had methodological limitations associated with interrelationships between neighborhood-level and individual-level characteristics or focused on the impact of either racial segregation or economic segregation on survival, rather than their joint effects.12–14 In other studies, women living in neighborhoods characterized by economic disadvantage experienced increased breast cancer mortality compared to those living in more economically privileged areas, even after controlling for individual-level characteristics. 7 However, even while accounting for individual-level race/ethnicity, these analyses do not consider neighborhood-level racial/ethnic segregation. 7
To assess the synergistic impact of economic and racial/ethnic segregation, public health researchers have used the Index of Concentration at the Extremes (ICE) as a measure of residential segregation and socio-spatial polarization. ICE was initially introduced as a tool to measure extreme economic segregation, but was adapted to incorporate racial and/or ethnic segregation as well.12, 13 Unlike other measures of residential segregation, ICE measures concentrations of privilege and deprivation simultaneously, and minimizes multicollinearity in models using measures of neighborhood poverty and wealth, as well as measures of racial/ethnic composition.14, 15 In doing so, it brings subtle social inequalities and polarization to the forefront and maps a critical dimension of social inequality not otherwise captured by metrics that characterize areas solely in terms of the proportion of the population at a specified socioeconomic level or identified as belonging to a particular racial/ethnic group.
This study assessed the relationships between neighborhood polarization (operationalized through economic, racial/ethnic, and racialized economic ICE measures) and breast cancer-specific survival in multilevel analyses. The primary objective was to identify the impact of neighborhood-level economic segregation and racial/ethnic segregation, while also investigating the joint impact of racialized economic segregation. In doing so, we capitalized on our unique Miami-Dade County population which includes two of the nations most segregated cities (Hialeah and Miami) and also allows for novel insight on economic and racial/ethnic segregation in a predominantly Hispanic/Latinx population by assessing these relationships in the context of Hispanic neighborhoods.
Methods
Study Site and Population
We used our local tumor registry, a single retrospectively maintained cancer registry across two hospital systems consisting of a South Florida National Cancer Institute (NCI) designated cancer center and partner safety-net hospital. Using this tumor registry, we identified patients diagnosed and treated for stage I-IV breast cancer between 2005–2017 at either our NCI-designated cancer center or affiliated safety-net hospital. Our catchment area includes Broward, Miami-Dade, Monroe, and Palm Beach counties. This region spans 10,000 square miles and is home to 6.2 million people, approximately 30% of South Florida’s total population. This study population is among the most diverse in the nation in terms of race/ethnicity, ancestry, and cultural identity with nearly half of South Florida residents born in Latin America or the Caribbean.16
Variables of Interest
Self-reported patient race/ethnicity was categorized into NHW, NHB, and Hispanic. We obtained census tract-level measures for income and race/ethnicity from American Community Survey (ACS) 5-year estimates from 2014 – 2018 to compute ICE and merged these estimates into our data. Census tracts served as proxies for neighborhoods because they are the smallest census unit for which racial/ethnic variables are considered statistically reliable (Table 1).17
Table 1:
Index of Concentration at the Extremes (ICE)
| Measure | Formula | ACS Table Numbers |
|---|---|---|
| ICE (high vs. low income) | (high-income households – low-income households)/Total households | B19001 |
| ICE (NHW vs. NHB) | (NHW persons – NHB persons)/Total population | B03002 |
| ICE (NHW vs. Hispanic) | (NHW persons – Hispanic persons)/Total population | B03003 |
| ICE (high-income NHW vs. low-income NHB) | (High-income NHW households – low-income NHB households)/Total households | B19001, B19001H, B19001B |
| ICE (high-income NHW vs. low-income Hispanic) | (High income NHW households – low-income Hispanic households)/Total households | B19001, 19001H |
Extreme marginalization is defined as neighborhoods having the lowest income levels and/or highest concentrations of NHB and Hispanics, while extreme privilege is defined as wealthier and/or more NHW-concentrated neighborhoods. ICE ranges from −1, where all households are considered marginalized, to 1, where all households are considered privileged. Zero would indicate neither extreme marginalization nor privilege, i.e. that these groups are equal in number in a given neighborhood. We examined both economic segregation, racial/ethnic segregation, and a segregation measure that incorporates both economic segregation and racial/ethnic segregation which was named racialized economic segregation. Five ICE variables were computed for this study: (1) ICEIncome representing high vs. low income concentration, (2) ICENHB representing NHW vs. NHB segregation, (3) ICEHispanic representing NHW vs. Hispanic segregation, (4) ICEIncomeNHB representing Black racialized economic segregation (low-income NHB neighborhoods vs. high-income NHW neighborhoods), and (5) ICEIncomeHispanic representing Hispanic racialized economic segregation (low-income Hispanic neighborhoods vs. high-income NHW neighborhoods). Census tract level medium household income was used as a proxy for neighborhood household income. We defined high and low household income on the national distribution of incomes.18 High income was defined as those earning $100,000 or more a year and low-income was defined as those earning less than $20,000 a year. We used these cutoffs to calculate the income ICE variable. All Census tract-level ICE metrics for our study were divided into quartiles based on all census tracts in Florida, where Q1 represents the most marginalized neighborhoods and Q4 represents the most advantaged neighborhoods. 15, 19, 20
Covariates included age at diagnosis, insurance status, risk factors, comorbidities, tumor characteristics (e.g, subtype), and National Comprehensive Cancer Network (NCCN) guideline-appropriate treatment. Age at diagnosis was treated as a continuous variable. Insurance status was categorized as either non-insured, government insured (Medicaid, Medicare, Military insurance), privately insured, insured not otherwise specified (NOS), or unknown insurance status. Risk factors included body-mass index (BMI), age at menarche, age at menopause, family history of breast cancer, and current or prior history of post-menopausal hormone replacement therapy. The most common comorbidities were included: hypertension (HTN), diabetes mellitus (DM), and coronary artery disease (CAD). Clinical stage at diagnosis was treated as a categorical variable and categorized as stage I to IV or unknown. Tumor subtype was categorized based on breast cancer receptors [estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2). To evaluate treatment, adherence to NCCN stage and receptor appropriate guideline treatment was determined by two surgical oncologists and treated as a dichotomous variable. breast cancer-specific survival was determined as time from primary diagnosis to point of death from local, regional or distant invasive breast cancer.
Statistical Analysis
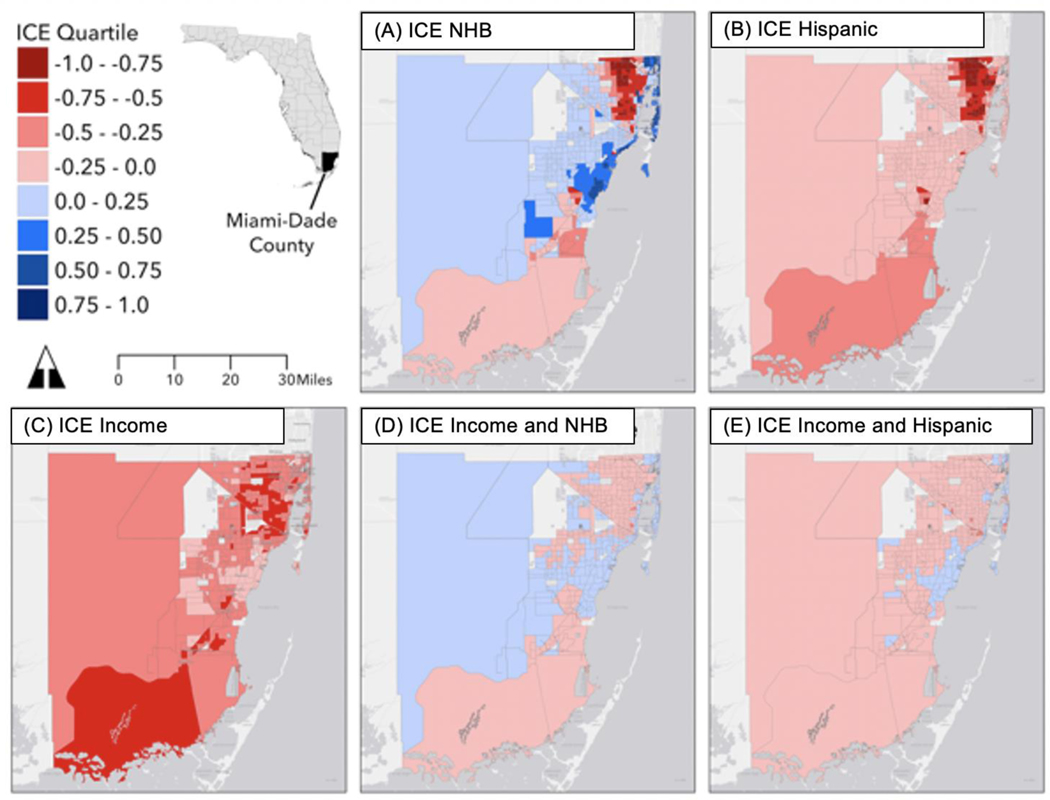
First, we mapped census tract-level ICE values for Miami-Dade County to qualitatively assess geographic patterns relative to previous studies of health disparities (Figure 1). Next, we analyzed demographic risk factors, tumor characteristics, and stage appropriate treatment by race/ethnicity using chi-square tests and analysis of variance (ANOVA). A multilevel analysis was conducted to account for the hierarchical nature of patients nested within census tracts. To evaluate the association of residential segregation on hazard of death from breast cancer, a multi-level Cox proportional hazard model was conducted. We fit separate models for each of the five ICE variables.
Figure 1: Census Tract-Level Index of Concentration at the Extremes (ICE)a in Miami-Dade County, Florida.
Caption: A) Non-Hispanic Black, (B) Hispanic, (C) Income, (D) Non-Hispanic Black racialized economic segregation, and (E) Hispanic racialized economic segregation. The most privileged and/or most NHW tracts appear in the darkest blue tones, and the least privileged and/or most NHB and Hispanic tracts appear in the darkest red tones.
a ICE ranges from −1, where all households are considered marginalized, to 1, where all households are considered privileged.
Then, we computed a bivariate (unadjusted) model to assess the association between each ICE variable and breast cancer-specific mortality. Model two adjusted for demographic variables. Model three adjusted further for receptor status and comorbidities. We did not include stage or treatment in our models as these could lie on the causal pathway between residential segregation and breast cancer-specific survival. Additionally, we conducted race/ethnic stratified frailty models to determine the effect of segregation on different race/ethnicities. All analyses were conducted using R 3.5.221 using survival version 2.3822, survminer version 0.4.323, and coxme version 2.2–1024 packages. All statistical tests were two-sided, and statistical significance was assessed at alpha less than 0.05.
Results
Population Characteristics
The study population was comprised of 5,909 breast cancer patients, who were primarily Hispanic (54.7%) (Table 2). Most patients had private insurance (43.5%) with significantly more Hispanic and NHB patients being uninsured (21.5% and 17.1%, respectively). Median income significantly differed by race/ethnicity with NHW patients having the highest median income (63, 446) followed by Hispanic patients (49, 383) and NHB patients (41, 761) (p < 0.001). NHB presented with more advanced stage disease (Stage III/IV vs. Stage I/II), more aggressive breast cancer subtypes (TNBC), and were less likely to receive NCCN-guideline concordance treatment compared to their NHW and Hispanic counterparts (Table 3).
Table 2:
Patient demographics and risk factors by Race/Ethnicity, 2005 – 2017
| Factor | NHW N = 1,615 26.3% |
NHB N = 1,060, 17.2% |
Hispanic N = 3,234, 54.7% |
All N = 5, 909 |
p-value |
|---|---|---|---|---|---|
| Age at diagnosis | < .001 | ||||
| <50 years | 443 (26.8) | 355 (33.5) | 1,020 (31.5) | 1,808 (30.6) | |
| 50–69 years | 877 (54.3) | 601 (56.7) | 1,831 (56.6) | 3,309 (56.0) | |
| 70–79 years | 203 (12.6) | 75 (7.1) | 293 (9.1) | 571 (9.7) | |
| 80+ years | 102 (6.3) | 29 (2.7) | 90 (2.8) | 221 (3.7) | |
| Birth Place | < .001 | ||||
| US-born | 931 (57.6) | 555 (52.4) | 664 (20.5) | 2,150 (36.4) | |
| Foreign-born | 188 (11.6) | 386 (36.4) | 2,009 (62.1) | 2,583 (43.7) | |
| Unknown | 496 (30.7 | 119 (11.2) | 561 (17.3) | 1,176 (19.9) | |
| Relationship | < 0.001 | ||||
| Married | 935 (57.9) | 351 (33.1) | 1,492 (46.3) | 2,782 (47.1) | |
| Single | 429 (26.6) | 526 (49.6) | 1,065 (32.9) | 2,020 (34.2) | |
| Divorced/Separated | 199 (12.3) | 158 (14.9) | 593 (18.3) | 950 (16.1) | |
| Unknown | 52 (3.2) | 25 (2.4) | 80 (2.5) | 157 (2.7) | |
| Median Income (mean SD) | 63,897 (23,414) | 42,251 (16,043) | 49,383 (19,327) | 52,094 (21,470) | <0.001 |
| Insurance | < 0.001 | ||||
| Private | 1,039 (64.3) | 395 (37.3) | 1,135 (35.1) | 2,569 (43.5) | |
| Government | 303 (18.8) | 368 (34.7) | 1,051 (32.5) | 1,722 (29.1) | |
| Insured, NOS | 73 ( 4.5) | 68 ( 6.4) | 201 (6.2) | 342 (5.7) | |
| Uninsured | 64 ( 4.0) | 181 (17.1) | 695 (21.5) | 940 (15.9) | |
| Unknown | 136 (8.4) | 152 (4.7) | 48 (4.5) | 336 (5.7) | |
| Tobacco Use | < 0.001 | ||||
| Never | 829 (58.9) | 790 (79.3) | 2,185 (71.7) | 3,804 (69.8) | |
| Active | 107 ( 7.6) | 64 ( 6.4) | 263 (8.6) | 434 (8.0) | |
| Former | 471 (33.5) | 142 (14.3) | 599 (19.7) | 1,212 (22.5) | |
| Alcohol Use | < 0.001 | ||||
| Never | 652 (46.5) | 796 (80.2) | 2,369 (77.9) | 3,817 (70.2) | |
| Active | 741 (52.8) | 189 (19.1) | 649 (21.3) | 1,579 (29.0) | |
| Former | 10 ( 0.7) | 7 ( 0.7) | 23 (0.80) | 40 (0.70) | |
| BMI (mean SD) | 26.9 (5.74) | 30.7 (6.83) | 28.8 (5.81) | 28.6 (6.12) | < 0.001 |
| Age at Menarche (mean SD) | 12.60 (1.54) | 13.08 (1.94) | 12.5 (1.76) | 12.6 (1.76) | < 0.001 |
| Age at Menopause (mean SD) | 48.4 (5.96) | 46.6 (7.29) | 47.3 (6.04) | 47.5 (6.28) | < 0.001 |
| Family History of Breast Cancer (% Yes) | 602 (45.1) | 326 (34.3) | 1,033 (35.0) | 1,961 (37.4) | < 0.001 |
| Current or Prior History of Hormone Replacement Therapy (HRT) | |||||
| Premenopausal Hormone Supplement Use (i.e. OCP, IUD) | 516 (55.7) | 185 (33.1) | 609 (33.3) | 1,310 (39.5) | < 0.001 |
| Postmenopausal HRT | 250 (28.3) | 39 (7.5) | 223 (12.6) | 512 (16.1) | < 0.001 |
| Comorbidities | |||||
| Hypertension | 410 (25.4) | 360 (34.0) | 776 (24.0) | 1,546 (26.2) | <0.001 |
| Diabetes | 86 (5.3) | 123 (11.6) | 235 (7.3) | 444 (7.5) | <0.001 |
| Coronary Artery Disease | 8 (0.5) | 7 (0.7) | 14 (0.4) | 29 (0.5) | 0.832 |
Table 3:
Tumor and Treatment Characteristics
| Factor | NHW N = 1,615 27.3% |
NHB N = 1,060 17.9% |
Hispanic N = 3,234 54.7% |
All N = 5,909 |
p-value |
|---|---|---|---|---|---|
| Clinical Stage | <0.001 | ||||
| I | 749 (46.4) | 280 (26.4) | 1,165 (36.0) | 2,194 (37.1) | |
| II | 503 (31.1) | 379 (35.8) | 1,158 (35.8) | 2,040 (34.5) | |
| III | 206 (12.8) | 220 (20.8) | 587 (18.2) | 1,013 (17.1) | |
| IV | 120 (7.4) | 141 (13.3) | 240 (7.4) | 501 (8.5) | |
| Unknown | 37 (2.3) | 40 (3.8) | 84 (2.6) | 161 (2.7) | |
| Tumor Grade | <0.001 | ||||
| Well diff./Moderately (0) | 1,025 (63.5) | 499 (47.1) | 1,931 (59.7) | 3,455 (58.5) | |
| Poorly diff. (1) | 583 (36.1) | 541 (51.0) | 1,282 (39.6) | 2,406 (40.7) | |
| Anaplastic/Undifferentiated | 7 ( 0.4) | 20 ( 1.9) | 21 (0.6) | 48 (0.8) | |
| Receptor Status | <0.001 | ||||
| ER+/HER2− | 1,026 (63.5) | 502 (47.4) | 1,992 (61.6) | 3,520 (59.6) | |
| ER+/HER2+ | 169 (10.5) | 107 (10.1) | 345 (10.9) | 639 (10.5) | |
| ER-/HER2+ | 83 ( 5.1) | 101 ( 9.5) | 244 (7.5) | 428 (7.2) | |
| ER-/HER2− | 194 (12.0) | 283 (26.7) | 467 (14.4) | 944 (16.0) | |
| Unknown | 143 (8.9) | 67 (6.3) | 177 (5.5) | 387 (6.5) | |
| Pathologic Stage | <0.001 | ||||
| I | 746 (46.2) | 280 (26.4) | 1,117 (34.5) | 2,143 (36.3) | |
| II | 398 (24.6) | 264 (24.9) | 885 (27.4) | 1,547 (26.2) | |
| III | 143 ( 8.9) | 105 ( 9.9) | 352 (10.9) | 600 (10.2) | |
| IV | 42 ( 2.6) | 37 ( 3.5) | 90 (2.8) | 169 (2.9) | |
| Unknown | 272 (16.8) | 368 (34.7) | 767 (23.7) | 1,407 (23.8) | |
| Treatment | <0.001 | ||||
| Surgery | 1,422(88.0) | 732 (69.1) | 2,608 (80.6) | 4,762 (80.6) | |
| Chemotherapy | 818 (50.7) | 626 (59.1) | 1,885 (58.3) | 3,329 (56.3) | |
| Radiation | 769 (47.6) | 418 (39.4) | 1,528 (47.2) | 2,715 (45.9) | |
| Endocrine Therapy | 1,054 (65.3) | 434 (40.9) | 1,832 (56.6) | 3,320 (56.2) | |
| NCCN Guideline Based Treatment | 1,285 (81.4) | 739 (72.5) | 2,443 (77.6) | 4,467 (77.7) | <0.001 |
Geospatial Visualization
Geospatial visualization of our ICE variables (ICEIncome, ICENHB, ICEHispanic, ICEIncomeNHB, and ICEIncomeHispanic) identified distinct regions of our catchment area for targeted interventions. In Figure 1C (ICE Income), the dark red tones represent areas with increased economic segregation, consistent with known Hispanic ethnic enclaves and NHB neighborhoods of Miami-Dade County. We observed the most extreme disparities in the ICE Black map (Figure 1A), which highlights the concentration of NHB households in a northern corridor of the county extending from downtown Miami north to Miami Gardens at the Broward County border, and a concentration of NHW households along the beaches south through Kendall and Palmetto Bay. The concentration of Hispanic households in the same NHB neighborhoods (Figure 1B) underscored the very small number of NHW households in these communities. These socio-spatial patterns are consistent with those observed in Miami-Dade County for other non-communicable public health issues such as colorectal cancer, gunshot injuries, and intimate partner violence.25–27
Breast Cancer-Specific Survival
During the study period, 724 deaths were attributable to breast cancer (12.2%). Number of events by segregation and race/ethnicity can be found in Supplemental Table 1. All models of breast cancer-specific survival using ICEIncome indicated an increased hazard of breast cancer-specific mortality for patients living in low-income neighborhoods compared to patients living in high-income neighborhoods (Table 4). This association remained after controlling for demographic and comorbidities (model 3: HR 1.67; 95% CI 1.31, 213) (Table 4).
Table 4:
Adjusted hazard ratios (HRs) for Breast Cancer Specific Mortality by Index of Concentration at the Extremes (ICE) in All Patients.
| Type of Segregation (ICE) | Quartilea | Unadjusted | Model 2 | Model 3 |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Economic Segregation | Q1 | 1.85 (1.47, 2.33)* | 1.63 (1.28, 2.06)* | 1.67 (1.31, 2.13)* |
| Economic Segregation | Q2 | 1.71 (1.32, 2.21)* | 1.56 (1.21, 2.02)* | 1.60 (1.23, 2.08)* |
| Economic Segregation | Q3 | 1.31 (1.01, 1.70)* | 1.19 (0.92, 1.54) | 1.20 (0.93, 1.56) |
| Economic Segregation | Q4 | REF | REF | REF |
| NHB Segregation | Q1 | 1.33 (0.85, 2.06) | 1.52 (0.94, 2.44) | 1.68 (1.04, 2.73)* |
| NHB Segregation | Q2 | 0.79 (0.49, 1.27) | 0.93 (0.57, 1.50) | 1.03 (0.63, 1.67) |
| NHB Segregation | Q3 | 0.63 (0.35, 1.15) | 0.70 (0.39, 1.27) | 0.75 (0.41, 1.37) |
| NHB Segregation | Q4 | REF | REF | REF |
| Hispanic Segregation | Q1 | 1.80 (1.49, 2.18)* | 1.40 (1.11, 1.77)* | 1.40 (1.10, 1.77)* |
| Hispanic Segregation | Q2 | 0.85 (0.64, 1.13) | 0.83 (0.62, 1.09) | 0.83 (0.62, 1.11) |
| Hispanic Segregation | Q3 | 0.90 (0.70, 1.17) | 0.91 (0.70, 1.17) | 0.92 (0.71, 1.19) |
| Hispanic Segregation | Q4 | REF | REF | REF |
| NHB Economic Segregation | Q1 | 2.62 (1.95, 3.51)* | 2.50 (1.79, 3.48)* | 2.64 (1.88, 3.70)* |
| NHB Economic Segregation | Q2 | 1.59 (1.18, 2.14)* | 1.87 (1.35, 2.59)* | 1.99 (1.44, 2.78)* |
| NHB Economic Segregation | Q3 | 1.37 (0.94, 1.98) | 1.47 (1.01, 2.13)* | 1.58 (1.08, 2.30)* |
| NHB Economic Segregation | Q4 | REF | REF | REF |
| Hispanic Economic Segregation | Q1 | 1.85 (1.34, 2.54)* | 1.96 (1.39, 2.77)* | 2.14 (1.51, 3.04) |
| Hispanic Economic Segregation | Q2 | 1.77 (1.21, 2.58)* | 1.68 (1.15, 2.48)* | 1.79 (1.21, 2.64)* |
| Hispanic Economic Segregation | Q3 | 1.23 (0.81, 1.86) | 1.25 (0.83, 1.89) | 1.34 (0.88, 2.02) |
| Hispanic Economic Segregation | Q4 | REF | REF | REF |
Q1: Most marginalized neighborhoods; Q4: Most advantaged neighborhoods (reference)
Model 1: Unadjusted
Model 2: Adjusts for ICE + age + race/ethnicity
Model 3: Adjusts for Model 2 + receptor status and comorbidities
In models using ICENHB and ICEHispanic, patients living in NHB neighborhoods had an increased hazard of death compared to patients living in more concentrated NHW neighborhoods (HR 1.68; 95% CI 1.04, 2.73) (Table 4). Patients living in Hispanic segregated neighborhoods (Table 4) had a statistically significant increased hazard of death compared to patients living in more concentrated NHW neighborhoods after controlling for demographic, comorbidity, tumor, and treatment characteristics (model 3: HR 1.40; 95% CI 1.10, 1.77).
In models using ICEIncomeNHB and ICEIncomeHispanic, patients living in either NHB or Hispanic economic segregation had an increased hazard of death compared to those living in neighborhoods characterized by more concentrated NHW high-income neighborhoods, after controlling for individual-level demographics, comorbidities, tumor, and treatment characteristics (model 3 for NHB: HR 2.64; 95% CI 1.88, 3.70 and for Hispanics: HR 2.14; 95% CI 1.51, 3.04) (Table 4).
Cox Models Stratified by Race/Ethnicity
In fully adjusted race-stratified models, NHW patients living in low-income and racially segregated neighborhoods had significantly increased hazard of breast cancer specific death compared to NHW patients living in NHW high-income neighborhoods (HRIncome: 2.40; 95% CI: (1.40, 4.13); HRNHB: 2.13 95% CI: (1.16, 3.92); HRHispanic: 2.24, 95% CI: 1.35, 2.70; HRIncomeNHB: 4.61 95% CI: (2.69, 7.85); HRincomeHispanic: 3.08 95% CI: (1.85, 5.09). NHB patients living in low-income neighborhoods had an increased hazard of death compared to NHB patients living in higher income neighborhoods (HR: 2.22, 95% CI: 1.10, 4.50). There was no significant association between any form of segregation and breast cancer specific mortality for Hispanic patients (Table 5).
Table 5.
Fully Adjusteda Race-Stratified Cox Models for Hazard of Breast Cancer-Specific Mortality Stratified by the Patient’s Race/Ethnicity
| Type of Segregation | Quartileb | NHW HR (95% CI) |
NHB (HR 95% CI) |
Hispanic (HR 95% CI) |
|---|---|---|---|---|
| Economic | Q1 | 2.40 (1.40, 4.13)* | 2.22 (1.10, 4.50 )* | 1.22 (0.91, 1.65) |
| Q2 | 2.79 (1.69, 4.62)* | 1.85 (0.88, 3.91) | 1.16 (0.91, 1.65) | |
| Q3 | 1.43 (0.88, 2.32) | 1.34 (0.63, 2.86) | 1.06 (0.74, 1.51) | |
| Q4 | Ref | Ref | Ref | |
| NHB | Q1 | 2.13 (1.16, 3.92)* | 0.79 (0.10, 6.12) | 2.38 (0.32, 17.7) |
| Q2 | 0.93 (0.51, 1.69) | 0.16 (0.01, 1.67) | 2.06 (0.27, 15.6) | |
| Q3 | 0.61 (0.28, 1.30( | 0.20 (0.01, 3.52) | 1.97 (0.24, 16.3) | |
| Q4 | Ref | Ref | Ref | |
| Hispanic | Q1 | 2.24 (1.35, 2.70)* | 1.15 (0.49, 2.69) | 1.27 (0.94, 1.72) |
| Q2 | 0.79 (0.45, 1.38) | 0.74 (0.27, 2.08) | 0.91 (0.63, 1.32) | |
| Q3 | 1.18 (0.71, 1.97) | 1.16 (0.41, 3.28) | 0.79 (0.58, 1.10) | |
| Q4 | Ref | Ref | Ref | |
| NHB Economic | Q1 | 4.61 (2.69, 7.85)* | 3.19 (0.76, 13.5) | 1.30 (0.80, 2.13) |
| Q2 | 2.80 (1.70, 4.63)* | 2.34 (0.52, 10.6) | 1.05 (0.66, 1.69) | |
| Q3 | 1.95 (1.14, 3.34)* | 1.19 (0.21, 6.9) | 1.01 (0.57, 1.81) | |
| Q4 | Ref | Ref | Ref | |
| Hispanic Economic | Q1 | 3.08 (1.85, 5.09)* | 2.99 (0.71, 12.6) | 1.14 (0.63, 2.07) |
| Q2 | 2.42 (1.32, 4.43) | 2.37 (0.55, 10.3) | 1.04 (0.52, 2.09) | |
| Q3 | 1.37 (0.76, 2.48) | 1.01 (0.19, 5.46) | 1.15 (0.56, 2.38) | |
| Q4 | Ref | Ref | Ref |
Adjusted for age, receptor status, and comorbidities.
Q1: Most marginalized neighborhoods; Q4: Most advantaged neighborhoods (reference)
Statistically significant at alpha < 0.05 level
Discussion
This study found important associations between measures of neighborhood economic segregation, racial residential segregation, racialized economic residential segregation, and breast cancer-specific survival. We identified that individuals of any race/ethnicity living in low-income NHB or Hispanic segregated neighborhoods experienced lower breast cancer-specific survival relative to those living in predominantly high-income or NHW neighborhoods, even after controlling for patient characteristics and tumor subtype More striking, we found that NHW patients living in low-income NHB or Hispanic segregated neighborhoods had an increased risk of mortality compared to those living in high-income NHW neighborhoods. Geovisualization of our findings provide additional insight into the spatial, residential, and economic segregation patterns in our catchment area, which coincide with Miami-Dade County’s most vulnerable communities, as well as historically redlined neighborhoods from the New Deal Era (Figure 1). These findings emphasize the pervasive nature of structural racism on breast cancer-specific survival across women living in disadvantaged neighborhoods. In doing so, we add insight to a growing body of literature that demonstrate how the ecological effects of structural racism—expressed through poverty and residential segregation—shape cancer survival across patients of all races/ethnicities.28–30
Breast Cancer Specific Mortality in Low-Income Neighborhoods (ICEIncome)for All Patients
When evaluating the impact of living in a low-income neighborhood, all patients, regardless of race/ethnicity, had lower breast cancer-specific survival, even after adjusting for covariates. Neighborhood economic status can shape cancer survival through several mechanisms such as access to healthcare, particularly through breast cancer screening leading to late-stage disease at presentation and lack of treatment.31 We found that a higher percentage of NHB patients lived in low-income neighborhoods, presented with late-stage disease (stage III-IV), and were less likely to receive NCCN-guideline concordant treatment. Wiese et al also found that neighborhood socioeconomic status was associated with lower breast cancer-specific survival; however, this study did not perform race/ethnicity stratified analysis, limiting understanding of the impact of low-income neighborhoods among patients of diverse races/ethnicities. 31
Breast Cancer Specific Mortality in Racially/Ethnically Segregated Neighborhoods (ICENHB and ICEHispanic) for All Patients
In terms of racial/ethnic residential segregation, we identified that patients of any race/ethnicity living in segregated Hispanic neighborhoods had shorter breast cancer-specific survival (Table 4). This contradicts previous studies showing that ethnic segregation may confer protective resources to historically marginalized residents who find social support and collective efficacy within their communities.32–41 Gomez et al. found protective effects on breast cancer survival associated with living in ethnic enclaves, with ethnic enclaves serving as a surrogate for low level of acculturation to unhealthy American eating habits.42 However, Pruitt et al. found that the increased mortality for breast cancer in residents of Hispanic neighborhoods differed on various levels of ethnicity, birthplace, and neighborhood poverty.8 Collectively, our findings, when positioned within the context of competing arguments in the literature, stress the need for a more nuanced approach to understand the influence of ethnic enclaves and ethnic residential segregation on breast cancer survival. The mixed results may be due to a key underlying factor driving people to live in ethnic enclaves compared to ethnically segregated neighborhoods—choice. Ethnic enclaves usually imply choice since immigrants often choose to live in these areas due to social support networks, but they (or their children) are likely to move out once they improve their socioeconomic status. On the other hand, Black-White segregation is often the result of historic racial oppression linked with unemployment, poor health outcomes, and limited social support. Our study brings to light the importance of understanding the dynamics of immigration-related segregation (enclaves) verses long-standing historic oppression-related among minorities on cancer outcomes.43 In doing so, we suggest that some component of the experience of racialized segregation—a form of structural racism—may be shaping cancer survival.
Breast Cancer Specific Mortality by Racialized Economic Segregation (ICEIncomeNHB and ICEIncomeHispanic) for All Patients
Few studies have examined racialized economic segregation. This novel ICE metric enables us to uniquely characterize complex measures of social inequity in a single metric by capturing socioeconomic and racial segregation. Furthermore, by design, it reduces multicollinearity by focusing on income as a relative measure of economic disadvantage, rather than absolute area-based measures of poverty which may be confounded by race/ethnicity and local living costs.12, 15 Our findings identified that breast cancer patients living in any low-income NHB or Hispanic neighborhood had an increased risk of breast cancer specific mortality. This emphasizes the importance of the combined effect of race and/or ethnicity and economic segregation in breast cancer mortality, consistent with the limited research assessing the impact of racialized economic segregation.31 Our study adds to the paucity of literature addressing the combined effect of economic and racial residential segregation on breast cancer-specific survival particularly in majority-Hispanic cities.
Race-Stratified Breast Cancer-Specific Mortality by ICE
Finally, in race-stratified models of breast-cancer specific mortality by ICE, we found that NHW patients living in any low-income segregated neighborhood had an increased risk of mortality compared to those living in high-income NHW neighborhoods. This important finding showing that even White women living in the same low-income neighborhoods as Black and Hispanic women experience shorter breast cancer-specific survival outcomes highlights the strong role that structural racism plays across races/ethnicities in breast cancer-specific survival. 30 We also show that NHBs living in low-income neighborhoods have significantly shorter breast-cancer specific survival; however, our inability to show that NHBs living in low-income NHB neighborhoods do not have shorter breast cancer-specific survival likely stems from the positivity assumption since we did not have enough NHB patients living in high-income NHW neighborhoods to statistically power for this race-specific evaluation (Figure 1, Table 5). This in and of itself is a striking disparity.
This study has several strengths and potential limitations. Along with inherent limitations of retrospective studies, we were unable to capture potential treatments received at other facilities, thus making guideline-appropriate care difficult to analyze in approximately 4% of cases. Despite this, each patient’s care was evaluated by a physician to determine if the patient met strict NCCN-guideline appropriate treatment based on tumor stage and subtype, which bridges a critical gap in previous literature.13, 15, 19, 20, 44, 45 A potential source of bias could be that the reviewers were not blinded to patient race if the charts commented on race; however, the reviewers were blinded to income and segregation as these were collected using the American Community Survey. Moreover, this two-institution study in South Florida, may not be generalizable to other health systems caring for similar populations. Nevertheless, these institutions consisted of an NCI designated cancer center and safety-net hospital which reflects two diverse racial/ethnic and socioeconomic populations. Also, although our study was limited as a two-institution study, the racial/ethnic and economic diversity of our population in one of the most segregated counties in the US allowed for novel examination of racialized economic segregation in a unique multicultural context, which likely reflects the future demographics of the US, particularly the Sun Belt.16 This has implications for better addressing breast cancer disparities and structural racism in other growing majority-minority cities across the US. The authors also note that recent studies have identified intra-ethnic differences between Hispanic Blacks and Hispanic Whites; however, this study was underpowered to evaluate the impact of ICE in this Hispanic subgroups.46, 47 Another strength of our study is the use of ICE to measure extreme race/ethnic and economic segregation. As ICE measures race/ethnicity and socioeconomic status simultaneously, it can help overcome established issues in neighborhood research such as multicollinearity and can capture the intersectionality of race/ethnicity and income while other measures of segregation such as the Index of Dissimilarity can only measure one dimension of segregation, specifically racial segregation.
Conclusion
This study illustrates that neighborhood-level structural racism predicts shorter breast cancer-specific survival, even after accounting for patient characteristics and tumor subtype. This study is also among the first to show that White women living in historically redlined Black or Hispanic neighborhoods also have shorter breast cancer-specific survival. This suggests potential gene-environment interactions as byproducts of structural racism that might be drivers of shorter breast cancer-specific survival in these historically redlined neighborhoods that affects patients living in these areas regardless of race.30, 45, 48 To address these survival disparities associated with economic and racial/ethnic residential segregation, a translational “cell to society” research framework integrating gene-environment interactions and social epidemiologic assessments of specific neighborhood-level characteristics detrimental to breast cancer-specific survival need to be conducted to comprehensively account for the individual and neighborhood-level contexts in which breast cancer patients are screened, diagnosed, and treated.
Supplementary Material
Acknowledgments
Funding: National Institute of Health Grant #K12CA226330
Footnotes
Design: Retrospective cohort study utilizing local tumor registry data from 2005–2017.
The authors declare no potential conflicts of interest.
References
- 1.Silber JH, Rosenbaum PR, Ross RN, et al. Disparities in Breast Cancer Survival by Socioeconomic Status Despite Medicare and Medicaid Insurance. Milbank Q. Dec 2018;96(4):706–754. doi: 10.1111/1468-0009.12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller JW, Smith JL, Ryerson AB, Tucker TC, Allemani C. Disparities in breast cancer survival in the United States (2001–2009): Findings from the CONCORD-2 study. Cancer. Dec 15 2017;123 Suppl 24(Suppl 24):5100–5118. doi: 10.1002/cncr.30988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelly KN, Hernandez A, Yadegarynia S, Ryon E, Franceschi D, Avisar E, Kobetz EN, Merchant N, Kesmodel S, Goel N. Overcoming disparities: Multidisciplinary breast cancer care at a public safety net hospital. Breast Cancer Res Treat. 2021. May;187(1):197–206. doi: 10.1007/s10549-020-06044-z. Epub 2021 Jan 25. PMID: 33495917. [DOI] [PubMed] [Google Scholar]
- 4.Bemanian A, Beyer KM. Measures Matter: The Local Exposure/Isolation (LEx/Is) Metrics and Relationships between Local-Level Segregation and Breast Cancer Survival. Cancer Epidemiol Biomarkers Prev. Apr 2017;26(4):516–524. doi: 10.1158/1055-9965.Epi-16-0926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey ZD, Feldman JM, Bassett MT. How Structural Racism Works — Racist Policies as a Root Cause of U.S. Racial Health Inequities. New England Journal of Medicine. 2020;384(8):768–773. doi: 10.1056/NEJMms2025396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massey DS, Rothwell J, Domina T. The Changing Bases of Segregation in the United States. Ann Am Acad Pol Soc Sci. Nov 1 2009;626(1)doi: 10.1177/0002716209343558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haan M, Kaplan GA, Camacho T. Poverty and health. Prospective evidence from the Alameda County Study. Am J Epidemiol. Jun 1987;125(6):989–98. doi: 10.1093/oxfordjournals.aje.a114637 [DOI] [PubMed] [Google Scholar]
- 8.Pruitt SL, Lee SJC, Tiro JA, Xuan L, Ruiz JM, Inrig S. Residential racial segregation and mortality among black, white, and Hispanic urban breast cancer patients in Texas, 1995 to 2009. Cancer. 2015;121(11):1845–1855. doi: 10.1002/cncr.29282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell EF, Kramer MR, Cooper HLF, Gabram-Mendola S, Senior-Crosby D, Jacob Arriola KR. Metropolitan area racial residential segregation, neighborhood racial composition, and breast cancer mortality. Cancer Causes & Control. 2012/09/01 2012;23(9):1519–1527. doi: 10.1007/s10552-012-0029-4 [DOI] [PubMed] [Google Scholar]
- 10.Beyer KMM, Zhou Y, Matthews K, Bemanian A, Laud PW, Nattinger AB. New spatially continuous indices of redlining and racial bias in mortgage lending: links to survival after breast cancer diagnosis and implications for health disparities research. Health & Place. 2016/07/01/ 2016;40:34–43. doi: 10.1016/j.healthplace.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 11.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between black and white women in California. J Community Health. Aug 2010;35(4):398–408. doi: 10.1007/s10900-010-9265-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Massey DS. The prodigal paradigm returns: Ecology comes back to sociology. Does it take a village. 2001:41–48. [Google Scholar]
- 13.Krieger N, Singh N, Waterman PD. Metrics for monitoring cancer inequities: residential segregation, the Index of Concentration at the Extremes (ICE), and breast cancer estrogen receptor status (USA, 1992–2012). Cancer Causes Control. Sep 2016;27(9):1139–51. doi: 10.1007/s10552-016-0793-7 [DOI] [PubMed] [Google Scholar]
- 14.Feldman JM, Waterman PD, Coull BA, Krieger N. Spatial social polarisation: using the Index of Concentration at the Extremes jointly for income and race/ethnicity to analyse risk of hypertension. J Epidemiol Community Health. Dec 2015;69(12):1199–207. doi: 10.1136/jech-2015-205728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krieger N, Waterman PD, Spasojevic J, Li W, Maduro G, Van Wye G. Public Health Monitoring of Privilege and Deprivation With the Index of Concentration at the Extremes. American journal of public health. Feb 2016;106(2):256–63. doi: 10.2105/ajph.2015.302955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bureau USC. QuickFacts Florida. Accessed May 5, 2021. https://www.census.gov/quickfacts/fact/table/FL,US/PST045219
- 17.Spielman SE, Folch D, Nagle N. Patterns and causes of uncertainty in the American Community Survey. Appl Geogr. Jan 2014;46:147–157. doi: 10.1016/j.apgeog.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Census Bureau. Table H-1. Income limits for each fifth and top 5 percent. Historical income tables: households. Accessed March 18, 2019, https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-income-households.html
- 19.Krieger N, Kim R, Feldman J, Waterman PD. Using the Index of Concentration at the Extremes at multiple geographical levels to monitor health inequities in an era of growing spatial social polarization: Massachusetts, USA (2010–14). Int J Epidemiol. Jun 1 2018;47(3):788–819. doi: 10.1093/ije/dyy004 [DOI] [PubMed] [Google Scholar]
- 20.Krieger N, Waterman PD, Batra N, Murphy JS, Dooley DP, Shah SN. Measures of Local Segregation for Monitoring Health Inequities by Local Health Departments. Am J Public Health. Jun 2017;107(6):903–906. doi: 10.2105/ajph.2017.303713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.R Core Team. R: A language and environment for statistical computing. https://www.R-project.org/
- 22.Therneau T. _A package for survival analysis in S_. Version 2.38. https://CRAN.R-project.org/package=survival
- 23.Kassambara A, Kosinski M. survminer: Drawing survival curves using ‘ggplot2’. R package version 0.4.3. https://CRAN.R-project.org/package=survminer
- 24.Therneau T. coxme: Mixed effects Cox models. R package version 2.2–10. https://CRAN.R-project.org/package=coxme
- 25.Hernandez MN, Roy Chowdhury R, Fleming LE, Griffith DA. Colorectal cancer and socioeconomic status in Miami-Dade County: Neighborhood-level associations before and after the Welfare Reform Act. Applied Geography. 2011/07/01/ 2011;31(3):1019–1025. doi: 10.1016/j.apgeog.2011.01.025 [DOI] [Google Scholar]
- 26.Zebib L, Stoler J, Zakrison TL. Geo-demographics of gunshot wound injuries in Miami-Dade county, 2002–2012. BMC Public Health. 2017/02/08 2017;17(1):174. doi: 10.1186/s12889-017-4086-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams J, Petersen N, Stoler J. Characterizing the spatial mismatch between intimate partner violence related healthcare services and arrests in Miami-Dade County, Florida. BMC Public Health. 2018/08/31 2018;18(1):1085. doi: 10.1186/s12889-018-5985-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haas JS, Earle CC, Orav JE, et al. Racial segregation and disparities in breast cancer care and mortality. Cancer. Oct 15 2008;113(8):2166–72. doi: 10.1002/cncr.23828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell E, Kramer MR, Cooper HL, Thompson WW, Arriola KR. Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. Journal of urban health : bulletin of the New York Academy of Medicine. Dec 2011;88(6):1117–29. doi: 10.1007/s11524-011-9612-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGhee H. The sum of us: What racism costs everyone and how we can prosper together. One World; 2021. [Google Scholar]
- 31.Wiese D, Stroup AM, Crosbie A, Lynch SM, Henry KA. The Impact of Neighborhood Economic and Racial Inequalities on the Spatial Variation of Breast Cancer Survival in New Jersey. Cancer Epidemiol Biomarkers Prev. Dec 2019;28(12):1958–1967. doi: 10.1158/1055-9965.Epi-19-0416 [DOI] [PubMed] [Google Scholar]
- 32.Markides KS, Coreil J. The health of Hispanics in the southwestern United States: an epidemiologic paradox. Public Health Rep. May–Jun 1986;101(3):253–65. [PMC free article] [PubMed] [Google Scholar]
- 33.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis. Autumn 2001;11(3):496–518. [PubMed] [Google Scholar]
- 34.Liao Y, Cooper RS, Cao G, et al. Mortality patterns among adult Hispanics: findings from the NHIS, 1986 to 1990. Am J Public Health. Feb 1998;88(2):227–32. doi: 10.2105/ajph.88.2.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abraído-Lanza AF, Dohrenwend BP, Ng-Mak DS, Turner JB. The Latino mortality paradox: a test of the “salmon bias” and healthy migrant hypotheses. American journal of public health. Oct 1999;89(10):1543–8. doi: 10.2105/ajph.89.10.1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turra CM, Elo IT. The Impact of Salmon Bias on the Hispanic Mortality Advantage: New Evidence from Social Security Data. Popul Res Policy Rev. 2008;27(5):515–530. doi: 10.1007/s11113-008-9087-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci. Oct 2005;60 Spec No 2:68–75. doi: 10.1093/geronb/60.special_issue_2.s68 [DOI] [PubMed] [Google Scholar]
- 38.Abraído-Lanza AF, Chao MT, Flórez KR. Do healthy behaviors decline with greater acculturation? Implications for the Latino mortality paradox. Soc Sci Med. Sep 2005;61(6):1243–55. doi: 10.1016/j.socscimed.2005.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osypuk TL, Diez Roux AV, Hadley C, Kandula NR. Are immigrant enclaves healthy places to live? The Multi-ethnic Study of Atherosclerosis. Soc Sci Med. Jul 2009;69(1):110–20. doi: 10.1016/j.socscimed.2009.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dubowitz T, Subramanian SV, Acevedo-Garcia D, Osypuk TL, Peterson KE. Individual and neighborhood differences in diet among low-income foreign and U.S.-born women. Women’s health issues : official publication of the Jacobs Institute of Women’s Health. May–Jun 2008;18(3):181–90. doi: 10.1016/j.whi.2007.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas JS, Phillips KA, Sonneborn D, et al. Variation in access to health care for different racial/ethnic groups by the racial/ethnic composition of an individual’s county of residence. Med Care. Jul 2004;42(7):707–14. doi: 10.1097/01.mlr.0000129906.95881.83 [DOI] [PubMed] [Google Scholar]
- 42.Keegan TH, Quach T, Shema S, Glaser SL, Gomez SL. The influence of nativity and neighborhoods on breast cancer stage at diagnosis and survival among California Hispanic women. BMC Cancer. Nov 4 2010;10:603. doi: 10.1186/1471-2407-10-603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iceland J. Residential Segregation: A Transatlantic Analysis 2014. [Google Scholar]
- 44.Shariff-Marco S, Yang J, John EM, et al. Intersection of Race/Ethnicity and Socioeconomic Status in Mortality After Breast Cancer. J Community Health. Dec 2015;40(6):1287–99. doi: 10.1007/s10900-015-0052-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shariff-Marco S, Yang J, John EM, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the Neighborhood and Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. May 2014;23(5):793–811. doi: 10.1158/1055-9965.Epi-13-0924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Champion CD, Thomas SM, Plichta JK, et al. Disparities at the Intersection of Race and Ethnicity: Examining Trends and Outcomes in Hispanic Women With Breast Cancer. JCO Oncol Pract. Oct 7 2020:Op2000381. doi: 10.1200/op.20.00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goel N, Yadegarynia S, Lubarsky M, et al. Racial and Ethnic Disparities in Breast Cancer Survival: Emergence of a Clinically Distinct Hispanic Black Population. Ann Surg. Sep 1 2021;274(3):e269–e275. doi: 10.1097/sla.0000000000005004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and Ethnic Disparities in Cancer Survival: The Contribution of Tumor, Sociodemographic, Institutional, and Neighborhood Characteristics. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. Jan 1 2018;36(1):25–33. doi: 10.1200/jco.2017.74.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.