Abstract
Cooked poultry cuts were inoculated with five-strain composite mixtures of either Listeria monocytogenes or Yersinia enterocolitica (1,000 CFU/150-g piece), packaged in 44:56 CO2-N2, and stored at 3.5, 6.5, or 10°C for up to 5 weeks. Both L. monocytogenes and Y. enterocolitica grew under all test conditions. The presence of a naturally occurring microbiota did not influence the growth of either pathogen. Addition of lactate with the shelf life extender ALTA 2341 lengthened the lag phases of L. monocytogenes and Y. enterocolitica but did not prevent their growth.
The extended shelf life of modified-atmosphere-packaged (MAP), ready-to-eat (RTE) refrigerated foods has increased concern about the growth of facultative anaerobic psychrotrophic pathogens such as Listeria monocytogenes and Yersinia enterocolitica. Both L. monocytogenes and Y. enterocolitica grow in RTE meat products under refrigeration with no apparent signs of spoilage (7, 11, 12, 16). Occasional postprocess contamination of cooked RTE foods has been documented for L. monocytogenes (6, 15, 22) and Y. enterocolitica (10, 21).
Acidification, reduction of water activity, addition of preservatives, and presence of a competitive microbiota, especially lactic acid bacteria, have been suggested as appropriate measures for preventing the growth of pathogens in nonfermented foods (8, 9, 14).
In a previous study, the shelf life for a hot- or cold-packaged, commercially processed RTE MAP poultry product was determined (2). Hot-packaged samples (packaged directly out of the oven) resulted in a product in which no background microbiota developed within a 2-month period. A reliable background microbiota predominated by lactic acid bacteria developed in cold-packaged samples (packaged at the end of the processing line). Even when the background microbiota reached 1010 CFU/piece, there were no perceptible sensory changes to the product. While hot packaging reduced the risk of postprocess contamination, it also eliminated a potential hurdle to pathogen growth. However, the development of a background microbiota in cold-packaged samples suggested the potential for postprocess contamination with other, potentially pathogenic, bacteria.
In the present study, the effect of a naturally occurring microbiota on the fate of the psychrotrophic pathogens L. monocytogenes and Y. enterocolitica was determined in a cooked MAP poultry product stored at refrigeration temperature (3.5°C) and under temperature abuse conditions (6.5 and 10°C). The antimicrobial effect of a combination of two approved additives, sodium lactate and ALTA 2341, was also investigated. Sodium lactate inhibits the growth of L. monocytogenes in cured and uncured meats (17, 20, 23). The commercial shelf life extender ALTA 2341 (1) has antilisterial activity similar to the bacteriocin pediocin (19).
Sample collection.
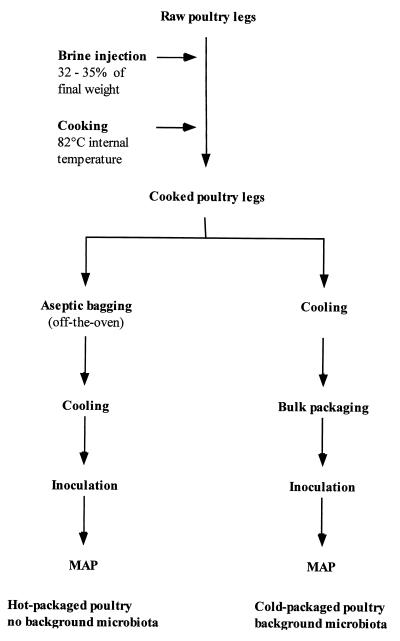
Poultry samples were prepared at a commercial poultry processing plant in southwestern Ontario (Fig. 1). Raw chicken legs (with skin) were injected, at 32 to 35% of their initial weight, with either a control commercial brine formulation (spices, sodium chloride, sodium triphosphate) or a test brine with added sodium lactate (60%, pH 7.0) (Wilke International, Inc., Olathe, Kans.) and the commercial shelf life extender ALTA 2341 (1) (Quest International, Sarasota, Fla.) at levels of 3% (wt/wt) and 0.5% (wt/wt), respectively, of the final cooked product. Leg quarters were oven roasted to a minimal internal temperature of 82°C, as measured at the thigh joint with a digital probe thermometer (PDT300; UEI, Beaverton, Oreg.). Cooked legs were aseptically collected directly out of the oven (hot packed) or after passing through a cooling tunnel (cold packed). Hot-packed samples were placed, five at a time, in sterile stomacher bags (Seward Medical, London, United Kingdom) that were closed and sent through the cooling tunnel. Cold-packed samples, collected after cooling to an internal temperature of 12 to 16°C at the end of the processing line, were bulk packed (40 at a time) in sterile autoclave bags (Fisher Scientific). Samples were immediately placed on ice following cooling and transported to the University of Guelph for inoculation, modified-atmosphere packaging, storage, and analysis.
FIG. 1.
Flow diagram for production of cooked MAP poultry.
Inoculum preparation.
Composite five-strain mixtures of L. monocytogenes and Y. enterocolitica were used (Table 1). Bacteria were individually propagated in brain heart infusion broth and incubated at 30°C. Overnight cultures (12 to 18 h) were pelleted and washed twice in 0.1% peptone. Cultures were diluted in 0.1% peptone to the same optical density at 600 nm, and equal volumes of the five strains were mixed to prepare the inoculum. These five-strain composites were further diluted to approximately 1,000 CFU/100 μl. The initial inoculum level was determined by plating the composite mixtures onto plate count agar (PCA) followed by incubation for 24 h at 30°C. The inoculum (100 μl) was applied dropwise onto the surface of each cooked chicken leg and spread over most of the top area with a flame-sterilized glass rod. The surface covered by the inoculum consisted mainly of skin and, to a lesser degree, meat.
TABLE 1.
Strains of L. monocytogenes and Y. enterocolitica used in inoculum preparation
| Organism | Strain | Serotype | Source |
|---|---|---|---|
| L. monocytogenesa | LI512 | 1/2b | Meat isolate |
| LI514 | 1/2b | Meat isolate | |
| LI521 (Murray B) | 4b | Patient/outbreak | |
| LI527 (Scott A) | 4b | Patient/outbreak | |
| LI549 | 4b | Foodborne illness case/cheese | |
| Y. enterocoliticab | R-69 | O:8 | Human |
| R-72 | O:3 | Pork | |
| RF18 | O:9 | Human | |
| ER-1261 | O:3 | Unknown | |
| PAA | O:8 | Human |
Strains of L. monocytogenes were obtained from A. Lammerding (Health of Animals Laboratory, Health Canada, Guelph, Ontario, Canada).
Strains of Y. enterocolitica were obtained from M. W. Griffiths (University of Guelph, Department of Food Science, Guelph, Ontario, Canada).
Sample packaging and storage.
Following inoculation, chicken legs were individually packaged in nylon-ethylene vinyl acetate bags (O2 transmission rate, 40 cm3/m2 in 24 h at 23°C and 0% relative humidity; moisture vapor transmission rate, 4.80 g/m2 in 24 h at 37.8°C and 90% relative humidity) (Winpak Technologies, Toronto, Ontario, Canada) under an atmosphere of 44:56 CO2-N2 (Canox, Guelph, Ontario, Canada) by using a tabletop Multivac A300 packaging machine (Sepp Hagenmüller KG, Wolfertschwenden, Germany). Air was evacuated from the package once, one gas flush was applied, and the package was sealed. Packages were stored at one of three temperatures: 3.5, 6.5, or 10 ± 0.5°C. Uninoculated controls were similarly packaged and stored. Duplicate samples were analyzed following inoculation and packaging and twice a week for a period of 2 to 5 weeks depending on the storage temperature. Each treatment was performed twice on two separate occasions, with the exception of the Y. enterocolitica test at 10°C, which was done once. Control samples (uninoculated) were packaged within 12 to 24 h of production, and test samples were inoculated and packaged within 15 to 24 h of production in the first trial and within 28 to 40 h in the second trial.
Microbiological analysis.
All media were supplied by Difco (Detroit, Mich.). The top of each package was swabbed with alcohol and opened with flame-sterilized scissors, and 100 ml of sterile 0.1% peptone was added to each bag. Chicken legs were hand massaged for 2 min, followed by a vigorous shaking 20 times. Serial dilutions were prepared, and 100 μl was surface plated onto PCA and Oxford agar containing the Oxford selective supplement when samples were inoculated with L. monocytogenes or onto PCA and cefsulodin-irgasan-novobiocin (CIN) agar when samples had been inoculated with Y. enterocolitica. PCA plates were incubated at 22°C for 48 h, and Oxford and CIN plates were incubated at 30°C for 48 and 18 h, respectively. Uninoculated control samples were plated on PCA, Oxford, and CIN agar. Dark colonies on Oxford agar were counted as L. monocytogenes, and small colonies on CIN agar with a dark red center surrounded by a transparent border were enumerated as Y. enterocolitica.
Immediately after inoculation and packaging, numbers of L. monocytogenes and Y. enterocolitica were assessed by a three-tube most probable number (MPN) procedure. L. monocytogenes MPN was determined by transferring 1-ml volumes of the wash and appropriate dilutions to 9 ml of Listeria enrichment broth followed by incubation at 30°C for 48 h. Samples (100 μl) from tubes showing growth were transferred to Fraser broth for 48 h at 30°C. Positive tubes of Fraser broth were streaked onto Oxford agar (30°C, 48 h) for confirmation. A similar procedure was used to determine initial levels of Y. enterocolitica. After 5 days at 22°C, positive tubes of irgasan-ticarcillin-cefsulodin broth were streaked onto CIN agar. Counts for all populations measured were reported as CFU per chicken leg. The identification of selected colonies enumerated as either Y. enterocolitica or L. monocytogenes was confirmed by Vitek Jr. (bioMérieux Vitek, Inc., Hazelwood, Mo.).
Identification of background microbiota.
In some instances, colonies were selected from the highest dilution of PCA plates from uninoculated samples. Colonies were purified on tryptic soy agar, and isolates were identified to the genus level based on Gram reaction, cell morphology, catalase, oxidase, motility, and, for gram-positive isolates, growth on selective agar (streptomycin thallous acetate agar, Rogosa SL agar, and MRS agar adjusted to pH 9.0). Selected gram-negative organisms were identified further by Vitek Jr.
Antimicrobial concentration.
The antimicrobial activity of ALTA 2341 was determined by a spot-on-lawn assay and quantified by the critical dilution assay, as previously described (4), with 5 μl of the prepared solution and L. monocytogenes LI512 or LI514 as the indicator culture. The activity, in arbitrary units (AU) per milliliter, was defined as the reciprocal of the highest dilution that inhibited the growth of the indicator culture lawn.
Headspace and pH.
Gas samples were withdrawn from uninoculated packages through sampling patches (Fisher Scientific) by using a gas-tight syringe. Gas composition (O2 and CO2) was determined by gas chromatography (GOW-MAC; Gow Mac Instruments Co., Bridgewater, N.J.) at the start of each trial, 24 h following packaging. At weekly intervals, uninoculated 11-g samples of chicken were homogenized for 2 min in 99 ml of 0.1% peptone by using a Stomacher Lab Blender 400 (Seward Medical). The pH of the slurry was measured with a Fisher Acumet 915 meter (Fisher Scientific).
Statistical analysis.
Values from the replicate trials were used for statistical analyses. Data were analyzed by ANOVA (analysis of variance) with the general linear model procedure of the SAS statistical package (SAS Institute Inc., Cary, N.C.). Replications, brine, packaging, and storage treatments were compared on the basis of log CFU per piece. Data reported are averages of duplicate samples and replicate trials.
Development of background microbiota.
With the exception of two packages that had approximately 105 CFU/piece (1,000 CFU/g), hot-packed samples (approximately 200 packages) did not develop a background microbiota within the storage period. Colonies from the two packages with counts had a homogeneous morphology on PCA and were identified, by standard biochemical tests, as enterococci. In contrast, a consistent background microbiota developed on cold-packaged samples. A total of 70 colonies were randomly selected from PCA plates of the highest dilution from samples that were stored at 3.5, 6.5, and 10°C. Gram staining and cellular morphology showed 69 of 70 of the isolates to be gram-positive rods and coccobacilli, with one gram-negative rod which was not further characterized. Based on catalase and oxidase reactions and growth on selective agar, gram-positive isolates were tentatively classified as Carnobacterium spp. (48 isolates), Leuconostoc spp. and Lactococcus spp. (11 isolates), and Brochothrix spp. (10 isolates).
In all control trials, the background microbiota grew on CIN agar at levels two or more log cycles lower than on PCA; however, none of the colonies had the typical appearance of yersiniae. Selected colonies were identified as either Serratia spp. or “not identified gram-negative” by Vitek Jr.
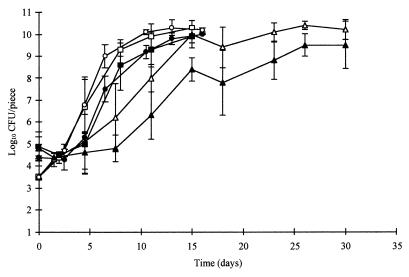
There were no significant differences between replicate trials for growth of the background microbiota. Counts of the background microbiota were similar at 6.5 and 10°C (P > 0.05); however, counts at both higher storage temperatures were significantly different (P < 0.01) from counts at 3.5°C (Fig. 2). The addition of sodium lactate and ALTA 2341 significantly affected counts of the background microbiota (P < 0.01). The effect was more pronounced at 3.5°C than at 6.5 or 10°C (Fig. 2).
FIG. 2.
Aerobic plate counts for cooked, modified-atmosphere, cold-packed poultry injected with regular brine (open symbols) or test brine containing sodium lactate and ALTA 2341 (solid symbols) and stored at 3.5°C (▴), 6.5°C (•), or 10°C (■).
The CO2 concentration decreased from 43 to 44% to approximately 33 to 34% within 24 h of packaging. Oxygen concentrations, when detected, were <1%. The pH of the chicken was 6.3 throughout the experiment and was not affected by brine treatments.
Growth of Y. enterocolitica and L. monocytogenes.
Y. enterocolitica or L. monocytogenes isolates were not recovered (<1,000 CFU/150-g piece) from control samples during the course of the experiment as determined by surface plating of initial dilutions onto selective media. No significant difference (P > 0.05) was observed in the growth of pathogens in hot- and cold-packed samples, indicating that the presence of a competitive microbiota did not influence the growth of either L. monocytogenes or Y. enterocolitica at any of the storage temperatures (data not shown). Levels of the background microbiota, although 1 to 2 log units higher than levels of pathogenic organisms, may have been insufficient to exert an effective inhibitory action. Alternatively, these results could be a reflection of the consistently high pH of the meat, the absence of a fermentable carbohydrate, or the type of organisms that predominated in the product.
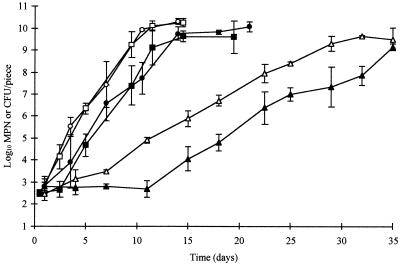
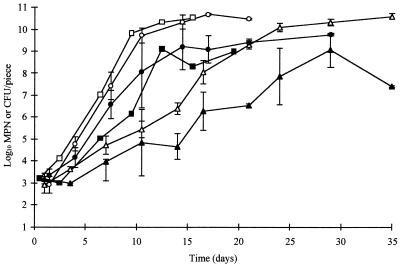
Addition of food-grade sodium lactate and ALTA 2341 to the brine did not prevent the growth of L. monocytogenes and Y. enterocolitica, but it significantly (P < 0.01) decreased counts of the pathogens. The preservatives extended the lag phase of L. monocytogenes by approximately 10 days in cold-packed samples stored at 3.5°C and by 1 to 2 days in samples stored at 6.5 or at 10°C (Fig. 3). The combined preservatives extended the lag phase of Y. enterocolitica by 3 days in samples stored at 3.5°C, but little effect was seen at the higher storage temperatures (Fig. 4). No significant difference (P > 0.05) was observed between replicate trials for growth of L. monocytogenes. However, inhibitory effects of the test brine on Y. enterocolitica were significantly greater (P < 0.01) in replicate two.
FIG. 3.
Growth of L. monocytogenes on cooked, modified-atmosphere, hot-packed poultry injected with regular brine (open symbols) or test brine containing sodium lactate and ALTA 2341 (solid symbols) and stored at 3.5°C (▴), 6.5°C (•), or 10°C (■). Counts below 1,000 CFU/piece were determined by MPN.
FIG. 4.
Growth of Y. enterocolitica on cooked, hot-packed poultry injected with regular brine (open symbols) or test brine containing sodium lactate and ALTA 2341 (solid symbols) and stored at 3.5°C (▴), 6.5°C (•), or 10°C (■). Data at 10°C are average counts from two packages from one trial. Counts below 1,000 CFU/piece were determined by MPN.
Two of five L. monocytogenes strains (LI512 and LI514) and none of the Y. enterocolitica strains used in this study were inhibited by 0.5% ALTA 2341. L. monocytogenes LI527 and LI549 were weakly inhibited at 2% ALTA 2341 but were not at lower concentrations. A heat treatment of 85°C for 30, 60, or 120 min did not reduce the inhibitory action of ALTA 2341. A 1% solution of ALTA 2341 was equivalent to 2,000 AU/ml, which is similar to that reported previously (5, 13).
The predominant spoilage microbiota in MAP products are lactic acid bacteria. The preservative action of lactic acid bacteria in fermented foods is largely due to the production of organic acids coupled with a drop in pH (8, 9). Other mechanisms of inhibition may be the decreased availability of nutrients, the formation of hydrogen peroxide, or the production of bacteriocins. The competitive ability of naturally occurring lactic acid bacteria in nonfermented RTE foods is much less clear. Most previous studies on the growth and survival of psychrotrophic pathogens in nonfermented RTE meats have used laboratory-prepared sterile or pasteurized aseptically handled meat (11, 12, 19). Lactic acid bacteria selected for production of specific bacteriocins have been coinoculated into heat-treated meats along with the pathogen of interest with variable degrees of success in inhibiting pathogen growth (3, 4, 18, 24). Cooked meat products with a naturally occurring background microbiota have occasionally been used for challenge studies (3, 7); however, the specific competitive action of background spoilage organisms on inoculated pathogens was not determined because a suitable bacterium-free control was not available.
In this study, the competitive ability of a naturally occurring population of lactic acid bacteria and Brochothrix spp. was evaluated in a commercially prepared product. The development of this background microbiota had no effect on the growth of L. monocytogenes or Y. enterocolitica. Temperature was the most important factor in inhibiting the growth of either pathogen. The preservatives sodium lactate and ALTA 2341, added at maximum recommended levels, provided a small additional barrier by extending the lag phase; however, their effectiveness diminished as the storage temperature increased.
Acknowledgments
This project was supported by contributions from J. M. Schneider, Inc., and the Ontario Ministry of Agriculture, Food and Rural Affairs. An Ontario Government Scholarship is also gratefully acknowledged.
REFERENCES
- 1.Anonymous. Product information sheet for ALTA 2341. Lachine, Quebec, Canada: Quest International; 1997. [Google Scholar]
- 2.Barakat R K, Harris L J. Abstracts of the 81st Annual Meeting of the International Association of Milk, Food and Environmental Sanitarians 1994, San Antonio, Tex. 1994. Shelf life and microbial ecology of precooked modified atmosphere packaged poultry product stored at 4°C, abstr. 135; p. 58. [Google Scholar]
- 3.Beumer R R, te Giffel M C, de Boer E, Rombouts F M. Growth of Listeria monocytogenes on sliced cooked meat products. Food Microbiol. 1996;13:333–340. [Google Scholar]
- 4.Degnan A J, Yousef A E, Luchansky J B. Use of Pediococcus acidilactici to control Listeria monocytogenes in temperature-abused vacuum-packaged wieners. J Food Prot. 1992;55:98–103. doi: 10.4315/0362-028X-55.2.98. [DOI] [PubMed] [Google Scholar]
- 5.Degnan A J, Kaspar C W, Otwell W S, Tamplin M L, Luchansky J B. Evaluation of lactic acid bacterium fermentation products and food-grade chemicals to control Listeria monocytogenes in blue crab (Callinectes sapidus) meat. Appl Environ Microbiol. 1994;60:3198–3203. doi: 10.1128/aem.60.9.3198-3203.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farber J M, Warburton D W, Gour L, Milling M. Microbiological quality of foods packaged under modified atmospheres. Food Microbiol. 1990;7:327–334. [Google Scholar]
- 7.Glass K A, Doyle M P. Fate of Listeria monocytogenes in processed meat products during refrigerated storage. Appl Environ Microbiol. 1989;55:1565–1569. doi: 10.1128/aem.55.6.1565-1569.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gombas D E. Biological competition as a preserving mechanism. J Food Saf. 1989;10:107–117. [Google Scholar]
- 9.Holzapfel W H, Geisen R, Schillinger U. Biological preservation of foods with reference to protective cultures, bacteriocins and food-grade enzymes. Int J Food Microbiol. 1995;24:343–362. doi: 10.1016/0168-1605(94)00036-6. [DOI] [PubMed] [Google Scholar]
- 10.Hudson J A, Mott S J, DeLacy K M, Edridge A L. Incidence and coincidence of Listeria spp., motile aeromonads and Yersinia enterocolitica on ready-to-eat fleshfoods. Int J Food Microbiol. 1992;16:99–108. doi: 10.1016/0168-1605(92)90002-k. [DOI] [PubMed] [Google Scholar]
- 11.Hudson J A, Mott S J. Growth of Listeria monocytogenes, Aeromonas hydrophila and Yersinia enterocolitica on cooked beef under refrigeration and mild temperature abuse. Food Microbiol. 1993;10:429–437. doi: 10.1016/0168-1605(93)90055-l. [DOI] [PubMed] [Google Scholar]
- 12.Hudson J A, Mott S J, Penney N. Growth of Listeria monocytogenes, Aeromonas hydrophila, and Yersinia enterocolitica on vacuum and saturated carbon dioxide controlled-atmosphere-packaged sliced roast beef. J Food Prot. 1994;57:204–208. doi: 10.4315/0362-028X-57.3.204. [DOI] [PubMed] [Google Scholar]
- 13.Jack R W, Wan J, Gordon J, Harmark K, Davisdon B E, Hillier A J, Wettenhall R E H, Hickey M W, Coventry M J. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl Environ Microbiol. 1996;62:2897–2903. doi: 10.1128/aem.62.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jay J M. Microorganisms in fresh ground meats: the relative safety of products with low versus high numbers. Meat Sci. 1996;43:S59–S66. doi: 10.1016/0309-1740(96)00055-1. [DOI] [PubMed] [Google Scholar]
- 15.Kerr K G, Rotowa N A, Hawkey P M, Lacey R W. Incidence of Listeria spp. in pre-cooked, chilled chicken products as determined by culture and enzyme-linked immunoassay (ELISA) J Food Prot. 1990;53:606–607. doi: 10.4315/0362-028X-53.7.606. [DOI] [PubMed] [Google Scholar]
- 16.Manu-Tawiah W, Myers D J, Olson D G, Molins R A. Survival and growth of Listeria monocytogenes and Yersinia enterocolitica in pork chops packaged under modified gas atmospheres. J Food Sci. 1993;58:475–479. [Google Scholar]
- 17.Qvist S, Sehested K, Zeuthen P. Growth suppression of Listeria monocytogenes in a meat product. Int J Food Microbiol. 1994;24:283–293. doi: 10.1016/0168-1605(94)90126-0. [DOI] [PubMed] [Google Scholar]
- 18.Schillinger U, Kaya M, Lücke F-K. Behaviour of Listeria monocytogenes in meat and its control by a bacteriocin-producing strain of Lactobacillus sake. J Appl Bacteriol. 1991;70:473–478. doi: 10.1111/j.1365-2672.1991.tb02743.x. [DOI] [PubMed] [Google Scholar]
- 19.Schlyter J H, Degnan A J, Loeffelholz J, Glass K A, Luchansky J B. Evaluation of sodium diacetate and ALTA™ 2341 on viability of Listeria monocytogenes in turkey slurries. J Food Prot. 1993;56:808–810. doi: 10.4315/0362-028X-56.9.808. [DOI] [PubMed] [Google Scholar]
- 20.Shelef L A, Yang Q. Growth suppression of Listeria monocytogenes by lactates in broth, chicken, and beef. J Food Prot. 1991;54:283–287. doi: 10.4315/0362-028X-54.4.283. [DOI] [PubMed] [Google Scholar]
- 21.Toora S, Budu-Amoako E, Ablett R F, Smith J. Isolation of Yersinia enterocolitica from ready-to-eat foods by a simple two step procedure. Food Microbiol. 1994;11:369–374. [Google Scholar]
- 22.Wang C, Muriana P M. Incidence of Listeria monocytogenes in packages of retail franks. J Food Prot. 1994;57:382–386. doi: 10.4315/0362-028X-57.5.382. [DOI] [PubMed] [Google Scholar]
- 23.Wederquist H J, Sofos J N, Schmidt G R. Listeria monocytogenes inhibition in refrigerated vacuum packaged turkey bologna by chemical additives. J Food Sci. 1994;59:498–500. , 516. [Google Scholar]
- 24.Winkowski K, Crandall A D, Montville T J. Inhibition of Listeria monocytogenes by Lactobacillus bavaricus MN in beef systems at refrigeration temperatures. Appl Environ Microbiol. 1993;59:2552–2557. doi: 10.1128/aem.59.8.2552-2557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]