Abstract
GATA transcription factors have been demonstrated to play key regulatory roles in plant growth, development, and hormonal response. However, the knowledge concerning the evolution of GATA genes in Eucalyptus urophylla and their trans-regulatory interaction is indistinct. Phylogenetic analysis and study of conserved motifs, exon structures, and expression patterns resolved the evolutionary relationships of these GATA proteins. Phylogenetic analysis showed that EgrGATAs are broadly distributed in four subfamilies. Cis-element analysis of promoters revealed that EgrGATA genes respond to light and are influenced by multiple hormones and abiotic stresses. Transcriptome analysis revealed distinct temporal and spatial expression patterns of EgrGATA genes in various tissues of E. urophylla S.T.Blake, which was confirmed by real-time quantitative PCR (RT-qPCR). Further research revealed that EurGNC and EurCGA1 were localized in the nucleus, and EurGNC directly binds to the cis-element of the EurGUN5 promoter, implying its potential roles in the regulation of chlorophyll synthesis. This comprehensive study provides new insights into the evolution of GATAs and could help to improve the photosynthetic assimilation and vegetative growth of E. urophylla at the genetic level.
Keywords: GATA, Eucalyptus, evolution, transcription factors, photosynthesis
1. Introduction
GATA transcription factors are a class of genes with type IV zinc finger conserved structural domains (C-X2-C-X17-20-C-X2-C) and are widely present in various plants and animals [1]. Type IV zinc finger structures in plants are usually present as C-X2-C-X18-C-X2-C or C-X2-C-X20-C-X2-C [2]. Based on their evolutionary relationship and gene structure, GATA family genes can be divided into four subgroups [3]. As a transcription factor, it mainly regulates the expression of downstream genes by binding to conserved sequences, thus affecting the biological phenotype. The normally recognized consensus sequence of the GATA transcription factor family is WGATAR (W stands for T or A, and R stands for G or A). Analysis of the GATA family information on the JASPAR plant database revealed that 11 GATA genes have been sequenced (Dap-seq or ChIP-seq) or experimented with to verify their consensus sequence (GATC). The first GATA gene was identified in tobacco [4]. To date, GATA members have been reported in numerous plant species, among which 30 GATA transcription factors have been identified in Arabidopsis, and 28, 64, 179, and 262 GATA genes have been identified in the genomes of rice (Oryza sativa), soybean (Glycine max), cotton (Gossypium hirsutum), and seven poplars (Populus), respectively [2,5,6,7].
GATA transcription factors are widely involved in the biological processes of plant growth, development, and stress resistance. In common wheat, the GATA family transcription factor ZIM-A1 can recognize the consensus sequence (TCKAG) in the promoter regions of the flowering genes, FLOWERING LOCUS T (FT) and CONSTANS (CO), and inhibit their expression [8]. GATA transcription factors are essential components of endogenous hormone pathways that govern a wide range of plant physiological processes. In Arabidopsis, GATA12, which has been identified as a downstream response factor of the DELLA protein RGL2, is inhibited by gibberellin and participates in the regulation of seed dormancy [9]. The GATA7 contributes to the regulation of plant shape and grain type in rice via regulation of brassinosteroids [10]. According to previous research, GATA23, which is driven by several auxin response factors (ARFs), is involved in the formation of lateral roots and the regeneration of roots [11]. In addition, ARF2 also directly affects the expression of GATA NITRATE-INDUCIBLE CARBON-METABOLISM-INVOLVED (GNC) and CYTOKININ-RESPONSIVE GATA FACTOR 1 (GNL/CGA1), thus regulating greening, flowering, and leaf senescence. Cytokinin can promote the expression of GNL/CGA, and gene mutations can result in the blocking of cytokinin-regulated growth processes, such as leaf senescence, hypocotyl growth, and branch formation [12]. Hence, GNL/CGA plays a key role in plant greening as it forms the junction between auxin and cytokinin [13].
Eucalypts are the main suppliers of high-quality woody biomass for the fiber, energy, and paper industry, with the added benefit of absorbing significant amounts of atmospheric carbon [14]. Improving the photosynthesis efficiency of Eucalyptus is of great significance for enhancing biomass accumulation and solving global warming problems. Chlorophylls, in conjunction with their binding proteins, play a key role in photosynthesis by capturing light energy and transmitting it to photosystem reaction centers [15,16]. To date, GATA transcription factors from a lot of species have been reported to control chloroplast development and chlorophyll synthesis [17]. In Arabidopsis, GNC and GNL/CGA1 accelerated chloroplast development and greening during photomorphogenesis by directly targeting transcription factor (TF) genes, such as PHYTOCHROME-INTERACTING FACTOR (PIF) and SPEECHLESS (SPCH) [13,18,19]. Overexpression of OsCGA1 or OsGATA12 in rice increased chloroplast biogenesis and chlorophyll content and inhibited the expression of genes related to chlorophyll degradation, whereas OsCGA1 knockdown resulted in reduced chlorophyll content [20,21]. In poplar, overexpression of GNC dramatically accelerated plant growth, chlorophyll accumulation, and photosynthesis, whereas its CRISPR-knockout lines showed retarded development [22]. Therefore, the significant involvement of the GATA family proteins in chlorophyll synthesis and various other biological processes necessitates a more in-depth examination of their roles and functions involvement in Eucalyptus.
In this study, we provide a complete analysis of the numbers, phylogeny, conserved motifs, exon–intron structure, cis-elements of the promoters, chromosome distribution, and expression of GATA genes in the published genome of Eucalyptus grandis (JGI, 2014) to better understand the dynamics of GATA gene evolution in Eucalyptus and facilitate future study on this critical TF family.
2. Results
2.1. Identification and Phylogenetic Analysis of Eucalyptus GATA Transcription Factors
A total of 23 genes were identified on the basis of the functional annotation in the Eucalyptus grandis genome. The length of GATA proteins ranged from 66 to 545 amino acids (Table S1). The isoelectric point of the proteins ranged from 4.87 to 10.61, and the molecular weight ranged from 72.96 kDa to 604.67 kDa.
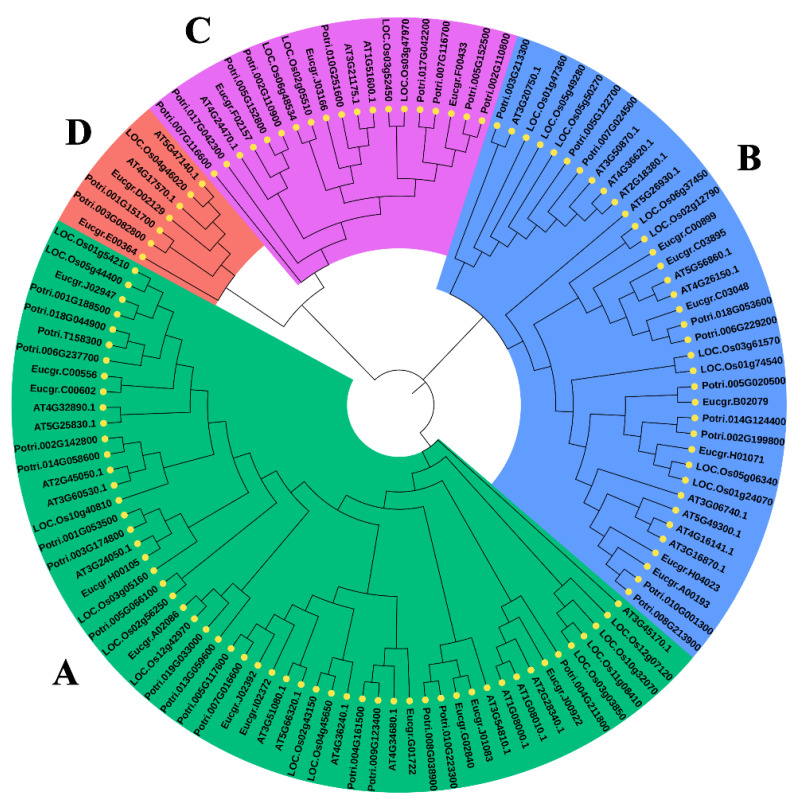
The protein sequences encoded by GATA genes were used to construct the phylogenetic tree to determine the evolutionary relationship between them. Results showed clustering of GATA proteins of Arabidopsis, Populus, rice, and Eucalyptus into four subfamilies (Figure 1). Preliminary classification of the phylogeny according to the four subfamilies identified by analysis of the GATA protein family from four species displayed good agreement with the topology in our study. Subfamily A was the largest, comprising 54 proteins, followed by 37, 18, and 7 GATA proteins belonging to Subfamilies B, C, and D, respectively. All Eucalyptus GATA members formed groups with GATA members of other species in each subfamily. In subfamilies, the gene phylogeny roughly followed species phylogeny, with Populus genes being closely related to the Eucalyptus genes. These genes may have similar evolutionary background and functions in woody trees.
Figure 1.
Phylogenetic relationships between GATA proteins from Arabidopsis, poplar, rice, and Eucalyptus. The GATA members of Eucalyptus grandis were classified into four subfamilies together with the homologous genes of other plants. A, B, C, and D represent the four subfamilies, respectively.
2.2. Gene Structure and Conserved Motifs of GATA Genes
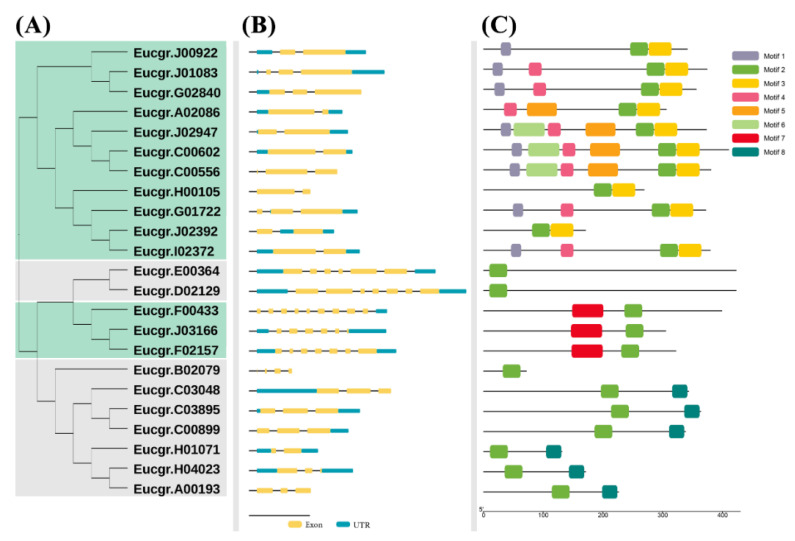
The relationship between 23 Eucalyptus GATA genes was determined by the construction of a phylogenetic tree using the CDS sequences. To elucidate the structural characteristics of the EgrGATA genes, the exon–intron structures and conserved motifs were analyzed (Figure 2). Genes of Subfamilies A and B contained two to three exons, whereas those of Subfamilies C and D contained five to seven exons.
Figure 2.
Predicted Eucalyptus grandis GATA (EgrGATA) protein phylogeny, conserved amino acid motifs, and gene structures. (A) Unrooted tree of EgrGATA proteins based on maximum-likelihood method. (B) Position of exons and UTRs in the EgrGATA gene models (yellow line represents exons, and blue line represents UTRs). (C) Composition and distribution of overrepresented amino acid motifs (sequences of conserved motifs are given in Figure S1).
Three genes of Subfamily A, Eucgr.J01083, Eucgr.G02840, and Eucgr.G01722, had three exons, whereas the other eight genes had only two exons. Only Eucgr.H01701 had two exons in Subfamily B, while the other six genes had three exons. The length and distribution of exons in the same subfamily were also relatively similar. Compared with the other three subfamilies, the length of exons in Subfamily C is shorter (1–1.5 kb). Significant variations in the number and distribution of exons suggest that distinct subfamilies have different evolutionary origins and chromosomal structural variations.
In the EgrGATA proteins, eight motifs were identified by the Multiple EM for Motif Elicitation (MEME) programs and defined as Motifs 1 to 8 (Figure 2C). The detailed sequence of each motif is provided in Figure S1. Motif 2, like the GATA domain, was detected in all EgrGATA proteins. In detail, Motifs 1, 3, and 4 were mainly involved in Subfamily A. Except for Motif 2, no other motifs were recognized in Subfamily B. Motifs 7 and 8 were identified in Subfamilies C and D, respectively. In Subfamily C, Motif 5 was located upstream of Motif 1. Proteins belonging to different subfamilies were found to have similar motif locations. In Subfamily A, Motifs 2 and 3 were located at the C-terminal, and Motif 2 was present upstream of Motif 3. Motif 1 was located at the N-terminal in proteins of Subfamily A, except for those encoded by Eucgr.A02086 and Eucgr.H00105. Proteins from different subfamilies had different motifs, but proteins from the same subfamily had similar motifs.
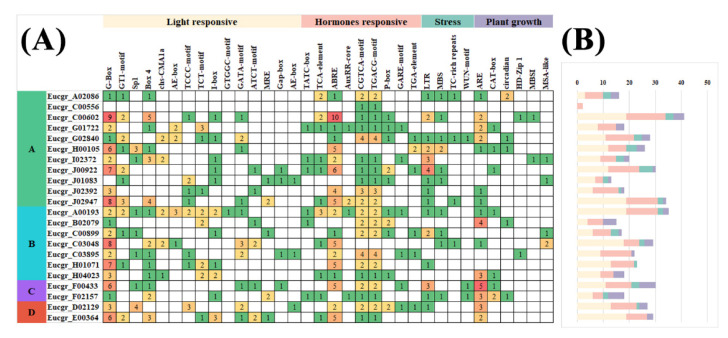
To understand gene regulation and function, it is necessary to identify putative cis-elements in promoter sequences of EgrGATA genes. The identified cis-elements were classified into four categories, including light-responsive, hormone-responsive, stress-responsive, and plant growth (Figure 3). There are 15 cis-elements that belong to the light-responsive category, such as G-box, GT1-motif, Sp1, Box 4, AE-box, GATA-motif, MRE, and AE-box. Among them, G-box is present in promoter regions of almost all EgrGATA genes, except for Eucgr.C00556 and Eucgr.J01083. The hormone-responsive category contained TATC-box (gibberellin-responsive), TCA-element (salicylic-acid-responsive), ABRE (abscisic-acid-responsive), AuxRR-core (auxin-responsive), CGTCA-motif (Methyl jasmonate-responsive), TGACG-motif (Methyl jasmonate-responsive), P-box (gibberellin-responsive), GARE-motif (gibberellin-responsive), and TGA-element (auxin-responsive).
Figure 3.
Analysis of cis-elements in the promoters of EgrGATA genes. (A) Overview of the types and numbers of cis-elements of the four subfamilies identified from the PLANTCARE database. (B) The number of light-responsive, hormone-responsive, stress-, and plant-growth-related elements present in the promoter region of each gene.
2.3. Chromosomal Localization and Collinearity Analysis
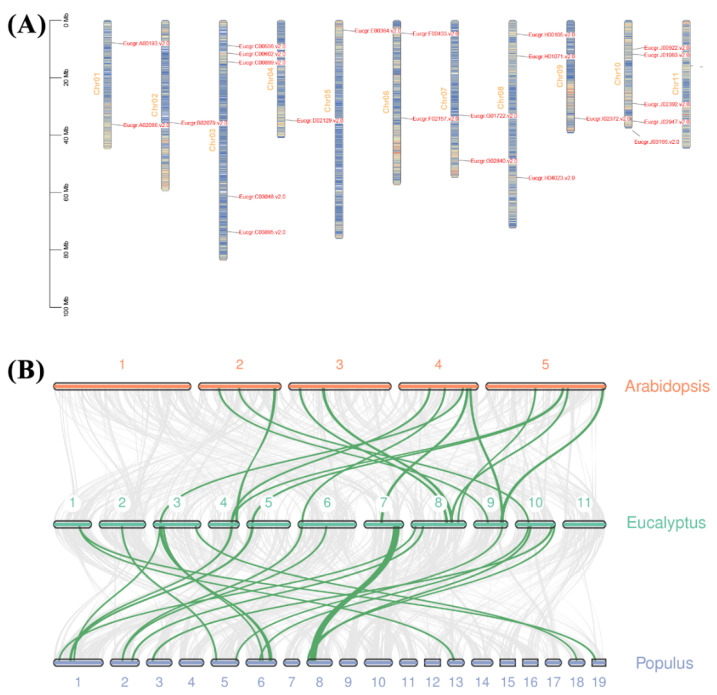
According to the EgrGATA annotation information, 23 genes were mapped to 10 chromosomes of Eucalyptus, and GATA family genes were not identified on Chr11 (Figure 4A). The distribution of the Eucalyptus GATA gene family on chromosomes is uneven. Gene number or density of the chromosome has no biological relevance to EgrGATA distribution. Chromosomes 5 and 10 contained the largest number of EgrGATA genes, and Chromosome 8 had three EgrGATA genes. The length of Chromosome 5 is second only to that of Chromosome 3, but it contains only one GATA gene.
Figure 4.
Distribution of EgrGATA genes on the chromosomes of Eucalyptus grandis and synteny analysis of GATA genes. (A) Chromosomal distributions of EgrGATA genes. Color gradient from blue to red on the chromosomes indicates low to high gene density, respectively. (B) Synteny analyses of GATA genes between Eucalyptus grandis and other module plant species (A. thaliana and P. trichocarpa). Green lines indicate the collinear blocks within Eucalyptus and other plant genomes.
To better understand the evolutionary relationships of GATA from Arabidopsis, Populus, and Eucalyptus grandis, we constructed a collinearity chart (Figure 4B). There are 14 and 25 GATA genes in Arabidopsis and poplar, respectively, which have a collinearity relationship with EgrGATAs. Among them, 13 genes are shared by Arabidopsis and poplar, which indicates that the GATA family has lost and reconstructed sequences in woody plants during evolution. Furthermore, the Ka/Ks ratio was calculated between Eucalyptus grandis and other species (Table S2). These results suggest that most homologous genes are under purifying selection in Eucalyptus.
2.4. Tissue-Specific Expression Patterns of GATA Genes
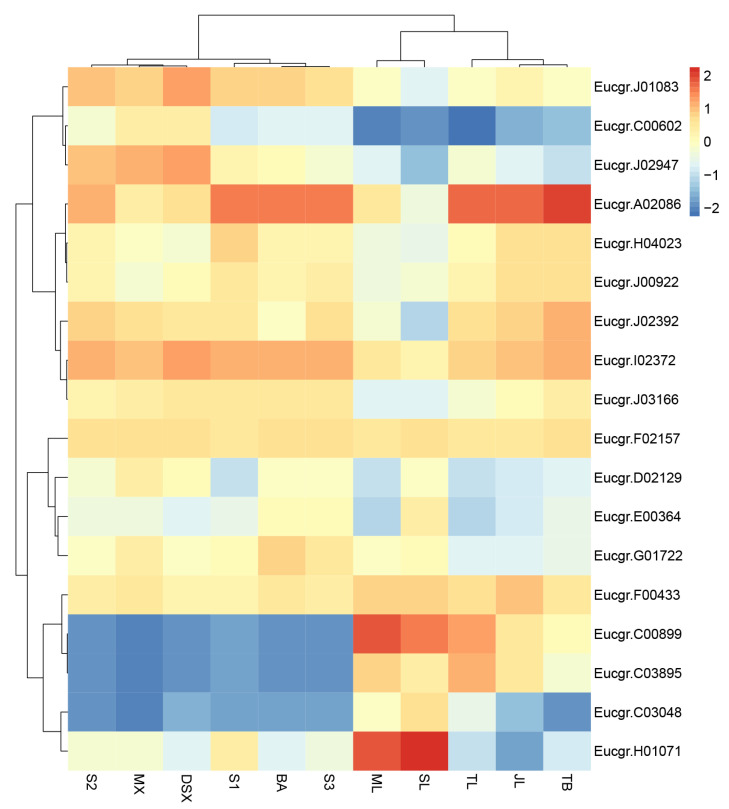
The expression patterns of EgrGATA genes were further analyzed based on RNA-seq data of 11 different tissues (terminal bud, spire leaves, transition leaves, mature leaves, senescent leaves, young stem, transition stem, mature stem, bark, developing secondary xylem, and mature xylem). A total of 22 EurGATA showed expression in different tissues and exhibited tissue-specific expression (Figure 5). Heat map and hierarchical cluster analysis showed that expression patterns of these genes were different in different tissues. In this study, Eucgr.C00602 and Eucgr.J02947 were highly expressed in the stem, while the other four GATA genes (Eucgr.C00899, Eucgr.C03895, Eucgr.C03048, and Eucgr.H01071) had high expression levels in the leaves. However, the expression levels of some EurGATA genes were similar in several tissues, such as Eucgr.F02157, Eucgr.F00433, Eucgr.J00922, and Eucgr.I02372.
Figure 5.
Tissue-specific gene expression patterns of 22 EgrGATA genes. The expression patterns of genes in terminal bud (TB), juvenile leaves (JL), transition leaves (TL), mature leaves (ML), senescent leaves (SL), young stem (S1), transition stem (S2), mature stem (S3), bark (BA), developing secondary xylem (DSX), and mature xylem (MX). The red and blue colors indicate the high and low transcript abundance, respectively. Expressions normalized by log2.
2.5. GATA Genes Are Involved in Chlorophyll Synthesis
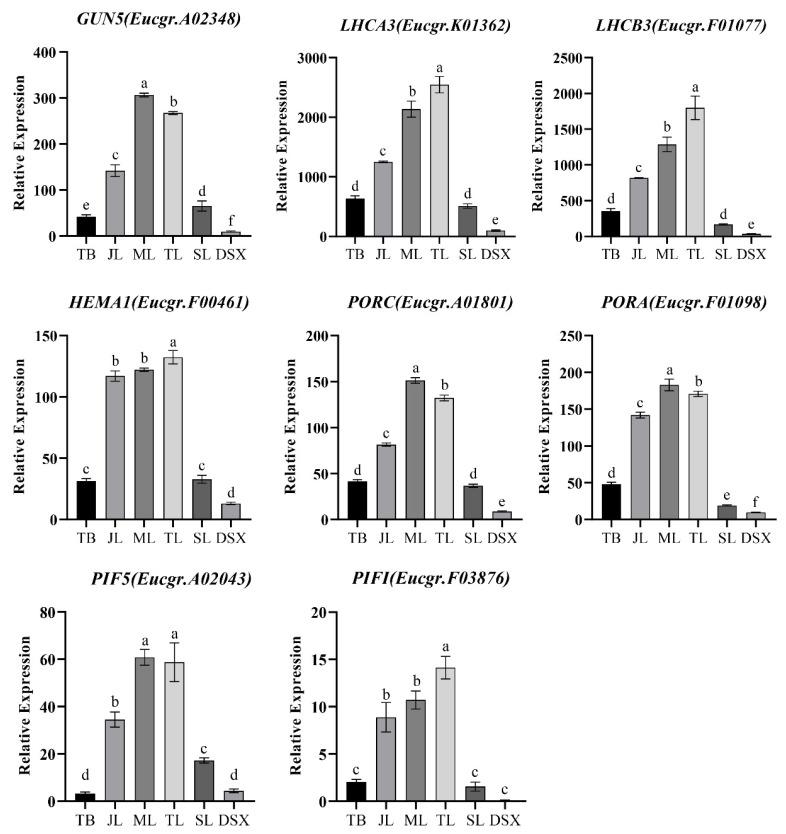
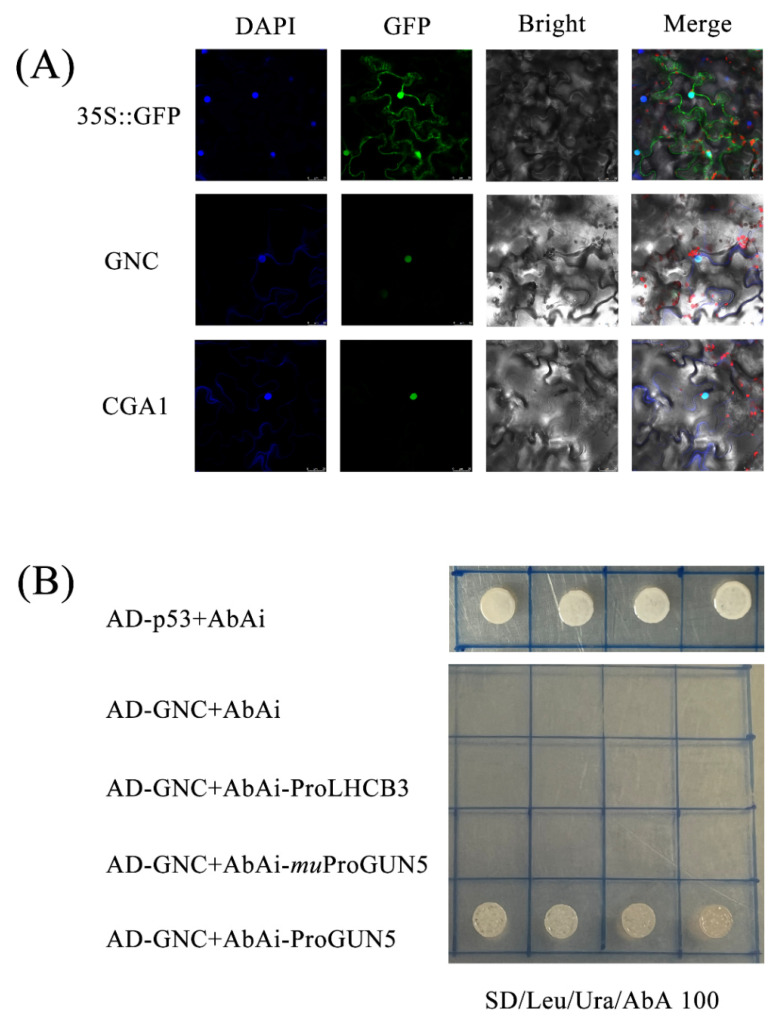
To investigate the relationship between EgrGATAs and the genes involved in chlorophyll synthesis, the transcription levels of those genes were detected by qRT-PCR (Figure 6). Like EurGNC (GATA, NITRATE-INDUCIBLE, CARBON-METABOLISM-INVOLVED), EurGUN5 (GENOMES UNCOUPLED 5) was also highly expressed in leaves and had low expression in stems. To detect the subcellular localization of the EurGNC and EurCGA1 (GNC-LIKE/CYTOKININ-RESPON-SIVE GATA FACTOR1), gene sequences were fused with the GFP reporter gene downstream of the CaMV 35S promoter. In Nicotiana benthamiana, reporter gene expression showed that GNC and CGA1-GFP fusion protein was predominant in the nucleus, indicating that CGA1 and GNC are nuclear-localized transcription factors (Figure 7A). The close relationship and similar expression pattern indicated that EurGNC may activate GUN5 or light-harvesting chlorophyll B-binding protein 3 (LHCB3); thus, it controls the expression of a series of chlorophyll-related genes.
Figure 6.
The qRT-PCR analyses of EgrGATA genes in leaves and stems. The qRT-PCR analyses of eight genes expressed in terminal bud (TB), spire leaves (SL), transition leaves (TL), mature leaves (ML), senescent leaves (SL), and developing secondary xylem (DSX). Photosystem I light-harvesting complex gene 3 (LHCA3). The different lowercase letters show statistically significant differences by the Tukey-Kramer multiple comparison test at p < 0.05.
Figure 7.
EurGNC is a nuclear-localized transcriptional regulator. (A) Subcellular localization of EurGNC and EurCGA1 proteins. Fluorescence signals of GFP were detected in tobacco leaf epidermal cells. Left panel, DAPI and GFP image; middle panel, bright field; and right panel, merging of GFP and bright field. Bar, 25 µm. (B) Yeast one-hybrid assay to detect whether EurGNC regulates downstream genes (EurGUN5 and EurLHCB3). AbA (100 ng/L) was used to repress the autoactivation. Empty pGADT7 (AD) and AbAi were used as negative controls, while pGADT7-p53 and pAbAi-53 were used as positive controls.
To verify the interaction between EurGNC and the upregulated GUN5, a yeast Y1H assay was conducted. EurGNC could not directly bind to the promoter of LHCB3 in the Y1H assay (Figure 7B), but it can bind to EurGUN5. EurGNC bound to the promoter fragments containing the cis-element (GATC), whereas it could not bind to the promoter fragments containing the mutated cis-element GATA.
3. Discussion
In previous studies, GATA family genes have been demonstrated to affect growth regulation, defense response, and stress tolerance in plants [10,23,24,25]. To date, GATA family genes have been identified and characterized in A. thaliana, rice [2], and Populus [7]. However, as a representative plant of the order Myrtales, genome-wide analysis of the GATA family has not been performed in Eucalyptus grandis, and the regulatory function of EgrGATA genes remains unclear. In this study, we systematically analyzed 23 GATA genes in the released Eucalyptus grandis genome. With the continuous development of gene-editing technology in Eucalyptus [26], research on growth-related TFs will provide candidate genes for genome editing and precision plant breeding.
3.1. Phylogenetic Analysis of EgrGATAs
Based on phylogenetic analysis, EgrGATA genes were classified into four subfamilies (Figure 1), which is consistent with classification in other plant species, such as that of A. thaliana and poplar. Therefore, GATA might have been present in the common ancestor of eudicotyledons. Surprisingly, OsGATA (LOC.Os04g46020) appeared on the branch of Subfamily D, which is contrary to the previous research which suggests, that Subfamily D GATAs originated after the differentiation of monocotyledon and dicotyledon, and no OsGATAs belonged to Subfamily D [2,22]. However, phylogenetic analyses of pepper, Gossypium, soybean, and cucumber revealed that OsGATAs occur in the Subfamily D branch [6,27,28,29]. Therefore, we speculated that due to the increase in the accuracy of genome sequencing, evolutionary analysis of the GATA family revealed that Subfamilies A, B, C, and D originated before the differentiation of monocots and dicots.
The number of GATAs identified in the Arabidopsis and poplar genomes were 30 and 39, respectively. Only 23 genes were identified in Eucalyptus, which belonged to the four subfamilies. We suspected that EgrGATAs were lost during evolution but found that sufficient genetic diversity was retained; a similar evolutionary loss event occurred in the WRKY transcription factor family [30]. According to the analysis of exon structure and motifs of EgrGATAs, different EgrGATAs from the same subfamily have similar numbers and patterns and tend to share the same evolutionary origins.
In the process of evolution, segmental and tandem duplications contributed to the formation and rapid expansion of gene families [31,32]. Previous research revealed that 28 EgrWRKYs participated in tandem duplication events without segmental duplication events in E. grandis [33]. Tandem duplication has occurred in several gene families, such as AP2, GRAS, MYB, p-coumarate 3-hydroxylase (C3H), caffeate/5-hydroxyferulate O-methyltransferase (COMT), and ROS [34,35,36,37]. Although EgrGATAs are slightly contracted compared to GATAs of most angiosperms studied hitherto, they lack traces of duplication events (Figure 2 and Figure 4), and a similar conclusion was reached upon the analysis of the Eucalyptus ARF family [38].
3.2. Structural and Functional Variation of EgrGATAs
The exon structures and motifs of EgrGATAs in the same branch had similar characteristics, thus implying that the genes in the same subfamily may have similar expression patterns and biological functions. During tissue development, the expression levels of EgrGATAs (EgrGATA12 (Eucgr.C00602) and EgrGATA9 (Eucgr.J02947)) increase in the xylem of the transition stem, developing secondary xylem, and mature xylem (Figure 5). The GATA12(Potri.006G237700) was predominantly expressed in developing xylem tissues and involved in the regulation of secondary cell wall component biosynthesis pathways in Populus trichocarpa [39]; it indicates a crucial role of EgrGATA12 in regulation of xylem differentiation. Additionally, the expression of EgrGNC (Eucgr.C03048) and CGA1/GNL (Eucgr.C00899) were upregulated in the leaves (Figure 5). In Arabidopsis, stomata formation is promoted by overexpression of GNC and CGA1 [40], which also regulate germination, greening, growth, and flowering time [24].
The cis-element is the short sequence located in the promoter regions of the genes that could be activated by trans-acting elements to regulate the activity of target genes [41]. The analysis of the cis-elements in the EgrGATA promoters showed that 21 EgrGATAs were related to light response (Figure 3A), and the G-box element was present most frequently in the promoters of those genes. In Catharanthus roseus, CrPIF1 repressed CrGATA1 promoter activity by binding to G-box elements, and the expression of CrGATA1 was significantly induced by light [42]. We found numerous cis-elements that are hormone-responsive, implying that EgrGATAs might form a hub for multiple hormones. Three gibberellic acid (GA)-responsive elements (TATC-box, P-box, and GARE-motif) were found in the promoter of 14 EgrGATAs. The promoters of 20 EgrGATAs had CGTCA and TGACG elements, which are MeJA-responsive, and the CGTCA-motif induces transcription of CICMO and CIBADH to accelerate the biosynthesis of glycine betaine in watermelon [43]. There are 63 abscisic-acid-responsive elements (ABREs) that span the promoter regions of 18 EgrGATAs; these elements, as positive regulators of cold tolerance, could bind with PsnICE1 in poplar [44]. Auxin-responsive element (TGA-element) was a key component for indole-3-acetic acid responsiveness [45], which is bound by ARFs to regulate plant architecture [46]. In our study, TGA-element was found in the promoter of six EgrGATAs.
3.3. EurGATAs Were Involved in the Biosynthesis of Chlorophyll
The essence of photosynthesis is the absorption and harvesting of light energy by chlorophyll in the plant leaves, and light absorption strongly depends on the chlorophyll content [47]. Studies in several species have documented that the GATA TF family affects chlorophyll synthesis directly or indirectly [19,48,49]. Overexpression of GATA in Arabidopsis increases the chlorophyll content and improves photosynthetic efficiency [49]. In this study, the expression levels of EurLHCB3 and EurGUN5 were positively correlated with the expression of EurGNC and EurCGA1 (Figure 4). GNC and CGA1 are the two master transcriptional regulators that could affect chlorophyll synthesis genes (GUN4, HEMA1, PORB, and PORC) localized both in the nucleus and chloroplast (GLUTAMATE SYNTHASE (GLU1/Fd-GOGAT)) [18]. Y1H assays showed that GNC could directly bind to the promoter of EurGUN5 (Figure 7B), thus revealing that EurGNC would directly activate EurGUN5 to accelerate chlorophyll biosynthesis. In addition, we found that GNC could not bind to the mutated GUN5 promoter sequence (‘GATC’ mutated to ‘GATA’). The GATA gene families were named because they contained ‘GATA’ sequences in the binding elements; however, subsequent studies have found that the GATA family motif can also recognize the GATC sequence [50].
4. Materials and Methods
4.1. Identification of GATA Family Genes in Eucalyptus
Arabidopsis thaliana, Eucalyptus grandis, rice, and poplar GATA nucleic acid and protein sequences were retrieved from the PlantTFDB 5.0 (http://planttfdb.cbi.pku.edu.cn, accessed on 5 October 2021). Multispecies GATA data were searched using the Hidden Markov Model (HMM) of HMMER 3.1 to identify whether or not it has the GATA zinc finger domain (PF00320) [51]. The theoretical molecular weight (MW) and isoelectric point (pI) of the encoded proteins were analyzed using the ProtParam tool (https://web.expasy.org/protparam/, accessed on 5 October 2021).
4.2. Analysis of Phylogenetic Relationships, Gene Structures, Conserved Motifs, and Protein
Multiple sequence alignment of amino acid sequences was performed using the Multiple Alignment using Fast Fourier Transform tool with default parameters. Subsequently, the results of multiple sequence alignment were trimmed using trimal, and a phylogenetic tree was generated using IQ-TREE. The phylogenetic tree generated was visualized using iTOL (http://itol.embl.de, accessed on 10 October 2021). Using the same method, a phylogenetic tree of Eucalyptus GATA proteins was constructed. Gene structure display server (http://gsds.cbi.pku.edu.cn, accessed on 10 October 2021) was used to map coding sequences to genomic sequences and visualize exon–intron structures. Eucalyptus GATA sequences were submitted to WebLogo (http://weblogo.berkeley.edu, accessed on 10 October 2021) for online analysis to map conserved structural domains. The genomic DNA sequences (2000 bp) present upstream to the 5′UTR were obtained from the genome of Eucalyptus grandis using TBtools. The promoters of EgrGATA were submitted to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html, accessed on 15 October 2021) for analysis of cis-acting elements. The diagram of cis-elements present in the promoter regions was visualized by TBtools.
4.3. Chromosomal Location and Cross-Species Collinearity Analysis
The chromosomal locations of the EgrGATA genes were derived from the gene annotation file and presented using TBtools. Cross-species collinearity analyses and plotting were performed using MCscan (Python version) of jcvi (v.0.8.12) and annotation files retrieved from Phytozome. TBtools was used to estimate nonsynonymous substitution rate (Ka), synonymous substitution rate (Ks), and Ka/Ks of the paralogous gene pair. When the Ka/Ks ratio was >1, <1, or =1, selection pressure on EgrGATA was considered positive, negative, or neutral, respectively.
4.4. Expression Profile Analysis of GATA Gene Family
Plants from the Guangxi Dongmen Forest Farm’s Eucalyptus urophylla were used in this study. Tissues (terminal bud, spire leaves, transition leaves, mature leaves, senescent leaves, young stem, transition stem, mature stem, bark, developing secondary xylem, mature xylem) from five months seedlings were used for analysis of tissue-specific expression (Figure S2). The total RNA was extracted and purified using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer’s instructions. Paired-end sequencing was performed on an Illumina Novaseq™ 6000, following the vendor’s recommended protocol. The FASTQ data were filtered using fastx-toolkit (http://hannonlab.cshl.edu/fastx_toolkit/index.html, accessed on 15 October 2021) based on a quality score criterion of Q20 and were then inspected with the FastQC program (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/, accessed on 15 October 2021). Fragments per kilobase of exon per million fragments mapped (FPKM) values of isoform genes were calculated by Cufflinks.
4.5. RNA Isolation and Quantitative Real-Time PCR (qRT-PCR) Validation
Total RNA from E. urophylla of different stages was the same as 4.4 used. The first-strand cDNA was generated using the FastQuant RT kit (Tiangen Biotech Co., Ltd., Beijing, China) with gDNase. A SuperReal PreMix Plus (SYBR Green) kit (Tiangen Biotech, Beijing, China) was used to prepare qRT-PCR reaction mixtures. Reactions were performed on the Applied Biosystems 7500 Fast Systems (AB Ltd., Lincoln, NE, USA) with Eucgr.H04673 as the reference gene. Primer sequences are listed in Table S3.
4.6. Cloning of EurGNC and EurCGA1 Coding Sequence (CDS) and Promoters of EurGUN5 and EurLHCB3
The full-length CDS of EurGNC and EurCGA1 were amplified by PCR using cDNA from 2.5. Promoters of EurGUN5 and EurLHCB3 were amplified from the genomic DNA.
4.7. Subcellular Location Analyses
To validate the position of GATA, the full-length CDS without a stop codon was cloned into the pBI121-eGFP vector. The verified plasmid vector was transformed into an LB4404 Agrobacterium competent cell, which was then used to invade and transform Nicotiana benthamiana. After 2 days, the fluorescence was detected using Olympus FV1000 confocal laser-scanning microscope (Olympus, Tokyo, Japan). GFP fluorescence was detected using an excitation filter at 450–490 nm.
4.8. Yeast One-Hybrid Assay
For yeast one-hybrid assay (Y1H), promoter fragments of EurGUN5 and EurLHCB3 were cloned into the pAbAi vector (Clontech, Beijing, China), and inserted upstream of the AbAr reporter gene (AUR1-C). The primers used are listed in Table S1. The ORFs of EurGNC were inserted into the pGADT7 vector to generate recombinant pGAD-EurGNC constructs. The pGADT7 and pAbAi-LHCB3 were used to represent negative controls. Pairs of plasmids were integrated into the yeast strain Y1HGold as described [9] and cultured on synthetic defined (SD) medium without Leu/Ura. After 3 days, the transformants were confirmed by colony PCR analysis. The resulting co-transformed yeast strains were then plated on the SD/-Leu/-Ura medium containing 50 ng/mL aureobasidin A to confirm TF–promoter interactions.
5. Conclusions
In conclusion, the GATA TFs are of critical importance for Eucalyptus development, and hence bear critical potential for the improvement of this highly economically relevant woody plant. Based on the phylogenetic and gene structure analyses, these genes were categorized into four subfamilies, which was consistent with the previously reported GATA families. Our study provides new insights into the evolution of the GATA gene family in angiosperms. Cis-element prediction and expression trends among the subfamilies of EgrGATAs showed they are modulated by various hormones and are involved in a range of physiological processes in different tissues. Moreover, qRT-PCR and Y1H assays demonstrated that EgrGNC acts as an activator and enhances the expression of EgrGUN5, which facilitates chlorophyll accumulation. Finally, our systematic analysis provides essential and beneficial knowledge for researchers to further study the function of different GATA TF family members in Eucalyptus and other plants.
Supplementary Materials
The following supporting information can be downloaded at: www.mdpi.com/article/10.3390/ijms23095251/s1.
Author Contributions
K.D.: Conceptualization, Writing—original draft. Y.X.: Investigation, Writing—original draft. D.Z.: Software, Validation. T.X.: Formal analysis. T.L.: Investigation, Formal analysis. J.Y.: Supervision, Methodology, Writing—review and editing. X.K.: Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (31901337) and the National Key R&D Program of China during the 14th Five-year Plan Period (2021YFD2200105).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patient R.K., McGhee J.D. The GATA family (vertebrates and invertebrates) Curr. Opin. Genet. Dev. 2002;12:416–422. doi: 10.1016/S0959-437X(02)00319-2. [DOI] [PubMed] [Google Scholar]
- 2.Reyes J.C., Muro-Pastor M.I., Florencio F.J. The GATA family of transcription factors in arabidopsis and rice. Plant Physiol. 2004;134:1718–1732. doi: 10.1104/pp.103.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim M., Xi H., Park J. Genome-wide comparative analyses of GATA transcription factors among 19 Arabidopsis ecotype genomes: Intraspecific characteristics of GATA transcription factors. PLoS ONE. 2021;16:e0252181. doi: 10.1371/journal.pone.0252181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniel-Vedele F., Caboche M. A tobacco cDNA clone encoding a GATA-1 zinc finger protein homologous to regulators of nitrogen metabolism in fungi. MGG Mol. Gen. Genet. 1993;240:365–373. doi: 10.1007/BF00280388. [DOI] [PubMed] [Google Scholar]
- 5.Zhu W., Guo Y., Chen Y., Wu D., Jiang L. Genome-wide identification, phylogenetic and expression pattern analysis of GATA family genes in Brassica napus. BMC Plant Biol. 2020;20:543. doi: 10.1186/s12870-020-02752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Z., Zou X., Huang Z., Fan S., Qun G., Liu A., Gong J., Li J., Gong W., Shi Y., et al. Genome-wide identification and analysis of the evolution and expression patterns of the GATA transcription factors in three species of Gossypium genus. Gene. 2019;680:72–83. doi: 10.1016/j.gene.2018.09.039. [DOI] [PubMed] [Google Scholar]
- 7.Kim M., Xi H., Park S., Yun Y., Park J. Genome-wide comparative analyses of GATA transcription factors among seven Populus genomes. Sci. Rep. 2021;11:16578. doi: 10.1038/s41598-021-95940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu H., Li T., Wang Y., Zheng J., Li H., Hao C., Zhang X. TaZIM-A1 negatively regulates flowering time in common wheat (Triticum aestivum L.) J. Integr. Plant Biol. 2019;61:359–376. doi: 10.1111/jipb.12720. [DOI] [PubMed] [Google Scholar]
- 9.Ravindran P., Verma V., Stamm P., Kumar P.P. A Novel RGL2–DOF6 Complex Contributes to Primary Seed Dormancy in Arabidopsis thaliana by Regulating a GATA Transcription Factor. Mol. Plant. 2017;10:1307–1320. doi: 10.1016/j.molp.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y.J., Zhang Y., Zhang L.L., Huang H.Y., Yang B.J., Luan S., Xue H.W., Lin W.H. OsGATA7 modulates brassinosteroids-mediated growth regulation and influences architecture and grain shape. Plant Biotechnol. J. 2018;16:1261–1264. doi: 10.1111/pbi.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olmo R., Cabrera J., Díaz-Manzano F.E., Ruiz-Ferrer V., Barcala M., Ishida T., García A., Andrés M.F., Ruiz-Lara S., Verdugo I., et al. Root-knot nematodes induce gall formation by recruiting developmental pathways of post-embryonic organogenesis and regeneration to promote transient pluripotency. New Phytol. 2020;227:200–215. doi: 10.1111/nph.16521. [DOI] [PubMed] [Google Scholar]
- 12.Ranftl Q.L., Bastakis E., Klermund C., Schwechheimer C. LLM-domain containing B-GATA factors control different aspects of cytokinin-regulated development in Arabidopsis thaliana. Plant Physiol. 2016;170:2295–2311. doi: 10.1104/pp.15.01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi K., Ohnishi A., Sasaki D., Fujii S., Iwase A., Sugimoto K., Masuda T., Wada H. Shoot removal induces chloroplast development in roots via cytokinin signaling. Plant Physiol. 2017;173:2340–2355. doi: 10.1104/pp.16.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vilasboa J., Da Costa C.T., Fett-Neto A.G. Rooting of eucalypt cuttings as a problem-solving oriented model in plant biology. Prog. Biophys. Mol. Biol. 2019;146:85–97. doi: 10.1016/j.pbiomolbio.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Meskauskiene R., Nater M., Goslings D., Kessler F., Op den Camp R., Apel K. FLU: A negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goslings D., Meskauskiene R., Kim C., Lee K.P., Nater M., Apel K. Concurrent interactions of heme and FLU with Glu tRNA reductase (HEMA1), the target of metabolic feedback inhibition of tetrapyrrole biosynthesis, in dark- and light-grown Arabidopsis plants. Plant J. 2004;40:957–967. doi: 10.1111/j.1365-313X.2004.02262.x. [DOI] [PubMed] [Google Scholar]
- 17.Cackett L., Luginbuehl L.H., Schreier T.B., Lopez-Juez E., Hibberd J.M. Chloroplast development in green plant tissues: The interplay between light, hormone, and transcriptional regulation. New Phytol. 2022;233:2000–2016. doi: 10.1111/nph.17839. [DOI] [PubMed] [Google Scholar]
- 18.Hudson D., Guevara D., Yaish M.W., Hannam C., Long N., Clarke J.D., Bi Y.M., Rothstein S.J. GNC and CGA1 modulate chlorophyll biosynthesis and glutamate synthase (GLU1/FD-GOGAT) expression in Arabidopsis. PLoS ONE. 2011;6:e26765. doi: 10.1371/journal.pone.0026765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zubo Y.O., Blakley I.C., Franco-Zorrilla J.M., Yamburenko M.V., Solano R., Kieber J.J., Loraine A.E., Schaller G.E. Coordination of chloroplast development through the action of the GNC and GLK transcription factor families. Plant Physiol. 2018;178:130–147. doi: 10.1104/pp.18.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson D., Guevara D.R., Hand A.J., Xu Z., Hao L., Chen X., Zhu T., Bi Y.-M., Rothstein S.J. Rice Cytokinin GATA Transcription Factor1 Regulates Chloroplast Development and Plant Architecture. Plant Physiol. 2013;162:132–144. doi: 10.1104/pp.113.217265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu G., Casaretto J.A., Ying S., Mahmood K., Liu F., Bi Y.M., Rothstein S.J. Overexpression of OsGATA12 regulates chlorophyll content, delays plant senescence and improves rice yield under high density planting. Plant Mol. Biol. 2017;94:215–227. doi: 10.1007/s11103-017-0604-x. [DOI] [PubMed] [Google Scholar]
- 22.An Y., Zhou Y., Han X., Shen C., Wang S., Liu C., Yin W., Xia X. The GATA transcription factor GNC plays an important role in photosynthesis and growth in poplar. J. Exp. Bot. 2020;71:1969–1984. doi: 10.1093/jxb/erz564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng W., Li W., Song N., Tang Z., Liu J., Wang Y., Pan S., Dai L., Wang B. Genome-wide characterization, evolution, and expression profile analysis of gata transcription factors in Brachypodium distachyon. Int. J. Mol. Sci. 2021;22:2026. doi: 10.3390/ijms22042026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richter R., Behringer C., Müller I.K., Schwechheimer C. The GATA-type transcription factors GNC and GNL/CGA1 repress gibberellin signaling downstream from DELLA proteins and phytochrome-interacting factors. Genes Dev. 2010;24:2093–2104. doi: 10.1101/gad.594910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo J., Bai X., Dai K., Yuan X., Guo P., Zhou M., Shi W., Hao C. Identification of GATA Transcription Factors in Brachypodium distachyon and Functional Characterization of BdGATA13 in Drought Tolerance and Response to Gibberellins. Front. Plant Sci. 2021;12:763665. doi: 10.3389/fpls.2021.763665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elorriaga E., Klocko A.L., Ma C., Du Plessis M., An X., Myburg A.A., Strauss S.H. Genetic containment in vegetatively propagated forest trees: CRISPR disruption of LEAFY function in Eucalyptus gives sterile indeterminate inflorescences and normal juvenile development. Plant Biotechnol. J. 2021;19:1743–1755. doi: 10.1111/pbi.13588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu C., Li N., Yin Y., Wang F., Gao S., Jiao C., Yao M. Genome-wide identification and function characterization of GATA transcription factors during development and in response to abiotic stresses and hormone treatments in pepper. J. Appl. Genet. 2021;62:265–280. doi: 10.1007/s13353-021-00618-3. [DOI] [PubMed] [Google Scholar]
- 28.Zhang C., Hou Y., Hao Q., Chen H., Chen L., Yuan S., Shan Z., Zhang X., Yang Z., Qiu D., et al. Genome-wide survey of the soybean GATA transcription factor gene family and expression analysis under low nitrogen stress. PLoS ONE. 2015;10:e0125174. doi: 10.1371/journal.pone.0125174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang K., Jia L., Yang D., Hu Y., Njogu M.K., Wang P., Lu X., Yan C. Genome-wide identification, phylogenetic and expression pattern analysis of gata family genes in cucumber (Cucumis sativus L.) Plants. 2021;10:1626. doi: 10.3390/plants10081626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen F., Hu Y., Vannozzi A., Wu K., Cai H., Qin Y., Mullis A., Lin Z., Zhang L. The WRKY Transcription Factor Family in Model Plants and Crops. Crit. Rev. Plant Sci. 2017;36:311–335. doi: 10.1080/07352689.2018.1441103. [DOI] [Google Scholar]
- 31.Qiao X., Li Q., Yin H., Qi K., Li L., Wang R., Zhang S., Paterson A.H. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019;20:38. doi: 10.1186/s13059-019-1650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cannon S.B., Mitra A., Baumgarten A., Young N.D., May G. The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol. 2004;4:10. doi: 10.1186/1471-2229-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan C., Yao H., Qiu Z., Ma H., Zeng B. Genome-wide analysis of Eucalyptus grandis WRKY genes family and their expression profiling in response to hormone and abiotic stress treatment. Gene. 2018;678:38–48. doi: 10.1016/j.gene.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Li Q., Yu H., Cao P.B., Fawal N., Mathé C., Azar S., Cassan-Wang H., Myburg A.A., Grima-Pettenati J., Marque C., et al. Explosive tandem and segmental duplications of multigenic families in Eucalyptus grandis. Genome Biol. Evol. 2015;7:1068–1081. doi: 10.1093/gbe/evv048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soler M., Camargo E.L.O., Carocha V., Cassan-Wang H., San Clemente H., Savelli B., Hefer C.A., Paiva J.A.P., Myburg A.A., Grima-Pettenati J. The Eucalyptus grandis R2R3-MYB transcription factor family: Evidence for woody growth-related evolution and function. New Phytol. 2015;206:1364–1377. doi: 10.1111/nph.13039. [DOI] [PubMed] [Google Scholar]
- 36.Carocha V., Soler M., Hefer C., Cassan-Wang H., Fevereiro P., Myburg A.A., Paiva J.A.P., Grima-Pettenati J. Genome-wide analysis of the lignin toolbox of Eucalyptus grandis. New Phytol. 2015;206:1297–1313. doi: 10.1111/nph.13313. [DOI] [PubMed] [Google Scholar]
- 37.Li Q., Clemente H.S., He Y., Fu Y., Dunand C. Global evolutionary analysis of 11 gene families part of reactive oxygen species (ROS) gene network in four Eucalyptus species. Antioxidants. 2020;9:257. doi: 10.3390/antiox9030257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu H., Soler M., Mila I., Clemente H.S., Savelli B., Dunand C., Paiva J.A.P., Myburg A.A., Bouzayen M., Grima-Pettenati J., et al. Genome-wide characterization and expression profiling of the AUXIN RESPONSE FACTOR (ARF) gene family in Eucalyptus grandis. PLoS ONE. 2014;9:e108906. doi: 10.1371/journal.pone.0108906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren M., Zhang Y., Liu C., Liu Y., Tian S., Cheng H., Zhang H., Wei H., Wei Z. Characterization of a High Hierarchical Regulator, PtrGATA12, Functioning in Differentially Regulating Secondary Wall Component Biosynthesis in Populus trichocarpa. Front. Plant Sci. 2021;12:657787. doi: 10.3389/fpls.2021.657787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klermund C., Ranftl Q.L., Diener J., Bastakis E., Richter R., Schwechheimer C. LLM-Domain B-GATA transcription factors promote stomatal development downstream of light signaling pathways in Arabidopsis thaliana hypocotyls. Plant Cell. 2016;28:646–660. doi: 10.1105/tpc.15.00783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Imagawa M. Negative regulation of gene expression in eukaryotes. Neurochem. Int. 1996;29:565–572. doi: 10.1016/S0197-0186(96)00058-7. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Patra B., Pattanaik S., Wang Y., Yuan L. GATA and phytochrome interacting factor transcription factors regulate light-induced vindoline biosynthesis in Catharanthus roseus. Plant Physiol. 2019;180:1336–1350. doi: 10.1104/pp.19.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu Z., Sun M., Jiang X., Sun H., Dang X., Cong H., Qiao F. Glycinebetaine biosynthesis in response to osmotic stress depends on jasmonate signaling in watermelon suspension cells. Front. Plant Sci. 2018;9:1469. doi: 10.3389/fpls.2018.01469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y.-M., Zhang Y.-M., Zhang X., Zhao X., Zhang Y., Wang C., Wang Y.-C., Wang L.-Q. Poplar PsnICE1 enhances cold tolerance by binding to different cis-acting elements to improve reactive oxygen species-scavenging capability. Tree Physiol. 2021;41:2424–2437. doi: 10.1093/treephys/tpab084. [DOI] [PubMed] [Google Scholar]
- 45.Xin S., Tao C., Li H. Cloning and functional analysis of the promoter of an Ascorbate oxidase gene from Gossypium hirsutum. PLoS ONE. 2016;11:e0161695. doi: 10.1371/journal.pone.0161695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xue Y., Wang J., Mao X., Li C., Li L., Yang X., Hao C., Chang X., Li R., Jing R. Association Analysis Revealed That TaPYL4 Genes Are Linked to Plant Growth Related Traits in Multiple Environment. Front. Plant Sci. 2021;12:641087. doi: 10.3389/fpls.2021.641087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brodersen C.R., Vogelmann T.C. Do changes in light direction affect absorption profiles in leaves? Funct. Plant Biol. 2010;37:403–412. doi: 10.1071/FP09262. [DOI] [Google Scholar]
- 48.Bi Y.M., Zhang Y., Signorelli T., Zhao R., Zhu T., Rothstein S. Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J. 2005;44:680–692. doi: 10.1111/j.1365-313X.2005.02568.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohnishi A., Wada H., Kobayashi K. Improved photosynthesis in Arabidopsis roots by activation of GATA transcription factors. Photosynthetica. 2018;56:433–444. doi: 10.1007/s11099-018-0785-9. [DOI] [Google Scholar]
- 50.Newton A., Mackay J., Crossley M. The N-terminal Zinc Finger of the Erythroid Transcription Factor GATA-1 Binds GATC Motifs in DNA. J. Biol. Chem. 2001;276:35794–35801. doi: 10.1074/jbc.M106256200. [DOI] [PubMed] [Google Scholar]
- 51.Eddy S.R. Accelerated profile HMM searches. PLoS Comput. Biol. 2011;7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Supplementary Materials.