Abstract
A PCR-based method was developed for the specific detection of Yersinia ruckeri in tissues of inoculated trout and naturally infected trout. No amplification products were obtained with other yersiniae, bacterial fish pathogens, or phylogenetically related bacteria (n = 34). The sensitivity of PCR detection was 60 to 65 bacterial cells per PCR tube, which was decreased to 10 to 20 cells by hybridization with a nonradioactive probe. The PCR assay proved to be as reliable as and faster than the conventional culture method for the detection of Y. ruckeri in infected trout tissues.
Yersiniosis or enteric redmouth disease (ERM), caused by Yersinia ruckeri, is a serious infectious disease in the rainbow trout farming industry that causes economic problems in this industry worldwide (2). The disease was first described in the rainbow trout Oncorhynchus mykiss in 1958 in the United States, and it has become endemic in North America (6). The first description in Europe occurred in 1981 in France, Germany, and the United Kingdom, but now ERM is present in most European countries (6). The disease is characterized by the development of a chronic or acute enterosepticemia with the presence of congestive or hemorrhagic zones on the skin of the gills, the mouth, and the intestines, and exophthalmos is also frequently evident. The disease usually occurs as an acute disease with high morbidity and mortality rates, which makes it advisable to have rapid and accurate methods for the specific detection of Y. ruckeri. PCR represents a widely used alternative to traditional identification methods (21). Although pathogenicity and virulence genes have been used as target regions, the rRNA molecule is now accepted as a very useful tool for identification purposes (5, 7, 8, 17). Recent phylogenetic studies of the genus Yersinia based on 16S rRNA sequencing have shown that this genus represents a coherent tight cluster within the family Enterobacteriaceae, with Y. ruckeri forming a separate subline within the Yersinia five-subline cluster (10). This fact suggests that by taking advantage of the differences at the level of 16S rRNA sequences, specific oligonucleotides that can be used in a PCR assay for diagnostic purposes can be designed. This approach has been used with different animal and fish pathogens (1, 4, 11, 15, 19, 20, 22). The present study reports an alternative PCR assay based on the selective amplification of a DNA fragment from the Y. ruckeri 16S rRNA gene.
Bacterial strains and preparation of bacterial DNAs.
The Y. ruckeri isolates, other Yersinia species, and other bacteria included in the family Enterobacteriaceae, as well as other phylogenetically related taxa (10) and other fish pathogenic bacteria used in this study, are listed in Table 1. Strains other than Vibrio spp. were grown at 22°C on Trypticase soy agar (TSA) (bioMerieux España s.a., Madrid, Spain). Vibrio strains were grown at the same temperature on TSA supplemented with 2.5% NaCl.
TABLE 1.
Bacterial species assayed in the PCR experiments
| Species | Straina | Source and/or geographic origin |
|---|---|---|
| Yersinia spp. | ||
| Y. ruckeri | CECT 955 | Rainbow trout, United States |
| CECT 956 | Fish isolation, United States | |
| CECT 4319 | Rainbow trout, United States | |
| 96/1897, 98/3650, A-824, A-898, A-903, A-923, A-978, A-1028, A-1147, A-1213, A-1261, A-1281, A-1286, 98/3906 | Trout kidney, Spain | |
| DK1, DK12, DK27, DK50, DK55, DK62 | Trout kidney, Denmark | |
| 93/03, 94/05, 95/548, 95/725, 96/1915, A-494, A-495, A-521, A-528, A-1015, A-1286, A-1335 | Trout liver, Spain | |
| 98/3900, 98/3926 | Trout intestine, Spain | |
| A-872, A-873 | Rainbow trout, Portugal | |
| Y. enterocolitica | CECT 4054 | Blood of human patient |
| CECT 4055 | Blood culture | |
| CECT 4315 | Cutaneous infection | |
| CECT 500 | Unknown | |
| CECT 559 | Chinchilla, Denmark | |
| CECT 754 | Human feces | |
| Y. frederiksenii | CECT 4316 | Sewage, Denmark |
| Y. intermedia | CECT 4317 | Human urine |
| Y. kristensenii | CECT 4318 | Human urine, Denmark |
| Y. aldovae | CECT 4314 | Drinking water, Czech Republic |
| Other genera | ||
| Vibrio parahaemolyticus | CECT 511 | Human food poisoning infection |
| Vibrio anguillarum | CECT 522 | Ulcerous lesions in cod, Norway |
| Vibrio vulnificus | CECT 529 | Human blood |
| Vibrio alginolyticus | 97/3157 | Fish clinical isolation, Spain |
| CECT 521 | Food infection, Japan | |
| Aeromonas salmonicida | CECT 894 | Salmon clinical isolation, United Kingdom |
| 98/3887 | Rainbow trout kidney, Spain | |
| Aeromonas caviae | CECT 838 | Guinea pig isolation |
| Aeromonas hydrophila | 97/3156 | Fish clinical isolation, Spain |
| Aeromonas encheleia | CECT 4340 | European eel, Spain |
| Pseudomonas cepacia | CECT 322 | Soil |
| Pseudomonas fluorescens | CECT 378 | Water, United Kingdom |
| Pasteurella haemolytica | CECT 924 | Unknown |
| Klebsiella pneumoniae | M5a1 | Supplied by M. J. Merrick, United Kingdom |
| Enterobacter cloacae | A-215 | Spanish clinical isolation |
| Proteus mirabilis | A-1118 | Dog urine, Spain |
| Escherichia coli | A-1641 | Dog urine, Spain |
| Cedacea davisae | CECT 842 | Human feces, United States |
| Kluyvera ascorbata | CECT 861 | Human sputum, United States |
| Providencia alcalifaciens | CECT 166 | Human feces |
| Serratia marcescens | CECT 846 | Unknown |
| Morganella morganii | CECT 173 | Diarrhea clinical case |
| Hafnia alvei | CECT 158 | Unknown |
| 98/3869 | Fish intestinal carrier, Bulgaria |
CECT, Colección Española Cultivos Tipo (Spanish Type Culture Collection), Valencia, Spain.
Bacterial chromosomal DNA was extracted by a modification of the method described by Lawson et al. (12). The bacterial strains were collected on agar plates, suspended in sterile distilled water, and centrifuged. The pellet was resuspended in 500 μl of TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid] buffer containing 50 μg of lysozyme (Sigma) and incubated at 37°C for 30 min. Then, 5 μl of a 10-mg/ml solution of proteinase K (Sigma) was added, and the mixture was incubated at 65°C in a water bath for 2 h. Subsequently, 50 μl of 20% sodium dodecyl sulfate (SDS) was added to the mixture, and it was returned immediately to the 65°C water bath for a further 10 min. The solution was treated twice with an equal volume of phenol-chloroform (Sigma), and the aqueous fraction was recovered. The DNA was precipitated with 2 volumes of 100% ethanol, centrifuged and dried under vacuum, and finally, dissolved in 50 μl of sterile distilled water containing 2 U of RNase (Boehringer Mannheim). All DNA samples were stored at −20°C.
Preparation of artificially contaminated trout tissue homogenates.
Samples of the spleen, liver, kidney, and feces from 25 fresh commercial rainbow trout were obtained aseptically. The organs were weighed and blended with the appropriate volume of 0.9% saline solution to obtain a 1/10 dilution of each organ. The tissue homogenates were then filtered with sterile cheesecloth and stored at −20°C until processing. The absence of Y. ruckeri in the tissue homogenates was determined by plating 100 μl onto TSA and MacConkey agar plates (bioMerieux), which were incubated at 22°C for 3 days. Samples of 1 ml of tissue homogenate were inoculated with Y. ruckeri CECT 956 to obtain a final concentration of 106 cells/ml. Then, 0.1 ml of the appropriate 10-fold dilutions of these homogenates were plated onto MacConkey agar for the enumeration of Y. ruckeri in the artificially contaminated tissues. Noninoculated tissue homogenates were used as controls.
Processing of inoculated and naturally infected trout.
Twenty-five rainbow trout were maintained in 200-liter-capacity tanks supplied with aerated flowthrough dechlorinated fresh water at an average temperature of 16°C. Each trout was inoculated intraperitoneally with a dose of 105 cells of Y. ruckeri CECT 956. A group of 10 noninoculated fish, maintained under identical conditions, were used as controls. Trout with clinical signs of yersiniosis were killed by an overdose of anesthetic (2-phenoxyethanol). The spleen, kidney, and liver of each trout were removed aseptically, and each organ was diluted and filtered as described above. Tissue homogenates of noninoculated trout were used as controls. Enumeration of Y. ruckeri in each organ from the inoculated trout and the absence of yersiniae in noninoculated trout were assayed as described above.
Naturally infected trout were obtained from two farms in Spain that were experiencing cases of yersiniosis. Fish were sacrificed, and tissues were removed and processed as described above. Isolation of Y. ruckeri from each organ was done by streaking a loop on TSA and MacConkey agar plates after 3 days of incubation at 22°C. Colonies suspected to be Y. ruckeri were subcultured on TSA and biochemically identified by using API 20E strips (bioMerieux España s.a.). Homogenates of liver, kidney, and feces from two naturally infected trout that were positive by PCR but bacteriologically negative were analyzed for the presence of Y. ruckeri. Tissue homogenates (1 ml) were added to 9 ml of enrichment broth. The enrichment broth was prepared by allowing the agar of the yersinia-selective agar base (Oxoid) to sediment, and the supernatant was recovered. The recommended selective supplement, SR109, was not added to the broth. After 48 h of incubation at 30°C, 100 μl of the enrichment broth was streaked onto TSA agar. Plates were incubated and Y. ruckeri colonies were identified as described above.
DNA extraction from tissues.
One hundred to 300 μl of tissue homogenates, previously filtered, were diluted to 500 μl with TES buffer and further processed by the method of Lawson et al. (12), described above. The total extracted DNA was dissolved in 20 μl of sterile distilled water and used for PCR experiments.
Primers design and PCR amplification.
Two forward primers, YER8 (5′-GCGAGGAGGAAGGGTTAAGTG-3′) and YER11 (5′-CACTTAACCCTTCCTCCTCGC-3′), and two reverse primers, YER10 (5′-GAAGGCACCAAGGCATCTCTG-3′) and YER12 (5′-AGTAATGTCTGGGGATCTGCC-3′), were designed from unconserved regions of the Y. ruckeri 16S rRNA gene sequence (accession no. X75275). The apparent specificity of each primer was determined by matching it to the Ribosomal Database Project microbial ribosomal small-subunit data set, using the Check-Probe function (14). Primers were tested for PCR amplification at two different annealing temperatures (57 and 60°C), with DNA from selected strains of Y. ruckeri, other Yersinia species, and other taxa included in Table 1.
The PCR amplification was conducted in a total volume reaction of 100 μl containing 50 to 70 ng of genomic bacterial DNA or 20 μl of DNA extracted from fish tissue, 1 mM each primer, 2 mM each deoxynucleotide triphosphate, 10 μl of Taq polymerase buffer (BioTools), and 1 U of Taq polymerase (BioTools). The amplification reaction was performed in a PT-100 thermal cycler (MJ Research, Inc.) by using 25 cycles of denaturation for 1 min at 92°C, annealing at the selected temperature for 1 min, and extension for 1 min at 72°C, followed by a final extension step of 72°C for 5 min. Negative (no template DNA) and positive (50 ng of purified DNA from Y. ruckeri CECT 956) controls were included in each batch of PCR mixtures. PCR-generated products were detected by using 1% agarose gels, with 10 μl of each amplification mixture subjected to electrophoresis.
To determine the detection limit of the PCR, a suspension of Y. ruckeri CECT 956 containing 104 CFU/ml was serially diluted twofold to 10 CFU/ml. Twenty microliters of each dilution was boiled for 10 min and added directly to the PCR mixture. The bacterial concentration was verified by plating 20 μl of each dilution onto TSA. The specificity of the PCR assay was evaluated by using the purified DNA extracted from all Y. ruckeri strains and other bacteria listed in Table 1 as a template.
Slot blot hybridization.
Bacterial suspensions of 101 to 105 cells were transferred to a nylon membrane, which was positively charged (Boehringer Mannheim) by a slot blot procedure using a Bio-Dot microfiltration apparatus (Bio-Rad). Bacterial DNA was extracted by alkaline lysis and fixed to the membrane by the hybridization colony method described by Sambrook et al. (18). The blot was assayed against the 575-bp 5′-end biotin product obtained from strain CECT 956 by PCR amplification with biotin YER8 and YER10 primers, labeled at the 5′ end. Prehybridization and hybridization were performed at 60°C for 3 h in 5× SSC–0.1% SDS (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The labeled probe concentration used was 30 ng/ml. Washes were performed at 65°C for high-stringency conditions (0.5× SSC–0.1% SDS). Hybridized DNA was detected with the CDP-Star procedure (Boehringer Mannheim), using a 1:5,000 dilution of streptavidin-peroxidase conjugate.
Development of the PCR assay.
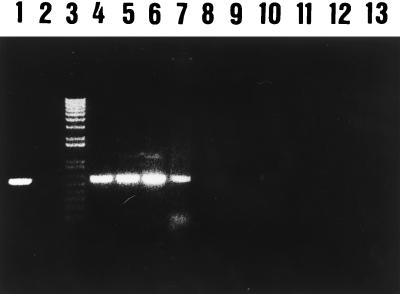
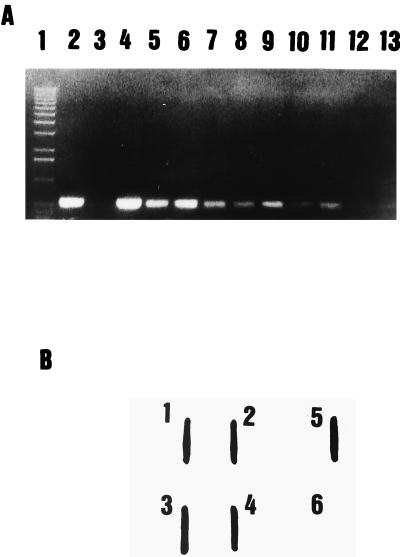
The primer combination YER10-YER11 gave an amplification product of 322 bp at both annealing temperatures, but amplification products were also observed with Yersinia enterocolitica and Aeromonas salmonicida (data not shown). The primer set YER8-YER10 gave a unique and specific amplification band with a 575-bp length at an annealing temperature of 60°C with Y. ruckeri strains only. However, at an annealing temperature of 57°C, nonspecific amplification products were observed with Y. enterocolitica. Identical amplification products were obtained when PCR was performed with whole bacterial cells boiled for 10 min instead of purified extracted DNA. On the basis of these results, the primer combination YER8-YER10 at an annealing temperature of 60°C was further used for all PCR assays. All Y. ruckeri strains showed a 575-bp amplification product, while no amplification products were found with any of the other Yersinia species, bacterial fish pathogens, or phylogenetically related bacteria listed in Table 1, at this annealing temperature (Fig. 1). These results indicated that the primer combination YER8-YER10, as well as the PCR procedure, were adequate for specific detection of Y. ruckeri. The PCR assay had a detection limit of 60 to 65 cells per PCR mixture (Fig. 2A), assuming that the lysate procedure was completed, since no viable cells were detected after the boiling treatment. This level equals 6.5 × 102 cells/ml. When the PCR amplification product was used as a nonradioactive probe in blotting hybridization experiments, we were able to detect at least to 10 to 20 bacterial cells (Fig. 2B).
FIG. 1.
Specificity of PCR assay for Y. ruckeri (specific amplification of the 575-bp fragment from the total DNA of Y. ruckeri, other Yersinia species, and other genera). Lane 1, Y. ruckeri CECT 4319 (positive control); lane 2, no template (negative control); lane 3, 1-kb plus DNA ladder (Amersham); lanes 4 to 7, Y. ruckeri CECT 956, 93/03, 94/30, and A-1261, respectively; lanes 8 to 13, Yersinia enterocolitica CECT 4315, Yersinia kristensenii CECT 4318, Yersinia intermedia CECT 4317, Aeromonas salmonicida CECT 894, Vibrio vulnificus CECT 529, and Hafnei alvei CECT 158.
FIG. 2.
(A) Sensitivity of detection of Y. ruckeri by PCR. Lane 1, 1-kb plus DNA ladder (Amersham); lane 2, Y. ruckeri CECT 4319; lane 3, no template (negative control); lanes 4 to 13, 3.32 × 104, 1.66 × 104, 8.3 × 103, 4.1 × 103, 2 × 103, 1 × 103, 520, 260, 130, and 65 cells of Y. ruckeri CECT 956, respectively. (B) Sensitivity of detection of Y. ruckeri by slot blot hybridization with a biotin-labeled 575-bp amplified product. Lanes 1 to 5, 2 × 104, 2 × 103, 2 × 102, 2 × 101, and 2 × 105 cells of Y. ruckeri CECT 956, respectively; lane 6, negative control.
Application of PCR for the detection of Y. ruckeri in contaminated tissue homogenates and experimentally inoculated and naturally infected trout tissues.
PCR amplification results were obtained for all feces and tissue homogenates used at a dilution rate of 1:4 in TES buffer (100 μl of tissue homogenate and 400 μl of TES buffer), and no differences were observed in the PCR amplification results regardless of the type of tissue homogenate. The detection limit in tissues was 2 × 104 CFU/g. When the amount of filtered homogenate was increased to over 100 μl (dilution rates of 2:3 or 3:2), an inhibitory effect on the PCR amplification was observed (data not shown); this could be explained by the presence of inhibitory substances in the tissue extracts, which have been reported to inhibit or reduce the sensitivity of the PCR (4, 16, 19). Although inhibition of the PCR has been reported when high concentrations of DNA or large populations of target cells are used (13), no differences were found in the PCR amplification results within a range of 104 to 109 Y. ruckeri cells.
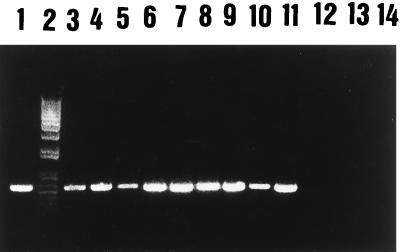
PCR consistently resulted in the amplification of target DNA in all tissues examined from experimentally inoculated trout (Fig. 3). Enumeration of Y. ruckeri in these inoculated trout ranged from 2 × 106 to 5 × 109 CFU/g. Noninoculated trout tissues were always PCR negative. In naturally infected trout, all tissue homogenates positive for Y. ruckeri by conventional culture method (streak of a loop onto TSA or MacConkey agar) were also positive by the PCR assay. Enumeration of Y. ruckeri in naturally infected trout ranged from 3 × 105 to 2 × 106 CFU/g. Samples from naturally infected trout that were negative by the culture method were also PCR negative, except for feces, kidney, and liver homogenates from two trout. The recovery of Y. ruckeri from these samples after selective enrichment suggests that the PCR assay could also be useful for the detection of asymptomatic infected fish, which are difficult to identify by using traditional bacteriological approaches (3, 9). The detection of these asymptomatic carriers is very important in order to prevent the transmission and diffusion of ERM (3).
FIG. 3.
Ethidium bromide-stained agarose gel of PCR-amplified DNA from Y. ruckeri in trout tissues. Lane 1, Y. ruckeri CECT 4319; lane 2, 1-kb plus DNA ladder (Amersham); lanes 3 to 5, DNA from kidney, liver, and spleen homogenates from intraperitoneally inoculated trout, respectively; lanes 6 and 7, 8 and 9, and 10 and 11, DNA from kidney, liver, and spleen homogenates from naturally infected trout, respectively; lanes 12 to 14, DNA from kidney, liver, and spleen homogenates from noninoculated trout, respectively.
The traditional microbiological approach (isolation plus identification) usually takes 2 to 3 days for the definitive identification of Y. ruckeri. In addition, different numerical profiles for Y. ruckeri can be obtained when commercial multisubstrate identification systems, particularly the API 20E system, are used; these profiles must be interpreted with caution (6). The entire PCR assay proposed in this work (tissue homogenization, DNA extraction, PCR amplification, and detection of amplified products) is more accurate, since it is totally specific for Y. ruckeri, and faster than the traditional microbiological approach, since it can be performed in an 8-h day. In summary, this PCR assay was found to be successful for the detection of Y. ruckeri not only in pure bacterial suspensions but also in tissue homogenates from inoculated trout and naturally infected trout, and therefore represents a useful alternative to the microbiological approach for the rapid and specific diagnosis of Y. ruckeri infection in fish farms.
Acknowledgments
The research described in this paper has been financially supported by Dibaq-Diproteg S. A.
We thank F. Uruburu (Director of the Spanish Type Culture Collection) for providing the collection strains and J. L. Larsen for providing the Danish Y. ruckeri clinical isolates.
A. Gibello and M. M. Blanco contributed equally to this work.
REFERENCES
- 1.Arias C R, Garay E, Aznar R. Nested PCR method for rapid and sensitive detection of Vibrio vulnificus in fish, sediments, and water. Appl Environ Microbiol. 1995;61:3476–3478. doi: 10.1128/aem.61.9.3476-3478.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin B, Austin D A. Bacterial fish pathogens: diseases in farmed and wild fish. 2nd ed. Chichester, United Kingdom: Ellis Horwood Limited; 1993. pp. 208–216. [Google Scholar]
- 3.Busch R A, Lingg A J. Establishment of an asymptomatic carrier state infection of enteric redmouth disease in rainbow trout (Salmo gairdneri) J Fish Res Board Can. 1975;32:2429–2432. [Google Scholar]
- 4.Coleman S S, Melanson D M, Biosca E G, Oliver J D. Detection of Vibrio vulnificus biotypes 1 and 2 in eels and oysters by PCR amplification. Appl Environ Microbiol. 1996;62:1378–1382. doi: 10.1128/aem.62.4.1378-1382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernández-Garayzábal J F. Molecular identification of pathogenic actinomycetes: a new approach. Microbiol SEM. 1995;11:403–404. [PubMed] [Google Scholar]
- 6.Furones M D, Rodgers C J, Munn C B. Yersinia ruckeri, the causal agent of enteric redmouth disease (ERM) in fish. Annu Rev Fish Dis. 1993;3:105–125. [Google Scholar]
- 7.Gendel S M. Computational analysis of the specificity of 16S rRNA-derived signature sequences for identifying food-related microbes. Food Microbiol. 1996;13:1–15. [Google Scholar]
- 8.Greisen K, Loeffelholz M, Purohit A, Leong D. PCR primers and probes for the 16S rRNA gene of most species of pathogenic bacteria, including bacteria found in cerebrospinal fluid. J Clin Microbiol. 1994;32:335–351. doi: 10.1128/jcm.32.2.335-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunter V A, Knittel M D, Fryer J L. Stress-induced transmission of Yersinia ruckeri infection from carriers to recipient steelhead trout Salmo gairdneri Richardson. J Fish Dis. 1980;3:467–472. [Google Scholar]
- 10.Ibrahim A, Goebel B M, Liesack W, Griffiths M, Stackebrandt E. The phylogeny of the genus Yersinia based on 16S rDNA sequences. FEMS Microbiol Lett. 1993;114:173–178. doi: 10.1111/j.1574-6968.1993.tb06569.x. [DOI] [PubMed] [Google Scholar]
- 11.Izumi S, Wakabayashi H. Use of PCR to detect Cytophaga psychrophila from apparently healthy juvenile ayu and coho salmon eggs. Fish Pathol. 1997;32:169–173. [Google Scholar]
- 12.Lawson P A, Gharbia S E, Shah H N, Clark D R. Recognition of Fusobacterium nucleatum subgroups Fn-1, Fn-2 and Fn-3 ribosomal RNA gene restriction patterns. FEMS Microbiol Lett. 1989;65:41–46. doi: 10.1016/0378-1097(89)90363-7. [DOI] [PubMed] [Google Scholar]
- 13.Lee C H, Pan S F, Chen C H. Sequence of a cloned pR72H fragment and its use for detection of Vibrio parahaemolyticus in shellfish with the PCR. Appl Environ Microbiol. 1995;61:1311–1317. doi: 10.1128/aem.61.4.1311-1317.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maidak B L, Larsen N, McCaughey M J, Overbeek R, Olsen G J, Fogel K, Blandy J, Woese C R. The ribosomal database project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntosh D, Meaden P G, Austin B. A simplified PCR-based method for the detection of Renibacterium salmoninarum utilizing preparations of rainbow trout (Oncorhynchus mykiss, Walbaum) lymphocytes. Appl Environ Microbiol. 1996;62:3929–3932. doi: 10.1128/aem.62.11.3929-3932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mooney J, Powell E, Clabby C, Powell R. Detection of Aeromonas salmonicida in wild Atlantic salmon using a specific DNA probe test. Dis Aquat Org. 1995;21:131–135. [Google Scholar]
- 17.Nübel U, Garcia-Pichel F, Muyzer G. PCR primers to amplify 16S rRNA genes from cyanobacteria. Appl Environ Microbiol. 1997;63:3327–3332. doi: 10.1128/aem.63.8.3327-3332.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 19.Saulnier D, de Kinkelin P. Polymerase chain reaction primers for investigations on the causative agent of proliferative kidney disease of salmonids. J Fish Dis. 1997;20:467–470. [Google Scholar]
- 20.Urdaci M C, Chakroun C, Fauré D, Bernardet J F. Development of a polymerase chain reaction assay for detection and identification of the fish pathogen Flavobacterium psychrophilum. Res Microbiol. 1998;149:519–530. doi: 10.1016/s0923-2508(98)80006-5. [DOI] [PubMed] [Google Scholar]
- 21.White T J, Madej R, Persing D H. The polymerase chain reaction:clinical applications. Adv Clin Chem. 1992;29:161–196. doi: 10.1016/s0065-2423(08)60224-3. [DOI] [PubMed] [Google Scholar]
- 22.Zlotkin A, Eldar A, Ghittino C, Bercovier H. Identification of Lactococcus garviae by PCR. J Clin Microbiol. 1998;36:983–985. doi: 10.1128/jcm.36.4.983-985.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]