Abstract
Simple Summary
Immune checkpoint inhibitors have been shown to improve survival in patients with advanced melanoma; however, a proportion of patients do not experience durable clinical benefit with these agents. Findings from a previous study suggested that the use of proton pump inhibitors while receiving immune checkpoint inhibitors may lead to worse clinical outcomes. To validate those results, we performed this retrospective analysis using data from three clinical trials involving patients with advanced melanoma treated with immune checkpoint inhibitors. We found that there is not enough evidence to conclude that proton pump inhibitors influence the efficacy of immune checkpoint inhibitors. Prospective studies are needed to conclusively determine if the use of proton pump inhibitors has any meaningful impact on the efficacy of immune checkpoint inhibitors in patients with advanced melanoma.
Abstract
The impact of proton pump inhibitors (PPIs) on clinical outcomes with first-line immune checkpoint inhibitors (ICIs) in patients with metastatic melanoma was previously analyzed in the phase II study, CheckMate 069. This retrospective analysis utilized data from three phase II/III studies of first-line ICI therapy in untreated advanced melanoma: CheckMate 066, 067, and 069. All randomized patients with PPI use ≤ 30 days before initiating study treatment were included in the PPI-use subgroup. Possible associations between baseline PPI use and efficacy were evaluated within each treatment arm of each study using multivariable modeling. Approximately 20% of 1505 randomized patients across the studies reported baseline PPI use. The median follow-up was 52.6–58.5 months. Objective response rate (ORR), progression-free survival (PFS), and overall survival analyses provided insufficient evidence of a meaningful association between PPI use and efficacy outcomes with nivolumab-plus-ipilimumab, nivolumab, or ipilimumab therapy. In five of the six ICI treatment arms, 95% confidence intervals for odds ratios or hazard ratios traversed 1. Significant associations were observed in the CheckMate 069 combination arm between PPI use and poorer ORR and PFS. This multivariable analysis found insufficient evidence to support meaningful associations between PPI use and ICI efficacy in patients with advanced melanoma.
Keywords: proton pump inhibitors, checkpoint inhibitors, melanoma, pooled analysis
1. Introduction
Immune checkpoint inhibitors (ICIs), such as the programmed death 1 (PD-1) inhibitor nivolumab, are well established as a treatment for patients with advanced melanoma [1], either alone or in combination with ipilimumab (a cytotoxic T-lymphocyte-associated protein 4 inhibitor), with durable survival rates demonstrated in randomized controlled phase III trials [2,3]. In the phase III CheckMate 066 trial, a 5-year overall survival (OS) rate of 39% was reported with nivolumab monotherapy in treatment-naive patients with wild-type BRAF advanced melanoma [3]. In the phase III CheckMate 067 trial, 5-year OS rates of 52% and 44% were demonstrated with nivolumab with or without ipilimumab, respectively, in previously untreated patients with advanced melanoma [2]. Despite these positive results in a disease that historically was regarded as having a very poor outcome, a proportion of patients with advanced melanoma do not experience durable clinical benefit with these agents [2,3].
The underlying reasons for heterogeneous responses to ICIs are not well understood. Indeed, reliable predictors of clinical benefits of these agents have yet to be identified, and approaches for overcoming innate and acquired tumor resistance to ICI therapies have yet to be developed [1]. Many factors may adversely affect ICI efficacy, such as the patient’s immune status [4,5,6,7,8] or co-administration of drugs, such as antibiotics, that reduce the diversity of the gut microbiome [7,8,9,10]. Both preclinical and clinical data suggest an association between the microbiome and the activity of ICIs against melanoma [7,8,11].
Proton pump inhibitors (PPIs) have also been shown to have an adverse effect on the gut microbiome [12,13]. A possible mechanism for this effect is the direct impact of PPIs on gastric pH, which is a major barrier to pathogens invading the GI tract. An effect of PPIs on the functionality of the immune system was also suggested in a study that found an increased risk of developing community-acquired pneumonia with PPI use [14].
As part of an effort to evaluate the impact of concomitant medications on clinical outcomes with ICIs, a previously presented retrospective analysis found that the use of PPIs reduced the efficacy of nivolumab and ipilimumab combination therapy but not ipilimumab alone, in 140 treatment-naive patients with metastatic melanoma [15]. That analysis utilized data from a randomized phase II study, CheckMate 069 [16,17]. Additionally, two large pooled retrospective analyses evaluated the impact of PPIs specifically on outcomes with ICIs using phase II/III trial data: one in non-small-cell lung cancer (NSCLC; n = 1512) and one in urothelial cancer (n = 1360) [10,18]. Both found that PPI use was associated with poor outcomes with ICIs. In order to validate the prior analysis of CheckMate 069 and evaluate the prognostic and predictive ability of PPI use in greater detail, in this study, we performed a retrospective analysis of the impact of PPI use on ICI outcomes across a total of 1505 treatment-naive patients with metastatic melanoma enrolled in CheckMate 066 and CheckMate 067, as well as CheckMate 069.
2. Materials and Methods
2.1. Patients, Study Design, and Treatment
This post hoc analysis utilized data from three multicenter, double-blinded, randomized studies of treatment-naive patients with advanced melanoma who had received ICI therapy: CheckMate 066, CheckMate 067, and CheckMate 069. Patient populations, study designs, and treatment regimens used in these studies have been described extensively [2,3,16,17,19,20,21,22,23] and are summarized in Table 1. All three studies were conducted in accordance with the provisions of the Declaration of Helsinki and the International Conference on Harmonisation Guidelines for Good Clinical Practice. All of the patients provided written informed consent.
Table 1.
Trial overview.
| Trial Characteristic | CheckMate 066 [3,19,20,24] | CheckMate 067 [2,21,22,23,25] | CheckMate 069 [16,17,26] |
|---|---|---|---|
| ClinicalTrials.gov no. | NCT01721772 | NCT01844505 | NCT01927419 |
| Study phase | III | III | II |
| Study design | Multicenter, randomized, double-blind |
Multicenter, randomized, double-blind |
Multicenter, randomized, double-blind |
| Key eligibility criteria | Unresected stage III/IV melanoma Previously untreated BRAF wild-type ECOG PS 0/1 |
Unresected stage III/IV melanoma Previously untreated BRAF wild-type or mutant ECOG PS 0/1 |
Unresected stage III/IV melanoma Previously untreated BRAF wild-type or mutant ECOG PS 0/1 |
| Randomization | 1:1 | 1:1:1 | 2:1 |
| Treatment groups | Nivolumab 3 mg/kg Q2W (n = 210) Dacarbazine 1000 mg/m2 Q3W (n = 208) |
Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W × 4 → nivolumab 3 mg/kg Q2W (n = 314) Nivolumab 3 mg/kg Q2W (n = 316) Ipilimumab 3 mg/kg Q3W × 4 (n = 315) |
Nivolumab 1 mg/kg + ipilimumab 3 mg/kg Q3W × 4 → nivolumab 3 mg/kg Q2W (n = 95) Ipilimumab 3 mg/kg Q3W × 4 (n = 47) |
| Primary endpoint(s) | OS | PFS OS |
ORR in BRAF wild-type population |
| Secondary/exploratory endpoints a | PFS ORR OS by PD-L1 expression Safety HRQoL |
ORR Efficacy by PD-L1 expression Safety HRQoL |
PFS in BRAF wild-type population ORR in BRAF mutant population PFS in BRAF mutant population HRQoL OS Safety |
| Tumor assessment | 9 weeks after randomization Then Q6W through year 1 Then Q12W until PD/treatment discontinuation |
12 weeks after randomization Then Q6W for 49 weeks Then Q12W until PD/treatment discontinuation |
12 weeks after first treatment Then Q6W through year 1 Then Q12W until PD/treatment discontinuation |
| Study period | 2013–2021 | 2013–ongoing | 2013–2021 |
a Key; not a comprehensive list. →: followed by; ECOG PS: Eastern Cooperative Oncology Group performance status; HRQoL: health-related quality of life; ORR: objective response rate; OS: overall survival; PD: progressive disease; PD-L1: programmed death-ligand 1; PFS: progression-free survival; Q2W: every 2 weeks; Q3W: every 3 weeks; Q6W: every 6 weeks; Q12W: every 12 weeks.
2.2. Assessments
Tumor response was assessed on the schedules shown in Table 1 using Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Objective response rate (ORR) per investigator was defined as the proportion of patients with a best overall response of partial or complete response, while progression-free survival (PFS) per investigator was defined as the time from randomization to first documented disease progression or death (whichever occurred first), and overall survival (OS) as the time from randomization to death.
2.3. Statistical Analysis
The aim of the statistical analysis was to evaluate the prognostic and predictive ability of baseline PPI use in patients who participated in CheckMate 066, 067, or 069. All analyses, which involved all randomized patients from each study, were performed based on separate database locks for each study. Baseline PPI use was determined using data entered on case report forms. The “PPI use” subgroup included patients who used any medication classified as a PPI (omeprazole, esomeprazole, pantoprazole, lansoprazole, rabeprazole, or dexlansoprazole) ≤ 30 days before the start of the study treatment. The “no PPI use” subgroup was defined as all other patients. The analysis did not consider PPI dosage, PPI treatment duration, or whether the patient continued to take a PPI during the study.
The association between PPI use at baseline and ORR, PFS, and OS was evaluated within each treatment arm of each study using multivariable modeling. Each model included PPI use at screening as a covariate in addition to a number of prognostic variables (i.e., age, sex, geographic region, race, Eastern Cooperative Oncology Group performance status [ECOG PS], metastasis stage, American Joint Committee on Cancer stage, history of brain metastases, PD-L1 status, lactate dehydrogenase level, and BRAF mutation status), which were based on clinical data from of each of the three studies; the particular prognostic variables utilized as covariates for each model varied between the studies and between efficacy outcomes (Supplementary Tables S1–S3). The analyses of ORR were performed using multivariable logistic regression models, in which odds ratios and corresponding two-sided 95% confidence intervals (CIs) were determined for all covariates in the model. The analyses of PFS and OS were performed using unstratified multivariable Cox proportional-hazards regression models, in which hazard ratios (HRs) and two-sided 95% CIs were determined for each covariate. In addition, PFS and OS distributions were estimated using the Kaplan–Meier method. Analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC, USA).
3. Results
The majority of the 1505 patients across CheckMate 066, 067, and 069 had melanoma stage IV, M1c, BRAF wild-type disease, and no history of brain metastases (Table 2). Approximately 20% of patients had used PPIs at baseline across all three studies, and higher proportions of these patients were at least 75 years of age and/or had a baseline ECOG PS of 1 or higher than patients who had not used PPIs at baseline. Slight imbalances were also seen in other parameters, such as baseline lactose dehydrogenase levels, which tended to be higher in patients on PPIs. Median follow-up, derived as median time from randomization to database lock, was 58.5 months in CheckMate 066, 53.0 months in CheckMate 067, and 52.6 months in CheckMate 069.
Table 2.
Baseline characteristics by PPI usage in CheckMate 066, 067, and 069.
| Baseline Characteristic |
CheckMate 066 (n = 418) | CheckMate 067 (n = 945) | CheckMate 069 (n = 142) | |||
|---|---|---|---|---|---|---|
| PPI Use | No PPI Use | PPI Use | No PPI Use | PPI Use | No PPI Use | |
| PPI use status a | 97 (23) | 321 (77) | 161 (17) | 784 (83) | 33 (23) | 109 (77) |
| Age (y) | ||||||
| <65 | 36 (37) | 163 (51) | 74 (46) | 491 (63) | 16 (48) | 52 (48) |
| ≥65–<75 | 38 (39) | 114 (36) | 55 (34) | 207 (26) | 12 (36) | 45 (41) |
| ≥75 | 23 (24) | 44 (14) | 32 (20) | 86 (11) | 5 (15) | 12 (11) |
| Sex | ||||||
| Female | 45 (46) | 127 (40) | 52 (32) | 283 (36) | 11 (33) | 36 (33) |
| Male | 52 (54) | 194 (60) | 109 (68) | 501 (64) | 22 (67) | 73 (67) |
| Region | ||||||
| Western Europe/ Canada |
62 (64) | 228 (71) | - | - | - | - |
| US | - | - | 31 (19) | 176 (22) | 29 (88) | 97 (89) |
| France | - | - | - | - | 4 (12) | 12 (11) |
| EU | - | - | 86 (53) | 431 (55) | - | - |
| Australia | - | - | 25 (16) | 90 (11) | - | - |
| Rest of world | 35 (36) | 93 (29) | 19 (12) | 87 (11) | - | - |
| Race | ||||||
| White | 97 (100) | 319 (99) | 157 (98) | 764 (97) | 32 (97) | 107 (98) |
| Asian | 0 | 1 (<1) | 0 | 10 (1) | 1 (3) | 0 |
| Other | 0 | 1 (<1) | 4 (2) | 10 (1) b | 0 | 2 (2) |
| ECOG PS | ||||||
| 0 | 41 (42) | 228 (71) | 97 (60) | 594 (76) | 22 (67) | 94 (86) |
| ≥1 c | 56 (58) | 92 (29) | 64 (40) | 189 (24) | 11 (33) | 15 (14) |
| Missing | 0 | 1 (<1) | 0 | 1 (<1) | 0 | 0 |
| M stage | ||||||
| M0 | 6 (6) | 22 (7) | 5 (3) | 42 (5) | 3 (9) | 10 (9) |
| M1a | 6 (6) | 37 (12) | 21 (13) | 113 (14) | 2 (6) | 21 (19) |
| M1b | 22 (23) | 72 (22) | 36 (22) | 171 (22) | 10 (30) | 29 (27) |
| M1c | 63 (65) | 190 (59) | 99 (61) | 458 (59) | 17 (52) | 48 (44) |
| Not reported | 0 | 0 | 0 | 0 | 1 (3) | 1 (1) |
| AJCC stage | ||||||
| III | 12 (12) | 37 (12) | 8 (5) | 55 (7) | 4 (12) | 15 (14) |
| IV | 85 (88) | 284 (88) | 153 (95) | 729 (93) | 29 (88) | 94 (86) |
| History of brain metastases | ||||||
| No | 94 (97) | 309 (96) | 152 (94) | 760 (97) | 33 (100) | 104 (95) |
| Yes | 3 (3) | 12 (4) | 9 (6) | 24 (3) | 0 | 4 (4) |
| Not reported | 0 | 0 | 0 | 0 | 0 | 1 (1) |
| PD-L1 status | ||||||
| Indeterminate/ negative |
50 (52) | 157 (49) | 76 (47) | 379 (48) | 13 (39) | 61 (56) |
| Positive | 47 (48) | 164 (51) | 85 (53) | 405 (52) | 20 (61) | 48 (44) |
| LDH | ||||||
| ≤ULN | 44 (45) | 200 (62) | 97 (60) | 493 (63) | 23 (70) | 83 (76) |
| >ULN | 47 (48) | 106 (33) | 62 (39) | 279 (36) | 10 (30) | 25 (23) |
| Not reported/ missing |
6 (6) | 15 (5) | 2 (1) | 12 (2) | 0 | 1 (1) |
| BRAF status | ||||||
| Wild-type | 96 (99) | 314 (98) | 123 (76) | 521 (66) | 25 (76) | 85 (78) |
| Mutant | 0 | 0 | 38 (24) | 263 (34) | 8 (24) | 24 (22) |
| Missing d | 1 (1) | 7 (2) | 0 | 0 | 0 | 0 |
Data are n (%). a Numbers of patients in this row represent the evaluable population for all subsequent percentages in the corresponding column. b Includes one patient with missing data. c Four patients in CheckMate 066, one in CheckMate 067, and two in CheckMate 069 were enrolled in each study despite having an ECOG PS of 2 [16,19,21]. d Two patients in the decarbazine arm of CheckMate 066 tested positive for BRAF V600 mutation following post-study biopsies [3]. AJCC: American Joint Committee on Cancer; ECOG PS: Eastern Cooperative Oncology Group performance status; EU: European Union; M stage: metastasis stage; LDH: lactate dehydrogenase; PD-L1: programmed death-ligand 1; PPI: proton pump inhibitor; ULN: upper limit of normal.
The overall results of the multivariable analyses of efficacy outcomes performed on each treatment group in each of the three studies are summarized in Figure 1. Detailed results showing the covariates included to control for confounding in the models are provided in Supplementary Tables S1–S3. Collectively, these results indicated that there is no evidence of a meaningful association between PPI use and outcomes with ICI therapy. In five of the six treatment arms involving ICIs in the three studies, the 95% CIs for odds ratios or HRs traversed 1. Significant associations were observed only in the nivolumab plus ipilimumab arm of CheckMate 069: between PPI use and poorer ORR and PFS outcomes as reported previously [15], with a trend toward poorer OS outcomes as well. Only seven patients in the ipilimumab arm of CheckMate 069 were on a PPI at baseline, resulting in multivariable analysis results for this arm that did not converge for ORR.
Figure 1.
Association between baseline PPI use and efficacy in CheckMate 066, 067, and 069. Forest plot of ORs for objective response rate and HRs for OS and PFS derived from multivariable models. Error bars indicate 95% CIs. Point estimates and CIs for the association between PPI use (yes vs. no) and each outcome for all covariates included in the multivariable models are shown in Supplementary Tables S1–S3. a The logistic regression model for the objective response rate failed to converge in this group. CI: confidence interval; DITC: dacarbazine; HR: hazard ratio; IPI: ipilimumab; NIVO: nivolumab; OR: odds ratio; OS: overall survival; PFS: progression-free survival; PPI: proton pump inhibitor.
Unadjusted results of Kaplan–Meier analyses of PFS by PPI use in the nivolumab-containing arms of the three studies are shown in Figure 2. In each of the treatment arms of CheckMate 066 and 067, PFS curves by PPI usage did not separate, although a trend toward improved PFS with PPI use was observed in the nivolumab arm of CheckMate 067. This trend was not confirmed in multivariable analysis after adjustment for other prognostic variables (Supplementary Table S2). In CheckMate 069, however, reduced PFS was observed with PPI usage in the nivolumab plus ipilimumab arm, which was confirmed in the multivariable analyses. Kaplan–Meier analysis of the dacarbazine and ipilimumab arms of the studies showed no discernible effect of PPI use on PFS (Supplementary Figure S1).
Figure 2.
Progression-free survival by baseline PPI use in the nivolumab-containing treatment arms of CheckMate 066, 067, and 069. Kaplan–Meier estimates of progression-free survival are shown in the nivolumab arms of CheckMate 066 (A) and CheckMate 067 (B) and in the nivolumab plus ipilimumab arms of CheckMate 067 (C) and CheckMate 069 (D). Shaded areas are 95% log–log confidence bands. CI: confidence interval; PFS: progression-free survival; PPI: proton pump inhibitor.
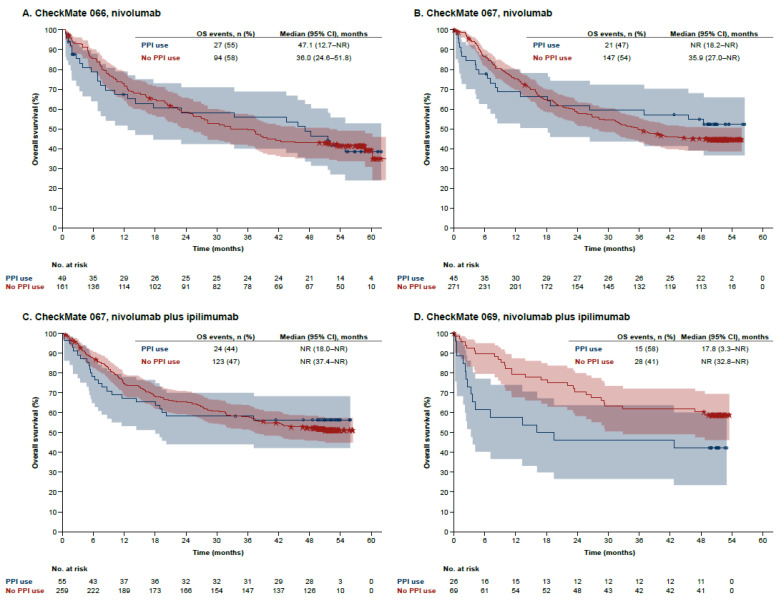
Univariate Kaplan–Meier analysis similarly showed no association between PPI use and OS with nivolumab in CheckMate 066, a possible association (small and negative) with nivolumab and nivolumab plus ipilimumab treatment in CheckMate 067, and a more pronounced negative association with nivolumab plus ipilimumab treatment in CheckMate 069 (Figure 3). Multivariate analysis showed that there was no significant evidence for associations in any of the studies, although a directional trend for poorer OS with nivolumab plus ipilimumab in CheckMate 069 was observed (Supplementary Table S3). Trends toward associations between PPI use and poorer OS in the dacarbazine arm of CheckMate 066 and the ipilimumab arm of CheckMate 067 suggested by Kaplan–Meier analysis (Supplementary Figure S2) were not supported after adjustment for other variables in the multivariate analyses (Supplementary Table S3).
Figure 3.
Overall survival by baseline PPI use in the nivolumab-containing treatment arms of CheckMate 066, 067, and 069. Kaplan–Meier estimates of overall survival are shown in the nivolumab arms of CheckMate 066 (A) and CheckMate 067 (B) and in the nivolumab plus ipilimumab arms of CheckMate 067 (C) and CheckMate 069 (D). Shaded areas are 95% log–log confidence bands. CI: confidence interval; OS: overall survival; PPI: proton pump inhibitor.
4. Discussion
The collective results of multivariable analyses of CheckMate 066, 067, and 069 involving a total of 1505 patients did not support a meaningful association between baseline PPI use and the efficacy of ICI treatment in patients with advanced melanoma. No significant associations were observed with any study drugs in the large global phase III studies CheckMate 066 (nivolumab or dacarbazine) or CheckMate 067 (nivolumab alone, nivolumab plus ipilimumab, or ipilimumab alone). However, PPI use by patients treated with nivolumab plus ipilimumab in the phase II CheckMate 069 study was associated with reduced ORR and PFS, with a directional trend observed for OS.
The negative association between PPI use and outcomes with ICIs found in CheckMate 069 in the present analysis confirms similar observations made by several of us in an earlier analysis in which PPIs were one of a number of concomitant medications that were evaluated [15]. These results differed substantially from those of CheckMate 066 and 067, however, and the precise reasons for this discrepancy are unclear. CheckMate 069 was carried out predominantly in the United States with some enrollment in France, while CheckMate 066 and 067 were global studies. Consistent with the diverse geographic distributions of the three studies, some characteristics of the study populations differed between the trials. Compared with patient populations in CheckMate 066 and 067, patients in CheckMate 069 tended to have better prognostic characteristics: proportions of patients with an ECOG PS of 0 or PD-L1–positive tumors were higher, and those with elevated levels of LDH or M1c disease were lower. A case–control analysis of data from matched patients in CheckMate 067 and 069 might potentially shed light on relevant prognostic factors, although this would be hypothesis-generating only. Additionally, of note is that, within the current analysis, the patient populations differed with respect to BRAF mutation status (a known prognostic factor in melanoma): CheckMate 066 enrolled patients with BRAF wild-type disease, whereas CheckMate 067 and 069 enrolled patients with both mutant and wild-type disease. However, with only 46 patients across CheckMate 067 and 069 having BRAF mutant melanoma, the data are insufficient to evaluate the impact of PPI use on ICI efficacy based on BRAF mutation status. Prospective studies are needed to conclusively determine the impacts of baseline prognostic characteristics on outcomes with ICIs in patients reporting PPI use.
A number of studies have examined the impact of PPIs on outcomes with ICI therapy in patients with various cancers outside of the present study; however, all were retrospective and involved patients with advanced disease [10,18,27,28,29,30,31,32,33,34,35,36]. Overall, the results were equivocal. The two large pooled retrospective analyses of phase II/III studies in NSCLC and urothelial cancer described earlier [10,18] found that PPI use was associated with poor outcomes with ICIs. A large, real-world, multicenter chart review of patients with NSCLC likewise found a negative impact of PPI use on clinical outcomes with pembrolizumab but also noted an association between PPI use and higher baseline ECOG PS, as was also seen in the current analysis [36]. The remainder of the studies were retrospective single-center chart reviews, each involving approximately 100 to 200 patients with a variety of advanced cancers, the majority of which found no association between PPI use and clinical outcomes with ICIs [29,30,32,33,34,35]. Several single-center chart reviews that focused on advanced melanoma found a favorable effect of PPI use on outcomes with ICIs in two cases and an unfavorable effect in one case [27,28,31]. Recent reviews of this subject were unable to make robust recommendations about PPI usage in patients being treated with ICIs, given the inconsistent data available from these studies, many of which were underpowered [37,38]. Our large analysis is consistent with both a lack of association between PPI use and ICI outcomes and the heterogeneity of the results obtained between studies. However, differences in trial design between the present study and previous analyses preclude broad conclusions from being made. For example, differences in the immune checkpoint inhibitors evaluated (e.g., anti–PD-1 monotherapy and combination of anti–PD-1 and anti–CTLA-4 therapy), particularly when comparing studies across tumor types, complicate the ability to generalize the results of these studies.
PPIs are selective inhibitors of H+/K+ ATPases, and they have multiple effects on the gastrointestinal (GI) microbiome [13]. PPIs reduce the diversity of GI microbiota and select for Lactobacilli, especially Streptococcaceae, in the upper GI tract. These pharyngeal commensals are able to move to the lower GI tract because PPIs disrupt the natural gastric acid barrier between the upper and lower GI tract. Although a causal link between aberrations in the GI microbiome, and the efficacy of immune checkpoint inhibitors has not yet been demonstrated, patients with advanced melanoma who have highly diverse GI microbiomes have superior systemic and antitumor immunological responses, compared with those in patients with low-diversity GI microbiomes [7,8]. The negative impact of PPIs on antitumor therapy observed in several studies has been suggested to be due to effects on the GI microbiome [29,30,31,32,33,35,39,40]. It has also been suggested that responses to ICIs can be negatively or positively influenced by the composition of the GI microbiome, which could explain the heterogeneous results observed in the studies cited above [8,9,11,37,41]. Unfortunately, data regarding patients’ microbiome profiles were not available for the current analysis within the scope of this manuscript.
The present analysis has some additional inherent limitations. It was an exploratory post hoc analysis among three studies differing in geographic location, design, and patient populations. As this analysis of patient subgroups based on PPI usage was not prespecified, some patient subgroups were too small for meaningful comparisons (e.g., the subgroup of patients who used PPIs in the ipilimumab arm of CheckMate 069 (n = 7). Additionally, PPI use was determined retrospectively using self-reported information from the prior medication pages of the patient case report form. The assumption that the absence of self-reported PPI use denotes the absence of PPI use at baseline carries the risk that a patient could be misclassified into the “no PPI use” subgroup due to a failure to report. Furthermore, the potential effects of PPI dosage, treatment duration, and discontinuation were not evaluated in this study. We also acknowledge that significant findings in the present post hoc analysis should be interpreted with caution, given that the relatively small sample sizes of patients reporting PPI use may have magnified differences in efficacy. This limitation is particularly relevant when considering outcomes observed in the “PPI use” subgroup of the phase II CheckMate 069 study (n = 33), which was substantially smaller than the corresponding subgroups of the phase III CheckMate 066 (n = 97) and 067 (n = 161) studies.
5. Conclusions
In conclusion, the results of our large multivariable analysis of three phase II/III studies found insufficient evidence to support a meaningful association between PPI use and efficacy outcomes with ICI therapy in patients with advanced melanoma. Ultimately, prospective studies will be needed to conclusively determine the impact of PPI usage on ICI efficacy in patients with melanoma or other cancers.
Acknowledgments
We thank the patients and investigators who participated in the CheckMate 066, 067, and 069 trials. We acknowledge Ono Pharmaceutical Company, Ltd. (Osaka, Japan) for contributions to nivolumab development and Dako, an Agilent Technologies, Inc. company (Santa Clara, CA, USA) for collaborative development of the PD-L1 immunohistochemistry 28-8 pharmDx assay. Professional medical writing and editorial assistance were provided by Wendy Sacks, and Michele Salernitano at Ashfield MedComms, an Ashfield Health Company, funded by Bristol Myers Squibb.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14092300/s1, Table S1: Multivariable logistic regression models of ORR by study and treatment, Table S2: Multivariable Cox proportional-hazards models of PFS by study and treatment, Table S3: Multivariable Cox proportional-hazards models of OS by study and treatment, Figure S1: Progression-free survival by baseline PPI use in the control arms of CheckMate 066, 067, and 069, Figure S2: Overall survival by baseline PPI use in the control arms of CheckMate 066, 067, and 069.
Author Contributions
Conceptualization, K.H. and O.M.; formal analysis, S.R., D.P. and D.D.; writing—original draft preparation, K.H., R.D., C.H., J.D.W., F.S.H., J.L., P.A.A., V.A., C.R., M.A.P., S.R., D.P., D.D. and O.M.; writing—review and editing, K.H., R.D., C.H., J.D.W., F.S.H., J.L., P.A.A., V.A., C.R., M.A.P., S.R., D.P., D.D. and O.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The analyzed studies were conducted in accordance with the Declaration of Helsinki, and associated study protocols were approved by the institutional review board at each study site.
Informed Consent Statement
Given that the current paper reports results obtained with previously published datasets, we suggest that informed consent forms from the three studies are not relevant.
Data Availability Statement
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html (accessed on 27 March 2022).
Conflicts of Interest
K.H. worked in a consulting/advisory role for Roche, Bristol Myers Squibb (BMS), and Amgen; received research/grant support from Roche, BMS, and Molecular Partners; and received honoraria from Roche, Novartis, BMS, and Amgen. R.D. worked in a consulting/advisory role for Roche, BMS, Merck Sharp & Dohme (MSD), Novartis, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Alligator Bioscience, Touch IME, MaxiVAX, and Regeneron; received honoraria from Roche, Novartis, BMS, MSD, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator Bioscience, MaxiVAX, and Touch IME; and received research/grant support from Roche, BMS, Novartis, MSD, and Amgen. C.H. worked in a consulting/advisory role for Almirall, Amgen, AstraZeneca, BMS, Inzyte, MSD, Novartis, Pierre Fabre, Roche, and Sanofi; received honoraria from Amgen, BMS, MSD, Novartis, Pierre Fabre, Roche, and Sanofi; and received research/grant support from Amgen. J.D.W. worked in a consulting/advisory role for BMS, Merck, BeiGene, Sellas Life Sciences, Eli Lilly, Amgen, Chugai Pharma, Ascentage Pharma, PsiOxus Therapeutics, F-Star Biotechnology, Surface Oncology, Apricity, Syndax, Trieza Therapeutics, Astellas Pharma, AstraZeneca, Truvax, Boehringer Ingelheim, Georgiamune, Imvaq Therapeutics, Kyowa Hakko Kirin, Syntalogic Pharmaceuticals, Werewolf Therapeutics, Idera Pharmaceuticals, Daiichi Sankyo, Dragonfly Therapeutics, Trishula Therapeutics, Maverick Therapeutics, and Recepta Biopharma; holds stock in Apricity, Tizona Therapeutics, Georgiamune, Imvaq Therapeutics, BeiGene, Linnaeus Therapeutics, and Arsenal IO; received research/grant support from BMS; is a co-inventor on patents for Newcastle Disease viruses for cancer therapy, CAR+T cells targeting differentiation antigens as means to treat cancer, anti-CD40 agonist mAb fused to monophosphoryl Lipid A (MPL) for cancer therapy, alphavirus replicon particles expressing TRP2, anti–PD-1 antibody, anti–CTLA-4 antibodies, anti-GITR antibodies, engineered vaccinia viruses for cancer immunotherapy, identifying and treating patients at risk for checkpoint blockade therapy associated colitis, immunosuppressive follicular helper-like T cells modulated by immune checkpoint blockade, phosphatidylserine targeting agents and uses thereof for adoptive T-cell therapies, heteroclitic cancer vaccines, recombinant poxviruses for cancer immunotherapy; and is a co-inventor and receives royalties for a blood test for monitoring myeloid derived suppressor cells, and xenogeneic DNA vaccines. F.S.H. worked in a consulting role for BMS, Corner Therapeutics, Eisai, EMD Serono, Genentech/Roche, Gossamer, Idera, Kairos, Merck, Novartis, Psioxus Therapeutics, Pieris Pharmaceutical, Takeda, and Sanofi; worked in an advisory role for 7 Hills Pharma, Aduro, Apricity, Bicara, Checkpoint Therapeutics, Pionyr, and Torque; received research/grant support from BMS and Novartis; received royalties from BMS and Novartis; and holds equity in Apricity. J.L. worked in a consulting role for Eisai, BMS, MSD, GlaxoSmithKline (GSK), Pfizer, Novartis, Roche/Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOncologi, Merck Sorono, Nektar, Vitaccess, Secarna, and Kymab; received travel/accommodations/expenses support from BMS, Pfizer, Novartis, Roche/Genentech, AstraZeneca, Boston Biomedical, Incyte, GSK, Pierre Fabre, and Merck Sorono; received honoraria from Eisai, BMS, MSD, GSK, Pfizer, Novartis, Roche/Genentech, Pierre Fabre, EUSA Pharma, Achilles Therapeutics, AstraZeneca, Boston Biomedical, Ipsen, Imugene, Incyte, iOncologi, Merck Serono, Nektar, Vitaccess, Kymab, and Secarna; and received research/grant support from Pfizer, Novartis, MSD, BMS, Achilles Therapeutics, Roche, Nektar, Covance, Immunocore, and AVEO. P.A.A. worked in a consulting role for BMS, Roche/Genentech, MSD, Novartis, Array BioPharma, Merck Serono, Pierre Fabre, Incyte, MedImmune, AstraZeneca, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Pfizer, OncoSec, Nouscom, Takis, and Lunaphore; received travel/accommodations/expenses support from MSD; holds stock in PrimeVax; and received research/grant support from BMS, Roche/Genentech, Array BioPharma, and Sanofi. V.A. worked in a consulting or advisory role for BMS, MSD, Novartis, Pierre Fabre, Merck Serono, and Roche; received honoraria from BMS, MSD, Novartis, Pierre Fabre, Merck, and Roche; and received travel/accommodations/expenses support from BMS and MSD. C.R. worked in a consulting/advisory role for BMS, Roche, Amgen, Novartis, Pierre Fabre, MSD, Sanofi, Biothera, CureVac, and Merck. MAP worked in a consulting/advisory role for BMS, Merck, Eisai, Novartis, Incyte, NewLink Genetics, Aduro, and Pfizer; received research/grant support from BMS, Merck, Novartis, Array BioPharma, RGenix, Infinity, and AstraZeneca. S.R., D.P., and D.D. are employees of BMS and hold BMS stock or stock options. O.M. worked in a consulting/advisory role for BMS, Roche, Amgen, MSD, Novartis, GSK, and Pierre Fabre; received research/grant support from BMS, MSD, Merck, and Amgen. BMS collaborated with K.H. and O.M. of Centre Hospitalier Universitaire Vaudois to design this study. Data were collected by BMS and analyzed in collaboration with the authors. Medical writing and editorial support were paid for by BMS.
Funding Statement
This study was sponsored by Bristol Myers Squibb and was supported in part by a National Institutes of Health/National Cancer Institute Cancer Center Support Grant (P30 CA008748 to J.D.W. and M.A.P.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Michielin O., Atkins M.B., Koon H.B., Dummer R., Ascierto P.A. Evolving impact of long-term survival results on metastatic melanoma treatment. J. Immunother. Cancer. 2020;8:e000948. doi: 10.1136/jitc-2020-000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larkin J., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Lao C.D., Cowey C.L., Schadendorf D., Wagstaff J., Dummer R., Ferrucci P.F., et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2019;381:1535–1546. doi: 10.1056/NEJMoa1910836. [DOI] [PubMed] [Google Scholar]
- 3.Robert C., Long G.V., Brady B., Dutriaux C., Di Giacomo A.M., Mortier L., Rutkowski P., Hassel J.C., McNeil C.M., Kalinka E.A., et al. Five-year outcomes with nivolumab in patients with wild-type BRAF advanced melanoma. J. Clin. Oncol. 2020;38:3937–3946. doi: 10.1200/JCO.20.00995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tumeh P.C., Harview C.L., Yearley Y.H., Shintaku I.P., Taylor E.J., Robert L., Chmielowski B., Spasic M., Henry G., Ciobanu V., et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaretsky J.M., Garcia-Diaz A., Shin D.S., Escuin-Ordinas H., Hugo W., Hu-Lieskovan S., Torrejon D.Y., Abril-Rodriguez G., Sandoval S., Barthly L., et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N. Engl. J. Med. 2016;375:819–829. doi: 10.1056/NEJMoa1604958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shin D.S., Zaretsky J.M., Escuin-Ordinas H., Garcia-Diaz A., Hu-Lieskovan S., Kalbasi A., Grasso C.S., Hugo W., Sandoval S., Torrejon D.Y., et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7:188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gopalakrishnan V., Spencer C.N., Nezi L., Reuben A., Andrews M.C., Karpinets T.V., Prieto P.A., Vicente D., Hoffman K., Wei S.C., et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matson V., Fessler J., Bao R., Chongsuwat T., Zha Y., Alegre M.L., Luke J.J., Gajewski T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li W., Deng Y., Chu Q., Zhang P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019;447:41–47. doi: 10.1016/j.canlet.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Chalabi M., Cardona A., Nagarkar D.R., Dhawahir Scala A., Gandara D.R., Rittmeyer A., Albert M.L., Powles T., Kok M., Herrera F.G., et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020;31:525–531. doi: 10.1016/j.annonc.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Sivan A., Corrales L., Hubert N., Williams J.B., Aquino-Michaels K., Earley Z.M., Benyamin F.W., Lei Y.M., Jabri B., Alegre M.L., et al. Commensal bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imhann F., Bonder M.J., Vich Vila A., Fu J., Mujagic Z., Vork L., Tigchelaar E.F., Jankipersadsing S.A., Cenit M.C., Harmsen H.J., et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–748. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jackson M.A., Goodrich J.K., Maxan M.E., Freedberg D.E., Abrams J.A., Poole A.C., Sutter J.L., Welter D., Ley R.E., Bell J.T., et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut. 2016;65:749–756. doi: 10.1136/gutjnl-2015-310861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laheij R.J.F., Sturkenboom M.C.J.M., Hassing R.-J., Dieleman J., Stricker B.H.C., Jansen J.B.M.J. Risk of community-acquired pneumonia and use of gastric acid-suppressive drugs. JAMA. 2004;292:1955–1960. doi: 10.1001/jama.292.16.1955. [DOI] [PubMed] [Google Scholar]
- 15.Homicsko K., Tsourti Z., Richtig G., Tsourti Z., Hanahan D., Coukos G., Wind-Rotolo M., Richtig E., Zygoura P., Holler C., et al. Proton pump inhibitors negatively impact survival of PD-1 inhibitor based therapies in metastatic melanoma patients. Ann. Oncol. 2018;29:x40. doi: 10.1093/annonc/mdy511.001. [DOI] [Google Scholar]
- 16.Postow M.A., Chesney J., Pavlick A.C., Robert C., Grossmann K., McDermott D., Linette G.P., Meyer N., Giguere J.K., Agarwala S.S., et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodi F.S., Chesney J., Pavlick A.C., Robert C., Grossmann K.F., McDermott D.F., Linette G.P., Meyer N., Giguere J.K., Agarwala S.S., et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016;17:1558–1568. doi: 10.1016/S1470-2045(16)30366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hopkins A.M., Kichenadasse G., Karapetis C.S., Rowland A., Sorich M.J. Concomitant proton pump inhibitor use and survival in urothelial carcinoma treated with atezolizumab. Clin. Cancer Res. 2020;26:5487–5493. doi: 10.1158/1078-0432.CCR-20-1876. [DOI] [PubMed] [Google Scholar]
- 19.Robert C., Long G.V., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 20.Ascierto P.A., Long G.V., Robert C., Brady B., Dutriaux C., Di Giacomo A.M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: Three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5:187–194. doi: 10.1001/jamaoncol.2018.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolchok J.D., Chiarion-Sileni V., Gonzalez R., Rutkowski P., Grob J.J., Cowey C.L., Lao C.D., Wagstaff J., Schadendorf D., Ferrucci P.F., et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodi F.S., Chiarion-Sileni V., Gonzalez R., Grob J.J., Rutkowski P., Cowey C.L., Lao C.D., Schadendorf D., Wagstaff J., Dummer R., et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1480–1492. doi: 10.1016/S1470-2045(18)30700-9. [DOI] [PubMed] [Google Scholar]
- 24.Study of Nivolumab (BMS-936558) Compared with Dacarbazine in Untreated, Unresectable, or Metastatic Melanoma (CheckMate 066) [(accessed on 19 April 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01721772.
- 25.Phase 3 Study of Nivolumab or Nivolumab Plus Ipilimumab versus Ipilimumab Alone in Previously Untreated Advanced Melanoma (CheckMate 067) [(accessed on 19 April 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01844505.
- 26.Study of Nivolumab (BMS-936558) plus Ipilimumab Compared with Ipilimumab Alone in the Treatment of Previously Untreated, Unresectable, or Metastatic Melanoma (CheckMate 069) [(accessed on 19 April 2022)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01927419.
- 27.Failing J.J., Finnes H.D., Kottschade L.A., Allred J.B., Markovic S.N. Effects of commonly used chronic medications on the outcomes of ipilimumab therapy in patients with metastatic melanoma. Melanoma Res. 2016;26:609–615. doi: 10.1097/CMR.0000000000000299. [DOI] [PubMed] [Google Scholar]
- 28.Afzal M.Z., Shirai K. What impact do the proton pump inhibitors have on the efficacy of immune check point inhibitors in metastatic malignant melanoma? J. Clin. Oncol. 2019;37:e21040. doi: 10.1200/JCO.2019.37.15_suppl.e21040. [DOI] [Google Scholar]
- 29.Kulkarni A., Kumar M., Pease D.F., Wang Y., DeFor T.E., Patel M. Impact of antibiotics and proton pump inhibitors on clinical outcomes of immune check point blockers in advanced non-small cell lung cancers and metastatic renal cell cancer. J. Clin. Oncol. 2019;37:e20520. doi: 10.1200/JCO.2019.37.15_suppl.e20520. [DOI] [Google Scholar]
- 30.Mukherjee S., Ibrahimi S., Khalid B., Roman D., Zhao D., Aljumaily R. Do proton pump inhibitors modulate the efficacy of anti-PD-1/PD-L1 therapy? A retrospective study. J. Oncol. Pharm. Pract. 2019;25:762–764. doi: 10.1177/1078155218771152. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen Q.P., Nomura M., Matsumoto S., Muto M. The effect of proton pump inhibitors on the efficacy of nivolumab monotherapy in different types of cancer. Ann. Oncol. 2019;30:vi115. doi: 10.1093/annonc/mdz338.107. [DOI] [Google Scholar]
- 32.Spakowicz D., Husain M., Tinoco G., Patel S.H., Burkat J.T., Verschraegen C.F., Kendra K.L., Hoffman S., Philippon J., Quiroga D.M., et al. Effect of concomitant medications on overall survival in patients with cancer undergoing immunotherapy. J. Clin. Oncol. 2019;37:94. doi: 10.1200/JCO.2019.37.8_suppl.94. [DOI] [Google Scholar]
- 33.Trabolsi A., Winter M., Rodriguez E. Proton pump inhibitors and response to immune check-point inhibitors: Single center study. J. Clin. Oncol. 2019;37:e14092. doi: 10.1200/JCO.2019.37.15_suppl.e14092. [DOI] [Google Scholar]
- 34.Zhao S., Gao G., Li W., Li X., Zhao C., Jiang T., Jia Y., He Y., Li A., Su C., et al. Antibiotics are associated with attenuated efficacy of anti-PD-1/PD-L1 therapies in Chinese patients with advanced non-small cell lung cancer. Lung Cancer. 2019;130:10–17. doi: 10.1016/j.lungcan.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 35.Iglesias-Santamaria A. Impact of antibiotic use and other concomitant medications on the efficacy of immune checkpoint inhibitors in patients with advanced cancer. Clin. Transl. Oncol. 2020;22:1481–1490. doi: 10.1007/s12094-019-02282-w. [DOI] [PubMed] [Google Scholar]
- 36.Cortellini A., Di Maio M., Nigro O., Leonetti A., Cortinovis D.L., Aerts J.G., Guaitoli G., Barbieri F., Giusti R., Ferrara M.G., et al. Differential influence of antibiotic therapy and other medications on oncological outcomes of patients with non-small cell lung cancer treated with first-line pembrolizumab versus cytotoxic chemotherapy. J. Immunother. Cancer. 2021;9:e002421. doi: 10.1136/jitc-2021-002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li M., Zeng C., Yao J., Ge Y., An G. The association between proton pump inhibitors use and clinical outcome of patients receiving immune checkpoint inhibitors therapy. Int. Immunopharmacol. 2020;88:106972. doi: 10.1016/j.intimp.2020.106972. [DOI] [PubMed] [Google Scholar]
- 38.Hussain N., Naeem M., Pinato D.J. Concomitant medications and immune checkpoint inhibitor therapy for cancer: Causation or association? Hum. Vaccin. Immunother. 2021;17:55–61. doi: 10.1080/21645515.2020.1769398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X., Liu C., Wang J., Fan Y., Wang Z., Wang Y. Proton pump inhibitors increase the chemosensitivity of patients with advanced colorectal cancer. Oncotarget. 2017;8:58801–58808. doi: 10.18632/oncotarget.18522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C., Xia Z., Li A., Meng J. The effect of proton pump inhibitor uses on outcomes for cancer patients treated with immune checkpoint inhibitors: A meta-analysis. Ann. Transl. Med. 2020;8:1655. doi: 10.21037/atm-20-7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vétizou M., Pitt J.M., Daillère R., Lepage P., Waldschmitt N., Flament C., Rusakiewicz S., Routy B., Roberti M.P., Duong C.P., et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Bristol Myers Squibb’s policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html (accessed on 27 March 2022).