Abstract
Proteostasis, a portmanteau of the words protein and homeostasis, refers to the ability of eukaryotic cells to maintain a stable proteome by acting on protein synthesis, quality control and/or degradation. Over the last two decades, an increasing number of disorders caused by proteostasis perturbations have been identified. Depending on their molecular etiology, such diseases may be classified into ribosomopathies, proteinopathies and proteasomopathies. Strikingly, most—if not all—of these syndromes exhibit an autoinflammatory component, implying a direct cause-and-effect relationship between proteostasis disruption and the initiation of innate immune responses. In this review, we provide a comprehensive overview of the molecular pathogenesis of these disorders and summarize current knowledge of the various mechanisms by which impaired proteostasis promotes autoinflammation. We particularly focus our discussion on the notion of how cells sense and integrate proteostasis perturbations as danger signals in the context of autoinflammatory diseases to provide insights into the complex and multiple facets of sterile inflammation.
Keywords: proteostasis, autoinflammation, ribosomopathies, proteinopathies, proteasomopathies
1. Introduction
Inflammation describes a highly conserved generally beneficial reaction—if controlled—of the body in response to a variety of danger signals [1,2,3]. It is triggered by the innate immune system and characterized by the rapid mobilization of specialized cells and humoral factors which combat the insult with a relatively low specificity [4,5,6]. Noxious signals capable of initiating innate responses are traditionally divided into exogenous and endogenous insults in order to facilitate self and non-self-discrimination [7,8]. External stimuli are typically derived from infectious agents and include a wide range of microbial products referred to as pathogen-associated molecular patterns (PAMPs) which are recognized by pathogen-recognition receptors (PRR) such as Toll-like receptors (TLR), RIG-I-like receptors (RLR), NOD-like receptors (NLR), among others [9,10]. The binding of PAMP to PRR triggers multiple intracellular signaling cascades ultimately resulting in the activation of the NF-κB and IRF3 transcription factors which in turn promote the expression of pro-inflammatory cytokines (i.e., TNFα, IL-1, IL-6) and type I and/or II interferons (IFN) [11,12]. Type I IFN is a large cytokine family consisting of 18 members with 14 IFN-α species and one single specimen each of IFN-β, IFN-κ, IFN-ω and IFN-ε which are produced by both immune and non-immune cells [13]. Following autocrine and/or paracrine binding to their receptor IFNAR1/2, type I IFN engage JAK/STAT signaling that results in the rapid transcription of a myriad of so-called IFN-stimulated genes (ISG) [14]. Prominent ISG include PKR and OAS1 whose products confer the cell an antiviral state by arresting protein translation and promoting RNA degradation, respectively [14]. Internal stimuli also known as damage-associated molecular patterns (DAMPs) are widely regarded as self-molecules that accidently reach undedicated compartments following tissue injury [15,16]. Prime examples of DAMPs include extracellular and/or cytosolic DNA as well as extracellular ATP or histones, only to name a few [17,18,19,20]. Like PAMPs, DAMPs use various PRR to elicit innate immune responses which are characterized by the prompt production of NF-κB- and IRF3-responsive genes [21,22]. As innate immunity triggered by DAMPs is pathogen-free, it is frequently categorized as sterile inflammation [23,24]. Importantly, sterile inflammation is not necessarily restricted to the unphysiological release of molecules from necrotic cells and/or damaged intracellular organelles. Disturbances in protein flux have also long been known to be immunostimulant and prominent hallmarks of a flurry of autoimmune diseases. It is indeed more than 60 years ago that extracellular protein aggregates initially called “rheumatoid factors” have been described in the plasma and synovial tissues of patients with rheumatoid arthritis (RA) [25]. It became rapidly evident that such aggregates were in fact large insoluble autoimmune complexes (IC) made up of self-reactive immunoglobulin (Ig) G [26]. It is their inefficient clearance by circulating proteases and/or phagocytes that results in their deposition in tissues and contributes to autoinflammation, notably through the stimulation of monocytes, macrophages and dendritic cells (DC) [27,28]. Herein, RA provided the first example of sterile inflammation triggered by loss of protein homeostasis. Meanwhile, over 40 autoinflammatory diseases have been reported to be associated with protein homeostasis (or more commonly referred to as “proteostasis”) perturbations.
2. Proteostasis Sensors and Their Roles in Triggering Sterile Inflammation
Imbalances of protein flux are not limited to the extracellular space but can also occur within the cell as a consequence of a disequilibrium between protein synthesis at ribosomes (i.e., translation) and degradation by the ubiquitin–proteasome system (UPS) and/or autophagy [29]. Regardless of localization, it is becoming increasingly clear that proteostasis perturbations behave as danger signals and have the potential to initiate sterile inflammation. Although our current knowledge on the mechanisms perceiving and integrating these signals are not fully understood, a growing body of evidence suggests a critical role of the endoplasmic reticulum (ER) in the surveillance of intracellular proteostasis. The mechanisms monitoring proteostasis outside the cell are less clear, albeit a couple of studies point to a potential involvement of PRR in this process, as discussed below.
2.1. The Unfolded Protein Response (UPR)
As shown in Figure 1, the ER-membrane resident proteins IRE1, ATF6 and PERK belong to the mechanisms by which the cell is capable of sensing intracellular proteostasis disruption. One estimates that approximately 30–40% of the newly synthetized proteins traffic through the endoplasmic reticulum (ER) [30]. It is further understood that up to 25% of these proteins fail to achieve final conformation and that such products are recognized by the protein quality control machinery in the ER and transported back to cytosol by the ER-associated degradation (ERAD) pathway for subsequent degradation by 26S proteasomes [31]. Given that retro-translocation is driven by degradation [32,33,34], proteasome dysfunction ultimately results in the accumulation of misfolded proteins within the ER lumen and subsequent activation of the IRE1, ATF6 and PERK receptors. This in turn engages the so-called unfolded protein response (UPR), a compensatory reaction which mostly aims to restore proteostasis by
Arresting global protein biosynthesis;
Upregulating specific genes coding for chaperones and ERAD components [35,36,37].
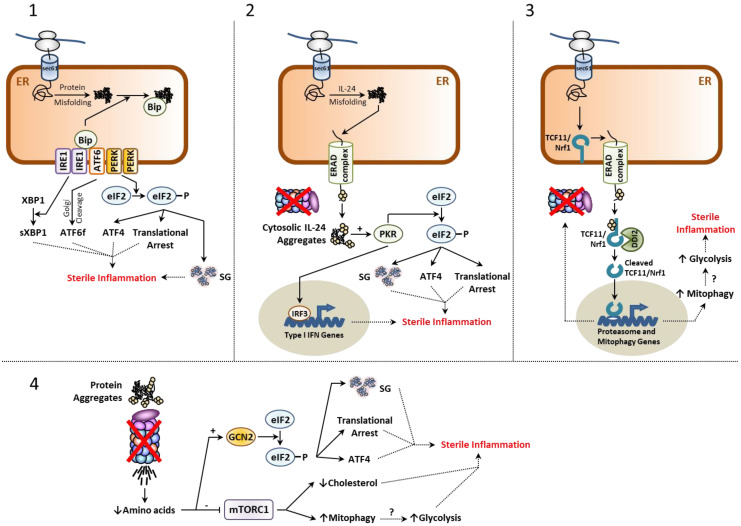
Figure 1.
Most inflammatory pathways engaged following proteostasis disruption originate from the ER. Proteostasis perturbations are typically caused by proteasome dysfunction and/or excessive protein misfolding, as indicated. (1) Sustained misfolding of secretory proteins results in their accumulation within the ER lumen and generates ER stress, which is sensed by the IRE1, ATF6 and PERK receptors upon dissociation of the chaperone protein Bip. This, in turn, initiates the UPR that comprises the formation of sXBP1, ATF6f as well as a translational arrest, the activation of ATF4 and the formation of SGs following phosphorylation of eIF2α by PERK. Persistent activation of all UPR branches have been reported to trigger sterile inflammation by various mechanisms (for more details, see main text). (2) Misfolding of IL-24 typically results in its subsequent retro-translocation back to the cytosol by the ERAD machinery. In case of proteasome dysfunction, IL-24 misfolded species accumulate in the cytosol and activate PKR to trigger a type I IFN response and inflammation via eIF2α phosphorylation. (3) Proteome imbalance is also sensed by the short-lived ER-membrane resident protein TCF11/Nrf1 whose turnover rate decreases with increased proteasome dysfunction. This results in its proteolytic cleavage by DDI2, thereby giving rise to a transcription factor inducing the expression of proteasome genes to restore protein homeostasis and mitophagy genes that may exacerbate inflammation through persistent glycolysis, as indicated. (4) Proteasome defects also reduce the intracellular pool of free amino acids and result in mTORC1 downregulation. This, in turn, promotes sterile inflammation by increasing mitophagy and blocking lipid synthesis (for more details, see the main text). Amino acid depletion in the cell also activates GCN2 of the integrated stress response (ISR) which facilitates inflammation following eIF2α phosphorylation.
While transient stimulations of the UPR are physiological reactions devoid of inflammation, excessive UPR activation is considered pathological and associated with persistent innate responses [35,38]. The mechanisms by which the UPR triggers sterile inflammation are diverse, complex, poorly understood and have been already discussed elsewhere [35,39]. Of particular interest is the PERK arm of the UPR which mediates the phosphorylation of eIF2α, an event that shuts down canonical translation of 5′ capped mRNA molecules and affects the steady-state level of short-lived proteins [40]. These proteins include notably IκBα whose increased turnover upon activation of the UPR facilitates the nuclear translocation of NF-κB and the subsequent production of inflammatory mediators [41,42]. As discussed later, the UPR has been proposed as a major contributor to autoinflammation in various proteinopathies [35].
2.2. The Integrated Stress Response (ISR)
As proteasome-mediated protein degradation produces peptides which are further degraded into amino acids by peptidases [43], it is legitimate to assume that the intracellular pool of amino acids is regulated—at least partially—by the UPS. This hypothesis, which has been proven to be true in yeast [43], implies that proteostasis perturbations caused by impaired protein breakdown are followed by a parallel depletion of intracellular amino acids.
A major sensor of amino acid deficiency in the cell is the general control nonderepressible 2 (GCN2) kinase which undergoes activation by uncharged tRNA that accumulate upon amino acid starvation [44]. As shown in Figure 1, like PERK, GCN2 promotes the phosphorylation of eIF2α in a pathway referred to as the integrated stress response (ISR) [45] which, by definition, intersects with the UPR. As both the ISR and PERK branch of the UPR converge to eIF2α, these pathways share similar consequences regarding their proinflammatory properties in case of sustained stimulus. Another kinase of the ISR capable of phosphorylating eIF2α is protein kinase R (PKR) [46], which is primarily activated by double-stranded (ds) RNA in infected cells during viral replication [47,48]. Besides phosphorylating eIF2α, PKR also triggers a signaling cascade resulting in the nuclear translocation of the transcription factor IRF3 and subsequent transcription of type I IFN genes [49,50,51,52]. Interestingly, the PKR activation spectrum is not restricted to RNA viruses and can be expanded to a flurry of non-microbial stimuli including proteostasis disruptors such as tunicamycin, oxidative stress and heat shock [53,54,55,56,57,58]. A recent study by Davidson et al. described cytosolic IL-24 as a potent PKR activator following proteasome dysfunction [59]. In this process, misfolding of newly synthetized IL-24 within the ER lumen results in its retro-translocation back to the cytosol by ERAD where it accumulates and activates PKR to engage a type I IFN response. Supporting the notion that abnormal cytosolic accumulation of IL-24 is a danger signal alerting the immune system via PKR, cells devoid of IL-24 and/or PKR fail to exhibit a type I IFN signature in response to proteasome inhibition [59]. The work of Davidson et al. therefore unambiguously identifies the IL-24/PKR axis as a new surveillance pathway capable of initiating sterile inflammation in response to perturbed proteostasis. This notion is fully in line with the observation that PKR is found constitutively phosphorylated/activated in the CNS of patients suffering from neurodegenerative proteinopathies [60,61,62,63] and that PKR inhibition is able to delay neuroinflammation in rats [64].
2.3. The mTORC1 Signaling Complex
Apart from GCN2, major amino acid sensors include the cytosolic SESN2 and CASTOR proteins which sense leucine and arginine levels, respectively [65]. Binding of these amino acids to their respective receptors activate GATOR2, which in turn inhibits the mTORC1 complex inhibitor GATOR1 [66]. Subsequently, mTORC1 itself undergoes activation and engages several downstream signaling pathways inhibiting autophagy, while promoting protein translation, lipid biosynthesis and mitochondria biogenesis [67]. Based on the assumption that the UPS regulates amino acid homeostasis, one would expect that impaired proteostasis result in downregulation of mTORC1 signaling (Figure 1). Indeed, our recent investigation on cells isolated from patients with PSMD12 haploinsufficiency confirmed that proteasome loss-of-function was associated with reduced activation of mTORC1 [68]. From the mTORC1 downstream pathways, lipid biosynthesis seems of particular interest regarding sterile inflammation. It was indeed recently suggested that a reduced cholesterol flux in the cell engages spontaneous type I IFN responses in a poorly understood process involving the cGAS/STING pathway [69]. Likewise, increased autophagy by reduced mTORC1 activation might be relevant with respect to sterile inflammation following proteotoxic stress. Elevated autophagy does not only imply increased lysosomal protein breakdown but also presupposes increased elimination of intracellular organelles including mitochondria, a process referred to as “mitophagy”. Supporting the notion that mTORC1 is a proteostasis sensor, increased mitophagy has been observed in patients with proteasomopathies [68]. Interestingly, sustained mitophagy implies a switch of the energetic metabolism from oxidative phosphorylation to glycolysis, the latter being associated with the accumulation of PEP, succinate, citrate and itaconate all exhibiting pro-inflammatory properties (i.e., M1 phenotype) by various and sometimes poorly understood mechanisms [70]. Whether mitophagy indeed participates in the initiation of sterile inflammation in response to impaired proteostasis is not known, but the observation that glycolysis is increased in microglia and astrocytes of patients in AD [70,71,72] supports this notion.
2.4. Stress-Induced Granulation
A cellular feature frequently shared by many neurodegenerative proteinopathies is the formation of so-called stress granules (SGs) in the CNS [73,74,75,76,77,78,79,80,81,82,83,84]. SGs are cytoplasmic liquid-like seemingly compartmentalized structures devoid of membranes mostly containing translation factors, RNA as well as RNA-binding proteins [85,86,87,88]. Importantly, SGs are not an exclusive feature of neurodegenerative diseases, and their formation may be induced in all cell types in response to various stress reactions driven by eIF2α phosphorylation, namely following activation of the UPR and/or ISR (Figure 1) [89]. Accordingly, SGs are induced in situations in which the proteostasis network is challenged such as viral infection and proteasome inhibition and [90,91,92]. Interestingly, while the role of SGs in the propagation of type I IFN responses during viral infection seems to be established [93,94,95], their contribution to inflammation following sterile proteostasis disruption is not known. Viral SGs are thought to amplify innate immune signaling by building a platform that facilitates the recruitment of the DNA/RNA sensors cGAS, PKR and RIG-1 [96,97,98]. It is unclear whether viral SGs are biochemically distinct from those observed in proteinopathies. In this regard, further investigations are warranted to answer the question whether SGs due to impaired proteostasis of non-viral origin predispose the cells to generate type I IFN responses.
2.5. The TCF11/Nrf1-NGLY1-DDI2 Axis
Another proteostasis sensor is the ER membrane-resident protein TCF11/Nrf1, also referred to as nuclear factor erythroid derived 2-related factor 1 (NFE2L1) and encoded by the NFE2L1 gene [99,100]. TCF11/Nrf1 is a highly glycosylated short-lived protein which under normal conditions is rapidly targeted to proteasome-mediated degradation by ERAD [99,100] following its de-glycosylation by the enzyme N-glycanase 1 (NGLY1) [101]. Consequently, in case of proteasome dysfunction, TCF11/Nrf1 becomes stabilized and prone to enzymatic processing by the aspartyl protease protein DDI1 homolog 2 (DDI2) at the ER membrane [102]. As shown in Figure 1, following proteolytic cleavage, processed TCF11/Nrf1 translocates into the nucleus and binds to ARE promoters to induce the transcription of 20S and 19S proteasome subunit genes and a subset of ubiquitin factors. The TCF11/Nrf1-DDI2 pathway is widely viewed as a compensatory mechanism ensuring the replacement of defective 26S complexes by newly synthetized ones. Like all proteostasis sensors, TCF11/Nrf1 seems to have the potential to engage proinflammatory programs. This assumption is based on the observation that TCF11/Nrf1 also up-regulates mitophagy genes [103]. While the safe elimination of damaged mitochondria by autophagy prevents the accidental leakage of immunostimulatory mitochondrial (mt) DNA into the cytosol, the removal of the healthy ones due to excessive mitophagy might reprogram the cells towards glycolytic processes and their inherent proinflammatory consequences. On the other hand, TCF11/Nrf1 has been shown to mitigate lipid-mediated inflammation acting as a sensor for cholesterol [104]. Altogether, these studies highlight that our current understanding of TCF11/Nrf1 in inflammation remains incomplete and warrants further investigation.
2.6. Pathogen Recognition Receptors (PRR)
Unexpectedly, works aimed to unravel the pathogenesis of neurodegenerative proteinopathies have revealed that PRR are able to sense intra- and extracellular proteostasis perturbations. Indeed, normally specialized in the recognition of PAMP and/or DAMPs, PRR are also capable of perceiving abnormal protein aggregates. For instance, extracellular α-syn oligomers and fibrils produced during the course of Parkinson disease (PD) bind to TLR4 and TLR2 expressed on microglia [105,106,107,108,109,110]. In a very similar fashion, Aβ aggregates from Alzheimer disease (AD) are captured by TLR2, 4, 6 and 9 [111,112,113,114,115]. In ALS, the inflammasome is activated by cytosolic TDP-43 aggregates [116,117,118]. These observations suggest a role for PRR beyond sensing PAMP and/or DAMP in surveilling both intra- and extracellular proteostasis disturbances. It remains however unclear whether this perception is limited to certain types of proteins and/or aggregates.
3. Causes of Proteostasis Perturbations and Associated Autoinflammatory Syndromes
Depending on the source of perturbation, proteostasis imbalances carry two possible consequences, namely
Protein aggregation or;
Protein depletion.
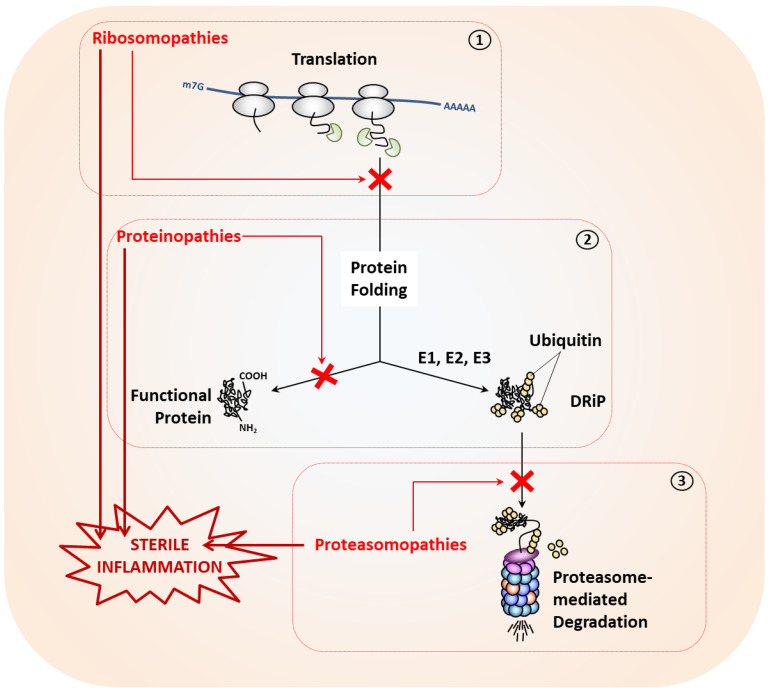
As illustrated in Figure 2, protein aggregation is frequently found in disorders such as proteinopathies or proteasomopathies following excessive protein misfolding or proteasome dysfunction, respectively. By contrast, intracellular protein depletion mostly occurs as a consequence of impaired translation in so-called ribosomopathies. The investigation of the molecular pathogenesis of such disorders have greatly improved our knowledge on how the innate immune respond reacts to disrupted proteostasis, as discussed below.
Figure 2.
Proteostasis perturbations may arise at any stage of the protein life cycle and typically result in the initiation of sterile inflammation. Depicted are three pathological situations causing impaired proteostasis in which translation (1), protein folding (2) and proteasome-mediated protein degradation of defective ribosomal products (DRiPs) following their polyubiquitination by E1, E2 and E3 enzymes of the ubiquitin-conjugation pathway (3) are affected.
3.1. Proteostasis Perturbations Caused by Translation Deficiency
Defects in protein synthesis are typically due to loss-of-function mutations in genes encoding components of the translation machinery. Prominent vulnerable genes in this category include those coding for proteins of the 40S and 60S ribosomal subunits (RPS and RPL, respectively). Genomic alterations of the RPS and/or RPL genes generally result in ribosome assembly disruption which itself causes a wide range of disorders traditionally referred to as “ribosomopathies” [119].
At the molecular level, this group of syndromes is essentially characterized by reduced protein synthesis due to persistently depressed translation [120]. Accordingly, ribosomopathies primarily affect cells with high protein demand, particularly those of the hematopoietic lineage. Major ribosomopathies include the various forms of Diamond-Blackfan anemia (DBA), which are inherited monogenic autosomal dominant genetic disorders characterized by ineffective erythropoiesis and caused by mutations in either RPS or RPL genes [121]. Here, insufficient protein synthesis leads to protein depletion and cell death. Specifically, it is understood that the ribosomal subunits that fail to become incorporated into ribosomes initiate a so-called nucleolar stress response that results in cell cycle arrest and apoptosis [122,123]. Paradoxically, ribosomopathies also increase cancer risk overtime [124]. This seemingly inconsistency can be however easily explained by the fact that cell proliferation defects exert a strong pressure that results in the progressive selection of transformed cells that have acquired compensatory mutations in proto-oncogenes [124].
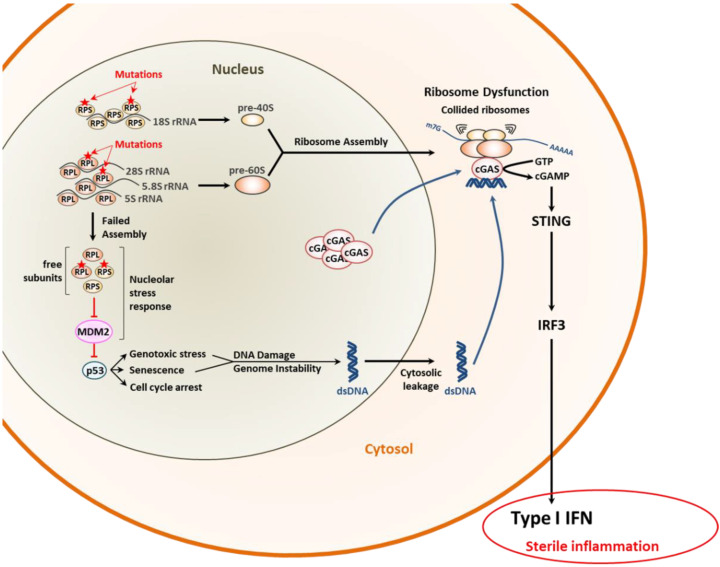
Most importantly, it was recently suggested that proteostasis disruption in DBA was associated with the generation of inflammatory gene signatures [125,126,127]. Autoinflammation could be confirmed in a DBA zebrafish model in which affected animals constitutively express type I IFN-stimulated genes (ISG) [128]. Further evidence for a functional cause-and-effect relationship between translation deficiency and sterile inflammation comes from the observation that mutations in RPL10 drive type I IFN responses in T cell leukemia [129]. The mechanisms by which ribosomopathies favor autoinflammation remain unclear, but it is conceivable that senescence due to prolonged cell cycle arrest might play a role in this process. Indeed, as shown in Figure 3, it was recently shown that senescence is intrinsically associated with genome instability and the subsequent leakage of DNA fragments into the cytosol which trigger type I IFN response by the cGAS/STING pathway [130,131]. Consistent with a potential role of cGAS in driving inflammatory responses upon ribosomal dysfunction, Wan et al. have shown that ribosome collision during translation results in the cytosolic accumulation of cGAS [132], thereby predisposing the cells to respond stronger to cytosolic, even background, DNA (Figure 3).
Figure 3.
Molecular pathogenesis of ribosomopathies and their potential inflammatory consequences. The failure of mutant ribosomal proteins of the small 40S (RPS) and/or large 60S (RPL) ribosomal subunits to assemble into ribosomes induces the nucleolar stress response leading to p53 stabilization via persistent inactivation of the MDM2 E3 ubiquitin ligase. This, in turn, results in genome instability and subsequent leakage of double-stranded (ds)DNA into the cytosol. Cytoplasmic dsDNA is then sensed by the cyclic GMP-AMP synthase (cGAS) which promotes a type I IFN response via the STING/IRF3 signaling pathway, as indicated. The recruitment of cGAS in the cytosol is itself facilitated by defective and collided ribosomes.
3.2. Proteostasis Perturbations Caused by Protein Misfolding
It is well established that protein biosynthesis (or mRNA translation) in eukaryotic cells is not an accurate process. Early works from Yewdell’s lab estimate that approximately 30% of newly synthetized proteins fail to reach their 3D structure because of damage, premature termination and/or incorporation of wrong amino acids [133,134]. As these improperly folded proteins (or DRiPs) are non-functional and, in most cases, prone to aggregation, they are rapidly sampled and sorted out by various protein quality control (PQC) systems. One major co-translational PQC mechanism relies on the action of the molecular chaperones Hsp70 and Hsp90 that are capable of sensing hydrophobicity exposed by all misfolded nascent proteins [135]. Both Hsp70 and Hsp90 recruit E3 ubiquitin ligases such as the carboxy-terminus of the Hsc70 interacting protein (CHIP), leucine-rich repeat and sterile alpha motif-containing 1 (LRSAM1) and/or E6-associated protein (E6-AP) which themselves mediate the ubiquitination of the presented protein substrates and target them for subsequent degradation by the 26S proteasome [136,137].
Besides aging, major causes for excessive misfolding include alterations in genes coding for
-
(i)
UPS components or;
-
(ii)
Highly translated host (or viral) proteins.
These events may result in the accumulation of insoluble protein aggregates, a typical feature of a heterogenous group of disorders known as proteinopathies [138,139]. As mentioned above, proteostasis disruption in proteinopathies is not necessarily due to UPS dysfunction. Indeed, virtually every protein variant with a particularly high synthesis rate may increase protein supply and/or misfolding, thereby surpassing UPS capacity. Prime examples of protein mutants predisposed to misfolding and aggregating include the HLA-B27 allele as well as various variants of the cystic fibrosis transmembrane conductance regulator (CFTR), transthyretin (TTR), α-1 antitrypsin (AAT), amyloid protein precursor (APP), α-synuclein (SNCA), huntingtin (HTT) and transactive response RNA/DNA-binding protein (TDP-43), just to name a few. With the exception of HLA-B27, CFTR and AAT, all these proteinopathies primarily affect CNS and promote typical neurodegenerative phenotypes. The reasons for this tissue selectivity remain obscure and intriguing in view of the wide distribution of these proteins throughout the body. An undebatable issue in this field, however, is that neurodegeneration has a sterile inflammatory component—commonly referred to as neuroinflammation—predominantly triggered by glia cells (i.e., microglia, astrocytes and/or oligodendrocytes). Likewise, non-neurodegenerative proteinopathies are strongly associated with autoinflammation. These observations unambiguously point to a cause-and-effect relationship between protein aggregation and innate immunity, as discussed below.
3.2.1. Non-Neurodegenerative Proteinopathies
These disorders are essentially caused by misfolding and subsequent aggregation of protein variants outside the CNS. These typically include ankylosing spondylitis (AS), cystic fibrosis (CF), alpha-1-antitrypsin deficiency (AATD) and hereditary transthyretin amyloidosis (ATTR) which are all associated with inflammatory reactions. As a large fraction of these mutant proteins (i.e., HLA-27, CFTR and AATD in AS, CF and AATD, respectively) typically misfold within the ER lumen, persistent activation of the UPR is thought to contribute to the disease inflammatory phenotype [140,141,142,143,144,145,146,147,148]. Nevertheless, additional pathways may synergize with the UPR to trigger autoinflammation in these patients.
For instance, ATTR is an autosomal dominant proteinopathy with neuronal and cardiac manifestations which provides an excellent example of extracellular proteostasis perturbation. It is characterized by the extracellular deposition of transthyretin (TTR)-derived amyloid fibrils, particularly in the peripheral nervous system (PNS) [149]. The pathologic basis of ATTR are single amino acid variations in the TTR protein, among which the Val30Met and Val142Ile substitutions are the most frequent ones [150]. The TTR protein is produced by the choroid plexus and liver and found in the cerebrospinal fluid (CSF) as well as in the bloodstream where it carries thyroxin and retinol [35,151]. TTR misfolding mutations typically result in the formation of extracellular precipitates resembling β-pleated sheet structures named amyloid fibrils which cause tissue dysfunction [152]. Although patients with ATTR exhibit no evidence of systemic inflammation, they present with elevated circulating inflammatory markers [153,154]. Recent evidence suggests that activation of the innate immune system in ATTR occurs extracellularly as well with TTR aggregates capable of activating immune cells, notably neutrophils and microglia to produce proinflammatory cytokines [155,156]. The receptors involved in this process remain however ill-defined.
3.2.2. Neurodegenerative Proteinopathies
This group of proteinopathies is by far the largest and includes prominent disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), spinocerebellar ataxias and polyglutamine (polyQ) diseases, among others. Although these disorders are clinically and phenotypically distinct, their molecular etiology is identical and based on the uncontrolled accumulation of protein variants mostly in the CNS. As alluded to earlier, one major mechanism by which proteostasis perturbations trigger neuroinflammation in these diseases involves the release of protein aggregates into the extracellular space and their subsequent binding to PRR such as TLR and/or TREM2 on microglia [105,106,107,108,109,110,111,112,113,114,115]. However, recent research on the molecular pathogenesis of amyotrophic lateral sclerosis (ALS) has expanded the spectrum of the molecular pathways initiating sterile inflammation in response to proteostasis disruption.
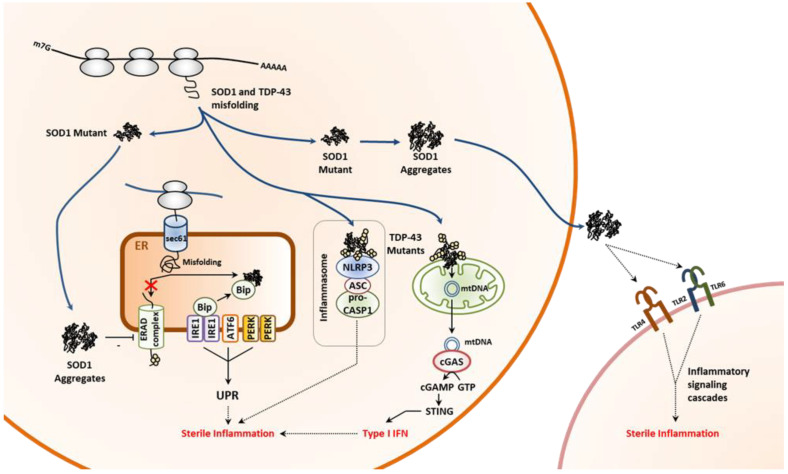
ALS is a neurodegenerative proteinopathy characterized by a progressive loss of motor neurons [157,158]. While most cases of ALS are sporadic, a dozen of predisposition genes have been identified over the past two decades [159,160]. These include notably SOD1 and TARDBP which code for the superoxide dismutase 1 (SOD1) and transactive response DNA binding protein 43 (TDP-43), respectively [161,162]. Both proteins tend to misfold and aggregate in neurons upon specific point mutations. In particular, abnormal accumulation and/or localization of TDP-43 accounts for 90% of ALS cases [163]. TDP-43 is a ribonucleoprotein highly expressed in the CNS with functions in RNA processing [164,165] and its deposition is favored by aging, variations in the TDP-43 gene itself and/or in the C9orf72, GRN or TBK1 genes. [166,167,168,169,170,171]. Not surprisingly, ALS is associated with constitutive activation of microglia and ongoing neuroinflammation [172,173]. The mechanisms involved in the inflammatory component of ALS are diverse and seem to depend on genetic lesion. For instance, TDP-43 or its variants, as cytosolic proteins not trafficking in the ER, do not generate ER stress and/or induce the UPR [174]. By contrast, SOD1 mutant species, although devoid of signal sequence, have been shown to impair ERAD and activate the UPR [175], which itself conceivably contributes to autoinflammation (Figure 4). In addition, SOD1 aggregates are released to activate microglia via binding to TLR2, TLR4 and/or CD14 (Figure 4) [176]. The neuroinflammatory phenotype of individuals with ALS is also thought to be a direct consequence of aggregation of TDP-43 or its mutant in the mitochondria [177,178]. Indeed, a recent study showed that mislocalization of TDP-43 variants in mitochondria results in mitochondrial DNA leakage into the cytosol, subsequent activation of the cGAS/STING innate pathway and IFN signaling in ALS. (Figure 4) [118]. In addition, cytosolic TDP-43 aggregates may promote innate immune responses by activating the inflammasome (Figure 4) [116,117]. Whether these processes occur simultaneously is unclear and their respective contributions to neuroinflammation may be also difficult to assess. In any case, these works reveal that the ability of the cells to react to impaired proteostasis due to protein misfolding is broad and probably even wider than initially assumed.
Figure 4.
Neuroinflammation caused by impaired proteostasis in ALS involves multiple pathways. Pathogenic variants of superoxide dismutase 1 (SOD1) facilitate the formation of intracellular protein aggregates which themselves promote sterile inflammation by causing persistent activation of the unfolded protein response (UPR) via inhibition of the ER-associated degradation machinery (ERAD), as indicated. In addition, extracellular SOD1 protein aggregates exert their pro-inflammatory effects by binding to phagocyte pattern recognition receptors such as Toll-like receptors (TLR)-2, 4 and 6. Pathogenic mutations within the TAR DNA-binding protein 43 (TDP-43) results in protein aggregation which activates the inflammasome and triggers the release of mitochondrial (mt) DNA. The latter, in turn, initiates a type I IFN response following sensing by the cytosolic cyclic GMP-AMP (cGAS) synthase and subsequent STING activation, as indicated. NLRP3: NOD-like receptor pyrin domain-containing-3; ASC: apoptosis associated speck-like protein containing a CARD; pro-CASP1: pro-caspase 1.
3.3. Proteostasis Perturbations Caused by Impaired Protein Degradation
As alluded to earlier, the breakdown of intracellular proteins in eukaryotic cells is ensured by two major conserved machineries from yeast to humans, namely
The ubiquitin–proteasome system (UPS) and;
The autophagy–lysosomal system [179].
The UPS allows the specific elimination of ubiquitin-tagged protein by the 26S proteasome [180]. Ubiquitination is a three-step process involving three groups of enzymes (i.e., E1, E2 and E3) that catalyze the transfer of ubiquitin moieties or chains on lysine, serine, threonine or cysteine residues of target proteins (a modus operandi described in excellent reviews) [181,182]. The 26S proteasome is formed by 20S core complex and a 19S regulatory particle [183,184]. While the 20S core complex contains the three different catalytic activities that permit degradation, the 19S regulatory particle is specialized in the recognition and unfolding of ubiquitin-modified substrates [185]. Specifically, the 20S proteasome is a large complex comprised of two outer α-subunits rings (α1–7) embracing two central head-to-head oriented rings containing β-subunits (β1–7). The catalytic activity is conferred by three of the β subunits, namely β1, β2 and β5 which exhibit caspase-like, trypsin-like and chymotrypsin-like activity, respectively [186]. The 19S regulatory particle is made up of a base containing six ATPase subunits (Rpt1–6) which unfold the protein substrate and a lid carrying subunits (i.e., Rpn10, Rpn13) capable of binding ubiquitin-modified proteins [187]. Importantly, the β1, β2 and β5 standard subunits may be replaced by the so-called β1i, β2i and β5i inducible ones in newly synthetized complexes to promote a switch from constitutive proteasomes to immunoproteasomes [188,189]. It is appreciated that immunoproteasomes are more effective than their standard counterparts at degrading ubiquitin-modified proteins, making them particularly important in situations in which protein homeostasis is challenged such as during infection or oxidative stress [190,191,192,193,194,195]. Due to its unique position at the intersection of multiple pathways [196], the UPS is involved in the regulation of myriad of cellular processes, and as such any dysfunction of one of its components may carry serious consequences on cell functioning or viability.
3.3.1. The UPS and its Dysfunctions
One major cause of proteasome dysfunction is aging [197]. Although not always consistent, investigations in animals and patients suffering from sporadic aging-related neurodegenerative diseases point to a decline ability of the UPS to eliminate undesirable proteins [198,199,200,201,202,203,204,205]. Other causes of UPS impairment are loss-of-function mutations in genes encoding proteasome subunits [35]. Surprisingly, as shown in Table 1, such genomic alterations lead to two clinical distinct phenotypes, namely autoinflammation (CANDLE/PRAAS) and neurodevelopmental disorders (NDD).
Table 1.
Summary of the genetic characteristics and associated clinical phenotypes of the proteasome variants identified so far.
| Proteasome | Gene | Variant | Genetic Model | Origin | Phenotype | Reference |
|---|---|---|---|---|---|---|
| 20S Complex | PSMB1 | p.Y103H | Homozygous, monogenic | Recessive inheritance | NDD | [206] |
| PSMB4 | 5′ UTR: c.–9G > A | Compound heterozygous, monogenic |
Recessive inheritance | PRAAS | [207] | |
| p.D212_V214del | ||||||
| PSMB4 | p.L78Wfs * 31 | Compound heterozygous, monogenic |
Recessive inheritance | PRAAS | [208] | |
| c.494 + 17A > G | ||||||
|
PSMB4/
PSMB8 |
p.Y222 * | Double heterozygous, digenic |
Recessive inheritance | PRAAS | [207] | |
| p.K105Q | ||||||
| PSMB8 | p.G179V | Homozygous, monogenic | Recessive inheritance | PRAAS | [209] | |
| PSMB8 | p.G201V | Homozygous, monogenic | Recessive inheritance | PRAAS | [210] | |
| PSMB8 | p.C135 * | Homozygous, monogenic | Recessive inheritance | PRAAS | [211] | |
| PSMB8 | p.T75M | Homozygous, monogenic | Recessive inheritance | PRAAS | [212] | |
| PSMB8 | p.R125C | Compound heterozygous, monogenic |
Recessive inheritance | PRAAS | [213] | |
| p.D119N | ||||||
| PSMB8 | p.Q55 * | Compound heterozygous, monogenic |
Recessive inheritance | PRAAS | [214] | |
| p.S118P | ||||||
| PSMB8 | p.A92V | Compound heterozygous, monogenic |
Recessive inheritance | PRAAS | [215] | |
| p.K105Q | ||||||
| PSMB8 | p.A92T | Homozygous, monogenic | Recessive inheritance | PRAAS | [216] | |
| PSMB8 | - | Homozygous, monogenic | Recessive inheritance | PRAAS | [217] | |
|
PSMB8/
PSMA3 |
p.T75M | Double heterozygous, digenic |
Recessive inheritance | PRAAS | [207] | |
| p.H111Ffs * 10 | ||||||
|
PSMB8/
PSMA3 |
p.T75M | Double heterozygous, digenic |
Recessive inheritance | PRAAS | [207] | |
| p.R233del | ||||||
|
PSMB9/
PSMB4 |
p.G165D | Double heterozygous, digenic |
Recessive inheritance | PRAAS | [207] | |
| p.P16Sfs * 45 | ||||||
| PSMB9 | p.G156D | Heterozygous, monogenic | de novo, dominant | PRAAS | [218] [219] |
|
| PSMB10 | p.F14S | Homozygous, monogenic | Recessive inheritance | PRAAS | [220] | |
| Assembly Factors |
POMP | p.E115Dfs * 20 | Heterozygous, monogenic | de novo, dominant | PRAAS | [207] |
| POMP | p.F114Lfs * 18 | Heterozygous, monogenic | de novo, dominant | PRAAS | [221] | |
| POMP | p.I112Wfs * 3 | Heterozygous, monogenic | de novo, dominant | PRAAS | [221] | |
| POMP | p.D109Efs * 2 | Heterozygous, monogenic | de novo, dominant | PRAAS | [222] | |
| PSMG4 | p.Y223Sfs * 2 | Compound heterozygous, monogenic | Recessive inheritance | PRAAS | [223] | |
| p.N225K | ||||||
| 19S Complex | PSMD12 | p.R123 * | Heterozygous, monogenic | de novo, dominant | NDD | [224] |
| PSMD12 | p.L425 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.R201 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | c.909−2A > G | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | Deletion | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.R201 * | Heterozygous, monogenic | de novo, dominant | NDD | [225] | |
| PSMD12 | p.R182 * | Heterozygous, monogenic | de novo, dominant | NDD | [68] | |
| PSMD12 | p.R357fs * 3 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.T146Kfs * 3 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.E313 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.Q170Gfs * 40 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.L149 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.Q106 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.Q345 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | c.1083 + 1G > A | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.Q416 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.S176Qfs * 15 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | c.1162−1G > A | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.S434Hfs * 2 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | c.795 + 1G > A | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.L50Gfs * 26 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.T146Kfs * 3 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.R182 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.L354Efs * 6 | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.Y302 * | Heterozygous, monogenic | de novo, dominant | NDD | ||
| PSMD12 | p.R289 * | Heterozygous, monogenic | de novo, dominant | NDD | [226] | |
| PSMC3 | p.S376Rfs15 * | Homozygous, monogenic | Recessive inheritance | NDD | [227] |
* Asterisks indicate termination codons.
3.3.2. Proteasome-Associated Autoinflammatory Syndromes (PRAAS)
From the mid-eighties, an increasing number of cases of rare autoinflammatory syndromes sharing similar clinical features such as lipodystrophy, skin lesions and recurrent fever have been reported. These conditions were referred to by a number of different names including Nakajo–Nishimura syndrome (NNS) [228,229,230], joint contractures, muscle atrophy and panniculitis-induced lipodystrophy (JMP) syndrome [230] and chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperatures (CANDLE) [231,232]. From 2010, it became evident that such syndromes were caused by loss-of-function mutations in genes encoding proteasome subunits and/or proteasome assembly factors and were subsequently renamed proteasome-associated autoinflammatory syndromes (PRAAS) [207,210,211,212,220,221,223,233] (Table 1). Two major hallmarks of PRAAS are the presence of a constitutive type I IFN gene signature in patients’ blood cells and a concomitant increased accumulation of ubiquitin-modified proteins in various cell types including fibroblasts. Recent studies have identified cells of the hematopoietic lineage as decisive mediators of type I IFN in PRAAS [208,234] and in view of increased eIF2α phosphorylation in B cells isolated from PRAAS subjects [221], a possible role of the UPR has been suggested in the induction of autoinflammation in these patients [35]. This notion was supported by in vitro studies showing that blocking the IRE1 arm of the UPR prevents the upregulation of IFN-stimulated genes (ISG) in response to proteasome inhibition in microglia cells [235]. Later studies however have shown that the IL-24/PKR axis of the ISR was the key contributor to type I IFN responses in ten unrelated PRAAS subjects [59,236]. These works unambiguously identify cytosolic IL-24 and PKR as a new DAMP/PRR pair involved in proteostasis surveillance. Whether other mechanisms contribute to sterile inflammation in PRAAS, notably in cells expressing no or low levels of IL-24, is currently not known.
3.3.3. Neurodevelopmental Disorders (NDD) Caused by Proteasome Variants
Surprisingly, the phenotypic spectrum of proteasome loss-of-function mutations is broader than initially assumed. In 2017, the identification of pathogenic variants of the PSMD12 gene in patients showing a predominant neuronal phenotype came as a surprise [224]. Subjects with PSMD12 mutations presented with short stature, facial dysmorphism, intellectual disability, limb anomalies and sometimes absent of speech. Later, similar manifestations were reported in patients carrying variants of the PSMB1 and PSMC3 genes [206,227], thereby confirming the causal relationship between proteasome loss-of-function and NDD. One particularly intriguing observation from these studies is that, in contrast to PRAAS patients, subjects with NDD were devoid of clinical signs of autoinflammation. However, recent investigation revealed that leukocytes isolated from NDD patients with PSMD12 haploinsufficiency do exhibit a typical type I IFN gene signature [68]. The reason why the upregulation of ISG in these cells is not associated with the development of clinical autoinflammation as observed in PRAAS patients is obscure, but this phenomenon has been described in other interferonopathies [237,238,239,240]. Interestingly, it was shown that PSMD12 haploinsufficiency was accompanied by a profound remodeling of mTORC1 and its downstream autophagy pathway. Specifically, the elimination of mitochondria by mitophagy in PSMD12-deficient cells was induced [68].
3.3.4. Disorders due to Deficient DUB and/or E3 Ubiquitin Ligases
A number of autoinflammatory syndromes have been described to be caused by genomic alterations in genes coding for DUB and E3 ubiquitin ligases. These include notably loss- or gain-of-function mutations in the TNFAIP3, USP18 and OTULIN, RNF31, RBCK1 genes [241,242,243,244,245,246,247]. However, in contrast to their proteasome counterparts, pathogenic variants from DUB and/or E3 ubiquitin ligases exert their proinflammatory effects mostly by interfering with specific PRR signaling cascades rather than by destabilizing the whole-cell proteome [248].
3.3.5. The Autophagy-Lysosomal System and Its Defects
The 2016 Nobel Prize in Physiology or Medicine went to Yoshinori Ohsumi for his discoveries of the “mechanisms of autophagy” [249,250]. Together with the UPS, autophagy is the main contributor to protein breakdown in the cell. Unlike the UPS, which is constitutive active, autophagy requires signals such as starvation, growth, etc. It relies on the formation of autophagosomes capable of sequestering intracellular material prior to delivery to lysosomes for subsequent degradation. Over the past few years, various types of autophagy have been described including micro-autophagy and macro-autophagy [251], the latter enabling the elimination of protein aggregates [252], a process frequently referred to as “aggrephagy” [253]. In this selective form of autophagy, ubiquitin-modified protein aggregates are recognized by the autophagy receptors p62, NBR1 and OPTN which themselves interact with lipidated LC3II on the inner phagophore membrane [254], thereby targeting them to autophagosomes. As such, protein homeostasis critically depends on functional autophagy under stress conditions and any and loss of autophagy has been early associated with the aggregation of abnormal proteins and neurodegeneration [255].
Another major cause of autophagy dysfunction and a fortiori protein homeostasis perturbations are loss-of-function mutations in genes encoding any one of the many components involved in this process. A particularly vulnerable gene in this regard is SQSTM1 encoding the autophagy receptor p62 and whose pathogenic variants are associated with the onset of various metabolic, myopathic and skeletal disorders as well as neurodegenerative disease including ALS. Strikingly, syndromes caused by SQSTM1 disruption are clearly associated with signs of autoinflammation [256,257,258]. However, given that p62 is also directly involved in the regulation of NF-κB signaling [259,260], the precise contribution of impaired aggrephagy to inflammation in these diseases is difficult to assess. Other ALS genes potentially causing imbalanced protein homeostasis include ATG5 and ATG7 [261,262]. Although clearly associated with protein aggregates, no inflammation was observed in these patients.
4. Conclusions and Future Directions
Altogether, these studies revealed that eukaryotic cells are equipped with various sensitive systems capable of sensing quantitative aberrations of their intra- and extracellular proteomes. Strikingly, these sensors are more or less directly connected to innate signaling pathways, and the continuous sampling of proteostasis perturbations consistently results in autoinflammation. The reason why the cell mostly responds to impaired proteostasis by the production of proinflammatory mediators is however not fully understood. One possible explanation is that inflammation might be part of autocrine program primarily aimed to help restoring proteostasis. This hypothesis is particularly based on the fact that many of the target genes (i.e., ISG) of type I IFN encode products that act on the proteostasis network at various levels. For instance, type I IFN limits the proteostatic burden by supporting a translation arrest via the induction of PKR. Conversely, by inducing immunoproteasome subunits and proteasome activators [263], type I IFN accelerates protein breakdown and as such help the cells to cope with proteotoxic stress. Given that inflammation has proliferation-promotive effects [264], it might also well be that the initiation of innate responses under these circumstances is intended to preserve integrity by generating new cellular space for the accumulating protein aggregates. Herein, when limited in time, sterile inflammation might represent a beneficial reaction rebalancing dysregulated proteostasis. However, the onset of sterile autoinflammation or neuroinflammation in patients with impaired proteostasis might signify a point of no return from which protein homeostasis cannot be rescued any longer. In this regard, a better comprehension of the mechanisms driving these processes is needed.
Author Contributions
Conceptualization: J.J.P., E.K. and F.E.; data curation: J.J.P., E.K. and F.E.; writing—original draft preparation: J.J.P. and F.E., writing—review and editing: E.K. and F.E.; funding acquisition: E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the German Research Foundation (DFG) Research Training Group (RTG) 2719 Pro to E.K. We also acknowledge support for the Article Processing Charge from the DFG and the Open Access Publication Fund of the University of Greifswald.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.A Series of Essays on Inflammation and Its Varieties. Essay, I. The Natural History of the Disease. Med. Chir. Rev. 1846;4:251–253. [PMC free article] [PubMed] [Google Scholar]
- 2.Plytycz B., Seljelid R. From inflammation to sickness: Historical perspective. Arch. Immunol. Exp. (Warsz) 2003;51:105–109. [PubMed] [Google Scholar]
- 3.Cavaillon J.M. Once upon a time, inflammation. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021;27:e20200147. doi: 10.1590/1678-9199-jvatitd-2020-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diamond G., Legarda D., Ryan L.K. The innate immune response of the respiratory epithelium. Immunol. Rev. 2000;173:27–38. doi: 10.1034/j.1600-065X.2000.917304.x. [DOI] [PubMed] [Google Scholar]
- 5.Shishido S.N., Varahan S., Yuan K., Li X., Fleming S.D. Humoral innate immune response and disease. Clin. Immunol. 2012;144:142–158. doi: 10.1016/j.clim.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenderman L., Buurman W., Daha M.R. The innate immune response. Immunol. Lett. 2014;162:95–102. doi: 10.1016/j.imlet.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Bianchi M.E. DAMPs, PAMPs and alarmins: All we need to know about danger. J. Leukoc. Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- 8.Skoberne M., Beignon A.S., Bhardwaj N. Danger signals: A time and space continuum. Trends Mol. Med. 2004;10:251–257. doi: 10.1016/j.molmed.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Kawai T., Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Sok S.P.M., Ori D., Nagoor N.H., Kawai T. Sensing Self and Non-Self DNA by Innate Immune Receptors and Their Signaling Pathways. Crit. Rev. Immunol. 2018;38:279–301. doi: 10.1615/CritRevImmunol.2018026540. [DOI] [PubMed] [Google Scholar]
- 11.Thaiss C.A., Levy M., Itav S., Elinav E. Integration of Innate Immune Signaling. Trends Immunol. 2016;37:84–101. doi: 10.1016/j.it.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int. Immunol. 2009;21:317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Capobianchi M.R., Uleri E., Caglioti C., Dolei A. Type I IFN family members: Similarity, differences and interaction. Cytokine Growth Factor Rev. 2015;26:103–111. doi: 10.1016/j.cytogfr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoggins J.W. Interferon-Stimulated Genes: What Do They All Do? Annu Rev. Virol. 2019;6:567–584. doi: 10.1146/annurev-virology-092818-015756. [DOI] [PubMed] [Google Scholar]
- 15.Nace G., Evankovich J., Eid R., Tsung A. Dendritic cells and damage-associated molecular patterns: Endogenous danger signals linking innate and adaptive immunity. J. Innate Immun. 2012;4:6–15. doi: 10.1159/000334245. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer L. Complexity of danger: The diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014;289:35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jounai N., Kobiyama K., Takeshita F., Ishii K.J. Recognition of damage-associated molecular patterns related to nucleic acids during inflammation and vaccination. Front. Cell Infect. Microbiol. 2012;2:168. doi: 10.3389/fcimb.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Denning N.L., Aziz M., Gurien S.D., Wang P. DAMPs and NETs in Sepsis. Front. Immunol. 2019;10:2536. doi: 10.3389/fimmu.2019.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 20.Allam R., Darisipudi M.N., Tschopp J., Anders H.J. Histones trigger sterile inflammation by activating the NLRP3 inflammasome. Eur. J. Immunol. 2013;43:3336–3342. doi: 10.1002/eji.201243224. [DOI] [PubMed] [Google Scholar]
- 21.Chen G.Y., Nunez G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mihm S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int. J. Mol. Sci. 2018;19:3104. doi: 10.3390/ijms19103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- 24.Feldman N., Rotter-Maskowitz A., Okun E. DAMPs as mediators of sterile inflammation in aging-related pathologies. Ageing Res. Rev. 2015;24:29–39. doi: 10.1016/j.arr.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Franklin E.C., Holman H.R., Muller-Eberhard H.J., Kunkel H.G. An unusual protein component of high molecular weight in the serum of certain patients with rheumatoid arthritis. J. Exp. Med. 1957;105:425–438. doi: 10.1084/jem.105.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mellors R.C., Heimer R., Corcos J., Korngold L. Cellular Origin of Rheumatoid Factor. J. Exp. Med. 1959;110:875–886. doi: 10.1084/jem.110.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blom A.B., Radstake T.R., Holthuysen A.E., Sloetjes A.W., Pesman G.J., Sweep F.G., van de Loo F.A., Joosten L.A., Barrera P., van Lent P.L., et al. Increased expression of Fcgamma receptors II and III on macrophages of rheumatoid arthritis patients results in higher production of tumor necrosis factor alpha and matrix metalloproteinase. Arthritis Rheum. 2003;48:1002–1014. doi: 10.1002/art.10871. [DOI] [PubMed] [Google Scholar]
- 28.Radstake T.R., Blom A.B., Sloetjes A.W., van Gorselen E.O., Pesman G.J., Engelen L., Torensma R., van den Berg W.B., Figdor C.G., van Lent P.L., et al. Increased FcgammaRII expression and aberrant tumour necrosis factor alpha production by mature dendritic cells from patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2004;63:1556–1563. doi: 10.1136/ard.2003.016550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brehm A., Kruger E. Dysfunction in protein clearance by the proteasome: Impact on autoinflammatory diseases. Semin. Immunopathol. 2015;37:323–333. doi: 10.1007/s00281-015-0486-4. [DOI] [PubMed] [Google Scholar]
- 30.Uhlen M., Fagerberg L., Hallstrom B.M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 31.Anton L.C., Yewdell J.W. Translating DRiPs: MHC class I immunosurveillance of pathogens and tumors. J. Leukoc. Biol. 2014;95:551–562. doi: 10.1189/jlb.1113599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mancini R., Fagioli C., Fra A.M., Maggioni C., Sitia R. Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: Evidence that retrotranslocation and degradation are coupled events. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000;14:769–778. doi: 10.1096/fasebj.14.5.769. [DOI] [PubMed] [Google Scholar]
- 33.Chillaron J., Haas I.G. Dissociation from BiP and retrotranslocation of unassembled immunoglobulin light chains are tightly coupled to proteasome activity. Mol. Biol. Cell. 2000;11:217–226. doi: 10.1091/mbc.11.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayer T.U., Braun T., Jentsch S. Role of the proteasome in membrane extraction of a short-lived ER-transmembrane protein. EMBO J. 1998;17:3251–3257. doi: 10.1093/emboj/17.12.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ebstein F., Poli Harlowe M.C., Studencka-Turski M., Kruger E. Contribution of the Unfolded Protein Response (UPR) to the Pathogenesis of Proteasome-Associated Autoinflammatory Syndromes (PRAAS) Front. Immunol. 2019;10:2756. doi: 10.3389/fimmu.2019.02756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 37.Dekker J., Strous G.J. Covalent oligomerization of rat gastric mucin occurs in the rough endoplasmic reticulum, is N-glycosylation-dependent, and precedes initial O-glycosylation. J. Biol. Chem. 1990;265:18116–18122. doi: 10.1016/S0021-9258(17)44725-9. [DOI] [PubMed] [Google Scholar]
- 38.Pahl H.L., Baeuerle P.A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grootjans J., Kaser A., Kaufman R.J., Blumberg R.S. The unfolded protein response in immunity and inflammation. Nat. Rev. Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaud M., Philippe C., Di Bella D., Tang W., Pyronnet S., Laurell H., Mazzolini L., Rouault-Pierre K., Touriol C. Translational Regulations in Response to Endoplasmic Reticulum Stress in Cancers. Cells. 2020;9:540. doi: 10.3390/cells9030540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Deng J., Lu P.D., Zhang Y., Scheuner D., Kaufman R.J., Sonenberg N., Harding H.P., Ron D. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol. Cell. Biol. 2004;24:10161–10168. doi: 10.1128/MCB.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaneko M., Niinuma Y., Nomura Y. Activation signal of nuclear factor-kappa B in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biol. Pharm. Bull. 2003;26:931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 43.Suraweera A., Munch C., Hanssum A., Bertolotti A. Failure of amino acid homeostasis causes cell death following proteasome inhibition. Mol. Cell. 2012;48:242–253. doi: 10.1016/j.molcel.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong J., Qiu H., Garcia-Barrio M., Anderson J., Hinnebusch A.G. Uncharged tRNA activates GCN2 by displacing the protein kinase moiety from a bipartite tRNA-binding domain. Mol. Cell. 2000;6:269–279. doi: 10.1016/S1097-2765(00)00028-9. [DOI] [PubMed] [Google Scholar]
- 45.Harding H.P., Novoa I., Zhang Y., Zeng H., Wek R., Schapira M., Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/S1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 46.Dar A.C., Dever T.E., Sicheri F. Higher-order substrate recognition of eIF2alpha by the RNA-dependent protein kinase PKR. Cell. 2005;122:887–900. doi: 10.1016/j.cell.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 47.Clemens M.J., Hershey J.W., Hovanessian A.C., Jacobs B.C., Katze M.G., Kaufman R.J., Lengyel P., Samuel C.E., Sen G.C., Williams B.R. PKR: Proposed nomenclature for the RNA-dependent protein kinase induced by interferon. J. Interferon Res. 1993;13:241. doi: 10.1089/jir.1993.13.241. [DOI] [PubMed] [Google Scholar]
- 48.Meurs E., Chong K., Galabru J., Thomas N.S., Kerr I.M., Williams B.R., Hovanessian A.G. Molecular cloning and characterization of the human double-stranded RNA-activated protein kinase induced by interferon. Cell. 1990;62:379–390. doi: 10.1016/0092-8674(90)90374-N. [DOI] [PubMed] [Google Scholar]
- 49.Gilfoy F.D., Mason P.W. West Nile virus-induced interferon production is mediated by the double-stranded RNA-dependent protein kinase PKR. J. Virol. 2007;81:11148–11158. doi: 10.1128/JVI.00446-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barry G., Breakwell L., Fragkoudis R., Attarzadeh-Yazdi G., Rodriguez-Andres J., Kohl A., Fazakerley J.K. PKR acts early in infection to suppress Semliki Forest virus production and strongly enhances the type I interferon response. J. Gen. Virol. 2009;90:1382–1391. doi: 10.1099/vir.0.007336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McAllister C.S., Toth A.M., Zhang P., Devaux P., Cattaneo R., Samuel C.E. Mechanisms of protein kinase PKR-mediated amplification of beta interferon induction by C protein-deficient measles virus. J. Virol. 2010;84:380–386. doi: 10.1128/JVI.02630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulz O., Pichlmair A., Rehwinkel J., Rogers N.C., Scheuner D., Kato H., Takeuchi O., Akira S., Kaufman R.J., Reis e Sousa C. Protein kinase R contributes to immunity against specific viruses by regulating interferon mRNA integrity. Cell Host Microbe. 2010;7:354–361. doi: 10.1016/j.chom.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh M., Fowlkes V., Handy I., Patel C.V., Patel R.C. Essential role of PACT-mediated PKR activation in tunicamycin-induced apoptosis. J. Mol. Biol. 2009;385:457–468. doi: 10.1016/j.jmb.2008.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pyo C.W., Lee S.H., Choi S.Y. Oxidative stress induces PKR-dependent apoptosis via IFN-gamma activation signaling in Jurkat T cells. Biochem. Biophys. Res. Commun. 2008;377:1001–1006. doi: 10.1016/j.bbrc.2008.10.103. [DOI] [PubMed] [Google Scholar]
- 55.Murtha-Riel P., Davies M.V., Scherer B.J., Choi S.Y., Hershey J.W., Kaufman R.J. Expression of a phosphorylation-resistant eukaryotic initiation factor 2 alpha-subunit mitigates heat shock inhibition of protein synthesis. J. Biol. Chem. 1993;268:12946–12951. doi: 10.1016/S0021-9258(18)31477-7. [DOI] [PubMed] [Google Scholar]
- 56.Ito T., Yang M., May W.S. RAX, a cellular activator for double-stranded RNA-dependent protein kinase during stress signaling. J. Biol. Chem. 1999;274:15427–15432. doi: 10.1074/jbc.274.22.15427. [DOI] [PubMed] [Google Scholar]
- 57.Patel C.V., Handy I., Goldsmith T., Patel R.C. PACT, a stress-modulated cellular activator of interferon-induced double-stranded RNA-activated protein kinase, PKR. J. Biol. Chem. 2000;275:37993–37998. doi: 10.1074/jbc.M004762200. [DOI] [PubMed] [Google Scholar]
- 58.Ito T., Warnken S.P., May W.S. Protein synthesis inhibition by flavonoids: Roles of eukaryotic initiation factor 2alpha kinases. Biochem. Biophys. Res. Commun. 1999;265:589–594. doi: 10.1006/bbrc.1999.1727. [DOI] [PubMed] [Google Scholar]
- 59.Davidson S., Yu C.H., Steiner A., Ebstein F., Baker P.J., Jarur-Chamy V., Hrovat Schaale K., Laohamonthonkul P., Kong K., Calleja D.J., et al. Protein kinase R is an innate immune sensor of proteotoxic stress via accumulation of cytoplasmic IL-24. Sci. Immunol. 2022;7:eabi6763. doi: 10.1126/sciimmunol.abi6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gal-Ben-Ari S., Barrera I., Ehrlich M., Rosenblum K. PKR: A Kinase to Remember. Front. Mol. Neurosci. 2018;11:480. doi: 10.3389/fnmol.2018.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchal J.A., Lopez G.J., Peran M., Comino A., Delgado J.R., Garcia-Garcia J.A., Conde V., Aranda F.M., Rivas C., Esteban M., et al. The impact of PKR activation: From neurodegeneration to cancer. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014;28:1965–1974. doi: 10.1096/fj.13-248294. [DOI] [PubMed] [Google Scholar]
- 63.Chukwurah E., Farabaugh K.T., Guan B.J., Ramakrishnan P., Hatzoglou M. A tale of two proteins: PACT and PKR and their roles in inflammation. FEBS J. 2021 doi: 10.1111/febs.15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ingrand S., Barrier L., Lafay-Chebassier C., Fauconneau B., Page G., Hugon J. The oxindole/imidazole derivative C16 reduces in vivo brain PKR activation. FEBS Lett. 2007;581:4473–4478. doi: 10.1016/j.febslet.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 65.Goberdhan D.C., Wilson C., Harris A.L. Amino Acid Sensing by mTORC1: Intracellular Transporters Mark the Spot. Cell Metab. 2016;23:580–589. doi: 10.1016/j.cmet.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Broer S., Broer A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017;474:1935–1963. doi: 10.1042/BCJ20160822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Condon K.J., Sabatini D.M. Nutrient regulation of mTORC1 at a glance. J. Cell Sci. 2019;132 doi: 10.1242/jcs.222570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Isidor B., Ebstein F., Hurst A., Vincent M., Bader I., Rudy N.L., Cogne B., Mayr J., Brehm A., Bupp C., et al. Stankiewicz-Isidor syndrome: Expanding the clinical and molecular phenotype. Genet. Med. 2021 doi: 10.1016/j.gim.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 69.York A.G., Williams K.J., Argus J.P., Zhou Q.D., Brar G., Vergnes L., Gray E.E., Zhen A., Wu N.C., Yamada D.H., et al. Limiting Cholesterol Biosynthetic Flux Spontaneously Engages Type I IFN Signaling. Cell. 2015;163:1716–1729. doi: 10.1016/j.cell.2015.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Soto-Heredero G., Gomez de Las Heras M.M., Gabande-Rodriguez E., Oller J., Mittelbrunn M. Glycolysis—A key player in the inflammatory response. FEBS J. 2020;287:3350–3369. doi: 10.1111/febs.15327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allaman I., Gavillet M., Belanger M., Laroche T., Viertl D., Lashuel H.A., Magistretti P.J. Amyloid-beta aggregates cause alterations of astrocytic metabolic phenotype: Impact on neuronal viability. J. Neurosci. 2010;30:3326–3338. doi: 10.1523/JNEUROSCI.5098-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ulland T.K., Song W.M., Huang S.C., Ulrich J.D., Sergushichev A., Beatty W.L., Loboda A.A., Zhou Y., Cairns N.J., Kambal A., et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell. 2017;170:649–663.e13. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Taylor J.P., Brown R.H., Jr., Cleveland D.W. Decoding ALS: From genes to mechanism. Nature. 2016;539:197–206. doi: 10.1038/nature20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramaswami M., Taylor J.P., Parker R. Altered ribostasis: RNA-protein granules in degenerative disorders. Cell. 2013;154:727–736. doi: 10.1016/j.cell.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y.R., King O.D., Shorter J., Gitler A.D. Stress granules as crucibles of ALS pathogenesis. J. Cell Biol. 2013;201:361–372. doi: 10.1083/jcb.201302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buchan J.R., Parker R. Eukaryotic stress granules: The ins and outs of translation. Mol. Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ash P.E., Vanderweyde T.E., Youmans K.L., Apicco D.J., Wolozin B. Pathological stress granules in Alzheimer’s disease. Brain Res. 2014;1584:52–58. doi: 10.1016/j.brainres.2014.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vanderweyde T., Apicco D.J., Youmans-Kidder K., Ash P.E.A., Cook C., Lummertz da Rocha E., Jansen-West K., Frame A.A., Citro A., Leszyk J.D., et al. Interaction of tau with the RNA-Binding Protein TIA1 Regulates tau Pathophysiology and Toxicity. Cell Rep. 2016;15:1455–1466. doi: 10.1016/j.celrep.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McAleese K.E., Walker L., Erskine D., Thomas A.J., McKeith I.G., Attems J. TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol. 2017;27:472–479. doi: 10.1111/bpa.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.St-Amour I., Turgeon A., Goupil C., Planel E., Hebert S.S. Co-occurrence of mixed proteinopathies in late-stage Huntington’s disease. Acta Neuropathol. 2018;135:249–265. doi: 10.1007/s00401-017-1786-7. [DOI] [PubMed] [Google Scholar]
- 81.Coudert L., Nonaka T., Bernard E., Hasegawa M., Schaeffer L., Leblanc P. Phosphorylated and aggregated TDP-43 with seeding properties are induced upon mutant Huntingtin (mHtt) polyglutamine expression in human cellular models. Cell Mol. Life Sci. 2019;76:2615–2632. doi: 10.1007/s00018-019-03059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryan V.H., Fawzi N.L. Physiological, Pathological, and Targetable Membraneless Organelles in Neurons. Trends Neurosci. 2019;42:693–708. doi: 10.1016/j.tins.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wolozin B., Ivanov P. Stress granules and neurodegeneration. Nat. Rev. Neurosci. 2019;20:649–666. doi: 10.1038/s41583-019-0222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dudman J., Qi X. Stress Granule Dysregulation in Amyotrophic Lateral Sclerosis. Front. Cell Neurosci. 2020;14:598517. doi: 10.3389/fncel.2020.598517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hofmann S., Kedersha N., Anderson P., Ivanov P. Molecular mechanisms of stress granule assembly and disassembly. Biochim Biophys Acta Mol. Cell Res. 2021;1868:118876. doi: 10.1016/j.bbamcr.2020.118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang P., Mathieu C., Kolaitis R.M., Zhang P., Messing J., Yurtsever U., Yang Z., Wu J., Li Y., Pan Q., et al. G3BP1 Is a Tunable Switch that Triggers Phase Separation to Assemble Stress Granules. Cell. 2020;181:325–345.e28. doi: 10.1016/j.cell.2020.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sanders D.W., Kedersha N., Lee D.S.W., Strom A.R., Drake V., Riback J.A., Bracha D., Eeftens J.M., Iwanicki A., Wang A., et al. Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell. 2020;181:306–324.e28. doi: 10.1016/j.cell.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guillen-Boixet J., Kopach A., Holehouse A.S., Wittmann S., Jahnel M., Schlussler R., Kim K., Trussina I., Wang J., Mateju D., et al. RNA-Induced Conformational Switching and Clustering of G3BP Drive Stress Granule Assembly by Condensation. Cell. 2020;181:346–361.e17. doi: 10.1016/j.cell.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pakos-Zebrucka K., Koryga I., Mnich K., Ljujic M., Samali A., Gorman A.M. The integrated stress response. EMBO Rep. 2016;17:1374–1395. doi: 10.15252/embr.201642195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazroui R., Di Marco S., Kaufman R.J., Gallouzi I.E. Inhibition of the ubiquitin-proteasome system induces stress granule formation. Mol. Biol. Cell. 2007;18:2603–2618. doi: 10.1091/mbc.e06-12-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mateju D., Franzmann T.M., Patel A., Kopach A., Boczek E.E., Maharana S., Lee H.O., Carra S., Hyman A.A., Alberti S. An aberrant phase transition of stress granules triggered by misfolded protein and prevented by chaperone function. EMBO J. 2017;36:1669–1687. doi: 10.15252/embj.201695957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rao L., Xu Y., Reineke L.C., Bhattacharya A., Tyryshkin A., Shin J.N., Eissa N.T. Post-Transcriptional Regulation of Alpha One Antitrypsin by a Proteasome Inhibitor. Int. J. Mol. Sci. 2020;21:4318. doi: 10.3390/ijms21124318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oh S.W., Onomoto K., Wakimoto M., Onoguchi K., Ishidate F., Fujiwara T., Yoneyama M., Kato H., Fujita T. Leader-Containing Uncapped Viral Transcript Activates RIG-I in Antiviral Stress Granules. PLoS Pathog. 2016;12:e1005444. doi: 10.1371/journal.ppat.1005444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Onomoto K., Jogi M., Yoo J.S., Narita R., Morimoto S., Takemura A., Sambhara S., Kawaguchi A., Osari S., Nagata K., et al. Critical role of an antiviral stress granule containing RIG-I and PKR in viral detection and innate immunity. PLoS ONE. 2012;7:e43031. doi: 10.1371/annotation/dcd836ee-9e23-4538-acb7-450560ba5c1d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoneyama M., Jogi M., Onomoto K. Regulation of antiviral innate immune signaling by stress-induced RNA granules. J. Biochem. 2016;159:279–286. doi: 10.1093/jb/mvv122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu S., Sun H., Yin L., Li J., Mei S., Xu F., Wu C., Liu X., Zhao F., Zhang D., et al. PKR-dependent cytosolic cGAS foci are necessary for intracellular DNA sensing. Sci Signal. 2019;12 doi: 10.1126/scisignal.aav7934. [DOI] [PubMed] [Google Scholar]
- 97.Liu Z.S., Cai H., Xue W., Wang M., Xia T., Li W.J., Xing J.Q., Zhao M., Huang Y.J., Chen S., et al. G3BP1 promotes DNA binding and activation of cGAS. Nat. Immunol. 2019;20:18–28. doi: 10.1038/s41590-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim S.S., Sze L., Liu C., Lam K.P. The stress granule protein G3BP1 binds viral dsRNA and RIG-I to enhance interferon-beta response. J. Biol. Chem. 2019;294:6430–6438. doi: 10.1074/jbc.RA118.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Radhakrishnan S.K., Lee C.S., Young P., Beskow A., Chan J.Y., Deshaies R.J. Transcription factor Nrf1 mediates the proteasome recovery pathway after proteasome inhibition in mammalian cells. Mol. Cell. 2010;38:17–28. doi: 10.1016/j.molcel.2010.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Steffen J., Seeger M., Koch A., Kruger E. Proteasomal degradation is transcriptionally controlled by TCF11 via an ERAD-dependent feedback loop. Mol. Cell. 2010;40:147–158. doi: 10.1016/j.molcel.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 101.Tomlin F.M., Gerling-Driessen U.I.M., Liu Y.C., Flynn R.A., Vangala J.R., Lentz C.S., Clauder-Muenster S., Jakob P., Mueller W.F., Ordonez-Rueda D., et al. Inhibition of NGLY1 Inactivates the Transcription Factor Nrf1 and Potentiates Proteasome Inhibitor Cytotoxicity. ACS Cent. Sci. 2017;3:1143–1155. doi: 10.1021/acscentsci.7b00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koizumi S., Irie T., Hirayama S., Sakurai Y., Yashiroda H., Naguro I., Ichijo H., Hamazaki J., Murata S. The aspartyl protease DDI2 activates Nrf1 to compensate for proteasome dysfunction. eLife. 2016;5 doi: 10.7554/eLife.18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang K., Huang R., Fujihira H., Suzuki T., Yan N. N-glycanase NGLY1 regulates mitochondrial homeostasis and inflammation through NRF1. J. Exp. Med. 2018;215:2600–2616. doi: 10.1084/jem.20180783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Widenmaier S.B., Snyder N.A., Nguyen T.B., Arduini A., Lee G.Y., Arruda A.P., Saksi J., Bartelt A., Hotamisligil G.S. NRF1 Is an ER Membrane Sensor that Is Central to Cholesterol Homeostasis. Cell. 2017;171:1094–1109.e15. doi: 10.1016/j.cell.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 105.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like receptor 4 is required for alpha-synuclein dependent activation of microglia and astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rannikko E.H., Weber S.S., Kahle P.J. Exogenous alpha-synuclein induces toll-like receptor 4 dependent inflammatory responses in astrocytes. BMC Neurosci. 2015;16:57. doi: 10.1186/s12868-015-0192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stefanova N., Fellner L., Reindl M., Masliah E., Poewe W., Wenning G.K. Toll-like receptor 4 promotes alpha-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 2011;179:954–963. doi: 10.1016/j.ajpath.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim C., Ho D.H., Suk J.E., You S., Michael S., Kang J., Joong Lee S., Masliah E., Hwang D., Lee H.J., et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nat. Commun. 2013;4:1562. doi: 10.1038/ncomms2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dzamko N., Gysbers A., Perera G., Bahar A., Shankar A., Gao J., Fu Y., Halliday G.M. Toll-like receptor 2 is increased in neurons in Parkinson’s disease brain and may contribute to alpha-synuclein pathology. Acta Neuropathol. 2017;133:303–319. doi: 10.1007/s00401-016-1648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim C., Rockenstein E., Spencer B., Kim H.K., Adame A., Trejo M., Stafa K., Lee H.J., Lee S.J., Masliah E. Antagonizing Neuronal Toll-like Receptor 2 Prevents Synucleinopathy by Activating Autophagy. Cell Rep. 2015;13:771–782. doi: 10.1016/j.celrep.2015.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang Y., Cella M., Mallinson K., Ulrich J.D., Young K.L., Robinette M.L., Gilfillan S., Krishnan G.M., Sudhakar S., Zinselmeyer B.H., et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160:1061–1071. doi: 10.1016/j.cell.2015.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lehnardt S., Massillon L., Follett P., Jensen F.E., Ratan R., Rosenberg P.A., Volpe J.J., Vartanian T. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc. Natl. Acad. Sci. USA. 2003;100:8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stewart C.R., Stuart L.M., Wilkinson K., van Gils J.M., Deng J., Halle A., Rayner K.J., Boyer L., Zhong R., Frazier W.A., et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu S., Liu Y., Hao W., Wolf L., Kiliaan A.J., Penke B., Rube C.E., Walter J., Heneka M.T., Hartmann T., et al. TLR2 is a primary receptor for Alzheimer’s amyloid beta peptide to trigger neuroinflammatory activation. J. Immunol. 2012;188:1098–1107. doi: 10.4049/jimmunol.1101121. [DOI] [PubMed] [Google Scholar]
- 115.Scholtzova H., Chianchiano P., Pan J., Sun Y., Goni F., Mehta P.D., Wisniewski T. Amyloid beta and Tau Alzheimer’s disease related pathology is reduced by Toll-like receptor 9 stimulation. Acta Neuropathol. Commun. 2014;2:101. doi: 10.1186/s40478-014-0101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhao W., Beers D.R., Bell S., Wang J., Wen S., Baloh R.H., Appel S.H. TDP-43 activates microglia through NF-kappaB and NLRP3 inflammasome. Exp. Neurol. 2015;273:24–35. doi: 10.1016/j.expneurol.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 117.Kadhim H., Deltenre P., Martin J.J., Sebire G. In-situ expression of Interleukin-18 and associated mediators in the human brain of sALS patients: Hypothesis for a role for immune-inflammatory mechanisms. Med. Hypotheses. 2016;86:14–17. doi: 10.1016/j.mehy.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 118.Yu C.H., Davidson S., Harapas C.R., Hilton J.B., Mlodzianoski M.J., Laohamonthonkul P., Louis C., Low R.R.J., Moecking J., De Nardo D., et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell. 2020;183:636–649.e18. doi: 10.1016/j.cell.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kampen K.R., Sulima S.O., Vereecke S., De Keersmaecker K. Hallmarks of ribosomopathies. Nucleic Acids Res. 2020;48:1013–1028. doi: 10.1093/nar/gkz637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berman I.R., Iliescu H., Stachura I. Pulmonary effects of blood container materials. Surg. Forum. 1977;28:182–184. [PubMed] [Google Scholar]
- 121.Da Costa L., Leblanc T., Mohandas N. Diamond-Blackfan anemia. Blood. 2020;136:1262–1273. doi: 10.1182/blood.2019000947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ellis S.R. Nucleolar stress in Diamond Blackfan anemia pathophysiology. Biochim. Biophys. Acta. 2014;1842:765–768. doi: 10.1016/j.bbadis.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 123.Chakraborty A., Uechi T., Kenmochi N. Guarding the ‘translation apparatus’: Defective ribosome biogenesis and the p53 signaling pathway. Wiley Interdiscip. Rev. RNA. 2011;2:507–522. doi: 10.1002/wrna.73. [DOI] [PubMed] [Google Scholar]
- 124.Kang J., Brajanovski N., Chan K.T., Xuan J., Pearson R.B., Sanij E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal. Transduct. Target. 2021;6:323. doi: 10.1038/s41392-021-00728-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kapralova K., Jahoda O., Koralkova P., Gursky J., Lanikova L., Pospisilova D., Divoky V., Horvathova M. Oxidative DNA Damage, Inflammatory Signature, and Altered Erythrocytes Properties in Diamond-Blackfan Anemia. Int. J. Mol. Sci. 2020;21:9652. doi: 10.3390/ijms21249652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pesciotta E.N., Lam H.S., Kossenkov A., Ge J., Showe L.C., Mason P.J., Bessler M., Speicher D.W. In-Depth, Label-Free Analysis of the Erythrocyte Cytoplasmic Proteome in Diamond Blackfan Anemia Identifies a Unique Inflammatory Signature. PLoS ONE. 2015;10:e0140036. doi: 10.1371/journal.pone.0140036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gazda H.T., Kho A.T., Sanoudou D., Zaucha J.M., Kohane I.S., Sieff C.A., Beggs A.H. Defective ribosomal protein gene expression alters transcription, translation, apoptosis, and oncogenic pathways in Diamond-Blackfan anemia. Stem Cells. 2006;24:2034–2044. doi: 10.1634/stemcells.2005-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Danilova N., Wilkes M., Bibikova E., Youn M.Y., Sakamoto K.M., Lin S. Innate immune system activation in zebrafish and cellular models of Diamond Blackfan Anemia. Sci. Rep. 2018;8:5165. doi: 10.1038/s41598-018-23561-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Girardi T., Vereecke S., Sulima S.O., Khan Y., Fancello L., Briggs J.W., Schwab C., de Beeck J.O., Verbeeck J., Royaert J., et al. The T-cell leukemia-associated ribosomal RPL10 R98S mutation enhances JAK-STAT signaling. Leukemia. 2018;32:809–819. doi: 10.1038/leu.2017.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dou Z., Ghosh K., Vizioli M.G., Zhu J., Sen P., Wangensteen K.J., Simithy J., Lan Y., Lin Y., Zhou Z., et al. Cytoplasmic chromatin triggers inflammation in senescence and cancer. Nature. 2017;550:402–406. doi: 10.1038/nature24050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frisch S.M., MacFawn I.P. Type I interferons and related pathways in cell senescence. Aging Cell. 2020;19:e13234. doi: 10.1111/acel.13234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wan L., Juszkiewicz S., Blears D., Bajpe P.K., Han Z., Faull P., Mitter R., Stewart A., Snijders A.P., Hegde R.S., et al. Translation stress and collided ribosomes are co-activators of cGAS. Mol. Cell. 2021;81:2808–2822.e10. doi: 10.1016/j.molcel.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yewdell J.W., Nicchitta C.V. The DRiP hypothesis decennial: Support, controversy, refinement and extension. Trends Immunol. 2006;27:368–373. doi: 10.1016/j.it.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 134.Schubert U., Anton L.C., Gibbs J., Norbury C.C., Yewdell J.W., Bennink J.R. Rapid degradation of a large fraction of newly synthesized proteins by proteasomes. Nature. 2000;404:770–774. doi: 10.1038/35008096. [DOI] [PubMed] [Google Scholar]
- 135.Wegele H., Muller L., Buchner J. Hsp70 and Hsp90—A relay team for protein folding. Rev. Physiol. Biochem. Pharm. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 136.Mishra A., Godavarthi S.K., Maheshwari M., Goswami A., Jana N.R. The ubiquitin ligase E6-AP is induced and recruited to aggresomes in response to proteasome inhibition and may be involved in the ubiquitination of Hsp70-bound misfolded proteins. J. Biol. Chem. 2009;284:10537–10545. doi: 10.1074/jbc.M806804200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mishra R., Amanullah A., Upadhyay A., Dhiman R., Dubey A.R., Singh S., Prasad A., Mishra A. Ubiquitin ligase LRSAM1 suppresses neurodegenerative diseases linked aberrant proteins induced cell death. Int. J. Biochem. Cell Biol. 2020;120:105697. doi: 10.1016/j.biocel.2020.105697. [DOI] [PubMed] [Google Scholar]
- 138.Inda C., Bolaender A., Wang T., Gandu S.R., Koren J., 3rd Stressing Out Hsp90 in Neurotoxic Proteinopathies. Curr. Top. Med. Chem. 2016;16:2829–2838. doi: 10.2174/1568026616666160413141350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Olzscha H. Posttranslational modifications and proteinopathies: How guardians of the proteome are defeated. Biol. Chem. 2019;400:895–915. doi: 10.1515/hsz-2018-0458. [DOI] [PubMed] [Google Scholar]
- 140.Mear J.P., Schreiber K.L., Munz C., Zhu X., Stevanovic S., Rammensee H.G., Rowland-Jones S.L., Colbert R.A. Misfolding of HLA-B27 as a result of its B pocket suggests a novel mechanism for its role in susceptibility to spondyloarthropathies. J. Immunol. 1999;163:6665–6670. [PubMed] [Google Scholar]
- 141.Dangoria N.S., DeLay M.L., Kingsbury D.J., Mear J.P., Uchanska-Ziegler B., Ziegler A., Colbert R.A. HLA-B27 misfolding is associated with aberrant intermolecular disulfide bond formation (dimerization) in the endoplasmic reticulum. J. Biol. Chem. 2002;277:23459–23468. doi: 10.1074/jbc.M110336200. [DOI] [PubMed] [Google Scholar]
- 142.Colbert R.A. HLA-B27 misfolding: A solution to the spondyloarthropathy conundrum? Mol. Med. Today. 2000;6:224–230. doi: 10.1016/S1357-4310(00)01699-3. [DOI] [PubMed] [Google Scholar]
- 143.Colbert R.A., Tran T.M., Layh-Schmitt G. HLA-B27 misfolding and ankylosing spondylitis. Mol. Immunol. 2014;57:44–51. doi: 10.1016/j.molimm.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kerbiriou M., Le Drevo M.A., Ferec C., Trouve P. Coupling cystic fibrosis to endoplasmic reticulum stress: Differential role of Grp78 and ATF6. Biochim. Biophys. Acta. 2007;1772:1236–1249. doi: 10.1016/j.bbadis.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 145.Trouve P., Ferec C., Genin E. The Interplay between the Unfolded Protein Response, Inflammation and Infection in Cystic Fibrosis. Cells. 2021;10:2980. doi: 10.3390/cells10112980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Segeritz C.P., Rashid S.T., de Brito M.C., Serra M.P., Ordonez A., Morell C.M., Kaserman J.E., Madrigal P., Hannan N.R.F., Gatto L., et al. hiPSC hepatocyte model demonstrates the role of unfolded protein response and inflammatory networks in alpha1-antitrypsin deficiency. J. Hepatol. 2018;69:851–860. doi: 10.1016/j.jhep.2018.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lawless M.W., Greene C.M., Mulgrew A., Taggart C.C., O’Neill S.J., McElvaney N.G. Activation of endoplasmic reticulum-specific stress responses associated with the conformational disease Z alpha 1-antitrypsin deficiency. J. Immunol. 2004;172:5722–5726. doi: 10.4049/jimmunol.172.9.5722. [DOI] [PubMed] [Google Scholar]
- 148.Carroll T.P., Greene C.M., O’Connor C.A., Nolan A.M., O’Neill S.J., McElvaney N.G. Evidence for unfolded protein response activation in monocytes from individuals with alpha-1 antitrypsin deficiency. J. Immunol. 2010;184:4538–4546. doi: 10.4049/jimmunol.0802864. [DOI] [PubMed] [Google Scholar]
- 149.Saraiva M.J., Magalhaes J., Ferreira N., Almeida M.R. Transthyretin deposition in familial amyloidotic polyneuropathy. Curr. Med. Chem. 2012;19:2304–2311. doi: 10.2174/092986712800269236. [DOI] [PubMed] [Google Scholar]
- 150.Quarta C.C., Buxbaum J.N., Shah A.M., Falk R.H., Claggett B., Kitzman D.W., Mosley T.H., Butler K.R., Boerwinkle E., Solomon S.D. The amyloidogenic V122I transthyretin variant in elderly black Americans. N. Engl. J. Med. 2015;372:21–29. doi: 10.1056/NEJMoa1404852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kanda Y., Goodman D.S., Canfield R.E., Morgan F.J. The amino acid sequence of human plasma prealbumin. J. Biol. Chem. 1974;249:6796–6805. doi: 10.1016/S0021-9258(19)42128-5. [DOI] [PubMed] [Google Scholar]
- 152.Sousa M.M., Saraiva M.J. Neurodegeneration in familial amyloid polyneuropathy: From pathology to molecular signaling. Prog. Neurobiol. 2003;71:385–400. doi: 10.1016/j.pneurobio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 153.Azevedo E.P., Guimaraes-Costa A.B., Bandeira-Melo C., Chimelli L., Waddington-Cruz M., Saraiva E.M., Palhano F.L., Foguel D. Inflammatory profiling of patients with familial amyloid polyneuropathy. BMC Neurol. 2019;19:146. doi: 10.1186/s12883-019-1369-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sousa M.M., Du Yan S., Fernandes R., Guimaraes A., Stern D., Saraiva M.J. Familial amyloid polyneuropathy: Receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J. Neurosci. 2001;21:7576–7586. doi: 10.1523/JNEUROSCI.21-19-07576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Azevedo E.P., Ledo J.H., Barbosa G., Sobrinho M., Diniz L., Fonseca A.C., Gomes F., Romao L., Lima F.R., Palhano F.L., et al. Activated microglia mediate synapse loss and short-term memory deficits in a mouse model of transthyretin-related oculoleptomeningeal amyloidosis. Cell Death Dis. 2013;4:e789. doi: 10.1038/cddis.2013.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Azevedo E.P., Guimaraes-Costa A.B., Torezani G.S., Braga C.A., Palhano F.L., Kelly J.W., Saraiva E.M., Foguel D. Amyloid fibrils trigger the release of neutrophil extracellular traps (NETs), causing fibril fragmentation by NET-associated elastase. J. Biol. Chem. 2012;287:37206–37218. doi: 10.1074/jbc.M112.369942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Williams D.B., Windebank A.J. Motor neuron disease (amyotrophic lateral sclerosis) Mayo Clin. Proc. 1991;66:54–82. doi: 10.1016/S0025-6196(12)61175-6. [DOI] [PubMed] [Google Scholar]
- 158.Rowland L.P. Amyotrophic lateral sclerosis. Curr. Opin. Neurol. 1994;7:310–315. doi: 10.1097/00019052-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 159.Chia R., Chio A., Traynor B.J. Novel genes associated with amyotrophic lateral sclerosis: Diagnostic and clinical implications. Lancet Neurol. 2018;17:94–102. doi: 10.1016/S1474-4422(17)30401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Maurel C., Dangoumau A., Marouillat S., Brulard C., Chami A., Hergesheimer R., Corcia P., Blasco H., Andres C.R., Vourc’h P. Causative Genes in Amyotrophic Lateral Sclerosis and Protein Degradation Pathways: A Link to Neurodegeneration. Mol. Neurobiol. 2018;55:6480–6499. doi: 10.1007/s12035-017-0856-0. [DOI] [PubMed] [Google Scholar]
- 161.Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J.P., Deng H.X., et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 162.Kabashi E., Valdmanis P.N., Dion P., Spiegelman D., McConkey B.J., Vande Velde C., Bouchard J.P., Lacomblez L., Pochigaeva K., Salachas F., et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat. Genet. 2008;40:572–574. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- 163.Ling S.C., Polymenidou M., Cleveland D.W. Converging mechanisms in ALS and FTD: Disrupted RNA and protein homeostasis. Neuron. 2013;79:416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Buratti E., Baralle F.E. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front. Biosci. 2008;13:867–878. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- 165.Buratti E., Baralle F.E. TDP-43: Gumming up neurons through protein-protein and protein-RNA interactions. Trends Biochem. Sci. 2012;37:237–247. doi: 10.1016/j.tibs.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 166.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H., Adamson J., et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–256. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Renton A.E., Majounie E., Waite A., Simon-Sanchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L., et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–268. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cook C.N., Wu Y., Odeh H.M., Gendron T.F., Jansen-West K., Del Rosso G., Yue M., Jiang P., Gomes E., Tong J., et al. C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy. Sci Transl Med. 2020;12 doi: 10.1126/scitranslmed.abb3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Neumann M., Sampathu D.M., Kwong L.K., Truax A.C., Micsenyi M.C., Chou T.T., Bruce J., Schuck T., Grossman M., Clark C.M., et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 170.Cirulli E.T., Lasseigne B.N., Petrovski S., Sapp P.C., Dion P.A., Leblond C.S., Couthouis J., Lu Y.F., Wang Q., Krueger B.J., et al. Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science. 2015;347:1436–1441. doi: 10.1126/science.aaa3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Freischmidt A., Wieland T., Richter B., Ruf W., Schaeffer V., Muller K., Marroquin N., Nordin F., Hubers A., Weydt P., et al. Haploinsufficiency of TBK1 causes familial ALS and fronto-temporal dementia. Nat. Neurosci. 2015;18:631–636. doi: 10.1038/nn.4000. [DOI] [PubMed] [Google Scholar]
- 172.Geloso M.C., Corvino V., Marchese E., Serrano A., Michetti F., D’Ambrosi N. The Dual Role of Microglia in ALS: Mechanisms and Therapeutic Approaches. Front. Aging Neurosci. 2017;9:242. doi: 10.3389/fnagi.2017.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Henkel J.S., Beers D.R., Zhao W., Appel S.H. Microglia in ALS: The good, the bad, and the resting. J. Neuroimmune Pharm. 2009;4:389–398. doi: 10.1007/s11481-009-9171-5. [DOI] [PubMed] [Google Scholar]
- 174.Shahheydari H., Ragagnin A., Walker A.K., Toth R.P., Vidal M., Jagaraj C.J., Perri E.R., Konopka A., Sultana J.M., Atkin J.D. Protein Quality Control and the Amyotrophic Lateral Sclerosis/Frontotemporal Dementia Continuum. Front. Mol. Neurosci. 2017;10:119. doi: 10.3389/fnmol.2017.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nishitoh H., Kadowaki H., Nagai A., Maruyama T., Yokota T., Fukutomi H., Noguchi T., Matsuzawa A., Takeda K., Ichijo H. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao W., Beers D.R., Henkel J.S., Zhang W., Urushitani M., Julien J.P., Appel S.H. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58:231–243. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Magrane J., Cortez C., Gan W.B., Manfredi G. Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum. Mol. Genet. 2014;23:1413–1424. doi: 10.1093/hmg/ddt528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang W., Li L., Lin W.L., Dickson D.W., Petrucelli L., Zhang T., Wang X. The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet. 2013;22:4706–4719. doi: 10.1093/hmg/ddt319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Varshavsky A. The Ubiquitin System, Autophagy, and Regulated Protein Degradation. Annu Rev. Biochem. 2017;86:123–128. doi: 10.1146/annurev-biochem-061516-044859. [DOI] [PubMed] [Google Scholar]
- 180.Cetin G., Klafack S., Studencka-Turski M., Kruger E., Ebstein F. The Ubiquitin-Proteasome System in Immune Cells. Biomolecules. 2021;11:60. doi: 10.3390/biom11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pickart C.M., Fushman D. Polyubiquitin chains: Polymeric protein signals. Curr. Opin. Chem. Biol. 2004;8:610–616. doi: 10.1016/j.cbpa.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 182.Pickart C.M., Eddins M.J. Ubiquitin: Structures, functions, mechanisms. Biochim. Biophys. Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 183.Tanaka K., Mizushima T., Saeki Y. The proteasome: Molecular machinery and pathophysiological roles. Biol. Chem. 2012;393:217–234. doi: 10.1515/hsz-2011-0285. [DOI] [PubMed] [Google Scholar]
- 184.Tanaka K. The proteasome: Overview of structure and functions. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009;85:12–36. doi: 10.2183/pjab.85.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bard J.A.M., Goodall E.A., Greene E.R., Jonsson E., Dong K.C., Martin A. Structure and Function of the 26S Proteasome. Annu. Rev. Biochem. 2018;87:697–724. doi: 10.1146/annurev-biochem-062917-011931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Dahlmann B. Proteasomes. Essays Biochem. 2005;41:31–48. doi: 10.1042/bse0410031. [DOI] [PubMed] [Google Scholar]
- 187.Greene E.R., Dong K.C., Martin A. Understanding the 26S proteasome molecular machine from a structural and conformational dynamics perspective. Curr. Opin. Struct. Biol. 2020;61:33–41. doi: 10.1016/j.sbi.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ebstein F., Kloetzel P.M., Kruger E., Seifert U. Emerging roles of immunoproteasomes beyond MHC class I antigen processing. Cell Mol. Life Sci. 2012;69:2543–2558. doi: 10.1007/s00018-012-0938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tubio-Santamaria N., Ebstein F., Heidel F.H., Kruger E. Immunoproteasome Function in Normal and Malignant Hematopoiesis. Cells. 2021;10:1577. doi: 10.3390/cells10071577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Seifert U., Bialy L.P., Ebstein F., Bech-Otschir D., Voigt A., Schroter F., Prozorovski T., Lange N., Steffen J., Rieger M., et al. Immunoproteasomes preserve protein homeostasis upon interferon-induced oxidative stress. Cell. 2010;142:613–624. doi: 10.1016/j.cell.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 191.Ebstein F., Voigt A., Lange N., Warnatsch A., Schroter F., Prozorovski T., Kuckelkorn U., Aktas O., Seifert U., Kloetzel P.M., et al. Immunoproteasomes are important for proteostasis in immune responses. Cell. 2013;152:935–937. doi: 10.1016/j.cell.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 192.Yun Y.S., Kim K.H., Tschida B., Sachs Z., Noble-Orcutt K.E., Moriarity B.S., Ai T., Ding R., Williams J., Chen L., et al. mTORC1 Coordinates Protein Synthesis and Immunoproteasome Formation via PRAS40 to Prevent Accumulation of Protein Stress. Mol. Cell. 2016;61:625–639. doi: 10.1016/j.molcel.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Niewerth D., Kaspers G.J., Assaraf Y.G., van Meerloo J., Kirk C.J., Anderl J., Blank J.L., van de Ven P.M., Zweegman S., Jansen G., et al. Interferon-gamma-induced upregulation of immunoproteasome subunit assembly overcomes bortezomib resistance in human hematological cell lines. J. Hematol. Oncol. 2014;7:7. doi: 10.1186/1756-8722-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Opitz E., Koch A., Klingel K., Schmidt F., Prokop S., Rahnefeld A., Sauter M., Heppner F.L., Volker U., Kandolf R., et al. Impairment of immunoproteasome function by beta5i/LMP7 subunit deficiency results in severe enterovirus myocarditis. PLoS Pathog. 2011;7:e1002233. doi: 10.1371/journal.ppat.1002233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kruger E., Kloetzel P.M. Immunoproteasomes at the interface of innate and adaptive immune responses: Two faces of one enzyme. Curr. Opin. Immunol. 2012;24:77–83. doi: 10.1016/j.coi.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 196.Goetzke C.C., Ebstein F., Kallinich T. Role of Proteasomes in Inflammation. J. Clin. Med. 2021;10:1783. doi: 10.3390/jcm10081783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chondrogianni N., Gonos E.S. Proteasome function determines cellular homeostasis and the rate of aging. Adv. Exp. Med. Biol. 2010;694:38–46. doi: 10.1007/978-1-4419-7002-2_4. [DOI] [PubMed] [Google Scholar]
- 198.Keller J.N., Huang F.F., Markesbery W.R. Decreased levels of proteasome activity and proteasome expression in aging spinal cord. Neuroscience. 2000;98:149–156. doi: 10.1016/S0306-4522(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 199.Tydlacka S., Wang C.E., Wang X., Li S., Li X.J. Differential activities of the ubiquitin-proteasome system in neurons versus glia may account for the preferential accumulation of misfolded proteins in neurons. J. Neurosci. 2008;28:13285–13295. doi: 10.1523/JNEUROSCI.4393-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Giannini C., Kloss A., Gohlke S., Mishto M., Nicholson T.P., Sheppard P.W., Kloetzel P.M., Dahlmann B. Poly-Ub-substrate-degradative activity of 26S proteasome is not impaired in the aging rat brain. PLoS ONE. 2013;8:e64042. doi: 10.1371/journal.pone.0064042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Keller J.N., Hanni K.B., Markesbery W.R. Impaired proteasome function in Alzheimer’s disease. J. Neurochem. 2000;75:436–439. doi: 10.1046/j.1471-4159.2000.0750436.x. [DOI] [PubMed] [Google Scholar]
- 202.Lopez Salon M., Morelli L., Castano E.M., Soto E.F., Pasquini J.M. Defective ubiquitination of cerebral proteins in Alzheimer’s disease. J. Neurosci. Res. 2000;62:302–310. doi: 10.1002/1097-4547(20001015)62:2<302::AID-JNR15>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 203.Jenner P., Olanow C.W. Understanding cell death in Parkinson’s disease. Ann. Neurol. 1998;44:72–84. doi: 10.1002/ana.410440712. [DOI] [PubMed] [Google Scholar]
- 204.McNaught K.S., Jenner P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 2001;297:191–194. doi: 10.1016/S0304-3940(00)01701-8. [DOI] [PubMed] [Google Scholar]
- 205.McNaught K.S., Belizaire R., Isacson O., Jenner P., Olanow C.W. Altered proteasomal function in sporadic Parkinson’s disease. Exp. Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- 206.Ansar M., Ebstein F., Ozkoc H., Paracha S.A., Iwaszkiewicz J., Gesemann M., Zoete V., Ranza E., Santoni F.A., Sarwar M.T., et al. Biallelic variants in PSMB1 encoding the proteasome subunit beta6 cause impairment of proteasome function, microcephaly, intellectual disability, developmental delay and short stature. Hum. Mol. Genet. 2020;29:1132–1143. doi: 10.1093/hmg/ddaa032. [DOI] [PubMed] [Google Scholar]
- 207.Brehm A., Liu Y., Sheikh A., Marrero B., Omoyinmi E., Zhou Q., Montealegre G., Biancotto A., Reinhardt A., Almeida de Jesus A., et al. Additive loss-of-function proteasome subunit mutations in CANDLE/PRAAS patients promote type I IFN production. J. Clin. Investig. 2015;125:4196–4211. doi: 10.1172/JCI81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Verhoeven D., Schonenberg-Meinema D., Ebstein F., Papendorf J.J., Baars P.A., van Leeuwen E.M.M., Jansen M.H., Lankester A.C., van der Burg M., Florquin S., et al. Hematopoietic stem cell transplantation in a patient with proteasome-associated auto-inflammatory syndrome (PRAAS) J. Allergy Clin. Immunol. 2021 doi: 10.1016/j.jaci.2021.07.039. [DOI] [PubMed] [Google Scholar]
- 209.Kitamura A., Maekawa Y., Uehara H., Izumi K., Kawachi I., Nishizawa M., Toyoshima Y., Takahashi H., Standley D.M., Tanaka K., et al. A mutation in the immunoproteasome subunit PSMB8 causes autoinflammation and lipodystrophy in humans. J. Clin. Investig. 2011;121:4150–4160. doi: 10.1172/JCI58414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Arima K., Kinoshita A., Mishima H., Kanazawa N., Kaneko T., Mizushima T., Ichinose K., Nakamura H., Tsujino A., Kawakami A., et al. Proteasome assembly defect due to a proteasome subunit beta type 8 (PSMB8) mutation causes the autoinflammatory disorder, Nakajo-Nishimura syndrome. Proc. Natl Acad Sci USA. 2011;108:14914–14919. doi: 10.1073/pnas.1106015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Liu Y., Ramot Y., Torrelo A., Paller A.S., Si N., Babay S., Kim P.W., Sheikh A., Lee C.C., Chen Y., et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012;64:895–907. doi: 10.1002/art.33368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Agarwal A.K., Xing C., DeMartino G.N., Mizrachi D., Hernandez M.D., Sousa A.B., Martinez de Villarreal L., dos Santos H.G., Garg A. PSMB8 encoding the beta5i proteasome subunit is mutated in joint contractures, muscle atrophy, microcytic anemia, and panniculitis-induced lipodystrophy syndrome. Am. J. Hum. Genet. 2010;87:866–872. doi: 10.1016/j.ajhg.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Jia T., Zheng Y., Feng C., Yang T., Geng S. A Chinese case of Nakajo-Nishimura syndrome with novel compound heterozygous mutations of the PSMB8 gene. BMC Med. Genet. 2020;21:126. doi: 10.1186/s12881-020-01060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Patel P.N., Hunt R., Pettigrew Z.J., Shirley J.B., Vogel T.P., de Guzman M.M. Successful treatment of chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome with tofacitinib. Pediatr. Derm. 2021;38:528–529. doi: 10.1111/pde.14517. [DOI] [PubMed] [Google Scholar]
- 215.Boyadzhiev M., Marinov L., Boyadzhiev V., Iotova V., Aksentijevich I., Hambleton S. Disease course and treatment effects of a JAK inhibitor in a patient with CANDLE syndrome. Pediatr. Rheumatol. Online J. 2019;17:19. doi: 10.1186/s12969-019-0322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Yamazaki-Nakashimada M.A., Santos-Chavez E.E., de Jesus A.A., Rivas-Larrauri F., Guzman-Martinez M.N., Goldbach-Mansky R., Espinosa-Padilla S., Saez-de-Ocariz M.D., Orozco-Covarrubias L., Blancas-Galicia L. Systemic Autoimmunity in a Patient With CANDLE Syndrome. J. Investig. Allergol. Clin. Immunol. 2019;29:75–76. doi: 10.18176/jiaci.0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cardis M.A., Montealegre Sanchez G.A., Goldbach-Mansky R., Richard Lee C.C., Cowen E.W. Recurrent fevers, progressive lipodystrophy, and annular plaques in a child. J. Am. Acad. Dermatol. 2019;80:291–295. doi: 10.1016/j.jaad.2018.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kataoka S., Kawashima N., Okuno Y., Muramatsu H., Miwata S., Narita K., Hamada M., Murakami N., Taniguchi R., Ichikawa D., et al. Successful treatment of a novel type I interferonopathy due to a de novo PSMB9 gene mutation with a Janus kinase inhibitor. J. Allergy Clin. Immunol. 2021 doi: 10.1016/j.jaci.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 219.Kanazawa N., Hemmi H., Kinjo N., Ohnishi H., Hamazaki J., Mishima H., Kinoshita A., Mizushima T., Hamada S., Hamada K., et al. Heterozygous missense variant of the proteasome subunit beta-type 9 causes neonatal-onset autoinflammation and immunodeficiency. Nat. Commun. 2021;12:6819. doi: 10.1038/s41467-021-27085-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sarrabay G., Mechin D., Salhi A., Boursier G., Rittore C., Crow Y., Rice G., Tran T.A., Cezar R., Duffy D., et al. PSMB10, the last immunoproteasome gene missing for PRAAS. J. Allergy Clin. Immunol. 2019 doi: 10.1016/j.jaci.2019.11.024. [DOI] [PubMed] [Google Scholar]
- 221.Poli M.C., Ebstein F., Nicholas S.K., de Guzman M.M., Forbes L.R., Chinn I.K., Mace E.M., Vogel T.P., Carisey A.F., Benavides F., et al. Heterozygous Truncating Variants in POMP Escape Nonsense-Mediated Decay and Cause a Unique Immune Dysregulatory Syndrome. Am. J. Hum. Genet. 2018;102:1126–1142. doi: 10.1016/j.ajhg.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Meinhardt A., Ramos P.C., Dohmen R.J., Lucas N., Lee-Kirsch M.A., Becker B., de Laffolie J., Cunha T., Niehues T., Salzer U., et al. Curative Treatment of POMP-Related Autoinflammation and Immune Dysregulation (PRAID) by Hematopoietic Stem Cell Transplantation. J. Clin. Immunol. 2021;41:1664–1667. doi: 10.1007/s10875-021-01067-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.de Jesus A.A., Brehm A., VanTries R., Pillet P., Parentelli A.S., Montealegre Sanchez G.A., Deng Z., Paut I.K., Goldbach-Mansky R., Kruger E. Novel proteasome assembly chaperone mutations in PSMG2/PAC2 cause the autoinflammatory interferonopathy CANDLE/PRAAS4. J. Allergy Clin. Immunol. 2019;143:1939–1943.e8. doi: 10.1016/j.jaci.2018.12.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kury S., Besnard T., Ebstein F., Khan T.N., Gambin T., Douglas J., Bacino C.A., Craigen W.J., Sanders S.J., Lehmann A., et al. De Novo Disruption of the Proteasome Regulatory Subunit PSMD12 Causes a Syndromic Neurodevelopmental Disorder. Am. J. Hum. Genet. 2017;100:352–363. doi: 10.1016/j.ajhg.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Khalil R., Kenny C., Hill R.S., Mochida G.H., Nasir R., Partlow J.N., Barry B.J., Al-Saffar M., Egan C., Stevens C.R., et al. PSMD12 haploinsufficiency in a neurodevelopmental disorder with autistic features. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2018;177:736–745. doi: 10.1002/ajmg.b.32688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Yan K., Zhang J., Lee P.Y., Tao P., Wang J., Wang S., Zhou Q., Dong M. Haploinsufficiency of PSMD12 causes proteasome dysfunction and subclinical autoinflammation. Arthritis Rheumatol. 2022 doi: 10.1002/art.42070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kroll-Hermi A., Ebstein F., Stoetzel C., Geoffroy V., Schaefer E., Scheidecker S., Bar S., Takamiya M., Kawakami K., Zieba B.A., et al. Proteasome subunit PSMC3 variants cause neurosensory syndrome combining deafness and cataract due to proteotoxic stress. EMBO Mol. Med. 2020;12:e11861. doi: 10.15252/emmm.201911861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kitano Y., Matsunaga E., Morimoto T., Okada N., Sano S. A syndrome with nodular erythema, elongated and thickened fingers, and emaciation. Arch. Derm. 1985;121:1053–1056. doi: 10.1001/archderm.1985.01660080107026. [DOI] [PubMed] [Google Scholar]
- 229.Tanaka M., Miyatani N., Yamada S., Miyashita K., Toyoshima I., Sakuma K., Tanaka K., Yuasa T., Miyatake T., Tsubaki T. Hereditary lipo-muscular atrophy with joint contracture, skin eruptions and hyper-gamma-globulinemia: A new syndrome. Intern. Med. 1993;32:42–45. doi: 10.2169/internalmedicine.32.42. [DOI] [PubMed] [Google Scholar]
- 230.Kasagi S., Kawano S., Nakazawa T., Sugino H., Koshiba M., Ichinose K., Ida H., Eguchi K., Kumagai S. A case of periodic-fever-syndrome-like disorder with lipodystrophy, myositis, and autoimmune abnormalities. Mod. Rheumatol. 2008;18:203–207. doi: 10.3109/s10165-008-0033-4. [DOI] [PubMed] [Google Scholar]
- 231.Torrelo A., Patel S., Colmenero I., Gurbindo D., Lendinez F., Hernandez A., Lopez-Robledillo J.C., Dadban A., Requena L., Paller A.S. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J. Am. Acad. Dermatol. 2010;62:489–495. doi: 10.1016/j.jaad.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 232.Ramot Y., Czarnowicki T., Maly A., Navon-Elkan P., Zlotogorski A. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome: A case report. Pediatr. Derm. 2011;28:538–541. doi: 10.1111/j.1525-1470.2010.01163.x. [DOI] [PubMed] [Google Scholar]
- 233.Kimura H., Usui F., Karasawa T., Kawashima A., Shirasuna K., Inoue Y., Komada T., Kobayashi M., Mizushina Y., Kasahara T., et al. Immunoproteasome subunit LMP7 Deficiency Improves Obesity and Metabolic Disorders. Sci. Rep. 2015;5:15883. doi: 10.1038/srep15883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Martinez C.A., Ebstein F., Nicholas S.K., De Guzman M., Forbes L.R., Delmonte O.M., Bosticardo M., Castagnoli R., Krance R., Notarangelo L.D., et al. HSCT corrects primary immunodeficiency and immune dysregulation in patients with POMP-related auto-inflammatory disease. Blood. 2021 doi: 10.1182/blood.2021011005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Studencka-Turski M., Cetin G., Junker H., Ebstein F., Kruger E. Molecular Insight Into the IRE1alpha-Mediated Type I Interferon Response Induced by Proteasome Impairment in Myeloid Cells of the Brain. Front. Immunol. 2019;10:2900. doi: 10.3389/fimmu.2019.02900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Minton K. Sensing proteotoxic stress. Nat. Rev. Immunol. 2022 doi: 10.1038/s41577-022-00702-7. [DOI] [PubMed] [Google Scholar]
- 237.Crow Y.J., Manel N. Aicardi-Goutieres syndrome and the type I interferonopathies. Nat. Rev. Immunol. 2015;15:429–440. doi: 10.1038/nri3850. [DOI] [PubMed] [Google Scholar]
- 238.Sullivan K.D., Evans D., Pandey A., Hraha T.H., Smith K.P., Markham N., Rachubinski A.L., Wolter-Warmerdam K., Hickey F., Espinosa J.M., et al. Trisomy 21 causes changes in the circulating proteome indicative of chronic autoinflammation. Sci. Rep. 2017;7:14818. doi: 10.1038/s41598-017-13858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Sullivan K.D., Lewis H.C., Hill A.A., Pandey A., Jackson L.P., Cabral J.M., Smith K.P., Liggett L.A., Gomez E.B., Galbraith M.D., et al. Trisomy 21 consistently activates the interferon response. eLife. 2016;5 doi: 10.7554/eLife.16220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Waugh K.A., Araya P., Pandey A., Jordan K.R., Smith K.P., Granrath R.E., Khanal S., Butcher E.T., Estrada B.E., Rachubinski A.L., et al. Mass Cytometry Reveals Global Immune Remodeling with Multi-lineage Hypersensitivity to Type I Interferon in Down Syndrome. Cell Rep. 2019;29:1893–1908.e4. doi: 10.1016/j.celrep.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhou Q., Wang H., Schwartz D.M., Stoffels M., Park Y.H., Zhang Y., Yang D., Demirkaya E., Takeuchi M., Tsai W.L., et al. Loss-of-function mutations in TNFAIP3 leading to A20 haploinsufficiency cause an early-onset autoinflammatory disease. Nat. Genet. 2016;48:67–73. doi: 10.1038/ng.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Damgaard R.B., Walker J.A., Marco-Casanova P., Morgan N.V., Titheradge H.L., Elliott P.R., McHale D., Maher E.R., McKenzie A.N.J., Komander D. The Deubiquitinase OTULIN Is an Essential Negative Regulator of Inflammation and Autoimmunity. Cell. 2016;166:1215–1230. doi: 10.1016/j.cell.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Damgaard R.B., Elliott P.R., Swatek K.N., Maher E.R., Stepensky P., Elpeleg O., Komander D., Berkun Y. OTULIN deficiency in ORAS causes cell type-specific LUBAC degradation, dysregulated TNF signalling and cell death. EMBO Mol. Med. 2019;11 doi: 10.15252/emmm.201809324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Meuwissen M.E., Schot R., Buta S., Oudesluijs G., Tinschert S., Speer S.D., Li Z., van Unen L., Heijsman D., Goldmann T., et al. Human USP18 deficiency underlies type 1 interferonopathy leading to severe pseudo-TORCH syndrome. J. Exp. Med. 2016;213:1163–1174. doi: 10.1084/jem.20151529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Boisson B., Laplantine E., Dobbs K., Cobat A., Tarantino N., Hazen M., Lidov H.G., Hopkins G., Du L., Belkadi A., et al. Human HOIP and LUBAC deficiency underlies autoinflammation, immunodeficiency, amylopectinosis, and lymphangiectasia. J. Exp. Med. 2015;212:939–951. doi: 10.1084/jem.20141130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Boisson B., Laplantine E., Prando C., Giliani S., Israelsson E., Xu Z., Abhyankar A., Israel L., Trevejo-Nunez G., Bogunovic D., et al. Immunodeficiency, autoinflammation and amylopectinosis in humans with inherited HOIL-1 and LUBAC deficiency. Nat. Immunol. 2012;13:1178–1186. doi: 10.1038/ni.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Oda H., Beck D.B., Kuehn H.S., Sampaio Moura N., Hoffmann P., Ibarra M., Stoddard J., Tsai W.L., Gutierrez-Cruz G., Gadina M., et al. Second Case of HOIP Deficiency Expands Clinical Features and Defines Inflammatory Transcriptome Regulated by LUBAC. Front. Immunol. 2019;10:479. doi: 10.3389/fimmu.2019.00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Di Donato G., d’Angelo D.M., Breda L., Chiarelli F. Monogenic Autoinflammatory Diseases: State of the Art and Future Perspectives. Int. J. Mol. Sci. 2021;22:6360. doi: 10.3390/ijms22126360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zimmermann A., Kainz K., Andryushkova A., Hofer S., Madeo F., Carmona-Gutierrez D. Autophagy: One more Nobel Prize for yeast. Microb. Cell. 2016;3:579–581. doi: 10.15698/mic2016.12.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tooze S.A., Dikic I. Autophagy Captures the Nobel Prize. Cell. 2016;167:1433–1435. doi: 10.1016/j.cell.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 251.Galluzzi L., Baehrecke E.H., Ballabio A., Boya P., Bravo-San Pedro J.M., Cecconi F., Choi A.M., Chu C.T., Codogno P., Colombo M.I., et al. Molecular definitions of autophagy and related processes. EMBO J. 2017;36:1811–1836. doi: 10.15252/embj.201796697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Kitada M., Koya D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021;17:647–661. doi: 10.1038/s41574-021-00551-9. [DOI] [PubMed] [Google Scholar]
- 253.Tan S., Wong E. Kinetics of Protein Aggregates Disposal by Aggrephagy. Methods Enzym. 2017;588:245–281. doi: 10.1016/bs.mie.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 254.Gatica D., Lahiri V., Klionsky D.J. Cargo recognition and degradation by selective autophagy. Nat. Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Hara T., Nakamura K., Matsui M., Yamamoto A., Nakahara Y., Suzuki-Migishima R., Yokoyama M., Mishima K., Saito I., Okano H., et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 256.Haack T.B., Ignatius E., Calvo-Garrido J., Iuso A., Isohanni P., Maffezzini C., Lonnqvist T., Suomalainen A., Gorza M., Kremer L.S., et al. Absence of the Autophagy Adaptor SQSTM1/p62 Causes Childhood-Onset Neurodegeneration with Ataxia, Dystonia, and Gaze Palsy. Am. J. Hum. Genet. 2016;99:735–743. doi: 10.1016/j.ajhg.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Roodman G.D., Kurihara N., Ohsaki Y., Kukita A., Hosking D., Demulder A., Smith J.F., Singer F.R. Interleukin 6. A potential autocrine/paracrine factor in Paget’s disease of bone. J. Clin. Investig. 1992;89:46–52. doi: 10.1172/JCI115584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Poloni M., Facchetti D., Mai R., Micheli A., Agnoletti L., Francolini G., Mora G., Camana C., Mazzini L., Bachetti T. Circulating levels of tumour necrosis factor-alpha and its soluble receptors are increased in the blood of patients with amyotrophic lateral sclerosis. Neurosci. Lett. 2000;287:211–214. doi: 10.1016/S0304-3940(00)01177-0. [DOI] [PubMed] [Google Scholar]
- 259.Wooten M.W., Geetha T., Seibenhener M.L., Babu J.R., Diaz-Meco M.T., Moscat J. The p62 scaffold regulates nerve growth factor-induced NF-kappaB activation by influencing TRAF6 polyubiquitination. J. Biol. Chem. 2005;280:35625–35629. doi: 10.1074/jbc.C500237200. [DOI] [PubMed] [Google Scholar]
- 260.Rea S.L., Walsh J.P., Layfield R., Ratajczak T., Xu J. New insights into the role of sequestosome 1/p62 mutant proteins in the pathogenesis of Paget’s disease of bone. Endocr. Rev. 2013;34:501–524. doi: 10.1210/er.2012-1034. [DOI] [PubMed] [Google Scholar]
- 261.Kim M., Sandford E., Gatica D., Qiu Y., Liu X., Zheng Y., Schulman B.A., Xu J., Semple I., Ro S.H., et al. Mutation in ATG5 reduces autophagy and leads to ataxia with developmental delay. eLife. 2016;5 doi: 10.7554/eLife.12245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Collier J.J., Guissart C., Olahova M., Sasorith S., Piron-Prunier F., Suomi F., Zhang D., Martinez-Lopez N., Leboucq N., Bahr A., et al. Developmental Consequences of Defective ATG7-Mediated Autophagy in Humans. N. Engl. J. Med. 2021;384:2406–2417. doi: 10.1056/NEJMoa1915722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Shin E.C., Seifert U., Urban S., Truong K.T., Feinstone S.M., Rice C.M., Kloetzel P.M., Rehermann B. Proteasome activator and antigen-processing aminopeptidases are regulated by virus-induced type I interferon in the hepatitis C virus-infected liver. J. Interferon Cytokine Res. 2007;27:985–990. doi: 10.1089/jir.2007.0039. [DOI] [PubMed] [Google Scholar]
- 264.Bousoik E., Montazeri Aliabadi H. “Do We Know Jack” About JAK? A Closer Look at JAK/STAT Signaling Pathway. Front. Oncol. 2018;8:287. doi: 10.3389/fonc.2018.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]