Abstract
Bacteria in drinking water, attached or not attached to goethite particles, were disinfected with chlorine. No additional protection was provided to the bacteria by their attachment to particles, and the limited efficiency of inactivation by chlorine was attributed to the presence of bacterial aggregates in both types of suspension.
Transport of drinking water in distribution systems generally requires the addition of disinfectants to the water in order to limit regrowth of bacteria. The bacteria found in the distribution water may occur as single, aggregated, or particle-attached bacteria. Such bacteria may originate from treatment plants (2), biofilm shearing (18, 21, 25), or resuspension of deposits (9, 14, 19). Concerns about the limited efficiency of the disinfection of particle-attached bacteria have resulted from the observation of colonized particles in treatment plant filter effluents (3, 12, 15–17, 24, 26) or in distribution system water (20). By analogy to biofilms (4, 8), survival of particle-attached bacteria has been mainly attributed to (i) high disinfectant reactivity of the particle surfaces coupled with (ii) limited mass transfer at the interface, these two factors leading to a low concentration of oxidant available for inactivation of bacterial cells. Thus, it is generally assumed that particle-attached bacteria are universally more resistant to disinfection than are suspended bacteria (15, 24). We have shown that this concept is not extrapolatable to the case of single bacteria attached to goethite (α-FeOOH) particles, which may represent classical corrosion particles with a low disinfectant demand.
Experiments were performed with a bacterial strain isolated from tap water (Sphingomonas sp.) which is slightly resistant to chlorine. We compared chlorination effects on suspended cells and cells attached to particles. The numbers of attached or aggregated bacteria were evaluated by culture of both sonicated and unsonicated subsamples of chlorinated and control samples.
A Sphingomonas sp. bacterial strain was isolated from Nancy distribution system tap water (after a 1-h exposure to 1 mg of Cl2 [free chlorine] liter−1 to select bacteria able to survive a brief disinfection). Suspensions of this pure strain were prepared by growing it in nutrient broth (Biomérieux, reference no. 51016) for 24 h at 30°C and harvesting cells by combining centrifugation (15,000 × g; 20 min; 20°C) and washing with commercial mineral water (CMW) to eliminate broth nutrients. Filter-sterilized, glass-bottled Evian CMW was used for all experiments to ensure a constant mineral composition of the water and a low organic content (total, 0.3 mg of C liter−1). Cells were resuspended in CMW and incubated for 24 h at 20°C to acclimate them to low-nutrient conditions. Organic polymers released by the microorganisms during incubation were eliminated by double centrifugation and CMW resuspension as described above. The final cell resuspension in CMW was used for inactivation and particle colonization experiments.
Particle colonization was achieved by mixing for 24 h (20°C, 220 rpm) in a chlorine demand-free flask (i) 200 ml of a suspension containing 625 mg (i.e., about 107) of goethite particles (Aldrich Chemical Co., Inc., reference no. 37,125-4; size range, 10 to 50 μm) liter−1 and (ii) 200 ml of a suspension of bacteria acclimated to low-nutrient conditions. Particles with firmly attached bacteria were washed by repeating the following cycle six times: (i) vigorous shaking by hand for 10 s, (ii) 5 min of sedimentation, (iii) siphoning off and discarding the supernatant (350 ml), and (iv) resuspension of the remaining 50 ml in 350 ml of CMW.
Inactivation experiments were performed by dosing suspended or particle-attached Sphingomonas suspensions with solutions of chlorine prepared from bleach (1.1 mg of Cl2 liter−1) in chlorine demand-free flasks at room temperature (pH 7.8 to 7.9). After 30 min, biocide action was stopped by adding sodium thiosulfate (10 mg liter−1) and mixing thoroughly. Free-chlorine measurements were performed by the DPD-colorimetric method (AFNOR T90-038) using a Hitachi U-2000 spectrophotometer. Enumeration of culturable bacteria was performed by pour plating 1-ml samples of appropriate dilutions into 15-ml volumes of glucose-free nutrient agar (AFNOR T90-402). Plates were incubated at 30°C for 15 days to allow enumeration of slow-growing bacteria adapted to oligotrophic conditions. Results were expressed as CFU, with 1 CFU representing a single cell, an aggregate, or a colonized particle. Dispersion of attached or aggregated bacteria was performed by sonicating subsamples (Labsonic 2000U sonicator; 19-mm probe, 20 W, 60 s, ice cooling). Average numbers of culturable bacteria per particle or aggregate were obtained by computing the numbers of CFU per milliliter for sonicated and nonsonicated samples.
During inactivation experiments with suspended bacteria, the concentration of free chlorine decreased from 1.1 to 0.2 mg of Cl2 liter−1 after 30 min. The disinfection efficiency was 99.99%, i.e., 4.0 log10, when bacteria were counted after sonication following chlorination (Table 1). The Sphingomonas bacteria that survived the chlorination were mainly forming bacterial aggregates, since ultrasound dispersion prior to pour plating increased the number of CFU by a factor of 200 (Table 1). Such aggregates were not detected before chlorination (Table 1), and this suggests that individual bacteria, which were the majority of the bacterial population before chlorination, were more readily inactivated than aggregated bacteria.
TABLE 1.
Impact of dispersion of samples on CFU counts of unchlorinated and chlorinated suspensions of suspended Sphingomonas cells
| Samples | Avg no. of CFU ml−1 (SD)a
|
|
|---|---|---|
| With no dispersion of aggregates by sonication | After sonication of samplesb | |
| Unchlorinated | 4.6 × 107 (3.9 × 107) | 3.8 × 107 (2.9 × 107) |
| Chlorinated | 2.0 × 101 (0.90 × 101) | 4.0 × 103 (0.90 × 103) |
Each value in parentheses indicates the standard deviation of three replicates.
Sonication was performed just before pour plating of the samples.
For inactivation experiments with bacteria attached to goethite particles, evidence of bacterial attachment was first determined by epifluorescence microscopic examination of the samples. Prior to chlorination, particles appeared colonized both with single cells and with aggregates of cells. This was quantified by measuring the difference in the bacterial counts of unchlorinated samples with and without sonication (Table 2); the 2.7 log10 increase observed after sonication was attributed to the dispersion of bacteria attached to particles (average of 540 CFU/colonized particle). Goethite particles generated little free chlorine demand: in inactivation experiments, a final concentration of 0.94 mg of Cl2 liter−1 was found for uncolonized particles after 30 min, while particles with attached bacteria resulted in a final concentration of 0.74 mg of Cl2 liter−1. Chlorination of colonized particles resulted in a 99.96% decay of CFU numbers counted after sonication, i.e., a 3.4 log10 reduction of culturable bacteria (Table 2).
TABLE 2.
Impact of dispersion of samples on CFU counts of unchlorinated and chlorinated suspensions of particle-attached Sphingomonas cells
| Samples | Avg no. of CFU g−1 (SD)a
|
|
|---|---|---|
| With no dispersion of attached bacteria by sonication | After sonication of samplesb | |
| Unchlorinated | 3.7 × 104 (4.0 × 104) | 2.0 × 107 (0.74 × 107) |
| Chlorinated | 1.3 × 101 (1.4 × 101) | 8.3 × 103 (3.7 × 103) |
Each value in parentheses indicates the standard deviation of three replicates.
Sonication was performed just before pour plating of the samples.
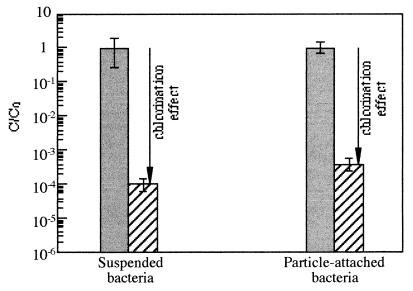
Comparison of the results obtained with suspended and attached bacteria (Fig. 1) showed similar disinfection efficiencies after exposure to 1.1 mg of Cl2 liter−1 (99.99 versus 99.96% reduction), and t-test statistics (27) confirmed that the difference between these values was not significant (P < 0.05). Thus, attachment to goethite particles did not provide Sphingomonas sp. with protection from disinfection under drinking water conditions.
FIG. 1.
Effect of chlorination on relative concentrations of Sphingomonas sp. cells suspended in CMW or attached to goethite particles in CMW.  , unchlorinated samples;
, unchlorinated samples;  , chlorinated samples; C0, initial concentration before chlorination (cf. Tables 1 and 2). CFU numbers were measured after dispersion of samples by sonication.
, chlorinated samples; C0, initial concentration before chlorination (cf. Tables 1 and 2). CFU numbers were measured after dispersion of samples by sonication.
These results are contrary to those obtained for inactivation experiments with granular activated carbon particles (12, 24), iron and manganese resuspended deposits (10), or wastewater particles (1). Nevertheless, all of these particles may exert a disinfectant demand that reduces the concentration of oxidant available for bacterial inactivation. This may also explain why Stringfellow et al. (26) and Pernitsky et al. (17) measured a lower disinfection efficiency for high granular activated carbon particle concentrations and why Berman et al. (1) observed better disinfection of bacteria fixed on small particles. In this study, a very low disinfectant reduction due to the particles was measured (about 10% after 30 min), and mass transfer was also enhanced by continuous agitation during experiments. Most of the surviving bacteria appeared within aggregates in suspended-bacterial inactivation experiments. On the other hand, only a few particles bore several hundred culturable bacteria on their surfaces, probably in the form of microcolonies or clumps; such formations were observed during microscopic examination of samples. In such a configuration, protection from disinfection resulted from limited diffusion (7, 13) coupled with disinfectant reduction inside bacterial aggregates (8, 23). The possibility of resistance due to exopolymers coating aggregated bacterial cells also cannot be excluded (5). The protection of single bacteria gained by attachment to goethite particles was probably negligible, as long as this material, which is often found in distribution system corrosion deposits (22), is not chlorine consuming. An apparent chlorine demand in such particles could result from their becoming coated with dissolved organic matter, since organic compounds are easily trapped at the surface of goethite particles (6, 11).
Thus, the general concern about water disinfection should be resolved on the whole without forgetting the simultaneous presence of bacterial clumps in water. Both clumps and particles can transport culturable bacteria throughout the water system despite the presence of residual disinfectant in the water.
Acknowledgments
This work was conducted as part of a larger research program entitled “Biofilm,” coordinated by the Centre International de l’Eau de Nancy (NANCIE; France). It was funded by the Agence de l’Eau Seine-Normandie (France), Anjou-Recherche (CGE; France), Communauté Urbaine du Grand Nancy (France), Office National de l’Eau Potable (Morocco), Syndicat des Eaux d’Ile de France, Pont-à-Mousson S.A., and NANCIE.
We thank Don Reasoner for kindly reviewing the manuscript.
REFERENCES
- 1.Berman D, Rice E W, Hoff J C. Inactivation of particle-associated coliforms by chlorine and monochloramine. Appl Environ Microbiol. 1988;54:507–512. doi: 10.1128/aem.54.2.507-512.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucklin K E, McFeters G A, Amirtharajah A. Penetration of coliforms through municipal drinking water filters. Water Res. 1991;25:1013–1017. [Google Scholar]
- 3.Camper A K, LeChevallier M W, Broadway S C, McFeters G A. Bacteria associated with granular activated carbon particles in drinking water. Appl Environ Microbiol. 1986;52:434–438. doi: 10.1128/aem.52.3.434-438.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C I, Griebe T, Characklis W G. Biocide action of monochloramine on biofilm systems of Pseudomonas aeruginosa. Biofouling. 1993;7:1–17. [Google Scholar]
- 5.Clark R M, Rice E W, Pierce B K, Johnson C H, Fox K R. Effect of aggregation on Vibrio cholerae inactivation. J Environ Eng. 1994;120:875–887. [Google Scholar]
- 6.Day G M, Hart B T, McKelvie I D, Beckett R. Influence of natural organic matter on the sorption of biocides onto goethite. I. Gamma-BHC and atrazine. Environ Technol. 1997;18:769–779. [Google Scholar]
- 7.De Beer D, Stoodley P, Lewandowski Z. Liquid flows in heterogeneous biofilms. Biotechnol Bioeng. 1994;44:636–641. doi: 10.1002/bit.260440510. [DOI] [PubMed] [Google Scholar]
- 8.De Beer D, Srinivasan R, Stewart P S. Direct measurement of chlorine penetration into biofilms during disinfection. Appl Environ Microbiol. 1994;60:4339–4344. doi: 10.1128/aem.60.12.4339-4344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthier, V., B. Gérard, J. M. Portal, J. C. Block, and D. Gatel. Organic matter as loose deposits in a drinking water distribution system. Water Res., in press.
- 10.Herson D S, McGonogle B, Payer M A, Baker K H. Attachment as a factor in the protection of Enterobacter cloacae from chlorination. Appl Environ Microbiol. 1987;53:1178–1180. doi: 10.1128/aem.53.5.1178-1180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korshin G V, Benjamin M M, Sletten R S. Adsorption of natural organic matter (NOM) on iron oxide: effects of NOM composition and formation of organohalide compounds during chlorination. Water Res. 1997;31:1643–1650. [Google Scholar]
- 12.LeChevallier M W, Hassenauer T S, Camper A K, McFeters G A. Disinfection of bacteria attached to granular activated carbon. Appl Environ Microbiol. 1984;48:918–923. doi: 10.1128/aem.48.5.918-923.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matson J V, Characklis W G. Diffusion into microbial aggregates. Water Res. 1976;10:877–885. [Google Scholar]
- 14.McMath S M, Delanoue A, Holt D M. Proceedings of the Water Quality Technology Conference of the American Water Works Association. 1997. “Clumps” shed from pipewalls in distribution systems, abstr. 5D5; p. 14. [Google Scholar]
- 15.Morin P, Camper A, Jones W, Gatel D, Goldman J C. Colonization and disinfection of biofilms hosting coliform-colonized carbon fines. Appl Environ Microbiol. 1996;62:4428–4432. doi: 10.1128/aem.62.12.4428-4432.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morin, P., V. Gauthier, S. Saby, and J. C. Block. Bacterial resistance to chlorine through attachment to particles and pipe surfaces in drinking water distribution systems, in press. In Proceedings of Biofilms in Aquatic Systems, a Conference of the Royal Society of Chemistry, Warwick University.
- 17.Pernitsky D J, Finch G R, Huck P M. Recovery of attached bacteria from GAC fines and implications for disinfection efficacy. Water Res. 1997;31:385–390. [Google Scholar]
- 18.Peyton B M, Characklis W G. Kinetics of biofilm detachment. Water Sci Technol. 1992;26:1995–1998. [Google Scholar]
- 19.Ridgway H F, Olson B H. Scanning electron microscope evidence for bacterial colonization of a drinking water distribution system. Appl Environ Microbiol. 1981;41:274–287. doi: 10.1128/aem.41.1.274-287.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridgway H F, Olson B H. Chlorine resistance patterns of bacteria from two drinking water distribution systems. Appl Environ Microbiol. 1982;44:972–987. doi: 10.1128/aem.44.4.972-987.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittmann B E. The effect of shear stress on biofilm loss rate. Biotechnol Bioeng. 1982;24:501–506. doi: 10.1002/bit.260240219. [DOI] [PubMed] [Google Scholar]
- 22.Smith S E, Bisset A, Colbourne J S, Holt D, Lloyd B J. The occurrence and significance of particles and deposits in a drinking water distribution system. J N Engl Water Works Assoc. 1997;111:135–150. [Google Scholar]
- 23.Stewart M H, Olson B H. Proceedings of the Water Quality Technology Conference of the American Water Works Association. 1986. Mechanisms of bacterial resistance to inorganic chloramines; pp. 577–590. [Google Scholar]
- 24.Stewart M H, Wolfe R L, Means E G. Assessment of the bacteriological activity associated with granular activated carbon treatment of drinking water. Appl Environ Microbiol. 1990;56:3822–3829. doi: 10.1128/aem.56.12.3822-3829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart P S, Peyton B M, Drury W J, Murga R. Quantitative observations of heterogeneities in Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 1993;59:327–329. doi: 10.1128/aem.59.1.327-329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stringfellow W T, Mallon K, Digiano F A. Enumerating and disinfecting bacteria associated with particles released from GAC filters-adsorbers. J Am Water Works Assoc. 1993;85:70–80. [Google Scholar]
- 27.Wonnacott T H, Wonnacott R J. Statistique: economie, gestion, sciences, médecine. 4th ed. Paris, France: Economica; 1991. [Google Scholar]