Abstract
Female APOE4 carriers have a greater predisposition to developing Alzheimer’s disease (AD) compared to their male counterparts, which may partly be attributed to menopause. We previously reported that a combination of menopause and APOE4 led to an exacerbation of cognitive and neurological deficits, which were associated with reduced brain DHA and DHA:AA ratio. Here, we explored whether DHA-enriched fish oil (FO) supplementation mitigated the detrimental impact of these risk factors. Whilst DHA-enriched fish oil improved recognition memory (NOR) in APOE4 VCD (4-vinylcyclohexene diepoxide)-treated mice (p < 0.05), no change in spatial working memory (Y-maze) was observed. FO supplementation increased brain DHA and nervonic acid and the DHA:AA ratio. The response of key bioenergetic and blood–brain barrier related genes and proteins provided mechanistic insights into these behavioural findings, with increased BDNF protein concentration as well as mitigation of aberrant Erβ, Cldn1 and Glut-5 expression in APOE4 mice receiving fish oil supplementation (p < 0.05). In conclusion, supplementation with a physiologically relevant dose of DHA-enriched fish oil appears to offer protection against the detrimental effects of menopause, particularly in “at-risk” APOE4 female carriers.
Keywords: Alzheimer’s disease, apolipoprotein E, arachidonic acid, docosahexaenoic acid, brain, BDNF, oestrogen, oestrogen receptor, Glut-5, 4-vinylcyclohexanediepoxide
1. Introduction
Developing interventions to prevent or delay onset of dementia is a key priority [1]. APOE4 is the strongest common genetic risk factor for the development of Alzheimer’s disease (AD) [2]; however, a fundamental understanding of its role in AD has been confounded by the pleiotropic nature of the APOE gene [3]. Evidence suggests that the AD risk associated with an APOE4 genotype is greater in females [4,5], particularly between the ages of 55 and 70 years [6], which may partly account for the greater overall incidence of AD in women [7]. The specific age at which this heightened risk occurs, coupled with the importance of oestrogens in cognition and the association between menopause and cognitive decline, is indicative of a potential menopausal involvement [8,9]. From the limited evidence currently available [10,11,12,13], neurological deficits associated with menopause may be exacerbated by an APOE4 genotype.
Docosahexaenoic acid (DHA) is the primary long-chain n-3 PUFA present in the brain, accounting for ~15% of total lipids [14]. Epidemiological studies have shown that higher DHA intake and status, primarily achieved through intake of oily fish, improves cognitive performance and reduces AD risk [15,16,17]. Although evidence from randomised control trials with DHA supplementation has been inconsistent [18,19,20], potentially due to variability in study designs (e.g., length of intervention, stage of disease progression and dose of intervention and DHA:EPA ratio), the benefits of DHA supplementation have been widely reported in cells and rodent models of AD [21,22,23]. Such models have revealed several brain structural and functional roles for DHA [24], including neuronal signalling, survival/neurogenesis, anti-amyloidogenic and anti-inflammatory effects [24,25,26]. In addition, human studies utilising 11C- and 13C-labelled carbon have identified APOE4-associated deficits in DHA metabolism and transport [27,28], which may account for APOE4-associated lower brain DHA status as we had previously reported [29]. As a result, APOE4 carriers may be more vulnerable to dietary n-3 PUFA deficiencies [30], which may in turn impact cognition, thus providing a rationale for higher intake [31]. There is evidence that both menopause and a high-fat (HF) diet disrupt n-3 PUFA status [32,33,34,35]. Therefore, postmenopausal APOE4 carriers consuming “Western-style” diets may be at particular risk of DHA deficiency and are likely to be responsive to intervention. We had previously reported that both APOE4 and menopause impact cognition, synaptic plasticity and brain DHA [13]. Here, we posit that supplementation with DHA-rich fish oil (FO) may restore DHA status and in turn ameliorate the cognitive deficits observed in postmenopausal APOE4 carriers. The effects of DHA supplementation were established by administering a physiologically relevant concentration of DHA-enriched FO to an APOE-TR mouse model where human menopause was induced by 4-vinylcyclohexane diepoxide (VCD) treatment. Behavioural tests of cognition were performed and were related to brain fatty acid, protein and gene expression profiles.
2. Materials and Methods
2.1. Study Approval
All experimental procedures and protocols used in this study were reviewed and approved by the Animal Welfare and Ethical Review Body (AWERB) and were conducted within the provisions of the Home Office Animals (Scientific Procedures) Act 1986.
2.2. Animal Model and Experimental Design
Female humanised APOE3 (B6.129P2-Apoetm2(APOE*3)Mae N8)- and APOE4 (B6.129P2-Apoetm2(APOE*4)Mae N8)-targeted replacement mice homozygous for the human APOE3 or APOE4 gene (Taconic, Germantown, NY, USA) were used in these experiments [29,36,37]. Mice were maintained in a controlled environment (21 ± 2 °C; 12 h light–dark cycle; light from 07:00 h) and fed ad libitum on a standard chow diet (RM3-P, Special Diet Services, Essex, UK) until the age of 4 months, ensuring normal development. Following this run-in period, mice were switched to one of two diets (Research Diets, New Brunswick, NJ, USA) for the remaining experimentation. The two diets were as follows: (1) high-fat diet (45 kCal% from fat) (HF) (2) and high-fat diet (45 kCal% from fat) with the addition of DHA-enriched fish oil (HFFO) (see Table S1 for full dietary composition). A HF diet was chosen to mimic a human “Western-style” diet, a risk factor prevalent in modern society that exacerbates AD-like age-related cognitive decline [38]. For the fish-oil-enriched diet, a bespoke blend of EPAX 1050 TGN + EPAX 6000 TGN (4:1 DHA:EPA fish oil) gifted from Epax® (Oslo, Norway) was added to the background diet. A 4:1 ratio (w/w) was selected based on previous studies [39], which state the importance of DHA in APOE4 carriers and the fact that the brain is highly enriched in DHA relative to the EPA. However, some EPA was retained on the basis that human oily fish sources provide both EPA and DHA in variable ratios depending on species and that EPA is important for glial cell function [40]. The enriched diet was designed to provide DHA + EPA at 4.7 g/kg of diet. Given that mean food intake in the mice was 3.2 g/day, this equated to a DHA + EPA human dose of ~2 g per day based on allometric scaling and body surface (BSA)-based calculations [41,42]. To prevent lipid oxidation, diets were stored at −20 °C until use, and fresh feed was provided every 3 days.
Menopause was induced using 14 injections of VCD (160 mg/kg) over a 3-week period beginning at 8 months of age [13], resulting in menopause being induced at 12 months of age, which roughly corresponds to middle age for a C57BL/6 mouse. Following completion of the final behavioural test, animals aged 12 months were sedated with a mixture of isoflurane (1.5%) in nitrous oxide (70%) and oxygen (30%) and transcardially perfused with an ice-cold PBS containing protease (SIGMAFASTTM protease inhibitor, Sigma, Devon, UK) and phosphatase (1 mM sodium pyrophosphate and 50 mM sodium fluoride, Sigma, Devon, UK) inhibitors. Sera were isolated via centrifugation at 2000× g for 10 min. Brains were rapidly removed, halved, snap frozen and stored at −80 °C until biochemical analysis. Animal numbers at study completion were as follows: APOE3 HF VCD, n = 12; APOE4 HF VCD, n = 12; APOE3 HF FO, n = 10; APOE4 HF FO, n = 7.
2.3. Behavioural Assessment
All behavioural tests were performed when mice reached 12 months of age. Prior to commencing, a visual placing test was performed on each animal to ensure animals were not visually impaired [43]. All behavioural tests were analysed using the Ethovision software (Tracksys Ltd., Nottingham, UK).
The novel object recognition (NOR), a measure of recognition memory was performed as described previously [44,45] with slight modifications. Briefly, on day 1, habituation was conducted in the empty maze for 10 min. On day 2, animals were conditioned to a single object for a 10 min period. On day 3, mice were exposed to 2 identical objects for 15 min. Following an intertrial interval of one hour, mice were placed back within the testing arena now containing one familiar object and one novel object. Videos were analysed for a 5 min period, after which if an accumulative object exportation of 8 s failed to be reached, analysis continued for the full 10 min or until 8 s was achieved. Those not achieving 8 s exploration were excluded from the analysis [46]. Preference index, the ratio of novel object exploration time divided by the total exploration time with both objects, was calculated.
The Y-maze spontaneous alternation test, a measure of spatial working memory, was performed as previously described [13]. Ethovision software was used to analyse each animal for 7 min, with zone transitioning and locomotor activity recorded. Spontaneous alternation was calculated using the following formula: (number of alternations/max number of alternations × 100).
2.4. Biochemical Analyses
Follicle-stimulating hormone (FSH) and brain-derived neurotrophic factor (BDNF) concentrations were determined by ELISA (Abnova, Taipei, Taiwan, ref KA2330 and R&D Systems, Minneapolis, MN, USA, ref DY248, respectively) in sera samples and cerebral cortex tissue, respectively, as per the manufacturer’s instructions. Total lipids were extracted from subcortical brain tissues (n = 5/6 per group), and the erythrocyte fraction using the Folch extraction method [47] as previously reported [29].
2.5. RNA Isolation and qRT-PCR
RNA isolation, cDNA synthesis and RT-qPCR were carried out as previously described [48]. Briefly, total RNA was isolated from the hippocampal samples using the Qiazol reagent (Qiagen, Manchester, UK). Here, 1 μg of total RNA was treated with DNase I (Invitrogen, Renfrew, UK) and used for cDNA synthesis using Invitrogen™ Oligo (dT) primers and M-MMLV reverse transcriptase. Quantitative real-time PCR (RT-qPCR) reactions were performed using SYBR green detection technology on the Roche light cycler 480 (Roche Life Science, Penzberg, Germany). Results are expressed as relative quantity scaled to the average across all samples per target gene and normalised to the reference gene glyceraldehyde-3-phosphate dehydrogenase (Gapdh), which was identified as the optimal housekeeping selection/combination using the Normfinder software [49]. The primer sequences are given in Table S2.
2.6. Statistical Analysis
All data are presented as mean ± SEM. Data analysis was performed in GraphPad Prism version 8 (GraphPad Software, San Diego, CA, USA). After checking for normality/equal variances and performing transformation where necessary, comparisons among groups were performed using either two-way or three-way ANOVA examining the impact of FO intervention and genotype (APOE3 and APOE4) as well as age (in months for body weight analysis) on the outcome variables. The p values were corrected for multiple testing using the Benjamini–Hochberg false discovery rate (FDR), with FDR 5% applied for comparison; p values of less than 0.05 were considered statistically significant.
3. Results
3.1. FO Supplementation or Ovarian Failure Does Not Significantly Modify Weight Gain
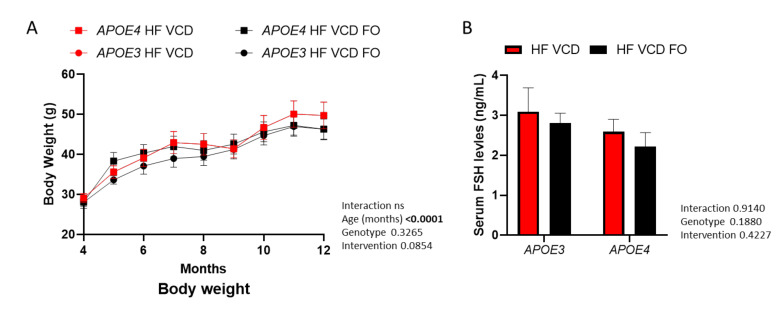
The results included here are part of a study series with our previous report, which detailed the impact of VCD treatment on phenotype relative to sham-injected animals [13]. The data for our reference VCD-treated animals have been previously reported [13]. Although body weight significantly increased over the 8-month intervention (p < 0.0001; Figure 1A), it did not differ across experimental groups. The effect of VCD treatment on serum FSH levels (see [13]) was not altered by FO intervention (p > 0.05; Figure 1B).
Figure 1.
Effects of fish oil (FO) supplementation on body weight and follicle-stimulating hormone (FSH) levels. (A) Although body weight significantly increased as animals aged, it was not altered across genotype or FO supplementation (three-way ANOVA n ≥ 7). (B) Serum FSH levels remained unchanged by FO intervention. Data are presented as mean ± SEM.
3.2. DHA-Rich FO Supplementation Restores APOE4-Induced Impairment in Recognition Memory
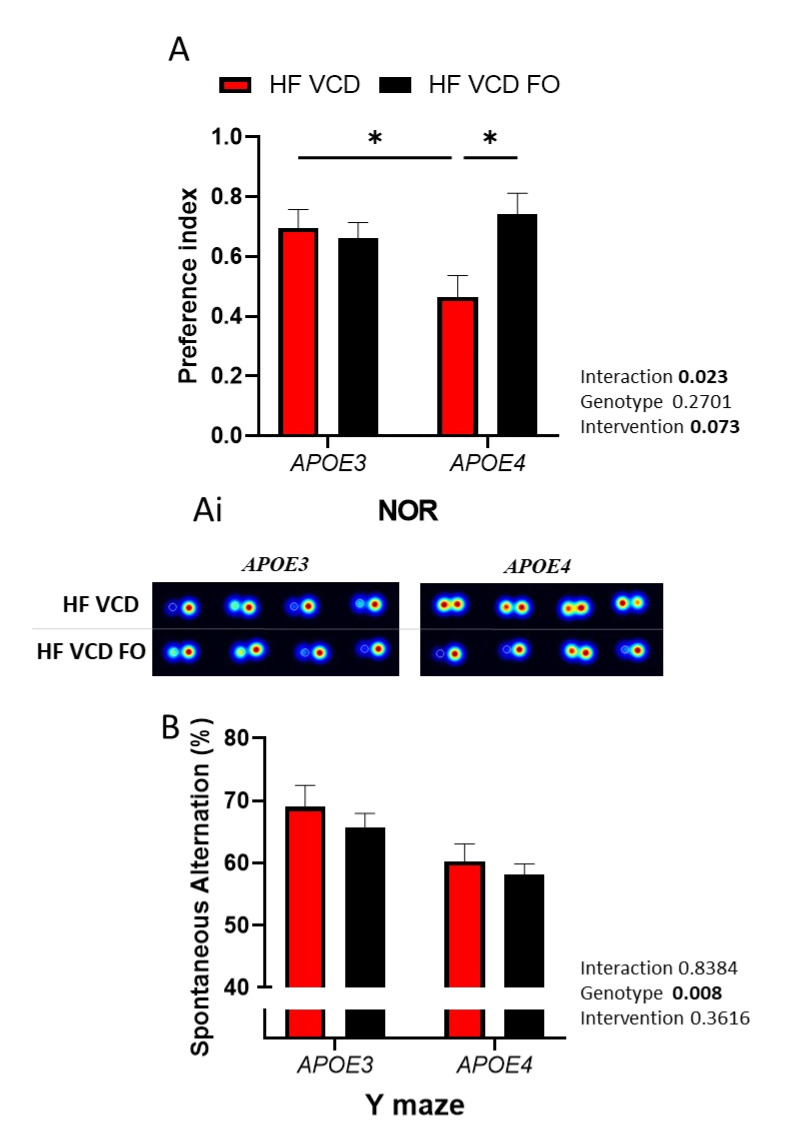
As previously reported, VCD treatment was detrimental to recognition memory performance in APOE4 animals [13]. Interestingly, supplementation with DHA-enriched FO improved recognition memory performance by 37% (p < 0.05; Figure 2A, with representative heatmaps shown in Figure 2Ai). Object recognition was not influenced by changes in locomotor activity (Figure S1A,B).
Figure 2.
The effect of fish oil (FO) supplementation on cognitive performance: (A) Recognition memory was improved via FO supplementation (n ≥ 6); (Ai) representative heatmaps of the performance of individual animals. (B) The Y-maze performance diminished under APOE4 genotype and was not influenced by FO supplementation. Data are presented as mean ± SEM; * p < 0.05.
Conversely, the Y-maze performance, a measure of spatial working memory, was reduced as a result of APOE4 genotype (p < 0.01) and was not improved by FO supplementation (Figure 2B).
3.3. DHA-Rich FO Supplementation Increases DHA Levels in the Brain of VCD-Treated Animals
FO supplementation significantly increased brain DHA levels (p < 0.01; Table 1). Furthermore, brain DHA:AA ratio, posited as an indicator of anti-inflammatory status, significantly increased in response to supplementation, with levels across both genotypes increasing by 30% (p < 0.0001; Table 1). In addition to n-3 PUFA status, changes in n-6 PUFA status were also apparent (Table 1). Furthermore, individual monounsaturated (MUFA) and saturated fatty acids (SFA) were modulated by FO intervention (Table S3), indicating broader fatty acid changes. Notably, levels of nervonic acid (24:1 n-9) which were diminished in APOE4 VCD-treated animals (reference), increased through FO supplementation (p < 0.05; Table 1).
Table 1.
Brain fatty acid composition.
| Fatty Acid % Total | APOE3 | APOE4 | |||||
|---|---|---|---|---|---|---|---|
| HF VCD |
HF VCD FO |
HF VCD |
HF VCD FO |
Genotype | Intervention | Interaction | |
| Total n-3 PUFA | 11.6 ± 0.70 | 13.6 ± 0.40 | 11.8 ± 0.50 | 13.5 ± 0.50 | 0.9991 | 0.0022 | 0.8036 |
| 20:5 n-3 (EPA) | 0.03 ± 0.01 | 0.06 ± 0.03 | 0.03 ± 0.01 | 0.09 ± 0.03 | 0.9242 | 0.3191 | 0.2855 |
| 22:5 n-3 | 0.10 ± 0.05 | 0.07 ± 0.03 | 0.06 ± 0.02 | 0.04 ± 0.01 | 0.2796 | 0.3508 | 0.9236 |
| 22:6 n-3 (DHA) | 11.2 ± 0.50 | 13.5 ± 0.40 | 11.7 ± 0.50 | 13.4 ± 0.50 | 0.7449 | 0.0006 | 0.5554 |
| Total n-6 | 12.6 ± 0.40 | 11.1 ± 0.30 | 13.2 ± 0.60 | 11.5 ± 0.40 | 0.2726 | 0.0036 | 0.8050 |
| 18:2 n-6 | 0.43 ± 0.07 | 0.43 ± 0.04 | 0.39 ± 0.06 | 0.43 ± 0.05 | 0.7696 | 0.7616 | 0.6878 |
| 20:2 n-6 | 0.08 ± 0.03 | 0.10 ± 0.05 | 0.07 ± 0.02 | 0.09 ± 0.02 | 0.7328 | 0.6002 | 0.9964 |
| 20:3 n-6 | 0.21 ± 0.02 | 0.42 ± 0.03 | 0.23 ± 0.02 | 0.40 ± 0.05 | 0.9581 | <0.0001 | 0.5492 |
| 20:4 n-6 (AA) | 8.10 ± 0.39 b | 7.5 ± 0.28 b | 8.67 ± 0.50 | 7.88 ± 0.26 | 0.2471 | 0.0892 | 0.7692 |
| 22:4 n-6 | 3.23 ± 0.14 | 2.57 ± 0.07 | 3.07 ± 0.15 | 2.64 ± 0.15 | 0.7537 | 0.0005 | 0.3814 |
| 22:5 n-6 | 0.54 ± 0.09 | 0.02 ± 0.01 | 0.77 ± 0.10 | 0.03 ± 0.02 | 0.1017 | <0.0001 | 0.1236 |
| DHA:AA | 1.39 ± 0.05 | 1.79 ± 0.02 | 1.36 ± 0.03 | 1.76 ± 0.04 | 0.3425 | <0.0001 | 0.9501 |
| SFA | 35.5 ± 0.90 | 37.0 ± 0.60 | 36.9 ± 1.20 | 36.6 ± 0.80 | 0.5870 | 0.5145 | 0.3146 |
| 14:0 | 0.24 ± 0.04 | 0.09 ± 0.03 | 0.18 ± 0.05 | 0.10 ± 0.03 | 0.5783 | 0.0052 | 0.2971 |
| 16:0 | 14.70 ± 0.70 | 16.40 ± 0.40 | 17.30 ± 0.90 | 16.30 ± 0.70 | 0.1000 | 0.6241 | 0.0809 |
| 18:0 | 18.90 ± 0.50 | 19.60 ± 0.30 | 18.30 ± 0.60 | 19.40 ± 0.30 | 0.4033 | 0.0600 | 0.6637 |
| 20:0 | 0.41 ± 0.03 | 0.38 ± 0.02 | 0.33 ± 0.03 | 0.35 ± 0.04 | 0.0966 | 0.7664 | 0.4637 |
| 22:0 | 0.38 ± 0.05 | 0.25 ± 0.09 | 0.19 ± 0.06 | 0.25 ± 0.09 | 0.2206 | 0.7141 | 0.2283 |
| 24:0 | 0.78 ± 0.18 | 0.26 ± 0.13 | 0.53 ± 0.14 | 0.39 ± 0.11 | 0.6621 | 0.0285 | 0.1980 |
| MFA | 30.5 ± 0.8 | 30.0 ± 0.8 | 27.9 ± 1.4 | 30.2 ± 0.9 | 0.2229 | 0.1262 | 0.0984 |
| 16:1 n-9 | 0.22 ± 0.06 | 0.36 ± 0.05 | 0.37 ± 0.06 | 0.37 ± 0.06 | 0.1805 | 0.2057 | 0.2327 |
| 16:1 n-7 | 0.42 ± 0.01 | 0.48 ± 0.04 | 0.46 ± 0.04 | 0.50 ± 0.02 | 0.4596 | 0.1600 | 0.6908 |
| 18:1 n-9 | 21.20 ± 0.80 | 20.60 ± 0.40 | 19.30 ± 1.20 | 20.20 ± 0.40 | 0.1337 | 0.8116 | 0.3281 |
| 18:1 n-7 | 3.58 ± 0.16 | 3.77 ± 0.12 | 3.39 ± 0.13 | 3.59 ± 0.11 | 0.1694 | 0.1470 | 0.9972 |
| 20:1 n-9 | 3.04 ± 0.19 | 2.73 ± 0.16 | 2.21 ± 0.21 | 2.82 ± 0.31 | 0.1248 | 0.5351 | 0.0603 |
| 22:1 n-9 | 0.21 ± 0.03 | 0.18 ± 0.04 | 0.18 ± 0.02 | 0.18 ± 0.04 | 0.7174 | 0.6213 | 0.6748 |
| 24:1 n-9 | 1.96 ± 0.18 a | 1.90 ± 0.16 a | 1.14 ± 0.24 b | 2.03 ± 0.23 a | 0.1094 | 0.058 | 0.0345 |
| Total DMA | 9.82 ± 0.47 | 8.23 ± 0.51 | 8.61 ± 0.44 | 8.48 ± 0.72 | 0.9861 | 0.1982 | 0.1098 |
PUFA, polyunsaturated fatty acid; 20:5 n-3 EPA, eicosapentaenoic acid; 22:5 n-3, docosapentaenoic acid; 22:6 n-3 DHA, docosahexaenoic acid; 18:2 n-6,, linoleic acid; 20:2 n-6, eicosadienoic acid; 20:3 n-6, dihomo-gamma-linolenic acid; 20:4 n-6 AA, arachidonic acid; 22:4 n-6, adrenic acid; 22:5 n-6, docosapentaenoic acid; DHA:AA, docosahexaenoic acid to arachidonic acid ratio; SFA, saturated fatty acid; 14:0, myristic acid; 16:0, palmitic acid; 18:0, stearic acid; 20:0, eicosanoic acid; 22:0, docosanoic acid; 24:0, tetracosanoic acid; MUFA, monounsaturated fatty acid; 16:1 n-9, palmitoleic acid; 16:1 n-7, palmitoleic acid; 18:1 n-9, oleic acid; 18:1 n-7, cis-vaccenic acid; 20:1 n-9, 11-eicosenoic acid; 22:1 n-9, erucic acid; 24:1 n-9, nervonic acid. Brain fatty acid composition of experimental animals (n = 5/6 per group) Data is % of total fatty acids and mean value ± SEM. Two-way ANOVA; a,b denote significant differences as analysed via Benjamini–Hochberg FDR correction if interaction effect was established. Bold numbers show significant p values.
3.4. FO Supplementation Improves Brain Deficits Induced by VCD and APOE4 Genotype
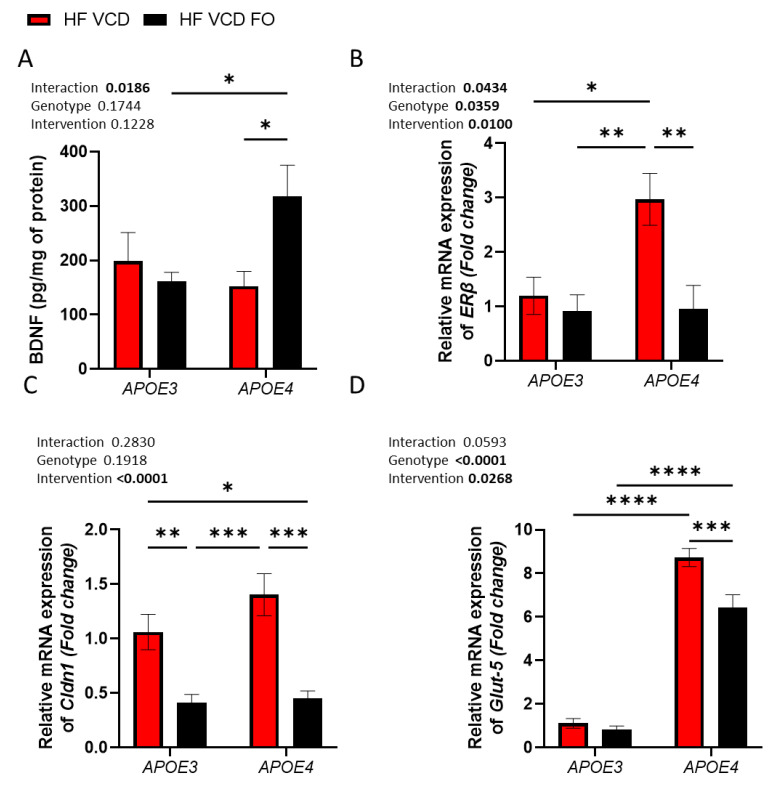
In APOE4 animals, FO supplementation resulted in a 2-fold increase in BDNF concentration (p < 0.05; Figure 3A). Expression of the oestrogenic receptor ERβ was distinctly increased in APOE4 VCD-treated animals, with the expression 2.5-fold greater than in APOE3 VCD-treated animals (p < 0.05; Figure 3B). The addition of FO prevented this change associated with APOE4 and VCD (p < 0.01; Figure 3B). Gene expression of the tight junction proteins Cldn1 and ZO-1 were similarly reduced by FO treatment, although only Cldn1 achieved significance (p < 0.0001 and p = 0.0526; Figure 3C and Table 2, respectively). Finally, we observed a large change in the expression of bioenergetic/metabolic-related genes (predominantly associated with fructose metabolism) within the brain. Notably, expression of the fructose transporter Glut-5 was increased 8-fold in APOE4 animals (p < 0.0001; Figure 3D). FO treatment significantly reduced Glut-5 expression by 14% compared to APOE4 VCD-treated animals (p < 0.001 Figure 3D). In contrast, Glut-1 remained constant, whilst Glut-3 was reduced in response to FO supplementation (p < 0.05, Table 2). Chrebp and Gsk3b were both similarly reduced by FO supplementation (p < 0.01, Table 2). Interestingly, the fructose-metabolising enzyme Aldob was expressed to a greater extent in APOE4 animals (p < 0.05; Table 2). As with Gsk3b and Chrebp, FO supplementation reduced Aldob expression (p < 0.05; Table 2). We observed no change in ERα (Table 2).
Figure 3.
The effect of fish oil (FO) supplementation on molecular targets in APOE4 and VCD-induced dysregulation. (A) Addition of FO supplementation increased BDNF levels in only APOE4 animals. (B) Increased Erβ expression observed in APOE4 VCD-treated animals was mitigated by FO supplementation. (C) Cldn1 was upregulated by VCD treatment; this expression was reduced across both genotypes through FO supplementation. (D) Expression of the fructose transporter Glut-5 was considerably increased in APOE4 animals compared to APOE3 and was partially recovered by FO treatment. Data are presented as mean ± SEM; * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Table 2.
RT-qPCR analysis of key bioenergetic and blood–brain barrier related genes in the hippocampus.
| Gene | Category | APOE3 | APOE4 | Genotype | Intervention | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| HF VCD | HF VCD FO |
HF VCD | HF VCD FO |
|||||
| ERα | Oestrogen receptor |
1.04 ± 0.15 | 0.91 ± 0.12 | 1.04 ± 0.15 | 1.06 ± 0.09 | 0.5836 | 0.6634 | 0.5687 |
| Zo-1 | Tight Junction |
1.04 ± 0.13 | 0.92 ± 0.05 | 1.30 ± 0.12 | 0.97 ± 0.12 | 0.1762 | 0.0669 | 0.3045 |
| Glut-1 | Metabolic/Bioenergetic | 1.01 ± 0.06 | 0.80 ± 0.06 | 0.87 ± 0.06 | 0.93 ± 0.08 | 0.9528 | 0.2605 | 0.0610 |
| Glut-3 | Metabolic/Bioenergetic | 1.01 ± 0.08 | 0.801 ± 0.09 | 1.11 ± 0.07 | 0.94 ± 0.08 | 0.1463 | 0.0243 | 0.8017 |
| Chrebp | Metabolic/Bioenergetic | 1.01 ± 0.08 | 0.81 ± 0.08 | 1.11 ± 0.04 | 0.81 ± 0.06 | 0.4712 | 0.0018 | 0.4648 |
| Gsk3b | Metabolic/Bioenergetic | 1.01 ± 0.06 | 0.80 ± 0.06 | 1.12 ± 0.04 | 0.81 ± 0.10 | 0.3549 | 0.0012 | 0.4690 |
| Aldob | Metabolic/Bioenergetic | 1.04 ± 0.13 | 0.81 ± 0.11 | 1.4 ± 0.11 | 1.01 ± 0.10 | 0.0237 | 0.0132 | 0.4912 |
Hippocampal RT-qPCR of key genes (n = 5/6 per group). Data is presented as mean ΔΔCt value ± SEM. Results were analysed by two-way ANOVA. Bold numbers show significant p values.
4. Discussion
APOE4 is pleiotropic and acts upon multiple physiological processes and pathological cascades [50]. The purported ability of DHA to modulate several of these pathways emphasises the potential of DHA as a lifestyle strategy to mitigate APOE4-associated AD risk through incorporation into the diet [51]. The present study follows on from our previous report, in which both APOE4- and VCD-mediated deficits in cognition were established [13]. Here, we showed that supplementation with DHA-enriched fish oil at a physiologically relevant dose improves recognition memory deficits in APOE4 VCD-treated animals. Gene expression and protein analysis supported these behavioural findings, with DHA-enriched fish oil supplementation increasing BDNF protein abundance and ameliorating tight junction and metabolic gene disturbances. Furthermore, brain DHA was increased by DHA-enriched fish oil supplementation for both genotypes, as was the DHA:AA ratio, both of which were diminished in APOE4 VCD-treated animals.
DHA-enriched fish oil supplementation mitigated the diminished recognition memory induced by the menopause mimic specifically in APOE4. There are limited reports evaluating the cognitive impact of n-3 PUFA supplementation in menopausal models. To the best of our knowledge, this is the first report in an APOE-TR menopause model and according to APOE genotype status. We previously identified reduced Y-maze and Barnes maze performance in APOE4 animals [13], indicating a genotype-mediated spatial memory impairment that is independent of VCD treatment and that may be sex-specific [52]. The absence of VCD influence may indicate a predisposition in middle-aged APOE4 females, which is uncoupled from menopause [52], although sex hormones may still be involved. The deficits in spatial working memory as assessed through Y-maze were not improved by FO supplementation, indicating subtleties between brain regions in their sensitivity to DHA supplementation. Supplementation in APOE3 animals had no effect on cognition, likely due to the lack of impairment in these animals [39]. This may change throughout the ageing process as they become more susceptible to cognitive decline associated with ageing. Utilising specific “brain-targeting” DHA (i.e., in phospholipid form), which is metabolised to LPC-DHA and reportedly more efficacious at crossing the blood–brain barrier (BBB) [53], may allow lower dose supplementation. Interestingly, Sugasini and colleagues reported an improvement in APOE4-mediated spatial memory deficits when utilising 45 mg DHA/kg LPC-DHA, further supporting this notion [54].
DHA-enriched fish oil increased brain DHA levels in both APOE3 and APOE4 VCD-treated animals. Allessandri and colleagues [55] observed that reduction in brain DHA resulting from ovariectomy was restored through 17β-estradiol (E2) treatment. Interestingly, they reported that 17β-estradiol treatment increased both hepatic and brain LC-PUFA-synthesising enzymes, such as Δ9-, Δ6- and Δ5-desaturase, restoring or maintaining brain DHA. Supplementation with DHA-rich n-3 PUFA in this study may similarly restore [56] or compensate for the dysregulation of these synthesising enzymes, subsequently improving the brain PUFA profile. It has been reported that transport of DHA into the brain (as free fatty acid) was impaired in humans and 13-month-old APOE4-TR mice (only when compared to APOE2 mice) [27,57]. However, in our model, DHA supplementation between 4 and 12 months increased DHA in a genotype-independent manner, suggesting that significant deficits in brain DHA uptake are not yet apparent at middle age. This is consistent with the findings of Yassine and colleagues, who conducted a PET study with [1-(11)C]-DHA and actually observed a higher DHA incorporation coefficient in several brain regions in middle-aged APOE4 carriers, with the authors concluding that this may compensate for higher APOE4-associated DHA turnover [28]. In addition, the dysregulated fatty acid profile caused by menopause reportedly leads to alterations in neuronal membrane lipid raft structure, which can in turn disrupt the signalosome [58]. Supplementation with DHA could mitigate such detrimental effects. Furthermore, the DHA:AA ratio in the brain improved through fish oil supplementation, which is in line with other reports [59,60]. The dysregulation and subsequent restoration of FA homeostasis may be partly responsible for the cognitive profile observed. Indeed, DHA:AA has been used to predict mild cognitive impairment/AD development [61], with alterations in this ratio profoundly impacting inflammation [29,60,62,63]. However, both APOE3 and APOE4 animals exhibited similar PUFA dysregulation in response to VCD and as such cannot completely explain the cognitive data. Further exploration is therefore warranted. Individual SFAs and MUFAs were altered by genotype and fish oil supplementation, highlighting broader fatty acid modulation. For example, the MUFA (nervonic acid) was increased in APOE4 animals receiving DHA-enriched fish oil. Believed to be a neuroprotective mediator [64,65], this alteration demonstrates the wider influence of DHA on other lipid entities that may contribute to brain health.
As with cognitive performance and brain DHA status, fish oil supplementation improved BDNF protein levels. Surprisingly, this was restricted to APOE4 animals, with the aetiology of the APOE genotype difference currently not understood. The neurological benefits of n-3 PUFA supplementation on the neurotrophic factor (BDNF) have been widely reported [66,67], and likely contributed to the improved cognitive function observed. Further gene expression analysis revealed changes in oestrogen receptors, tight junction proteins and metabolic gene profiles. Firstly, dysregulation of the oestrogen receptor ERβ was apparent in APOE4 VCD-treated animals, with DHA-enriched fish oil supplementation mitigating this increase. Disturbances in oestrogen receptor signalling has been highly connected with brain ageing and neurodegenerative disease as these receptors influence several neural processes, including proliferation, neuroinflammation, cholesterol metabolism and synaptic plasticity [68]. Furthermore, oestrogen receptors may directly modulate APOE expression in the brain [69], which one might speculate could lead to the exacerbation of the APOE4 genotype. Expression of the tight junction proteins Cldn1 and ZO-1 was reduced by FO supplementation. Interestingly, Cldn1 has been reported to be expressed in pathological conditions, specifically during BBB leakage, and is an indicator of tight junction complex disorganization [70]. If so, it may be the case that menopause exacerbates BBB leakiness and should be the focus of future research endeavours. Conversely, the increased expression may indicate loss of protein and therefore increased expression to compensate for this. Nevertheless, DHA-enriched fish oil supplementation altered this gene expression, reaffirming the involvement of DHA in maintaining tight junction and potentially BBB integrity and function. Finally, FO treatment altered metabolic gene expression in the APOE-TR VCD model. Most notably, the dramatic increase in Glut-5 expression in APOE4 animals was partially mitigated by FO treatment. To our knowledge, there are no reports currently highlighting such an interaction. We therefore speculate that this may indicate impaired glucose metabolism and a subsequent switch to the use of fructose. This is potentially supported by the concomitant changes in Aldob, Chrebp and Gsk3b, which further support the notion that DHA has the capacity to prevent metabolic disturbances in the brain [71]. On the other hand, it may be related to inflammation representing microglia activation because this is where Glut-5 is primarily expressed in the central nervous system [72] as a constituent of the microglia “sensome” of genes [73].
5. Conclusions
Female APOE4 carriers are at greater risk of AD, which may in part be explained by a menopause–APOE4 interaction. The results of this study suggest that FO supplementation at 2 g/day attenuates multiple deleterious processes in the brain, ameliorating the impact of APOE4 and menopause. As such, FO supplementation may offer a viable strategy to mitigate the deleterious APOE4–menopause interaction. Further investigation is warranted to establish if this is the case in humans.
Acknowledgments
The authors thank the staff of the Disease Modelling Unit at the University of East Anglia for expertise and help conducting the rodent studies.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14091698/s1, Table S1: Full dietary information, Table S2: Primer sequences used for qPCR analysis, Table S3: Full correlation analysis results, Figure S1: Pearson’s correlation indicates that NOR behavioural test was not influenced by locomotor activity.
Author Contributions
A.-M.M., D.V., M.G.P. and A.M. conceptualised and designed the experiments and analytical approaches; D.V. provided the Home Office Animal License; M.G.P. and A.M. performed the research and subsequent sample processing; G.H. contributed to animal husbandry; M.G.P. and R.N.M.S. performed the fatty acid analysis; M.G.P. performed all other analysis and analysed the data; M.G.P., A.-M.M. and D.V. wrote the manuscript with contributions from all authors; M.M. and C.F. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Alzheimer’s Society, grant number AS-PhD-2015-023.
Institutional Review Board Statement
Experimental procedures and protocols were reviewed and approved by the Animal Welfare and Ethical Review Board (AWERB) and were conducted within the pro-visions of the Home Office Animals (Scientific Procedures) Act 1986.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Steyaert J., Deckers K., Smits C., Fox C., Thyrian R., Jeon Y.H., Vernooij-Dassen M., Köhler S., Interdem Taskforce on Prevention of Dementia Putting primary prevention of dementia on everybody’s agenda. Aging Ment. Health. 2021;25:1376–1380. doi: 10.1080/13607863.2020.1783514. [DOI] [PubMed] [Google Scholar]
- 2.Michaelson D.M. APOE epsilon4: The most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimers Dement. 2014;10:861–868. doi: 10.1016/j.jalz.2014.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Safieh M., Korczyn A.D., Michaelson D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019;17:64. doi: 10.1186/s12916-019-1299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farrer L.A., Cupples L.A., Haines J.L., Hyman B., Kukull W.A., Mayeux R., Myers R.H., Pericak-Vance M.A., Risch N., van Duijn C.M. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: A meta-analysis. JAMA. 1997;278:1349–1356. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 5.Snyder H.M., Asthana S., Bain L., Brinton R., Craft S., Dubal D.B., Espeland M.A., Gatz M., Mielke M.M., Raber J., et al. Sex biology contributions to vulnerability to Alzheimer’s disease: A think tank convened by the Women’s Alzheimer’s Research Initiative. Alzheimers Dement. 2016;12:1186–1196. doi: 10.1016/j.jalz.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neu S.C., Pa J., Kukull W., Beekly D., Kuzma A., Gangadharan P., Wang L.S., Romero K., Arneric S.P., Redolfi A., et al. Apolipoprotein E Genotype and Sex Risk Factors for Alzheimer Disease: A Meta-analysis. JAMA Neurol. 2017;74:1178–1189. doi: 10.1001/jamaneurol.2017.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Podcasy J.L., Epperson C.N. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin. Neurosci. 2016;18:437–446. doi: 10.31887/DCNS.2016.18.4/cepperson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arevalo M.A., Azcoitia I., Gonzalez-Burgos I., Garcia-Segura L.M. Signaling mechanisms mediating the regulation of synaptic plasticity and memory by estradiol. Horm. Behav. 2015;74:19–27. doi: 10.1016/j.yhbeh.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 9.Ryan J., Scali J., Carrière I., Amieva H., Rouaud O., Berr C., Ritchie K., Ancelin M.L. Impact of a premature menopause on cognitive function in later life. BJOG Int. J. Obstet. Gynaecol. 2014;121:1729–1739. doi: 10.1111/1471-0528.12828. [DOI] [PubMed] [Google Scholar]
- 10.Karim R., Koc M., Rettberg J.R., Hodis H.N., Henderson V.W., St. John J.A., Allayee H., Brinton R.D., Mack W.J. Apolipoprotein E4 genotype in combination with poor metabolic profile is associated with reduced cognitive performance in healthy postmenopausal women: Implications for late onset Alzheimer’s disease. Menopause. 2019;26:7–15. doi: 10.1097/GME.0000000000001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bojar I., Stasiak M., Cyniak-Magierska A., Raczkiewicz D., Lewiński A. Cognitive Function, APOE Gene Polymorphisms, and Thyroid Status Associations in Postmenopausal Women in Poland. Dement. Geriatr. Cogn. Disord. 2016;42:169–185. doi: 10.1159/000449373. [DOI] [PubMed] [Google Scholar]
- 12.Porrello E., Monti M.C., Sinforiani E., Cairati M., Guaita A., Montomoli C., Govoni S., Racchi M. Estrogen receptor alpha and APOEepsilon4 polymorphisms interact to increase risk for sporadic AD in Italian females. Eur. J. Neurol. 2006;13:639–644. doi: 10.1111/j.1468-1331.2006.01333.x. [DOI] [PubMed] [Google Scholar]
- 13.Pontifex M.G., Martinsen A., Saleh R.N.M., Harden G., Tejera N., Müller M., Fox C., Vauzour D., Minihane A.M. APOE4 genotype exacerbates the impact of menopause on cognition and synaptic plasticity in APOE-TR mice. FASEB J. 2021;35:e21583. doi: 10.1096/fj.202002621RR. [DOI] [PubMed] [Google Scholar]
- 14.Bradbury J. Docosahexaenoic acid (DHA): An ancient nutrient for the modern human brain. Nutrients. 2011;3:529–554. doi: 10.3390/nu3050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y., Zhuang P., He W., Chen J.N., Wang W.Q., Freedman N.D., Abnet C.C., Wang J.B., Jiao J.J. Association of fish and long-chain omega-3 fatty acids intakes with total and cause-specific mortality: Prospective analysis of 421 309 individuals. J. Intern. Med. 2018;284:399–417. doi: 10.1111/joim.12786. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y., Chen J., Qiu J., Li Y., Wang J., Jiao J. Intakes of fish and polyunsaturated fatty acids and mild-to-severe cognitive impairment risks: A dose-response meta-analysis of 21 cohort studies. Am. J. Clin. Nutr. 2016;103:330–340. doi: 10.3945/ajcn.115.124081. [DOI] [PubMed] [Google Scholar]
- 17.Saleh R.N.M., Minihane A.M. Fish, n-3 fatty acids, cognition and dementia risk: Not just a fishy tale. Proc. Nutr. Soc. 2021;11:1–14. doi: 10.1017/S0029665121003700. [DOI] [PubMed] [Google Scholar]
- 18.Bo Y., Zhang X., Wang Y., You J., Cui H., Zhu Y., Pang W., Liu W., Jiang Y., Lu Q. The n-3 Polyunsaturated Fatty Acids Supplementation Improved the Cognitive Function in the Chinese Elderly with Mild Cognitive Impairment: A Double-Blind Randomized Controlled Trial. Nutrients. 2017;9:54. doi: 10.3390/nu9010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiser M.J., Butt C.M., Mohajeri M.H. Docosahexaenoic Acid and Cognition throughout the Lifespan. Nutrients. 2016;8:99. doi: 10.3390/nu8020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao J., Li Q., Chu J., Zeng W., Yang M., Zhu S. Effect of n-3 PUFA supplementation on cognitive function throughout the life span from infancy to old age: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2014;100:1422–1436. doi: 10.3945/ajcn.114.095315. [DOI] [PubMed] [Google Scholar]
- 21.Takeyama E., Islam A., Watanabe N., Tsubaki H., Fukushima M., Mamun M.A., Sato S., Sato T., Eto F., Yao I., et al. Dietary Intake of Green Nut Oil or DHA Ameliorates DHA Distribution in the Brain of a Mouse Model of Dementia Accompanied by Memory Recovery. Nutrients. 2019;11:2371. doi: 10.3390/nu11102371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang G., Shi B., Wu K., Chen S., Gao X., Xiao S., Kang J.X., Li W., Huang R. The protective role of endogenous n-3 polyunsaturated fatty acids in Tau Alzheimer’s disease mouse model. Int. J. Neurosci. 2019;129:325–336. doi: 10.1080/00207454.2018.1533824. [DOI] [PubMed] [Google Scholar]
- 23.Teng E., Taylor K., Bilousova T., Weiland D., Pham T., Zuo X., Yang F., Chen P.P., Glabe C.G., Takacs A., et al. Dietary DHA supplementation in an APP/PS1 transgenic rat model of AD reduces behavioral and Abeta pathology and modulates Abeta oligomerization. Neurobiol. Dis. 2015;82:552–560. doi: 10.1016/j.nbd.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belkouch M., Hachem M., Elgot A., Lo Van A., Picq M., Guichardant M., Lagarde M., Bernoud-Hubac N. The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J. Nutr. Biochem. 2016;38:1–11. doi: 10.1016/j.jnutbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Lim G.P., Calon F., Morihara T., Yang F., Teter B., Ubeda O., Salem N., Jr., Frautschy S.A., Cole G.M. A diet enriched with the omega-3 fatty acid docosahexaenoic acid reduces amyloid burden in an aged Alzheimer mouse model. J. Neurosci. 2005;25:3032–3040. doi: 10.1523/JNEUROSCI.4225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calon F., Lim G.P., Morihara T., Yang F., Ubeda O., Salem N., Jr., Frautschy S.A., Cole G.M. Dietary n-3 polyunsaturated fatty acid depletion activates caspases and decreases NMDA receptors in the brain of a transgenic mouse model of Alzheimer’s disease. Eur. J. Neurosci. 2005;22:617–626. doi: 10.1111/j.1460-9568.2005.04253.x. [DOI] [PubMed] [Google Scholar]
- 27.Chouinard-Watkins R., Rioux-Perreault C., Fortier M., Tremblay-Mercier J., Zhang Y., Lawrence P., Vohl M.C., Perron P., Lorrain D., Brenna J.T., et al. Disturbance in uniformly 13C-labelled DHA metabolism in elderly human subjects carrying the apoE epsilon4 allele. Br. J. Nutr. 2013;110:1751–1759. doi: 10.1017/S0007114513001268. [DOI] [PubMed] [Google Scholar]
- 28.Yassine H.N., Croteau E., Rawat V., Hibbeln J.R., Rapoport S.I., Cunnane S.C., Umhau J.C. DHA brain uptake and APOE4 status: A PET study with [1-11C]-DHA. Alzheimers Res. Ther. 2017;9:23. doi: 10.1186/s13195-017-0250-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinsen A., Tejera N., Vauzour D., Harden G., Dick J., Shinde S., Barden A., Mori T.A., Minihane A.M. Altered SPMs and age-associated decrease in brain DHA in APOE4 female mice. FASEB J. 2019;33:10315–10326. doi: 10.1096/fj.201900423R. [DOI] [PubMed] [Google Scholar]
- 30.Nock T.G., Chouinard-Watkins R., Plourde M. Carriers of an apolipoprotein E epsilon 4 allele are more vulnerable to a dietary deficiency in omega-3 fatty acids and cognitive decline. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1068–1078. doi: 10.1016/j.bbalip.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 31.Yassine H.N., Braskie M.N., Mack W.J., Castor K.J., Fonteh A.N., Schneider L.S., Harrington M.G., Chui H.C. Association of Docosahexaenoic Acid Supplementation With Alzheimer Disease Stage in Apolipoprotein E epsilon4 Carriers: A Review. JAMA Neurol. 2017;74:339–347. doi: 10.1001/jamaneurol.2016.4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Witt P.M., Christensen J.H., Ewe M., Aardestrup I.V., Schmidt E.B. The incorporation of marine n-3 PUFA into platelets and adipose tissue in pre- and postmenopausal women: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2010;104:318–325. doi: 10.1017/S0007114510000371. [DOI] [PubMed] [Google Scholar]
- 33.Jin Y., Kim T.H., Park Y. Association between erythrocyte levels of n-3 polyunsaturated fatty acids and depression in postmenopausal women using or not using hormone therapy. Menopause. 2016;23:1012–1018. doi: 10.1097/GME.0000000000000667. [DOI] [PubMed] [Google Scholar]
- 34.Colangelo L.A., Ouyang P., Golden S.H., Szklo M., Gapstur S.M., Vaidya D., Liu K. Do sex hormones or hormone therapy modify the relation of n-3 fatty acids with incident depressive symptoms in postmenopausal women? The MESA Study. Psychoneuroendocrinology. 2017;75:26–35. doi: 10.1016/j.psyneuen.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma S., Zhuang Y., Gomez-Pinilla F. High-fat diet transition reduces brain DHA levels associated with altered brain plasticity and behaviour. Sci. Rep. 2012;2:431. doi: 10.1038/srep00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Knouff C., Hinsdale M.E., Mezdour H., Altenburg M.K., Watanabe M., Quarfordt S.H., Sullivan P.M., Maeda N. Apo E structure determines VLDL clearance and atherosclerosis risk in mice. J. Clin. Investig. 1999;103:1579–1586. doi: 10.1172/JCI6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullivan P.M., Mezdour H., Aratani Y., Knouff C., Najib J., Reddick R.L., Quarfordt S.H., Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 38.Thériault P., ElAli A., Rivest S. High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget. 2016;7:67808–67827. doi: 10.18632/oncotarget.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chouinard-Watkins R., Vandal M., Léveillé P., Pinçon A., Calon F., Plourde M. Docosahexaenoic acid prevents cognitive deficits in human apolipoprotein E epsilon 4-targeted replacement mice. Neurobiol. Aging. 2017;57:28–35. doi: 10.1016/j.neurobiolaging.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Bazinet R.P., Metherel A.H., Chen C.T., Shaikh S.R., Nadjar A., Joffre C., Layé S. Brain eicosapentaenoic acid metabolism as a lead for novel therapeutics in major depression. Brain Behav. Immun. 2020;85:21–28. doi: 10.1016/j.bbi.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Sharma V., McNeill J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009;157:907–921. doi: 10.1111/j.1476-5381.2009.00267.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 43.Pinto L.H., Enroth-Cugell C. Tests of the mouse visual system. Mamm. Genome. 2000;11:531–536. doi: 10.1007/s003350010102. [DOI] [PubMed] [Google Scholar]
- 44.Davis K.E., Eacott M.J., Easton A., Gigg J. Episodic-like memory is sensitive to both Alzheimer’s-like pathological accumulation and normal ageing processes in mice. Behav. Brain Res. 2013;254:73–82. doi: 10.1016/j.bbr.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Leger M., Quiedeville A., Bouet V., Haelewyn B., Boulouard M., Schumann-Bard P., Freret T. Object recognition test in mice. Nat. Protoc. 2013;8:2531–2537. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 46.Denninger J.K., Smith B.M., Kirby E.D. Novel Object Recognition and Object Location Behavioral Testing in Mice on a Budget. J. Vis. Exp. 2018;141:e58593. doi: 10.3791/58593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 48.Vauzour D., Rodriguez-Ramiro I., Rushbrook S., Ipharraguerre I.R., Bevan D., Davies S., Tejera N., Mena P., de Pascual-Teresa S., Del Rio D., et al. n-3 Fatty acids combined with flavan-3-ols prevent steatosis and liver injury in a murine model of NAFLD. Biochim. Biophys. Acta Mol. Basis Dis. 2018;1864:69–78. doi: 10.1016/j.bbadis.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Day P.E.L., Chambers K.F., Winterbone M.S., García-Blanco T., Vauzour D., Kroon P.A. Validation of control genes and a standardised protocol for quantifying gene expression in the livers of C57BL/6 and ApoE−/− mice. Sci. Rep. 2018;8:8081. doi: 10.1038/s41598-018-26431-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhong N., Weisgraber K.H. Understanding the association of apolipoprotein E4 with Alzheimer disease: Clues from its structure. J. Biol. Chem. 2009;284:6027–6031. doi: 10.1074/jbc.R800009200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frautschy S.A., Cole G.M. Why Pleiotropic Interventions are Needed for Alzheimer’s Disease. Mol. Neurobiol. 2010;41:392–409. doi: 10.1007/s12035-010-8137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bour A., Grootendorst J., Vogel E., Kelche C., Dodart J.C., Bales K., Moreau P.H., Sullivan P.M., Mathis C. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 2008;193:174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Patrick R.P. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J. 2018;33:1554–1564. doi: 10.1096/fj.201801412R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugasini D., Thomas R., Yalagala P.C.R., Tai L.M., Subbaiah P.V. Dietary docosahexaenoic acid (DHA) as lysophosphatidylcholine, but not as free acid, enriches brain DHA and improves memory in adult mice. Sci. Rep. 2017;7:11263. doi: 10.1038/s41598-017-11766-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alessandri J.M., Extier A., Al-Gubory K.H., Langelier B., Baudry C., LePoupon C., Lavialle M., Guesnet P. Ovariectomy and 17β-estradiol alter transcription of lipid metabolism genes and proportions of neo-formed n-3 and n-6 long-chain polyunsaturated fatty acids differently in brain and liver. J. Nutr. Biochem. 2011;22:820–827. doi: 10.1016/j.jnutbio.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Chouinard-Watkins R., Pinçon A., Coulombe J.D., Spencer R., Massenavette L., Plourde M. A Diet Rich in Docosahexaenoic Acid Restores Liver Arachidonic Acid and Docosahexaenoic Acid Concentrations in Mice Homozygous for the Human Apolipoprotein E epsilon4 Allele. J. Nutr. 2016;146:1315–1321. doi: 10.3945/jn.116.230052. [DOI] [PubMed] [Google Scholar]
- 57.Vandal M., Alata W., Tremblay C., Rioux-Perreault C., Salem N., Jr., Calon F., Plourde M. Reduction in DHA transport to the brain of mice expressing human APOE4 compared to APOE2. J. Neurochem. 2014;129:516–526. doi: 10.1111/jnc.12640. [DOI] [PubMed] [Google Scholar]
- 58.Marin R., Diaz M. Estrogen Interactions With Lipid Rafts Related to Neuroprotection. Impact of Brain Ageing and Menopause. Front. Neurosci. 2018;12:128. doi: 10.3389/fnins.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hashimoto M., Hossain S., Shimada T., Sugioka K., Yamasaki H., Fujii Y., Ishibashi Y., Oka J., Shido O. Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer’s disease model rats. J. Neurochem. 2002;81:1084–1091. doi: 10.1046/j.1471-4159.2002.00905.x. [DOI] [PubMed] [Google Scholar]
- 60.Hashimoto M., Tanabe Y., Fujii Y., Kikuta T., Shibata H., Shido O. Chronic administration of docosahexaenoic acid ameliorates the impairment of spatial cognition learning ability in amyloid beta-infused rats. J. Nutr. 2005;135:549–555. doi: 10.1093/jn/135.3.549. [DOI] [PubMed] [Google Scholar]
- 61.Abdullah L., Evans J.E., Emmerich T., Crynen G., Shackleton B., Keegan A.P., Luis C., Tai L., LaDu M.J., Mullan M., et al. APOE ε4 specific imbalance of arachidonic acid and docosahexaenoic acid in serum phospholipids identifies individuals with preclinical Mild Cognitive Impairment/Alzheimer’s Disease. Aging. 2017;9:964–985. doi: 10.18632/aging.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zárate R., El Jaber-Vazdekis N., Tejera N., Pérez J.A., Rodríguez C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017;6:25. doi: 10.1186/s40169-017-0153-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duvall M.G., Levy B.D. DHA- and EPA-derived resolvins, protectins, and maresins in airway inflammation. Eur. J. Pharmacol. 2016;785:144–155. doi: 10.1016/j.ejphar.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Umemoto H., Yasugi S., Tsuda S., Yoda M., Ishiguro T., Kaba N., Itoh T. Protective Effect of Nervonic Acid Against 6-Hydroxydopamine-Induced Oxidative Stress in PC-12 Cells. J. Oleo. Sci. 2021;70:95–102. doi: 10.5650/jos.ess20262. [DOI] [PubMed] [Google Scholar]
- 65.Hu D., Cui Y., Zhang J. Nervonic acid amends motor disorder in a mouse model of Parkinson’s disease. Transl. Neurosci. 2021;12:237–246. doi: 10.1515/tnsci-2020-0171. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Dong Y., Xu M., Kalueff A.V., Song C. Dietary eicosapentaenoic acid normalizes hippocampal omega-3 and 6 polyunsaturated fatty acid profile, attenuates glial activation and regulates BDNF function in a rodent model of neuroinflammation induced by central interleukin-1beta administration. Eur. J. Nutr. 2018;57:1781–1791. doi: 10.1007/s00394-017-1462-7. [DOI] [PubMed] [Google Scholar]
- 67.Sun G.Y., Simonyi A., Fritsche K.L., Chuang D.Y., Hannink M., Gu Z., Greenlief C.M., Yao J.K., Lee J.C., Beversdorf D.Q. Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases. Prostaglandins Leukot. Essent. Fat. Acids. 2018;136:3–13. doi: 10.1016/j.plefa.2017.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maioli S., Leander K., Nilsson P., Nalvarte I. Estrogen receptors and the aging brain. Essays Biochem. 2021;65:913–925. doi: 10.1042/EBC20200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang J.M., Irwin R.W., Brinton R.D. Activation of estrogen receptor alpha increases and estrogen receptor beta decreases apolipoprotein E expression in hippocampus in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2006;103:16983–16988. doi: 10.1073/pnas.0608128103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sladojevic N., Stamatovic S.M., Johnson A.M., Choi J., Hu A., Dithmer S., Blasig I.E., Keep R.F., Andjelkovic A.V. Claudin-1-Dependent Destabilization of the Blood-Brain Barrier in Chronic Stroke. J. Neurosci. 2019;39:743–757. doi: 10.1523/JNEUROSCI.1432-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sinclair A.J. Docosahexaenoic acid and the brain- what is its role? Asia Pac. J. Clin. Nutr. 2019;28:675–688. doi: 10.6133/apjcn.201912_28(4).0002. [DOI] [PubMed] [Google Scholar]
- 72.Mizuno T.M., Lew P.S., Jhanji G. Regulation of the Fructose Transporter Gene Slc2a5 Expression by Glucose in Cultured Microglial Cells. Int. J. Mol. Sci. 2021;22:12668. doi: 10.3390/ijms222312668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baufeld C., Osterloh A., Prokop S., Miller K.R., Heppner F.L. High-fat diet-induced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathol. 2016;132:361–375. doi: 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon request.