ABSTRACT
Eliminating diarrheal diseases as a leading cause of childhood morbidity and mortality in low- and middle-income countries (LMICs) will require multiple intervention strategies. In this review, we spotlight a series of preclinical studies investigating the potential of orally administered monoclonal secretory IgA (SIgA) antibodies (MAbs) to reduce disease associated with three enteric bacterial pathogens: Campylobacter jejuni, enterotoxigenic Escherichia coli (ETEC), and invasive Salmonella enterica serovar Typhimurium. IgA MAbs targeting bacterial surface antigens (flagella, adhesins, and lipopolysaccharide) were generated from mice, humanized mice, and human tonsillar B cells. Recombinant SIgA1 and/or SIgA2 derivates of those MAbs were purified from supernatants following transient transfection of 293 cells with plasmids encoding antibody heavy and light chains, J-chain, and secretory component (SC). When administered to mice by gavage immediately prior to (or admixed with) the bacterial challenge, SIgA MAbs reduced infection C. jejuni, ETEC, and S. Typhimurium infections. Fv-matched IgG1 MAbs by comparison were largely ineffective against C. jejuni and S. Typhimurium under the same conditions, although they were partially effective against ETEC. While these findings highlight future applications of orally administered SIgA, the studies also underscored the fundamental challenges associated with using MAbs as prophylactic tools against enteric bacterial diseases.
KEYWORDS: Enteric, infection, antibody, prophylactic
Diarrheal diseases remain a leading cause of morbidity and mortality in young children in low- and middle-income countries (LMICs). In 2013, the landmark Global Enteric Multicenter Study (GEMS) identified the pathogens most frequently associated with moderate-to-severe diarrhea (MSD) in infants and young children under the age of five at seven sites across Africa and Asia.1 Topping the list were rotavirus, Cryptosporidium, enterotoxigenic Escherichia coli (ETEC) and Shigella, followed by an array of other agents, including Vibrio cholerae O1 and Campylobacter jejuni. The same culprits were implicated in a separate multinational and multidisciplinary birth cohort study called MAL-ED, which reported on the incidence and etiology of diarrhea in children under the age of two in eight low resource locations around the globe.2 While a leading cause of MSD, the food-and water-borne pathogen, Salmonella enterica serovar Typhimurium (STm), also deserves attention because it has emerged in parts of Saharan and sub-Saharan Africa as a cause of fatal bloodstream infections in young children.3,4
For newborns, breastfeeding is one of the most effective defenses against diarrheal diseases.5–7 Colostrum and breast milk each contain an array of bioactive compounds, including cytokines, defensins, lactoferrin, and human milk oligosaccharides that collectively hinder opportunistic and pathogenic bacteria from colonizing the neonatal intestinal epithelium.6,8 Human colostrum and breast milk are also enriched in secretory IgA (SIgA), with concentrations ranging from 10 to 50 mg/ml in colostrum and 0.5–1 mg/ml in breastmilk.8–10 SIgA is the primary class of antibodies in mucosal secretions, including the fluids that coat the human gastrointestinal tract, where it acts locally to protect epithelial surfaces from viral and bacterial infections. SIgA also plays an important role in sculpting the gut microbiome and promoting intestinal homeostasis.11 SIgA, in colostrum and breastmilk, originates from IgA-antibody secreting cells that were primed at distal mucosal sites (e.g., intestinal mucosa), then preferentially homed to the lactating mammary gland.12,13 As such, maternally derived SIgA has immunological specificity for an array of enteric pathogens. Therefore, SIgA plays an important role in protecting the newborn gastrointestinal tract from a range of diarrheal diseases during the first years of life.
Recognizing the impact of enteric diseases on childhood health in LMICs, the Bill and Melinda Gates Foundation (BMGF) has invested in efforts to develop effective vaccines against leading causes of childhood diarrhea, including rotavirus.14 In addition, the Innovative Technology Solutions group recently awarded a series of research grants to develop low-cost supplements to human colostrum to combat MSD within at-risk populations. Of particular interest is the prospect of oral delivery of recombinant human SIgA monoclonal antibodies (MAbs) to target the handful of bacterial pathogens most frequently associated with MSD.2,15,16 The prospect of preventing infections by C. jejuni, ETEC, and STm in infants with SIgA MAbs is a tall order, considering the myriad of challenges that have been encountered over the past decades of enteric vaccine development.17,18 Nonetheless, as proof of concept, Virdi and colleagues reported that monomeric immunoglobulin A (IgA)-like antibody was sufficient to prevent ETEC-like infection in piglets.19 In this brief review, we highlight a first series of reports from BMGF’s so-called “synthetic colostrum” investment.
Secretory IgA (SIgA) in mucosal immunity
The mucosal surfaces that line the upper and lower airways, the female genital tract, and the entire length of the alimentary tract, represent points of entry for viral and bacterial pathogens. As a defense mechanism, humans secrete a myriad of complex proteinaceous and gelatinous substances that form a physical barrier against particles and pathogenic agents. In addition, a network of lymphoid tissues and specialized leukocyte populations form the so-called mucosal immune system that gives rise to pathogen-specific cellular and humoral responses associated with clearing active infections and preventing future recurrences. From the perspective of mucosal antibodies, SIgA is the most well recognized because of its sole distribution in external secretions and its unique biological attributes.
SIgA is an assemblage of two or more IgA monomers linked at their C-termini by joining (J) chain and associated with secretory component (SC) (Figure 1).20–22 Humans have two IgA subclasses (IgA1, IgA2) that differ in the length of the hinge regions (Figure 1).23 B cells that express J chain and, therefore, secrete dimeric (dIgA) and/or polymeric IgA (pIgA) are induced specifically within mucosa-associated lymphoid tissues (MALT), such as Peyer’s patches of the small intestine.24 MALT-derived plasmablasts home specifically to distant mucosal tissues and the lactating mammary gland.12,13 Dimeric and pIgA are transported across polarized epithelia in the gut and mammary glands by the polymeric immunoglobulin receptor (pIgR) and then released into intestinal secretions and breast milk, respectively. Prior to release, the pIgR is proteolytically cleaved to liberate an ~80 kDa fragment known as secretory component (SC), which remains associated with IgA after its release and, by definition, gives rise to SIgA (Figure 1).9 While the overall organization of SIgA has been known for decades, only recently has high-resolution cryo-electron microscopy revealed the molecular interactions between IgA, J chain and SC.20–22
Figure 1.
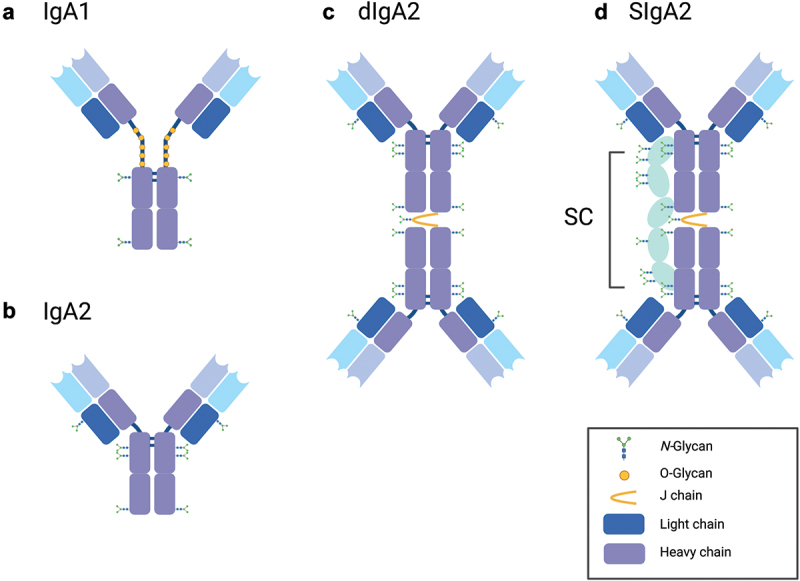
Structure of human IgA and SIgA.
Cartoons depicting human (a) monomeric IgA1, (b) monomeric IgA2, (c) dimeric IgA2, and (d) SIgA2. SIgA2 contains multiple N-glycans, with one N-glycan found on J chain. Graphic generated using BioRender.com.
A multitude of functional activities have been ascribed to SIgA, with immune exclusion at the top of the list. Immune exclusion refers to the ability of SIgA to crosslink (agglutinate) antigens, entrap them in mucus or other matrices, and promote their clearance from the intestinal lumen through peristalsis.25,26 Immune exclusion occurs in the context of the gut and the airways.27 SIgA’s other activities include toxin neutralization, inhibition of virus uptake, suppression of bacterial virulence factors, and interference with bacterial division processes (“enchained growth”).28,29 In addition to its effects against pathogens, SIgA also shapes the composition of the commensal microbiota30 and is postulated to play an important role in maintaining stability and microbial diversity on mucosal surfaces.31 However, the precise mechanisms by which SIgA influences the host microbiome remains unclear.32
Inhibition of C. jejuni infection with recombinant SIgA MAbs
C. jejuni is a primary etiological agent of MSD in children under the age of five in the developing world, according to the GEMS.1,16,33 C. jejuni infection is associated with multiple post-infectious sequelae, including reactive arthritis, Guillain-Barré syndrome, and irritable bowel disease.34 Recently published longitudinal studies conducted in seven developing countries have implicated Campylobacter as causing permanent growth stunting, a finding that has intensified the call by public health officials for measures to control Campylobacter in regions where the disease remains endemic.15,35 At the same time, there is considerable evidence that SIgA is important in immunity to C. jejuni. For example, fecal IgA antibody responses were associated with reduced illness in human subjects that underwent a primary and secondary challenge with C. jejuni strain 81–176.36 In children, immunity to C. jejuni infection correlated with levels of anti-Campylobacter SIgA in breast milk.37
C. jejuni virulence factors include capsular polysaccharide (CPS),38 cytolethal distending toxin (CDT),39 and lipo-oligosaccharide (LOS). In addition, infection of the human intestinal mucosa by C. jejuni is dependent on the bacterium’s two polar flagella, which contribute to motility, as well as adherence to and invasion of intestinal epithelial cells (Figure 2).40–44 In cell and animal models, strains of C. jejuni lacking flagella are unable to colonize the intestinal mucosa,44–46 while motility-deficient strains are severely attenuated in human subjects.47 The C. jejuni flagellar filament consists of a major flagellin subunit, FlaA, and a minor subunit, FlaB, and is capped by FliD.42 The flagellin subunits of C. jejuni are unusual in that they are heavily glycosylated, possibly to evade host innate and adaptive immunity.48 The flagellar-capping protein FliD, also known as hook-associated protein 2 (HAP2), is a 70-kDa protein with high sequence conservation across the C. jejuni species, making it an appealing target for MAbs.49,50
Figure 2.
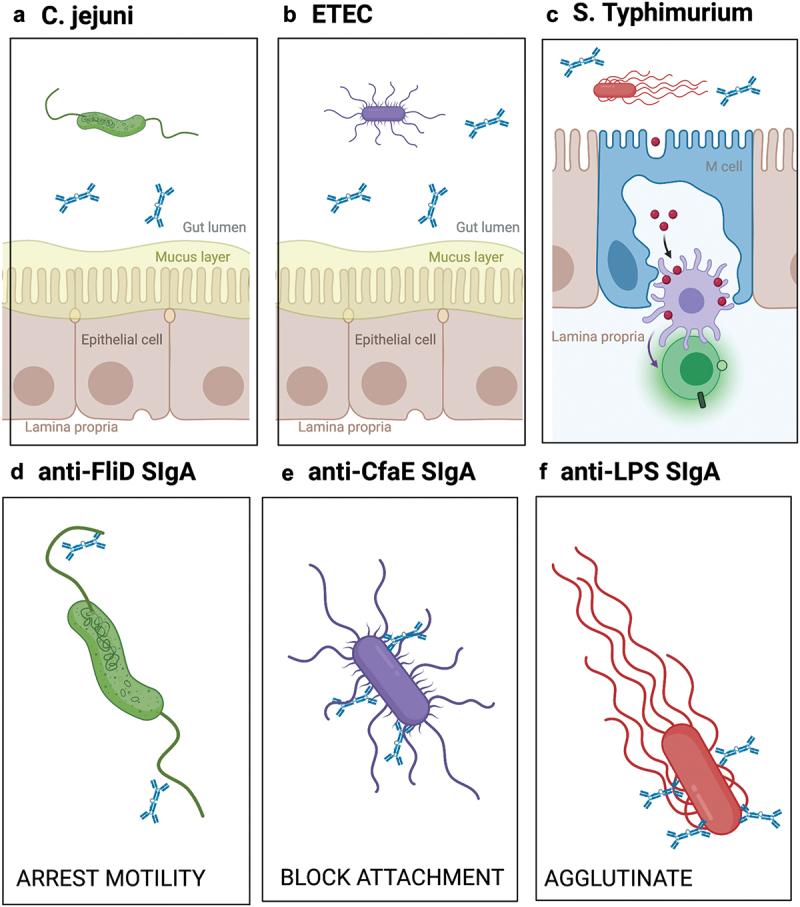
Proposed mechanisms by which SIgA MAbs prevent C. jejuni, ETEC and STm from interacting with the intestinal epithelium.
Schematic showing interactions of SIgA and (a) C. jejuni, (b) ETEC, (c) and STm with the context of the intestinal lumen, mucus, enterocytes and M cells. The lower panels (d–f) depict recombinant SIgA interactions with their target antigens and proposed modes of action. Graphic generated using BioRender.com.
Perruzza and colleagues isolated FliD-reactive MAbs from IgA+ and IgG+ B memory cells from 50 human tonsillar samples.50 B memory cells were immortalized and screened for clones expressing FliD-reactive antibodies, and the corresponding VH regions were cloned into human IgA1 or IgA2 vectors that were used to transiently co-transfect Expi293 cells in conjunction with vectors encoding VL, J-chain and SC. Properly assembled SIgA was purified from cell supernatants by affinity and size exclusion chromatography. In the end, the investigators evaluated two MAbs, CAA1 and CCG4, targeting different epitopes on FliD in a mouse model of C. jejuni colonization.
In a twenty-one-day old mouse model, animals were gavaged with 200 μg of CAA1 or CCG4 as SIgA1 or SIgA2 and challenged 2 h later with 10^8 CFU of virulent C. jejuni 81–176. Readouts of C. jejuni infection included bacterial shedding in stool and mucosal inflammation in the cecum, as measured by lipocalin-2 release, neutrophil infiltration, and a 24-point histopathology scoring system (e.g., crypt hyperplasia, goblet cell depletion, epithelial desquamation). The investigators found that CAA1 and CCG4 expressed as SIgA1 or SIgA2 were equally as effective at reducing bacterial shedding and suppressing intestinal inflammation in the mouse model. At early times points (i.e., 24 h and 48 h), shedding of C. jejuni from SIgA-treated mice was greater than the control mice, suggesting that targeting the bacterial flagella accelerates the clearance of C. jejuni from the gut lumen. At later time points (i.e., 72 h), C. jejuni shedding in the stools of CAA1 and CCG4 SIgA-treated mice were lower than controls, indicative of SIgA treatment having prevented the bacterium from establishing a niche in the cecum. Unfortunately, neither dose response or time course experiments were conducted, so it is unclear whether SIgA1 and SIgA2 variants of CAA1 and CCG4 have different efficacies when antibody levels are limiting.
One additional notable finding by Peruzza and colleagues relates the role of antibody isotype. The authors reported that 2 h pre-treatment of mice with IgG1 versions of CAA1 and CCG4 (200 μg each) had no demonstrable effect on C. jejuni colonization or campylobacter-induced inflammation, compared to the same amount of SIgA2.50 Although the reasons why IgG1 failed to confer mucosal immunity against C. jejuni were not investigated, they did report that intestinal clearance (“half-life”) of IgG1 was faster than SIgA.
Targeting ETEC colonization factors with SIgA
Enterotoxigenic E. coli (ETEC) is a motile Gram-negative bacterium that is transmitted via the fecal-oral route. ETEC is ubiquitous in LMICs and infections can occur across all age groups. For tourists and military personnel traveling or stationed in countries where ETEC is endemic, the disease presents as an acute, self-limiting bout of severe watery diarrhea commonly known as Traveler’s diarrhea. Unfortunately, things are more problematic for young children who live in these same regions. A recent global survey of LMICs implicated ETEC as a leading cause of MSD in children under the age of five, with evidence that prolonged or repeated bouts of ETEC and/or other leading causative agents of diarrhea can have repercussions for growth and cognitive development.2,51
ETEC disease pathogenesis is attributed to a handful of virulence factors (Figure 2).52 In humans, ETEC adheres to the proximal small intestinal epithelium using a panoply of adhesins (pili or fimbriae) and colonization factors, after which the bacterium secretes a cholera-like toxin known as heat-labile toxin (LT) and/or a small heat stable (ST) toxin.52 While the exact correlates of protection are not known, inhibition of colonization and toxin-neutralization are considered important determinants. Moreover, as ETEC is noninvasive and resides within the gut lumen, it is safe to assume that SIgA is important (and possibly indispensable) in preventing and clearing ETEC infections.53
In a series of studies, orally administered recombinant SIgA MAbs were evaluated in mouse and NHP models for the ability to limit the severity and duration of experimental ETEC infection.54–58 A collection of MAbs reactive with the ETEC adhesin CfaE were isolated from humanized mice and a subset expressed as recombinant SIgA or IgG1. Following down-selection based on in vitro functional assays, three SIgA MAbs were tested in mice. In those studies, ETEC (10^7) were incubated for 1 h with the equivalent of 10 mg/kg of each MAb as SIgA1 and SIgA2, then administered to mice by gavage as a single bolus. Bacterial burden was assessed 24 h later by measuring ETEC colony forming units (CFUs) from intestinal homogenates. The investigators found that irrespective of IgA subclass, each MAb reduced bacterial load by 10–100-fold in the mouse model.
One of those MAbs, 68–61 SIgA2, was also shown to afford a degree of protection against intragastric ETEC challenge when administered to non-human primates (Aotus nancymaae) at a dose of 9 mg/kg on days −1, 0 and +1. The benefit of 68–61 SIgA2 was apparent in terms of reduced severity of diarrhea, even though antibody treatment did not affect shedding of ETEC in stool. These results demonstrate the that a single orally administered SIgA MAb is able to at least partially protect mice and NHPs against ETEC colonization and disease.
When assessing ETEC vaccine efficacy in human Phase I clinical trials, the primary endpoint is defined as a reduction in episodes of moderate to severe diarrhea.59 It should be noted that bacterial shedding in stool samples does not necessarily correlate with disease severity (e.g., individuals that do not experience MSD can still shed ETEC in high numbers), an observation that can confound interpretation of preclinical studies in mice and NHPs.
Inhibition of invasive S. Typhimurium by recombinant anti-LPS SIgA
STm is a leading cause of enteric disease in children and adults worldwide. While infection normally manifests as self-limiting gastroenteritis, the emergence invasive non-typhoidal STm (iNTS) isolates such as sequence type 313 (ST313) in sub-Saharan Africa capable of causing fatal systemic infections in children and immunocompromised individuals has raised alarms.3,4,60 Furthermore, the increase of iNTS isolates carrying resistance to one or more commonly used antibiotics has prompted investigations into vaccines and alternative biologics, such as SIgA, to prevent Salmonella infections.
STm is a highly versatile pathogen that employs a range of metabolic pathways and virulence factors to successfully colonize and invade the intestinal mucosa.61 In mice, STm initially breaches the intestinal barrier by invading M cells (Figure 2), a specialized epithelial cell type overlying gut-associated lymphoid tissues such as Peyer’s patches in the ileum.62 M cell invasion is an active process that involves flagella-based motility and a type-three secretion system (T3SS) encoded by a specialized genomic island called SPI-1.63 Due to the rarity of M cells along the length of the GI tract, M cell uptake is considered a bottleneck in STm infection process.64 Following M cell uptake, STm resides within macrophages and dendritic cells as it spreads systemically, largely hidden from circulating antibodies. With this in mind, blocking STm invasion of M cells constitutes the most desirable point in which to interfere with infection.
In two recent reports, Richards and colleagues investigated the ability of orally administered mouse and human SIgA MAbs to prevent invasion of Peyer’s patch tissues by STm in a mouse model.65,66 The antibody of choice for these studies was Sal4, a well-studied murine IgA MAb directed against the immunodominant O5-antigen of STm lipopolysaccharide.67–71 In pioneering studies, Michetti and colleagues demonstrated that Sal4 IgA alone was sufficient, when transported into intestinal secretions as the result of a backpack tumor implant, to significantly reduce STm entry into Peyer’s patch tissues.70,71 To evaluate whether oral administration of Sal4 is also effective, Richards and colleagues purified dimeric Sal4 mouse IgA, complexed it with recombinant SC in vitro, and delivered it in the form of SIgA across a range of doses (0.4–50 μg per mouse; 0.02–2.5 mg/kg) to mice by gavage.65 Mice were euthanized 24 h later and bacterial numbers within the Peyer’s patch tissues were determined. When admixed with STm (10^7 CFUs), Sal4 SIgA reduced bacterial uptake into Peyer’s patch tissues in a dose-dependent manner. At the equivalent of 2.5 mg/kg Sal4 SIgA, for example, bacterial uptake was reduced by several orders of magnitude. While not unexpected, a class-switched IgG1 variant of Sal4 had no demonstrable effect on STm uptake into Peyer’s patch tissues, even at relatively high doses (10 mg/kg). It was proposed that the stark difference in efficacy between Sal4 SIgA and IgG1 was due to differences in antibody stability in the gastrointestinal environment and/or functionality due to SIgA multivalency.
In a follow-up study, Richards and colleagues generated a human Sal4 IgA2 variant and expressed it in Expi293 cells as a monomer, dimer or SIgA.66 All three forms of Sal4 IgA2 were able to reduce STm uptake into Peyer’s patch tissues when administered to mice by gavage at the time of challenge, although at lower doses SIgA and dIgA proved superior to mIgA, possibly revealing the importance of avidity and cross-linking in antibody functionality in vivo. The ability of Sal4 SIgA to promote large and densely packed aggregates of STm within the intestinal lumen was cited as the culprit in limiting bacterial uptake via M cells.
Summary and perspectives
Combatting diarrheal diseases on a global scale will require a holistic approach that includes improved water and sanitation, vaccine deployment, and targeted preventative measures for high-risk individuals. With the successful implementation of highly effective oral vaccines against rotavirus over the past decade, attention is now turned toward the other etiologic agents of MSD in certain populations, the incidence of many enteric bacterial pathogens is unabated with significant public health consequences.
The demonstration in mice and NHPs that orally administered recombinant SIgA MAbs targeting single epitopes on C. jejuni, ETEC, and STm were able to curtail intestinal infection contributes to an emerging field aimed at the development of effective prophylactics against diarrheal diseases.19,72 Nonetheless, notable challenges remain. Foremost is the need for sustained or repeated delivery of SIgA to afford protection for prolonged periods. In the mouse studies highlighted in this review, protection was achieved only when SIgA MAbs were administered shortly before or at the time of bacterial challenge. For example, Perruzza demonstrated protection against C. jejuni infection within 2 h following SIgA MAb treatment,50 while Richards reported that the window for STm was <20 min after Sal4 SIgA treatment.65 In the case of ETEC, a reduction in intestinal ETEC burden was only observed when the bacteria were premixed with anti-CfaE HuMAbs prior to oral delivery.54 Moreover, dose-response experiments were not conducted, except in the case of Sal4.65 Therefore, the exact amount of SIgA required for protection against ETEC and C. jejuni and the frequency of SIgA dosing for the three pathogens remains to be determined.
The economics of SIgA production and formulation will be major determinants of the practicability of prophylactic oral antibody treatment. Existing mammalian cell-based expression systems are considered prohibitively expensive for the purpose of manufacturing SIgA at-scale, although advances in continuous downstream processes are changing the landscape to some degree.73 Options for SIgA include a range of yeast- and plant-based systems.58,72,74,75 As a case in point, Virdi and colleagues reported that camelid single-chain-derived IgA antibodies could be produced in soybean seeds or secreted from the yeast Pichia pastoris, freeze- or spray-dried, and orally delivered within food in a pig model.19 One non-traditional platform being pursued (at least for single chain antibody production) is Spirulina.76 Alternative strategies such as transgenic animals that express human MAbs including human IgA in colostrum and breast milk are also worthy of investigation, especially considering their track record with other biologics.77,78
While oral administration of MAbs and MAb cocktails has obvious benefits over intravenous and subcutaneous routes of delivery, the pharmacokinetics and stability of SIgA in the human gastric and intestinal environments remain unknown and need to be taken in consideration.55,57 Within the context of the gut, the benefit of SIgA over IgG is obvious and is likely due to SIgA glycosylation and resistance to intestinal proteases.79 Considering the importance of IgA MAb stability to ensure adequate local concentrations in the gut, antibody engineering approaches have been employed to increase the serum half-life of polymeric IgA for a systemic administration strategy, assuming efficient luminal transport via pIgR.80 Other strategies include extending SIgA’s retention time within the protective mucus barrier using SIgA carrying bacterial-derived mucin-binding proteins.81 One caveat to that approach is that it has yet to be determined whether mucus affinity promotes or hinders mucosal protection, as recent studies on IgG in cervical vaginal mucus demonstrate that the ability to rapidly diffuse through mucus is advantageous over entrapment.82
Another approach is the use of multivalent or combination MAb cocktails to target a single pathogen of interest or to broaden the efficacy of a single prophylactic formulation. Shrestha and colleagues demonstrated that multivalent IgGs had enhanced sperm agglutinating activity in a mucin matrix designed to mimic human cervix environment.83 Others have opted to engineer camelid-derived single-chain antibodies carrying IgA Fc regions, with great success.19,72 In summary, the prospect of oral antibody prophylaxis, especially with SIgA, is of great interest as an adjunct to vaccination or antibiotic treatment. Even short-term interventions have the potential to have long-term impacts on in childhood health in LMICs. The studies highlighted in this review constitute a first step toward a new and targeted applications in combating enteric diseases.
Acknowledgments
We thank Dr. Omar Vandal (Bill and Melinda Gates Foundation) for his insight and technical comments in preparing the review.
Funding Statement
This work was supported by awards from the National Institutes of Allergy and Infectious Diseases (AI119647) and the Bill and Melinda Gates Foundation (OPP1176017). The funders had no role in the decision to publish, or preparation of the manuscript.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–8. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Platts-Mills JA, Liu J, Rogawski ET, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health. 2018;6(12):e1309–e18. doi: 10.1016/S2214-109X(18)30349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kasumba IN, Pulford CV, Perez-Sepulveda BM, Sen S, Sayed N, Permala-Booth J, Livio S, Heavens D, Low R, Hall N, et al. Characteristics of Salmonella recovered from stools of children enrolled in the global enteric multicenter study. Clin Infect Dis. 2021. doi: 10.1093/cid/ciab051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pulford CV, Perez-Sepulveda BM, Canals R, Bevington JA, Bengtsson RJ, Wenner N, Rodwell EV, Kumwenda B, Zhu X, Bennett RJ, et al. Stepwise evolution of Salmonella Typhimurium ST313 causing bloodstream infection in Africa. Nat Microbiol. 2021;6(3):327–38. doi: 10.1038/s41564-020-00836-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandtzaeg P. Mucosal immunity: integration between mother and the breast-fed infant. Vaccine. 2003;21:3382–88. doi: 10.1016/S0264-410X(03)00338-4. [DOI] [PubMed] [Google Scholar]
- 6.Hennet T, Borsig L. Breastfed at Tiffany’s. Trends Biochem Sci. 2016;41:508–18. doi: 10.1016/j.tibs.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Labbok MH, Clark D, Goldman AS. Breastfeeding: maintaining an irreplaceable immunological resource. Nat Rev Immunol. 2004;4:565–72. doi: 10.1038/nri1393. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz L, Espinosa-Martos I, García-Carral C, Manzano S, McGuire MK, Meehan CL, McGuire MA, Williams JE, Foster J, Sellen DW, et al. What’s Normal? Immune Profiling of Human Milk from Healthy Women Living in Different Geographical and Socioeconomic Settings. Front Immunol. 2017;8. doi: 10.3389/fimmu.2017.00696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brandtzaeg P. The mucosal immune system and its integration with the mammary glands. J Pediatr. 2010;156(2):S8–15. doi: 10.1016/j.jpeds.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 10.McGuire MK, Randall AZ, Seppo AE, Jarvinen KM, Meehan CL, Gindola D, Williams JE, Sellen DW, Kamau-Mbuthia EW, Kamundia EW, et al. Multipathogen analysis of IgA and IgG antigen specificity for selected pathogens in milk produced by women from diverse geographical regions: the INSPIRE study. Front Immunol. 2020;11:614372. doi: 10.3389/fimmu.2020.614372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gopalakrishna KP, Hand TW. Influence of maternal milk on the neonatal intestinal microbiome. Nutrients. 2020;12(3):823. doi: 10.3390/nu12030823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roux ME, McWilliams M, Phillips-Quagliata JM, Weisz-Carrington P, Lamm ME. Origin of IgA-secreting plasma cells in the mammary gland. J Exp Med. 1977;146:1311–22. doi: 10.1084/jem.146.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson E, Butcher EC. CCL28 controls immunoglobulin (Ig)A plasma cell accumulation in the lactating mammary gland and IgA antibody transfer to the neonate. J Exp Med. 2004;200:805–09. doi: 10.1084/jem.20041069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirkwood CD, Ma LF, Carey ME, Steele AD. The rotavirus vaccine development pipeline. Vaccine. 2019;37:7328–35. doi: 10.1016/j.vaccine.2017.03.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amour C, Gratz J, Mduma E, Svensen E, Rogawski ET, McGrath M, Seidman, JC, McCormick, BJ, Shrestha, S, Samie, A, Mahfuz, M, Qureshi, S, Hotwani, A, Babji, S, Trigoso, DR, Lima, AA, Bodhidatta, L, Bessong, P, Ahmed, T, Shakoor, S, Kang, G, Kosek, M, Guerrant, RL, Lang, D, Gottlieb, M, Houpt, ER, Platts-Mills, JA. Epidemiology and impact of campylobacter infection in children in 8 low-resource settings: results from the MAL-ED study. Clin Infect Dis. 2016;63:1171–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Platts-Mills JA, Kosek M. Update on the burden of Campylobacter in developing countries. Curr Opin Infect Dis. 2014;27:444–50. doi: 10.1097/QCO.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riddle MS, Chen WH, Kirkwood CD, MacLennan CA. Update on vaccines for enteric pathogens. Clin Microbiol Infect. 2018;24:1039–45. doi: 10.1016/j.cmi.2018.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Walker R, Kaminski RW, Porter C, Choy RKM, White JA, Fleckenstein JM, Cassels F, Bourgeois L. Vaccines for protecting infants from bacterial causes of diarrheal disease. Microorganisms. 2021;9. doi: 10.3390/microorganisms9071382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Virdi V, Palaci J, Laukens B, Ryckaert S, Cox E, Vanderbeke E, Depicker A, Callewaert N. Yeast-secreted, dried and food-admixed monomeric IgA prevents gastrointestinal infection in a piglet model. Nat Biotechnol. 2019;37:527–30. doi: 10.1038/s41587-019-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar N, Arthur CP, Ciferri C, Matsumoto ML. Structure of the secretory immunoglobulin A core. Science. 2020;367(6481):1008–14. doi: 10.1126/science.aaz5807. [DOI] [PubMed] [Google Scholar]
- 21.Kumar Bharathkar S, Parker BW, Malyutin AG, Haloi N, Huey-Tubman KE, Tajkhorshid E, Stadtmueller BM. The structures of Secretory and dimeric Immunoglobulin A. Elife. 2020;9. doi: 10.7554/eLife.56098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadtmueller BM, Huey-Tubman KE, Lopez CJ, Yang Z, Hubbell WL, Bjorkman PJ. The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins. Elife. 2016;5. doi: 10.7554/eLife.10640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Sousa-Pereira P, Woof JM. IgA: structure, function, and developability. Antibodies (Basel). 2019;8(4):57. doi: 10.3390/antib8040057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandtzaeg P, Johansen FE. Mucosal B cells: phenotypic characteristics, transcriptional regulation, and homing properties. Immunol Rev. 2005;206:32–63. doi: 10.1111/j.0105-2896.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 25.Stokes CR, Soothill JF, Turner MW. Immune exclusion is a function of IgA. Nature. 1975;255:745–46. doi: 10.1038/255745a0. [DOI] [PubMed] [Google Scholar]
- 26.Mantis NJ, Rol N, Corthesy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–11. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Binsker U, Lees JA, Hammond AJ, Weiser JN. Immune exclusion by naturally acquired secretory IgA against pneumococcal pilus-1. J Clin Invest. 2020;130:927–41. doi: 10.1172/JCI132005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forbes SJ, Bumpus T, McCarthy EA, Corthesy B, Mantis NJ. Transient suppression of Shigella flexneri type 3 secretion by a protective O-antigen-specific monoclonal IgA. mBio. 2011;2:e00042–11. doi: 10.1128/mBio.00042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moor K, Diard M, Sellin ME, Felmy B, Wotzka SY, Toska A, Bakkeren E, Arnoldini M, Bansept F, Co AD. High-avidity IgA protects the intestine by enchaining growing bacteria. Nature. 2017;544:498–502. doi: 10.1038/nature22058. [DOI] [PubMed] [Google Scholar]
- 30.Ding M, Yang B, Ross RP, Stanton C, Zhao J, Zhang H, Chen W. Crosstalk between sIgA-Coated Bacteria in Infant Gut and Early-Life Health. Trends Microbiol. 2021;29:725–35. doi: 10.1016/j.tim.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Catanzaro JR, Strauss JD, Bielecka A, Porto AF, Lobo FM, Urban A, Schofield WB, Palm NW. IgA-deficient humans exhibit gut microbiota dysbiosis despite secretion of compensatory IgM. Sci Rep. 2019;9(1):13574. doi: 10.1038/s41598-019-49923-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang C, Chen-Liaw A, Moran TM, Cerutti A, Faith JJ. Immunoglobulin a antibody composition is sculpted to bind the self gut microbiome. bioRxiv 2020:2020.11.30.405332. [DOI] [PMC free article] [PubMed]
- 33.Schnee AE, Petri WA Jr.. Campylobacter jejuni and associated immune mechanisms: short-term effects and long-term implications for infants in low-income countries. Curr Opin Infect Dis. 2017;30(3):322–28. doi: 10.1097/QCO.0000000000000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirkpatrick BD, Tribble DR. Update on human Campylobacter jejuni infections. Curr Opin Gastroenterol. 2011;27(1):1–7. doi: 10.1097/MOG.0b013e3283413763. [DOI] [PubMed] [Google Scholar]
- 35.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, Operario DJ, Uddin J, Ahmed S, Alonso PL. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388(10051):1291–301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tribble DR, Baqar S, Scott DA, Oplinger ML, Trespalacios F, Rollins D, Walker RI, Clements JD, Walz S, Gibbs P. Assessment of the duration of protection in campylobacter jejuni experimental infection in humans. Infect Immun. 2010;78(4):1750–59. doi: 10.1128/IAI.01021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruiz-Palacios GM, Calva JJ, Pickering LK, Lopez-Vidal Y, Volkow P, Pezzarossi H, West MS. Protection of breast-fed infants against Campylobacter diarrhea by antibodies in human milk. J Pediatr. 1990;116(5):707–13. doi: 10.1016/S0022-3476(05)82652-6. [DOI] [PubMed] [Google Scholar]
- 38.Bacon DJ, Szymanski CM, Burr DH, Silver RP, Alm RA, Guerry P. A phase-variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol Microbiol. 2001;40(3):769–77. doi: 10.1046/j.1365-2958.2001.02431.x. [DOI] [PubMed] [Google Scholar]
- 39.Hickey TE, Majam G, Guerry P. Intracellular survival of campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect Immun. 2005;73(8):5194–97. doi: 10.1128/IAI.73.8.5194-5197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barrero-Tobon AM, Hendrixson DR. Flagellar biosynthesis exerts temporal regulation of secretion of specific Campylobacter jejuni colonization and virulence determinants. Mol Microbiol. 2014;93:957–74. doi: 10.1111/mmi.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guerry P. Campylobacter flagella: not just for motility. Trends Microbiol. 2007;15:456–61. doi: 10.1016/j.tim.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 42.Lertsethtakarn P, Ottemann KM, Hendrixson DR. Motility and chemotaxis in Campylobacter and Helicobacter. Annu Rev Microbiol. 2011;65:389–410. doi: 10.1146/annurev-micro-090110-102908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tegtmeyer N, Sharafutdinov I, Harrer A, Soltan Esmaeili D, Linz B, Backert S. Campylobacter Virulence Factors and Molecular Host-Pathogen Interactions. Curr Top Microbiol Immunol. 2021;431:169–202. [DOI] [PubMed] [Google Scholar]
- 44.Yao R, Burr DH, Doig P, Trust TJ, Niu H, Guerry P. Isolation of motile and non-motile insertional mutants of Campylobacter jejuni: the role of motility in adherence and invasion of eukaryotic cells. Mol Microbiol. 1994;14:883–93. doi: 10.1111/j.1365-2958.1994.tb01324.x. [DOI] [PubMed] [Google Scholar]
- 45.Takata T, Fujimoto S, Amako K. Isolation of nonchemotactic mutants of Campylobacter jejuni and their colonization of the mouse intestinal tract. Infect Immun. 1992;60:3596–600. doi: 10.1128/iai.60.9.3596-3600.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pavlovskis OR, Rollins DM, Haberberger RL Jr., Green AE, Habash L, Strocko S, Walker, RI. Significance of flagella in colonization resistance of rabbits immunized with Campylobacter spp. Infect Immun. 1991;59:2259–64. doi: 10.1128/iai.59.7.2259-2264.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Black RE, Levine MM, Clements ML, Hughes TP, Blaser MJ. Experimental Campylobacter jejuni infection in humans. J Infect Dis. 1988;157:472–79. doi: 10.1093/infdis/157.3.472. [DOI] [PubMed] [Google Scholar]
- 48.Thibault P, Logan SM, Kelly JF, Brisson JR, Ewing CP, Trust TJ, Guerry, P. Identification of the carbohydrate moieties and glycosylation motifs in Campylobacter jejuni flagellin. J Biol Chem. 2001;276:34862–70. doi: 10.1074/jbc.M104529200. [DOI] [PubMed] [Google Scholar]
- 49.Chintoan-Uta C, Cassady-Cain RL, Stevens MP. Evaluation of flagellum-related proteins FliD and FspA as subunit vaccines against Campylobacter jejuni colonisation in chickens. Vaccine. 2016;34:1739–43. doi: 10.1016/j.vaccine.2016.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Perruzza L, Jaconi S, Lombardo G, Pinna D, Strati F, Morone D, Seehusen F, Hu Y, Bajoria S, Xiong J, Kumru OS, Joshi SB, Volkin DB, Piantanida R, Benigni F, Grassi F, Corti D, Pizzuto MS. Prophylactic activity of orally administered FliD-Reactive monoclonal siga against campylobacter infection. Front Immunol. 2020;11:1011. doi: 10.3389/fimmu.2020.01011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AA. The impoverished gut–a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–29. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleckenstein JM, Kuhlmann FM. Enterotoxigenic Escherichia coli infections. Curr Infect Dis Rep. 2019;21:9. doi: 10.1007/s11908-019-0665-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riaz S, Steinsland H, Hanevik K. Human mucosal IgA immune responses against enterotoxigenic Escherichia coli. Pathogens. 2020;9(9):714. doi: 10.3390/pathogens9090714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Giuntini S, Stoppato M, Sedic M, Ejemel M, Pondish JR, Wisheart D, Schiller ZA, Thomas WD Jr, Barry EM, Cavacini LA, Klempner MS, Wang Y. Identification and characterization of human monoclonal antibodies for immunoprophylaxis against enterotoxigenic Escherichia coli infection. Infect Immun. 2018;86(8):e00355-18. doi: 10.1128/IAI.00355-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu Y, Kumru OS, Xiong J, Antunez LR, Hickey J, Wang Y, Cavacini L, Klempner M, Joshi SB, Volkin DB. Preformulation characterization and stability assessments of secretory IgA monoclonal antibodies as potential candidates for passive immunization by oral administration. J Pharm Sci. 2020;109(1):407–21. doi: 10.1016/j.xphs.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stoppato M, Gaspar C, Regeimbal J, Nunez RG, Giuntini S, Schiller ZA, Gawron MA, Pondish JR, Martin JC, Schneider MI, et al. Oral administration of an anti-CfaE secretory IgA antibody protects against Enterotoxigenic Escherichia coli diarrheal disease in a nonhuman primate model. Vaccine. 2020;38(10):2333–39. doi: 10.1016/j.vaccine.2020.01.064. [DOI] [PubMed] [Google Scholar]
- 57.Wallace AL, Schneider MI, Toomey JR, Schneider RM, Klempner MS, Wang Y, Cavacini LA. IgA as a potential candidate for enteric monoclonal antibody therapeutics with improved gastrointestinal stability. Vaccine. 2020;38:7490–97. doi: 10.1016/j.vaccine.2020.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teh AY, Cavacini L, Hu Y, Kumru OS, Xiong J, Bolick DT, Joshi SB, Grünwald-Gruber C, Altmann F, Klempner M. Investigation of a monoclonal antibody against enterotoxigenic Escherichia coli, expressed as secretory IgA1 and IgA2 in plants. Gut Microbes. 2021;13(1):1–14. doi: 10.1080/19490976.2020.1859813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darsley MJ, Chakraborty S, DeNearing B, Sack DA, Feller A, Buchwaldt C, Bourgeois AL, Walker R, Harro CD. The oral, live attenuated enterotoxigenic Escherichia coli vaccine ACE527 reduces the incidence and severity of diarrhea in a human challenge model of diarrheal disease. Clin Vaccine Immunol. 2012;19(12):1921–31. doi: 10.1128/CVI.00364-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kariuki S, Mbae C, Van Puyvelde S, Onsare R, Kavai S, Wairimu C, Ngetich R, Clemens J, Dougan G. High relatedness of invasive multi-drug resistant non-typhoidal Salmonella genotypes among patients and asymptomatic carriers in endemic informal settlements in Kenya. PLoS Negl Trop Dis. 2020;14(8):e0008440. doi: 10.1371/journal.pntd.0008440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rivera-Chavez F, Baumler AJ. The pyromaniac inside you: Salmonella metabolism in the host gut. Annu Rev Microbiol. 2015;69:31–48. doi: 10.1146/annurev-micro-091014-104108. [DOI] [PubMed] [Google Scholar]
- 62.Carter PB, Collins FM. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rivera-Chavez F, Lopez CA, Zhang LF, Garcia-Pastor L, Chavez-Arroyo A, Lokken KL, Tsolis RM, Winter SE, Bäumler AJ. Energy Taxis toward Host-Derived Nitrate Supports a Salmonella Pathogenicity Island 1-Independent Mechanism of Invasion. mBio. 2016;7(4). doi: 10.1128/mBio.00960-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lim CH, Voedisch S, Wahl B, Rouf SF, Geffers R, Rhen M, Pabst O. Independent bottlenecks characterize colonization of systemic compartments and gut lymphoid tissue by Salmonella. PLoS Pathog. 2014;10(7):e1004270. doi: 10.1371/journal.ppat.1004270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Richards AF, Doering JE, Lozito SA, Varrone JJ, Willsey GG, Pauly M, Whaley K, Zeitlin L, Mantis NJ. Inhibition of invasive Salmonella by orally administered IgA and IgG monoclonal antibodies. PLoS Negl Trop Dis. 2020;14(3):e0007803. doi: 10.1371/journal.pntd.0007803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Richards AF, Baranova DE, Pizzuto MS, Jaconi S, Willsey GG, Torres-Velez FJ, Doering JE, Benigni F, Mantis NJ. Recombinant human secretory iga induces Salmonella typhimurium agglutination and limits bacterial invasion into gut-associated lymphoid tissues. ACS Infect Dis. 2021;7(5):1221–35. doi: 10.1021/acsinfecdis.0c00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Forbes SJ, Eschmann M, Mantis NJ. Inhibition of Salmonella enterica serovar typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin a antibody. Infect Immun. 2008;76(9):4137–44. doi: 10.1128/IAI.00416-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forbes SJ, Martinelli D, Hsieh C, Ault JG, Marko M, Mannella CA, Mantis NJ. Association of a protective monoclonal IgA with the O antigen of Salmonella enterica serovar Typhimurium impacts type 3 secretion and outer membrane integrity. Infect Immun. 2012;80(7):2454–63. doi: 10.1128/IAI.00018-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amarasinghe JJ, D’Hondt RE, Waters CM, Mantis NJ. Exposure of Salmonella enterica Serovar typhimurium to a protective monoclonal IgA triggers exopolysaccharide production via a diguanylate cyclase-dependent pathway. Infect Immun. 2013;81:653–64. doi: 10.1128/IAI.00813-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992;60:1786–92. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Michetti P, Porta N, Mahan MJ, Slauch JM, Mekalanos JJ, Blum AL, Kraehenbuhl J-P, Neutra MR. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology. 1994;107(4):915–23. doi: 10.1016/0016-5085(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 72.Virdi V, Juarez P, Boudolf V, Depicker A. Recombinant IgA production for mucosal passive immunization, advancing beyond the hurdles. Cell Mol Life Sci. 2016;73(3):535–45. doi: 10.1007/s00018-015-2074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khanal O, Lenhoff AM. Developments and opportunities in continuous biopharmaceutical manufacturing. mAbs. 2021;13:1903664. doi: 10.1080/19420862.2021.1903664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakanishi K, Matsuda M, Ida R, Hosokawa N, Kurohane K, Niwa Y, Kobayashi H, Imai Y. Lettuce-derived secretory IgA specifically neutralizes the Shiga toxin 1 activity. Planta. 2019;250(4):1255–64. doi: 10.1007/s00425-019-03215-1. [DOI] [PubMed] [Google Scholar]
- 75.Stoger E, Fischer R, Moloney M, Ma JK. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu Rev Plant Biol. 2014;65(1):743–68. doi: 10.1146/annurev-arplant-050213-035850. [DOI] [PubMed] [Google Scholar]
- 76.Jester B, Zhao H, Gewe M, Adame T, Perruzza L, Bolick D, Agosti, J, Khuong, N, Kuestner, R, Gamble C, Cruickshank K, Ferrara J, Lim R, Paddock T, Brady C, Ertel S, Zhang M, Tasch M, Saveria T, Doughty D, Marshall J, et al. Expression and manufacturing of protein therapeutics in spirulina. bioRxiv 2021:2021.01.25.427910.
- 77.Baranova DE, Chen L, Destrempes M, Meade H, Mantis NJ. Passive immunity to vibrio cholerae O1 afforded by a human monoclonal IgA1 antibody expressed in milk. Pathogens and Immunity. 2020;5(1):89–116. doi: 10.20411/pai.v5i1.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu X, Pollock D, Duval M, Lewis C, Joseph K, Meade H, Stoger E, Fischer R, Moloney M. Neutralization of HIV by milk expressed antibody. J Acquir Immune Defic Syndr. 2013;62(1):10–16. doi: 10.1097/QAI.0b013e318271c450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bioley G, Monnerat J, Lotscher M, Vonarburg C, Zuercher A, Corthesy B. Plasma-derived polyreactive secretory-like IgA and IgM opsonizing Salmonella enterica typhimurium reduces invasion and gut tissue inflammation through agglutination. Front Immunol. 2017;8:1043. doi: 10.3389/fimmu.2017.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lombana TN, Rajan S, Zorn JA, Mandikian D, Chen EC, Estevez A, Yip V, Bravo DD, Phung W, Farahi F. Production, characterization, and in vivo half-life extension of polymeric IgA molecules in mice. MAbs. 2019;11(6):1122–38. doi: 10.1080/19420862.2019.1622940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muscariello L, De Siena B, Marasco R. Lactobacillus cell surface proteins involved in interaction with mucus and extracellular matrix components. Curr Microbiol. 2020;77(12):3831–41. doi: 10.1007/s00284-020-02243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang YY, Schroeder HA, Nunn KL, Woods K, Anderson DJ, Lai SK, Cone RA. Diffusion of immunoglobulin g in shed vaginal epithelial cells and in cell-free regions of human cervicovaginal mucus. PLoS One. 2016;11:e0158338. doi: 10.1371/journal.pone.0158338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shrestha B, Schaefer A, Chavez EC, Kopp AJ, Jacobs TM, Moench TR, Lai SK. Engineering tetravalent IgGs with enhanced agglutination potencies for trapping vigorously motile sperm in mucin matrix. Acta Biomater. 2020;117:226–34. doi: 10.1016/j.actbio.2020.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]


