Abstract
We attempted to mimic in small upflow anaerobic sludge bed (UASB) bioreactors the metabolic association found in nature between methanogens and methanotrophs. UASB bioreactors were inoculated with pure cultures of methanotrophs, and the bioreactors were operated by using continuous low-level oxygenation in order to favor growth and/or survival of methanotrophs. Unlike the reactors in other similar studies, the hybrid anaerobic-aerobic bioreactors which we used were operated synchronously, not sequentially. Here, emphasis was placed on monitoring various methanotrophic populations by using classical methods and also a PCR amplification assay based on the mmoX gene fragment of the soluble methane monooxygenase (sMMO). The following results were obtained: (i) under the conditions used, Methylosinus sporium appeared to survive better than Methylosinus trichosporium; (ii) the PCR method which we used could detect as few as about 2,000 sMMO gene-containing methanotrophs per g (wet weight) of granular sludge; (iii) inoculation of the bioreactors with pure cultures of methanotrophs contributed greatly to increases in the sMMO-containing population (although the sMMO-containing population decreased gradually with time, at the end of an experiment it was always at least 2 logs larger than the initial population before inoculation); (iv) in general, there was a good correlation between populations with the sMMO gene and populations that exhibited sMMO activity; and (v) inoculation with sMMO-positive cultures helped increase significantly the proportion of sMMO-positive methanotrophs in reactors, even after several weeks of operation under various regimes. At some point, anaerobic-aerobic bioreactors like those described here might be used for biodegradation of various chlorinated pollutants.
In nature, methane-oxidizing bacteria (methanotrophs), as expected, live in close association with methane-producing microorganisms (methanogens); as reported by Buchholz et al. (5), methanotrophs are strongly associated with the oxic-anoxic interface in water columns or sediments. In eutrophic lakes, maximal rates of methane oxidation have been shown to occur in the oxic-anoxic interfaces of the water column, where the oxygen concentrations may not exceed 1 ppm (14, 15).
In a few studies workers have attempted to mimic in bioreactors the close spatial association of the two trophic microbial groups by using sequential anaerobic-aerobic processes, and in some cases, there has been an attempt to apply this association to the biodegradation of some pollutants (11, 20, 30). This concept could be applied to the biodegradation of tetrachloroethylene (PCE) and trichloroethylene (TCE). Biodegradation of PCE to TCE can occur in anaerobic environments (10, 29), whereas TCE derived from PCE can be further degraded aerobically by methanotrophic bacteria that contain the soluble methane monooxygenase (sMMO) (4, 6, 7, 9, 16, 22, 27). In simple bioprocessing terms, the procedure involves transferring the effluent of an anaerobic reactor into an aerobic reactor enriched with a methanotrophic consortium and supplying oxygen and methane to the latter reactor to sustain and maximize the methanotrophic metabolic activity.
Upflow anaerobic sludge blanket (UASB) reactors have been characterized well, and the usefulness of these reactors for treatment of municipal and industrial wastes has been well-documented (28). UASB reactors can accommodate low concentrations of oxygen without deleterious effects on the integrity or metabolic activity of the granular biomass (13, 23). Thus, a partially aerated UASB reactor contains the substrates required by methanotrophic bacteria (i.e., indigenously produced methane and exogenously added oxygen) and may, therefore, be an ideal system for maintaining consortia composed of methanogens and methanotrophs. A bioprocess in which a hybrid aerobic-anaerobic sludge reactor is used has been designed recently in our laboratory (23).
In this study, as a further step in the development of a generic bioprocess, we evaluated the ability of methanotrophic bacteria to grow and/or survive in UASB reactors. To do this, coaggregation of methanotrophs with anaerobic granular sludge dominated by methanogens was studied in laboratory-scale UASB reactors under different oxygenation conditions and with different hydraulic retention times (HRTs). Granular sludge was inoculated with methanotrophic cultures known to contain the sMMO.
In this work, special emphasis was put on monitoring various methanotrophic populations, particularly those associated with the granular sludge. The density of the methanotrophic population that contained the sMMO gene cluster was monitored by using molecular biology tools developed in a previous study (21) and other more traditional methods. The results obtained in these investigations are described below.
MATERIALS AND METHODS
Bacterial strains and media.
Methylosinus trichosporium OB3b (= ATCC 35070) and Methylosinus sporium (= ATCC 35069) were obtained from the American Type Culture Collection, Rockville, Md.). These strains were grown on copper-free low-nitrate mineral (LN-NMS) medium (3) at 30°C in an atmosphere containing 50% methane and 50% air.
UASB reactors.
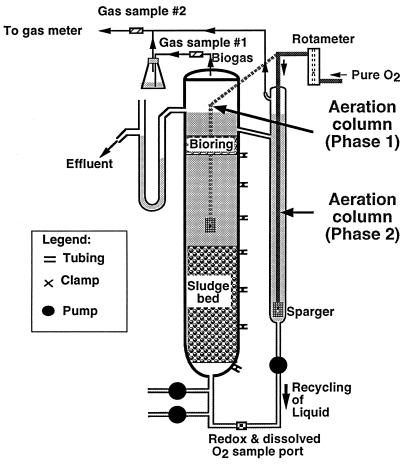
The design of the UASB reactors used in this study has been described by Shen and Guiot (23). Recycled effluent was oxygenated by placing an oxygen-bearing diffuser on top of the granular bed or by incorporating an aeration column coupled to the UASB reactor (Fig. 1). Reactors of different sizes (1 and 5 liters) were used in this study. The 1-liter reactors were inoculated with 200 ml of granular sludge (about 13.4 g of volatile suspended solids [VSS]) originating from a full-scale UASB reactor treating wastewater from a baby food plant (Champlain Industry, Cornwall, Ontario, Canada), whereas the 5-liter reactors were inoculated with 1,000 ml (about 50 g of VSS) of the same sludge. After the granular sludge was added, the reactors were acclimatized to predetermined oxygenation levels for 5 days at an HRT of 72 h. The operation of the reactor, the chemical composition of the feed, and rate of addition of the feed were as described by Shen and Guiot (23).
FIG. 1.
Schematic diagram of the bioreactor setup used. The bioreactor volume was 1 liter during phase I and 5 liters during phase II. The culture was aerated with an immersed sparger during phase I, whereas the culture was aerated by using a separate aeration column (with a sparger) during phase II.
Inoculation of reactors with M. trichosporium and M. sporium.
The cultures used to inoculate reactors were grown in 6-liter Erlenmeyer flasks containing 3 liters of LN-NMS medium. Each inoculum consisted of cells obtained after growth on LN-NMS medium plates (one plate per liter of medium) in modified “anaerobic jars” flushed with a 50% methane–50% air mixture and incubated at 30°C. The inocula used for the flasks were obtained by recovering the biomass on LN-NMS medium plates with about 5 ml of LN-NMS medium. During growth, the headspaces of the cultures were flushed twice daily with a 50% methane–50% air mixture for 3 days; the cultures were agitated at 250 rpm and incubated at 30°C, and then they were harvested and concentrated 100-fold by using a 0.1-μm-pore-size polysulfone hollow fiber membrane filter (Amicon).
Viable bacterial populations in granular sludge.
Granular sludge was added to a preweighed sterile tube containing 3.5 g of 3-mm-diameter glass beads. The weight of each sample was determined, and a volume of a sterile saline solution (0.85% [wt/vol] NaCl) equivalent to three times the weight of the granular sample was added. The samples were kept on ice, vortexed vigorously for 2 min, and then serially diluted (10-fold dilutions) in the sterile saline solution. Aliquots (0.1 ml) of each dilution were plated onto solid LN-NMS agar plates, and the plates were incubated for 2 to 3 weeks at 30°C either under a 50% methane–50% air atmosphere or under a 100% air atmosphere. All determinations were made in triplicate. Colonies obtained from the serial dilutions were tested for the ability to express sMMO activity and the presence of the sMMO gene. Colonies that exhibited sMMO activity were identified by using the naphthalene oxidation assay (4). Colonies that expressed the sMMO genotype were identified by a colony hybridization technique (12). The colonies were transferred onto nylon membrane filters and were examined by performing a Southern analysis with gene probes specific for the mmoX gene of the sMMO gene cluster, essentially as described by Sotsky et al. (25). The sMMO gene probe was prepared as described by Miguez et al. (21).
Determination of the numbers of strictly aerobic and facultatively aerobic microorganisms.
The numbers of cells were determined by using the Standard Methods for the Examination of Water and Wastewater (1). Sludge (granular material) samples obtained from predetermined ports in the reactor were removed, mixed, and blended in a sterile Kinematica blender. Aliquots of the disrupted sludge were then serially diluted, plated onto plate count agar (Difco), and incubated at 30°C for 24 to 48 h. The colonies were counted, and the number of colonies was considered to represent the total number of facultatively aerobic microorganisms. Colonies obtained from plates that were incubated aerobically and contained 50 to 100 CFU were transferred onto sterile plate count agar and incubated anaerobically in an anaerobic jar (GasPack system) at 30°C for 1 month. Growth under these conditions revealed the presence of facultatively aerobic microorganisms. The colonies that were not able to grow represented the strictly aerobic microorganisms.
DNA extraction from granular sludge for PCR.
DNA was extracted from 1-g (wet weight) granule samples by using the freeze-thaw method of Tsai and Olson (26). DNA was further purified by using Sephadex G-200 saturated with TE buffer (21). All PCR procedures were performed as previously described (21).
Sensitivity of detection of the sMMO gene in granular sludge by PCR.
The sMMO gene in granular sludge was detected by PCR amplification of the mmoX gene fragment (21). The sensitivity of the method was estimated as follows: (i) serial 10-fold dilutions of a 48-h culture of M. sporium ATCC 35069 were prepared by using 120 mM sodium phosphate buffer; (ii) prior to DNA extraction (see above), a 1-ml aliquot of each serial dilution was added to 1 g of a granular sludge sample; and (iii) PCR amplification was performed as described previously (21).
RESULTS
Detection of sMMO activity and of the sMMO gene in granular sludge.
Before inoculation of the reactors, efforts were made to evaluate the concentration of sMMO-containing methanotrophic bacteria in the granular sludge originating from a full-scale UASB reactor treating wastewater from the baby food plant. The results indicated that the granular sludge contained at least 2,000 indigenous methanotrophic cells having sMMO activity per g (wet weight) of granular sludge, as determined by the naphthalene oxidation assay (4).
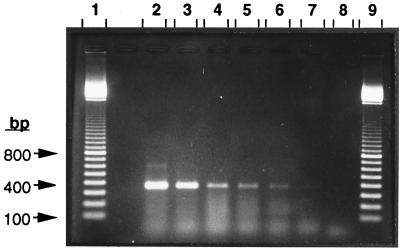
As a second step, we attempted to detect the sMMO gene in the granular sludge by the PCR method. Early investigations involving inoculation of granular sludge with a serially diluted culture of M. sporium (1.4 × 108 viable cells/ml) showed that the PCR method could detect as few as about 2,000 sMMO gene-containing methanotrophic cells per g (wet weight) of granular sludge (Fig. 2, lanes 6 and 7). Considering that 1 g (wet weight) of granular sludge may contain between 1011 and 1012 viable cells, this detection limit represented a detection capability of approximately 2 sMMO gene-containing cells per 108 to 109 viable microbial cells. Interestingly, autoclaving granular sludge for 40 min did not prevent amplification of the extracted DNA by the mmoX primer set (results not shown).
FIG. 2.
Sensitivity of detection of the sMMO gene in enriched granular sludge by PCR when the mmoX1-mmoX2 primer set was used. For details see Materials and Methods. Identical granular sludge samples were spiked with different numbers of viable M. sporium ATCC 35069 cells, and the samples were analyzed by PCR. Lanes 1 and 9, 100-bp size ladder marker; lane 2, granular sludge sample containing 1.4 × 108 cells of M. sporium; lane 3, granular sludge sample containing 1.4 × 107 cells; lane 4, granular sludge sample containing 1,400 cells; lane 5, granular sludge sample containing 14 cells; lane 6, granular sludge sample containing 1.4 M. sporium cells; lane 7, granular sludge sample containing no M. sporium cells (there is a faint band); lane 8, water. Since the granular sludge sample used was found to contain about 2,000 sMMO-positive cells per g (wet weight), the sensitivity of our PCR method was calculated to be about 2,000 mmoX probe-positive cells per g (wet weight) of sludge.
Isolation of indigenous sMMO-containing methanotrophs.
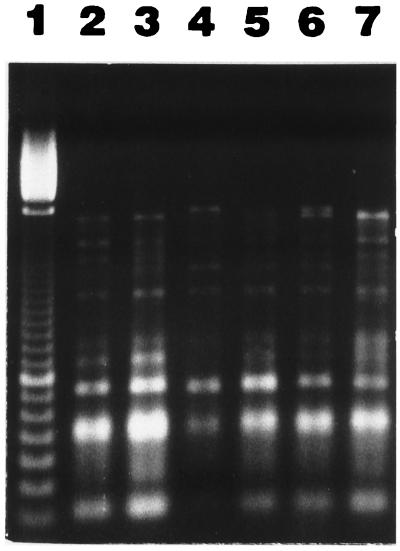
In order to obtain initial information on the natural population of sMMO-containing methanotrophs in the granular sludge, the methanotrophic colonies obtained from the initial granular sludge samples were divided into subgroups on the basis of differences in colony appearance and morphology, and representative cultures were further purified to homogeneity and maintained at 4°C. The fresh isolates obtained were then fingerprinted by using a modified randomly amplified polymorphic DNA (RAPD)-PCR method to verify their genetic similarity to some well-characterized methanotrophic cultures. The results (Fig. 3) revealed strong similarities among the fresh isolates and M. sporium ATCC 35069.
FIG. 3.
RAPD-PCR analysis of representative fresh methanotrophic isolates obtained early in this study. Lane 1, 100-bp size ladder marker; lane 2, M. sporium ATCC 35069; lane 3, UASB isolate M5; lane 4, Ville Mercier isolate M1; lane 5, UASB isolate M6; lane 6, Ville Mercier isolate M10; lane 7, UASB isolate M3.
Consequently, M. sporium ATCC 35069 and another closely related sMMO-containing methanotroph, M. trichosporium OB3b (= ATCC 35070), were used as test organisms in studies aimed at determining the feasibility of coupling sMMO-containing methanotrophs with methanogens in UASB reactors under O2-limiting conditions.
Survival of M. trichosporium and M. sporium in UASB reactors operated with various levels of oxygenation.
This study was done in two phases, phase I and phase II (Table 1). In phase I we used 1-liter reactors, and the reactors were inoculated with either M. trichosporium OB3b (= ATCC 35070) or M. sporium ATCC 35069. In phase II we used 5-liter reactors, and the reactors were inoculated with only M. sporium ATCC 35069. As indicated in Table 1, growth and survival of methanotrophs was evaluated under various oxygen feeding and hydraulic regimes.
TABLE 1.
Experimental design
| Phase | Reac-tor vol (liters) | No. of reactors | Oxygen supply rate (liters/day) | HRT regime | Duration (days) | Hydraulic loading rate (liters/day) | HRT (days) |
|---|---|---|---|---|---|---|---|
| Ia | 1 | 3c | 1.5 or 3.0e | I | 0–24 | 0.3 | 3 |
| II | 25–41 | 1 | 1 | ||||
| III | 42–72 | 2 | 0.5 | ||||
| IIb | 5 | 6d | 9, 16, or 24f | I | 35 | 2.5 | 2 |
One of the two reactors aerated at a rate of 1.5 liters/day and the reactor aerated at a rate of 3.0 liters/day were inoculated with M. trichosporium Ob3b ATCC 35070). The second reactor aerated at a rate of 1.5 liters/day was inoculated with M. sporium ATCC 35069.
Reactors were inoculated only with M. sporium and were operated at three different dissolved oxygen levels.
No control reactor was included in this experiment.
Three control reactors were included in this experiment.
In all cases, no dissolved oxygen was detected, indicating that all of the inlet oxygen was consumed.
The inlet dissolved oxygen concentration at the bottom of the granule bed was 2, 5, or 8.5 ppm.
Phase I.
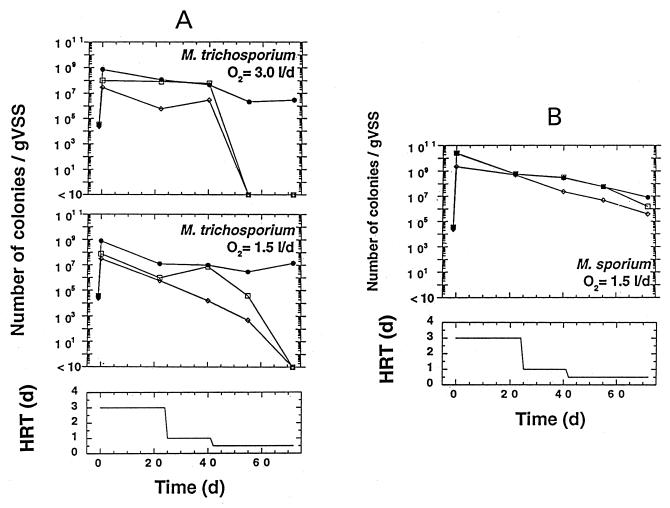
The objective of phase I was to determine which of the two well-characterized sMMO-containing methanotrophs used exhibited greater growth and/or survival potential during cocultivation with anaerobic consortia (granular sludge) in the UASB reactors. M. trichosporium (6.9% inoculum based on the total sludge VSS) was inoculated into two 1-liter UASB reactors that were operated under two different oxygenation regimes, 1.5 and 3 liters of O2/day. M. sporium (6.5% inoculum based on the total sludge VSS), on the other hand, was inoculated into only one 1-liter UASB reactor that was oxygenated at a rate of 1.5 liters/day; therefore, M. sporium was not tested at the 3.0-liter/day oxygenation rate as M. trichosporium was. Equal numbers of M. trichosporium viable cells were inoculated into the two reactors to a final concentration of 2 × 107 cells/g of VSS. M. sporium was inoculated to a concentration of 109 cells/g of VSS (Fig. 4). The reactors were operated for 72 days (Table 1) at three different HRTs. The results obtained with M. trichosporium OB3b are shown in Fig. 4A. With the two reactors, as expected, an immediate 5-log increase in the total number of methanotrophs was observed. The size of the methanotrophic population gradually decreased during reactor operation and with changes in the HRT until it was around 107 cells/g of VSS, which was about 2 logs less than the size of the methanotrophic population right after inoculation. In spite of this significant decrease, the size of the total methanotrophic population in the granular sludge after 72 days of reactor operation was at least 2 logs greater than the size of the population prior to inoculation, suggesting that inoculation with M. trichosporium contributed to establishing a significant methanotrophic population. The level of oxygenation (either 1.5 or 3.0 liters/day) did not appear to influence this finding. The size of the population of methanotrophs that exhibited sMMO activity (i.e., had the ability to oxidize naphthalene) followed a pattern very similar to that of the total methanotrophic population for the first 40 days of operation (Fig. 4A). However, the size of this population decreased rapidly after 40 days to almost undetectable levels, probably in part as a result of the change in the HRT from 1 to 0.5 day. The decrease in the number of methanotrophs that exhibited sMMO activity appeared to be more rapid in the reactor aerated at a rate of 3.0 liters/day than in the other reactor. Not surprisingly, with the two reactors, the profile obtained for sMMO probe-positive methanotrophs generally resembled the profile obtained for methanotrophs that exhibited sMMO activity.
FIG. 4.
Phase I study. (A) Survival of various methanotrophic populations in granular sludge in 1-liter reactors aerated at two different oxygenation levels and inoculated with M. trichosporium OB3b (= ATCC 35070). Symbols: •, total methanotrophic population; □, methanotrophs exhibiting sMMO activity; ◊, methanotrophs containing the sMMO gene. For details see Materials and Methods. The graph at the bottom shows the HRT profile used in these experiments. (B) Survival of various methanotrophic populations (see above) in granular sludge in a 1-liter reactor aerated at a rate of 1.5 liters of oxygen per day and inoculated with M. sporium ATCC 35069. l, liters; d, days.
The results obtained for M. sporium are presented in Fig. 4B. As expected, the size of the total methanotrophic population increased by at least 5 logs immediately after inoculation with M. sporium and, as described above for M. trichosporium, decreased gradually during reactor operation until it was about 107 cells/g of VSS after 72 days, a value that still was at least 2 logs greater than the value prior to inoculation. The numbers of methanotrophs that exhibited sMMO activity and the numbers of methanotrophs that contained the sMMO gene were quite similar; the profile for each of these two populations resembled the profile for the total methanotrophic population. In contrast to the results obtained with the reactors inoculated with M. trichosporium (Fig. 4A), the number of methanotrophs that exhibited sMMO activity or contained the sMMO gene did not decreased significantly after the change in the HRT from 1 to 0.5 day. The sizes of these populations were approximately 106 cells/g of VSS by day 72.
Interestingly, for several granular sludge samples, the number of methanotrophs that exhibited sMMO activity was greater than the number of methanotrophs that contained the sMMO gene. This might indicate that some of the colonies that exhibited sMMO activity did not hybridize with our mmoX probe.
Finally, with all three reactors, visual observation indicated that the physical characteristics of the granular sludge changed during reactor operation; the granular sludge became more fluffy upon exposure to oxygen, suggesting that colonization by a different microbial population occurred. In spite of these changes in physical characteristics, the overall performance of all of the bioreactors, as measured by the volumetric production of methane, was identical to that of control bioreactors not fed any oxygen (results not shown).
During phase I, efforts were made to estimate the sizes of the populations of mmoX probe-positive methanotrophic bacteria present in the aqueous phases of the reactors (Table 2). A general pattern was observed; following inoculation of the three reactors, a significant increase in the size of the extragranular methanotrophic population was always observed, although the significance varied greatly. With all three reactors, the size of the extragranular methanotrophic population decreased very significantly with time but generally remained greater than 105 cells/g of VSS for the first 40 days of operation in spite of the changes in reactor operation. The effluent from all UASB type reactors, including ours, is rarely a clear homogeneous aqueous solution. It is usually murky and full of particulate suspended matter which adheres to glass walls and tubing. Periodically and unpredictably, the suspended matter is released and is discharged with the rest of the effluent. For these reasons, the numbers of mmoX probe-positive methanotrophs in the effluent varied. Our results show that during operation of the reactors mmoX probe-positive methanotrophs were indeed lost in the effluent.
TABLE 2.
Concentration of mmoX probe-positive methanotrophic bacteria not associated with granular sludge in Phase I
| Reactor no. | Methanotrophic inoculum | Oxygen feeding rate (liters/day) | Time of sampling (day) | No. of mmoX probe-positive methano-trophic bacteria per g of VSS |
|---|---|---|---|---|
| 1 | M. trichosporium OB3b (= ATCC 35070) | 3 | 0 | 3 × 105 |
| 0.5 | 7.6 × 106 | |||
| 22 | 4.1 × 107 | |||
| 40 | 1.5 × 105 | |||
| 55 | NDa | |||
| 72 | ND | |||
| 2 | M. trichosporium OB3b (= ATCC 35070) | 1.5 | 0 | ND |
| 0.5 | 2 × 108 | |||
| 22 | 2 × 104 | |||
| 40 | 1.9 × 107 | |||
| 55 | ND | |||
| 72 | ND | |||
| 3 | M. sporium (= ATCC 35069) | 1.5 | 0 | ND |
| 0.5 | 1.61 × 1011 | |||
| 22 | 1.9 × 108 | |||
| 40 | 4.8 × 106 | |||
| 55 | 2.8 × 105 | |||
| 72 | 6.9 × 104 |
ND, not detected.
Phase II.
The main objective of phase II was to improve the survival and retention of sMMO-containing methanotrophs in the granular biomass. To do this, certain modifications to the design and operation of the reactors were introduced, and 5-liter reactors were used instead of 1-liter reactors. To ensure that the dissolved oxygen concentration in the influent reaching the granular sludge was proper and constant, the 5-liter reactors were connected to aeration columns as described by Shen and Guiot (23). Three dissolved oxygen concentrations were tested, 2, 5, and 8.5 ppm. In addition, the nitrogen components of the feed, NH4HCO3 and (NH4)2SO4, were replaced with urea (109 mg/liter) and NaNO3 (425 mg/liter) in order to provide sufficient nitrogen for methanotrophic growth (2) while avoiding the potential inhibition of methane oxidation by ammonium ions (18). Finally, since M. sporium ATCC 35069 appeared to exhibit better survival characteristics during phase I (Fig. 4), this organism was used as the inoculum in the phase II investigations.
As shown in Table 1 and discussed above, six 5-liter reactors that contained 1,000 ml of granular sludge (50 g of VSS) each were first acclimatized to predetermined levels of dissolved oxygen (i.e., 2, 5, and 8.5 ppm) for 5 days prior to inoculation with M. sporium. Three of the six reactors were each inoculated with a culture of M. sporium (109 cells/g of VSS) at a level equivalent to a 2.4% inoculum based on the total sludge VSS. The three control reactors (which were not inoculated with methanotrophs) were operated as follows: one reactor was maintained under strictly anaerobic conditions, and the remaining two reactors were maintained at influent dissolved oxygen concentrations of 5 and 8.5 ppm. The following results were obtained (Fig. 5).
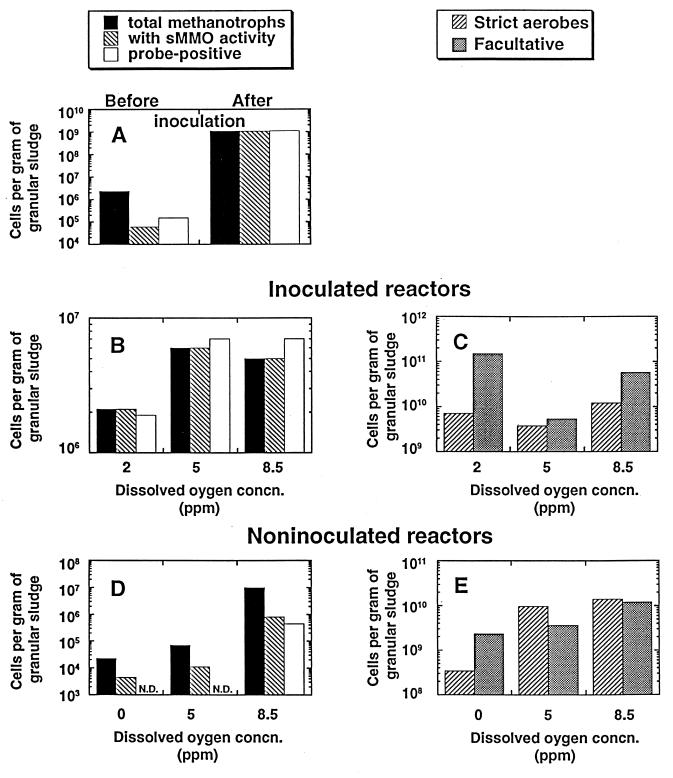
FIG. 5.
Phase II study: survival of various microbial populations in granular sludge in 5-liter reactors aerated at various oxygenation levels and inoculated or not inoculated with M. sporium ATCC 35069. (A) Various methanotrophic populations right before and after inoculation. (B through E) Results obtained after 35 days of reactor operation. (B) Various methanotrophic populations in the granular sludge of the inoculated reactors. (C) strictly aerobic and facultatively aerobic microbial populations associated with the granular sludge in the inoculated reactors. (D) Various methanotrophic populations in the noninoculated reactors. (E) Strictly aerobic and facultatively aerobic microbial populations in the granular sludge of the noninoculated reactors. N.D., not detected.
(i) Just prior to inoculation with M. sporium, the granular sludge contained 2.3 × 106 methanotrophs per g of VSS; only 2.6% of these methanotrophs (0.6 × 105 cells/g of VSS) exhibited sMMO activity, and 6.5% (1.5 × 105 cells/g of VSS) proved to be probe positive (Fig. 5A).
(ii) Right after inoculation, the granular sludge contained approximately 109 methanotrophs per g of VSS; all of these methanotrophs exhibited sMMO activity and responded positively to the mmoX probe (Fig. 5A). Inoculation, therefore, increased the size of the total methanotrophic population about 500-fold.
(iii) After 35 days of operation (Fig. 5B), the total number of viable methanotrophs in the inoculated reactors had decreased by at least 2 logs and varied between 2.1 × 106 and 7 × 106 cells per g of granular sludge. A similar pattern was observed for the number of bacteria that exhibited sMMO activity or were probe positive (Fig. 5B).
(iv) After 35 days of operation of all three inoculated reactors, the numbers of probe-positive bacteria and bacteria with sMMO activity were very similar to the total methanotroph numbers, unlike the situation observed in the reactors before inoculation. It was obvious that inoculation was instrumental in helping establish a relatively stable population of methanotrophic bacteria that exhibited sMMO activity.
(v) Maintaining the dissolved oxygen concentration at either 5 or 8.5 ppm increased the size of the methanotrophic population by a factor of about three compared with the population incubated in the presence of 2 ppm of dissolved oxygen (Fig. 5B).
(vi) Figure 5C shows that the number of strictly aerobic microorganisms per gram of granular sludge was relatively constant at each level of dissolved oxygen but that the size of the population of facultative microorganisms varied more with the dissolved oxygen concentration and, in particular, increased at the lowest oxygen level used (2 ppm).
(vii) In all cases, the size of the total population of methanotrophs measured on day 35 never exceeded 0.7 × 107 cells per g of granular sludge, while the size of the total strictly aerobic microbial population ranged from 109 to 1010 cells per g. Such population sizes for methanotrophs may be considered significant, although they are relatively small.
The results obtained with the control (uninoculated) reactors are shown in Fig. 5D and E. These results may be summarized as follows.
(i) After 35 days of operation of the control reactors, the numbers of methanotrophs were highly variable. The numbers of viable methanotrophs were approximately 104 cells per g of granular sludge in reactors maintained at either 0 or 5 ppm of dissolved oxygen but reached 107 cells per g in the reactor maintained at 8.5 ppm of dissolved oxygen (Fig. 5D).
(ii) Although measurable populations of methanotrophs with sMMO activity were detected in the reactors maintained at 0 or 5 ppm of dissolved oxygen, interestingly, no methanotrophs that responded to our probe were detected (Fig. 5D). However, there was a very significant population of probe-positive methanotrophs in the reactor maintained at 8.5 ppm of dissolved oxygen.
(iii) In the control (uninoculated) reactors, the size of the population of strictly aerobic microorganisms, as expected, increased greatly with the level of dissolved oxygen (Fig. 5E). Similar, although less pronounced, results were obtained for the population of facultative microorganisms.
As was the case during phase I, the granular sludge present in the aerated (oxygenated) reactors acquired floccular characteristics during operation of the reactors. This was probably attributable to significant changes in the microbial composition of the populations in the oxygenated reactors, such as a significant increase in the size of the population of strictly aerobic and facultative microorganisms (Fig. 5E) and the establishment of a relatively small but significant methanotrophic population.
Aeration of the anaerobic reactors used during the phase II study did not seriously affect methanogenesis, as shown previously (24). Whether the bioreactors were inoculated with methanotrophs or not and whether the dissolved oxygen concentration maintained in the bioreactors was 2 or 8.5 mg/liter (Table 1), the methane production rate was 61 to 65% of the rate measured in the bioreactor that was operated completely anaerobically.
DISCUSSION
This study had the following two main goals: (i) to evaluate the ability of well-known methanotrophic cultures to grow and/or survive in UASB reactors in the form of coaggregates containing methanogens and methanotrophs as part of the granular sludge; and (ii) to validate the use of PCR and catabolic gene probes for monitoring the populations of sMMO-related methanotrophs associated with the granular sludge in hybrid anaerobic-aerobic bioreactors operated under various regimes. In the PCR method used in this study we used a primer set designated mmoX1-mmoX2 which has been described recently (21) and was derived from the mmoX gene encoding the alpha subunit of the sMMO.
sMMO-containing methanotrophs associated with granular sludge.
As indicated above, the source of the granular sludge inoculum used for bioreactor inoculation was a full-scale UASB reactor treating wastewater from a baby food plant. Our results indicated that before inoculation this anaerobic granular sludge contained at least 2,000 indigenous methanotrophic bacteria exhibiting sMMO activity per g (wet weight) of sludge; however, the number of bacteria was generally 104 to 105 cells per g. Given the industrial nature of the granular sludge (i.e., anaerobic granular sludge that was present in an open system with methane and was exposed to wastewater containing some dissolved oxygen), it was not surprising to find such levels of sMMO-containing methanotrophs. Consistent with this finding, Kato et al. (17) obtained strong evidence that methane-oxidizing microorganisms were present in granular sludge intentionally exposed to oxygen in laboratory experiments.
The PCR method used in this study could detect as few as about 2,000 sMMO gene-containing methanotrophs per g (wet weight) of granular sludge (Fig. 2). This detection limit was, therefore, equivalent to the lowest population levels of sMMO-related methanotrophs measured in our granular sludge (see above) by the traditional plate count method. According to our calculations, our PCR method was able to detect approximately 2 sMMO gene-containing cells per 108 to 109 viable microbial cells. This detection ability is very interesting; it is equivalent to detecting one “contaminating” bacterial cell in a 1-ml aliquot of a low-density bacterial culture.
The cultivable sMMO-containing methanotrophs obtained from the granular sludge exhibited significant genetic similarities to M. sporium ATCC 35069 after RAPD-PCR analysis. Based on the fact that methanotrophs are known to be difficult to isolate from environmental samples, we concluded that a variable and sometimes significant population of M. sporium-like bacteria was present in the granular sludge without necessarily assuming that this population was the dominant sMMO-containing population. Nevertheless, the results justified our decision to inoculate anaerobic reactors with M. sporium ATCC 35069 and with another closely related methanotroph, M. trichosporium OB3b (= ATCC 35070), since similar organisms were naturally present in the granular sludge.
Inoculation of reactors with known methanotrophic cultures.
Our results show conclusively that anaerobic reactors operated at various levels of aeration can maintain for up to 72 days a significant sMMO-containing microbial population following inoculation of the reactors with known methanotrophic cultures (Fig. 4 and 5). These initial results are promising. In all of the cases studied, the size of the total methanotrophic population at the end of the reactor experiment was always at least 2 logs greater than the size of the initial population. In this regard, the sMMO-containing populations resulting from inoculation with M. sporium appeared to be more stable and more resistant to changes in the HRT than the equivalent populations associated with reactors inoculated with M. trichosporium (Fig. 4).
Correlation between the various population levels.
In general, there was a good correlation between the sMMO-containing population levels measured with molecular tools (colony hybridization to an sMMO gene-specific probe) and the population levels measured with the naphthalene oxidation assay. This indicated that most of the sMMO hybridizing colonies also produced an active sMMO. At times, the number of methanotrophs that exhibited sMMO activity was greater than the number of methanotrophs that contained the sMMO gene. This could indicate that some methanotrophs did not respond to our mmoX gene probe, a possibility that should not be forgotten.
Methanotrophs not associated with the granular sludge.
As indicated in Table 2, significant populations of sMMO probe-positive methanotrophs that were not associated with the granular sludge were present following inoculation of the reactors during phase I. The size of the methanotrophic population decreased significantly with time and with changes in the HRT but nevertheless always remained greater than 105 cells per g for the first 40 days of reactor operation. Since the reactors were operated in a continuous mode, this suggested either that the extragranular methanotrophic fraction was able to survive and grow up to a certain point and/or that there was continuous release of sMMO probe-positive bacteria from the granules. Note that for two of the three reactors inoculated (Table 2) no sMMO probe-positive methanotrophic population was detected in the extragranular fraction before inoculation. This suggests that inoculation of the reactors may also have been beneficial to the extragranular methanotrophic fraction, a phenomenon that could also help increase reactor performance in subsequent biotreatment studies.
Methanogenic-methanotrophic hybrid reactors.
In recent years, the concept of coupling methanogens and methanotrophs, either sequentially or synchronously, in order to enhance the biodegradation of pollutants has attracted the attention of a few groups of scientists (8, 11, 19, 24). This concept is relatively new, and process development based on this concept is still in its infancy. In this study, we show that significant methanotrophic populations can be maintained in aerobic-anaerobic bioreactors that are initially inoculated with a pure culture of a methanotrophic bacterium and are operated under oxygen-limited conditions. Most of the cultivable methanotrophic bacteria obtained from our reactors not only were sMMO probe positive but also, and more importantly, exhibited sMMO activity. Our initial results appear quite promising. In this study, the bioreactors were inoculated with a methanotrophic culture only once. It is likely that larger populations of methanotrophic bacteria could be maintained in hybrid bioreactors if repeated pulse inoculation was used, a hypothesis that should be tested in the near future.
ACKNOWLEDGMENTS
We thank the following former or present Biotechnology Research Institute colleagues for their collaboration: J. Al-Hawari for scientific support in analytical chemistry, Alain Corriveau and Louise Paquet for analytical technical support, and Jennifer Sealy, a former co-op student, for technical and scientific assistance.
REFERENCES
- 1.American Public Health Association. Standard methods for the examination of water and wastewater. 16th ed. Washington, D.C: American Public Health Association; 1985. [Google Scholar]
- 2.Bowman J P, Sayler G S. Optimization and maintenance of soluble methane monooxygenase activity in Methylosinus trichosporium OB3b. Biodegradation. 1994;5:1–11. doi: 10.1007/BF00695208. [DOI] [PubMed] [Google Scholar]
- 3.Bowman J P, Jiménez L, Rosario I, Hazen T C, Sayler G S. Characterization of the methanotrophic bacterial community present in a trichloroethylene-contaminated groundwater site. Appl Environ Microbiol. 1993;59:2380–2387. doi: 10.1128/aem.59.8.2380-2387.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brusseau G A, Tsien H C, Hanson R S, Wackett L P. Optimization of trichloroethylene oxidation by methanotrophs and the use of a colorimetric assay to detect soluble monooxygenase activity. Biodegradation. 1990;1:19–29. doi: 10.1007/BF00117048. [DOI] [PubMed] [Google Scholar]
- 5.Buchholz L A, Klump J V, Collins M L P, Brantner C A, Remsen C C. Activity of methanotrophic bacteria in Green Bay sediments. FEMS Microbiol Ecol. 1995;16:1–8. [Google Scholar]
- 6.Chu K H, Alvarez-Cohen L. Trichloroethylene degradation by methane-oxidizing cultures grown with various nitrogen sources. Water Environ Res. 1996;68:76–82. [Google Scholar]
- 7.Duba A G, Jackson K J, Jovanovich M C, Knapp R B, Taylor R T. TCE remediation using in situ, resting-state bioaugmentation. Environ Sci Technol. 1996;30:1982–1989. [Google Scholar]
- 8.Fathepure B Z, Vogel T M. Complete degradation of polychlorinated hydrocarbons by a two-stage biofilm reactor. Appl Environ Microbiol. 1991;57:3418–3422. doi: 10.1128/aem.57.12.3418-3422.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitch M W, Weissman D, Phelps P, Georgiou G, Speitel G E., Jr Trichloroethylene degradation by Methylosinus trichosporium OB3B mutants in a sequencing biofilm reactor. Water Res. 1996;30:2655–2664. [Google Scholar]
- 10.Freedman D L, Gossett J M. Biological reductive dechlorination of tetrachloroethylene and trichloroethylene to ethylene under methanogenic conditions. Appl Environ Microbiol. 1989;55:2144–2151. doi: 10.1128/aem.55.9.2144-2151.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerritse J, Renard V, Visser J, Gottschal J C. Complete degradation of tetrachloroethene by combining anaerobic dechlorinating and aerobic methanotrophic enrichment cultures. Appl Microbiol Biotechnol. 1995;43:920–928. doi: 10.1007/BF02431929. [DOI] [PubMed] [Google Scholar]
- 12.Greer C, Masson L, Comeau Y, Brousseau R, Samson R. Application of molecular biology techniques for isolating and monitoring pollutant-degrading bacteria. Water Pollut Res J Can. 1993;28:275–287. [Google Scholar]
- 13.Guiot S R. Process coupling of anaerobic and aerobic biofilms for treatment of contaminated waste liquids. In: Wise D L, editor. Global environmental biotechnology. Proceedings of the 3rd International Symposium of the International Society for Environmental Biotechnology. Studies in environmental sciences. Vol. 66. Amsterdam, The Netherlands: Elsevier Science; 1997. pp. 591–601. . pp., 591–601. [Google Scholar]
- 14.Hanson R S, Hanson T E. Methanotrophic bacteria. Microbiol Rev. 1996;60:439–471. doi: 10.1128/mr.60.2.439-471.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson R S, Wattenberg E V. Ecology of methylotrophic bacteria. In: Goldberg I, Rokem J S, editors. Biology of methylotrophs. London, United Kingdom: Butterworth-Heinemann; 1991. pp. 325–348. [DOI] [PubMed] [Google Scholar]
- 16.Henrysson T, McCarty P L. Influence of the endogenous storage lipid poly-β-hydroxybutyrate on the reducing power availability during cometabolism of trichloroethylene and naphthalene by resting methanotrophic mixed cultures. Appl Environ Microbiol. 1993;59:1602–1606. doi: 10.1128/aem.59.5.1602-1606.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato M T, Field J A, Lerttinga G. Methanogenesis in granular sludge exposed to oxygen. FEMS Microbiol Lett. 1993;114:317–324. [Google Scholar]
- 18.King G M, Schnell S. Ammonium and nitrite inhibition of methane oxidation by Methylobacter albus BG8 and Methylosinus trichosporium OB3b at low methane concentrations. Appl Environ Microbiol. 1994;60:3508–3513. doi: 10.1128/aem.60.10.3508-3513.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Long J L, Stensel H D, Ferguson J F, Strand S E, Ongerth J E. Anaerobic and aerobic treatment of chlorinated aliphatic compounds. J Environ Eng. 1993;119:300–320. [Google Scholar]
- 20.McFarland M J, Vogel C M, Spain J C. Methanotrophic cometabolism of trichlorroethylene in a two stage bioreactor system. Water Res. 1992;26:259–265. [Google Scholar]
- 21.Miguez C B, Bourque D, Sealy J A, Greer C W, Groleau D. Detection and isolation of methanotrophic bacteria possessing soluble methane monooxygenase (sMMO) genes using the polymerase chain reaction (PCR) Microb Ecol. 1997;33:21–31. doi: 10.1007/s002489900004. [DOI] [PubMed] [Google Scholar]
- 22.Oldenhuis R, Vink R L J M. Degradation of chlorinated aliphatic hydrocarbons by Methylosinus trichosporium OB3b expressing soluble methane monooxygenase. Appl Environ Microbiol. 1989;55:2819–2826. doi: 10.1128/aem.55.11.2819-2826.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen C F, Guiot S R. Long-term impact of dissolved oxygen on the activity of anaerobic granules. Biotechnol Bioeng. 1996;49:611–620. doi: 10.1002/(SICI)1097-0290(19960320)49:6<611::AID-BIT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 24.Shen C F, Miguez C B, Bourque D, Groleau D, Guiot S R. Methanotroph and methanogen coupling in granular biofilm under O2-limited conditions. Biotechnol Lett. 1996;18:495–500. [Google Scholar]
- 25.Sotsky J B, Greer C W, Atlas R M. Frequency of genes in aromatic and aliphatic hydrocarbon biodegradation pathways within bacterial populations from Alaskan sediments. Can J Microbiol. 1994;40:981–985. doi: 10.1139/m94-157. [DOI] [PubMed] [Google Scholar]
- 26.Tsai Y-L, Olson B H. Rapid method for direct extraction of DNA from soils and sediments. Appl Environ Microbiol. 1991;57:1070–1074. doi: 10.1128/aem.57.4.1070-1074.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsien H-C, Brusseau G A, Hanson R S, Wackett L P. Biodegradation of trichloroethylene by Methylosinus trichosporium OB3b. Appl Environ Microbiol. 1989;55:3155–3161. doi: 10.1128/aem.55.12.3155-3161.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vochten P, Schowanek S, Schowanek W, Verstraete W. Aerobic versus anaerobic wastewater treatment. In: Hall E R, Hobson P N, editors. Proceedings of the 5th International Symposium on Anaerobic Digestion. Advances in water pollution control. Oxford, United Kingdom: Pergamon Press; 1988. pp. 91–104. [Google Scholar]
- 29.Wild A P, Winkelbauer W, Leisinger T. Anaerobic dechlorination of trichloroethene, tetrachloroethene and 1,2-dichloroethane by an acetogenic mixed culture in a fixed-bed reactor. Biodegradation. 1995;6:309–318. doi: 10.1007/BF00695261. [DOI] [PubMed] [Google Scholar]
- 30.Zitomer D H, Speece R E. Sequential environments for enhanced biotransformation of aqueous contaminants. Environ Sci Technol. 1993;27:227–244. [Google Scholar]