Abstract
Simple Summary
Hyperthermia (HT) is a cancer treatment which locally heats the tumor to supraphysiological temperature, and it is an effective sensitizer for radiotherapy (RT) and chemotherapy. HT is further capable of modulating the immune system. Thus, a better understanding of its effect on the immune phenotype of tumor cells, and particularly when combined with RT, would help to optimize combined anti-cancer treatments. Since in clinics, no standards about the sequence of RT and HT exist, we analyzed whether this differently affects the cell death and immunological phenotype of human breast cancer cells. We revealed that the sequence of HT and RT does not strongly matter from the immunological point of view, however, when HT is combined with RT, it changes the immunophenotype of breast cancer cells and also upregulates immune suppressive immune checkpoint molecules. Thus, the additional application of immune checkpoint inhibitors with RT and HT should be beneficial in clinics.
Abstract
Hyperthermia (HT) is an accepted treatment for recurrent breast cancer which locally heats the tumor to 39–44 °C, and it is a very potent sensitizer for radiotherapy (RT) and chemotherapy. However, currently little is known about how HT with a distinct temperature, and particularly, how the sequence of HT and RT changes the immune phenotype of breast cancer cells. Therefore, human MDA-MB-231 and MCF-7 breast cancer cells were treated with HT of different temperatures (39, 41 and 44 °C), alone and in combination with RT (2 × 5 Gy) in different sequences, with either RT or HT first, followed by the other. Tumor cell death forms and the expression of immune checkpoint molecules (ICMs) were analyzed by multicolor flow cytometry. Human monocyte-derived dendritic cells (moDCs) were differentiated and co-cultured with the treated cancer cells. In both cell lines, RT was the main stressor for cell death induction, with apoptosis being the prominent cell death form in MCF-7 cells and both apoptosis and necrosis in MDA-MB-231 cells. Here, the sequence of the combined treatments, either RT or HT, did not have a significant impact on the final outcome. The expression of all of the three examined immune suppressive ICMs, namely PD-L1, PD-L2 and HVEM, was significantly increased on MCF-7 cells 120 h after the treatment of RT with HT of any temperature. Of special interest for MDA-MB-231 cells is that only combinations of RT with HT of both 41 and 44 °C induced a significantly increased expression of PD-L2 at all examined time points (24, 48, 72, and 120 h). Generally, high dynamics of ICM expression can be observed after combined RT and HT treatments. There was no significant difference between the different sequences of treatments (either HT + RT or RT + HT) in case of the upregulation of ICMs. Furthermore, the co-culture of moDCs with tumor cells of any treatment had no impact on the expression of activation markers. We conclude that the sequence of HT and RT does not strongly affect the immune phenotype of breast cancer cells. However, when HT is combined with RT, it results in an increased expression of distinct immune suppressive ICMs that should be considered by including immune checkpoint inhibitors in multimodal tumor treatments with RT and HT. Further, combined RT and HT affects the immune system in the effector phase rather than in the priming phase.
Keywords: hyperthermia, radiotherapy, immune phenotype, hyperthermia treatment sequence, breast cancer, immune checkpoint molecules, dendritic cell activation
1. Introduction
Breast cancer is the most commonly diagnosed cancer amongst women with 23% of total cancer cases and 14% of cancer-related deaths, which makes it the leading cause of cancer-related deaths in women [1]. About 30% of those who are diagnosed with early-stage breast cancer develop distant metastasis later on [2]. Therefore, the goal of anti-cancer therapy should be local tumor control as well as a focus on systemic effects to detain the cancer cells and avoid metastasis. This can be achieved by combining standard cancer therapies, namely radiotherapy (RT) and chemotherapy (CT), with further immune modulators. Immune checkpoint inhibitors (ICIs) have shown some effectiveness in triple negative breast cancers here [3].
In recent years it has become obvious that hyperthermia (HT) is also capable of modulating the immune system [4]. HT is commonly used as an adjuvant therapy with standard cancer treatments like RT and CT [5,6,7,8,9]. HT causes increased blood flow and oxygenation of the tissue, and it affects the cellular repair of DNA damage caused by irradiation, making it one of the most potent radiosensitizers [10,11]. Besides its radio- and chemo-sensitizing properties, HT can create favorable conditions for anti-tumor immune responses that can be further improved by immunotherapies [12]. It has further been shown that HT selectively induces apoptosis in hypoxic cancer cells and increases the cytotoxicity of immune cells against target cancer cells, making it less harmful to normal tissue [13].
One can conclude that HT has both direct effects on the tumor cells and systemic effects, which are mainly immune-mediated. A key focus has been set on the activation of dendritic cells (DCs) by HT- and/or RT-treated tumor cells. Danger-associated molecular patterns (DAMPs), such as high mobility group box 1 protein (HMGB1) and heat shock proteins (HSPs), activate immune cells when being released by the cancer cells after HT [14,15,16,17,18]. Tumor antigens that are bound to HSPs are taken up by antigen presenting cells (APCs) such as DCs, which further cross-present them to CD8+ T-cells, ideally leading to their activation and subsequent T cell-mediated eradication of tumor cells [18,19]. Furthermore, natural killer cells are also activated by HSPs [20]. HT induces not only the release of HSPs but also cytokines and chemokines, resulting in an improved trafficking of immune cells into the tumor and an increased cytotoxicity of immune cells [18,21]. Together, these HT-induced modulations have been preclinically shown to contribute to tumor regressions [15,22].
However, these beneficial local and systemic effects of HT highly depend on numerous factors such as temperature level, timing, and time interval between treatments [23]. Although there are several publications that have suggested standardization of thermal dosing and timing of the HT application [24,25], this is still lacking for HT. In some studies, HT was performed once or twice a week, but the frequency of HT was not the same for all patients, and the total number of the HT treatment session differed in each patient [23]. According to the quality assurance guidelines for HT [26,27], the general duration time of the HT treatment should be 30–60 min with a goal temperature of 40–44 °C, and the interval between HT and RT usually ranges from some minutes to 4 h [28]. However, most of these suggestions have until now not been evaluated for the immune effects of HT and, particularly, the influence of the sequence of HT and RT application on the immune phenotype of tumor cells is still unknown.
The combination of HT and RT has been studied in clinical trials for different cancer entities and showed positive results compared to RT alone [29,30,31,32,33]. Even though there are several preclinical studies and some clinical trials [34] that evaluated immune alterations after HT and RT, the optimal sequence of HT and RT and its effects on the immunophenotype of the cancer cells need more investigation. When HT is applied before RT, it is believed that HT sensitizes tumor cells for RT, and when HT is applied after RT, it exacerbates irradiation-induced damage to the tumor [13,19].
Reirradiation and hyperthermia for recurrent breast cancer in previously irradiated areas is performed with dose concepts such as 8 × 4 Gy or 10 × 3 Gy [35]. Clinical studies with thermography-controlled, water-filtered infrared-A achieving good results have focused on 5 × 4 Gy as the RT schedule, in combination with HT [36]. Furthermore, extreme hypofractionated RT protocols are currently evaluated for breast cancer [37]. For translational biological research, the optimal RT dose has not been established by now [38]. We decided to use 2 × 5 Gy, as this is a well-accepted hypofractionation RT schedule in preclinical studies which was shown to be particularly effective when combined with HT [39].
Some preclinical studies suggest that applying HT after irradiation achieves better results [40,41]. However, the effect of the treatment sequences varies depending on the read-out system and the tumor entity [11,42]. Thus, whether HT should be applied before or after RT is still controversial [13,42]. Furthermore, the impact of distinct temperatures clinically used in HT on the immunological effects in these settings needs further investigation.
Thus, the aim of this work was to evaluate whether the sequence of HT with 39, 41 or 44 °C and hypo-fractionated RT affects the immunophenotype of breast cancer cells differently, and whether this plays a role in the initiation of an immune response, namely in the activation of DCs. Therefore, two human breast cancer cell lines were treated in different sequences of HT with different temperatures and RT. Afterwards, tumor cell death and the expression of prominent ICMs on the cancer cells was analyzed. Finally, co-culture experiments with human monocyte-derived DCs (moDCs) were performed. Our findings indicate for the first time that when HT is combined with RT, it modulates the expression of several immune checkpoint molecules, but the sequence of application has only a minor influence on it. Further, the combination of HT with RT of the examined breast cancer cells rather modulates the immune system in the effector phase, and not in the priming phase, as the co-incubation of the treated tumor cells with moDCs did not significantly alter the activation state of these central APCs.
2. Materials and Methods
2.1. Ex Vivo Heating System for Hyperthermia Treatment of Tumor Cells
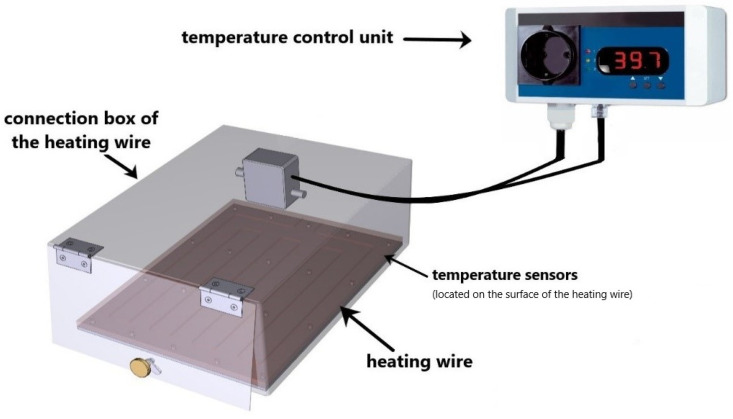
The heat treatment of the tumor cells was performed in a self-designed heating chamber under sterile conditions. The heating chamber was designed and built in collaboration with the Keylab Glass Technology (fabrication in the workshop of the Faculty of Engineering) of the University of Bayreuth. The heating chamber consists of the temperature control unit type TS125 (H-Tronic GmbH, Hirschau, Germany), a heating wire type HST 2.0 m 50 W (Horst GmbH, Lorsch, Germany), a junction box for the resistance heating wire (Horst GmbH, Lorsch, Germany) and a temperature sensor (H-Tronic GmbH, Hirschau, Germany). During the 60 min heating session, the temperature was constantly set to distinct temperatures (39 °C, 41 °C, and 44 °C) and automatically controlled, with no more than ±0.1 °C deviation from the target temperature. In the electric heating chamber, the temperature inside the system was measured and monitored by a surface temperature gauge. We checked the temperature accuracy in our cell culture flasks using dummy flasks by positioning several temperature sensors on the contact surface to the heating chamber as well as into the cell culture medium. It was shown that the measured temperatures were very homogeneous and had very small deviations (±0.2 °C) from the specified temperature in the heating chamber. A graphical illustration of the electrical heating chamber is shown in Figure 1.
Figure 1.
Graphical illustration of the heating device. The heating device was mostly made of stainless steel. The device consists of a temperature control unit, heating wire, temperature sensor, and the connection box for the heating wire. The heating chamber is automatically self-controlled and the target temperature was set to 39 °C, 41 °C, or 44 °C. The temperature deviation was not more than ±0.1 °C.
2.2. Cell Lines and Cell Culture
The two human breast cancer cell lines MCF-7 (Merck KGaA, Darmstadt, Germany) and MDA-MB-231 (Merck KGaA, Darmstadt, Germany) were cultivated at 37 °C in 5% CO2 and 90% humidity under sterile conditions. Both cell lines were grown in Dulbecco’s modified Eagle’s serum (DMEM, PAN-Biotech GmbH, Aidenbach, Germany) supplemented with 10% fetal bovine serum (FBS, Biochrome AG, Berlin, Germany) and 1% Penicillin-Streptomycin (PenStrep, Gibco, Carlsbad, CA, USA). The cell lines were tested to be free of mycoplasma.
2.3. Treatments and Sampling
As shown in Figure 2, on the day before treatment, cells of the respective cell lines were seeded in 25 cm2 cell culture flasks. The confluency of the cells did not exceed 90%. On the treatment day (day 0), each flask was handled as follows: hyperthermia treatment for 60 min was conducted using an electrical heating chamber with three different clinically relevant temperatures (39 °C, 41 °C, and 44 °C). Irradiation of the cells was performed with hypofractionation of 2 times 5 Gy (120 kV, 21.5 mA for 0.7 min) using an X-ray chamber (Isovolt Titan series, GE Technologies, Hürth, Germany). The interval between the irradiation and hyperthermia treatment was within 1–2 h. This treatment schedule was adopted from the clinical hyperthermia guidelines that indicate that the time interval between the radiation therapy and hyperthermia treatments should be close to each other, and that the time interval is mostly around 1 h and less than 4 h [43].
Figure 2.
Treatment set-up. On the day before the start of the treatment, the cells of the respective cell line (either MDA-MB-231 or MCF-7) were seeded (displayed with the cell culture flasks). In hyperthermia-only treatment (HT), or HT followed by radiotherapy (HT + RT), HT treatment was performed on day 0 in the heating chamber system for 60 min with three different respective temperatures (39 °C, 41 °C, and 44 °C). After the HT treatment, irradiation was performed at the latest within 2 h for HT + RT. For RT + HT, the respective treatments were performed in the same manner but in reverse order. RT was performed in clinically relevant doses of 2 × 5 Gy. Irradiation in the respective RT + HT arms was always performed 2 h after the initial treatment at the latest. Sampling in all arms was performed on day 1 (24 h), d2 (48 h), d3 (72 h) and d5 (120 h) after the last irradiation.
2.4. Detection of Tumor Cell Death Forms by Annexin V/PI Staining
The cell death forms of tumor cells after irradiation and hyperthermia treatment were analyzed by multicolor flow cytometry, using Annexin V/propidium-iodide (PI) staining [39]: 100,000 cells/well resuspended in Ringer’s solution (B. Braun, Melsungen, Germany) were stained with 1 µg/mL of PI (Sigma Aldrich, Munich, Germany) and 0.5 µg/mL FITC-labeled AnnexinV (Geneart, Life Technologies, Regensburg, Germany), incubated for 30 min, at 4 °C, in the dark. The cells were analyzed on a Cytoflex S flow cytometer (Beckman Coulter, Krefeld, Germany). The gating strategy for the detection of cell death forms using Annexin V/PI staining is illustrated in Figure 3.
Figure 3.
Gating strategy for the detection of cell death forms by AnnexinV/PI staining. Exemplarily shown are data of MCF-7 breast cancer cells. The cells were first gated on singlets (a,d) by FSC-A vs. FSC-H gating, followed by the exclusion of debris in the FSC/SSC plot (b,e). Viable cells were defined as Annexin negative/PI negative, apoptotic cells as Annexin positive/PI negative and necrotic cells as Annexin positive/PI positive (c,f). Data of cultured control samples (a–c) and of 2 × 5 Gy irradiated cells (d–f) are shown exemplarily.
2.5. Detection of Immune Checkpoint Molecules by Multicolor Flow Cytometry
After the treatments at respective time points, the tumor cells were harvested and 1 × 105 tumor cells per well of a 96-well plate were incubated for 30 min at 4 °C, with no light exposure, with 100 µL of the staining solution (Table 1) in a FACS buffer (PBS, Dulbecco’s Phosphate Buffered Saline (Sigma-Aldrich, Munich, Germany), 2% FBS and 2 mM EDTA (Carl Roth, Karlsruhe, Germany)). For an autofluorescence control, only Zombie NIR was put into the FACS buffer. The mean fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence intensity of autofluorescence control samples from fully stained samples. The samples were measured on a Cytoflex S flow cytometer (Beckman Coulter, Krefeld, Germany) and analyzed using Kaluza 2.0 (Beckman Coulter, Brea, CA, USA).
Table 1.
List of antibodies and dyes for the immune checkpoint molecule analysis on the surface of tumor cells via multicolor flow cytometry.
| Marker | Fluorochrome | Manufacturer |
|---|---|---|
| PD-L1 (CD274) | BV 605 | Biolegend |
| PD-L2 (CD273) | APC | Biolegend |
| ICOS-L (CD275) | BV 421 | BD Horizon |
| HVEM (CD270) | APC | Biolegend |
| TNFRSF9 (CD137-L) | BV 421 | Biolegend |
| OX40-L (CD252) | PE | Biolegend |
| Live/Dead | Zombie NIR | Biolegend |
2.6. Isolation of Human Peripheral Blood Mononuclear Cells (PBMCs), Monocyte Enrichment and Differentiation into Immature Dendritic Cells (imm. moDCs)
Peripheral blood mononuclear cells (PBMC) were isolated from leukoreduction system chambers (LRSC) of healthy, anonymous donors having undergone a strict health check by the Transfusion Medicine and Hemostaseology Department of the Universitätsklinikum Erlangen, Germany. The permission to use these LRSCs was given by the ethics committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg (ethical approval no. 180_13 B and 48_19 B), according to the rules of the Declaration of Helsinki in its current form.
Mononuclear cells were separated by using density gradient solution Lymphoflot (Bio-Rad Medical Diagnostics GmbH, Dreieich, Germany) and centrifugation in Sepmate PBMC isolation tubes (SepmateTM, Stemcell Technologies Inc., Vancouver, BC, Canada). Afterwards, the cell suspension was washed several times at 4 °C (2 times with PBS and 2 times with RPMI-1640 medium) and in-between, the centrifugation was performed from high force to low force; this way, cells that are larger and lighter than mononuclear cells would be eliminated by centrifugation. For imm. moDC differentiation, an optimized protocol was used according to Lühr and colleagues [44]: 30 × 106 monocytes were seeded in IgG (Human IgG, Sigma Aldrich, Taufkirchen, Germany)-precoated cell culture dishes with 10 mL of moDC medium (RPMI-1640 (Merck, Darmstadt, Germany)) supplemented with 1% Pen/Strep (Gibco, Carlsbad, CA, USA), 1% L-Glutamine (Gibco, Carlsbad, CA, USA), 1% Hepes buffer 1M (Biochrom GmbH, Berlin, Germany) and 1% heat-inactivated human serum (Gibco, Carlsbad, CA, USA). After 1 h of incubation, cells that did not attach at the bottom of the plates were rinsed away. Therefore, mainly monocytes which are able to bind to the plate-bound IgG via FcγRs remained, and 10 mL of fresh, pre-warmed moDC medium was added. For the differentiation of monocytes into moDCs, on day 1, old culture medium was removed and 10 mL of fresh moDC medium was added to each cell culture dish containing the following cytokines: 800 U/mL of GM-CSF (MACS Miltenyi Biotec, Bergisch Gladbach, Germany) and 500 U/mL of IL-4 (ImmunoTools, Friesoythe, Germany). On day 3, 4 mL of moDC medium containing the same concentration of cytokines was added. On day 5, 4 mL of moDC medium with half of the previously used concentration of GM-CSF (400 U/mL) and IL-4 (250 U/mL) was added.
2.7. Co-Culture of moDCs with Treated and Untreated MCF-7 Cancer Cells and Detection of DC Activation Markers on the Surface of moDCs
On day 6, the obtained moDCs were harvested from the cell culture dish mechanically by using a serological pipette. After counting the harvested cells, 1 × 105 moDCs were seeded in 6-well plates. For the co-culture, 2 × 105 of differently treated MCF-7 cancer cells were added to the moDCs with 2 mL of moDC medium and 2 mL supernatant of the treated cancer cells. As a positive control, 2 mL of moDC medium with a maturation cocktail containing 13.16 ng/mL of IL-1β (ImmunoTools, Friesoythe, Germany), 1000 U/mL of IL-6 (ImmunoTools, Friesoythe, Germany), 10 ng/mL of TNF-α (ImmunoTools, Friesoythe, Germany) and 1 µg/mL of PGE-2 (Pfizer, Berlin, Germany) were added to the moDCs to generate mature moDCs.
After 24 h and 48 h of co-incubation with untreated and treated MCF-7 cancer cells, the expression of co-stimulatory molecules and activation markers was analyzed on moDCs by using multicolor flow cytometry (Figure 4). Therefore, moDCs in suspension were harvested mechanically using a serological pipette. Then, each condition of moDCs was divided into 2 duplicates, one stained with staining solution (Table 2), while the other duplicate served as an autofluorescence control with only Zombie Yellow in the FACS Buffer.
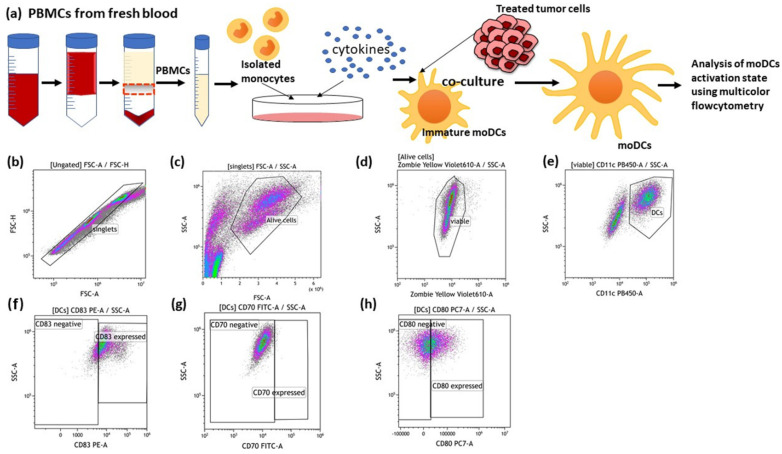
Figure 4.
Generation of human monocyte-derived DCs (moDCs) from PBMCs and the detection of DC activation markers after co-incubation with treated cancer cells. (a) PBMCs were isolated from buffy coat and seeded into an IgG pre-coated cell culture dish. On day 6 after differentiation, moDCs were co-cultured with differently treated MCF-7 breast cancer cells. After 24 h and 48 h of co-incubation, the activation markers of the moDCs were analysed using multicolor flow cytometry. The gating strategies for flow cytometry are shown (b–h). (b) After pre-gating on the singlets, the viable cells were detected (c,d). Then, gating on CD11c positive cells identified moDCs (e). Dot plots of CD83 (f), CD70 (g) and CD80 (h) expression on the cell surface of moDCs are exemplarily presented.
Table 2.
List of antibodies and dyes used to analyze the expression of various activation markers on the surface of moDCs via multicolor flow cytometry.
| Marker | Fluorochrome | Manufacturer |
|---|---|---|
| CD70 | FITC | Biolegend |
| CD83 | PE-Cy7 | eBioscience |
| CD80 | APC | Miltenyi Biotec (MACS) |
| Live/Dead | Zombie Yellow | Biolegend |
| CD11c | V450 | Biolegend |
The cells were gated according to Figure 4. The mean fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence intensity of autofluorescence control samples from the fully stained samples. The samples were measured on a Cytoflex S flow cytometer (Beckman Coulter, Krefeld, Germany) and analyzed using Kaluza 2.0 (Beckman Coultier, Brea, CA, USA).
2.8. Statistical Analysis
For statistical analysis, the software Prism 7 (Graph Pad, San Diego, CA, USA) was used. Separate Kruskal–Wallis tests with Dunn’s correction for multiple testing were used to compare the treatments within one HT temperature to the untreated control. Further, the combinatorial treatments were compared to RT only with a Kruskal–Wallis test with Dunn’s correction for multiple testing. To compare the sequence of the combined treatments of one HT temperature (RT + HT vs. HT + RT), a Mann–Whitney U test was used. Results were considered statistically significant for * p < 0.1, ** p < 0.01, *** p < 0.001.
3. Results
3.1. Radiotherapy in Combination with Hyperthermia Significantly Induces Apoptosis in MCF-7 Cells Andboth Apoptosis and Necrosis in MDA-MB-231 Breast Cancer Cells
In order to elucidate the effect of HT on the immune phenotype of human breast cancer cells, three different clinically relevant HT temperatures were used (39 °C, 41 °C, 44 °C). MCF-7 and MDA-MB-231 cells were treated with either HT or RT alone, and in a combinational setting in different sequences with either HT followed by RT (HT + RT), or RT followed by HT (RT + HT).
3.1.1. Radiotherapy in Combination with Hyperthermia Regardless of the Treatment Sequence Significantly Induces Apoptosis in MCF-7 Breast Cancer Cells
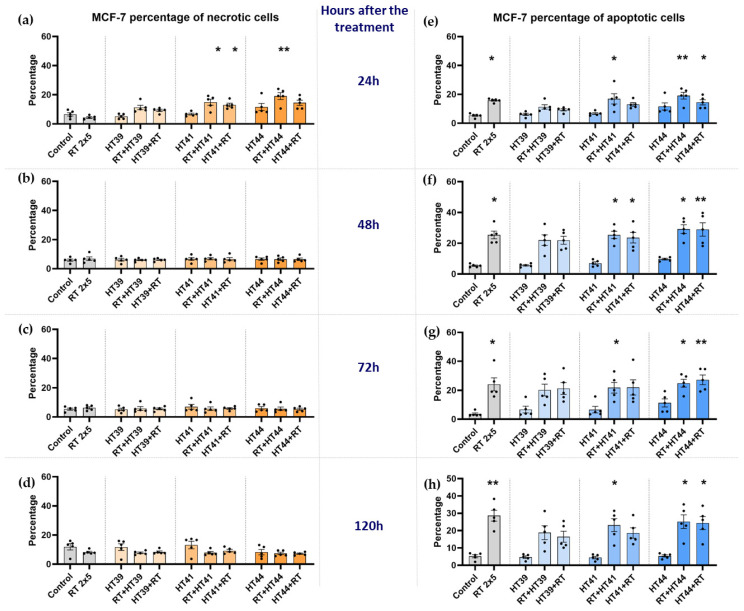
Necrosis and apoptosis of MCF-7 cells were determined 24 h, 48 h, 72 h, and 120 h after the respective treatment (Figure 5).
Figure 5.
Radiotherapy alone and in combination with hyperthermia regardless of the treatment sequence induces apoptosis in MCF-7 breast cancer cells. The percentage of necrotic MCF-7 cells are shown in graphs (a) 24 h, (b) 48 h, (c) 72 h and (d) 120 h after the treatment. The percentage of apoptotic MCF-7 cells is shown in graphs (e) 24 h, (f) 48 h, (g) 72 h and (h) 120 h after the treatment. MCF-7 cells were irradiated 2 times with 5 Gy (RT) or treated with HT of different temperatures (39 °C, 41 °C, 44 °C) and combinations of both, either HT followed by RT (HT (39 °C, 41 °C, 44 °C) + RT) or vice versa (RT + HT (39 °C, 41 °C, 44 °C)). The time interval between HT and RT was less than 2 h. The cell death forms were analyzed by AnxV/PI staining using multicolor flow cytometry. Mean ± SD are presented from at least five independent experiments. Statistical significance is calculated by using a Kruskal–Wallis test with Dunn’s correction to compare the percentage of necrotic and apoptotic cells of each group of a respective temperature to the untreated control, and a Mann–Whitney U test to compare the different sequences of HT and RT. * (p < 0.1), ** (p < 0.01) for Kruskal–Wallis test with Dunn’s correction.
As shown in Figure 5, in MCF-7 cells at an early time point (24 h) after the treatment, a slight increase of necrotic cells was observed, particularly after combinations of RT with HT. However, at later time points, the cancer cell death is dominantly in the form of apoptosis. The key inductor of apoptosis was RT alone (Figure 5e–h). In contrast, neither necrosis nor apoptosis was induced in MCF-7 breast cancer cells by HT as a single treatment, even with up to 44 °C.
As observed for RT alone, a combination of HT and RT induced significantly more apoptosis regardless of the treatment sequence (Figure 5e–h). HT of 44 °C significantly induced apoptotic cancer cell death at all time points when it was combined with radiation therapy (Figure 5e–h). In contrast, 39 °C HT with RT resulted in a slight decrease of apoptosis compared to RT alone.
In any case, the sequences of the combined treatments, either RT or HT first, were not significantly different from each other in the induction of cancer cell death, with the exception of a tendency for less apoptosis when HT of 41 °C was given before RT, as compared to afterwards.
3.1.2. Radiotherapy in Combination with Hyperthermia Regardless of the Treatment Sequence Significantly Induce Apoptosis and Necrosis in MDA-MB-231 Breast Cancer Cells
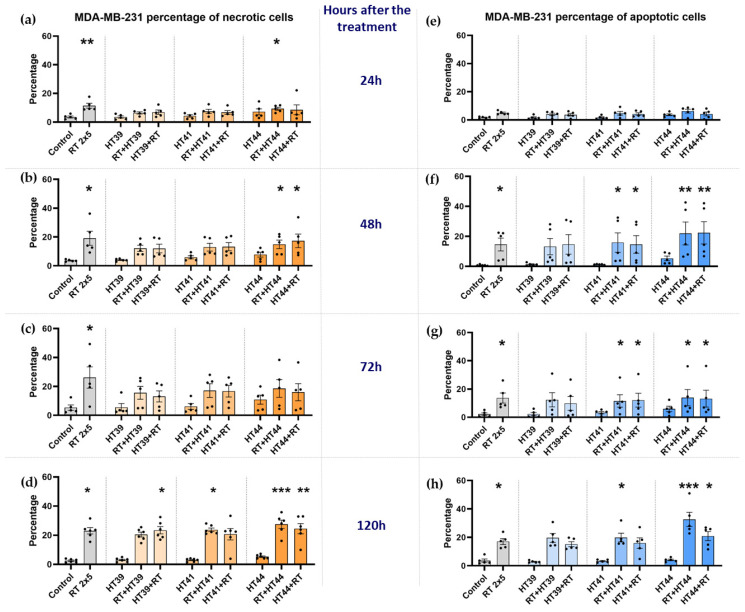
MDA-MB-231 cells were treated with the respective treatments similar to MCF-7 cells, and afterwards cell death forms were analyzed (Figure 6).
Figure 6.
Radiotherapy alone and in combination with hyperthermia regardless of the treatment sequence significantly induces apoptosis and necrosis in MDA-MB-231 breast cancer cells. The percentage of necrotic MDA-MB-231 cells are shown in graphs (a) 24 h, (b) 48 h, (c) 72 h and (d) 120 h after the treatment. The percentage of apoptotic MDA-MB-231 cells is shown in graphs (e) 24 h, (f) 48 h, (g) 72 h and (h) 120 h after the treatment. MDA-MB-231 breast cancer cells were irradiated 2 times with 5 Gy (RT) or treated with HT of different temperatures (39 °C, 41 °C, 44 °C) and combinations of both, either HT followed by RT (HT (39 °C, 41 °C, 44 °C) + RT) or vice versa (RT + HT (39 °C, 41 °C, 44 °C)). Mean ± SD are presented from at least five independent experiments. Statistical significance was calculated by using a Kruskal–Wallis test with Dunn’s correction to compare the percentage of necrotic and apoptotic cells of each group of a respective temperature to the untreated control, and a Mann–Whitney U test to compare the different sequences of HT and RT. * (p < 0.1), ** (p < 0.01), *** (p < 0.001) for Kruskal–Wallis test with Dunn’s correction.
In contrast to MCF-7 breast cancer cells, RT alone could significantly induce apoptosis and necrosis of MDA-MB-231 cells (Figure 6a–d,f–h), but again, HT alone did not induce significantly increased apoptosis or necrosis at all of the examined timepoints.
Again, the percentages of apoptotic cells were slightly lower when HT of 39 °C was combined with RT, compared to 41 °C and 44 °C. The highest induction of necrosis was observed when RT was combined with HT of 44 °C (Figure 6d).
Again, the sequences of the combined treatments, either RT or HT first, were similar in the induction of breast cancer cell death.
3.2. Hyperthermia in Combination with Radiotherapy Affects the Expression of Immune Checkpoint Molecules on Breast Cancer Cells
Next, we investigated the impact of HT, RT, and HT in combination with RT on the expression of immune inhibitory ICMs (PD-L1, PD-L2, HVEM) and on one immune stimulatory ICM (OX40-L) on MCF-7 and MDA-MB-231 breast cancer cells.
3.2.1. Hyperthermia in Combination with Radiotherapy Upregulates the Expression of Several Inhibitory Immune Checkpoint Molecules on MCF-7 Breast Cancer Cells
Regarding RT alone, the well-known inhibitory ICM PD-L1 was significantly upregulated up to 72 h after treatment. (Figure 7a–c). However, PD-L2 and HVEM were also increased following RT (Figure 7e–l).
Figure 7.
Hyperthermia in combination with radiotherapy affects the expression of inhibitory immune checkpoint molecules (PD-L1, PD-L2, and HVEM) on MCF-7 breast cancer cells. MCF-7 cells were irradiated 2 times with 5 Gy (RT) or treated with HT of different temperatures (39 °C, 41 °C, 44 °C) and combination of both, either HT followed by RT (HT (39 °C, 41 °C, 44 °C) + RT) or vice versa (RT + HT (39 °C, 41 °C, 44 °C)). The time interval between HT and RT was less than 2 h. The expression of ICMs ((a–d): PD-L1, (e–h): PD-L2 and (i–l): HVEM) were analyzed by multicolor flow cytometry. The mean fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence intensity of unstained samples from stained samples. Mean ± SD are presented from at least five independent experiments. Statistical significance is calculated by using Kruskal–Wallis tests with Dunn’s correction by comparing the ΔMFI of cells after the treatment to untreated control of the corresponding timepoint, and Mann–Whitney U tests to compare the ΔMFI of different sequences of HT and RT. * (p < 0.1), ** (p < 0.01). Further, RT alone was compared with combinational treatments (HT + RT and RT + HT); # (p < 0.1).
When adding HT to RT, the time point 120 h is particularly of interest here: all of the three immune suppressive ICMs examined, namely PD-L1, PD-L2 and HVEM, were significantly increased when RT was combined with HT of any temperature. At this time, some of the observed increased expressions of ICMs were significantly enhanced even when compared to RT-only treatment (Figure 7d,h,l).
Generally, a high dynamic of ICM expression can be observed after combined RT and HT treatments. There was no significant difference between the different sequences of treatments (either HT + RT or RT + HT) in case of the upregulation of inhibitory ICMs.
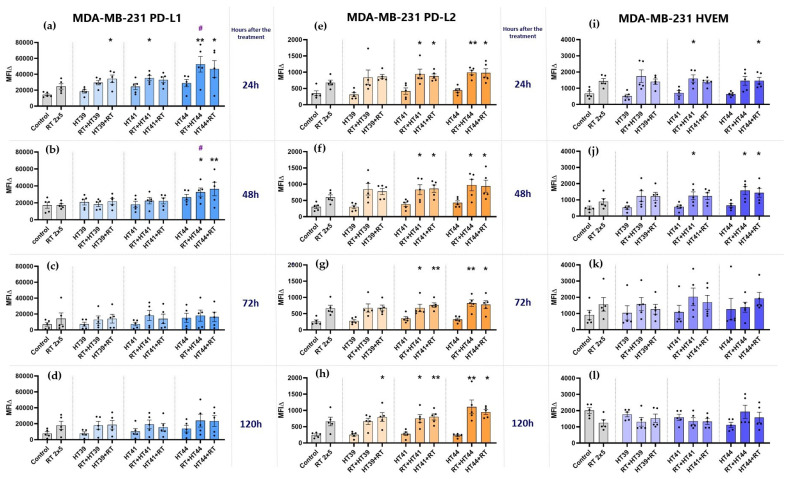
3.2.2. Hyperthermia in Combination with Radiotherapy Upregulates the Expression of Several Inhibitory Immune Checkpoint Molecules on MDA-MB-231 Breast Cancer Cells
In contrast to MCF-7 cells, RT alone did not significantly increase the expression of PD-L1 on MDA-MB-231 cells. However, the combination of RT with HT at 44 °C in particular significantly increased PD-L1 expression at earlier time points after treatment (Figure 8a,b). This increase was even significantly higher when compared to RT alone when RT was given before HT. As for PD-L1, the expression of PD-L2 and HVEM was also not significantly increased by RT only.
Figure 8.
Hyperthermia in combination with radiotherapy affects the expression of inhibitory immune checkpoint molecules (PD-L1, PD-L2, and HVEM) on MDA-MB-231 breast cancer cells. MDA-MB-231 cells were irradiated 2 times with 5 Gy (RT) or treated with HT of different temperatures (39 °C, 41 °C, 44 °C) and a combination of both, either HT followed by RT (HT (39 °C, 41 °C, 44 °C) +RT) or vice versa (RT + HT (39 °C, 41 °C, 44 °C)). The time interval between HT and RT was less than 2 h. The expression of ICMs ((a–d): PD-L1, (e–h): PD-L2 and (i–l): HVEM) was analyzed by multicolor flow cytometry. The mean fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence intensity of unstained samples from stained samples. Mean ± SD are presented from at least five independent experiments. Statistical significance is calculated by using Kruskal–Wallis tests with Dunn’s correction by comparing the ΔMFI of cells after the treatment to untreated control of the corresponding timepoint, and Mann–Whitney U tests to compare the ΔMFI of different sequences of HT and RT. * (p < 0.1), ** (p < 0.01). RT alone was compared with combinational treatments (HT + RT and RT + HT); # (p < 0.1).
Of special interest for MDA-MB-231 cells is that only the combinations of RT with HT of both 41 and 44 °C induced a significant increased expression of PD-L2 at all examined time points (Figure 8e–h), while the expression of HVEM was only slightly altered at earlier time points, but again with combinations of RT with HT of 41 or 44 °C (Figure 8i,j). At later time points, its expression was even slightly decreased after RT or a combination of RT with HT of 39 or 41 °C (Figure 8l).
Generally, as for MCF-7 cells, a high dynamicity of ICM expression can be observed after combined RT and HT treatments. There was again no significant difference between the tested sequences of treatments (either HT + RT or RT + HT) in case of the upregulation of inhibitory ICMs.
3.2.3. Hyperthermia in Combination with Radiotherapy Only Slightly Affects the Expression of Stimulatory Immune Checkpoint Molecules on MCF-7 and MDA-MB-231 Breast Cancer Cells
The expression of the immune stimulatory ICMs ICOS-L, CD27-L, CD137-L (not shown) and of OX40-L (Figure 9) was determined on MCF-7 and MDA-MB-231 breast cancer cells.
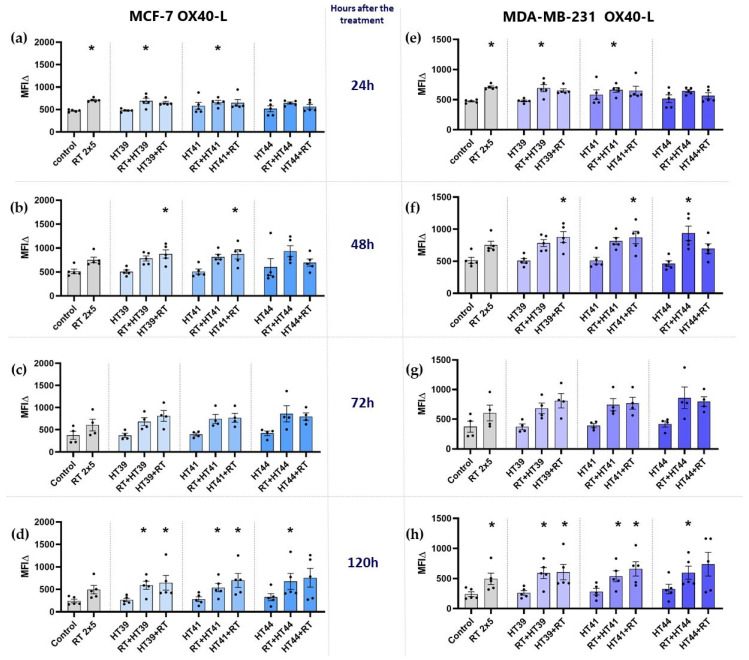
Figure 9.
Expression of the immune stimulatory ICM OX40-L on MCF-7 and MDA-MB-231 cells at different timepoints after the treatment. (a–d) MCF-7 and (e–h) MDA-MB-231 cells were irradiated 2 times with 5 Gy (RT) or treated with HT of different temperatures (39 °C, 41 °C, 44 °C) and combinations of both, either HT followed by RT (HT (39 °C, 41 °C, 44 °C) + RT) or vice versa (RT + HT (39 °C, 41 °C, 44 °C)). The time interval between HT and RT was less than 2 h. The expression of OX40-L was analyzed by multicolor flow cytometry (a,e) 24 h, (b,f) 48 h, (c,g) 72 h, or (d,h) 120 h later. The mean fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence intensity of unstained samples from stained samples. Mean ± SD are presented from at least five independent experiments. Statistical significance is calculated by using Kruskal–Wallis tests with Dunn’s correction by comparing the ΔMFI of cells after the treatment to untreated control of the corresponding timepoint, and Mann–Whitney U tests to compare the ΔMFI of different sequences of HT and RT. * (p < 0.1).
In both cell lines, HT alone at any temperature did not significantly affect the expression of OX40-L, and RT increased it only slightly.
The combination of HT with RT led to a significantly increased expression of this immune-stimulatory ICM particularly at 120 h after the treatment (Figure 9d,h). There was no significant difference observed with regard to different combinational treatment sequences (whether RT or HT came first).
3.3. The Impact of HT- and RT-Treated Breast Cancer Cells on the Activation State of moDCs
To get first hints whether the immune phenotype characterized by ICM expression of treated breast cancer cells affects the activation state of moDCs, MCF-7 breast cancer cells treated with different sequence of HT and RT were co-cultured with immature moDCs. For this, HT of 44 °C was chosen, alone and in combination with RT, as the most prominent alterations were observed here (Figure 7). The DC activation markers, CD80, CD83 and CD70, were analyzed using multicolor flow cytometry (Figure 10).
Figure 10.
Expression of activation markers on moDCs after contact with hyperthermia- and radiotherapy treated MCF-7 breast cancer cells. Displayed is the expression of DC activation markers after 24 h (a)—CD83, (b)—CD70, (c)—CD80, and 48 h (d)—CD83, (e)—CD70, (f)—CD80 after co-incubation of immature moDCs with untreated MCF-7 tumor cells or with differently treated MCF-7 tumor cells. The tumor cells were treated with 2 × 5 Gy RT, HT of 44 °C, and first HT of 44 °C and then RT or RT followed by HT of 44 °C. As a positive control, immature moDCs were activated with the standard maturation cocktail (MC), and as negative control, immature moDCs were kept in moDC medium without the maturation cocktail (w/o MC). The expression of DC activation markers was analyzed by multicolor flow cytometry. The mean fluorescence intensity (ΔMFI) was calculated by subtracting the fluorescence intensity of unstained samples from stained samples. Mean ± SD are presented from at least four independent experiments. Statistical significance is calculated by using Kruskal–Wallis tests with Dunn’s correction by comparing the ΔMFI of the treatments to untreated controls at the corresponding timepoint, and Mann–Whitney U tests to compare the ΔMFI of different sequence of HT and RT. * (p < 0.1).The arm without maturation cocktail was compared to the arm with maturation cocktail with a Mann–Whitney U test # (p < 0.1), ## (p < 0.01).
As expected, the incubation of immature moDCs with a maturation cocktail induced upregulation of all of the DC activation markers analyzed after 24 h (Figure 10a–c) and 48 h (Figure 10d–f), respectively. Only immature moDCs which were incubated with tumor cells treated with HT of 44 °C significantly upregulated CD70 at 24 h (Figure 10b). Otherwise, moDCs co-incubated with treated breast cancer cells did not show any significant upregulation of their activation markers. Only the expression of CD80 (Figure 10c,f) tended to be higher, when DCs co-cultured with tumor cells are compared to the DCs without the maturation cocktail. However, this was irrespective of the treatment of the breast cancer cells.
4. Discussion
4.1. In Human Breast Cancer Cells, Radiotherapy Rather Than Moderate Hyperthermia Is the Key Trigger for Cell Death Induction
RT has been used as a standard anticancer therapy for decades and its effect on the immune system has been studied extensively in recent years. It has become obvious that besides its local killing effects on cancer cells, ionizing radiation also has a strong impact on the immune system [45]. Furthermore, it has been recognized that HT can modulate the immune system and thereby affect the anti-tumor immune response, mostly in combination with RT [46]. This may result in both local and systemic anti-tumor immune responses, also in breast cancer [47]. Combinations of RT with HT [8,48,49], with or without immunotherapy such as ICIs [50,51], are promising multimodal treatments for breast cancer, which however need further optimization. It is still not clear which treatment sequence would have a better effect. Thus, in our preclinical approach, we investigated whether it is better to use HT first and then RT, or RT followed by HT, regarding breast cancer cell death induction, immune checkpoint molecule expression and the activation of DCs after co-incubation with the treated tumor cells.
Immunogenic cancer cell death (ICD) is one of the key triggers that has been reported by several studies to induce anti-tumor immune responses [52]. Indeed, ICD can be induced by a combination of RT with, e.g., graphene-induced HT [53], but also with conventional heat application, as shown, e.g., for colorectal cancer cells [17]. ICD mainly triggers the activation of DCs via the release of danger signals and consecutive cytotoxic T cell priming [54]. However, knowledge about HT-induced immune alterations independently of ICD is scarce, and theoretical evaluations even suggest that HT rather boosts the RT-induced cell killing, but does not fundamentally change the anti-tumor immune response [55].
Our in vitro examinations indicate that RT is the key trigger for cell death induction in breast cancer cells, with apoptosis being prominent in MCF-7 cells and both apoptosis and necrosis in MDA-MB-231 cells. The co-culture experiment with immature moDCs further showed that HT in combination with RT did not induce enough ICD to mature moDCs with the examined breast cancer cells. We therefore conclude that, besides ICD with danger signal release, the immune phenotype of the tumor cell surface plays an additional role in triggering RT-plus-HT-induced anti-tumor immune responses, mainly in the effector phase [56]. Furthermore, with regard to inducing breast cancer cell death, there was no significant difference between HT and RT combinational sequences, i.e., whether to apply HT first or RT first. This finding confirms the analyses by Mei et al. [57] who report no significant difference in the case of cell death induction regarding whether HT is used before or after RT. One has to stress that in these analyses, the focus was set on the temperature effects of HT alone and in combination with RT. However, different heating methods can have different outcomes, as we have recently shown that microwave heating more effectively induced cell death compared to conventional warm bath heating systems [39]. In the current study, we intentionally focused on conventional heating to set the focus on HT-induced immune alterations on the tumor cell surface, rather than on sole ICD induction. However, in addition to performing similar analyses in the future with microwave-based heating for further elucidating synergistic effects of RT plus HT treatments, preclinical in vivo models will have to be used for ICM expression and tumor cell death analyses with a setting closely resembling the clinical situation. This has already been performed for the analyses of the immunogenicity of B16 melanoma tumors following combined RT and HT treatment [19].
4.2. The Combination of Hyperthermia and Radiotherapy Affects Particularly the Expression of Immune Suppressive Immune Checkpoint Molecules of Breast Cancer Cells, but Independently of the Treatment Sequence
Identifying ICM expression profiles of cancer cells is crucial, because ICMs are responsible for tumor evasion from the immune system and strongly regulate the effector phase of the immune response. A high expression of immune inhibitory ICMs is linked to worse clinical outcomes, and ICIs are becoming more and more promising approaches in anti-cancer treatment, either alone or in combination with other treatments like RT and HT [58,59].
It has already become obvious that RT enhances the expression of PD-L1 [60,61], either alone or in combination with other treatments [61,62]. How RT and its combination with HT affect the expression of other ICMs, specifically, which sequence of HT and RT treatment alters the ICMs, is not clear yet. We revealed that the expression of ICMs follows a high dynamic which is strongly time-dependent: in MCF-7 breast cancer cells, not earlier than 72 h after treatment and most pronouncedly after 120 h, three immunosuppressive ICMs, namely PD-L1, PD-L2 and HVEM, were upregulated in their expression following combined RT plus HT treatments, independently of the temperature. This calls for an inhibition of the PD-1 receptor which binds both PD-L1 and PD-L2, rather than sole PD-L1 inhibition, in breast cancer immunotherapies. Further, the inhibition of HVEM should be considered too, as it has already been shown that breast cancer patients have a worse prognosis when low amounts of tumor-infiltrating lymphocytes are present and HVEM is expressed by the tumor cells [63]. Future preclinical in vivo experiments will have to elucidate the best combination of RT, HT and immune checkpoint inhibition. Recent preclinical data of the combination regimens of hyperthermia and ICIs have already demonstrated their combined efficacy [51]. Our in vitro data suggest that the sequence of RT and HT has a minor role in this, as HT in combination with RT, regardless of the sequence, induced the upregulation of inhibitory ICMs, unlike HT alone. It would be of additional interest to examine the effects on immune checkpoints in normal epithelial cells and whether these effects will impact the cytotoxic effects of ICIs in normal breast epithelial cells.
Besides the factor “time after treatment”, the characteristics of suppressive ICMs are important. In MDA-MB-231 cells, PD-L2 was upregulated at all examined times when HT of 41 °C or 44 °C was combined with RT. This calls again for targeting the PD-1 receptor in multimodal RT- and HT-based tumor therapies, rather than only PD-L1, as, additionally, the utility of PD-L1 as a predictive biomarker in most of the breast cancer subtypes remains elusive [64].
It has to be further considered that the upregulation of PD-L1 differed in MDA-MB-231 and MCF-7 cells in a time-dependent manner. MCF-7 cells showed an increased expression of PD-L1 at later timepoints (72–120 h), while on the surface of MDA-MB-231 cells, it occurred earlier (24–48 h) after treatments with RT plus HT. This should be also considered for the design of multimodal breast cancer therapies. For lung cancer, it has already been proven in preclinical and clinical studies that the timing of ICI affects the clinical outcome [65,66]. Once again, instead of focusing on one ICM, several other ICMs also should be monitored at different timepoints. Similarly to the findings of Hader et al. [39], we also detected that combinations of RT with HT induced higher expressions of immune-suppressive ICMs compared to HT or RT alone. Li et al. stressed that HT can create a tumor microenvironment with high PD-L1 expression and lymphocyte infiltration, making the tumor more likely to respond to anti-PD1 therapy [51].
Not only inhibitory ICMs were changed, but also the immune-stimulatory ICM OX40-L was significantly upregulated particularly at 120 h after the treatment with RT plus HT. This might offer an opportunity to strengthen the immunostimulatory properties of HT at distinct time points during therapy with OX40-L-agonistic antibodies. The latter has already been used preclinically in combination with other anti-cancer treatments and has shown promising results [67]. However, besides OX40-L, the other immune-activating ICMs (ICOS-L, CD27-L, CD137-L) examined were not strongly affected by RT and HT (not shown). This is in contrast to other tumor entities, such as, e.g., head and neck cancer, where ICOS-L is upregulated on HPV-positive cancer cells after RT [68].
We conclude that in the area of multimodal tumor therapies, the temporal expression of several ICMs should be monitored closely, and personalized therapy approaches will become more and more important. Here, HT will find a new place as an immunomodulator and as a combination partner with RT and ICIs. Even though cell death induction tends to be higher when HT of 44 °C is combined with RT, the expression of ICMs is modulated by even lower temperatures, such as 41 °C and 39 °C. This highlights that the immunomodulating effects of HT are manifold, and besides focusing on the level of temperature with regard to tumor cell death induction [69], the immunomodulating phenotype of tumor cells has to be considered in a timely manner after treatment. Nevertheless, precise monitoring of the temperature in the tumor during the treatment will not only improve the efficacy of the local treatment, but also gives a chance to predict the changes in immune phenotype of the cancer cells. Besides monitoring of local immune alterations inside and around the treated tumor, systemic effects should be complementarily considered in the future to increase knowledge about immune modulations induced by RT alone and in combination with HT and ICIs [70,71].
Even though the sequence of application might affect several cellular processes [28,72], it does not significantly impact on the immune phenotype of the surface of breast cancer cells. In addition to the sequence, different time intervals between RT and HT should be analyzed in the future in vitro and particularly in vivo, also taking into account the oxygenation status of the tissues [73]. In clinics, still no consensus regarding the sequence of application of RT with HT has been reached, and at least from the immunological points of view that were analyzed in this work, it does not matter very much. However, immune factors have to be included in considerations of thermometric parameters to guide HT in the future and to finally validate them in prospective clinical trials [74].
4.3. The Co-Incubation of RT- and HT-Treated Breast Cancer Cells Does Not Affect the Activation State of Dendritic Cells
An immune response consists of a priming and an effector phase. In the priming phase, antigens are taken up by DCs, which have to additionally be stimulated by adjuvants such as danger signals. The latter can be released by stressed tumor cells and mostly in connection with ICD [54]. However, anti-tumor immune responses are not only triggered by the initial priming of T cells against the tumor, but also by restoring anti-tumor immunity in the effector phase. Both modes of action have already been proven to be involved in RT-induced anti-tumor immune responses [75].
Our data now show for the first time that the combination of RT and HT in breast cancer treatment affects the expression of several ICMs in a time-dependent manner. However, there was no significant difference between the different treatments and sequences in regard to the upregulation of activation markers on moDCs. Matsumoto et al. observed that treatment of tumor cells with HT and consecutive co-incubation with murine bone marrow DCs also did not induce activation of these APCs. In order to improve this, they suggest to additionally expose the DCs themselves to mild HT [76]. Thus, future research should analyze this with in vivo systems, but taking both the priming and effector phases of the anti-tumor immune response into consideration [77].
5. Conclusions
The Combination of HT of 39 °C, 41 °C and 44 °C with hypofractionated RT particularly affects the surface immune phenotype of human breast cancer cells. Mainly, immune suppressive ICMs are upregulated following combined treatments, in dependence of the tumor cells, the time after treatment and the nature of the ICM. Besides PD-L1, further suppressive ICMs such as PD-L2 and HVEM should be considered for clinicians when treating breast cancer patients in multimodal settings including RT and HT.
One has to stress that the sequence of the application of RT and HT has no significant impact on the breast cancer cell immune phenotype, and from the immunological point of view, it does not matter very much how this is currently handled in distinct clinics/institutes. For the first time it was shown here, at least preclinically, that rather the immune effector than the immune priming phase is modulated by combination treatments of RT with HT. Besides the induction of ICD, the modulation of the cancer cell’s surface immune phenotype has to be considered for the design of innovative prospective clinical trials for breast cancer, including HT. In multimodal treatment settings it might be beneficial to add distinct ICIs in the combinational therapy of HT and RT.
Acknowledgments
We thank the staff and particularly Erwin Strasser of the Transfusion Medicine and Hemostaseology Department of the Universitätsklinikum Erlangen, Germany, for co-operation in the generation of primary immune cells from biomaterial of leukoreduction system chambers (LRSC) of healthy, anonymous donors. We further acknowledge the support by the German Research Foundation and the Friedrich-Alexander-Universität Erlangen-Nürnberg within the funding program Open Access Publishing. This work has been supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 955625, Hyperboost.
Author Contributions
The structure and the content of the manuscript was conceptualized by U.S.G., M.R., M.H. and A.S. A.S. performed most of the experiments with support of L.H. and D.D. regarding the dendritic cell experiments. The drafts of the manuscripts were written by A.S., M.H., U.S.G. and B.F. The final manuscript was written by A.S., M.R., U.S.G., B.F., M.H., R.F., O.J.O., S.S. and S.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 955625, Hyperboost. Further funding was awarded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—RTG2599 (421758891) to D.D. (P1) and U.G. (P10) and the SFB TRR 305—B05 to D.D.
Institutional Review Board Statement
Peripheral blood mononuclear cells (PBMC) were isolated from leukoreduction system chambers (LRSC) of healthy, anonymous donors having undergone a strict health check by the Transfusion Medicine and Hemostaseology Department of the Universitätsklinikum Erlangen, Germany. The permission to use these LRSCs was given by the ethics committee of the Friedrich-Alexander-Universität Erlangen-Nürnberg (ethical approval no. 180_13 B and 48_19 B), according to the rules of the Declaration of Helsinki in its current form.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest with regard to the work presented here.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jemal A., Bray F., Center M.M., Ferlay J., Ward E., Forman D. Global cancer statistics. CA Cancer J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.O’Shaughnessy J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncologist. 2005;10:20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 3.Villacampa G., Tolosa P., Salvador F., Sánchez-Bayona R., Villanueva L., Dienstmann R., Ciruelos E., Pascual T. Addition of immune checkpoint inhibitors to chemotherapy versus chemotherapy alone in first-line metastatic triple-negative breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2022;104:102352. doi: 10.1016/j.ctrv.2022.102352. [DOI] [PubMed] [Google Scholar]
- 4.Frey B., Rückert M., Deloch L., Rühle P.F., Derer A., Fietkau R., Gaipl U.S. Immunomodulation by ionizing radiation-impact for design of radio-immunotherapies and for treatment of inflammatory diseases. Immunol. Rev. 2017;280:231–248. doi: 10.1111/imr.12572. [DOI] [PubMed] [Google Scholar]
- 5.Issels R., Kampmann E., Kanaar R., Lindner L. Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: Translation into clinical application. Int. J. Hyperth. 2016;32:89–95. doi: 10.3109/02656736.2015.1119317. [DOI] [PubMed] [Google Scholar]
- 6.Hurwitz M., Stauffer P. Hyperthermia, Radiation and Chemotherapy: The Role of Heat in Multidisciplinary Cancer Care. Semin. Oncol. 2014;41:714–729. doi: 10.1053/j.seminoncol.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Issels R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer. 2008;44:2546–2554. doi: 10.1016/j.ejca.2008.07.038. [DOI] [PubMed] [Google Scholar]
- 8.De-Colle C., Weidner N., Heinrich V., Brucker S., Hahn M., Macmillan K., Lamprecht U., Gaupp S., Voigt O., Zips D. Hyperthermic chest wall re-irradiation in recurrent breast cancer: A prospective observational study. Strahlenther. Onkol. 2019;195:318–326. doi: 10.1007/s00066-018-1414-z. [DOI] [PubMed] [Google Scholar]
- 9.Oldenborg S., Rasch C.R.N., Van Os R., Kusumanto Y.H., Oei B.S., Venselaar J.L., Heymans M., Vörding P.J.Z.V.S., Crezee J., Van Tienhoven G. Reirradiation + hyperthermia for recurrent breast cancer en cuirasse. Strahlenther. Onkol. 2017;194:206–214. doi: 10.1007/s00066-017-1241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song C.W., Shakil A., Osborn J.L., Iwata K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int. J. Hyperth. 2009;12:367–373. doi: 10.3109/02656739609022525. [DOI] [PubMed] [Google Scholar]
- 11.Horsman M.R., Overgaard J. Hyperthermia: A Potent Enhancer of Radiotherapy. Clin. Oncol. 2007;19:418–426. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 12.Tsang Y.-W., Huang C.-C., Yang K.-L., Chi M.-S., Chiang H.-C., Wang Y.-S., Andocs G., Szasz A., Li W.-T., Chi K.-H. Improving immunological tumor microenvironment using electro-hyperthermia followed by dendritic cell immunotherapy. BMC Cancer. 2015;15:708. doi: 10.1186/s12885-015-1690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rao W., Deng Z.-S., Liu J. A Review of Hyperthermia Combined With Radiotherapy/Chemotherapy on Malignant Tumors. Crit. Rev. Biomed. Eng. 2010;38:101–116. doi: 10.1615/CritRevBiomedEng.v38.i1.80. [DOI] [PubMed] [Google Scholar]
- 14.Chen T., Guo J., Han C., Yang M., Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J. Immunol. 2009;182:1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- 15.Datta N.R., Gómez Ordóñez S., Gaipl U.S., Paulides M.M., Crezee H., Gellermann J., Marder D., Puric E., Bodis S. Local hyperthermia combined with radiotherapy and-/or chemotherapy: Recent advances and promises for the future. Cancer Treat. Rev. 2015;41:742–753. doi: 10.1016/j.ctrv.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 16.Schildkopf P., Frey B., Mantel F., Ott O.J., Weiss E.-M., Sieber R., Janko C., Sauer R., Fietkau R., Gaipl U.S. Application of hyperthermia in addition to ionizing irradiation fosters necrotic cell death and HMGB1 release of colorectal tumor cells. Biochem. Biophys. Res. Commun. 2010;391:1014–1020. doi: 10.1016/j.bbrc.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Schildkopf P., Frey B., Ott O.J., Rubner Y., Multhoff G., Sauer R., Fietkau R., Gaipl U.S. Radiation combined with hyperthermia induces HSP70-dependent maturation of dendritic cells and release of pro-inflammatory cytokines by dendritic cells and macrophages. Radiother. Oncol. 2011;101:109–115. doi: 10.1016/j.radonc.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 18.Skitzki J.J., A Repasky E., Evans S.S. Hyperthermia as an immunotherapy strategy for cancer. Curr. Opin. Investig. Drugs. 2009;10:550–558. [PMC free article] [PubMed] [Google Scholar]
- 19.Werthmöller N., Frey B., Rückert M., Lotter M., Fietkau R., Gaipl U.S. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int. J. Hyperth. 2016;32:23–30. doi: 10.3109/02656736.2015.1106011. [DOI] [PubMed] [Google Scholar]
- 20.Schmid T.E., Multhoff G. Radiation-induced stress proteins—The role of heat shock proteins (HSP) in anti-tumor responses. Curr. Med. Chem. 2012;19:1765–1770. doi: 10.2174/092986712800099767. [DOI] [PubMed] [Google Scholar]
- 21.Peer A.J., Grimm M.J., Zynda E.R., Repasky E.A. Diverse immune mechanisms may contribute to the survival benefit seen in cancer patients receiving hyperthermia. Immunol. Res. 2009;46:137–154. doi: 10.1007/s12026-009-8115-8. [DOI] [PubMed] [Google Scholar]
- 22.Knippertz I., Stein M.F., Dörrie J., Schaft N., Müller I., Deinzer A., Steinkasserer A., Nettelbeck D.M. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int. J. Hyperth. 2011;27:591–603. doi: 10.3109/02656736.2011.589234. [DOI] [PubMed] [Google Scholar]
- 23.Crezee J., Oei A.L., Franken N.A.P., Stalpers L.J.A., Kok H.P. Response: Commentary: The Impact of the Time Interval between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2020;10:10. doi: 10.3389/fonc.2020.00528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bakker A., Van Der Zee J., Van Tienhoven G., Kok H.P., Rasch C.R.N., Crezee H. Temperature and thermal dose during radiotherapy and hyperthermia for recurrent breast cancer are related to clinical outcome and thermal toxicity: A systematic review. Int. J. Hyperth. 2019;36:1024–1039. doi: 10.1080/02656736.2019.1665718. [DOI] [PubMed] [Google Scholar]
- 25.Crezee H., Kok H.P., Oei A.L., Franken N.A.P., Stalpers L.J.A. The Impact of the Time Interval between Radiation and Hyperthermia on Clinical Outcome in Patients with Locally Advanced Cervical Cancer. Front. Oncol. 2019;9:412. doi: 10.3389/fonc.2019.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dobšíček Trefná H., Schmidt M., van Rhoon G.C., Kok H.P., Gordeyev S.S., Lamprecht U., Marder D., Nadobny J., Ghadjar P., Abdel-Rahman S., et al. Quality assurance guidelines for interstitial hyperthermia. Int. J. Hyperth. 2019;36:276–293. doi: 10.1080/02656736.2018.1564155. [DOI] [PubMed] [Google Scholar]
- 27.Lagendijk J.J.W., Van Rhoon G.C., Hornsleth S.N., Wust P., De Leeuw A.C.C., Schneider C.J., Van Ddk J.D.P., Van Der Zee J., Van Heek-Romanowski R., Rahman S.A., et al. Esho Quality Assurance Guidelines for Regional Hyperthermia. Int. J. Hyperth. 2009;14:125–133. doi: 10.3109/02656739809018219. [DOI] [PubMed] [Google Scholar]
- 28.Elming P.B., Sørensen B.S., Oei A.L., Franken N.A.P., Crezee J., Overgaard J., Horsman M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers. 2019;11:60. doi: 10.3390/cancers11010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vernon C.C., Hand J.W., Field S.B., Machin B., Whaley J.B., van der Zee J., van Putten W.L., van Rhoon G.C., van Dijk J.D., González González D., et al. Radiotherapy with or without hyperthermia in the treatment of superficial localized breast cancer: Results from five randomized controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 1996;35:731–744. doi: 10.1016/0360-3016(96)00154-x. [DOI] [PubMed] [Google Scholar]
- 30.Overgaard J., Bentzen S.M., Overgaard J., Gonzalez D.G., Hulshof M.C.C.M., Arcangeli G., Dahl O., Mella O. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. Lancet. 1995;345:540–543. doi: 10.1016/S0140-6736(95)90463-8. [DOI] [PubMed] [Google Scholar]
- 31.Van Der Zee J., González D.G. The Dutch Deep Hyperthermia Trial: Results in cervical cancer. Int. J. Hyperth. 2009;18:1–12. doi: 10.1080/02656730110091919. [DOI] [PubMed] [Google Scholar]
- 32.van der Zee J., González D., van Rhoon G.C., van Dijk J.D., van Putten W.L., Hart A.A. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: A prospective, randomised, multicentre trial. Lancet. 2000;355:1119–1125. doi: 10.1016/S0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 33.Ott O.J., Schmidt M., Semrau S., Strnad V., Matzel K.E., Schneider I., Raptis D., Uter W., Grützmann R., Fietkau R. Chemoradiotherapy with and without deep regional hyperthermia for squamous cell carcinoma of the anus. Strahlenther. Onkol. 2018;195:607–614. doi: 10.1007/s00066-018-1396-x. [DOI] [PubMed] [Google Scholar]
- 34.Issels R.D., Noessner E., Lindner L.H., Schmidt M., Albertsmeier M., Blay J.-Y., Stutz E., Xu Y., Buecklein V., Altendorf-Hofmann A., et al. Immune infiltrates in patients with localised high-risk soft tissue sarcoma treated with neoadjuvant chemotherapy without or with regional hyperthermia: A translational research program of the EORTC 62961-ESHO 95 randomised clinical trial. Eur. J. Cancer. 2021;158:123–132. doi: 10.1016/j.ejca.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 35.Schouten D., van Os R., Westermann A.M., Crezee H., van Tienhoven G., Kolff M.W., Bins A.D. A randomized phase-II study of reirradiation and hyperthermia versus reirradiation and hyperthermia plus chemotherapy for locally recurrent breast cancer in previously irradiated area. Acta Oncol. 2022:441–448. doi: 10.1080/0284186X.2022.2033315. [DOI] [PubMed] [Google Scholar]
- 36.Notter M., Stutz E., Thomsen A.R., Vaupel P. Radiation-Associated Angiosarcoma of the Breast and Chest Wall Treated with Thermography-Controlled, Contactless wIRA-Hyperthermia and Hypofractionated Re-Irradiation. Cancers. 2021;13:3911. doi: 10.3390/cancers13153911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray Brunt A., Haviland J.S., Wheatley D.A., Sydenham M.A., Alhasso A., Bloomfield D.J., Chan C., Churn M., Cleator S., Coles C.E., et al. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet. 2020;395:1613–1626. doi: 10.1016/S0140-6736(20)30932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demaria S., Guha C., Schoenfeld J., Morris Z., Monjazeb A., Sikora A., Crittenden M., Shiao S., Khleif S., Gupta S., et al. Radiation dose and fraction in immunotherapy: One-size regimen does not fit all settings, so how does one choose? J. Immunother. Cancer. 2021;9:e002038. doi: 10.1136/jitc-2020-002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hader M., Savcigil D.P., Rosin A., Ponfick P., Gekle S., Wadepohl M., Bekeschus S., Fietkau R., Frey B., Schlücker E., et al. Differences of the Immune Phenotype of Breast Cancer Cells after Ex Vivo Hyperthermia by Warm-Water or Microwave Radiation in a Closed-Loop System Alone or in Combination with Radiotherapy. Cancers. 2020;12:1082. doi: 10.3390/cancers12051082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillette E.L., Ensley B.A. Effect of heating order on radiation response of mouse tumor and skin. Int. J. Radiat. Oncol. 1979;5:209–213. doi: 10.1016/0360-3016(79)90721-1. [DOI] [PubMed] [Google Scholar]
- 41.Hill S.A., Denekamp J. The response of six mouse tumours to combined heat and X rays: Implications for therapy. Br. J. Radiol. 1979;52:209–218. doi: 10.1259/0007-1285-52-615-209. [DOI] [PubMed] [Google Scholar]
- 42.van der Zee J., de Bruijne M., van Rhoon G.C. Thermal medicine, heat shock proteins and cancer. Int. J. Hyperth. 2006;22 doi: 10.1080/02656730600768589. [DOI] [PubMed] [Google Scholar]
- 43.Trefná H.D., Crezee H., Schmidt M., Marder D., Lamprecht U., Ehmann M., Hartmann J., Nadobny J., Gellermann J., Van Holthe N., et al. Quality assurance guidelines for superficial hyperthermia clinical trials: I. Clinical requirements. Int. J. Hyperth. 2017;33:471–482. doi: 10.1080/02656736.2016.1277791. [DOI] [PubMed] [Google Scholar]
- 44.Lühr J.J., Alex N., Amon L., Kräter M., Kubánková M., Sezgin E., Lehmann C.H.K., Heger L., Heidkamp G.F., Smith A.-S., et al. Maturation of Monocyte-Derived DCs Leads to Increased Cellular Stiffness, Higher Membrane Fluidity, and Changed Lipid Composition. Front. Immunol. 2020;11:11. doi: 10.3389/fimmu.2020.590121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rückert M., Deloch L., Fietkau R., Frey B., Hecht M., Gaipl U.S. Immune modulatory effects of radiotherapy as basis for well-reasoned radioimmunotherapies. Strahlenther. Onkol. 2018;194:509–519. doi: 10.1007/s00066-018-1287-1. [DOI] [PubMed] [Google Scholar]
- 46.Hader M., Frey B., Fietkau R., Hecht M., Gaipl U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020;69:293–306. doi: 10.1007/s00262-019-02460-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kolberg H.C., Hoffmann O., Baumann R. The Abscopal Effect: Could a Phenomenon Described Decades Ago Become Key to Enhancing the Response to Immune Therapies in Breast Cancer? Breast Care. 2020;15:443–449. doi: 10.1159/000511431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zagar T.M., Oleson J.R., Vujaskovic Z., Dewhirst M.W., Craciunescu O.I., Blackwell K.L., Prosnitz L.R., Jones E.L. Hyperthermia combined with radiation therapy for superficial breast cancer and chest wall recurrence: A review of the randomised data. Int. J. Hyperth. 2010;26:612–617. doi: 10.3109/02656736.2010.487194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arslan S.A., Ozdemir N., Sendur M.A., Eren T., Ozturk H.F., Aral I.P., Soykut E.D., Inan G.A. Hyperthermia and radiotherapy combination for locoregional recurrences of breast cancer: A review. Breast Cancer Manag. 2017;6:117–126. doi: 10.2217/bmt-2017-0011. [DOI] [Google Scholar]
- 50.Ho A.Y., Barker C.A., Arnold B.B., Powell S.N., Hu Z.I., Gucalp A., Lebron-Zapata L., Wen H.Y., Kallman C., D’Agnolo A., et al. A phase 2 clinical trial assessing the efficacy and safety of pembrolizumab and radiotherapy in patients with metastatic triple-negative breast cancer. Cancer. 2020;126:850–860. doi: 10.1002/cncr.32599. [DOI] [PubMed] [Google Scholar]
- 51.Li Z., Deng J., Sun J., Ma Y. Hyperthermia Targeting the Tumor Microenvironment Facilitates Immune Checkpoint Inhibitors. Front. Immunol. 2020;11:11. doi: 10.3389/fimmu.2020.595207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroemer G., Galassi C., Zitvogel L., Galluzzi L. Immunogenic cell stress and death. Nat. Immunol. 2022;23:487–500. doi: 10.1038/s41590-022-01132-2. [DOI] [PubMed] [Google Scholar]
- 53.Podolska M.J., Shan X., Janko C., Boukherroub R., Gaipl U.S., Szunerits S., Frey B., Muñoz L.E. Graphene-Induced Hyperthermia (GIHT) Combined with Radiotherapy Fosters Immunogenic Cell Death. Front. Oncol. 2021;11:664615. doi: 10.3389/fonc.2021.664615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galluzzi L., Vitale I., Warren S., Adjemian S., Agostinis P., Martinez A.B., Chan T.A., Coukos G., Demaria S., Deutsch E., et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J. Immunother. Cancer. 2020;8:e000337. doi: 10.1136/jitc-2019-000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheidegger S., Barba S.M., Gaipl U.S. Theoretical Evaluation of the Impact of Hyperthermia in Combination with Radiation Therapy in an Artificial Immune—Tumor-Ecosystem. Cancers. 2021;13:5764. doi: 10.3390/cancers13225764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andersen M.H. The Balance Players of the Adaptive Immune System. Cancer Res. 2018;78:1379–1382. doi: 10.1158/0008-5472.CAN-17-3607. [DOI] [PubMed] [Google Scholar]
- 57.Mei X., Ten Cate R., Van Leeuwen C.M., Rodermond H.M., De Leeuw L., Dimitrakopoulou D., Stalpers L.J.A., Crezee J., Kok H.P., Franken N.A.P., et al. Radiosensitization by hyperthermia: The effects of temperature, sequence, and time interval in cervical cell lines. Cancers. 2020;12:582. doi: 10.3390/cancers12030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mondini M., Levy A., Meziani L., Milliat F., Deutsch E. Radiotherapy–immunotherapy combinations—Perspectives and challenges. Mol. Oncol. 2020;14:1529–1537. doi: 10.1002/1878-0261.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ibuki Y., Takahashi Y., Tamari K., Minami K., Seo Y., Isohashi F., Koizumi M., Ogawa K. Local hyperthermia combined with CTLA-4 blockade induces both local and abscopal effects in a murine breast cancer model. Int. J. Hyperth. 2021;38:363–371. doi: 10.1080/02656736.2021.1875059. [DOI] [PubMed] [Google Scholar]
- 60.Kordbacheh T., Honeychurch J., Blackhall F., Faivre-Finn C., Illidge T. Radiotherapy and anti-PD-1/PD-L1 combinations in lung cancer: Building better translational research platforms. Ann. Oncol. 2018;29:301–310. doi: 10.1093/annonc/mdx790. [DOI] [PubMed] [Google Scholar]
- 61.Derer A., Spiljar M., Bäumler M., Hecht M., Fietkau R., Frey B., Gaipl U.S. Chemoradiation Increases PD-L1 Expression in Certain Melanoma and Glioblastoma Cells. Front. Immunol. 2016;7:610. doi: 10.3389/fimmu.2016.00610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim Y.J., Koh J., Kim S., Jeon S.-R., Chie E.K., Kim K., Kang G.H., Han S.-W., Kim T.-Y., Jeong S.-Y., et al. Chemoradiation-Induced Alteration of Programmed Death-Ligand 1 and CD8 + Tumor-Infiltrating Lymphocytes Identified Patients With Poor Prognosis in Rectal Cancer: A Matched Comparison Analysis. Int. J. Radiat. Oncol. 2017;99:1216–1224. doi: 10.1016/j.ijrobp.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 63.Tsang J.Y.S., Chan K.-W., Ni Y.-B., Hlaing T., Hu J., Cheung S.-Y., Tse G.M. Expression and Clinical Significance of Herpes Virus Entry Mediator (HVEM) in Breast Cancer. Ann. Surg. Oncol. 2017;24:4042–4050. doi: 10.1245/s10434-017-5924-1. [DOI] [PubMed] [Google Scholar]
- 64.Abad M.N., Calabuig-Fariñas S., de Mena M.L., Torres-Martínez S., González C.G., García J., Ángel G., González-Cruz V.I., Herrero C.C. Programmed Death-Ligand 1 (PD-L1) as Immunotherapy Biomarker in Breast Cancer. Cancers. 2022;14:307. doi: 10.3390/cancers14020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breen W.G., Leventakos K., Dong H., Merrell K.W. Radiation and immunotherapy: Emerging mechanisms of synergy. J. Thorac. Dis. 2020;12:7011–7023. doi: 10.21037/jtd-2019-cptn-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao X., Li J., Zheng L., Yang Q., Chen X., Chen X., Yu Y., Li F., Cui J., Sun J. Immune Response on Optimal Timing and Fractionation Dose for Hypofractionated Radiotherapy in Non–Small-Cell Lung Cancer. Front. Mol. Biosci. 2022;9:786864. doi: 10.3389/fmolb.2022.786864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aspeslagh S., Postel-Vinay S., Rusakiewicz S., Soria J.-C., Zitvogel L., Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur. J. Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 68.Wimmer S., Deloch L., Hader M., Derer A., Grottker F., Weissmann T., Hecht M., Gostian A.-O., Fietkau R., Frey B., et al. Hypofractionated Radiotherapy Upregulates Several Immune Checkpoint Molecules in Head and Neck Squamous Cell Carcinoma Cells Independently of the HPV Status While ICOS-L Is Upregulated Only on HPV-Positive Cells. Int. J. Mol. Sci. 2021;22:9114. doi: 10.3390/ijms22179114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kok H.P., Wust P., Stauffer P.R., Bardati F., van Rhoon G.C., Crezee J. Current state of the art of regional hyperthermia treatment planning: A review. Radiat. Oncol. 2015;10:196. doi: 10.1186/s13014-015-0503-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frey B., Mika J., Jelonek K., Cruz-Garcia L., Roelants C., Testard I., Cherradi N., Lumniczky K., Polozov S., Napieralska A., et al. Systemic modulation of stress and immune parameters in patients treated for prostate adenocarcinoma by intensity-modulated radiation therapy or stereotactic ablative body radiotherapy. Strahlenther. Onkol. 2020;196:1018–1033. doi: 10.1007/s00066-020-01637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou J.-G., Donaubauer A.-J., Frey B., Becker I., Rutzner S., Eckstein M., Sun R., Ma H., Schubert P., Schweizer C., et al. Prospective development and validation of a liquid immune profile-based signature (LIPS) to predict response of patients with recurrent/metastatic cancer to immune checkpoint inhibitors. J. Immunother. Cancer. 2021;9:e001845. doi: 10.1136/jitc-2020-001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Oei A., Kok H., Oei S., Horsman M., Stalpers L., Franken N., Crezee J. Molecular and biological rationale of hyperthermia as radio- and chemosensitizer. Adv. Drug Deliv. Rev. 2020;163–164:84–97. doi: 10.1016/j.addr.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 73.Overgaard J., Grau C., Lindegaard J., Horsman M. The potential of using hyperthermia to eliminate radioresistant hypoxic cells. Radiother. Oncol. 1991;20:113–116. doi: 10.1016/0167-8140(91)90197-O. [DOI] [PubMed] [Google Scholar]
- 74.Ademaj A., Veltsista D.P., Ghadjar P., Marder D., Oberacker E., Ott O.J., Wust P., Puric E., Hälg R.A., Rogers S., et al. Clinical Evidence for Thermometric Parameters to Guide Hyperthermia Treatment. Cancers. 2022;14:625. doi: 10.3390/cancers14030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Demaria S., Golden E.B., Formenti S.C. Role of Local Radiation Therapy in Cancer Immunotherapy. JAMA Oncol. 2015;1:1325–1332. doi: 10.1001/jamaoncol.2015.2756. [DOI] [PubMed] [Google Scholar]
- 76.Yamamoto N., Matsumoto K., Hagiwara S., Saito M., Furue H., Shigetomi T., Narita Y., Mitsudo K., Tohnai I., Kobayashi T., et al. Optimization of hyperthermia and dendritic cell immunotherapy for squamous cell carcinoma. Oncol. Rep. 2011;25:1525–1532. doi: 10.3892/or.2011.1232. [DOI] [PubMed] [Google Scholar]
- 77.Rückert M., Flohr A.-S., Hecht M., Gaipl U.S. Radiotherapy and the immune system: More than just immune suppression. Stem Cells. 2021;39:1155–1165. doi: 10.1002/stem.3391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.