Abstract
In the last decade, bioprinting has emerged as a facile technique for fabricating tissues constructs mimicking the architectural complexity and compositional heterogeneity of native tissues. Amongst different bioprinting modalities, extrusion-based bioprinting (EBB) is the most widely used technique. Coaxial bioprinting, a type of EBB, enables fabrication of concentric cell-material layers and enlarges the scope of EBB to mimic several key aspects of native tissues. Over the period of development of bioprinting, tissue constructs integrated with vascular networks, have been one of the major achievements made possible largely by coaxial bioprinting. In this review, current advancements in biofabrication of constructs with coaxial bioprinting are discussed with a focus on different bioinks that are particularly suitable for this modality. This review also expounds the properties of different bioinks suitable for coaxial bioprinting and then analyses the key achievements made by the application of coaxial bioprinting in tissue engineering, drug delivery and in-vitro disease modelling. The major limitations and future perspectives on the critical factors that will determine the ultimate clinical translation of the versatile technique are also presented to the reader.
Keywords: Bioprinting, coaxial bioprinting, vasculature, bioink
1. Bioprinting and its role in tissue engineering and drug development
Bioprinting is an additive manufacturing technique for fabrication of constructs to be applied in tissue engineering (TE) for the treatment and repair of tissue or organ damage or as in-vitro models for studying disease biology and drug actions [1]. It is a computer-aided transfer process for fabrication of tissue constructs and scaffolds via the organized layer-by-layer stacking of biomaterials and living cells [2] . This process provides the ability to deposit cells with high resolutions comparable to that of native tissue organization [3][4]. Other advantages of bioprinting include the ability to accurately control cell distribution simultaneously providing a scalable, cost-effective process [5].
Bioprinting of biological components can be achieved by three major modalities, namely, droplet-based bioprinting (DBB), laser-based bioprinting (LBB), and extrusion-based bioprinting (EBB). Each of these methods further encompass several process variants under a similar deposition mechanism, for example, inkjet and electro-hydrodynamic jetting based bioprinting are methods which are covered under the broad term of DBB strategy [6][7]. Among all bioprinting modalities, a large number of studies have been conducted with EBB mainly attributed to several advantages of EBB over the other modalities, namely, 1) the ability to fabricate tissue constructs with a wide range of bioinks, 2) fabricating constructs containing physiologically-relevant cell densities, 3) causing relatively lesser cell damage during the bioprinting process compared to the other modalities, 4) fabricating scalable structures with anatomically accurate geometries, and 5) perform the fabrication in a cost-effective manner using accessible bioprinters. On the other hand, lower resolution and limited feature size, have been some of the major limitations of EBB [8].
2. Working Mechanisms of Extrusion-based Bioprinting
Extrusion-based bioprinting is carried out by loading desired bioinks of interest into cartridges, which are then subsequently extruded out onto a surface through a nozzle via either pneumatic pressure or mechanical forces [9]. EBB can be achieved by three distinct methods-pneumatic-based extrusion, screw-based extrusion, and piston-based extrusion. The instrumentation setup allows for three degrees of translational movements in the cartesian space thus facilitating the deposition of bioinks onto a substrate in a layer-by-layer fashion. Parameters, such as pressure and temperature (for pneumatic-based extrusion) or rotational or piston speed (for screw- and piston-based extrusion or combination of them), or movement of piston (positive displacement pumps) are controlled by computer algorithms [9]. Pneumatic-based extrusion is one of the most widely used method and it works by employing compressed air moving through a syringe and nozzle to drive the bioink out at a controlled flowrate. This is a straightforward process but delays in dispensing times are observed due to the compressed air and the accuracy of deposition is highly dependent on rheological properties of utilized bioinks [10], [11]. In piston-based extrusion, a linearly moving piston provides the force to push the bioink out onto the deposition surface. This process allows for a direct control of bioink release from the nozzle. Screw-based extrusion works via a rotating screw to drive the bioink out onto the deposition surface. This process enables the deposition of highly viscous bioinks; however, a major drawback is that of harming cells during the extrusion process; however, this can be circumvented by additional modifications to the screw mechanism [10]. It can be inferred that this process has been successfully employed for the fabrication of multi-material constructs and has potential future applications in fabrication of constructs with variable material composition [12][13]. For extrusion of highly viscous bioinks, mechanical extrusion process is employed due to larger pressure drop needed between the syringe inlet and nozzle outlet [11].
3. Crosslinking mechanisms in extrusion-based bioprinting with a focus on coaxial bioprinting
In addition to its working mechanisms, EBB can also be classified based on the crosslinking mechanism of the utilized bioink. Different bioink combinations require a different extrusion setup. These can be classified into five, namely, 1) direct extrusion process, 2) extrusion into a coagulation bath, 3) extrusion into a support bath, and 4) extrusion onto a framework and 4) coaxial extrusion [14]-[16]. Direct extrusion utilizes bioinks possessing sheer-thinning properties. This allows for the bioink to behave like a fluid during the extrusion process and then transition to a gel state upon extrusion. Using a coagulation bath may eliminate the need for shear-thinning bioinks that are essential in direct extrusion. Here, the bath contains certain crosslinker agents, such as calcium chloride (for sodium alginate) or Schiff base crosslinkers [17]. Clogging of the nozzle tip during bioprinting is a major limitation of the method. The third method, extrusion into a support bath, also known as embedded bioprinting, entails extrusion of bioink into a granular or colloidal support bath composed of soft micro/nanoparticles in a high volume fraction, which enables omnidirectional deposition of cell-laden bioinks [18]. The fourth method is extrusion onto a pre-constructed scaffold, wherein the extrusion of various hydrogels, such as alginate or collagen, are deposited onto a prefabricated mechanically strong scaffold, preferably made of thermoplastic biocompatible materials such as polycaprolactone (PCL). This approach overcomes the inadequate mechanical stability of hydrogel-based scaffolds [19]. The fifth method is the coaxial bioprinting process. The term ‘coaxial’ is used to represent the concentric arrangement of two separate nozzles through which the bioink solution and crosslinker solution (coagulation solution) are extruded. Based on the combination used, two types of fibers can be bioprinted, 1) solid fibers, where the bioink is extruded through the core and the crosslinker surrounds it or 2) hollow fibers, where the bioink is extruded from the outer nozzle. This process enables for a simple single-step generation of tissue constructs. Jin et al have also shown that a double layered capsule can be formed wherein the extruded structure is arranged in core-shell-shell morphology for a potential application in controlled drug release [20]. Along the same lines, Attalla et al have showcased the fabrication of concentric human umbilical vein endothelial cell (HUVEC) and mouse 3T3 fibroblast laden tri-layered hollow fibers to mimic the compartmentalization in native blood vessels [21] similar to the work of Silva et al on the in-silico and in-vitro assessment of coaxial nozzles for the fabrication of perfusable constructs with dimensions similar to that of human arteries [22].
4. Instrumentation aspects of coaxial bioprinting
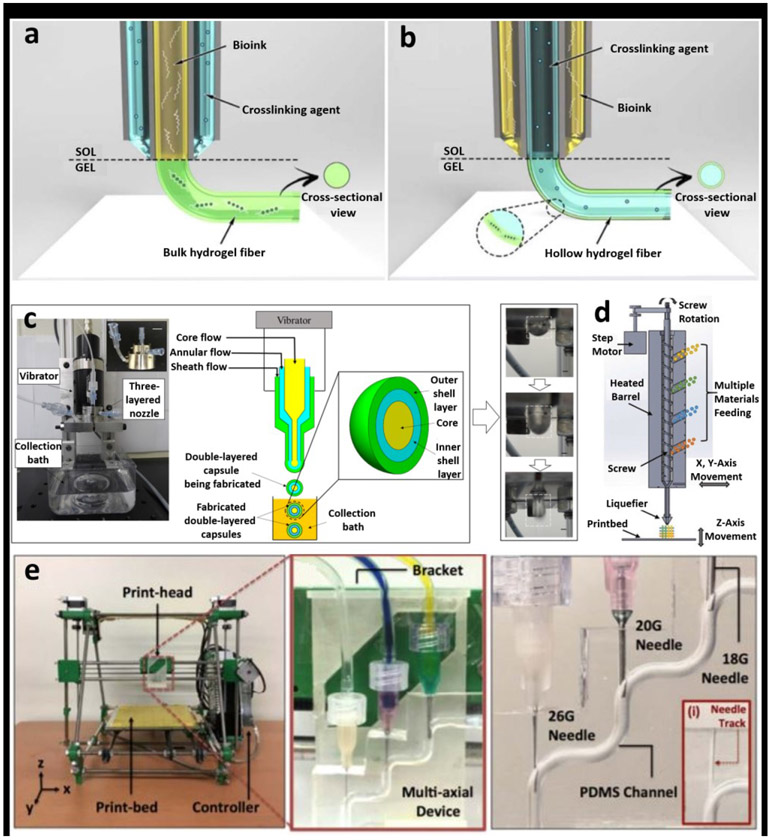
A representation of different configurations for coaxial bioprinting is presented in Figure 1. Figure 1a highlights a configuration wherein the bioink within the core is encapsulated by a shell crosslinker and Figure 1b demonstrates the opposite configuration for deposition of hollow fibers. Inner nozzle is preferred to be longer enabling the crosslinker flowing along the outside surface of the nozzle preventing nozzle clogging. Figures 1d and 1e illustrates the creation of fibers with dual nozzles and multi-channels, respectively, which is advantageous as it enables the fabrication of dual-shell constructs to mimic native blood vessels.
Figure 1:
a) Various configurations for coaxial bioprinting. a) The method wherein the bioink is the core encapsulated by a shell crosslinker and b) a configuration enabling the deposition of hollow fibers. Adapted with permission from [15]. c) A triple nozzle coaxial setup for fabrication of dual-shell constructs enabling better mimicry of native blood vessels. Adapted with permission from [20]. d) Configuration whereby deposition of multi-material fibers is possible. Adapted with permission from reference [13] e) A configuration allowing for creation of fibers with multiple channels. Adapted with permission from [21] .
A key aspect that is often required to be optimized is the nozzle diameter. As the diameter decreases, the resolution improves, but higher shear stresses could be detrimental to cell viability. Zhang et al fabricated a human umbilical vein endothelial cells (HUVECs)-laden vascular network generated with an inner nozzle diameter of 210 μm, providing a potential lower limit to the nozzle diameter [23]. The core and shell bioinks are independently controlled by a different pneumatic extrusion process thereby allowing different flowrates for shell and core, which in turn affect the dimensions of the extruded fibers. Larger flowrates in the shell lead to thicker fibers and this knowledge has enabled researchers to produce fibers with different configurations, such as helical, spiral, wavy or straight fibers [24].
The general method for fabrication of hollow tubes would be the use of a core and shell configuration. The shell is a stable material crosslinked around a liquid core. The core is removed during post-processing, leaving behind a stable hollow tube. This method shows immense applications in tissue engineering by fabricating structures, which can mimic the native blood vessel anatomy and other tubular-like structures [25].
5. Bioinks for coaxial bioprinting
One of the most critical aspects in 3D bioprinting is the selection of an appropriate bioink such that tissue constructs can be mimicked effectively and with ease. Usually the most desirable properties that any biomaterial should possess to become a bioink for EBB are shear thinning, rapid gelation or solidification, and strong stiffness such that soft constructs can be fabricated using low concentration of bioink concentrations [26]. Among all bioinks, hydrogels are most widely used as they can be processed in mild conditions, are compatible with most bioprinting techniques and more importantly, are biocompatible mimicking the structural properties of the extracellular matrix [27]-[29]. Crosslinking is the process of gelation for hydrogels ensuring that bioprinted constructs retain their shape over time. This can be achieved either by physical (non-covalent bonding, reversible bonding) or chemical (covalent bonding, irreversible bonding) means [30]. Physical crosslinking is achieved by hydrophobic interactions, hydrogen bonding, stereocomplexation, electrostatic interactions, and guest–host interactions [31]. Chemical crosslinking processes are carried out via free radical polymerization, enzymatic reaction, condensation reaction, high energy irradiation or Schiff’s base reactions [14][31]. Photoirradiation crosslinking is also commonly employed, however; precautions are needed to be exercised to minimize the risk of photo-induced damage to cells. Due to its rapid crosslinking characteristics, coaxial bioprinting usually utilizes ionic crosslinking. Previously, reported reviews [32] and [14] have provided a detailed insight into various bioinks utilized in 3D bioprinting, such as but not limited to, agarose, alginate, chitosan, collagen, fibrin, gelatin, GelMa (gelatin methacryloyl), hyaluronic acid, Pluronic F-127 and poly(ethylene)-glycol. The most commonly used hydrogel bioinks in coaxial bioprinting include, alginate, and its composite form with the addition of other hydrogels. A detailed description for the bioinks utilized in coaxial bioprinting are provided in the ensuing sections.
5.1. Alginate
Alginate is one of the most commonly used biopolymers for hydrogel formation, mainly due to its low cost, rapid ionic crosslinking capabilities, tuneable gelation kinetics, and biocompatible nature [33]-[35]. Physical crosslinking of alginate with divalent cations allows gelation and this mechanism has been widely exploited in the domain of bioprinting [36].
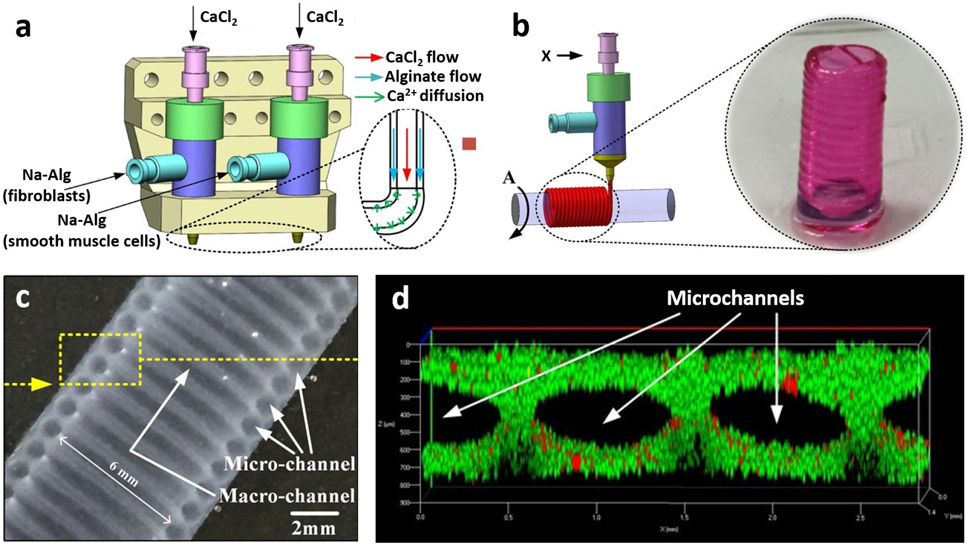
Our group was the first to publish on coaxial bioprinting [37]-[44], which was then utilized by various researchers. At that time, alginate was employed due to its rapid crosslinking and elastic properties, where tubular vascular constructs and microfluidic channels were bioprinted, both in free-standing and embedded (in a bulk gel construct) forms. More recently, He et al [45] fabricated vascular constructs containing HUVECs and human umbilical vein smooth muscle cells (HUVSMCs) via a four-layer coaxial nozzle arrangement with alginate bioink as the shell. Results indicated that there was an improved mechanical strength with lower elasticity in four-layer constructs as compared to single-layer constructs. Fabricated constructs permitted cell proliferation and viability after perfusion. Wang et al [46] described the formation of microfibers that mimicked glioma microenvironment using alginate as a human glioma stem cell (GSC23) (shell)/ human glioma cell line (U118)(core) laden bioink. U118 Cells obtained from the microfiber core showed increased chemoresistivity properties speculated to be due to signalling interactions between GSC23 and U118 cells. These properties demonstrated the potential of fabricated constructs for glioma drug screening and resistance studies. Skeletal muscle myofibers were also fabricated using alginate fibers and C2C12 (muscle precursor cells-laden PEG-fibrinogen methacrylate biopolymer [47]. Here, alginate acted as a template for successful deposition of C2C12 cell-laden fibers. Gao et al [48] was successful in generation of alginate based multi-level fluidic channels, where bioprinting was performed over a horizontal rod (figure 2). Mouse smooth muscle cell–laden alginate based shell bioink was coaxially deposited followed by the deposition of a layer of mouse fibroblasts (L929) – laden alginate over the muscle filament and finally the construct was seeded with HUVECs to achieve the multi-level fluidic channels.
Figure 2:
a) Schematic representation of coaxial bioprinting and b) spiral deposition of channels with the aid of a support rod. c) Structural features of the construct as viewed under a microscope and d) visualization of micro-channels with live (green) and dead (red) L292 cells. Adapted with permission from Reference [48].
Although commonly used, alginate has certain limitations such as, poor cell recognition and adhesion properties, slow degradation and low cell proliferation [33]. Therefore, later studies demonstrated blending of other bioinks with alginate in order to improve the biological properties.
5.2. Chitosan
Chitosan is a semi-crystalline, linear polymer obtained by the partial deacetylation of chitin, a natural polymer. Various methods are available for the gelation of chitosan such as via Schiff base formation or crosslinking with the aid of dialdehyde crosslinkers or Genipin (a naturally occurring crosslinker) [49][50].
Very limited work has been performed on coaxial bioprinting of chitosan. For example, Zhang et al [41] described a detailed comparative study between alginate and chitosan-based perfusable vascular constructs and the results indicated that although chitosan could be successfully used to form uniform constructs, it suffered from ruptures due to poor mechanical properties and structural integrity. On the other hand, alginate showed superior mechanical properties when compared to the fragile chitosan for fabrication of vascular constructs.
5.3. Composite bioinks
Due to the previously mentioned limitations of chitosan and alginate, there is a great need for development of novel bioinks to achieve enhanced tissue formation for a particular application. For example, Dolati et al [51] utilized carbon nanotubes to reinforce alginate bioink for fabrication of vascular constructs with improved mechanical properties. A study by Zhang et al [52] reported similar results on enhancement of mechanical properties due to the incorporation of multiwall carbon nanotubes (MWCNTs) the vascular structures. MNCNT-reinforced vascular constructs exhibited improved burst pressure and tensile strength. Though the constructs showed biocompatibility in short term studies, noticeable drop in cell motility and proliferation was observed in long term studies. Milojević et al [25] described a process of fabricating a construct that resembles native ECM using alginate and carboxymethylcellulose (CMC) reinforced with cellulose nanofibers for fabrication of HUVEC-laden constructs. Results indicated that the constructs were suitable for cell attachment and proliferation with improved mechanical stability.
To obtain tissue constructs with good cytocompatability and to support proliferation of encapsulated cells, incorporation of GelMA (gelatin methacryloyl) into alginate has also been investigated [53]. In this regard, Wang et al [54] demonstrated a one-step process to fabricate stable, continuous and perfusable hollow constructs using coaxial bioprinting. Similarly, Liu et al [26] demonstrated improved biological compatibility of constructs fabricated using HUVEC-laden GelMA and calcium chloride core encapsulated by an alginate shell bioink. This method allowed for the reduction in the use of bioinks in core during the fabrication process and permitted a large degree of control over the microenvironments for bioprinted cells. Taymour et al [35] demonstrated the use of a composite bioink composed of human hepatocellular carcinoma cells (HepG2), alginate, methylcellulose and Matrigel. Constructs printed with this bioink improved cell viability concomitant to reduction in bioink viscosity without compromising on the bioprintability. Colosi et al [55] demonstrated the use of coaxial bioprinting with a low viscous bioink consisting of GelMA, alginate and photoinitiator loaded with HUVECs as the core bioink, which was encapsulated by a calcium chloride shell bioink. Cardiomyocytes seeded on the constructs after bioprinting exhibited synchronous beating, which could imply the flexibility of constructs allowing the migration of HUVECs to the scaffold periphery and supporting the beating of cardiomyocytes. Future studies still need to be conducted to confirm the growth of various other cell types over HUVEC-laden constructs [55]. Turner et al [56] demonstrated the use a blend bioink for application in wound healing. Briefly, the bioink shell consisted of GelMA loaded with human bone-marrow-derived mesenchymal stems cells (hBMSCs), which surrounded a cell-adhesive and proteolytic peptide-functionalized, succinylated chitosan/dextran aldehyde core bioink loaded with HUVECs. Here, the shell layer provided the required structural support during the extrusion process. The constructs were able to facilitate cell growth, proliferation, and formation of vascular networks. To improve bioink properties for bone tissue engineering, Liu et al illustrated the use of chitosan and hydroxyapatite (HA) composite bioink to produce biocompatible constructs [57]. HA was chosen as it increased the mechanical properties of fabricated fibers. Moreover, results indicate that the fiber material exhibits high cell viability and vascularization through the formation of microchannels.
Further research on various bioinks, such as GelMA, polyethylene glycol-diacrylate (PEGDA), hyaluronic acid and Pluronic F-127, have been carried out to characterize the use of multiple cell types and bioinks in the shell to fabricate vascular constructs to better mimic native blood vessel anatomy [58]. Coaxial bioprinting offers a unique opportunity to work with two different bioinks by using them as either a shell or core bioinks. Table 1 provides an overview of various bioink combinations used in coaxial bioprinting.
Table 1:
Summary of various bioink combinations used to tailor constructs for various different applications
| Shell Bioink or Crosslinker |
Core Bioink or Crosslinker | Cell Type Used | Applications | Ref. |
|---|---|---|---|---|
| GelMA | Peptide-functionalized, succinylated chitosan (C)/dextran aldehyde (D) | Shell - hBMSCs Core - HUVECs | Wound healing applications | [56] |
| GelMA | Gelatin | Shell: HUVECs, MC3T3-E1 or MDA-MB-231 Core: ECs | Large-scale vascularized tissue constructs | [76] |
| Alg/SF | Pluronic F127 and calcium ions | C3A liver cancer cells | 3D bioprinting for tissue and organ regeneration | [77] |
| Gelatin-PEG-tyramine (GPT) prepolymer | Gelatin | Shell: HDFs Core: HUVECs | In vitro disease modelling | [61] |
| Sodium alginate | Gelatin and alginate | Core: NE-4C | Neural research | [81] |
| Gelatin-GelMA | PVA | Shell: HUVECs | Tissue modelling and regenerative medicine | [54] |
| ALG- CMC- NFC | Calcium chloride | HUVECs | Fabrication of a perfusable and hollow construct | [25] |
| Calcium chloride | ALG, GelMA and β-tricalcium phosphate (TCP) | Core: BM-hMSCs | Development of osteochondral constructs | [68] |
| Sodium alginate | GelMA and calcium chloride | Core: GFP-HUVECs | Organ repair and tissue engineering | [63] |
| GelMA | Alginate | Shell: EPC Core: islets cells | Islet transplantation for Type I diabetes treatment | [86] |
| Calcium alginate | GelMA | Core: HUVECs | Drug delivery vehicles in tissue engineering | [70] |
| Alginate | Cell suspension | Shell: GSC23 Core: U118 | Microfiber constructs for drug development and screening | [46] |
| GelMA, alginate and eight-arm poly(ethylene glycol) acrylate with a tripentaerythritol core | Calcium chloride | Shell: Various cell types | Fabrication and creation of human cannular tissues | [78] |
| HA-GelMA and photoinitiator (VA-086) | HA-GelMa | Core: MSCs | Treatment of musculoskeletal conditions | [64] |
| Alginate | GelMA and calcium chloride | Core: HUVECs | High degree of control of the microenvironment of 3D bioprinted scaffolds and constructs | [26] |
| Alginate/gelatin | Fibrinogen | Core: RFP-GSCs and GFP-MSCs | In vitro analysis and interactions of tumor microenvironment | [87] |
| Calcium chloride | Alginate, GelMA, chondroitin sulfate amino ethyl methacrylate (CS-AEMA) and HAMA (optional) | Core: BM-MSCs | Cartilage tissues engineering | [69] |
| Calcium chloride | GelMA, alginate and a photoinitiator (LAP) | Core: HUVECs | Tissue engineering and tissue model development for drug delivery | [55] |
| algMC + Matrigel | algMC, algMC + fibrin or algMC + plasma | Shell: HepG2 Core: NIH 3T3 | Tissue engineering for the tailoring of scaffold microenvironments for artificial liver. | [35] |
6. Applications of coaxial bioprinting
The coaxial bioprinting process provides certain unique advantages, such as controlled simultaneous deposition of different bioinks and improved bioprinting resolution over single nozzle bioprinting and the ability to fabricate constructs in a single step including a one-step deposition of sacrificial bioink layers [59]. A key property, which sets coaxial bioprinting apart from the remaining extrusion processes, is the ability to fabricate vascularized constructs [60] [59]. These properties attract the use of this method in the development and fabrication of various applications ranging from tissue engineering to drug screening and regenerative medicine.
6.1. Tissue Engineering
One of the key bottlenecks in the aspect of tissue engineering is formation of vascular networks. Turner et al, as mentioned earlier, were able to, with a single step process, develop pre-vascularized scaffolds for wound care applications [56]. This was achieved by employing core-shell bioprinting and a custom cell-responsive composite bioink. The unique blend was chosen due to its ability to provide stability and excellent cell viability to designed constructs during extrusion over a chilled surface. In essence, the shell and core bioinks acted as “delivery vehicles” for the two cell types and bioprinted constructs had the ability to provide appropriate environment for cell growth, propagation, differentiation, and early-stage tube-like vascular network development. In-vitro studies confirmed a two-fold increased rate of wound healing when compared to untreated cells. This, and the ability to deliver the two cell types, suggests the use of these construct as “living dressings” which can provide regenerative properties to nonhealing or chronic wound regions. Another key takeaway would be the use of this method for large scale fabrication of prevascularized tissues as it overcomes the use of complex microfabrication and ink removal strategies.
As a proof-of-concept study, Hong et al [61] described the use of a cell-laden gelatin bioink for a simple, one-step coaxial bioprinting of vascularized tissue constructs. They utilized human dermal fibroblasts (HDFs)-laden gelatin-PEG-tyramine (GPT) prepolymer as a shell bioink surrounding a core bioink made of HUVECs-laden gelatin. GPT utilized PEG as a spacer between tyramine and gelatin enabling a rapid gelation rate of ~ 4.5 s. A key aspect of this study was the creation of perfusable vascular constructs lined up with both cell types maintained for up to eight days in vitro.
The use of hydrogel-based scaffolds and decellularized extracellular matrix (dECM) have found limited success, due to their low efficiency in the treatment of volumetric muscle losses (VML), an irrecoverable injury as a result of a loss of 20% or more muscle [62]. Choi et al created volumetric muscle constructs with the help of a coaxial nozzle having skeletal muscle dECM-based bioink in the core which was surrounded by a shell bioink made up of dECM and a granule-based printing reservoir. [62]. The bioprinted constructs induced muscle fiber generation, innervation and vascularization leading to a functional recovery rate of 85% in case of VML injuries in rats. These results showcase the ability to use these constructs in pre-clinical studies and development of human-scale muscle tissues for VML and other studies such as that on development of drugs and toxicity assessment.
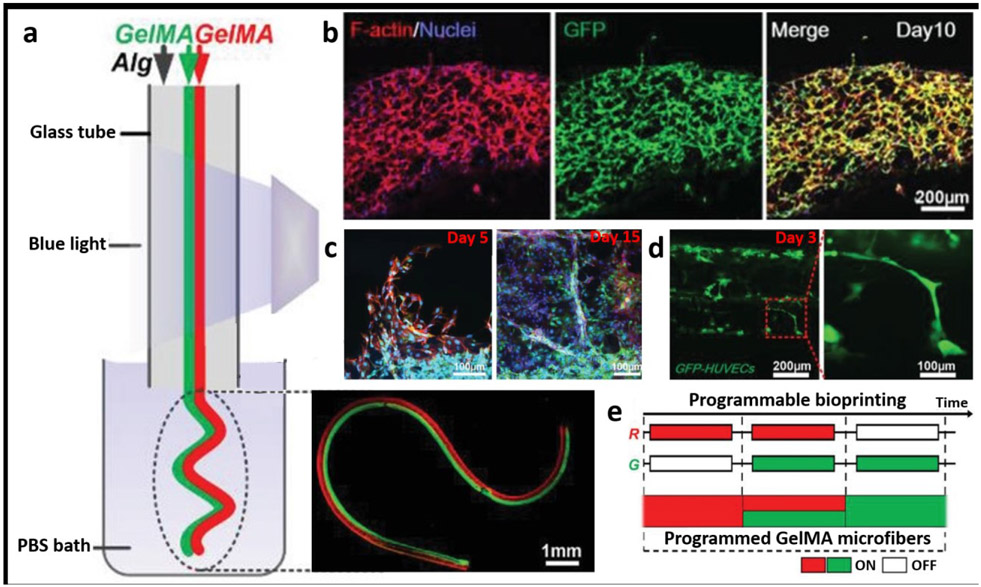
One of the most versatile microfibers would be hydrogel microfibers. Due to their simple structures, they find numerous applications such as microcarriers for cells, drug molecules, micro-organs, and transplantation therapy. In general, an ideal microfiber will have three key features. First amongst them is to allow the desired biological function of the target tissue, which will be characterized by the functional microenvironment-specific to the tissue type and expression of appropriate phenotypic markers by the constituent cells. Secondly, the method should enable mass production, and thirdly, should support long-term cell viability. In this regard, Shao et al demonstrated the potential of coaxial bioprinting for fabrication of vascular organoids as well as fabricated constructs recapitulating angiogenic sprouting and tumor angiogenic environments, as shown in Figure 3 [63]. The microfibers were created using a core/shell arrangement with the core being composed of green fluorescent protein (GFP)+HUVECs-laden GelMA and calcium chloride, which was surrounded by a shell bioink consisting of sodium alginate. An essential feature of these microfibers was that the composition of microfibers can be controlled by switching the extrusion of each bioink “ON” and “OFF” as shown in Figure 3e.
Figure 3:
a) Schematic depiction of the fabrication process for heterogeneous GelMA microfibers (Janus structures), b) 10 day culture of GFP+HUVEC-laden GelMA microfibers showing f-actin/nuclei staining in the constructs, c) Visualization of angiogenic sprouting in cell-laden constructs at different time points – it was noted that HUVEC-laden microfibers and MDA-MB-231-laden microfiber encapsulated in GelMA showed that there was an increase in sprout length with successive co–cultures, d) Visualization of vascular organoids and sprouting, e) a program layout for bioprinting of GelMA microfibers. Adapted with permission from reference [63].
Coaxial bioprinting was also studied for the purpose of repairing osteochondral defects. For example, Bella et al [64] conducted intraoperative bioprinting to treat cartilage defects with the use of a biopen, a hand-held bioprinting device [64]. Intraoperative bioprinting which is a process enabling direct bioprinting into a defect site under surgical settings, has recently become popular [65] [66]. This biopen worked on the principle of coaxial bioprinting containing a shell bioink composed of HA-GelMA and hyaluronic acid methacrylate (HAMA) hydrogel with a photoinitiator (VA-086), that would surround the core bioink composed of allogeneic adipose-derived mesenchymal stem cells, (ADSC - laden HA-GelMA). The biopen was tested against preconstructed bench-based printed scaffolds for the treatment of chondral defects in sheep. The results indicated that the biopen based treatment led to early regeneration of cartilage. These results pave way for a new avenue with the use of biopen for real-time intraoperative bioprinting of cells leading to advancements in tissue engineering for the treatment of musculoskeletal conditions. Along the same lines, Hu et al [67] described the fabrication of multi-material and multi-gradient constructs, encapsulating HUVECs for the repair of osteochondral defects [67]. In another study, Kosik-Kozioł et al described the use of coaxial bioprinting, wherein the core bioink consists of a blend of BM-hMSCs (bone marrow - derived human mesenchymal stem cells)-laden alginate, GelMA and β-tricalcium phosphate (TCP) with a calcium chloride shell to assist the crosslinking of alginate [68]. Through molecular techniques and immunological assays, formation of calcified zone of osteochondral tissue caused by the differentiation of BM-hMSCs was confirmed. In another study, Costantini et al [69] demonstrated the use of a unique blend of bioinks to produce hydrogel constructs [69]. The team utilized a core bioink composed of bone marrow-derived human MSCs (BM-MSCs) laden in alginate, GelMA, chondroitin sulfate amino ethyl methacrylate (CS-AEMA) and, an optional, HAMA, as well as CaCl2 crosslinker in the shell. It was observed that the bioink combination excluding HAMA led to neocartilage formation with high ratios of collagen Type II: collagen Type I and collagen Type II: collagen Type X, whereas the inclusion of HAMA lead to differentiation of BM-MSCs to form hypertrophic cartilage, as a result of extensive crosslinking.
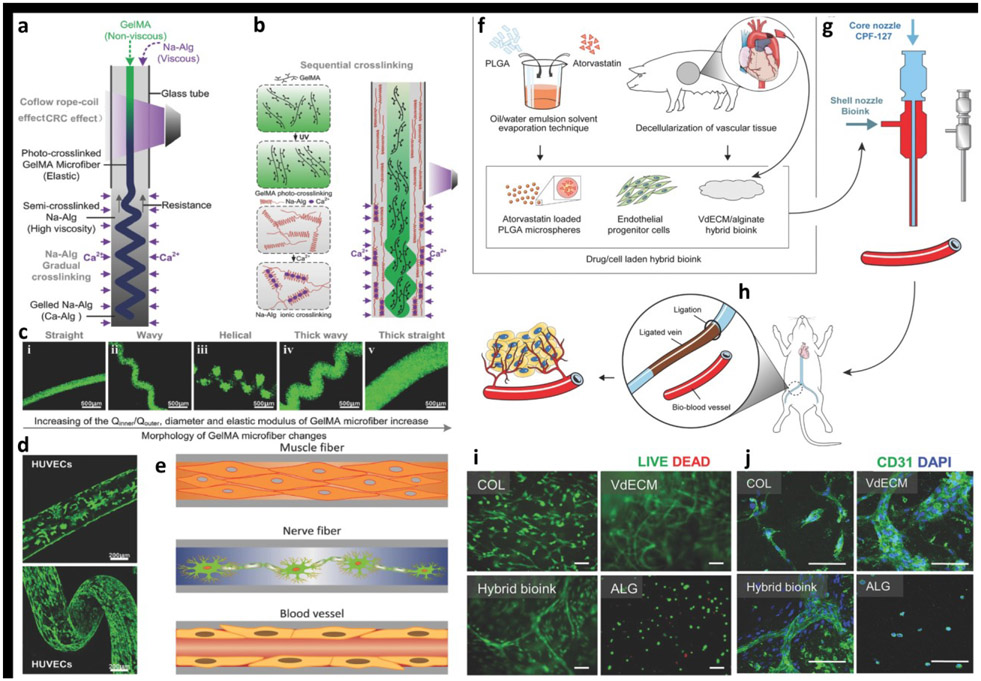
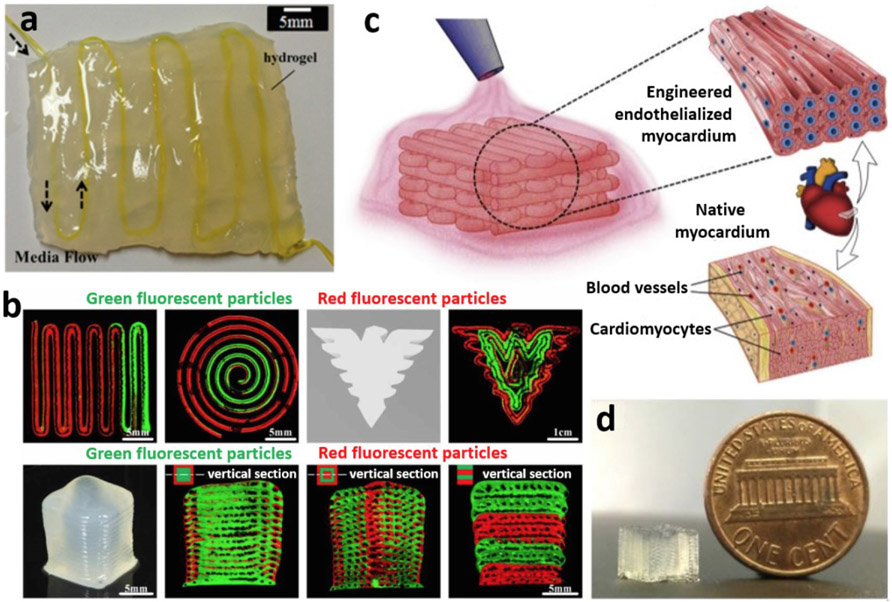
A significant progress has also been made in the area of vascular tissue engineering. For example, fabrication of blood-vessel resembling constructs has been described by Shao et al using GelMA bioink with different coaxial nozzle and shell/core arrangements. Fabrication of microfiber configurations, such as helical and Janus structures, were demonstrated as shown in Figures 4a-d [70]. Utilizing HUVECs-laden GelMA core bioink surrounded by calcium alginate shell led to the development of vessel-resembling structures because of cell migration in microfibers. The constructs showed structural diversity, cytocompatibility and mechanical tenability, which can be used for regenerative applications such as muscles, nerves and blood vessels (Figure 4e). Gao et al [71] developed a hybrid bioink consisting of vascular-tissue-derived decellularized extracellular matrix (VdECM) – alginate mixed with EPCs and atorvastatin/PLGA microspheres (a proangiogenic drugs), which showed to promote differentiation, proliferation, and neovascularization of EPCs and even enabled the direct fabrication of blood-vessel structures (Figures 4f-j). Studies with a nude mouse ischemic model concluded that these substitutes could have potential application in the treatment of ischemic disorders, as grafts for bypass surgery as well as the replacement of injured blood vessels.
Figure 4:
a) The schematic of fabrication of the heterotypic GelMA microfibers based on the co-flow rope-coil effect. b) The sequential cross-linking strategy based on ionically crosslinked Na-Alg and photo-cross-linked GelMA. c) The morphology of GelMA microfibers changed with change in the flow rate ratio between core and sheath section of the nozzle, and the morphological change process of the GelMA microfibers was straight–wavy–helical–thick wavy–thick straight. d) Confocal laser-scanning microscopy images of the cell-laden GelMA microfibers revealed the cellular morphology after 10 days of culture. e) The potential applications of GelMA microfibers in fiber-based tissue engineering, such as muscle fibers, nerve fibers, and blood vessels. Adapted with permission from Reference [70]. f) A hybrid bioink was prepared by mixing VdECM and sodium alginate, which was used to encapsulate atorvastatin/PLGA microspheres and endothelial progenitor cells (EPCs). g) Coaxial bioprinting was applied to fabricate cell/drug-laden vascular constructs, h) which were evaluated in a mouse model by transplanting the structure to the vicinity of the ligated limb vein to treat ischemic disease. i) Relatively few dead cells were detected at Day 7 proving that the hybrid bioink provided a friendly environment to cells (scale bar: 100 μm). j) Formation of vasculature was detected in the hybrid bioink at Day 7 (Green: CD31 and Blue: 4′6-diamidino-2-phenylindole (DAPI); scale bar: 100 μm. Adapted with permission from Reference [71]).
6.2. Drug Delivery and Controlled Release
A key objective in drug delivery is the ability to produce devices that can release drugs in a controlled and sustained manner. One of the major advantages of coaxially bioprinted constructs is the ability to load two different or more materials and drugs in a concentric manner. In this regard, Do et al [72] studied the controlled release of fluorophores by coaxially printed poly(lactic-co-glycolic acid) (PLGA)/ alginate core/shell tubes. The results indicated the ability of constructs to sequentially release fluorophores while still not being cytotoxic. These results could lead to development of constructs to aid in the drug delivery for various disease as well as maintaining a high dose efficacy. Along the same lines, Liu et al explored a combination of multi-nozzle electrospinning and coaxial bioprinting for the preparation of functionally-graded osteochondral scaffolds [73]. The electrospun gentamycin sulfate GS/polyvinyl alcohol (PVA) and coaxial electrospun core (PVA-DFO)/shell (PCL) allowed for the loading and release of biomolecules, such as GS and desferrioxamine. The results indicated that loaded biomolecules could be delivered from constructs via various release profiles over space and time. This shows the possibility of fabricating mechanically-strong constructs exhibiting spatiotemporal release of multiple biomolecules.
Akkineni et al [74] were able to achieve controlled and tuneable release of drugs-gentamicin, clindamycin, and vancomycin, from a coaxially bioprinted scaffold. The drugs were loaded as the core portion and encapsulated by a high viscous shell component composed of alginate, methylcellulose and Laponite. Results indicated that drug release kinetics was highly dependent on the shell composition. Moreover, incorporation of Laponite slowed the drug release rate. Therefore, a tuneable drug eluting construct was effectively fabricated by alteration of shell composition and thickness. Similarly, Kilian et al [75], employed the use of a differentiation factor (TGF-β3 for human articular chondrocytes (hChon) and BMP-2 for human pre-osteoblasts (hOB)) laden Laponite bioink core and cell laden polysaccharide shell arrangement for the co-differentiation of the two cell types within the same zonal construct. Alterations of the Laponite layer thickness enabled a control over the rate of delivery of the differentiation factors from the core to the cells present in the shell.
6.3. Vascularized Tissue Fabrication
Two major blockades for bioprinting of scalable tissues are 1) inefficient methods to bioprint fine (< mm level) nutrient delivery channels (NDCs) within the cell-laden structures and 2) inability to feasibly vascularize NDCs. An ideal bioprinted construct suitable for transplantation would require a network of functional blood vessels, which mimics the native blood vessel anatomy and would eventually be able to form anastomosis at the surgical site. Three key factors in bioprinting of vascularized, and functionalized tissue constructs will thus include 1) an efficient strategy for prefabrication of vascularized networks, 2) a productive method to endothelialize these vascular networks, and 3) long-term perfusion culture for vascularization and biological function. Accordingly, Zhang et al, introduced the integration of coaxially bioprinted vascular microfluidic channels in bulk hydrogels for its applications in thick tissue and organ fabrication (Figure 5a) [42]. This method provided some major advantages such as, the ability to control the microenvironment architecture dimensions of the fabricated constructs, omission of the scaffold post processing steps and reduced biomaterial requirement when compared to traditional scaffold fabrication processes. This proof of study laid the foundation for follow up studies. For example, Shao et al [76] used coaxial bioprinting to perform direct fabrication of vascularized NDCs. The shell bioink consisted of GelMA mixed with target specific tissue cells, such as mouse osteoblast cell line (MC3T3-E1) or human breast cell line (MDA-MB-231) that surrounded HUVECs-laden gelatin core bioink [76]. This gave rise to a bioprinted tissue constructs with shell-core fibers and heterogeneous constructs with vascular channels (Figure 5b). Vascularization of these constructs was achieved by dissolving the core fibers leading to the deposition and adherence of HUVECs over the channels. In essence, a self-seeding process for HUVECs was obtained. This method enabled the development of cancer or osteogenic tissue constructs over 1 cm in length having a culture period over 20 days. Further studies need to be conducted to study the promotion of vascularization and biological function with long-term perfusion culture combined with perfusion device or chip. Vascularized and neurotized tissue constructs have also been created by the introduction of nerve cells into the coaxial bioprinting process. In another study, Zhang et al [23] was successful in the fabrication of vascular endothelialized tissues seeded with myocardial cells (Figures 5c-d). Utilizing a bioink formulation of alginate- GelMa in a core-shell fashion, with HUVECs-laden core, a cell-laden microfiber scaffold was demonstrated enabling the perfusion of cells towards the peripheries of the microfiber forming a confluent layer of endothelium, which resembles the structure of a blood vessel. Further seeding cardiomyocytes showcased the formation of myocardium with the ability to perform synchronous and spontaneous contraction. These accomplishments all lead to the conclusion that coaxial bioprinting could be potentially used for fabrication of large-scale vascularized tissue constructs for applications in tissue engineering, organ repair and regenerative medicine [76].
Figure 5:
a) Coaxially bioprinted scalable perfusable hydrogel constructs (adapted with permission from Reference [42]) and b) multi-material coaxial bioprinting of tubular channels in various configurations (adapted with permission from Reference [76]). c) Concept of coaxial bioprinting of vascularized myocardium and d) a sample thick tissue construct bioprinted using such a concept (adapted with permission from reference [23]).
For the development of scalable vascularized tissues and organs constructs, one key criterion is the appropriate selection of bioinks which can enable the efficient creation of vascular network microchannels with perfusion ability and sufficient mechanical properties. Hence, Li et al described the use of a hybrid bioink made of C3A liver cancer cells mixed with silk fibroin and alginate (Alg/SF) as the sheath, which surrounded a core bioink made of a crosslinker blend of Pluronic F127 and calcium ions [77]. This combination of shell and core bioinks led to the formation of a built-in vascular network with a regular structure and smooth microporous wall. The shell bioink was selected owing to its desirable rheological properties such as improved shear thinning and viscosity. It also maintained storage and loss modulus in a wide range of shear frequency, compared to pure Alg- or SF-only bioink. Moreover, the composite shell could form a strong double crosslinked network and preserve high cell viability and growth when compared to the construct with pure Alg network.
One of the major challenges to be tackled in conventional single-nozzle 3D bioprinting is the fabrication of multilayer tubular constructs with heterocellular composition, more specifically, resembling the anatomy of blood vessels, intestine, trachea, colon, etc. In this respect, by employing coaxial bioprinting Pi et al has described the fabrication of perfusable constructs [78]. This was performed using a custom bioink combination of GelMA, alginate and eight-arm poly (ethylene glycol) acrylate with a tripentaerythritol core. It acted as the shell bioink, which surrounded a calcium chloride core crosslinker. Three types of tubular constructs were fabricated with different cell lines, viz; cannular urothelial tissue constructs were bioprinted, using human urothelial cells and human bladder smooth muscle cells, as well as vascular endothelial and muscle tissue constructs, using HUVECs and hSMCs. These constructs attributed high cell viability and growth mainly as a result of the perfusable nature of the bioprinted constructs, allowing the flow of cell media. The constructs also showcased features of native human tubular tissues such as the continuous perfusion of fluids enabling the growth and interaction of different cell types in the fabricated layers.
6.4. In-vitro Tissue Models
Tissue models provide an in vitro environment to study cells response to various stimuli such as growth factors and drugs. Bioprinting of cells aid in the development of in-vitro platforms primarily to study fundamental biological questions, disease development and toxicological effects of drugs on cells [79] [80]. Within this content, Li et al [81] built neural tissue, predominantly for cell distribution and differentiation purposes. Here, the shell was fabricated of sodium alginate that encompassed a core consisting of mouse neural progenitor cell (NE-4C) laden-gelatin and alginate. The coaxial nozzle immersed in CaCl2 solution for crosslinking of the shell leading to the formation of a NE-4C encapsulated hydrogel. Utilizing molecular biology techniques, cell differentiation was studied, and the results indicated that NE-4Cs had a stronger differentiation tendency compared to cells grown in 2D or 3D bioprinted grid structures.
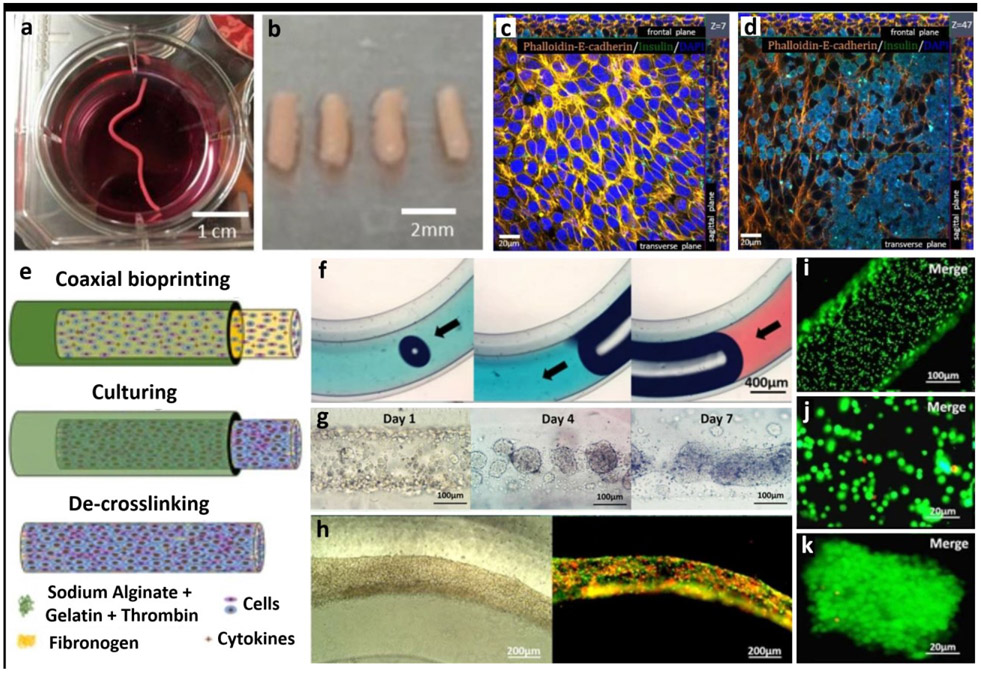
Scaffold-free fabrication is a novel method to fabricate tissue models by the process of self-assembly. This is done by taking advantage of the fact that cells tend to aggregate and form tissue-like structures, which then aggregate to shape organ-like structures. Few studies have employed the use of coaxial bioprinting to generate capsules for growing tissues in a scaffold-free manner such as cartilage tissue and human adipose-derived stem cells based porous tissue strands. These studies have shown increased cell viability and proliferation compared to solid tissue strands [82] [83]. Using this approach, heterocellular tissue strands of rat dermal fibroblasts and beta-TC-3 insulinoma cells were fabricated, which were stained positive to insulin [84] (Figures 6a-d).
Figure 6:
Scaffold-free fabrication of tissue models. (a-b) Fabrication of tissue strands made of rat dermal fibroblasts (RDFs), (c-d) which were then further cultured with beta-TC-3 insulinoma cells. Beta-TC-3 cells adhered on top of RDF strands showing positive to insulin in the outer layer of strands. Adapted with permission from Reference [84]. e) Schematic for the fabrication steps of multicellular heterogeneous tumor fibers: coaxial bioprinting, in vitro culturing and de-crosslinking. f) Integrity and continuity testing of the fiber by passing dye through the filament – no leaks is indicative of a continuous fiber. g) Images of the cultured tumour fibers after 1, 4 and 7 days, h) tumor/stroma cell fibers traced with RFP/GFP - cell fibers composed of GFP-expressing MSCs and RFP-expressing tumor cells after bioprinting and cultured over a period of 3 days, i-k) cell viability testing for the cell laden fibers immediately after bioprinting performed using LIVE/DEAD assay, h) Assessment of cell viability five days post bioprinting and culturing. Adapted with permission from Reference [87].
Exogenous insulin dose has been the most frequently used method for the treatment of Type I diabetes (T1D), but this method has its own disadvantages/side effects such as hyper- and hypoglycaemia leading to tissue and organ damage. For a couple of decades, the most promising treatment method for the T1D had been the pancreatic islet transplantation process [85]. This method has some major drawbacks such as low islet graft survival, activation of an immune response and low vascularization thus leading to low survival rates of the transplanted cells. To combat these downfalls, studies are conducted towards encapsulation of these cells for a more efficient and successful transplant process. Following this path, Liu at al described the use of a coaxial bioprinting technique for the encapsulation of islet cells [86]. Here the islet cells were mixed with alginate forming the core bioink which was surrounded by an endothelial progenitor cell (EPC)-laden GelMA shell bioink. The main outcome of this study was the ability to isolating the islet cells from eliciting an immune response.
Currently, there is a need for innovation towards the treatment and research of glioblastoma (GBM), the most common tumor found in the central nervous system. Conventional therapy utilizing surgery, chemotherapy and radiotherapy show unsatisfactory results due to the cancer cells’ aptitude to resist alkylating agents leading to recurrence of GBM. Hence, development of in-vitro tissue models could shed light into the mechanism of drug resistance for these cells. In this respect, Wang et al described fabrication of glioma cell (GSC23)-laden hydrogel microfibers via the process of coaxial bioprinting [46]. The combination of cell-laden alginate acted as the shell bioink that contained a human glioma cell line (U118)-laden bioink in the core. The fiber-like aggregates of cells led to an increased cell-cell and cell-ECM interaction. These results indicate the reproduction of native microenvironment of glial cells and thus can be used as models for drug resistance studies. It can also be noted that the cells on the core exhibited increased chemoresistance caused by certain core-shell cell interactions. In addition, the drug resistance mechanism was confirmed because of lower degree of O6-methylguanine-DNA methyltransferase promoter methylation.
In another study illustrated in Figures 6e-k, coaxial bioprinting was implemented to imitate tumor microenvironment with respect to tumour-stromal interactions [87]. The heterogeneous and multicellular self-assembled brain tumor model was formed using alginate/gelatin as the shell bioink, which had a cell-laden core bioink consisting of red fluorescence protein (RFP)-expressing glioma stem cells (GSCs) and GFP+MSCs and fibrinogen. The authors observed that fabricated constructs showed higher transcription of RFP when compared to 2D culture models. The fusion of tumor-stroma cells was also observed. Consequently, the fabricated tumor model could potentially be used for in-vitro analysis of various types of interactions in tumor microenvironment.
7. Limitations of Coaxial Bioprinting
Tissue engineering and regenerative medicine have a strict requirement of constructs that are mechanically stable and biocompatible. At the same time, they should also be anatomically accurate with the ability for supporting tissue growth. EBB has grown as an ideal fabrication technique to build suitable constructs as it permits the fabrication of vascularized and perfusable tissues [88]; however, this method presents certain limitations. Examples are limited microenvironmental complexity, inability to precisely control cell deposition and certain cell damage experienced during extrusion. The other notable limitation include the lack of a broad range of bioink materials. For instance, though ionic, physical and covalent crosslinking schemes can be employed, rapid crosslinking of hydrogels during coaxial bioprinting most often requires the constant use of an ionically-crosslinked hydrogel as a bioink. Coaxial bioprinting faces certain drawbacks such as inability to effectively fabricate constructs that are anatomically bifurcated besides fabricating constructs that are hierarchically stacked [89]. Bifurcated vascular constructs have been done using manual approaches after coaxial extrusion; however automated techniques are essential to directly generate hierarchically stacked vascular networks. In the future, coaxial bioprinting of vascular networks can be performed using embedded bioprinting, where a secondary needle can be used to patch up bifurcation points.
In addition, coaxial bioprinting is found to have limited capability to promote capillary sprouting. Ability to induce sprouting angiogenesis is highly crucial for multi-scale vascularization of tissues and new bioinks should be designed to promote sprouting angiogenesis from coaxially bioprinted vascular network. For embedding coaxially-extruded vascular networks in larger scale bulk hydrogels, properties of the bulk hydrogel should match with that of the vasculature so that no interface is generated between the vasculature and bulk hydrogel. Interface formation can trigger the excessive accumulation of media after perfusion through the vascular network.
Other limitations include the limitation in achievable dimensions of vascular constructs as the smallest lumen size is in the order of a few hundred microns, which require higher resolution. As the lumen of vasculature is open, it is also difficult to control precise deposition of vascular network as sharp turns cannot be made as such may generate occlusion of the lumen. This limits the vascular network design as the space between adjacent vascular segments cannot be too tight. Although a number of studies have demonstrated coaxially bioprinted vascular constructs, fabrication of vascular constructs that are truly similar to native blood vessels is still a challenge. Therefore, further research is needed to better resemble the anatomical, histological, biomechanical and functional properties of native blood vessels.
8. Conclusion and Future Perspectives
The innovations in the field of 3D bioprinting, especially in coaxial bioprinting, were all stemmed from the necessity for development of a method to rapidly and efficiently fabricate vascularized tissues that will not only aid in tissue engineering and regenerative medicine, but also in disease modelling and drug screening This has been made possible by the collaborative efforts of researchers from various disciplines, all culminating in the development of 3D bioprinting. Recent advancements in the field of mechatronics (to create robust and efficient bioprinting machines), cell culture, biomaterial science (to enable the utilization of various bioink combinations for better scaffold preparation) and microfluidics could secure coaxial bioprinting as the ‘go to method’ for tissue construction as this process provides the opportunity to form constructs in a single step without the need for additional post-extrusion processing. Based on the nozzle employed, conventional or multi-nozzle arrangement, one may be able to generate tissue constructs with different properties. Since this is an emerging field, additional studies demand to further improve on the areas with limitations. The possibility of in-situ crosslinking enhances the resolution of constructs and hence improves cell-cell and cell-material interactions. A promising and exciting aspect of coaxial bioprinting is in the development of bioinks for fabrication of scalable laboratory-grown organs [90], which will enable the repair in diseases or improve upon the native capabilities of the human physiology. Coaxial bioprinting also enables the use of drug and biomolecule loaded constructs that could be useful in various cell culture and drug screening analysis. Combined with advances in automation and organoid bioprinting platforms [91], drug assays are expected to be much accelerated.
Acknowledgements
This work was supported by the National Institute of Health Award #R01DE028614 (I.T.O.). P.D. acknowledges the INSPIRE Faculty Award from the Department of Science and Technology of the Government of India.
Footnotes
Competing interests
I.T.O. serves as a board member for Biolife4D Company exploring the use of 3D bioprinting technologies for fabrication of cardiac models. The remaining authors declare no competing interests.
References:
- [1].Li J, Chen M, Fan X, and Zhou H, “Recent advances in bioprinting techniques: approaches, applications and future prospects,” J. Transl. Med, vol. 14, no. 1, p. 271, 2016, doi: 10.1186/s12967-016-1028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ozbolat IT, “3D Bioprinting: Fundementals, Principles and Applications, Ed. Oxford: Academic Press, 2017. [Google Scholar]
- [3].Groll J et al. , “Biofabrication: Reappraising the definition of an evolving field,” Biofabrication, vol. 8, no. 1, 2016, doi: 10.1088/1758-5090/8/1/013001. [DOI] [PubMed] [Google Scholar]
- [4].Huang Y, Zhang X-F, Gao G, Yonezawa T, and Cui X, “3D bioprinting and the current applications in tissue engineering,” Biotechnol. J, vol. 12, no. 8, p. 1600734, 2017, doi: 10.1002/biot.201600734. [DOI] [PubMed] [Google Scholar]
- [5].Mandrycky C, Wang Z, Kim K, and Kim D-H, “3D bioprinting for engineering complex tissues,” Biotechnol. Adv, vol. 34, no. 4, pp. 422–434, 2016, doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Vijayavenkataraman S, Yan W-C, Lu WF, Wang C-H, and Fuh JYH, “3D bioprinting of tissues and organs for regenerative medicine.,” Adv. Drug Deliv. Rev, vol. 132, pp. 296–332, Jul. 2018, doi: 10.1016/j.addr.2018.07.004. [DOI] [PubMed] [Google Scholar]
- [7].Ozbolat IT and Yu Y, "Bioprinting toward organ fabrication: challenges and future trends," IEEE. Trans. Biomed. Eng, vol. 60, no. 3, pp. 691–699, 2013, doi. 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- [8].Askari M, Afzali Naniz M, Kouhi M, Saberi A, Zolfagharian A, and Bodaghi M, “Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: a comprehensive review with focus on advanced fabrication techniques,” Biomater. Sci, p., 2020, doi: 10.1039/D0BM00973C. [DOI] [PubMed] [Google Scholar]
- [9].Jiang T, Munguia-Lopez JG, Flores-Torres S, Kort-Mascort J, and Kinsella JM, “Extrusion bioprinting of soft materials: An emerging technique for biological model fabrication,” Appl. Phys. Rev, vol. 6, no. 1, p. 11310, 2019, doi: 10.1063/1.5059393. [DOI] [Google Scholar]
- [10].Pati F, Jang J, Lee JW, and Cho D-W, “Chapter 7 - Extrusion Bioprinting,” Atala A and J. J. B. T.-E. of 3D B and Yoo T, Eds. Boston: Academic Press, 2015, pp. 123–152. [Google Scholar]
- [11].Ning L and Chen X, “A brief review of extrusion-based tissue scaffold bio-printing,” Biotechnol. J, vol. 12, no. 8, p. 1600671, 2017, doi: 10.1002/biot.201600671. [DOI] [PubMed] [Google Scholar]
- [12].Rafiee M, Farahani RD, and Therriault D, “Multi-Material 3D and 4D Printing: A Survey,” Adv. Sci, vol. 7, no. 12, p. 1902307, 2020, doi: 10.1002/advs.201902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhou Z, Salaoru I, Morris P, and Gibbons GJ, “Development of a direct feed fused deposition modelling technology for multi-material manufacturing,” AIP Conf. Proc, vol. 1769, no. 1, p. 190004, Oct. 2016, doi: 10.1063/1.4963614. [DOI] [Google Scholar]
- [14].Cui X et al. , “Advances in Extrusion 3D Bioprinting: A Focus on Multicomponent Hydrogel-Based Bioinks,” Adv. Healthc. Mater, vol. 9, no. 15, p. 1901648, 2020, doi: 10.1002/adhm.201901648. [DOI] [PubMed] [Google Scholar]
- [15].Costantini M, Colosi C, Świ\keszkowski W, and Barbetta A, “Co-axial wet-spinning in 3D bioprinting: state of the art and future perspective of microfluidic integration,” Biofabrication, vol. 11, no. 1, p. 12001, Nov. 2018, doi: 10.1088/1758-5090/aae605. [DOI] [PubMed] [Google Scholar]
- [16].Hong N, Yang G-H, Lee J, and Kim G, “3D bioprinting and its in vivo applications,” J. Biomed. Mater. Res. Part B Appl. Biomater, vol. 106, no. 1, pp. 444–459, 2018, doi: 10.1002/jbm.b.33826. [DOI] [PubMed] [Google Scholar]
- [17].Heo DN et al. , “3D Bioprinting of Carbohydrazide-Modified Gelatin into Microparticle-Suspended Oxidized Alginate for the Fabrication of Complex-Shaped Tissue Constructs.,” ACS Appl. Mater. Interfaces, vol. 12, no. 18, pp. 20295–20306, May 2020, doi: 10.1021/acsami.0c05096. [DOI] [PubMed] [Google Scholar]
- [18].Hinton TJ et al. , “Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels,” Sci. Adv, vol. 1, no. 9, 2015, doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shim J-H, Lee J-S, Kim JY, and Cho D-W, “Bioprinting of a mechanically enhanced three-dimensional dual cell-laden construct for osteochondral tissue engineering using a multi-head tissue/organ building system,” J. Micromechanics Microengineering, vol. 22, no. 8, p. 85014, Jul. 2012, doi: 10.1088/0960-1317/22/8/085014. [DOI] [Google Scholar]
- [20].Jin Y, Zhao D, and Huang Y, “Fabrication of Double-Layered Alginate Capsules Using Coaxial Nozzle,” J. Micro Nano-Manufacturing, vol. 5, no. 4, 2017, doi: 10.1115/1.4037646. [DOI] [Google Scholar]
- [21].Attalla R, Puersten E, Jain N, and Selvaganapathy PR, “3D bioprinting of heterogeneous bi- and tri-layered hollow channels within gel scaffolds using scalable multi-axial microfluidic extrusion nozzle,” Biofabrication, vol. 11, no. 1, p. 15012, Dec. 2018, doi: 10.1088/1758-5090/aaf7c7. [DOI] [PubMed] [Google Scholar]
- [22].Silva C, Cortés-Rodriguez CJ, Hazur J, Reakasame S, and Boccaccini AR, “Rational Design of a Triple-Layered Coaxial Extruder System: in silico and in vitro Evaluations Directed Toward Optimizing Cell Viability.,” Int. J. bioprinting, vol. 6, no. 4, p. 282, 2020, doi: 10.18063/ijb.v6i4.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Zhang YS et al. , “Bioprinting 3D microfibrous scaffolds for engineering endothelialized myocardium and heart-on-a-chip.,” Biomaterials, vol. 110, pp. 45–59, Dec. 2016, doi: 10.1016/j.biomaterials.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kjar A, McFarland B, Mecham K, Harward N, and Huang Y, “Engineering of tissue constructs using coaxial bioprinting,” Bioact. Mater, vol. 6, no. 2, pp. 460–471, 2021, doi: 10.1016/j.bioactmat.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].AU - Milojević M et al. , “Core/shell Printing Scaffolds For Tissue Engineering Of Tubular Structures,” JoVE, no. 151, p. e59951, 2019, doi: doi: 10.3791/59951. [DOI] [PubMed] [Google Scholar]
- [26].Liu W et al. , “Coaxial extrusion bioprinting of 3D microfibrous constructs with cell-favorable gelatin methacryloyl microenvironments.,” Biofabrication, vol. 10, no. 2, p. 24102, Jan. 2018, doi: 10.1088/1758-5090/aa9d44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Drury JL and Mooney DJ, “Hydrogels for tissue engineering: scaffold design variables and applications.,” Biomaterials, vol. 24, no. 24, pp. 4337–4351, Nov. 2003, doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- [28].Van Vlierberghe S, Dubruel P, and Schacht E, “Biopolymer-based hydrogels as scaffolds for tissue engineering applications: a review.,” Biomacromolecules, vol. 12, no. 5, pp. 1387–1408, May 2011, doi: 10.1021/bm200083n. [DOI] [PubMed] [Google Scholar]
- [29].Tibbitt MW and Anseth KS, “Hydrogels as extracellular matrix mimics for 3D cell culture.,” Biotechnol. Bioeng, vol. 103, no. 4, pp. 655–663, Jul. 2009, doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Duchi S et al. , “Handheld Co-Axial Bioprinting: Application to in situ surgical cartilage repair,” Sci. Rep, vol. 7, no. 1, p. 5837, 2017, doi: 10.1038/s41598-017-05699-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Akhtar MF, Hanif M, and Ranjha NM, “Methods of synthesis of hydrogels … A review.,” Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc, vol. 24, no. 5, pp. 554–559, Sep. 2016, doi: 10.1016/j.jsps.2015.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hospodiuk M, Dey M, Sosnoski D, and Ozbolat IT, “The bioink: A comprehensive review on bioprintable materials,” Biotechnol. Adv, vol. 35, no. 2, pp. 217–239, 2017, doi: 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [33].Axpe E and Oyen ML, “Applications of Alginate-Based Bioinks in 3D Bioprinting.,” Int. J. Mol. Sci, vol. 17, no. 12, Nov. 2016, doi: 10.3390/ijms17121976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jungst T, Smolan W, Schacht K, Scheibel T, and Groll J, “Strategies and Molecular Design Criteria for 3D Printable Hydrogels,” Chem. Rev, vol. 116, no. 3, pp. 1496–1539, Feb. 2016, doi: 10.1021/acs.chemrev.5b00303. [DOI] [PubMed] [Google Scholar]
- [35].Taymour R, Kilian D, Ahlfeld T, Gelinsky M, and Lode A, “3D bioprinting of hepatocytes: core–shell structured co-cultures with fibroblasts for enhanced functionality,” Sci. Rep, vol. 11, no. 1, p. 5130, 2021, doi: 10.1038/s41598-021-84384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sun J and Tan H, “Alginate-Based Biomaterials for Regenerative Medicine Applications.,” Mater. (Basel, Switzerland), vol. 6, no. 4, pp. 1285–1309, Mar. 2013, doi: 10.3390/ma6041285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu Y, Zhang Y, Martin JA, and Ozbolat IT, “Evaluation of cell viability and functionality in vessel-like bioprintable cell-laden tubular channels,” J. Biomech. Eng, vol. 135, no. 9, p. 91011, Sep. 2013, doi: 10.1115/1.4024575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang Y, Yu Y, and Ozbolat IT, “Characterization of Printable Micro-Fluidic Channels for Organ Printing.” Jun. 10, 2013, doi: 10.1115/MSEC2013-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Chen H and Ozbolat IT, “Development of a Multi-Arm Bioprinter for Hybrid Tissue Engineering.” Jun. 10, 2013, doi: 10.1115/MSEC2013-1025. [DOI] [Google Scholar]
- [40].Yu Y and Ozbolat IT, “Bioprinting Induced Cell Damage in Cellular Micro-Fluidic Channel Fabrication.” Jun. 10, 2013, doi: 10.1115/MSEC2013-1081. [DOI] [Google Scholar]
- [41].Zhang Y, Yu Y, and Ozbolat IT, “Direct Bioprinting of Vessel-Like Tubular Microfluidic Channels,” J. Nanotechnol. Eng. Med, vol. 4, no. 2, pp. 210011–210017, May 2013, doi: 10.1115/1.4024398. [DOI] [Google Scholar]
- [42].Zhang Y, Yu Y, Chen H, and Ozbolat IT, “Characterization of printable cellular micro-fluidic channels for tissue engineering,” Biofabrication, vol. 5, no. 2, 2013, doi: 10.1088/1758-5082/5/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang Y and Ozbolat IT, “Characterization of vessel-like printable cellular microfluidic channel,” IIE Annu. Conf. Expo 2013, no. May 2013, pp. 2291–2298, 2013. [Google Scholar]
- [44].Yu Y and Ozbolat I, “Cell viability characterization of bioprintable blood-vessel-like cellular channels towards 3D organ fabrication,” IIE Annu. Conf. Expo 2013, pp. 2299–2304, 2013. [Google Scholar]
- [45].He J, Shao J, Li X, Huang Q, and Xu T, “Bioprinting of Coaxial Multicellular Structures for a 3D Co-culture Model,” Bioprinting, vol. 11, p. e00036, 2018, doi: 10.1016/j.bprint.2018.e00036. [DOI] [Google Scholar]
- [46].Wang X et al. , “Coaxial extrusion bioprinted shell-core hydrogel microfibers mimic glioma microenvironment and enhance the drug resistance of cancer cells.,” Colloids Surf. B. Biointerfaces, vol. 171, pp. 291–299, Nov. 2018, doi: 10.1016/j.colsurfb.2018.07.042. [DOI] [PubMed] [Google Scholar]
- [47].Costantini M et al. , “Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo,” Biomaterials, vol. 131, pp. 98–110, 2017, doi: 10.1016/j.biomaterials.2017.03.026. [DOI] [PubMed] [Google Scholar]
- [48].Gao Q et al. , “3D Bioprinting of Vessel-like Structures with Multilevel Fluidic Channels,” ACS Biomater. Sci. Eng, vol. 3, no. 3, pp. 399–408, Mar. 2017, doi: 10.1021/acsbiomaterials.6b00643. [DOI] [PubMed] [Google Scholar]
- [49].Muzzarelli RAA, El Mehtedi M, Bottegoni C, Aquili A, and Gigante A, “Genipin-Crosslinked Chitosan Gels and Scaffolds for Tissue Engineering and Regeneration of Cartilage and Bone.,” Mar. Drugs, vol. 13, no. 12, pp. 7314–7338, Dec. 2015, doi: 10.3390/md13127068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Croisier F and Jérôme C, “Chitosan-based biomaterials for tissue engineering,” Eur. Polym. J, vol. 49, no. 4, pp. 780–792, 2013, doi: 10.1016/j.eurpolymj.2012.12.009. [DOI] [Google Scholar]
- [51].Dolati F, Yu Y, Zhang Y, De Jesus AM, Sander EA, and Ozbolat IT, “In vitroevaluation of carbon-nanotube-reinforced bioprintable vascular conduits,” Nanotechnology, vol. 25, no. 14, p. 145101, Mar. 2014, doi: 10.1088/0957-4484/25/14/145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Zhang Y, Yu Y, Dolati F, and Ozbolat IT, “Effect of multiwall carbon nanotube reinforcement on coaxially extruded cellular vascular conduits,” Mater. Sci. Eng. C, vol. 39, pp. 126–133, 2014, doi: 10.1016/j.msec.2014.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Ying G, Jiang N, Yu C, and Zhang YS, “Three-dimensional bioprinting of gelatin methacryloyl (GelMA),” Bio-Design Manuf., vol. 1, no. 4, pp. 215–224, 2018, doi: 10.1007/s42242-018-0028-8. [DOI] [Google Scholar]
- [54].Wang Y, Kankala RK, Zhu K, Wang S-B, Zhang YS, and Chen A-Z, “Coaxial Extrusion of Tubular Tissue Constructs Using a Gelatin/GelMA Blend Bioink,” ACS Biomater. Sci. Eng, vol. 5, no. 10, pp. 5514–5524, Oct. 2019, doi: 10.1021/acsbiomaterials.9b00926. [DOI] [PubMed] [Google Scholar]
- [55].Colosi C et al. , “Microfluidic Bioprinting of Heterogeneous 3D Tissue Constructs Using Low-Viscosity Bioink,” Adv. Mater, vol. 28, no. 4, pp. 677–684, Jan. 2016, doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Turner PR, Murray E, McAdam CJ, McConnell MA, and Cabral JD, “Peptide Chitosan/Dextran Core/Shell Vascularized 3D Constructs for Wound Healing.,” ACS Appl. Mater. Interfaces, vol. 12, no. 29, pp. 32328–32339, Jul. 2020, doi: 10.1021/acsami.0c07212. [DOI] [PubMed] [Google Scholar]
- [57].Liu C et al. , “Bioprinted Chitosan and Hydroxyapatite Micro-Channels Structures Scaffold for Vascularization of Bone Regeneration,” J. Biomater. Tissue Eng, vol. 7, pp. 28–34, 2017, doi: 10.1166/jbt.2017.1535. [DOI] [Google Scholar]
- [58].Tomasina C, Bodet T, Mota C, Moroni L, and Camarero-Espinosa S, “Bioprinting Vasculature: Materials, Cells and Emergent Techniques.,” Mater. (Basel, Switzerland), vol. 12, no. 17, Aug. 2019, doi: 10.3390/ma12172701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Kjar A, McFarland B, Mecham K, Harward N, and Huang Y, “Engineering of tissue constructs using coaxial bioprinting,” Bioact. Mater, vol. 6, no. 2, pp. 460–471, 2021, doi: 10.1016/j.bioactmat.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gu Z, Fu J, Lin H, and He Y, “Development of 3D bioprinting: From printing methods to biomedical applications.,” Asian J. Pharm. Sci, vol. 15, no. 5, pp. 529–557, Sep. 2020, doi: 10.1016/j.ajps.2019.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hong S, Kim JS, Jung B, Won C, and Hwang C, “Coaxial bioprinting of cell-laden vascular constructs using a gelatin–tyramine bioink,” Biomater. Sci, vol. 7, no. 11, pp. 4578–4587, 2019, doi: 10.1039/C8BM00618K. [DOI] [PubMed] [Google Scholar]
- [62].Choi Y-J et al. , “A 3D cell printed muscle construct with tissue-derived bioink for the treatment of volumetric muscle loss.,” Biomaterials, vol. 206, pp. 160–169, Jun. 2019, doi: 10.1016/j.biomaterials.2019.03.036. [DOI] [PubMed] [Google Scholar]
- [63].Lei S, Gao Q, Xie C, Jianzhong F, and Xiang M-X, “Bioprinting of Cell-Laden Microfiber: Can It Become a Standard Product?,” 2019. [DOI] [PubMed] [Google Scholar]
- [64].Di Bella C et al. , “In situ handheld three-dimensional bioprinting for cartilage regeneration.,” J. Tissue Eng. Regen. Med, vol. 12, no. 3, pp. 611–621, Mar. 2018, doi: 10.1002/term.2476. [DOI] [PubMed] [Google Scholar]
- [65].Wu Y, Ravnic DJ, and Ozbolat IT, “Intraoperative Bioprinting: Repairing Tissues and Organs in a Surgical Setting,” Trends Biotechnol., vol. 38, no. 6, pp. 594–605, 2020, doi: 10.1016/j.tibtech.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Moncal KK et al. , “Intra-Operative Bioprinting of Hard, Soft, and Hard/Soft Composite Tissues for Craniomaxillofacial Reconstruction,” Adv. Funct. Mater, vol. 31, no. 29, p. 2010858, 2021, doi: 10.1002/adfm.202010858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Hu Q, Jiang B, and Zhang H, “Method for Novel 3d Bioprinting of Gradient Scaffold For Osteochondral Regeneration Using a Coaxial Multi-nozzle and Software,” J. Biomater. Tissue Eng, vol. 9, pp. 24–31, 2019. [Google Scholar]
- [68].Kosik-Kozioł A et al. , “3D bioprinted hydrogel model incorporating β-tricalcium phosphate for calcified cartilage tissue engineering.,” Biofabrication, vol. 11, no. 3, p. 35016, May 2019, doi: 10.1088/1758-5090/ab15cb. [DOI] [PubMed] [Google Scholar]
- [69].Costantini M et al. , “3D bioprinting of BM-MSCs-loaded ECM biomimetic hydrogels for in vitro neocartilage formation.,” Biofabrication, vol. 8, no. 3, p. 35002, Jul. 2016, doi: 10.1088/1758-5090/8/3/035002. [DOI] [PubMed] [Google Scholar]
- [70].Shao L et al. , “Fiber-Based Mini Tissue with Morphology-Controllable GelMA Microfibers.,” Small, vol. 14, no. 44, p. e1802187, Nov. 2018, doi: 10.1002/smll.201802187. [DOI] [PubMed] [Google Scholar]
- [71].Gao G et al. , “Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3D Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease,” Adv. Funct. Mater, vol. 27, no. 33, p. 1700798, 2017, doi: 10.1002/adfm.201700798. [DOI] [Google Scholar]
- [72].Do AV et al. , “Controlled and Sequential Delivery of Fluorophores from 3D Printed Alginate-PLGA Tubes,” Ann. Biomed. Eng, vol. 45, no. 1, pp. 297–305, 2017, doi: 10.1007/s10439-016-1648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Liu Y-Y, Yu H-C, Liu Y, Liang G, Zhang T, and Hu Q-X, “Dual drug spatiotemporal release from functional gradient scaffolds prepared using 3D bioprinting and electrospinning,” Polym. Eng. Sci, vol. 56, no. 2, pp. 170–177, 2016, doi: 10.1002/pen.24239. [DOI] [Google Scholar]
- [74].Akkineni AR et al. , “Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections,” Pharmaceutics, vol. 13, no. 12, 2021, doi: 10.3390/pharmaceutics13122151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kilian D et al. , “Core{\textendash}shell bioprinting as a strategy to apply differentiation factors in a spatially defined manner inside osteochondral tissue substitutes,” Biofabrication, vol. 14, no. 1, p. 14108, Jan. 2022, doi: 10.1088/1758-5090/ac457b. [DOI] [PubMed] [Google Scholar]
- [76].Shao L, Gao Q, Xie C, Fu J, Xiang M, and He Y, “Directly coaxial 3D bioprinting of large-scale vascularized tissue constructs.,” Biofabrication, vol. 12, no. 3, p. 35014, May 2020, doi: 10.1088/1758-5090/ab7e76. [DOI] [PubMed] [Google Scholar]
- [77].Li H et al. , “Three-Dimensional Bioprinting of Perfusable Hierarchical Microchannels with Alginate and Silk Fibroin Double Cross-linked Network,” 3D Print. Addit. Manuf, vol. 7, no. 2, pp. 78–84, Apr. 2020, doi: 10.1089/3dp.2019.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Pi Q et al. , “Digitally Tunable Microfluidic Bioprinting of Multilayered Cannular Tissues,” Adv. Mater, vol. 30, no. 43, p. 1706913, 2018, doi: 10.1002/adma.201706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Peng W, Datta P, Ayan B, Ozbolat V, Sosnoski D, and Ozbolat IT, “3D bioprinting for drug discovery and development in pharmaceutics.,” Acta Biomater., vol. 57, pp. 26–46, Jul. 2017, doi: 10.1016/j.actbio.2017.05.025. [DOI] [PubMed] [Google Scholar]
- [80].Satpathy A, Datta P, Wu Y, Ayan B, Bayram E, and Ozbolat IT, “Developments with 3D bioprinting for novel drug discovery,” Expert Opin. Drug Discov, vol. 13, no. 12, pp. 1115–1129, Dec. 2018, doi: 10.1080/17460441.2018.1542427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Li X et al. , “A comparative study of the behavior of neural progenitor cells in extrusion-based in vitro hydrogel models.,” Biomed. Mater, vol. 14, no. 6, p. 65001, Sep. 2019, doi: 10.1088/1748-605X/ab3b4b. [DOI] [PubMed] [Google Scholar]
- [82].Yu Y et al. , “Three-dimensional bioprinting using self-assembling scalable scaffold-free ‘tissue strands’ as a new bioink,” Sci. Rep, vol. 6, no. 1, p. 28714, 2016, doi: 10.1038/srep28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wu Y et al. , “Porous tissue strands: Avascular building blocks for scalable tissue fabrication,” Biofabrication, vol. 11, no. 1, 2019, doi: 10.1088/1758-5090/aaec22. [DOI] [PubMed] [Google Scholar]
- [84].Akkouch A, Yu Y, and Ozbolat IT, “Microfabrication of scaffold-free tissue strands for three-dimensional tissue engineering,” Biofabrication, vol. 7, no. 3, p. 31002, 2015, doi: 10.1088/1758-5090/7/3/031002. [DOI] [PubMed] [Google Scholar]
- [85].Ravnic DJ, Leberfinger AN, and Ozbolat IT, “Bioprinting and Cellular Therapies for Type 1 Diabetes.,” Trends Biotechnol., vol. 35, no. 11, pp. 1025–1034, Nov. 2017, doi: 10.1016/j.tibtech.2017.07.006. [DOI] [PubMed] [Google Scholar]
- [86].Liu X et al. , “Development of a Coaxial 3D Printing Platform for Biofabrication of Implantable Islet-Containing Constructs.,” Adv. Healthc. Mater, vol. 8, no. 7, p. e1801181, Apr. 2019, doi: 10.1002/adhm.201801181. [DOI] [PubMed] [Google Scholar]
- [87].Dai X et al. , “Coaxial 3D bioprinting of self-assembled multicellular heterogeneous tumor fibers,” Sci. Rep, vol. 7, no. 1, p. 1457, 2017, doi: 10.1038/s41598-017-01581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Hospodiuk M, Moncal K, Dey M, and Ozbolat I, “Extrusion-Based Biofabrication in Tissue Engineering and Regenerative Medicine,” 2018, pp. 255–281. [Google Scholar]
- [89].Jeong H-J, Nam H, Jang J, and Lee S-J, “3D Bioprinting Strategies for the Regeneration of Functional Tubular Tissues and Organs.,” Bioeng. (Basel, Switzerland), vol. 7, no. 2, Mar. 2020, doi: 10.3390/bioengineering7020032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wu Y et al. , “Hybrid Bioprinting of Zonally-stratified Human Articular Cartilage Using Scaffold-free Tissue Strands as Building Blocks, Adv. Healthc. Mater, vol. 9, no. 22, 2001657, doi: 10.1002/adhm.202001657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ayan B et al. , “Aspiration-assisted Bioprinting for Precise Positioning of Biologics” Sci. Adv, vol. 6, no. 10, eaaw5111, 2020, doi: 10.1126/sciadv.aaw5111. [DOI] [PMC free article] [PubMed] [Google Scholar]