Abstract
Several filamentous fungi are ecologically and economically important plant pathogens that infect a broad variety of crops. They cause high annual yield losses and contaminate seeds and fruits with mycotoxins. Not only powerful infection structures and detrimental toxins, but also cell organelles, such as peroxisomes, play important roles in plant infection. In this review, we summarize recent research results that revealed novel peroxisomal functions of filamentous fungi and highlight the importance of peroxisomes for infection of host plants. Central for fungal virulence are two primary metabolic pathways, fatty acid β‐oxidation and the glyoxylate cycle, both of which are required to produce energy, acetyl‐CoA, and carbohydrates. These are ultimately needed for the synthesis of cell wall polymers and for turgor generation in infection structures. Most novel results stem from different routes of secondary metabolism and demonstrate that peroxisomes produce important precursors and house various enzymes needed for toxin production and melanization of appressoria. All these peroxisomal functions in fungal virulence might represent elegant targets for improved crop protection.
Keywords: filamentous fungi, melanin, mycotoxins, peroxisomes, primary and secondary metabolism, virulence
Essential for virulence of phytopathogenic fungi are peroxisomes and their metabolic pathways of both primary and secondary metabolism.
1. INTRODUCTION
Filamentous fungi perform a multitude of important functions in diverse ecosystems, from organic matter decomposition to facilitating the uptake of nutrients by plant roots (Grossart et al., 2019; Lindahl et al., 2007). Filamentous fungi are characterized by their typical tubular structures called hyphae that extend into a fine network, the mycelium, and often live in symbiotic or parasitic relationships with animals and plants. Hyphae further differentiate into specialized structures such as asexual spores (conidia), fruiting bodies, sexual spores, and structures required to infect animal and plant hosts.
Filamentous plant‐pathogenic fungi comprise a large polyphyletic group and primarily belong to the divisions of ascomycetes and basidiomycetes. Together with other pathogens, fungi cause severe yield losses every year, estimated to 21% and 30% of the global wheat and rice production, respectively (Savary et al., 2019). Moreover, several fungi contaminate edible seeds and fruits with minute concentrations of mycotoxins (Puntscher et al., 2019; Streit et al., 2013). Therefore, plant‐pathogenic fungi constitute a highly relevant factor for food security and supply.
The ability to infect a host is called pathogenicity. Filamentous fungi employ diverse infection strategies with different types of morphological and metabolic adaptations, depending on the site of penetration, on the infected host plant organ, and on their nutritional lifestyle (necro‐, hemibio‐, or biotrophic). Upon host penetration, fungi exploit nutrients either by killing host cells (necrotrophic lifestyle) or by establishing a long‐term feeding relationship with the host (biotrophic lifestyle). Fungal conidia are wind‐ or water‐dispersed and germinate on plant surfaces (leaves, fruits) by forming a germ tube (Figure 1). Hyphal penetration of the plant surface can either occur directly through the strong physical barriers formed by the cuticle and cell wall, or the fungus can enter the plant via natural openings, such as stomata and wounds. Some fungi, such as species of Magnaporthe, Colletotrichum, and Phakopsora, form a special dome‐shaped infection structure called the appressorium at the tip of the germ tube (Figure 1). To be able to penetrate the cuticle and cell wall, these fungi generate strong turgor pressure in the cytosol of the appressorium and form a penetration peg or penetration hypha. Once inside the host cell, the apex of the penetration peg then enlarges to form a haustorium or an analogous feeding structure (e.g., bulbous and branched intracellular invasive hyphae) within an epidermal cell to absorb nutrients from the host (Figure 1). Fungal virulence factors are essential for plant infection and fungal growth and proliferation. According to the classical definition, they are proteins or metabolites, both of which are generally secreted, into either the plant apoplast or the cytoplasm. The molecular functions of biomolecules as virulence factors are (a) to kill the host (necrotrophic lifestyle), (b) to suppress the plant immune system (biotrophic lifestyle), or (c) to evade plant defence mechanisms. Examples of fungal virulence factors include cell wall‐degrading enzymes, effector proteins, and toxins (Figure 1; Pontes et al., 2020). Small RNAs can also be exchanged between fungi and the plant host to modulate the gene expression of the other interaction partner (Figure 1; Cai et al., 2020). According to a newer broader definition, the spectrum of virulence factors can be extended to whole organelles, such as mitochondria and peroxisomes. The role of the latter organelle in fungal pathogenicity is discussed below.
FIGURE 1.

Basic concept of the early stage of plant infection by an appressorium‐forming filamentous fungus. To penetrate the plant cuticle and cell wall, a germinating conidium forms an appressorium that has a thick melanin‐enriched cell wall to withstand the high turgor pressure generated by glycerol. By the development of a haustorium or a related feeding structure, a close contact site between both organisms is formed that facilitates the secretion of fungal effectors into the plant cytosol and the capture of plant nutrients by the fungus in the biotrophic stage of its life cycle. An invasive hypha mediates further colonization of the plant.
Peroxisomes are small and versatile organelles surrounded by a single membrane that are found in most eukaryotes. A variety of metabolic processes are compartmentalized in peroxisomes such as fatty acid β‐oxidation, the glyoxylate cycle, and the metabolism of reactive oxygen species (ROS; Maruyama & Kitamoto, 2013; Reumann & Bartel, 2016). In fungal development, peroxisomes are involved in meiosis and in the maturation and germination of sexual spores (Navarro‐Espíndola et al., 2020). All peroxisomal proteins are nuclear‐encoded and are synthesized on free cytosolic ribosomes with specific signals that direct them either to the peroxisomal matrix or to the membrane. Matrix proteins are directed posttranslationally to their destination by one of two major peroxisome targeting signals (PTSs). Most possess a PTS1, which consists of a C‐terminal tripeptide, such as SKL, combined with auxiliary upstream residues (Brocard & Hartig, 2006; Gould et al., 1989). Few matrix proteins, such as 3‐ketoacyl thiolase, are synthesized with a conserved nonapeptide in the N‐terminal domain, the PTS2 (e.g., RLx5HL, Kunze, 2020; Swinkels et al., 1991). Peroxisome biogenesis is mediated by 37 peroxins (PEX proteins) described to date (Distel et al., 1996; Jansen et al., 2021; Kiel et al., 2006).
PTS1 and PTS2 proteins are recognized in the cytosol by two receptors, PEX5 and PEX7, respectively (Figure 2, Table 1). A second PTS1 receptor, PEX9, has recently been identified in Saccharomyces cerevisiae and is an in‐paralog of PEX5 (Effelsberg et al., 2016; Walter & Erdmann, 2019). In fungi, PEX7 requires coreceptors of the PEX20 family (PEX18/20/21) for PTS2 protein import. The receptor docking site at the peroxisomal surface is formed by PEX13 and PEX14, assisted by PEX17 (or PEX33) in fungi (Jansen et al., 2021; Kiel et al., 2006). Together PEX5 and PEX14 build a transient pore for PTS1 protein import (Meinecke et al., 2010), while a distinct pore is formed by PEX14, PEX17, and PEX18 for PTS2 proteins (Montilla‐Martinez et al., 2015). After import, the PTS1/2 proteins are released into the peroxisomal lumen by largely unknown mechanisms, involving the cargo release factor PEX8 (Table 1). To allow for another round of cargo import, PEX5 is monoubiquitinated, extracted from the peroxisomal membrane, and recycled. In brief, this mechanism involves PEX4 as the ubiquitin‐conjugating (E2) enzyme, which is anchored in the membrane by PEX22, and three RING finger peroxins (PEX2, PEX10, and PEX12) that have ubiquitin (E3) ligase activity (Jansen et al., 2021; Kiel et al., 2006; Walter & Erdmann, 2019). Two AAA+ ATPases, PEX1 and PEX6, which are membrane‐associated by PEX15 or PEX26, form a heterohexameric complex that extracts PEX5 from the membrane in an ATP‐dependent manner (Ciniawsky et al., 2015). After de‐ubiquitination of PEX5 by a cytosolic enzyme that remains unknown, one cargo import cycle by PEX5 is completed and the receptor is ready for new rounds of PTS1 protein import. Peroxisomal membrane proteins are directed to their destination either directly or indirectly via the endoplasmic reticulum (ER). Three PEX proteins (PEX19 and PEX3, plus PEX16 in plants and animals) are major players in these pathways (Table 1).
FIGURE 2.
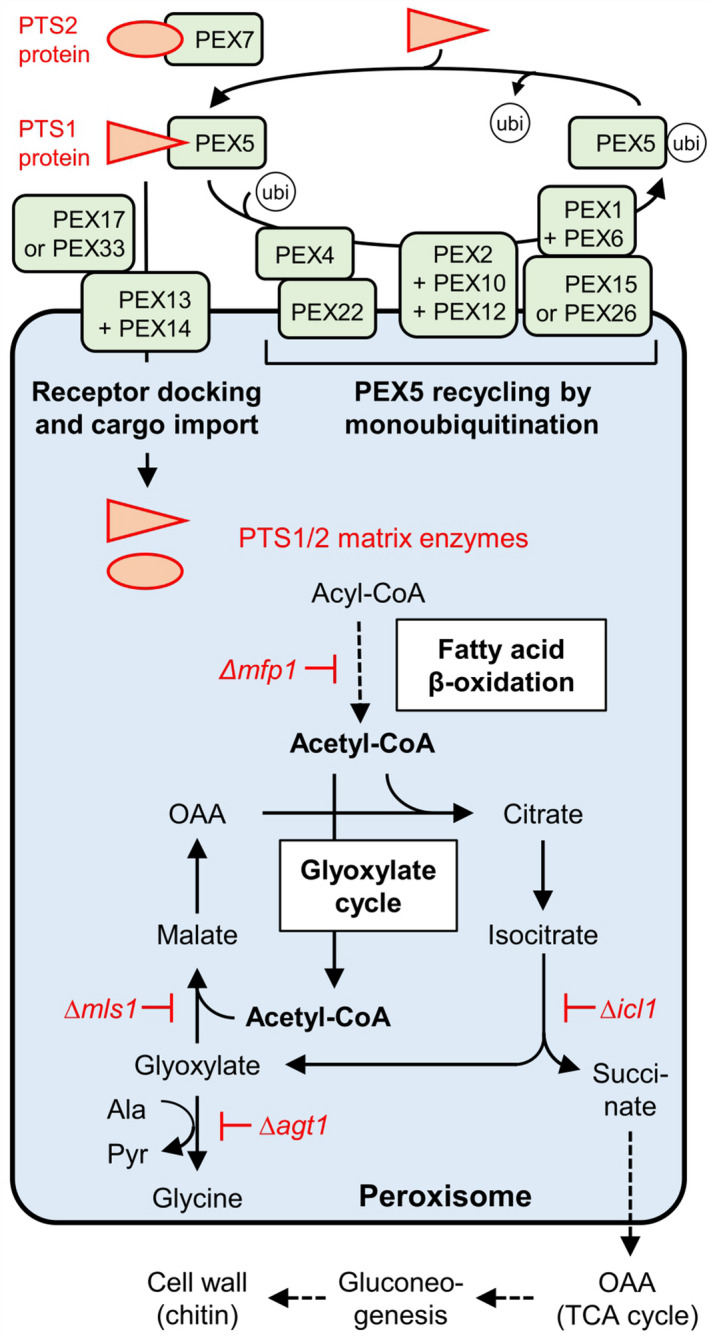
Peroxisomal import mechanism and matrix enzymes involved in fatty acid β‐oxidation and the glyoxylate cycle and their importance for the virulence of filamentous plant‐pathogenic fungi. Fungal deletion mutants altered in plant pathogenicity were investigated in different fungi (Δagt1, Magnaporthe oryzae; Δicl1, Colletotrichum lagenarium, Leptosphaeria maculans, Magnaporthe grisea, M. oryzae; Δmfp1, M. grisea; Δmls1, Sclerotinia sclerotiorum) and are indicated in red. Ala, alanine; OAA, oxaloacetate; Pyr, pyruvate; Ubi, ubiquitin
TABLE 1.
Overview of selected fungal peroxins (PEX proteins) important for peroxisome biogenesis and fungal virulence
| Name | Function | Description |
|---|---|---|
| PEX5, PEX9 | PTS1 receptors | PTS1 matrix protein binding and transport to peroxisomes; import pore formation (by PEX5) |
| PEX7, PEX18, PEX20, PEX21 | PTS2 receptor (PEX7) and PTS2 coreceptors (PEX18, 20, 21) | PTS2 matrix protein binding and transport to peroxisomes |
| PEX13, PEX14, PEX17, PEX33 | PEX5 docking and pore formation | Docking complex formation in the peroxisomal membrane; binding of the PEX5–cargo complex; import pore formation for PTS1 proteins (by PEX14 and PEX5) and PTS2 proteins (by PEX14, PEX17, PEX18) |
| PEX4, PEX22 | PEX5 recycling | Monoubiquitination of PEX5 by PEX4 acting as ubiquitin‐conjugating enzyme (E2); PEX4 is membrane‐anchored by PEX22 |
| PEX2, PEX10, PEX12 | PEX5 recycling | RING finger‐containing ubiquitin ligase (E3) complex for mono‐ubiquitination of PEX5 |
| PEX1, PEX6, PEX15, PEX26 | PEX5 recycling | Formation of a heterohexameric AAA+ ATPase complex by PEX1 and PEX6 that mediates ATP‐dependent extraction of PEX5 from the membrane; the complex is membrane‐anchored by PEX15 or PEX26 |
| PEX8 | PTS1 cargo release factor | Dissociation of PTS1 cargo from PEX5 in the matrix |
| PEX19, PEX3 | PMP import | Receptor for PMPs (PEX19), anchored by PEX3 in the peroxisomal membrane |
As revealed by the corresponding knockout mutants, PEX proteins in boldface have relevant protein functions in virulence in different fungal species.
In the past few years, peroxisomes of filamentous plant‐pathogenic fungi have been implicated in important novel functions in the infection and colonization of plants, and the first molecular mechanisms have been elucidated. For instance, the Woronin body is a specialized peroxisome variant uniquely found in filamentous ascomycetes and serves to seal septal pores upon physical damage. Dysfunctional Woronin bodies lead to severe morphological defects in infection structures in Magnaporthe grisea (Soundararajan et al., 2004). Woronin body biogenesis and infection‐related functions have been previously reviewed in detail (van der Klei & Beenhuis, 2013; Kubo, 2013; Maruyama & Kitamoto, 2019).
In this review, we summarize state‐of‐the‐art research results and recent progress in deciphering the role of peroxisomes in plant infection with a focus on primary and secondary metabolism. The classical and well‐known functions of peroxisomal fatty acid β‐oxidation and the glyoxylate cycle are lipid breakdown and the generation of substrates for gluconeogenesis. These pathways were newly found to be indirectly important or even essential for fungal virulence because they generate precursors for mycotoxin biosynthesis, melanization of infection structures, turgor pressure, and cell wall biosynthesis. Many molecular mechanisms and metabolic networks have been elucidated and are reviewed below.
2. PRIMARY METABOLISM: PEROXISOMAL FATTY ACID β‐OXIDATION, THE GLYOXYLATE CYCLE, AND ROS HOMEOSTASIS ARE CRUCIAL FOR FUNGAL VIRULENCE
2.1. Fatty acid β‐oxidation
In fungi, fatty acid β‐oxidation is exclusively located in peroxisomes (Figure 2; van den Bosch et al., 1992), and the pathway is crucial for conidial germination. On leaf surfaces, conidia lack access to nutrients and depend solely on endogenous energy mobilization. Triacylglycerides stored in lipid droplets need to deliver the energy and carbon skeletons required for tip growth of the germination tube and can be mobilized solely by peroxisomal β‐oxidation. Fatty acid‐derived energy is also required for the development of infection structures. The roles of lipid bodies and peroxisomal fatty acid β‐oxidation in appressorium formation have been elucidated by elegant studies in Magnaporthe spp. and Colletotrichum spp. In the rice blast fungus M. grisea, which represents a species complex that also includes M. oryzae, lipid droplets are translocated through septa from the conidial cells to the appressorium, to facilitate fatty acid breakdown and energy mobilization at the site of need (Thines et al., 2000). In the wild type, lipid droplets normally vanish during appressorium maturation due to the high rate of fatty acid degradation by peroxisomal β‐oxidation. In the Δpex6 mutant, appressoria were still formed but their lipid droplets persisted and were even enlarged (Wang et al., 2007). The same phenotype was also observed in the loss‐of‐function mutant of multifunctional protein 1 (MFP1), which catalyses the second and third steps of fatty acid β‐oxidation (Figure 2; Wang et al., 2007), and in the Δpex13 mutant of Colletotrichum orbiculare (Fujihara et al., 2010). C. orbiculare is the causal agent of anthracnose disease on cucumber and relies on appressoria for host penetration, similar to M. grisea. The likely explanation for this phenotype is the feedback inhibition of impaired fatty acid β‐oxidation on lipase activity (Figure 2). In transmission electron micrographs of developing appressoria of wild‐type C. orbiculare, peroxisomes are located in very close proximity to lipid bodies with intensive physical contact sites, further strengthening the organelle's coordinated functions in the mobilization of storage lipids. Growth of the Δpex13 mutant was strongly reduced when cultivated on media containing long‐chain fatty acids as the sole carbon source. Although the mutant still formed appressoria on cucumber cotyledons, it had lost its virulence, and both phenotypes could be complemented by PEX13 (Fujihara et al., 2010). Also, the Δmfp1 and Δpex13 mutants of M. grisea were strongly reduced in virulence, as indicated by a much lower size and number of lesions on rice plants inoculated with M. grisea, altogether implying that peroxisomal fatty acid β‐oxidation is crucial for appressorium functionality (Wang et al., 2007). Further progress was made when investigating the infectivity of M. grisea mutants independent of leaf penetration. Upon manual leaf wounding, some pex mutants still had reduced virulence compared with the wild‐type strains (Wang et al., 2019), indicating that peroxisomal fatty acid degradation is important for the infection process beyond appressorium functions and leaf penetration.
If peroxisomes had only a single virulence function in plant infection (i.e., in appressorium functionality), pex mutants should not be compromised in virulence in appressoria‐lacking pathogens. This question was addressed in recent comprehensive studies using Fusarium graminearum, the causal agent of the disease Fusarium head blight on wheat and barley. F. graminearum enters the leaves either through natural openings or by direct penetration through the cuticle. To multiply the number of direct penetration sites, several infection hyphae are merged to form infection cushions (Boenisch & Schäfer, 2011; Mary Wanjiru et al., 2002; Mentges et al., 2020; Pritsch et al., 2000). F. graminearum pex mutants have been instrumental in revealing the significance of peroxisomal functions for virulence. The mutants were defective either in a docking complex protein (Δpex13 or Δpex14), in the RING finger complex (Δpex2, ∆pex10, or Δpex12), or in the AAA+ ATPase (∆pex1), or they lacked another PEX protein required for matrix protein (∆pex4 or Δpex33) or membrane protein import (∆pex3). Several pex mutants exhibited reduced growth on agar plates supplemented with fatty acids of different chain lengths as the sole carbon source, confirming that fatty acid β‐oxidation is impaired in the mutants (Chen et al., 2018; Kong et al., 2019; Wang et al., 2020; Zhang, Liu, et al., 2019; Zhang, Wang, et al., 2019). Interestingly, in this appressoria‐lacking pathogen, all pex mutants were reduced in virulence, as demonstrated by weaker disease symptoms on wheat, maize, Brachypodium, and tomato (Figure 2; Chen et al., 2018; Kong et al., 2019; Wang et al., 2020; Zhang, Liu, et al., 2019; Zhang, Wang, et al., 2019). Fungal virulence is most probably reduced due to dysfunctional infection hyphae caused by inefficient degradation of fatty acids not only from endogenous lipid droplets but also from exogenous sources (liberated from the host plants). The precise connection between peroxisomal functions and virulence in appressoria‐lacking pathogens remains to be deciphered.
Taken together, plant‐pathogenic fungi require high activities of peroxisomal fatty acid catabolism for full functionality of appressoria and infection hyphae. The pathway provides energy and acetyl‐CoA for subsequent pathways. As described below, not only acetyl‐CoA metabolization by the glyoxylate cycle but also its processing pathways for the biosynthesis of mycotoxins, melanin, and glycerol are tightly connected to fungal pathogenicity.
2.2. The glyoxylate cycle
The glyoxylate cycle is closely connected to fatty acid β‐oxidation both metabolically and functionally because it is also compartmentalized in peroxisomes and because acetyl‐CoA is removed by reacting with oxaloacetate and glyoxylate to citrate and malate, respectively. The cycle product succinate (C4), generated by the key enzyme isocitrate lyase (ICL), is further converted to carbohydrates and cell wall polymers by the mitochondrial tricarboxylic acid (TCA) cycle and cytosolic gluconeogenesis (Figure 2).
The unexpected role of the glyoxylate cycle in fungal virulence in a plant pathosystem was first investigated for the fungus Leptosphaeria maculans on Brassica napus (Idnurm & Howlett, 2002). The icl1 insertion mutant of L. maculans showed reduced virulence on Brassica leaves and is blocked in fatty acid catabolism (Figure 2). Growth of the mutant was completely inhibited on medium supplemented with Tween 20 (a C12 fatty acid) as the sole carbon source (Idnurm & Howlett, 2002), indicating that glyoxylate activity is a prerequisite for fatty acid catabolism. In M. grisea, ICL1 expression was up‐regulated during the development of infection structures (Wang et al., 2003). The ∆icl1 mutant was less virulent than the wild type, which correlated with compromised appressorium formation and reduced leaf penetration ability. Acetyl‐CoA accumulation in the icl1 mutant most probably causes feedback inhibition of fatty acid catabolism. Hence, also for M. grisea the glyoxylate cycle is important for virulence and successful leaf infection (Figure 2).
Likewise, the icl1 knockout mutant of Colletotrichum lagenarium was less virulent on cucumber leaves than the wild type, but interestingly it was still able to cause macroscopic lesions (Asakura et al., 2006). On wounded leaves, the mutant was able to grow as invasively as the wild type. This result further strengthened the conclusion that high glyoxylate cycle activity is important for fatty acid catabolism during early appressorium maturation and prior to plant colonization (Wang et al., 2003). The addition of glucose and sucrose partially restored the virulence of the C. lagenarium ∆icl1 mutant, consistent with the need of succinate for gluconeogenesis and for fungal virulence (Asakura et al., 2006). Additionally, glucose supplementation probably fulfils the energy demand of the fungus due to the impaired primary metabolic pathways. In contrast, the ∆icl mutant of F. graminearum maintained full virulence on wheat spikes, consistent with the major role of ICL in appressoria formation and their absence in this fungus (Lee et al., 2009).
Sclerotinia sclerotiorum is another appressorium‐forming pathogen with a broad host spectrum that includes soybean and tomato. The knockout of malate synthase (MLS1) in S. sclerotiorum led to reduced virulence of the mutant on tomato if no additional carbon source was supplied to the fungus on the leaf surface (Liberti et al., 2007). Only after the addition of glucose (and not by oleic acid alone) to the leaf surface was virulence restored, further supporting the important function of the glyoxylate cycle in appressorium formation and fungal virulence (Liberti et al., 2007).
Peroxisomal alanine glyoxylate aminotransferase (AGT1) transaminates glyoxylate with the amino group of alanine, yielding pyruvate and glycine, and the enzyme is thereby closely associated with the glyoxylate cycle. When the gene was deleted in M. oryzae, the mutant showed aberrant appressorium formation and reduced virulence on rice and barley reminiscent of a glyoxylate cycle defect (Bhadauria et al., 2012). Pyruvate can be further reduced to glycerol, lactate, or ethanol by NADH (produced by fatty acid β‐oxidation), thereby generating NAD+ to maintain redox homeostasis in peroxisomes for continuous fatty acid β‐oxidation and lipid mobilization. In the agt1 mutant of M. oryzae, pyruvate synthesis is blocked, which results in feedback inhibition of β‐oxidation and the glyoxylate cycle, as the reoxidation of NADH is impaired (Bhadauria et al., 2012). Hence, due to its relationship with the glyoxylate cycle, the peroxisomal aminotransferase AGT1 is also important for fungal virulence.
Taken together, the glyoxylate cycle is a very important primary metabolic pathway of peroxisomes that is required for successful infection of the host and full virulence of plant‐pathogenic fungi. Beyond the direct metabolic functions of the glyoxylate cycle discussed above, the pathway provides carbohydrates for subsequent pathways connected to virulence, for instance to synthesize cell wall components and glycerol in appressoria, as presented below.
2.3. Peroxisomal functions required for cell wall integrity
The cell wall of plant‐pathogenic fungi is crucial not only for morphogenesis and growth but also for infection and virulence. Successful penetration fundamentally depends on a rigid cell wall (Geoghegan et al., 2017). The fungal cell wall contains the polysaccharides β‐(1,3)‐ and β‐(1,6)‐glucan and chitin as the main components and can dynamically adapt to environmental requirements by changing its form and composition (Geoghegan et al., 2017).
Peroxisomal defects lead to an altered cell wall composition and reduced virulence of plant‐pathogenic fungi. Peroxisomal PTH2 is a carnitine acyltransferase in M. grisea; the Δpth2 mutant exhibited cell wall defects, appressoria with lost penetration ability, and overall reduced virulence (Bhambra et al., 2006; Ramos‐Pamplona & Naqvi, 2006). Knockout of PTH2 in S. sclerotiorum also resulted in reduced virulence (Liberti et al., 2013). In M. grisea the cell wall was reduced in chitin content and sensitive to cell wall‐perturbing agents. The mutant also lacked invasive hyphae (Bhambra et al., 2006). A similar phenotype was observed for the Δpex6 mutant in the same study. The reason the appressoria lost their penetration functionality was attributed to the weakened cell wall (Ramos‐Pamplona & Naqvi, 2006). Pex mutants in general show defects in cell wall synthesis due to their inability to import peroxisomal enzymes required for β‐oxidation and the glyoxylate cycle, and cannot synthesize succinate for gluconeogenesis as a prerequisite for cell wall biosynthesis (see above, Figure 2). The cell wall of the ∆pex13 mutant of C. orbiculare is much thinner and contains fewer layers in appressoria than the wild type (Fujihara et al., 2010). The conidia and hyphae of several F. graminearum mutants (∆pex1, ∆pex2, ∆pex4, ∆pex10, and ∆pex12) were prone to rupture and the cell walls were more sensitive to the cell wall‐perturbing agent Congo red and to cell wall‐degrading enzymes compared with the wild type, all indicating a reduction in cell wall integrity (Wang et al., 2020; Zhang, Liu, et al., 2019; Zhang, Wang, et al., 2019). Similarly, calcofluor white, another cell wall‐perturbing agent, negatively affected the growth of the Δpex6 mutant of the tangerine pathotype of Alternaria alternata and its cell wall was also thinner than that of the wild type (Wu et al., 2020). Likewise, the M. oryzae mutant Δpex19, which is compromised in membrane protein import, was more sensitive to Congo red and showed reduced growth (Li et al., 2014).
2.4. Peroxisomal impact on turgor generation and morphology of appressoria
Appressoria of Magnaporthe spp. and Colletotrichum spp. are equipped with a particularly strong chitin‐enriched cell wall supplemented with a pronounced melanin layer, both of which are important for appressorial turgor generation (de Jong et al., 1997). Glycerol is the main osmolyte for turgor generation. Appressorial glycerol production requires lipid mobilization from the conidia, peroxisomal fatty acid β‐oxidation, and the glyoxylate cycle (Thines et al., 2000). In detail, glycerol can be produced (a) directly by hydrolysis of triacylglycerol by lipases, (b) from the glycolytic intermediate dihydroxyacetone phosphate, or from the final product pyruvate, and (c) from pyruvate synthesized by the abovementioned peroxisomal AGT (Figure 2; Foster et al., 2017).
An additional peroxisomal pathway has been proposed, in which the carbon atoms of acetyl‐CoA are channelled via the glyoxylate cycle into gluconeogenesis to generate glycerol (Thines et al., 2000). To address whether peroxisomal activities are crucial for sufficient glycerol accumulation and turgor generation in appressoria for successful fungal virulence, studies have focused on Magnaporthe spp. and Colletotrichum spp. To determine the turgor pressure in appressoria, the method of choice is incipient cytorrhysis, in which appressoria are exposed to solutions of different osmolarities. The osmotic potential of the solution in which the number of cell collapses remains zero is then equal to the appressorial turgor pressure. In the Δicl1 mutants of M. grisea, turgor generation was delayed compared with the wild type, as indicated by a higher number of cell collapses at a certain osmolarity (Wang et al., 2003). More dramatic turgor reductions were observed in pex mutants of M. oryzae (Δpex5, Δpex6, ∆pex7, ∆pex13, ∆pex14, Δpex14/17, and Δpex19), M. grisea Δpex6, and C. orbiculare Δpex13 (Fujihara et al., 2010; Goh et al., 2011; Li et al., 2014; Ramos‐Pamplona & Naqvi, 2006; Wang et al., 2007, 2013). For example, in the nonvirulent Δpex19 mutant of M. oryzae, reduced glycerol synthesis and increased glycerol leakage were detected, correlating with decreased melanization of appressoria and reduced cell wall integrity, caused by impaired fatty acid β‐oxidation and glyoxylate cycle activity (Li et al., 2014). In comparison to the nonvirulent mutants of Δpex19, Δpex5, Δpex6, and ∆pex7, only Δpex14/17 had a relatively mild, persistent virulence. While Δpex14/17 only lacked appressorial turgor but was fully melanized (see above), other pex mutants additionally lacked appressorial melanization. In contrast to delayed turgor generation in M. oryzae Δicl, no decrease in appressorial turgor was observed in the M. grisea Δmfp1 mutant, arguing against an interdependence of both pathways in this species (Wang et al., 2007). Hence, peroxisomes are involved in cell wall biosynthesis, melanization, and turgor generation of appressoria, and are indispensable for the virulence of appressoria‐forming fungi.
2.5. Peroxisome‐mediated scavenging of host‐derived ROS
ROS fulfil several functions in fungi; for instance, they are involved in signalling pathways during development and in responses to the environment. Due to their very high reactivity, ROS levels need to be tightly controlled in fungi to prevent cell death. In pathogen–plant interactions, ROS generation and scavenging play important roles on both sides (reviewed by Segal & Wilson, 2018). On the host side, the so‐called oxidative burst is initiated upon infection and is generally characterized by localized ROS accumulation, which is harmful to both infection partners and is central to the hypersensitive response. Hence, successful scavenging of plant‐derived ROS by pathogenic fungi is essential for survival and virulence (reviewed by Lehmann et al., 2015).
Independent of host infection, pex mutants of F. graminearum and M. oryzae accumulated higher cellular H2O2 levels, and many mutants exhibited reduced growth on H2O2 or other oxidative media compared with the wild type (Chen et al., 2018; Kong et al., 2019; Li et al., 2017; Wang et al., 2019; Zhang, Liu, et al., 2019; Zhang, Wang, et al., 2019). The studies documented that the mutants had lost the ability to control their endogenous ROS balance and that they were more sensitive to oxidative stress. When investigating whether ROS scavenging was accomplished primarily by fungal peroxisomes rather than the cytosol or mitochondria, the less virulent F. graminearum mutants (Δpex13, Δpex14, and Δpex33) were indeed more sensitive to ROS generated by the oxidative burst in planta (Chen et al., 2018). The main ROS‐scavenging enzymes, catalase and peroxidases (e.g., glutathione peroxidase), are PTS1 proteins, leading to reduced peroxisomal scavenging activities in Δpex mutants and increased ROS susceptibility (Wang et al., 2015). Barley infected by the Δpex14/17 mutant of M. oryzae showed a similar phenotype. The mutant did not scavenge ROS as effectively as the wild type, as indicated by reduced lesion formation (Li et al., 2017). When ROS production by the host was temporarily impaired by heat treatment, however, virulence of the Δpex13 and Δpex14 mutants on barley could be partially restored (Wang et al., 2019). These studies demonstrate a leading role of fungal peroxisomes in scavenging host ROS. Thus, the detoxification of host‐derived ROS adds another element to the list of peroxisomal functions that contribute to fungal virulence.
3. SECONDARY METABOLISM: DISTINCT ROLES OF PEROXISOMES AND THEIR CONTRIBUTIONS TO VIRULENCE
Plant‐pathogenic fungi produce a large variety of secondary metabolites that often confer ecological advantages to the fungus and are critical for plant colonization and infection but are not essential for survival. These secondary metabolites include mycotoxins and pigments (e.g., melanin; Keller et al., 2005). Rather unexpectedly, peroxisomes play essential roles in several of the underlying biosynthetic pathways.
3.1. Peroxisomes are essential for mycotoxin biosynthesis
Mycotoxins are secondary metabolites that are produced by crop‐colonizing fungi and are toxic to animals and humans (Turner et al., 2009). Mycotoxins are of major economic relevance not only because they contaminate and poison crops used as human food and animal feed but also because they act as important virulence factors in plant–fungi interactions. Fungi synthesize mycotoxins that show activity against a broad range of plant hosts, a single specific host, or a very narrow host range (Hof, 2008). Plant‐pathogenic fungi use mycotoxins to alter plant metabolism to their advantage at different nodes (Pusztahelyi et al., 2015). Some of the most important mycotoxins include four structurally unrelated groups, namely (a) trichothecenes (TRI), (b) epoxy‐decatrienoic esters, (c) aflatoxins, and (d) fumonisins. In recent years, a role of peroxisomes in toxin biosynthesis has been revealed for the first two groups. Fusarium and Alternaria species are the best‐studied model organisms to decipher the underlying peroxisomal biosynthesis pathways.
3.1.1. The mycotoxin deoxynivalenol of the group of trichothecenes from F. graminearum
Trichothecenes are triterpenes of three nonaromatic C6 rings synthesized from six isoprene units. Due to their relatively small size and amphipathic nature, they can diffuse passively across membranes (Proctor et al., 2002). The group comprises over 200 mycotoxins that often form a C‐12, C‐13 epoxide (Figure 3a; McCormick et al., 2011). The predominant mycotoxin of cereal grains worldwide and the agronomically most important triterpene is deoxynivalenol (DON), also referred to as vomitoxin (Audenaert et al., 2014). DON is a virulence factor that is synthesized by F. graminearum for plant infection and colonization (Boddu et al., 2007). Fusarium head blight is recognizable by premature bleaching of spikelets and is responsible for severe yield losses worldwide. Grains contaminated with minute amounts of DON are toxic to humans and animals. DON inhibits protein elongation and termination during translation (Perincherry et al., 2019). Upon plant infection, DON biosynthesis is induced in F. graminearum (Goswami & Kistler, 2005) and is accompanied by a proliferation of peroxisomes (Chen et al., 2018). Biosynthesis starts in the cytosol, involving the so‐called toxisome, which is an ovoid ER‐derived organelle formed specifically for DON biosynthesis (Boenisch et al., 2017; Menke et al., 2013). Pathway intermediates are transported either by vesicular trafficking to the vacuole or by exocytosis into the apoplast for DON release (Chen et al., 2019). This compartmentalization, particularly of the last steps of DON biosynthesis, prevents inhibition of the organism’s own protein biosynthesis.
FIGURE 3.
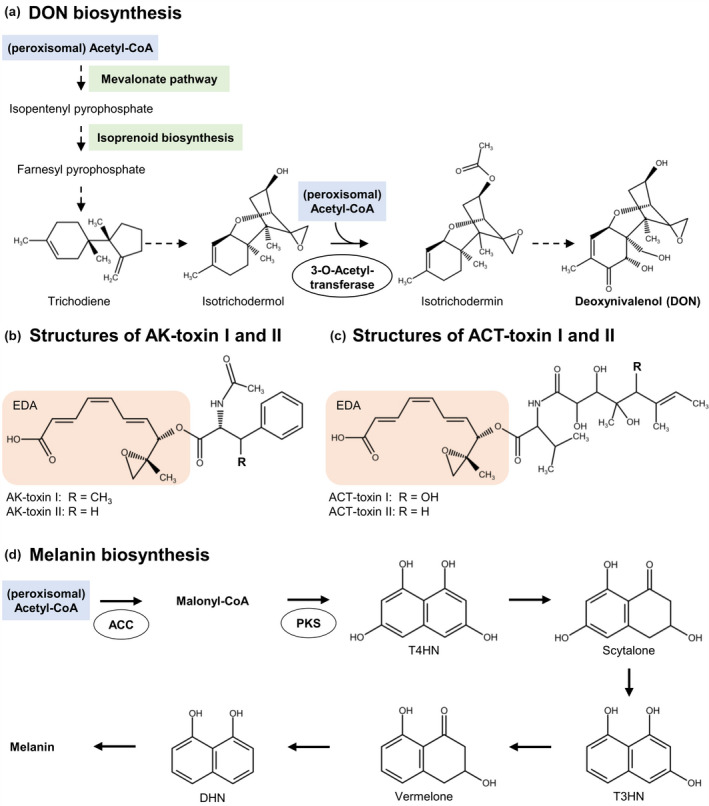
Peroxisomal functions in the biosynthesis of mycotoxins and melanin. (a) Biosynthesis of the mycotoxin deoxynivalenol (DON, [(3α,7α)‐3,7,15‐trihydroxy‐12,13‐epoxytrichothec‐9‐en‐8‐one]) from Fusarium graminearum requires peroxisomal acetyl‐CoA at two important steps. (b) The structures of relevant AK toxins of Alternaria species are shown. AK toxin I ((2E,4Z,6E)‐8‐(2‐acetamido‐3‐phenylbutanoyl)oxy‐8‐(2‐methyloxiran‐2‐yl)octa‐2,4,6‐trienoic acid) is very similar to AK toxin II. (c) ACT toxins I and II are AK toxin derivatives. The orange boxes indicate the common moiety of 9,10‐epoxy‐8‐hydroxy‐9‐methyl‐decatrienoic acid (EDA). (d) DHN‐melanin is synthesized by the polyketide pathway in filamentous fungi. ACC, acetyl‐CoA carboxylase; DHN, 1,8‐dihydroxynaphthalene; PKS, polyketide synthase; T3HN, 1,3,8‐trihydroxynaphthalene; T4HN, 1,3,6,8‐tetrahydroxynaphthalene
DON biosynthesis from farnesyl pyrophosphate involves 15 trichothecene biosynthesis (TRI) genes encoding 10 biosynthetic enzymes, one DON transporter localized in the plasma membrane, two regulatory proteins, and two proteins of unknown function (Figure 3a; Zheng et al., 2017). Although none of these DON enzymes are localized in peroxisomes, an unexpected function of this organelle in DON biosynthesis was first revealed when analysing a peroxisomal carnitine acetyltransferase (CAT) mutant of F. graminearum that was deficient in acetyl‐CoA export across the peroxisomal membrane (Son et al., 2012). The enzyme converts acetyl‐CoA to acetyl‐carnitine for subsequent export to the cytoplasm (Elgersma et al., 1995; van Roermund et al., 1995). Surprisingly, the cat1 mutant was considerably reduced in DON synthesis and was less virulent on wheat (Son et al., 2012), suggesting that peroxisomal acetyl‐CoA is required for DON biosynthesis. This hypothesis was further supported when F. graminearum mutants lacking PEX proteins of the docking machinery (PEX13 and PEX14) or PEX33 not only formed dysfunctional peroxisomes but also had reduced DON levels and virulence compared with the wild type (Chen et al., 2018). In the cat1 and pex mutants, reduced DON biosynthesis correlated with lower concentrations of cellular acetyl‐CoA, suggesting that reduced acetyl‐CoA export across the peroxisomal membrane (in cat1) and/or reduced acetyl‐CoA production by fatty acid β‐oxidation (in the pex mutants) lowers DON biosynthesis. In the same three pex mutants, expression of important TRI genes for DON biosynthesis was also significantly decreased (Chen et al., 2018). This second, more indirect, function of peroxisomes in regulating the expression of DON biosynthesis genes needs to be unravelled in future studies.
Similar results showing reduced DON production and virulence were obtained for other pex mutants of F. graminearum that were compromised in matrix protein import. For instance, three F. graminearum pex mutants that were impaired in PEX5 recycling (∆pex1, ∆pex4, and ∆pex10) produced significantly less DON, showed lower TRI gene expression levels, and (for ∆pex1 and ∆pex10) had reduced acetyl‐CoA concentrations compared with the wild type (Zhang, Liu, et al., 2019; Zhang, Wang, et al., 2019). Likewise, the F. graminearum Δpex2 and Δpex12 mutants also showed down‐regulation of selected TRI genes and reduced DON production compared with the wild type (Wang et al., 2020). Detailed studies of the mevalonate pathway and DON biosynthesis demonstrated that peroxisomal acetyl‐CoA is required at two steps. First, acetyl‐CoA represents the precursor for isoprenoid biosynthesis via the mevalonate pathway (Vranová et al., 2012). Second, acetyl‐CoA is needed for acetylation of the first tricyclic pathway intermediate, isotrichodermol, a reaction catalysed by 3‐O‐acetyl transferase (Figure 3a, Desjardins et al., 1993; Kimura et al., 1998).
All the abovementioned fungal pex mutants with dysfunctional peroxisomes and impaired DON synthesis had strongly reduced virulence. This finding is consistent with previous results demonstrating that the disruption of DON biosynthesis reduced wheat head infection and the earlier conclusion that DON is an important virulence factor in wheat pathogenicity (Boddu et al., 2007). F. graminearum virulence was even more reduced in pex mutants than in DON mutants (Chen et al., 2018), due to impaired fatty acid β‐oxidation (see Section 1.1). Taken together, acetyl‐CoA produced by peroxisomal fatty acid β‐oxidation is required for isoprenoid biosynthesis and for acetylation of isotrichodermol, the precursor of DON biosynthesis.
DON biosynthesis is induced by independent mitogen‐activated protein kinase (MAPK) cascades, including the high‐osmolarity glycerol (HOG) pathway, which is triggered by osmotic and oxidative stress (Chen et al., 2019). Theoretically, host‐derived, secreted ROS, which are primarily meant to combat plant‐pathogenic fungi (see above), might simultaneously stimulate DON biosynthesis via the same signal transduction pathway (Ponts, 2015). In this scenario, the scavenging function of fungal peroxisomes in destroying host‐derived ROS would reduce their own DON production and virulence. This speculative negative function of peroxisomes apparently contradicts the organelle's predominantly positive regulatory functions in the detoxification of host‐derived ROS and in acetyl‐CoA provision for DON biosynthesis. More detailed future studies are needed to decipher whether natural concentrations of host‐derived ROS indeed trigger fungal DON biosynthesis to enhance host cell death, that is, also in natural plant–pathogen interactions, and whether the rather small compartment of fungal peroxisomes is indeed capable of reducing host‐derived ROS levels below the concentration required to trigger DON biosynthesis.
3.1.2. AK toxin of A. alternata
A. alternata is the causal agent of severe necrotic diseases and has a very broad host range, including pear, strawberry, tomato, and citrus fruits. A. alternata produces a variety of host‐specific toxins to kill crops. During the infection of pears and when causing the black spot disease on the fruits, A. alternata synthesizes two so‐called AK toxins (I and II), which are epoxy derivatives of an unsaturated medium‐chain fatty acid (decatrienoic acid, Figure 3b). Both toxins kill host plants at nanomolar concentrations (Otani et al., 1985). The mode of AK toxin action in plants is not fully understood, but epoxy fatty acids are known to irreversibly depolarize the host plasma membrane (Park & Ikeda, 2008; Tsuge et al., 2013). The biosynthetic pathway of AK toxin has not been fully deciphered to date, but four clustered genes (AKT1, AKT2, AKT3, and AKTR) have been linked with AK toxin production. Initially, AKT1 and AKT2 were identified by restriction enzyme‐mediated integration mutagenesis, and AKT3 and AKTR were soon later identified by analysing adjacent downstream genome sequences. AKT3 and AKTR are clustered in multiple copies in the genome. When specific gene copies were deleted, the mutants were unable to produce AK toxin and its precursor, epoxy‐decatrienoic acid (EDA, 9,10‐epoxy‐8‐hydroxy‐9‐methyl‐decatrienoic acid), and lost their virulence (Figure 3b; Tanaka et al., 1999; Tanaka & Tsuge, 2000). Surprisingly, three of the encoded proteins were found to carry a PTS1 (AKT1, SKI>; AKT2, SKL>; AKT3, PKL>) and the respective N‐terminal green fluorescent protein (GFP) fusions were indeed localized in A. alternata peroxisomes (Imazaki et al., 2010; Tanaka & Tsuge, 2000). Similar to the AKT mutants, the Δpex6 mutant had lost its ability to produce the precursor EDA and was less infectious on pear, further demonstrating that PTS1 protein import into peroxisomes is required for AK toxin biosynthesis and for full virulence of A. alternata on pear (Imazaki et al., 2010).
The precise enzymatic activities of the peroxisomal enzymes AKT1–3 are still unknown; however, sequence homology allowed some predictions. AKT1 belongs to the group of acyl‐CoA ligases that activate straight‐chain fatty acids prior to β‐oxidation. AKT2 is a hydrolase and AKT3 an enoyl‐CoA hydratase. Interestingly, orthologs of the AKT genes were also identified in the strawberry pathotype of A. alternata that produces the AF toxin and in the tangerine pathotype that synthesizes the ACT toxin (see below). These findings were fully consistent with the enzymatic functions in the synthesis of the same EDA precursor and backbone of these structurally similar toxins.
3.1.3. ACT toxin of A. alternata
The tangerine pathotype of A. alternata synthesizes the ACT toxin, which is toxic to tangerine and grapefruit plants (Akimitsu et al., 2003) and represents an essential virulence factor (Miyamoto et al., 2008). ACT and AK toxins share the same EDA moiety (Figure 3b) and both disrupt the host membrane and cause electrolyte leakage (Kohmoto et al., 1993). Very recently, ACT toxin biosynthesis was linked to peroxisomes based on infection studies using the A. alternata ∆pex6 mutant of the tangerine pathotype. After treating leaves from the citrus hybrid calamondin with cell‐free extracts of this pathovar, the lesion sizes were significantly smaller and necrosis was hardly detectable for the ∆pex6 mutant compared with the wild type and a complementation strain. High‐performance liquid chromatography revealed a significant reduction in the ACT toxin concentration (by 40%) in the mutant (Wu et al., 2020). The addition of purified ACT toxin to A. alternata on calamondin leaves enabled the ∆pex6 mutant to partially restore lesion formation, demonstrating that the mutant was compromised in ACT toxin biosynthesis and that peroxisomes played an important role in this pathway. Three predicted peroxisomal orthologs of enzymes mediating EDA production for AK toxin biosynthesis were detected in the genome, including the abovementioned AKT1, a hydrolase (ACTT2, SKL>, an AKT2 ortholog), and an enoyl‐CoA hydrolase (ACTT3, PKL>, an AKT3 ortholog; Wu et al., 2020).
3.2. Peroxisomal functions required for melanin biosynthesis
Melanins are a group of natural pigments that are found in most organisms, including humans, fungi, and plants. In fungi, melanin is generally produced intracellularly and subsequently incorporated into the cell wall for protection against ionizing UV radiation and desiccation (reviewed by Cordero & Casadevall, 2017). A major function of melanin in plant‐pathogenic fungi is the enhancement of their infection ability. To succeed in leaf penetration, a very high osmotic pressure needs to be built up in appressoria for penetration hyphae to overcome the plant cell wall (Howard et al., 1991; de Jong et al., 1997). This turgor is sustained by cell wall reinforcement and melanization. Even in fungi that do not form appressoria, cell wall melanization represents an important virulence factor. This process was demonstrated in fungi impaired in melanin biosynthesis, because they were frequently compromised in their infection ability (Kawamura et al., 1997; Steiner & Oerke, 2007; Zhang et al., 2017).
The exact chemical structure of melanin is still unknown. Melanin is produced by three alternative biochemical pathways, referred to as melanogenesis, namely the biosynthesis of 1,8‐dihydroxynaphthalene (DHN)‐melanin, l‐3,4‐dihydroxyphenylalanine (DOPA)‐melanin, and glutaminyl‐4‐hydroxybenzene (GHB)‐melanin. DHN is a product of the polyketide synthase pathway starting with malonyl‐CoA, which is derived from carboxylation of acetyl‐CoA by acetyl‐CoA carboxylase (ACC; Figure 3c). Polyketide synthase (PKS) converts malonyl‐CoA to a hydroxylated naphthalene derivative (1,3,6,8‐tetrahydroxynaphthalene [T4HN]) in the cytosol (Figure 3c, Fujii et al., 2000). This intermediate is further converted to the naphthalene derivative DHN carrying two hydroxyl groups, followed by polymerization of DHN. In ascomycetes, such as Magnaporthe spp., DHN‐melanin accumulates in conidia, hyphae, and appressoria (Belozerskaya et al., 2016).
A role of peroxisomal metabolism in melanin biosynthesis was initially not expected. In the Δpex6 mutant of C. lagenarium, which infects cucumber, melanin production was strongly reduced in appressoria compared with the wild type (Kimura et al., 2001). Similarly, several other pex deletion mutants from different fungi contained reduced amounts of melanin in infection structures or lacked the pigment completely. The Δpex13 mutant of C. orbiculare completely lacked melanin, as did the Δpks1 mutant of the same species. Both mutants were reduced in leaf penetration ability and were less virulent on cucumber leaves as compared with the wild type (Fujihara et al., 2010). External addition of scytalone to the pex13 mutant, an intermediate of melanin biosynthesis (downstream of the PKS1 reaction), indeed restored appressorial melanization. Nevertheless, because other peroxisomal functions are required for full penetration ability (β‐oxidation, glyoxylate cycle, and glycerol biosynthesis, see above), scytalone supplementation alone could not restore the mutant's penetration ability (Fujihara et al., 2010).
Similar to the C. orbiculare pex13 mutant, the appressoria of the M. grisea Δpex6 mutant exhibited a lower degree of melanization and showed reduced virulence on rice and barley leaves as compared with the wild type (Ramos‐Pamplona & Naqvi, 2006; Wang et al., 2007). Further peroxisome biogenesis mutants of M. oryzae (Δpex1, Δpex5, Δpex7, and Δpex19) on rice or barley also lacked melanin (Deng et al., 2016; Goh et al., 2011; Li et al., 2014; Wang et al., 2013) while the Δpex14/17 mutant was surprisingly not impaired in melanin biosynthesis (Li et al., 2017). The precise peroxisome function involved in melanin biosynthesis could be identified by deletion of peroxisomal MFP1 in M. grisea, which resulted in similarly reduced appressorial melanization, demonstrating that peroxisomal acetyl‐CoA is required for melanin biosynthesis and for plant infection. This finding was further supported by deletion of two CAT isoforms in M. grisea, which abolished acetyl‐CoA export from peroxisomes (see above). The appressoria of the double mutant were no longer melanized, and its pathogenicity was lost (Ramos‐Pamplona & Naqvi, 2006), demonstrating and confirming that peroxisome biogenesis and peroxisomal acetyl‐CoA are required for melanin biosynthesis and for melanin‐dependent virulence.
4. SUMMARY AND CONCLUSIONS
Fatty acid β‐oxidation and the glyoxylate cycle are classical functions of peroxisomes that were unexpectedly found to be crucial for fungal virulence because they provide energy, acetyl‐CoA, and succinate for carbohydrate synthesis, appressorium formation, and turgor generation (Figure 4). Moreover, peroxisomal acetyl‐CoA is an important precursor for diverse nonperoxisomal pathways, for instance, leading to melanization of fungal cell walls (Figure 4). Newly discovered peroxisome functions important for fungal virulence include the synthesis of mycotoxin precursors as part of secondary metabolism. In ongoing attempts to develop biological control agents against fungal diseases, specific peroxisomal enzymes essential for virulence represent elegant targets. However, single gene targets bear the risk of rapid evolution of resistance mechanisms by phytopathogenic fungi. With their numerous essential roles in fungal pathogenicity, the entire peroxisome has now emerged as an important virulence factor and thereby offers the possibility to block multiple biogenesis pathways of diffferent virulence factors simultaneously. This might be feasible by disrupting one PEX gene that is essential for peroxisome biogenesis and for the import of all PTS1 proteins. The first attempts toward this goal have already revealed the potential of this approach. Tomato plants expressing a small interfering RNA against PEX6 of Fusarium oxysporum were indeed more resistant to Fusarium infection (Tetorya & Rajam, 2021). To avoid the usage of GMOs, the same mechanism could be exploited by spray application of exogenous small interfering RNAs as fungicides. These environmentally friendly RNA interference‐based fungicides are expected to find applications in crop protection in the next decade (Cai et al., 2020).
FIGURE 4.
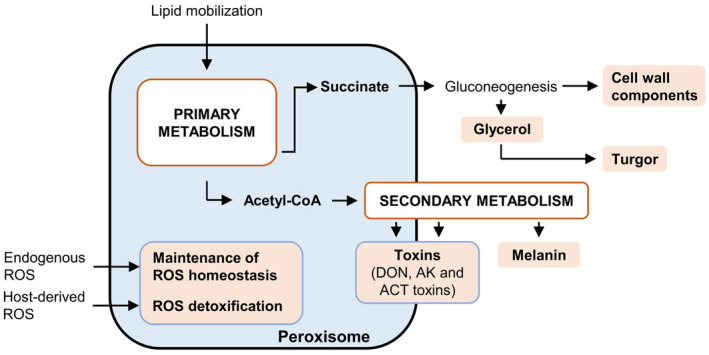
Overview of peroxisomal functions and metabolic pathways that are crucial for fungal virulence. Functions and metabolites relevant for virulence are indicated in orange.
ACKNOWLEDGEMENTS
We thank Dr. Imke de Grahl and Stefan Wirling for critical manuscript reading and for providing valuable input. The authors declare no conflict of interest.
Falter, C. & Reumann, S. (2022) The essential role of fungal peroxisomes in plant infection. Molecular Plant Pathology, 23, 781–794. 10.1111/mpp.13180
REFERENCES
- Akimitsu, K. , Peever, T.L. & Timmer, L.J.M.P.P. (2003) Molecular, ecological and evolutionary approaches to understanding Alternaria diseases of citrus. Molecular Plant Pathology, 4, 435–446. [DOI] [PubMed] [Google Scholar]
- Asakura, M. , Okuno, T. & Takano, Y. (2006) Multiple contributions of peroxisomal metabolic function to fungal pathogenicity in Colletotrichum lagenarium . Applied and Environmental Microbiology, 72, 6345–6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audenaert, K. , Vanheule, A. , Höfte, M. & Haesaert, G. (2014) Deoxynivalenol: a major player in the multifaceted response of Fusarium to its environment. Toxins, 6, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belozerskaya, T.A. , Gessler, N.N. & Aver‘yanov, A.A. (2016) Melanin pigments of fungi. In: Merillon, J.‐M. & Ramawat, K.G. (Eds.) Fungal metabolites. Cham: Springer International Publishing, pp. 263–291. [Google Scholar]
- Bhadauria, V. , Banniza, S. , Vandenberg, A. , Selvaraj, G. & Wei, Y. (2012) Peroxisomal alanine: glyoxylate aminotransferase AGT1 is indispensable for appressorium function of the rice blast pathogen, Magnaporthe oryzae . PLoS One, 7, e36266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhambra, G.K. , Wang, Z.Y. , Soanes, D.M. , Wakley, G.E. & Talbot, N.J. (2006) Peroxisomal carnitine acetyl transferase is required for elaboration of penetration hyphae during plant infection by Magnaporthe grisea . Molecular Microbiology, 61, 46–60. [DOI] [PubMed] [Google Scholar]
- Boddu, J. , Cho, S. & Muehlbauer, G.J. (2007) Transcriptome analysis of trichothecene‐induced gene expression in barley. Molecular Plant‐Microbe Interactions, 20, 1364–1375. [DOI] [PubMed] [Google Scholar]
- Boenisch, M.J. , Broz, K.L. , Purvine, S.O. , Chrisler, W.B. , Nicora, C.D. , Connolly, L.R. et al. (2017) Structural reorganization of the fungal endoplasmic reticulum upon induction of mycotoxin biosynthesis. Scientific Reports, 7, 44296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boenisch, M.J. & Schäfer, W. (2011) Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biology, 11, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bosch, H. , Schutgens, R.B. , Wanders, R.J. & Tager, J.M. (1992) Biochemistry of peroxisomes. Annual Review of Biochemistry, 61, 157–197. [DOI] [PubMed] [Google Scholar]
- Brocard, C. & Hartig, A. (2006) Peroxisome targeting signal 1: is it really a simple tripeptide? Biochimica et Biophysica Acta, 1763, 1565–1573. [DOI] [PubMed] [Google Scholar]
- Cai, Q. , He, B. , Weiberg, A. , Buck, A.H. & Jin, H. (2020) Small RNAs and extracellular vesicles: new mechanisms of cross‐species communication and innovative tools for disease control. PLoS Pathogens, 15, e1008090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Kistler, H.C. & Ma, Z. (2019) Fusarium graminearum trichothecene mycotoxins: biosynthesis, regulation, and management. Annual Review of Phytopathology, 57, 15–39. [DOI] [PubMed] [Google Scholar]
- Chen, Y. , Zheng, S. , Ju, Z. , Zhang, C. , Tang, G. , Wang, J. et al. (2018) Contribution of peroxisomal docking machinery to mycotoxin biosynthesis, pathogenicity and pexophagy in the plant pathogenic fungus Fusarium graminearum . Environmental Microbiology, 20, 3224–3245. [DOI] [PubMed] [Google Scholar]
- Ciniawsky, S. , Grimm, I. , Saffian, D. , Girzalsky, W. , Erdmann, R. & Wendler, P. (2015) Molecular snapshots of the Pex1/6 AAA+ complex in action. Nature Communications, 6, 7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero, R.J. & Casadevall, A. (2017) Functions of fungal melanin beyond virulence. Fungal Biology Reviews, 31, 99–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, S. , Gu, Z. , Yang, N. , Li, L. , Yue, X. , Que, Y. et al. (2016) Identification and characterization of the peroxin 1 gene MoPEX1 required for infection‐related morphogenesis and pathogenicity in Magnaporthe oryzae . Scientific Reports, 6, 36292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins, A.E. , Hohn, T.M. & McCormick, S.P. (1993) Trichothecene biosynthesis in Fusarium species: chemistry, genetics, and significance. Microbiology Reviews, 57, 595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel, B. , Erdmann, R. , Gould, S.J. , Blobel, G. , Crane, D.I. , Cregg, J.M. et al. (1996) A unified nomenclature for peroxisome biogenesis factors. Journal of Cellular Biology, 135, 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effelsberg, D. , Cruz‐Zaragoza, L.D. , Schliebs, W. & Erdmann, R. (2016) Pex9p is a new yeast peroxisomal import receptor for PTS1‐containing proteins. Journal of Cell Science, 129, 4057–4066. [DOI] [PubMed] [Google Scholar]
- Elgersma, Y. , van Roermund, C.W. , Wanders, R.J. & Tabak, H.F. (1995) Peroxisomal and mitochondrial carnitine acetyltransferases of Saccharomyces cerevisiae are encoded by a single gene. The EMBO Journal, 14, 3472–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, A.J. , Ryder, L.S. , Kershaw, M.J. & Talbot, N.J. (2017) The role of glycerol in the pathogenic lifestyle of the rice blast fungus Magnaporthe oryzae . Environmental Microbiology, 19, 1008–1016. [DOI] [PubMed] [Google Scholar]
- Fujihara, N. , Sakaguchi, A. , Tanaka, S. , Fujii, S. , Tsuji, G. , Shiraishi, T. et al. (2010) Peroxisome biogenesis factor PEX13 is required for appressorium‐mediated plant infection by the anthracnose fungus Colletotrichum orbiculare . Molecular Plant‐Microbe Interactions, 23, 436–445. [DOI] [PubMed] [Google Scholar]
- Fujii, I. , Mori, Y. , Watanabe, A. , Kubo, Y. , Tsuji, G. & Ebizuka, Y. (2000) Enzymatic synthesis of 1,3,6,8‐tetrahydroxynaphthalene solely from malonyl coenzyme A by a fungal iterative type I polyketide synthase PKS1. Biochemistry, 39, 8853–8858. [DOI] [PubMed] [Google Scholar]
- Geoghegan, I. , Steinberg, G. & Gurr, S. (2017) The role of the fungal cell wall in the infection of plants. Trends in Microbiology, 25, 957–967. [DOI] [PubMed] [Google Scholar]
- Goh, J. , Jeon, J. , Kim, K.S. , Park, J. , Park, S.Y. & Lee, Y.H. (2011) The PEX7‐mediated peroxisomal import system is required for fungal development and pathogenicity in Magnaporthe oryzae . PLoS One, 6, e28220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami, R.S. & Kistler, H.C. (2005) Pathogenicity and in planta mycotoxin accumulation among members of the Fusarium graminearum species complex on wheat and rice. Phytopathology, 95, 1397–1404. [DOI] [PubMed] [Google Scholar]
- Gould, S.J. , Keller, G.A. , Hosken, N. , Wilkinson, J. & Subramani, S. (1989) A conserved tripeptide sorts proteins to peroxisomes. Journal of Cell Biology, 108, 1657–1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossart, H.‐P. , Van den Wyngaert, S. , Kagami, M. , Wurzbacher, C. , Cunliffe, M. & Rojas‐Jimenez, K. (2019) Fungi in aquatic ecosystems. Nature Reviews Microbiology, 17, 339–354. [DOI] [PubMed] [Google Scholar]
- Hof, H. (2008) Mycotoxins: pathogenicity factors or virulence factors? Mycoses, 51, 93–94. [DOI] [PubMed] [Google Scholar]
- Howard, R.J. , Ferrari, M.A. , Roach, D.H. & Money, N.P. (1991) Penetration of hard substrates by a fungus employing enormous turgor pressures. Proceedings of the National Academy of Sciences of the United States of America, 88, 11281–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idnurm, A. & Howlett, B. (2002) Isocitrate lyase is essential for pathogenicity of the fungus Leptosphaeria maculans to canola (Brassica napus). Eukaryotic Cell, 1, 719–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imazaki, A.I. , Tanaka, A. , Harimoto, Y. , Yamamoto, M. , Akimitsu, K. , Park, P. et al. (2010) Contribution of peroxisomes to secondary metabolism and pathogenicity in the fungal plant pathogen Alternaria alternata . Eukaryotic Cell, 9, 682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, R.L.M. , Santana‐Molina, C. , van den Noort, M. , Devos, D.P. & van der Klei, I.J. (2021) Comparative genomics of peroxisome biogenesis proteins: making sense of the PEX proteins. Frontiers in Cellular and Developmental Biology, 9, 654163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong, J.C. , McCormack, B.J. , Smirnoff, N. & Talbot, N.J. (1997) Glycerol generates turgor in rice blast. Nature, 389, 244. [Google Scholar]
- Kawamura, C. , Moriwaki, J. , Kimura, N. , Fujita, Y. , Fuji, S. , Hirano, T. et al. (1997) The melanin biosynthesis genes of Alternaria alternata can restore pathogenicity of the melanin‐deficient mutants of Magnaporthe grisea . Molecular Plant‐Microbe Interactions, 10, 446–453. [DOI] [PubMed] [Google Scholar]
- Keller, N.P. , Turner, G. & Bennett, J.W. (2005) Fungal secondary metabolism—from biochemistry to genomics. Nature Reviews Microbiology, 3, 937–947. [DOI] [PubMed] [Google Scholar]
- Kiel, J.A.K.W. , Veenhuis, M. & van der Klei, I.J. (2006) PEX genes in fungal genomes: common, rare or redundant. Traffic, 7, 1291–1303. [DOI] [PubMed] [Google Scholar]
- Kimura, A. , Takano, Y. , Furusawa, I. & Okuno, T. (2001) Peroxisomal metabolic function is required for appressorium‐mediated plant infection by Colletotrichum lagenarium . The Plant Cell, 13, 1945–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura, M. , Kaneko, I. , Komiyama, M. , Takatsuki, A. , Koshino, H. , Yoneyama, K. et al. (1998) Trichothecene 3‐O‐acetyltransferase protects both the producing organism and transformed yeast from related mycotoxins. Cloning and characterization of Tri101 . Journal of Biological Chemistry, 273, 1654–1661. [DOI] [PubMed] [Google Scholar]
- van der Klei, I.J. & Veenhuis, M. (2013) The versatility of peroxisome function in filamentous fungi. Subcellular Biochemistry, 69, 135–152. [DOI] [PubMed] [Google Scholar]
- Kohmoto, K. , Itoh, Y. , Shimomura, N. , Kondoh, Y. , Otani, H. , Kodama, M. et al. (1993) Isolation and biological activities of two host‐specific toxins from the tangerine pathotype of Alternaria alternata . Journal of Phytopathology, 83, 495–502. [Google Scholar]
- Kong, X. , Zhang, H. , Wang, X. , van der Lee, T. , Waalwijk, C. , van Diepeningen, A. et al. (2019) FgPex3, a peroxisome biogenesis factor, is involved in regulating vegetative growth, conidiation, sexual development, and virulence in Fusarium graminearum . Frontiers in Microbiology, 10, 2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo, Y. (2013) Function of peroxisomes in plant–pathogen interactions. Subcellular Biochemistry, 69, 329–345. [DOI] [PubMed] [Google Scholar]
- Kunze, M. (2020) The type‐2 peroxisomal targeting signal. Biochimica et Biophysica Acta Molecular Cell Research, 1867, 118609. [DOI] [PubMed] [Google Scholar]
- Lee, S.H. , Han, Y.K. , Yun, S.H. & Lee, Y.W. (2009) Roles of the glyoxylate and methylcitrate cycles in sexual development and virulence in the cereal pathogen Gibberella zeae . Eukaryotic Cell, 8, 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann, S. , Serrano, M. , L’Haridon, F. , Tjamos, S.E. & Metraux, J.P. (2015) Reactive oxygen species and plant resistance to fungal pathogens. Phytochemistry, 112, 54–62. [DOI] [PubMed] [Google Scholar]
- Li, L. , Wang, J. , Chen, H. , Chai, R. , Zhang, Z. , Mao, X. et al. (2017) Pex14/17, a filamentous fungus‐specific peroxin, is required for the import of peroxisomal matrix proteins and full virulence of Magnaporthe oryzae . Molecular Plant Pathology, 18, 1238–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, L. , Wang, J. , Zhang, Z. , Wang, Y. , Liu, M. , Jiang, H. et al. (2014) MoPex19, which is essential for maintenance of peroxisomal structure and Woronin bodies, is required for metabolism and development in the rice blast fungus. PLoS One, 9, e85252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberti, D. , Grant, S.J. , Benny, U. , Rollins, J.A. & Dobinson, K.F. (2007) Development of an Agrobacterium tumefaciens‐mediated gene disruption method for Sclerotinia sclerotiorum . Canadian Journal of Plant Pathology, 29, 394–400. [Google Scholar]
- Liberti, D. , Rollins, J.A. & Dobinson, K.F. (2013) Peroxysomal carnitine acetyl transferase influences host colonization capacity in Sclerotinia sclerotiorum . Molecular Plant‐Microbe Interactions, 26, 768–780. [DOI] [PubMed] [Google Scholar]
- Lindahl, B.D. , Ihrmark, K. , Boberg, J. , Trumbore, S.E. , Högberg, P. , Stenlid, J. et al. (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytologist, 173, 611–620. [DOI] [PubMed] [Google Scholar]
- Maruyama, J. & Kitamoto, K. (2013) Expanding functional repertoires of fungal peroxisomes: contribution to growth and survival processes. Frontiers in Physiology, 4, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, J. & Kitamoto, K. (2019) The Woronin body: a fungal organelle regulating multicellularity. In: Hoffmeister, D. & Gressler, M. (Eds.) Biology of the fungal cell. Cham: Springer International Publishing, pp. 3–14. [Google Scholar]
- Mary Wanjiru, W. , Zhensheng, K. & Buchenauer, H. (2002) Importance of cell wall degrading enzymes produced by Fusarium graminearum during infection of wheat heads. European Journal of Plant Pathology, 108, 803–810. [Google Scholar]
- McCormick, S.P. , Stanley, A.M. , Stover, N.A. & Alexander, N.J. (2011) Trichothecenes: from simple to complex mycotoxins. Toxins, 3, 802–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinecke, M. , Cizmowski, C. , Schliebs, W. , Krüger, V. , Beck, S. , Wagner, R. et al. (2010) The peroxisomal importomer constitutes a large and highly dynamic pore. Nature Cell Biology, 12, 273–277. [DOI] [PubMed] [Google Scholar]
- Menke, J. , Weber, J. , Broz, K. & Kistler, H.C. (2013) Cellular development associated with induced mycotoxin synthesis in the filamentous fungus Fusarium graminearum . PLoS One, 8, e63077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentges, M. , Glasenapp, A. , Boenisch, M. , Malz, S. , Henrissat, B. , Frandsen, R.J.N. et al. (2020) Infection cushions of Fusarium graminearum are fungal arsenals for wheat infection. Molecular Plant Pathology, 21, 1070–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto, Y. , Masunaka, A. , Tsuge, T. , Yamamoto, M. , Ohtani, K. , Fukumoto, T. et al. (2008) Functional analysis of a multicopy host‐selective ACT‐toxin biosynthesis gene in the tangerine pathotype of Alternaria alternata using RNA silencing. Molecular Plant‐Microbe Interactions, 21, 1591–1599. [DOI] [PubMed] [Google Scholar]
- Montilla‐Martinez, M. , Beck, S. , Klümper, J. , Meinecke, M. , Schliebs, W. , Wagner, R. et al. (2015) Distinct pores for peroxisomal import of PTS1 and PTS2 proteins. Cell Reports, 13, 2126–2134. [DOI] [PubMed] [Google Scholar]
- Navarro‐Espíndola, R. , Suaste‐Olmos, F. & Peraza‐Reyes, L. (2020) Dynamic regulation of peroxisomes and mitochondria during fungal development. Journal of Fungi, 6, 302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani, H. , Kohmoto, K. , Nishimura, S. , Nakashima, T. , Ueno, T. & Fukami, H. (1985) Biological activities of AK‐toxins I and II, host‐specific toxins from Alternaria alternata Japanese pear pathotype. Annals of the Phytopathological Society of Japan, 51, 285–293. [Google Scholar]
- Park, P. & Ikeda, K.‐I. (2008) Ultrastructural analysis of responses of host and fungal cells during plant infection. Journal of General Plant Pathology, 74, 2–14. [Google Scholar]
- Perincherry, L. , Lalak‐Kanczugowska, J. & Stepien, L. (2019) Fusarium‐produced mycotoxins in plant–pathogen interactions. Toxins, 11, 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontes, J.G.M. , Fernandes, L.S. , Dos Santos, R.V. , Tasic, L. & Fill, T.P. (2020) Virulence factors in the phytopathogen–host interactions: an overview. Journal of Agricultural and Food Chemistry, 68, 7555–7570. [DOI] [PubMed] [Google Scholar]
- Ponts, N. (2015) Mycotoxins are a component of Fusarium graminearum stress‐response system. Frontiers in Microbiology, 6, 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritsch, C. , Muehlbauer, G.J. , Bushnell, W.R. , Somers, D.A. & Vance, C.P. (2000) Fungal development and induction of defense response genes during early infection of wheat spikes by Fusarium graminearum . Molecular Plant‐Microbe Interactions, 13, 159–169. [DOI] [PubMed] [Google Scholar]
- Proctor, R.H. , Desjardins, A.E. , McCormick, S.P. , Plattner, R.D. , Alexander, N.J. & Brown, D.W. (2002) Genetic analysis of the role of trichothecene and fumonisin mycotoxins in the virulence of Fusarium . European Journal of Plant Pathology, 108, 691–698. [Google Scholar]
- Puntscher, H. , Cobankovic, I. , Marko, D. & Warth, B. (2019) Quantitation of free and modified Alternaria mycotoxins in European food products by LC‐MS/MS. Food Control, 102, 157–165. [Google Scholar]
- Pusztahelyi, T. , Holb, I. & Pócsi, I. (2015) Secondary metabolites in fungus–plant interactions. Frontiers in Plant Science, 6, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos‐Pamplona, M. & Naqvi, N.I. (2006) Host invasion during rice‐blast disease requires carnitine‐dependent transport of peroxisomal acetyl‐CoA. Molecular Microbiology, 61, 61–75. [DOI] [PubMed] [Google Scholar]
- Reumann, S. & Bartel, B. (2016) Plant peroxisomes: recent discoveries in functional complexity, organelle homeostasis, and morphological dynamics. Current Opinion in Plant Biology, 34, 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Roermund, C.W. , Elgersma, Y. , Singh, N. , Wanders, R.J. & Tabak, H.F. (1995) The membrane of peroxisomes in Saccharomyces cerevisiae is impermeable to NAD(H) and acetyl‐CoA under in vivo conditions. The EMBO Journal, 14, 3480–3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savary, S. , Willocquet, L. , Pethybridge, S.J. , Esker, P. , McRoberts, N. & Nelson, A. (2019) The global burden of pathogens and pests on major food crops. Nature Ecology and Evolution, 3, 430–439. [DOI] [PubMed] [Google Scholar]
- Segal, L.M. & Wilson, R.A. (2018) Reactive oxygen species metabolism and plant–fungal interactions. Fungal Genetics and Biology, 110, 1–9. [DOI] [PubMed] [Google Scholar]
- Son, H. , Min, K. , Lee, J. , Choi, G.J. , Kim, J.‐C. & Lee, Y.‐W. (2012) Mitochondrial carnitine‐dependent acetyl coenzyme A transport is required for normal sexual and asexual development of the ascomycete Gibberella zeae . Eukaryotic Cell, 11, 1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soundararajan, S. , Jedd, G. , Li, X. , Ramos‐Pamploña, M. , Chua, N.H. & Naqvi, N.I. (2004) Woronin body function in Magnaporthe grisea is essential for efficient pathogenesis and for survival during nitrogen starvation stress. The Plant Cell, 16, 1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner, U. & Oerke, E.‐C. (2007) Localized melanization of appressoria is required for pathogenicity of Venturia inaequalis . Phytopathology, 97, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Streit, E. , Schwab, C. , Sulyok, M. , Naehrer, K. , Krska, R. & Schatzmayr, G. (2013) Multi‐mycotoxin screening reveals the occurrence of 139 different secondary metabolites in feed and feed ingredients. Toxins, 5, 504–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinkels, B.W. , Gould, S.J. , Bodnar, A.G. , Rachubinski, R.A. & Subramani, S. (1991) A novel, cleavable peroxisomal targeting signal at the amino‐terminus of the rat 3‐ketoacyl‐CoA thiolase. The EMBO Journal, 10, 3255–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, A. , Shiotani, H. , Yamamoto, M. & Tsuge, T. (1999) Insertional mutagenesis and cloning of the genes required for biosynthesis of the host‐specific AK‐toxin in the Japanese pear pathotype of Alternaria alternata . Molecular Plant‐Microbe Interactions, 12, 691–702. [DOI] [PubMed] [Google Scholar]
- Tanaka, A. & Tsuge, T. (2000) Structural and functional complexity of the genomic region controlling AK‐toxin biosynthesis and pathogenicity in the Japanese pear pathotype of Alternaria alternata . Molecular Plant‐Microbe Interactions, 13, 975–986. [DOI] [PubMed] [Google Scholar]
- Tetorya, M. & Rajam, M.V. (2021) RNAi‐mediated silencing of PEX6 and GAS1 genes of Fusarium oxysporum f. sp. lycopersici confers resistance against Fusarium wilt in tomato. 3 Biotech, 11, 443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thines, E. , Weber, R.W.S. & Talbot, N.J. (2000) MAP kinase and protein kinase A–dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea . The Plant Cell, 12, 1703–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge, T. , Harimoto, Y. , Akimitsu, K. , Ohtani, K. , Kodama, M. , Akagi, Y. et al. (2013) Host‐selective toxins produced by the plant pathogenic fungus Alternaria alternata . FEMS Microbiology Reviews, 37, 44–66. [DOI] [PubMed] [Google Scholar]
- Turner, N.W. , Subrahmanyam, S. & Piletsky, S.A. (2009) Analytical methods for determination of mycotoxins: a review. Analytica Chimica Acta, 632, 168–180. [DOI] [PubMed] [Google Scholar]
- Vranová, E. , Coman, D. & Gruissem, W. (2012) Structure and dynamics of the isoprenoid pathway network. Molecular Plant, 5, 318–333. [DOI] [PubMed] [Google Scholar]
- Walter, T. & Erdmann, R. (2019) Current advances in protein import into peroxisomes. Protein Journal, 38, 351–362. [DOI] [PubMed] [Google Scholar]
- Wang, J.Y. , Li, L. , Chai, R.Y. , Qiu, H.P. , Zhang, Z. , Wang, Y.L. et al. (2019) Pex13 and Pex14, the key components of the peroxisomal docking complex, are required for peroxisome formation, host infection and pathogenicity‐related morphogenesis in Magnaporthe oryzae . Virulence, 10, 292–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Zhang, Z. , Wang, Y. , Li, L. , Chai, R. , Mao, X. et al. (2013) PTS1 peroxisomal import pathway plays shared and distinct roles to PTS2 pathway in development and pathogenicity of Magnaporthe oryzae . PLoS One, 8, e55554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhang, L.I. , Liu, C. , Sun, S. , Liu, A. , Liang, Y. et al. (2020) The roles of FgPEX2 and FgPEX12 in virulence and lipid metabolism in Fusarium graminearum . Fungal Genetics and Biology, 135, 103288. [DOI] [PubMed] [Google Scholar]
- Wang, X. , Li, S. , Liu, Y. & Ma, C. (2015) Redox regulated peroxisome homeostasis. Redox Biology, 4, 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z.Y. , Soanes, D.M. , Kershaw, M.J. & Talbot, N.J. (2007) Functional analysis of lipid metabolism in Magnaporthe grisea reveals a requirement for peroxisomal fatty acid β‐oxidation during appressorium‐mediated plant infection. Molecular Plant‐Microbe Interactions, 20, 475–491. [DOI] [PubMed] [Google Scholar]
- Wang, Z.Y. , Thornton, C.R. , Kershaw, M.J. , Debao, L. & Talbot, N.J. (2003) The glyoxylate cycle is required for temporal regulation of virulence by the plant pathogenic fungus Magnaporthe grisea . Molecular Microbiology, 47, 1601–1612. [DOI] [PubMed] [Google Scholar]
- Wu, P.C. , Chen, C.W. , Choo, C.Y.L. , Chen, Y.K. , Yago, J.I. & Chung, K.R. (2020) Proper functions of peroxisomes are vital for pathogenesis of citrus brown spot disease caused by Alternaria alternata . Journal of Fungi, 6, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L.I. , Liu, C. , Wang, L. , Sun, S. , Liu, A. , Liang, Y. et al. (2019) FgPEX1 and FgPEX10 are required for the maintenance of Woronin bodies and full virulence of Fusarium graminearum . Current Genetics, 65, 1383–1396. [DOI] [PubMed] [Google Scholar]
- Zhang, L. , Wang, L. , Liang, Y. & Yu, J. (2019) FgPEX4 is involved in development, pathogenicity, and cell wall integrity in Fusarium graminearum . Current Genetics, 65, 747–758. [DOI] [PubMed] [Google Scholar]
- Zhang, T. , Zhang, B. , Hua, C. , Meng, P. , Wang, S. , Chen, Z. et al. (2017) VdPKS1 is required for melanin formation and virulence in a cotton wilt pathogen Verticillium dahliae . Science China Life Sciences, 60, 868–879. [DOI] [PubMed] [Google Scholar]
- Zheng, X. , Zhang, X. , Zhao, L. , Apaliya, M. , Yang, Q. , Sun, W. et al. (2017) Screening of deoxynivalenol producing strains and elucidation of possible toxigenic molecular mechanism. Toxins, 9, 184. [DOI] [PMC free article] [PubMed] [Google Scholar]


