Abstract
Arnebiae Radix (dried root of Arnebia euchroma (Royle) Johnst.) has been used in traditional Chinese medicine (TCM) to treat macular eruptions, measles, sore throat, carbuncles, burns, skin ulcers, and inflammation. Previous studies have shown that shikonins and shikonofurans are two of their main bioactive ingredients. However, systematic investigations of their constituents have rarely been conducted. It is necessary to establish a rapid and effective method to identify the chemical constituents of Arnebiae Radix. This will help to further improve the effective resource utilization rate of this plant. In this study, a rapid and effective UHPLC-Q-Exactive Orbitrap mass spectrometry method was established to simultaneously analyze chemical ingredients in Arnebiae Radix within a short period of time. Based on the results of a full scan MS, the MS2 database (mzVault and mzCloud), the diagnostic fragment ions, the retention time, and the bibliography, a total of 188 compounds were identified, with 114 of those being reported from Arnebiae Radix for the first time. The results of this study lay the foundation for obtaining a thorough understanding of the active ingredients in Arnebiae Radix and its quality control. This method may be widely used for the chemical characterization of different samples.
Keywords: Arnebiae Radix, identification, chemical constituents, UHPLC-Q-Exactive Orbitrap MS
1. Introduction
Arnebiae Radix, commonly called known as “Zicao” in traditional Chinese medicine (TCM), is the root of Arnebia euchroma (Royle) Johnst. It is primarily distributed in Mongolia, Xinjiang, and Northeast China [1,2]. It has been widely used as a folk medicine for clearing heat (Qingre) and for detoxification (Jiedu) by oral administration and for promoting blood circulation in local wounds via external application [3,4]. Arnebiae Radix has been used for many years for the treatment of macular eruptions, measles, sore throat, carbuncles, burns, skin ulcers, inflammation, allergic contact dermatitis(ACD) [5,6,7,8] and recently, for the treatment of cancer [9,10]. Previous studies have acknowledged the richness and complexity of its chemical composition. Its main active ingredients are the naphthoquinone compound, which has exhibited extensive antioxidant, antimicrobial, anti-inflammatory, and antitumor activities [11,12,13]. Many compounds have been reported to exist in Arnebiae Radix, including shikonins and shikonofurans [14,15]. However, the characterization of the constituents of Arnebiae Radix is still insufficient. Therefore, it is necessary to develop a systematic strategy for the rapid detection and identification of the constituents of Arnebiae Radix, as this will be very helpful for understanding its material basis and quality control.
The complexity of chemicals contained in TCM has presented a significant challenge regarding the rapid identification and characterization of components. Liquid chromatography-mass spectrometry (LC-MS), especially ultra-high performance liquid chromatography-high resolution mass spectrometry (UHPLC-HRMS), has been used extensively for qualitative analysis, quantitative analysis, and quality control of TCM due to its validity, sensitivity, and specificity [16,17]. HRMS provides high mass accuracy measurements for fragment ions with fast scan speeds. These features help with the identification of constituents with excellent accuracy and high reproducibility [18,19,20,21]. Cai et al. [22] used UHPLC-HRMS with parallel reaction monitoring (PRM) mode to unanimously and tentatively identify 149 chlorogenic acid derivatives from D. nervosa, which widely extended the knowledge on the chemical constituents of D. nervosa, facilitating the understanding of effective substances and quality control. Xiong et al. [23] used UHPLC-Q-Exactive Orbitrap mass spectrometry to systematically identify 106 constituent tannins in Paeoniae Radix Alba in negative ion mode. A systematic strategy was proposed for the rapid detection and identification of the chemical constituents of Arnebiae Radix using UHPLC-Q-Exactive Orbitrap mass spectrometry based on the expected compound and diagnosis fragmentation ion techniques.
The aim of the present investigation was to detect and identify the chemical constituents of Arnebiae Radix by UHPLC-Q-Exactive Orbitrap MS. In total, 188 compounds were identified in Arnebiae Radix, 114 of which are reported for the first time here. This result will improve the in-depth understanding of the pharmacological actions of Arnebiae Radix and lay a foundation for quality control of the drug for future clinical use.
2. Results and Discussion
2.1. Optimization of the UHPLC-Q-Exactive Orbitrap MS Condition
In order to acquire a better chromatographic peak shape and separation resolution, various factors were set to carry out a detection and identification process, including the column (Thermo Scientific Hypersil GOLDTM aQ 100 mm × 2.1 mm, 1.9 μm and Waters ACQUITY BEH C18 column, 100 mm × 2.1 mm, 1.7 μm), column temperature (30, 35, 40 °C), and mobile phase gradient. The chemical constituents showed a high resolution and high sensitivity level based on the LC-MS conditions of “Section 3.3”.
2.2. Establishment of Diagnostic Fragment Ions (DFIs)
The DFI filter is a rapid screening method that is used to identify traditional Chinese medicine chemical components based on the accurate ion mass information provided by high-resolution mass spectrometry and the mass spectrometry fragmentation characteristics of similar chemical components. Thus, it is suitable for the identification of structural analogs. Different from a previous method of referring to the literature to establish a chemical database, the diagnostic fragment ions overcome the limitation of finding potential new compounds and deduce the overall structure of the compounds from the fragmentation information of the mass spectrum of the compound [24,25,26]. Generally, it is well-known that chemical constituents in the same category possess identical carbon skeletons and homologous biosynthetic pathways. It is easily understood that shikonin derivatives, phenolic acids, and flavonoids with the same carbon skeletons will generate similar fragmentation patterns, and these can be defined as DFIs for screening and characterization.
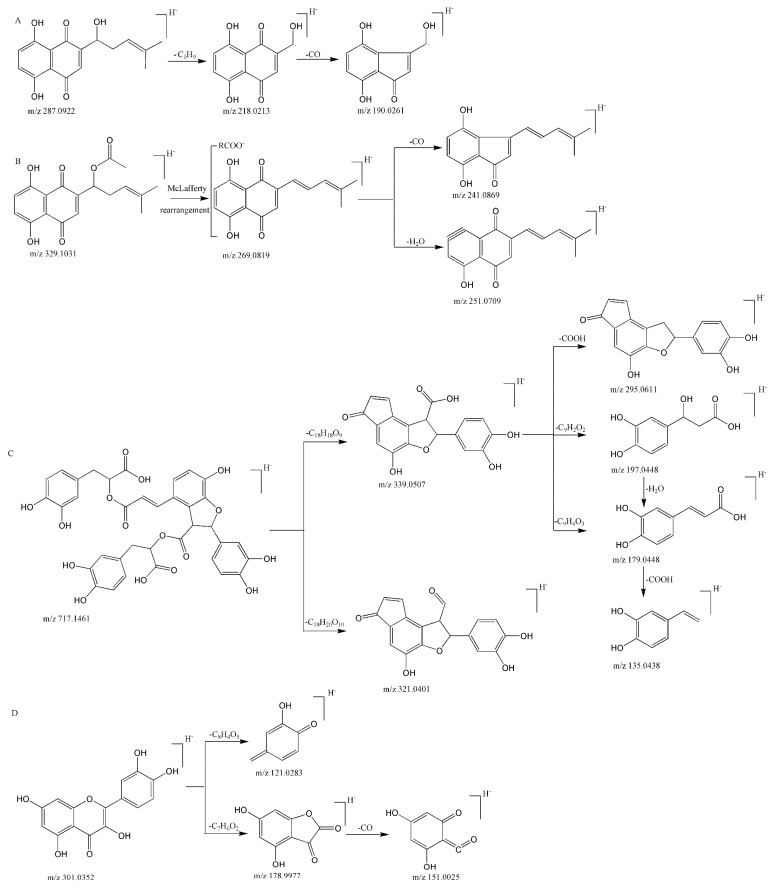
In this study, the fragmentation patterns of 14 reference standards were investigated by UHPLC-Q-Exactive Orbitrap MS in negative mode to establish the DFIs, and the selected fragmentation patterns of the components are shown in Figure 1. In shikonin, the deprotonated molecular ion [M − H]− produced m/z 287.0925 (C16H15O5) m/z 218.0213 and 190.0261 as the predominant fragment ions by the loss of C5H9 and CO, respectively. For acetylshikoninor β,β′-dimethylacrylalkannin, the deprotonated molecular ion [M − H]− at m/z 329.1031 (C18H17O6) and 369.1344 (C21H21O6) produced m/z 269.0819, 251.0709 and 241.0869 as the predominant fragment ions.
Figure 1.
Fragmentation routes of the reference standards: shikonin (A); acetylshikonin (B); salvianolic acid B (C); quercetin (D).
The quasi-molecular ion of the reference standards salvianolic acid B is m/z 717.14610 in negative mode. The parent ion yielded the fragment ions m/z 339.0507 (C18H11O7) and 321.0401 (C18H9O6) by the loss of C18H18O9 and C18H20O10, respectively. With the breaking of the ester bond between the carbonyl group and the oxygen atom, 197.0446 (C9H9O5) was obtained. In addition, m/z 197.0446 (C9H9O5) produced the fragment ions m/z 179.0448 (C9H7O4) and 135.0439 (C8H7O2) by the loss of H2O and COOH. All of the above ions can be used as DFIs of phenolic acid.
Quercetin yielded a deprotonated molecular ion [M − H]− at m/z 301.0359 (C15H9O7), which initially produced 151.0025 (C7H6O2) and 178.9977 (C8H4O5) by Retro Diels–Alder (RDA) rearrangement. The diagnostic ion 178.9977 was shown to eliminate a molecule of CO2 to yield relative fragment ions of 121.0283 (C7H4O4).
2.3. Characterization of the Chemical Constituents in Arnebiae Radix
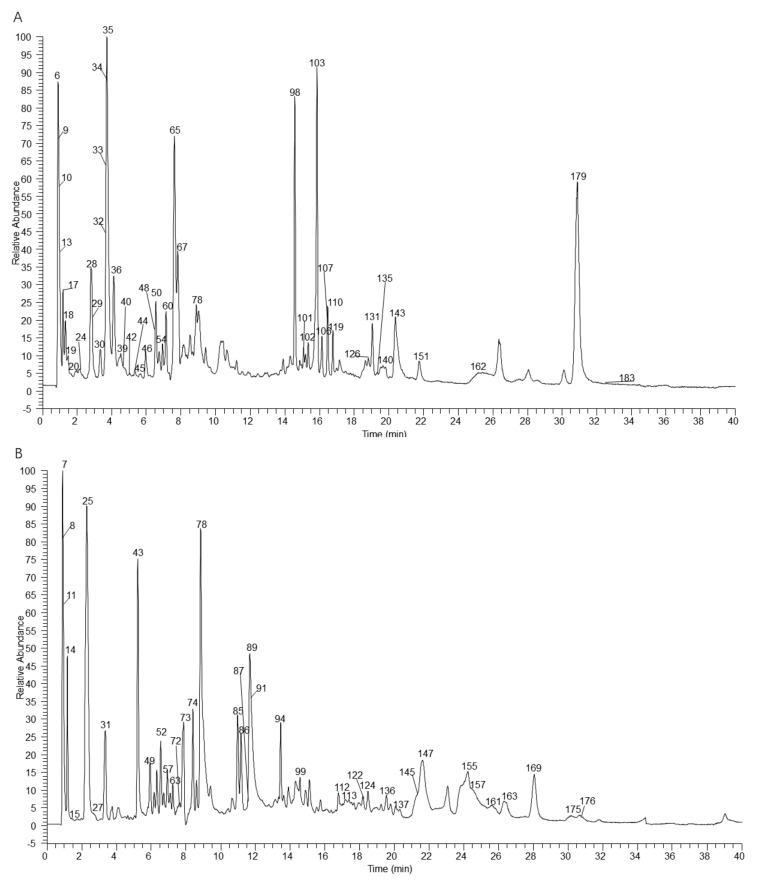
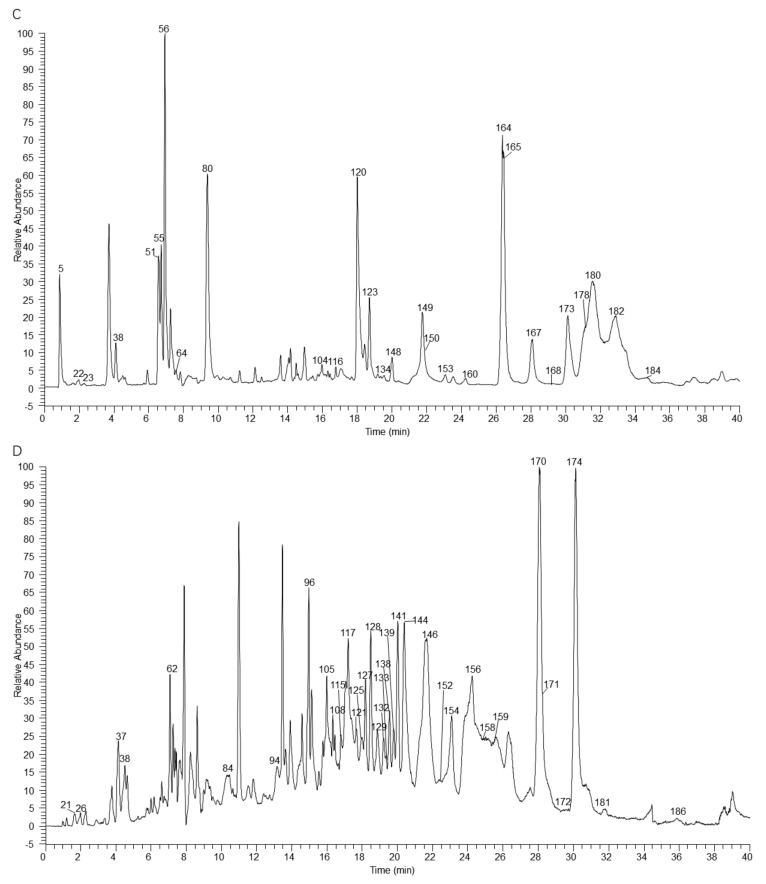
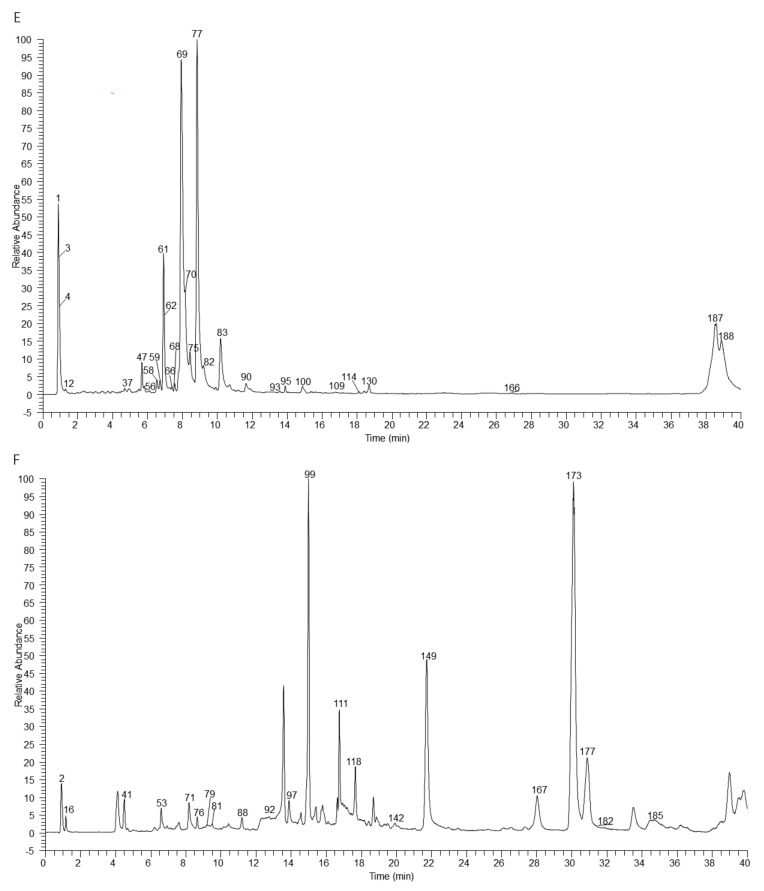
The table lists all the chemical constituents detected in the extracted Arnebiae Radix sample by UHPLC-Q Exactive Orbitrap mass spectrometry based on the diagnostic fragment ions, retention time, MS2 database (mzVault and mzCloud), and bibliographical identification (Table 1). A total of 188 chemical constituents (114 first report) were accurately or tentatively identified. The extracted ion chromatogram (EIC) in negative ion mode was obtained, as shown in Figure 2. Large differences in chemical constituents were seen in the different batches of Arnebiae Radix samples (A, B, and C), indicating that the chemical constituents of Arnebiae Radix differ significantly in the current Chinese medicine market, possibly due to the different growth environments, growth periods, and medicinal material storage times used [27]. Generally, the samples from batch C from southern Xinjiang showed obvious advantages in terms of their peak intensity and number of peaks.
Table 1.
Chromatographic and mass data of the components detected in Arnebiae Radix though UHPLC-Q-Exactive Orbitrap MS.
| No. | Batch | tR | Theoretical Mass m/z | Experimental Mass m/z | Error (ppm) | Formula | MS/MS Fragment (-) |
Identification |
|---|---|---|---|---|---|---|---|---|
| 1 | A, B, C | 0.83 # | 131.0462 | 131.0450 | −9.05 | C4H8N2O3 | MS2[131]: 114.0183(100), 113.0343(65), 70.0284(37), 95.0237(25), 131.0449(22) | Asparagine |
| 2 | A, B, C | 0.86 # | 145.0619 | 145.0606 | −8.80 | C5H10N2O3 | MS2[145]: 127.0500(100), 128.0340(73), 145.0606(97), 102.0546(48), 109.0394(40) | Glutamine |
| 3 | A, B, C | 0.88 | 387.1144 | 387.1139 | −1.44 | C13H24O13 | MS2[387]: 89.0229(100), 119.0336(45), 179.0550(42), 341.1084(33), 161.0444(16), 221.0658(5) | 2,3,4,5,6-pentahydroxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyheptanoic acid |
| 4 | A, B, C | 0.88 # | 341.1089 | 341.1084 | −1.66 | C12H22O11 | MS2[341]: 89.0230(100), 59.0124(48), 71.0124(40), 101.0230(33), 119.0336(29), 113.0231(25) | α,α-Trehalose |
| 5 | A, B, C | 0.88 # | 179.0561 | 179.0550 | −6.27 | C6H12O6 | MS2[341]: 59.0124(100), 89.0230(68), 71.0124(63), 75.0073(48), 101.0230(33), 119.0336(29) | Mannose |
| 6 | A, B, C | 0.88 # | 503.1618 | 503.1612 | −1.19 | C18H32O16 | MS2[503]: 89.0230(100), 101.0230(50), 113.0230(38), 179.0551(26) | Raffinose |
| 7 | A, B, C | 0.89 # | 135.0299 | 135.0286 | −9.68 | C4H8O5 | MS2[135]: 75.0073(100), 135.0287(51), 72.9917(13), 89.0230(11), 59.0124(10) | Threonic acid |
| 8 | A, B, C | 0.90 # | 195.0510 | 195.0500 | −5.26 | C6H12O7 | MS2[195]: 195.0501(100), 75.0073(82), 129.0180(76), 99.0074(22), 87.0073(21), 59.0124(12) | Gluconic acid |
| 9 | A, B, C | 0.90 # | 191.0561 | 191.0551 | −5.25 | C7H12O6 | MS2[191]: 111.0074(100), 87.0073(35), 85.0280(28), 191.0551(12) | Quinic acid |
| 10 | A, B, C | 0.91 # | 149.0455 | 149.0443 | −8.17 | C5H10O5 | MS2[149]: 149.0443(100), 89.0230(76), 59.0124(32), 75.0073(32) | Arabinose |
| 11 | A, B, C | 0.92 # | 193.0354 | 193.0344 | −5.16 | C6H10O7 | MS2[193]: 103.0023(100), 59.0124(29), 85.0280(16), 193.0708(10) | β-D-Glucopyranuronic acid |
| 12 | A, B, C | 0.93 # | 133.0142 | 133.0130 | −9.60 | C4H6O5 | MS2[133]: 115.0023(100), 71.0124(44), 133.0130(32) | Malic acid |
| 13 | A, B, C | 0.93 # | 177.0405 | 177.0395 | −5.60 | C6H10O6 | MS2[177]: 59.0124(100), 129.0181(42), 99.0074(34), 89.0230(32), 177.0397(31) | δ-Gluconic acidδ-lactone |
| 14 | A, B, C | 0.94 # | 191.0197 | 191.0188 | −5.01 | C6H8O7 | MS2[191]: 111.0074(100), 87.0073(35), 85.0280(28), 191.0188(11) | Citric acid isomer |
| 15 | A, B, C | 1.17 # | 191.0197 | 191.0188 | −5.01 | C6H8O7 | MS2[191]: 111.0074(100), 87.0073(34), 85.0281(23), 191.0188(10) | Citric acid |
| 16 | A, B, C | 1.17 # | 147.0299 | 147.0287 | −8.35 | C5H8O5 | MS2[147]: 129.0180(100), 85.0280(28), 103.0386(20), 147.0287(18), 101.0230(17) | α-Hydroxyglutaric acid |
| 17 | A, B, C | 1.17 # | 243.0623 | 243.0617 | −2.35 | C9H12N2O6 | MS2[243]: 110.0234(100), 128.0340(24), 200.0556(21), 152.0342(16), 140.0341(11) | Uridine |
| 18 | A, B, C | 1.17 | 167.0211 | 167.0199 | −6.79 | C5H4N4O3 | MS2[167]: 167.0200(100), 124.0140(38) | Uric acid |
| 19 | A, B, C | 1.31 # | 161.0455 | 161.0444 | −7.37 | C6H10O5 | MS2[161]: 99.0437(100), 57.0332(63), 101.0230(33), 161.0444(29), 59.0124(22) | 3-Hydroxy-3-methylglutaric acid |
| 20 | A, B, C | 1.42 # | 145.0506 | 145.0495 | −8.02 | C6H10O4 | MS2[145]: 145.0494(100), 101.0594(50) | 3-Methylglutaric acid |
| 21 | A, B, C | 1.66 # | 169.0142 | 169.0133 | −5.49 | C7H6O5 | MS2[169]: 125.0231(100) | Gallic acid |
| 22 | A, B, C | 1.92 | 164.0717 | 164.0707 | −6.23 | C9H11NO2 | MS2[164]: 147.0440(100), 164.0706(59), 72.0077(28), 96.9587(13) | Phenylalanine |
| 23 | A, B, C | 1.96 # | 137.0244 | 137.0232 | −8.74 | C7H6O3 | MS2[137]: 93.0331(100), 137.0231(37) | Salicylic acid |
| 24 | A, B, C | 2.14 # | 218.1034 | 218.1028 | −2.60 | C9H17NO5 | MS2[218]: 88.0389(100), 146.0810(50), 218.1027(13), 71.0124(9) | Pantothenic acid |
| 25 | A, B, C | 2.28 # | 197.0455 | 197.0447 | −4.40 | C9H10O5 | MS2[167]: 72.9917(100), 135.0439(67), 179.0340(58), 123.0439(50), 197.0445(11) | Danshensu |
| 26 | A, B, C | 2.28 # | 417.0827 | 417.0800 | −6.62 | C20H18O10 | MS2[417]: 219.0268(100), 197.0445(21), 179.0339(5) | Salvianolic acid D |
| 27 | A, B, C | 2.35 # | 167.0350 | 167.0339 | −6.36 | C8H8O4 | MS2[167]: 167.0339(100), 123.0439(87) | Vanillic acid |
| 28 | A, B, C | 2.81 # | 153.0193 | 153.0183 | −3.10 | C7H6O4 | MS2[153]: 109.0281(100), 153.0182(30), 126.0911(19) | Protocatechuic acid |
| 29 | B, C | 2.96 # | 158.0823 | 158.0811 | −7.38 | C7H13NO3 | MS2[158]: 116.0704(100), 158.0811(21) | N-Acetylvaline |
| 30 | A, B, C | 3.33 # | 181.0506 | 181.0497 | −5.43 | C9H10O4 | MS2[181]: 163.0389(100), 181.0496(68), 135.0439(54), 137.0231(42), 119.0489(34) | Ethyl 3,4-dihydroxybenzoate |
| 31 | A, B, C | 3.35 # | 167.0350 | 167.0339 | −6.24 | C8H8O4 | MS2[167]: 123.0438(100), 167.0339(10) | Isovanillic acid |
| 32 | A, B, C | 3.46 # | 203.0826 | 203.0818 | −3.85 | C11H12N2O2 | MS2[203]: 116.0492(100), 203.0816(70), 74.0233(36), 72.0077(32), 159.0916(31), 142.0650(29) | Tryptophan |
| 33 | A, B, C | 3.66 # | 175.0612 | 175.0601 | −6.16 | C7H12O5 | MS2[175]: 146.9600(100), 115.0387(80), 175.0601(69), 113.0594(32), 85.0644(31) | 2-Isopropylmalic acid |
| 34 | A, B, C | 3.77 # | 161.0244 | 161.0231 | −8.25 | C9H6O3 | MS2[161]: 161.0233(100), 133.0282(66) | 7-Hydroxycoumarin |
| 35 | A, B, C | 3.80 # | 153.0193 | 153.0182 | −3.82 | C7H6O4 | MS2[153]: 109.0282(100), 153.0181(57) | Gentisic acid |
| 36 | A, B, C | 4.16 # | 188.0353 | 188.0345 | −4.51 | C10H7NO3 | MS2[188]: 144.0443(100), 188.0343(4) | Kynurenic acid |
| 37 | C | 4.51 # | 465.1038 | 465.1038 | −0.20 | C21H22O12 | MS2[465]: 285.0403(100), 125.0232(85), 275.0563(53), 177.0189(26), 151.0033(21), 303.0518(19) | Taxifolin-glucoside |
| 38 | B, C | 4.62 *# | 289.0718 | 289.0721 | 1.00 | C15H14O6 | MS2[289]: 245.0816(100), 289.0721(94), 125.0233(62), 109.0283(58), 179.0340(48), 151.0390(30), 161.0594(20) | Catechin/Catechin hydrate |
| 39 | A, B, C | 4.78 # | 163.0401 | 163.0390 | −6.61 | C9H8O3 | MS2[163]: 163.0389(100), 120.0522(32) | 3-Coumaric acid |
| 40 | A, B, C | 5.02 # | 163.0401 | 163.0390 | −6.74 | C9H8O3 | MS2[163]: 119.0489(100), 163.0390(16) | p-Coumaric acid |
| 41 | A, C | 5.08 # | 465.1038 | 465.1037 | −0.26 | C21H22O12 | MS2[465]: 285.0403(100), 125.0232(39), 275.0558(12), 177.0183(14), 151.0033(21), 303.0507(8) | Taxifolin-glucoside isomer |
| 42 | B, C | 5.17 # | 172.0979 | 172.0969 | −5.85 | C8H15NO3 | MS2[172]: 130.0861(100), 172.0969(16), 128.1068(2) | N-Acetyl-L-leucine |
| 43 | A, B, C | 5.22 | 179.0350 | 179.0340 | −5.49 | C9H8O4 | MS2[179]: 135.0439(100), 179.0340(29) | Caffeic acid |
| 44 | B, C | 5.33 # | 151.0401 | 151.0390 | −7.14 | C8H8O3 | MS2[151]: 107.0489(100), 151.0387(5) | 2-Hydroxyphenylacetic acid |
| 45 | A, B, C | 5.41 # | 193.0506 | 193.0497 | −4.68 | C10H10O4 | MS2[193]: 134.0361(100), 149.0596(34), 193.0499(13) | Ferulic acid |
| 46 | A, B, C | 5.94 # | 375.1310 | 375.1307 | −0.96 | C17H20N4O6 | MS2[375]: 255.0884(100), 212.0821(18), 151.0388(18), 161.0234(14) | Riboflavin |
| 47 | C | 6.01 # | 449.1089 | 449.1090 | 0.17 | C21H22O11 | MS2[449]: 259.0608(100), 59.0124(94), 269.0455(78), 125.0233(37), 287.0564(32), 178.9974(18) | Eriodictyol-glucoside |
| 48 | A, B, C | 6.01 # | 206.0823 | 206.0817 | −3.00 | C11H13NO3 | MS2[206]: 164.0706(100), 147.0440(28), 58.0285(24), 206.0814(21), 70.0285(14) | N-Acetyl-L-phenylalanine |
| 49 | A, B, C | 6.16 # | 167.0350 | 167.0340 | −5.88 | C8H8O4 | MS2[167]: 167.0339(100), 152.0103(19) | 4-Methoxysalicylic acid |
| 50 | A, B, C | 6.49 # | 173.0819 | 173.0809 | −5.79 | C8H14O4 | MS2[173]: 111.0802(100), 173.0809(55), 129.0908(6), 112.0835(5) | Suberic acid |
| 51 | A, B, C | 6.54 # | 537.1038 | 537.1036 | 0.52 | C27H22O12 | MS2[537]: 339.0504(100), 229.0137(64), 295.0609(56), 197.0446(31), 135.0439(25), 179.0338(14) | Salvianolic acid U |
| 52 | A, B, C | 6.54 # | 163.0401 | 163.0390 | −6.61 | C9H8O3 | MS2[163]: 119.0490(100), 163.0389(14), 120.0522(6) | 4-Coumaric acid isomer |
| 53 | A, B, C | 6.61 # | 313.0718 | 313.0715 | −0.74 | C17H14O6 | MS2[313]: 109.0281(100), 147.0439(38), 159.0440(27), 269.0816(14) | Salvianolic acid F |
| 54 | A, B, C | 6.67 # | 174.0561 | 174.0550 | −5.82 | C10H9NO2 | MS2[174]: 146.9600(100), 174.0550(67), 130.0650(35) | Indole-3-acetic acid |
| 55 | A, B, C | 6.71 # | 537.1038 | 537.1036 | 0.52 | C27H22O12 | MS2[537]: 197.0446(100), 135.0439(80), 339.0505(68), 229.0137(64), 295.0609(63), 179.0340(42) | Salvianolic acid T |
| 56 | A, B, C | 6.91 # | 537.1038 | 537.1033 | −0.97 | C27H22O12 | MS2[537]: 197.0447(100), 135.0439(71), 339.0506(64), 295.0609(58), 229.0137(47), 179.0340(41) | Salvianolic acid J |
| 57 | B, C | 6.91 # | 313.0718 | 313.0711 | −2.09 | C17H14O6 | MS2[313]: 269.0818(100), 313.0716(46), 203.0341(44), 159.0443(31), 109.0281(22) | Salvianolic acid F isomer |
| 58 | A, B, C | 6.91 # | 493.1140 | 493.1135 | −1.08 | C26H22O10 | MS2[493]: 197.0446(100), 135.0439(63), 295.0609(34), 179.0339(33), 72.9917(24), 269.0818(21) | Salvianolic acid A isomer |
| 59 | C | 7.03 # | 433.1140 | 433.1140 | −0.17 | C21H22O10 | MS2[433]: 271.0609(100), 151.0030(30), 98.9477(74), 119.0489(11) | Naringenin-glucoside |
| 60 | A, B, C | 7.13 # | 193.0506 | 193.0497 | −4.83 | C10H10O4 | MS2[193]: 134.0361(100), 193.0496(13), 149.0596(7) | Isoferulic acid |
| 61 | C | 7.22 # | 449.1089 | 449.1087 | −0.53 | C21H22O11 | MS2[449]: 151.0025(100), 287.0558(65), 135.0439(46), 98.9477(41), 96.9587(12) | Eriodictyol hexoside 1 |
| 62 | C | 7.30 # | 433.1140 | 433.1134 | −1.44 | C21H22O10 | MS2[433]: 271.0610(100), 151.0025(33), 98.9476(10), 119.0492(6) | Naringenin-glucoside isomer |
| 63 | A, B, C | 7.50 *# | 609.1461 | 609.1456 | −0.79 | C27H30O16 | MS2[609]: 300.0273(100), 301.0345(57) | Rutin |
| 64 | A, B, C | 7.57 # | 137.0244 | 137.0231 | −9.40 | C7H6O3 | MS2[137]: 93.0332(100), 137.0232(52) | Salicylic acid isomer |
| 65 | B, C | 7.60 # | 715.1305 | 715.1303 | −0.30 | C36H28O16 | MS2[715]: 197.0446(100), 151.0390(54), 135.0437(40), 177.0182(39), 179.0339(35) | DidehydioSalvianolic acid B |
| 66 | C | 7.77 *# | 463.0882 | 463.0881 | −0.28 | C21H20O12 | MS2[463]: 300.0272(100), 301.0346(56), 151.0025(4), 178.9980(2) | Isoquercitrin |
| 67 | A, B, C | 7.81 # | 163.0401 | 163.0390 | −6.74 | C9H8O3 | MS2[163]: 163.0389(100), 137.0596(91), 119.0489(46), 162.8380(17) | 4-Coumaric acid |
| 68 | A, B, C | 7.88 # | 551.1195 | 551.1198 | 0.46 | C28H24O12 | MS2[551]: 197.0447(100), 135.0440(74), 59.0124(62), 353.0659(50), 179.0341(45), 309.0770(41) | Monomethyl lithospermate isomer |
| 69 | A, B, C | 7.92 # | 717.1461 | 717.1456 | −0.75 | C36H30O16 | MS2[717]: 339.0505(100), 321.0760(40), 295.0611(20), 197.0449(26), 135.0440(16), 179.03469(6) | Salvianolic acid B isomer |
| 70 | A, B, C | 8.02 # | 537.1038 | 537.1034 | −0.86 | C27H22O12 | MS2[537]: 295.0609(100), 339.0504(44), 109.0282(41), 185.0233(31), 277.0504(13) | Salvianolic acid isomer |
| 71 | A, B, C | 8.19 # | 357.0616 | 357.0611 | −1.46 | C18H14O8 | MS2[357]: 135.0439(100), 229.0135(31), 197.0448(29), 179.0341(23), 109.0280(17) | Salvianolic acid H |
| 72 | B, C | 8.31 # | 277.1445 | 277.1443 | −0.99 | C16H22O4 | MS2[277]: 259.1336(100), 247.1335(88), 174.0675(82), 121.0282(76), 241.1230(47) | De-O-Methyllasiodiplodin |
| 73 | A, B, C | 8.37 *# | 593.1512 | 593.1509 | −0.43 | C27H30O15 | MS2[593]: 285.0401(100), 284.0324(49) | Nicotiflorin |
| 74 | A, B, C | 8.40 # | 187.0976 | 187.0966 | −5.25 | C9H16O4 | MS2[187]: 125.0959(100), 187.0966(50), 97.0645(4), 169.0859(3) | Azelaic acid |
| 75 | A, B, C | 8.50 # | 551.1195 | 551.1201 | 1.13 | C28H24O12 | MS2[551]: 327.0827(100), 135.0439(37), 197.0446(33), 217.0499(24), 229.0137(22), 353.0664(22) | Monomethyl lithospermate |
| 76 | A, B | 8.65 *# | 609.1825 | 609.1829 | 1.61 | C28H34O15 | MS2[609]: 301.0714(100), 302.0744(8) | Hesperidin |
| 77 | A, B, C | 8.84 * | 359.0772 | 359.0769 | −0.95 | C18H16O8 | MS2[359]: 161.0233(100), 197.0446(37), 179.0339(16), 72.9917(11), 135.0440(6) | Rosmarinic acid |
| 78 | A, B, C | 8.88 # | 731.1618 | 731.1613 | −0.60 | C37H32O16 | MS2[731]: 109.0282(100), 335.0921(89), 353.0670(70), 197.0446(61), 489.1185(45) | 9″-Methyl salvianolate B isomer |
| 79 | A, B, C | 9.00 # | 731.1618 | 731.1620 | 0.32 | C37H32O16 | MS2[731]: 367.0821(100), 353.0667(97), 109.0282(74), 197.0446(58), 335.0924(53), 489.1198(35) | 9″-Methyl salvianolate B |
| 80 | A, B, C | 9.22 * | 537.1038 | 537.1036 | −0.51 | C27H22O12 | MS2[537]: 339.0511(100), 197.0447(82), 135.0440(74), 295.0613(66), 179.0338(25) | Lithospermic acid |
| 81 | A, B, C | 9.22 # | 493.1140 | 493.1138 | −0.45 | C26H22O10 | MS2[493]: 185.0235(100), 109.0281(94), 295.0608(77), 203.0343(21), 159.0440(20), 135.0439(19), 197.0448(17), 179.0343(12) | Salvianolic acid A |
| 82 | C | 9.68 # | 493.1140 | 493.1140 | −0.09 | C26H22O10 | MS2[493]: 197.0446(100), 135.0439(82), 295.0608(55), 179.0338(38), 185.0234(33), 109.0281(33), 269.0817(30) | Salvianolic acid A isomer |
| 83 | C | 10.19 * | 717.1461 | 717.1460 | −0.15 | C36H30O16 | MS2[717]: 321.0401(100), 339.0507(29), 295.0609(16), 185.0237(10), 197.0444(3), 135.0438(2), 179.0339(2) | Salvianolic acid B |
| 84 | A, B, C | 10.33 # | 357.0616 | 357.0616 | −0.12 | C18H14O8 | MS2[357]: 135.0439(100), 337.0353(56), 72.9917(27), 179.0339(26), 197.0446(25), 321.0403(18) | Salvianolic acid I |
| 85 | A, B, C | 10.66 # | 731.1618 | 731.1625 | 1.07 | C37H32O16 | MS2[731]: 229.0136(100), 339.0506(76), 313.0716(59), 203.0340(47), 267.0659(32) | 9″-Methyl salvianolate B isomer |
| 86 | A, B, C | 11.04 # | 277.1445 | 277.1441 | −1.56 | C16H22O4 | MS2[277]: 277.1443(100), 233.1541(74), 203.1433(11) | Dibutylphthalate |
| 87 | B, C | 11.16 # | 373.0929 | 373.0926 | −0.73 | C19H18O8 | MS2[373]: 135.0439(100), 175.0391(65), 197.0447(57), 179.0340(27), 72.9917(23), 161.0235(15) | Methyl rosmarinate |
| 88 | A, B, C | 11.20 # | 201.1132 | 201.1125 | −3.79 | C10H18O4 | MS2[201]: 139.1116(100), 201.1123(92), 183.1017(47) | 3-tert-Butyladipic acid |
| 89 | A, B, C | 11.65 * | 491.0984 | 491.0980 | −0.84 | C26H20O10 | MS2[491]: 311.0559(100), 135.0439(35), 197.0447(4), 179.0341(2) | Salvianolic acid C |
| 90 | C | 11.87 # | 551.1195 | 551.1193 | −0.44 | C28H24O12 | MS2[551]: 321.0400(100), 231.0292(22), 109.0281(21), 293.0455(17), 135.0440(11), 197.0447(8), 179.0337(4) | Monomethyl lithospermate isomer |
| 91 | B, C | 11.92 # | 731.1618 | 731.1627 | 1.32 | C37H32O16 | MS2[731]: 335.0560(100), 353.0666(60), 309.0762(38), 135.0439(33), 197.0446(20) | 9″-Methyl salvianolate B isomer |
| 92 | A | 12.49 *# | 301.0354 | 301.0353 | −0.22 | C15H10O7 | MS2[301]: 151.0026(100), 301.0359(89), 178.9977(53), 121.0283(19) | Quercetin |
| 93 | C | 13.01 # | 731.1618 | 731.1630 | 1.73 | C37H32O16 | MS2[731]: 339.0507(100), 229.0137(42), 295.0607(30), 359.0772(19) | 9″-Methyl salvianolate B isomer |
| 94 | A, B, C | 13.13 # | 363.1085 | 363.1086 | 0.03 | C18H20O8 | MS2[363]: 218.0214(100), 190.0262(79), 303.0872(74), 227.0343(70), 219.0251(5) | Shikonin derivative |
| 95 | C | 13.25 *# | 271.0612 | 271.0613 | 0.31 | C15H12O5 | MS2[271]: 151.0025(100), 271.0612(62), 119.0490(42), 227.1071(23), 107.0125(14), 93.0332(14), 177.0185(11) | Naringenin |
| 96 | A, B, C | 14.54 # | 285.0405 | 285.0408 | 1.30 | C15H10O6 | MS2[285]: 285.0406(100), 227.0711(16), 241.0508(11), 215.0701(11) | Kaempferol |
| 97 | A, B, C | 14.56 | 253.0870 | 253.0869 | −0.67 | C16H14O3 | MS2[253]: 237.0551(100), 238.0615(31), 270.0533(20), 253.0505(14) | Rhizonone |
| 98 | A, B, C | 14.57 | 315.1238 | 315.1239 | 0.43 | C18H20O5 | MS2[315]: 241.0865(100), 256.1102(65), 300.1002(27), 271.0973(22), 285.0762(11) | Ethylshikonin |
| 99 | A, B, C | 14.61 # | 299.0561 | 299.0561 | 0.03 | C16H12O6 | MS2[301]: 284.0323(100), 255.0293(16), 299.0560(12), 285.0359(10) | Hispidulin |
| 100 | C | 15.35 # | 583.2562 | 583.2564 | 0.24 | C33H36N4O6 | MS2[583]: 285.1242(100), 297.1239(15), 241.1341(9), 213.1030(3) | Bilirubin |
| 101 | A, B, C | 15.35 # | 187.1340 | 187.1331 | −4.53 | C10H20O3 | MS2[187]: 59.0124(100), 125.0960(21), 141.8672(14), 187.1324(14) | 3-Hydroxydecanoic acid |
| 102 | A, B, C | 15.74 # | 371.1500 | 371.1497 | −0.87 | C21H24O6 | MS2[371]: 271.0970(100), 253.0863(52), 99.0439(49), 241.0868(40), 225.0916(38) | Valerylshikonin isomer |
| 103 | A, B, C | 15.85 # | 403.1398 | 403.1398 | −0.03 | C21H24O8 | MS2[403]: 303.0875(100), 218.0215(96), 227.0345(65), 190.0258(75), 99.0435(83) | Shikonin derivative |
| 104 | B, C | 15.96 | 391.1762 | 391.1762 | −0.20 | C21H28O7 | MS2[391]: 255.1024(100), 273.1133(92), 190.0262(18), 117.0544(17), 227.0340(14) | Hydroxyshikonofuran J |
| 105 | A, B, C | 15.99 # | 391.1398 | 391.1399 | 0.21 | C20H24O8 | MS2[363]: 218.0215(100), 303.0872(98), 227.0343(87), 190.0262(82), 87.0437(71) | Shikonin derivative |
| 106 | A, B, C | 16.05 | 269.0819 | 269.0819 | −0.09 | C16H14O4 | MS2[269]: 136.0153(100), 251.0712(58), 223.0753(44), 269.0817(41), 241.0870(31) | Dehydratedshikonin |
| 107 | A, B, C | 16.13 | 333.1344 | 333.1341 | −0.82 | C18H22O6 | MS2[333]: 255.1023(100), 273.1129(66), 219.0292(15), 254.0937(2), 237.0917(1) | Hydroxyshikonofuran A |
| 108 | B, C | 16.31 | 391.1762 | 391.1763 | 0.27 | C21H28O7 | MS2[391]: 255.1023(100), 273.1133(91), 117.0545(25) | Hydroxyshikonofuran K |
| 109 | C | 16.36 # | 283.0612 | 283.0611 | −0.45 | C16H12O5 | MS2[283]: 283.0608(100), 240.0423(27), 257.0451(19), 239.0373(3) | Glycitein |
| 110 | A, B, C | 16.46 # | 333.1344 | 333.1343 | −0.07 | C18H22O6 | MS2[333]: 255.1023(100), 273.1130(65), 315.1576(30), 254.0945(2), 237.0918(2) | Hydroxyshikonofuran A isomer |
| 111 | A, B, C | 16.76 | 315.1238 | 315.1238 | −0.06 | C18H20O5 | MS2[315]: 255.1023(100), 59.0125(29), 227.1077(13), 237.0922(11), 121.0281(7), 187.0390(4) | Shikonofuran A |
| 112 | A, B, C | 16.76 | 347.1136 | 347.1134 | −0.57 | C18H20O7 | MS2[347]: 241.0867(100), 287.0922(73), 269.0824(69), 59.0124(68), 227.1079(23) | Shikonin acetate |
| 113 | A, B, C | 16.79 # | 305.1758 | 305.1758 | 1.63 | C18H26O4 | MS2[305]: 135.0803(100), 249.1492(62), 174.9551(46), 235.0195(26), 146.9600(26) | Octyl ferulate |
| 114 | C | 16.87 *# | 283.0612 | 283.0612 | −0.03 | C16H12O5 | MS2[283]: 283.0608(100), 268.0377(67), 265.1805(10) | Baicalein |
| 115 | A, B, C | 17.67 # | 349.1293 | 349.1292 | −0.37 | C18H22O7 | MS2[349]: 255.1023(100), 227.1071(43), 237.0921(13) | Shikonofurans derivative |
| 116 | A, B, C | 17.00 | 405.1555 | 405.1558 | 0.74 | C21H26O8 | MS2[405]: 303.0870(100), 218.0214(59), 190.0262(58), 227.0343(56), 245.0451(54), 101.0594(53) | Dihydrohydroxyshikonin tiglate |
| 117 | A, B, C | 17.11 * | 299.0561 | 299.0560 | −0.38 | C16H12O6 | MS2[299]: 299.0558(100), 254.0581(27), 237.0556(25), 281.0452(22), 284.0333(13) | Kaempferide |
| 118 | A, B, C | 17.14 # | 313.0718 | 313.0716 | −0.46 | C17H14O6 | MS2[313]: 298.0482(100), 202.1159(82), 283.0243(53), 312.2260(47), 255.0294(24) | Pectolinarigenin |
| 119 | B, C | 17.19 | 345.0980 | 345.0975 | −1.47 | C18H18O7 | MS2[345]: 285.0766(100), 267.0674(32), 257.0807(25), 59.0123(20) | Lithospermidin C |
| 120 | A, B, C | 18.04 * | 287.0925 | 287.0923 | −0.62 | C16H16O5 | MS2[287]: 218.0214(100), 219.0255(9), 190.0261(2) | Shikonin |
| 121 | B, C | 18.15 | 433.1868 | 433.1871 | 0.65 | C23H30O8 | MS2[433]: 255.1023(100), 273.1131(84), 273.9557(16), 59.0126(5) | Hydroxyshikonofuran F |
| 122 | A, B, C | 18.17 | 361.1657 | 361.1655 | −0.54 | C20H26O6 | MS2[361]: 255.1022(100), 273.1129(73), 259.0607(15), 87.0439(4) | Hydroxyshikonofuran G |
| 123 | B, C | 18.45 | 433.1868 | 433.1866 | −0.56 | C23H30O8 | MS2[433]: 255.1023(100), 273.1131(81), 59.0123(6), 237.0913(3) | Hydroxyshikonofuran H |
| 124 | A, B, C | 18.47 | 361.1657 | 361.1658 | 0.39 | C20H26O6 | MS2[361]: 255.1022(100), 273.1128(72), 218.0214(10), 87.0438(3) | Hydroxyshikonofuran D |
| 125 | A, B, C | 18.57 # | 269.0455 | 269.0454 | 3.57 | C15H10O5 | MS2[301]: 269.0454(100), 149.0229(1) | Apigenin |
| 126 | A, B, C | 18.65 # | 345.0980 | 345.0975 | −1.47 | C18H18O7 | MS2[345]: 285.0766(100), 267.0659(14), 257.0817(8), 59.0124(4), 239.0709(1) | Lithospermidin C isomer |
| 127 | A, B, C | 18.83 | 343.1551 | 343.1551 | −0.03 | C20H24O5 | MS2[343]: 283.0974(100), 266.0824(14), 255.1023(35), 87.0438(16), 227.1078(3) | Shikonofuran D |
| 128 | A, B, C | 18.89 | 343.1187 | 343.1184 | −0.80 | C19H20O6 | MS2[343]: 283.0974(100), 255.1023(35), 87.0438(16), 266.0824(14) | 1-Methoxyacetylshikonin |
| 129 | A, B, C | 18.92 | 373.1657 | 373.1658 | 0.37 | C21H26O6 | MS2[373]: 255.1022(100), 273.1131(61), 174.9551(35) | Hydroxyshikonofuran I |
| 130 | C | 18.99 # | 357.2071 | 357.2071 | −0.04 | C22H30O4 | MS2[357]: 357.2070(100), 339.1964(69), 295.2061(31), 327.1965(30), 269.0819(13) | Cannabidiolic acid |
| 131 | A, B, C | 19.04 # | 269.0819 | 269.0812 | −0.20 | C16H14O4 | MS2[269]: 269.0816(100), 254.0582(80), 149.0231(42), 133.0646(37), 210.0677(35) | Medicarpin |
| 132 | A, B, C | 19.16 | 373.1657 | 373.1655 | −0.36 | C21H26O6 | MS2[373]: 255.1025(100), 273.1130(67), 174.9550(24) | Hydroxyshikonofuran E |
| 133 | B, C | 19.33 | 375.1813 | 375.1812 | 1.03 | C21H28O6 | MS2[375]: 255.1025(100), 273.1132(71) | Hydroxyshikonofuran B |
| 134 | A, B, C | 19.33 | 343.1187 | 343.1185 | −0.53 | C19H20O6 | MS2[343]: 283.0973(100), 255.1021(13) | 1-methoxyacetylshikonin isomer |
| 135 | A, B, C | 19.49 | 375.1813 | 375.1810 | 0.72 | C21H28O6 | MS2[375]: 255.1023(100), 273.1132(74) | Hydroxyshikonofuran L |
| 136 | A, B, C | 19.52 | 355.1551 | 355.1546 | −1.49 | C21H24O5 | MS2[355]: 255.1025(100), 355.3217(70), 99.0438(58), 218.0218(30), 227.1070(15), 237.0919(4) | Shikonofuran E |
| 137 | B, C | 19.67 | 375.1813 | 375.1784 | −7.74 | C21H28O6 | MS2[375]: 255.1024(100), 273.1131(69), 101.0594(3) | Hydroxyshikonofuran C |
| 138 | A, B, C | 19.74 | 359.1136 | 359.1139 | 0.82 | C19H20O7 | MS2[359]: 299.0921(100), 284.0687(57), 359.1137(11), 161.0234(9), 271.0973(7) | 1/4-methoxylithospermidin C |
| 139 | A, B, C | 19.80 | 425.1242 | 425.1245 | 1.90 | C23H22O8 | MS2[425]: 321.1494(100), 178.9977(59), 227.1074(58), 271.0969(27), 363.1226(21), 245.1178(16), 345.1130(12) | Unknown |
| 140 | A, B, C | 19.80 | 375.1813 | 375.1811 | −0.52 | C21H28O6 | MS2[375]: 255.1023(100), 273.1131(72), 101.0594(3) | Hydroxyshikonofuran M |
| 141 | A, B | 20.06 | 357.1707 | 357.1705 | −0.61 | C21H26O5 | MS2[357]: 255.1023(100), 101.0594(47), 297.1130(18), 227.1069(9), 121.0281(7), 237.0916(6) | Shikonofuran B |
| 142 | A, B, C | 20.15 | 357.1707 | 357.1704 | −0.95 | C21H26O5 | MS2[357]: 255.1023(100), 101.0594(45), 227.1068(10), 237.0921(8), 121.0283(8), 172.0517(2), 143.0497(2) | Shikonofuran C |
| 143 | A, B, C | 20.16 | 371.1500 | 371.1497 | −0.79 | C21H24O6 | MS2[371]: 269.0817(100), 241.0866(16), 251.0706(7) | α,α-dimethylpropionylshikonin |
| 144 | A, B, C | 20.50 | 387.1449 | 387.1448 | −0.30 | C21H24O7 | MS2[387]: 117.0544(100), 269.0813(31), 251.0711(31), 59.0123(21), 241.0867(13) | β-hydroxyisovalerylshikonin |
| 145 | A, B, C | 20.94 | 401.1606 | 401.1605 | 1.16 | C22H26O7 | MS2[401]: 299.0923(100), 255.1027(57), 121.0284(15), 313.0705(10), 237.0908(10) | 1-Methoxy-β-hydroxyisovalerylshikonin |
| 146 | A, B, C | 21.33 | 387.1449 | 387.1448 | −0.23 | C21H24O7 | MS2[387]: 101.0594(100), 189.0184(76), 285.0765(60), 217.0135(49), 257.0814(28), 267.0659(19) | Lithospermidin A |
| 147 | B, C | 21.69 | 459.1661 | 459.1664 | 0.71 | C24H28O9 | MS2[459]: 299.0916(100), 59.0124(58), 271.0966(36), 281.0820(21) | 1/4-methoxylithospermidin H |
| 148 | B, C | 21.70 | 445.1504 | 445.1503 | −0.18 | C23H26O9 | MS2[445]: 285.0766(100), 257.0819(9), 59.0124(8), 267.0657(7) | Lithospermidin D |
| 149 | A, B | 21.76 # | 271.0976 | 271.0974 | −0.53 | C16H16O4 | MS2[271]: 253.0867(100), 271.0968(73), 203.0342(58), 256.0739(44), 238.0630(39) | Deoxyshikonin isomer |
| 150 | A, B, C | 21.89 * | 329.1031 | 329.1030 | −0.19 | C18H18O6 | MS2[329]: 269.0818(100), 251.0710(81), 241.0868(48), 59.0124(47) | Acetylshikonin |
| 151 | A, B, C | 22.80 | 387.1449 | 387.1449 | 0.01 | C21H24O7 | MS2[387]: 299.0923(100), 270.0893(94), 285.0756(68), 271.0954(66), 87.0438(63), 253.0864(24) | 1/4-methoxylithospermidin I |
| 152 | A, B, C | 22.84 | 373.1293 | 373.1293 | 0.15 | C20H22O7 | MS2[373]: 285.0768(100), 267.0663(10), 257.0817(9), 174.9552(8), 87.0438(6) | Lithospermidin E |
| 153 | B, C | 23.03 # | 445.1504 | 445.1497 | −1.61 | C23H26O9 | MS2[445]: 285.0768(100), 257.0817(49), 59.0123(18), 267.0655(7) | Lithospermidin D isomer |
| 154 | A, B, C | 23.13 | 387.1449 | 387.1447 | −0.56 | C21H24O7 | MS2[387]: 299.0903(100), 285.0767(78), 270.0897(74), 87.0438(58), 271.0974(50) | 1/4-methoxylithospermidin E |
| 155 | A, B, C | 24.03 | 285.0768 | 285.0767 | −0.66 | C16H14O5 | MS2[285]: 285.0766(100), 267.0659(55), 227.0344(50), 73.0281(8), 257.0817(6), 239.0708(2) | Sakuranetin |
| 156 | B, C | 24.23 # | 445.1504 | 445.1504 | −0.11 | C23H26O9 | MS2[445]: 285.0771(100), 257.0809(93), 59.0124(35) | Lithospermidin D isomer |
| 157 | A, B, C | 24.57 | 399.1449 | 399.1445 | −1.07 | C22H24O7 | MS2[399]: 270.0891(100), 299.0921(98), 271.0978(68), 99.0437(56), 281.0811(21) | 1/4-methoxylithospermidin J |
| 158 | A, B, C | 24.63 | 385.1293 | 385.1292 | −0.33 | C21H22O7 | MS2[385]: 285.0767(100), 257.0817(11), 99.0438(9), 267.0651(8) | Lithospermidin F |
| 159 | A, B, C | 24.81 | 399.1449 | 399.1445 | −1.07 | C22H24O7 | MS2[399]: 299.0921(100), 271.0957(63), 99.0438(53), 281.0811(25) | 1/4-methoxylithospermidin F |
| 160 | A, B, C | 24.99 | 343.1187 | 343.1193 | 1.69 | C19H20O6 | MS2[343]: 57.0332(100), 343.2271(79), 269.0806(55), 251.0713(51), 285.1859(50), 73.0281(43), 241.0872(33) | Propionylshikonin |
| 161 | A, B, C | 25.48 | 401.1606 | 401.1603 | −0.67 | C22H26O7 | MS2[401]: 299.0919(100), 270.0900(92), 101.0594(61), 271.0976(53), 281.0826(21), 253.0871(17) | 1/4-methoxylithospermidin A |
| 162 | A, B, C | 25.60 | 387.1449 | 387.1448 | −0.30 | C21H24O7 | MS2[387]: 285.0766(100), 257.0816(9), 101.0594(9), 267.0658(8) | Lithospermidin B |
| 163 | B, C | 26.29 | 459.1661 | 459.1654 | −1.41 | C24H28O9 | MS2[459]: 299.0922(100), 271.0971(10), 59.0124(9) | 1/4-methoxylithospermidin D |
| 164 | B | 26.38 # | 445.1504 | 445.1500 | −0.94 | C23H26O9 | MS2[445]: 285.0768(100), 257.0815(54), 59.0125(21), 267.0662(6) | Lithospermidin D isomer |
| 165 | B | 26.38 # | 271.0976 | 271.0974 | −0.86 | C16H16O4 | MS2[271]: 253.0867(100), 271.0966(71), 203.0341(57), 256.0736(48), 238.0629(39) | Deoxyshikonin isomer |
| 166 | A, B, C | 26.60 | 429.1555 | 429.1552 | −0.59 | C23H26O8 | MS2[429]: 269.0822(100), 251.0710(65), 59.0125(65), 241.0866(35) | 5-acetoxy-valerylshikonin |
| 167 | A, B | 28.04 # | 271.0976 | 271.0974 | −0.53 | C16H16O4 | MS2[271]: 253.0866(100), 271.0967(66), 203.0341(54), 256.0738(45), 238.0629(42) | Deoxyshikonin isomer |
| 168 | B, C | 28.07 | 429.1555 | 429.1551 | −0.87 | C23H26O8 | MS2[429]: 269.0822(100), 251.0706(63), 59.0124(61), 241.0871(37) | β-acetoxyisovalerylshikonin |
| 169 | A, B, C | 28.08 # | 425.1242 | 425.1227 | −2.13 | C23H22O8 | MS2[425]: 269.0818(100), 251.0710(27), 87.0437(17), 241.0966(10) | Shikonin derivative |
| 170 | A, B, C | 28.16 | 357.1344 | 357.1342 | −0.40 | C20H22O6 | MS2[357]: 269.0819(100), 251.0713(70), 87.0438(63), 241.0863(50), 223.0820(4) | Butyrylshikonin |
| 171 | A, B, C | 29.19 | 357.1344 | 357.1342 | −0.40 | C20H22O6 | MS2[357]: 269.0819(100), 251.0707(66), 87.0437(72), 241.0866(43) | Isobutyrylshikonin |
| 172 | A, B, C | 29.53 | 399.1449 | 339.1999 | 9.71 | C22H24O7 | MS2[399]: 299.0923(100), 99.0439(11), 281.0812(10), 271.0970(9) | 1/4-Methoxylithospermidin L |
| 173 | A, B, C | 30.11 # | 271.0976 | 271.0974 | −0.64 | C16H16O4 | MS2[271]: 253.0867(100), 271.0967(74), 203.0341(54), 256.0736(45), 238.0631(41) | Deoxyshikonin isomer |
| 174 | A, B, C | 30.19 * | 369.1344 | 369.1342 | −0.39 | C21H22O6 | MS2[369]: 269.0818(100), 251.0710(71), 99.0438(68), 270.0888(57), 241.0867(49) | β,β-dimethylacrylshikonin |
| 175 | A, B, C | 30.44 | 401.1606 | 401.1603 | −0.59 | C22H26O7 | MS2[401]: 299.0924(100), 121.0283(15), 271.0959(12), 101.0592(11), 281.0879(10) | 1/4-methoxylithospermidin B |
| 176 | A, B, C | 30.64 | 599.1923 | 599.1926 | 1.52 | C34H32O10 | MS2[599]: 426.1099(100), 412.0935(42), 102.9554(26), 132.4304(21), 116.5537(21), 59.0124(21) | 7-(11′-Deoxyalkannin)-Acetylshikonin |
| 177 | A | 30.72 | 369.1344 | 369.1340 | −1.07 | C21H22O6 | MS2[369]: 269.0817(100), 251.0709(68), 99.0438(61), 241.0864(52) | α-methylene-butenoylshikonin |
| 178 | A, B, C | 30.89 # | 271.0976 | 271.0974 | −0.53 | C16H16O4 | MS2[271]: 253.0868(100), 271.0968(77), 203.0343(64), 256.0738(47), 238.0630(40) | Deoxyshikonin isomer |
| 179 | A, B, C | 30.98 | 371.1500 | 371.1497 | −0.95 | C21H24O6 | MS2[371]: 269.0818(100), 251.0710(66), 101.0594(65), 241.0865(43) | α-methylbutyrylshikonin |
| 180 | A, B, C | 31.62 | 555.1661 | 555.1657 | −0.63 | C32H28O9 | MS2[555]: 486.0952(100), 555.1646(11) | Shikometabolin B |
| 181 | A, B, C | 31.78 | 599.1923 | 599.1936 | 3.14 | C34H32O10 | MS2[599]: 412.0944(100), 426.1105(77) | 7-(11′-Deoxyalkannin)-Acetylalkannin |
| 182 | A | 32.05 | 369.1344 | 369.1343 | −0.14 | C21H22O6 | MS2[369]: 269.0816(100), 251.0705(48), 99.0439(43), 241.0871(37) | Tigloylshikonin |
| 183 | A, B, C | 33.05 | 371.1500 | 371.1497 | −0.95 | C21H24O6 | MS2[371]: 269.0819(100), 101.0594(71), 251.0710(64), 241.0866(47) | Isovalerylshikonin |
| 184 | A, B, C | 34.64 # | 271.0976 | 271.0973 | −1.08 | C16H16O4 | MS2[271]: 253.0866(100), 271.0974(66), 203.0341(58), 256.0735(45), 238.0630(37) | Deoxyshikonin isomer |
| 185 | A | 35.15 | 369.1344 | 369.1342 | −0.47 | C21H22O6 | MS2[369]: 269.0829(100), 251.0721(46), 99.0443(54), 241.0867(40) | Angeloylshikonin |
| 186 | A, B, C | 35.84 | 627.2236 | 627.2235 | 0.69 | C36H36O10 | MS2[627]: 426.1100(100), 412.0944(80), 87.0437(45), 495.1801(24), 349.1006(12), 290.5349(12) | 7-(11′-Deoxyalkannin)-Isobutyrylshikonin |
| 187 | A, B, C | 38.59 | 639.2236 | 639.2231 | 0.19 | C37H36O10 | MS2[639]: 537.1556(100), 639.2231(5), 509.1626(4), 519.1473(2), 101.0590(2) | 7-(11′-Deoxyalkannin)-β,β-dimethylacrylshikonin |
| 188 | A, B, C | 38.89 | 639.2236 | 639.2232 | 0.30 | C37H36O10 | MS2[639]: 537.1553(100), 639.2230(4), 519.1443(2), 101.0589(1) | 7-(11′-Deoxyalkannin)-β,β-dimethylacrylalkannin |
* Identified by comparison with standards. # First reported in Arnebiae Radix.
Figure 2.
The high-resolution extracted ion chromatogram (HREIC) in 10 ppm for multiple compounds in Arnebiae Radix. (A) m/z 313.07176, 131.04621, 145.06186, 503.16175, 191.05611, 149.04554, 177.04046, 147.02989, 243.06225, 731.16175, 493.11402, 357.06159, 201.11323, 253.08701, 315.12379, 299.05611, 583.25620, 175.06119, 153.01933, 188.03531, 172.09791, 151.04006, 193.05063, 375.13100, 163.04006, 206.08226, 173.08193, 174.05605, 449.10893, 137.02441, 715.13045, 551.11949, 277.14453, 167.02106, 161.04554, 145.05063, 164.07170, 218.10339, 158.08226, 181.05063, 203.08260, 187.13396, 371.15001, 269.08193, 333.13436, 345.09797, 357.20713, 375.18131, 387.14492; (B) m/z 491.09837, 609.14610, 731.16175, 135.02989, 195.05102, 193.03537, 191.01972, 197.04554, 167.03498, 161.02441, 179.03498, 163.04006, 493.11402, 463.08819, 593.15119, 187.09758, 277.14453, 373.09289, 305.17583, 313.07176, 363.10854, 283.06119, 347.11362, 349.12927, 299.05611, 361.16566, 269.04554, 355.15509, 425.12419, 357.17074, 459.16605, 373.12927, 445.15040, 285.07684, 399.14492, 385.12927, 401.16057, 599.19227, 465.10384, 433.11402; (C) m/z 179.05611, 287.09249, 555.16605, 405.15549, 137.02441, 329.10306, 369.13436, 627.22357, 537.10384, 429.15549, 445.15040, 271.09758, 343.15509, 433.18679, 285.04046, 343.11871, 373.16566, 357.13436, 357.06159, 391.13984, 169.01424, 417.08271, 465.10384, 289.07176, 433.11402, 403.13984, 391.17622; (D) m/z 363.10854, 343.15509, 433.18679, 285.04046, 343.11871, 373.16566, 357.13436, 357.06159, 391.13984, 169.01424, 417.08271, 465.10384, 289.07176, 433.11402, 403.13984, 391.17622, 283.06119, 347.11362, 349.12927, 405.15549, 299.05611, 361.16566, 269.04554, 355.15509, 359.11362, 425.12419, 357.17074, 387.14492, 459.16605, 373.12927, 445.15040, 285.07684, 399.14492, 385.12927, 401.16057, 369.13436, 599.19227, 627.22357; (E) m/z 131.04621, 465.10384, 433.11402, 449.10893, 463.08819, 493.11402, 551.11949, 731.16175, 271.06119, 583.25620, 283.06119, 357.20713, 537.10384, 133.01424, 429.15549, 341.10893, 387.14492, 359.11362, 387.11441, 639.22357, 329.10306, 717.14610,359.07724; (F) m/z 609.18249, 301.03537, 357.17074, 271.09758, 369.13436, 313.07176, 131.04621, 145.06186, 503.16175, 191.05611, 149.04554, 177.04046, 147.02989, 243.06225, 731.16175, 493.11402, 357.06159, 201.11323, 253.08701, 315.12379, 299.05611 ((A–D,E,F) correspond to the EIC of Samples B, C, A, respectively).
2.3.1. Identification of Shikonins
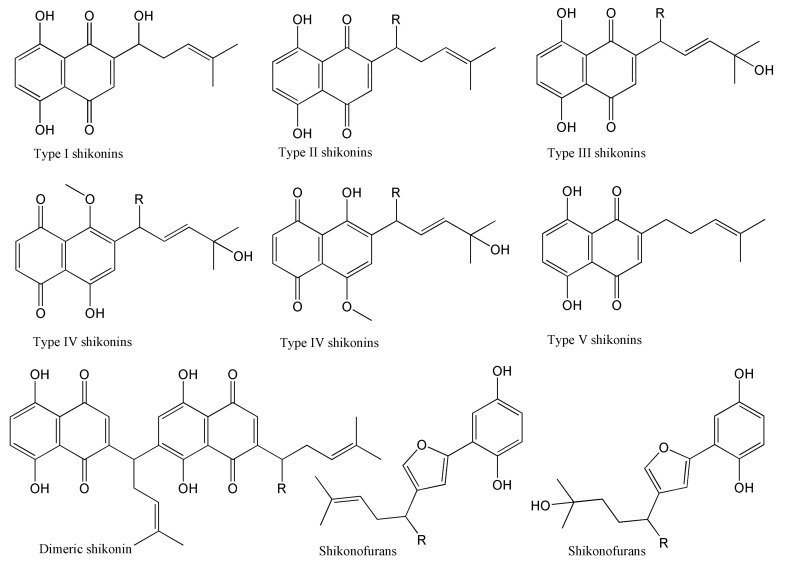
It is well-known that the common structural features of most shikonins are a skeleton of naphthoquinone combined with an isohexenyl side chain. Based on a comparison with fragment ions detailed in the literature and reference standards, this study identified seven different types of shikonin compounds in the crude extract, and the chemical structures of these are shown in Figure 3.
Figure 3.
Chemical structures of 7 different types of shikonin compounds found in Arnebiae Radix.
Type I Shikonins
Shikonins, composed of a naphthoquinone moiety and an isohexenyl side chain, were identified based on the DFIs at m/z 218.0213 and 190.0261. In total, five type I shikonins (94, 103, 105, 116, and 120) were unambiguously or tentatively identified. For instance, shikonin (120, R.T. = 18.04 min) was unambiguously identified by comparing the listed diagnostic ions and retention times with reference standards. Compounds 94, 103, and 105 were found at 13.13, 15.85, and 15.99 min, and these generated similar fragment ions as the shikonins. Thus, they were tentatively characterized as shikonin derivatives. Compound 116, which was observed at 17.00 min yielded a deprotonated ion [M − H]− m/z 405.15549 and was tentatively identified as dihydrohydroxyshikonin tiglate [28].
Type II Shikonins
Compounds 150 and 174 found at 21.89 and 30.19 min, yielded deprotonated ions [M − H]− m/z 329.10306 (C18H17O6, −0.19 ppm) and 369.13436 (C21H21O6, −0.39 ppm), respectively. Through a comparison with the reference standards, they were identified as acetylshikonin and β,β′-dimethylacrylshikonin. Compounds 102, 143, 179, and 183, which were found at 15.74, 20.16, 30.98, and 33.05 min, showed a common precursor ion at [M − H]− m/z 371.1500 (C21H24O6). They mainly yielded fragment ions at m/z 269.0819, 241.0869, and 251.0709 and were tentatively characterized as α,α-dimethylpropionylshikonin, α-methylbutyrylshikonin, isovalerylshikonin, and isovalerylshikonin isomer through a comparison with the literature and MS2 fragmentation [27,28]. Compound 144, found at 20.50 min, showed a precursor ion at [M − H]− m/z 387.14492. Therefore, it was tentatively characterized as β-hydroxyisovalerylshikonin [27,28]. Compounds 166 and 168 appeared at retention times (tR) of 26.60 min and 28.07 min, respectively. The parent ion m/z 429.15549 generated the characteristic fragments m/z 269.0822, 251.0709, and 241.0869, which were tentatively identified as 5-acetoxy-valerylshikonin and β-acetoxyisovalerylshikonin [27]. Similarly, compounds 170 and 171 were tentatively characterized as butyrylshikonin and isobutyrylshikonin. Overall, compounds 177, 182, and 185 demonstrated highly similar product ion spectra and MS/MS fragmentation behaviors but had different retention times, suggesting that they are isobaric compounds. Therefore, they were tentatively identified as α-methylene-butenoylshikonin, tigloylshikonin, and angeloylshikonin, as previously reported [27].
Type III Shikonins
Due to the presence of a double bond between C-12 and C-13, the α-cleavage mechanism of the ester carbonyl is the main fragmentation pathway of the type III shikonins producing product ion at m/z 285.0766. This ion formed the diagnostic generation product ions 257.0807 and 267.0674 by successive neutral loss of H2O and CO, respectively [27]. Compounds 146 and 162 had the same molecular formula C21H24O7. In addition, diagnostic products at m/z 285.0766, 267.0663, and 257.0815 were observed through MS2 fragmentation ion. Based on a literature search of all shikonins reported [29], compounds 146 and 162 were tentatively identified as lithospermidin A and lithospermidin B, respectively. The ESI-MS of compounds 119, 148, 152, and 158 gave deprotonated molecular ions at m/z 345.09797, 445.15040, 373.12927, and 385.12927, which matched the molecular formulas C18H18O7, C23H26O9, C20H22O7, and C21H22O7, respectively. Consequently, these compounds were tentatively identified as lithospermidin C, lithospermidin D, lithospermidin E, and lithospermidin F [27]. Compound 126 was eluted at 18.65 min and generated the same MS and MS2 fragmentation results as compound 119. Thus, this compound was tentatively identified as the lithospermidin C isomer. Compounds 153, 156, and 164 appeared at retention times (tR) of 23.03 min, 24.23 min, and 26.38 min, respectively. They had the same MS and MS2 fragmentation ions as compound 148 and were tentatively identified as lithospermidin D isomers.
Type IV Shikonins
Diagnostic ions were identified at m/z 299.0921, 284.0687, and 271.0973 for type IV shikonins, and fragmentation ions were obtained at m/z 284.0687 and at m/z 271.0973 via the neutral loss of CH3 and CO from 299.0921, respectively [27]. As an example, compound 138 with a retention time at 19.74 min showed a deprotonated molecular ion at m/z 359.11362 (C19H19O7, 0.82 ppm) by ESI-MS. Diagnostic product ions were located at 299.0921, 284.0697, and 271.0973 in MS2 and the compound was tentatively identified as 1/4-methoxylithospermidin C [27,28]. According to the literature [29,30] and the different retention times, isomers in each group were eluted based on a predefined order. Compounds 147, 151, 154, 157, 159, 161, 163, 172, and 175 were predicted to be 1/4-methoxylithospermidin H, 1/4-methoxylithospermidin I, 1/4-methoxylithospermidin E, 1/4-methoxylithospermidin J, 1/4-methoxylithospermidin F, 1/4-methoxylithospermidin A, 1/4-methoxylithospermidin D, 1/4-methoxylithospermidin L, and 1/4-methoxylithospermidin B from the experimental chromatographic peaks [27,28]. By comparing MS and MS2 fragmentation in the literature, compound 145 was tentatively assigned as 1-methoxy-β-hydroxyisovalerylshikonin [28].
Type V Shikonins
Deoxyshikonin, a precursor for the biosynthesis of other shikonins, was not classified as any type of shikonin or shikonofuran due to the absence of a carboxylic group. The deprotonated molecular ion at m/z 271.0968 for deoxyshikonin gave a product ion base peak at m/z 203.0342. In addition, a product ion at m/z 253.0867 was derived from the neutral loss of H2O from the deprotonated molecular ion [27]. Compounds 149, 165, 167, 173, 178, and 184 were eluted at 21.76, 26.38, 28.04, 30.11, 30.89, and 34.64 min and showed a deprotonated molecular ion [M − H]− at m/z 271.098 (C16H15O4). According to a previously published paper [27,28] and the MS2 fragmentation results, these compounds were tentatively assigned as deoxyshikonin or its isomers based on the different base peak ions in the MS2 spectrum.
Shikonofurans
According to previous studies [27], m/z 255.1024 is the DFIs of shikonofurans, and it generates fragment ions at m/z 237.0917 and m/z 227.0340 through the neutral loss of H2O and CO, respectively. The compounds 111, 127, 136, 141, and 142 were eluted at 16.76, 18.83, 19.52, 20.06, and 20.15 min and showed deprotonated molecular ions [M − H]− at m/z 315.12379 (−0.06 ppm, C18H19O5), 343.15509 (−0.03 ppm, C20H23O5), 355.15509 (−1.49 ppm, C21H23O5), 357.17074 (−0.61 ppm, C37H35O10), and 357.17074 (−0.95 ppm, C37H35O10), suggesting that they are shikonofurans. Therefore, compounds 111, 127, 136, 141, and 142 were identified as shikonofuran A, shikonofuran D, shikonofuran E, shikonofuran B, and shikonofuran C, respectively, by comparing their respective MS2 data and data presented in the literature [27]. According to the DFIs, compound 115 was tentatively identified as a shikonofuran derivative. In addition, DFIs at m/z 273.1133 were used for the classification of another shikonofuran indicating that the hydration was likely on the double bond of the side chain. A peak at m/z 255.1024 was observed due to neutral losses of H2O from DFI 273.1133 [27]. Compounds 104, 107, 108, 110, 121, 122, 123, 124, 129, 132, 133, 135, 137 and 140 eluted at 15.96, 16.13, 16.31, 16.46, 18.15, 18.17, 18.45, 18.47, 18.92, 19.16, 19.33, 19.49, 19.67, and 19.80 were predicted to be hydroxyshikonofurans due to the fragment ions yielded at m/z 273.1133 and m/z 255.1024. Therefore, they were tentatively identified as hydroxyshikonofuran J, hydroxyshikonofuran A, hydroxyshikonofuran K, hydroxyshikonofuran A isomer, hydroxyshikonofuran F, hydroxyshikonofuran G, hydroxyshikonofuran H, hydroxyshikonofuran D, hydroxyshikonofuran I, hydroxyshikonofuran E, hydroxyshikonofuran B, hydroxyshikonofuran L, hydroxyshikonofuran C, and hydroxyshikonofuran M [27,28]. Compounds 128 and 134 were found at 18.89 and 19.33 min and showed a common precursor ion at [M − H]− m/z 343.11871. They were tentatively characterized as 1-methoxyacetylshikonin and its isomer, respectively [28].
Dimeric Shikonin
Compounds 176, 181, 186, 187, and 188 were eluted at 30.64, 31.78, 35.84, 38.59, and 38.89 min and showed deprotonated molecular ions [M − H]− at m/z 599.19227 (C34H31O10), 599.19227 (C34H31O10), 627.22357 (C36H35O10), 639.22357 (C37H35O10), and 639.22357 (C37H35O10). According to a previously published paper [28], they were tentatively assigned as 7-(11′-deoxyalkannin)-acetylshikonin,7-(11′-deoxyalkannin)-acetylalkannin, 7-(11′-deoxyalkannin)-isobutyrylshikonin, 7-(11′-deoxyalkannin)-β,β-dimethylacrylshikonin, and 7-(11′-deoxyalkannin)-β,β-dimethylacrylalkannin based on the different base peak ions in the MS2 spectrum listed in Table 1.
2.3.2. Identification of Phenolic Acids
Four compounds—rosmarinicacid (77), lithospermic acid (80), salvianolic acid B (83), and salvianolic acid C (89)—were unambiguously identified by comparing their MS and MS2 fragmentation ion and retention times with the reference standards in negative ionization mode.
Compound 69 produced the same MS and MS2 fragmentation results as compound 83 (salvianolic acid B). Thus, this compound was tentatively identified as salvianolic acid B isomer. Compounds 51, 55, 56, and 70 were found at 6.54, 6.71, 6.91, and 8.02 min and showed a common precursor ion at [M − H]− at m/z 537.1039 (C27H21O12). They yielded m/z 197.0446 by the breaking of the ester bond between the carbonyl group and the oxygen atom, m/z 179.0448 (C9H7O4) due to the loss of H2O units, and m/z 135.0439 (C8H7O2) by the loss of COOH. According to a previously published paper [31], they were tentatively assigned as salvianolic acid U, salvianolic acid T, salvianolic acid J, and salvianolic acid isomer based on the different base peak ions in the MS2 spectrum. Compounds 25, 26, 43, 65, 71, 84, 87, and 130 were eluted at 2.28, 2.28, 5.22, 7.60, 8.19, 10.33, 11.16, and 18.99 min with deprotonated ions [M − H]− at m/z 197.04554 (C9H9O5), 417.08271 (C20H17O10), 179.03498 (C9H7O4), 359.07724 (C18H15O8), 357.06159 (C18H13O8), 357.06159 (C18H13O8), 373.09289 (C19H17O8), and 357.20713 (C22H29O4). They were tentatively identified as danshensu, salvianolic acid D, caffeic acid, didehydiosalvianolic acid B, methyl rosmarinate, and cannabidiolic acid [31,32,33,34,35]. Likewise, compounds 53 and 57; compounds 58, 81, and 82; compounds 68, 75, and 90; and compounds 78, 79, 85, 91, and 93 produced similar diagnostic ions and MS2 fragmentation behaviors. They were provisional identified as salvianolic acid F, salvianolic acid A, monomethyl lithospermate, 9″-methyl salvianolate B, and their isomers, respectively.
Compound 3 was eluted at 0.88 min with deprotonated ions [M − H]− at m/z 387.11441 (−1.44 ppm, C13H23O13), and the presence of fragment ions at m/z 341.1084 and 179.0550 was used to tentatively identify it as 2,3,4,5,6-pentahydroxy-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyheptanoic acid [28]. Compounds 4, 5, and 6 were eluted at the same time (0.88 min) and produced the same fragment ions (89.0230, 101.0230, 113.0231), which were initially identified as α, α-trehalose, mannose, and raffinose. Compound 10 was eluted at 0.91 min with deprotonated ions [M − H]− at m/z 149.04554 and was initially identified as arabinose. By searching in the databases (ChemSpider, mzVault, and mzCloud), compounds 7, 8, 11, 12, 13, 16, 19, and 101 were eluted at 0.89, 0.90, 0.92, 0.93, 0.93, 1.17, 1.31, and 15.35 min with deprotonated ions [M − H]− at m/z 135.02989 (C4H7O5), 195.05102 (C6H11O7), 193.03537(C6H9O7), 133.01424 (C4H5O5), 177.04046 (C6H9O6), 147.02989(C5H7O5), 161.04554(C6H9O5), and 187.13396 (C10H19O3), respectively. They were tentatively identified as threonic acid, gluconic acid, β-D-glucopyranuronic acid, malic acid, δ-gluconic acid δ-lactone, α-hydroxyglutaric acid, 3-hydroxy-3-methylglutaric acid, and 3-hydroxydecanoic acid. According to the literature [36,37], compounds 9, 14, and 15, which were eluted at 0.90, 0.94, and 1.17 min were identified as quinic acid, citric acid isomer, and citric acid. Based on the literature [28,31,32,33,34,35,36,37,38] and a database search (ChemSpider, mzVault and mzCloud), compounds 18, 20, 21, 23, 24, 27, 28, 31, 33, 35, 36, 39, 40, 44, 45, 49, 50, 52, 54, 60, 64, 67, 74, 88, and 113 were tentatively assigned as uric acid, 3-methylglutaric acid, gallic acid, salicylic acid, pantothenic acid, vanillic acid, protocatechuic acid, isovanillic acid, 2-isopropylmalic acid, gentisic acid, kynurenic acid, 3-coumaric acid, p-coumaric acid, 2-hydroxyphenylacetic acid, ferulic acid, 4-methoxysalicylic acid, suberic acid, 4-coumaric acid isomer, indole-3-acetic acid, isoferulic acid, salicylic acid isomer, 4-coumaric acid, azelaic acid, 3-tert-butyladipic acid, and octyl ferulate based on the different base peak ions in the MS2 spectrum listed in Table 1.
2.3.3. Identification of Flavonoids
A total of 24 individual flavonoid constituents were putatively identified in Arnebiae Radix extract using UHPLC-Q-Exactive Orbitrap MS. Eight compounds, including rutin (63), isoquercitrin (66), nicotiflorin (73), hesperidin (76), quercetin (92), naringenin (95), kaempferol (96), and baicalein (114) were identified as having a pseudomolecular ion [M − H]− at m/z 609.14610 (−0.79 ppm, C27H29O16), 463.08819 (−0.28 ppm, C21H19O12), 593.15119 (−0.43 ppm, C27H29O15), 609.18249 (1.61 ppm, C28H33O15), 301.03537 (−0.22 ppm, C15H9O7), 271.06119 (0.31 ppm, C15H11O5), 285.04046 (1.30 ppm, C15H9O6), and 283.06119 (−0.03 ppm, C16H11O5), respectively. These were unambiguously identified by comparing their accurate mass information and chromatography retention times with reference standards in negative ionization mode. Compounds 37, 41, 47, 59, 61, 62, and 72 yielded deprotonated molecular ions [M − H]− at m/z 465.10384 (−0.20 ppm, C21H21O12), 465.10384 (−0.26 ppm, C21H21O12), 449.10893 (0.17 ppm, C21H21O11), 433.11402 (−0.17 ppm, C21H21O10), 449.10893 (0.17 ppm, C21H21O11), 433.11402 (−0.17 ppm, C21H21O10) and 277.14453 (−0.99 ppm, C16H21O4), which initially produced 151.0025 (C7H6O2), and 178.9977 (C8H4O5) by Retro Diels–Alder (RDA) rearrangement. According to a previous study [39,40] and the DFIs, they were tentatively characterized as taxifolin-glucoside, taxifolin-glucoside, eriodictyol-glucoside, naringenin-glucoside, eriodictyol-glucoside, naringenin-glucoside and De-O-methyllasiodiplodin. Compounds 99, 100, 109, 118, 125, and 131 were tentatively assigned as hispidulin, bilirubin, glycitein, pectolinarigenin, apigenin, and medicarpin by searching in the databases such as the chemical structure database (ChemSpider) and MS2 database (mzVault and mzCloud). Peaks 38, 46, and 117 were eluted at 4.62, 5.94, and 19.11 min with deprotonated ions [M − H]− at m/z 289.07176 (C15H13O6), 375.13100 (C17H19N4O6), and 345.09797 (C18H17O7). They were tentatively characterized as catechin, riboflavin, and kaempferide [39,40,41,42,43].
2.3.4. Identification of Amino Acids
Compounds 1, 2, 22, 29, 32, 42, and 48 yielded a quasi-molecular ions [M − H]− at m/z 131.04621 (C4H7N2O3), 145.06186 (C5H9N2O3), 164.07170 (C9H10NO2), 158.08226 (C7H12NO3), 203.08260 (C11H11N2O2), 172.09791 (C8H14NO3), and 206.08226 (C11H12NO3) and were eluted at 0.83, 0.86, 1.92, 2.96, 3.46, 5.17, and 6.01 min, respectively. Comparing the MS2 fragment ions with data from the bibliography [37,44,45], compounds 1, 2, 22, 29, 32, 42, and 48 were tentatively identified as asparagine, glutamine, phenylalanine, N-acetylvaline, tryptophan, N-acetyl-L-leucine, and N-acetyl-L-phenylalanine, respectively.
2.3.5. Others
Compounds 7, 30, 34, and 86 were tentatively identified as threonic acid, ethyl 3,4-dihydroxybenzoate, 7-hydroxycoumarin, and dibutylphthalate by searching in databases such as the chemical structure database (ChemSpider) and MS2 database (mzVault and mzCloud).
Compound 97, found at 14.56 min, possessed a quasi-molecular ion [M − H]− at m/z 253.08701 and was tentatively identified as Rhizonone [28].
3. Materials and Methods
3.1. Chemicals and Reagents
MS grade formic acid and acetonitrile were purchased from Thermo Fisher Scientific Co., Ltd. (Waltham, MA, USA). Ultra-pure water was obtained from Guangzhou Watsons Food & Beverage Co., Ltd. (Guangzhou, China). Other solvents were of analytical grade and were supplied by the Aladdin Industrial Corporation (Shanghai, China). The chemical reference standards of shikonin, acetylshikonin, β,β-dimethylacrylshikonin, lithospermic acid, rosmarinic acid, salvianolic acid B, and salvianolic acid C were purchased from Chengdu Pufei De Biotech Co., Ltd. (Chengdu, China), while those of nicotiflorin, rutin, isoquercitrin, quercetin, hesperidin, naringenin, kaempferol, and baicalein were provided by Cheng Du Herbpurify Co., Ltd. (Chengdu, China). The purities of all reference standards were above 98% according to an HPLC-UV analysis. The brand and model of the sonication device was KQ-300DE from Kunshan Ultrasonic Instrument Co., Ltd. (Kunshan China).
3.2. Sample and Standard Preparation
A total of three batches of Arnebiae Radix were used in this study. Batch A samples were collected from Bortala Mongol Autonomous Prefecture, Xinjiang Uygur Autonomous Region, China; batch B samples (1703060112) were purchased from the herbal medicine markets; and batch C samples were obtained from Zhaosu County and authenticated by Professor Shengjun Ma. The voucher specimens were deposited in the School of Pharmaceutical Sciences, Hunan University of Medicine.
Arnebiae Radix herbs were ground into powder before sample preparation and sieved through No. 40 mesh. Dried Arnebiae Radix powder (10 g) was sonication-extracted (300 W, 40 KHz) in 200 mL of methanol for 1 h at room temperature (16–24 °C), and then the extracted solution was filtered and dried by rotary evaporation. The extracts of these samples were redissolved and centrifuged at 12,000 rpm for 10 min to obtain the supernatant. A volume of 2 μL of supernatant was injected into UHPLC-Q-Exactive Orbitrap MS for analysis.
Standard solutions were prepared in methanol at a concentration of 1.00 mg/mL. The stock solutions of the reference standards were further diluted to obtain working solutions, and then these solutions were stored at 4 °C before analysis.
3.3. Instruments and Conditions
In order to acquire a better chromatographic peak shape and separation resolution, various factors were set in the detection and identification process, including a column (Thermo Scientific Hypersil GOLDTM aQ 100 mm × 2.1 mm, 1.9 μm and Waters ACQUITY BEH C18 column, 100 mm × 2.1 mm, 1.7 μm), column temperature (30, 35, 40 °C), and the mobile phase gradient.
Each LC-MS analysis was exercised on a Q-Exactive Focus Orbitrap MS connected to a Thermo Scientific Dionex Ultimate 3000 RS through an ESI source. Chromatographic separation was performed at 35 °C using a Thermo Scientific Hypersil GOLDTM aQ (100 mm × 2.1 mm, 1.9 μm). The mobile phase was composed of 0.1% formic acid (A) and acetonitrile (B), and the flow rate was 0.3 mL/min. The following gradient was used: 0–2 min, 95–90% A; 2–5 min, 90–80% A; 5–10 min, 80–75% A;10–15 min, 75–50% A; 15–25 min, 50–45% A; 25–40 min, 45–20% A; 40–45 min, 20–5% A; 45–45.1 min, 5–95% A; 45–50 min, 95% A.
All samples were analyzed in negative mode using the following tuning method. In terms of the mass spectrometry conditions, the spray voltage was 3.2 kV, the sheath gas and auxiliary gas operated at flow rates of 35 arb and 10 arb, respectively the temperature of the capillary was 320 °C and that of the auxiliary gas heater was 350 °C, and the S-lens RF level is 60. In the mass range of m/z 100–1200, a high-resolution mass spectrum was obtained at a resolution of 70,000, which was detected by the Orbitrap analyzer. MS2 data at a resolution of 17,500 were obtained by data-dependent MS2 scanning or parallel reaction monitoring (PRM) mode. Nitrogen (purity ≥ 99.999%) served as the collision gas, which generated the fragment ions, and the energy level was set as a normalized collision energy of 30%.
3.4. Data Processing and Analysis
Xcalibur software version 4.2 (Thermo Fisher Scientific, San Jose, CA, USA) was used to obtain all high-resolution data including the full-scan MS and MS2 data. Peaks detected with intensities over 10,000 were selected for identification. The chemical formulas for all parent and fragment ions of the selected peaks were calculated from the accurate mass using a formula predictor by setting the parameters as follows: C [0–60], H [0–120], O [0–60], and N [0–10]. The mass tolerance of MS and MS2 was within 10 ppm.
4. Conclusions
In this research, an efficient strategy based on UHPLC Q-Exactive Orbitrap MS in negative ion mode was established to detect chemical components in Arnebiae Radix. A total of 188 constituents were identified, of which 114 were reported in Arnebiae Radix for the first time here, including shikonins, phenolic acids, and flavonoids. These were detected and identified based on their chromatographic retention, MS and MS2, and bibliography data. These results are very useful references for understanding the bioactive compounds of Arnebiae Radix and their utilization. Overall, the results lay the foundation for in-depth research on the pharmacodynamic material basis of Arnebiae Radix.
Author Contributions
L.Z. and S.M. contributed equally; L.Z.: writing—original draft, performed experiments and sorted the data. S.M.: conceptualization, analyzed the validation data. K.L.: data curation. P.X. and S.Q.: performed experiments and analyzed the data. W.C.: writing—review and editing, conceptualization, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Xinjiang Uygur Autonomous Region (2020D03002) and the Graduate Student Innovation Project of the Xinjiang Uygur Autonomous Region (XJ2021G175).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be provided upon request.
Conflicts of Interest
All the authors declare that they have no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhan Z.L., Hu J., Liu T., Kang L.P., Nan T.G., Guo L.P. Advances in studies on chemical compositions and pharmacological activities of Arnebiae Radix. China J. Chin. Mater. Med. 2015;40:4127–4135. [PubMed] [Google Scholar]
- 2.Zhang J., Li J., Zhang P., Huang Y.Y. Herbal Textual Analysis of Medicinal Plant Arnebia. J. Anhui Agric. Sci. 2019;47:199–202. [Google Scholar]
- 3.Andujar I., Rios J.L., Giner R.M., Recio M.C. Pharmacological properties of shikonin—A review of literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 4.Wada N., Kawano Y., Fujiwara S., Kikukawa Y., Okuno Y., Tasaki M., Ueda M., Ando Y., Yoshinaga K., Ri M. Shikonin, dually functions as a proteasome inhibitor and a necroptosis inducer in multiple myeloma cells. Int. J. Oncol. 2015;46:963–972. doi: 10.3892/ijo.2014.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee J.H., Jung K.M., Bae I.H., Cho S., Seo D.B., Lee S.J., Park Y.H., Lim K.M. Anti-inflammatory and barrier protecting effect of Lithospermum erythrorhizon extracts in chronic oxazolone-induced murine atopic dermatitis. J. Dermatol. Sci. 2009;56:64–66. doi: 10.1016/j.jdermsci.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 6.Ashkani-Esfahani S., Imanieh M.H., Khoshneviszadeh M., Meshksar A., Noorafshan A., Geramizadeh B., Ebrahimi S., Handjani F., Tanideh N. The healing effect of arnebia euchroma in second degree burn wounds in rat as an animal model. Iran. Red Crescent Med. J. 2012;14:70–74. [PMC free article] [PubMed] [Google Scholar]
- 7.Kaith B.S., Kaith N.S., Chauhan N.S. Anti-inflammatory effect of Arnebia euchroma root extracts in rats. J. Ethnopharmacol. 1996;55:77–80. doi: 10.1016/S0378-8741(96)01477-8. [DOI] [PubMed] [Google Scholar]
- 8.Li H., Lim E., Ang G., Lim Z.Q., Cai M.H., Loh J.A., Ng C., Seetoh P., Tian E., Goh L.B. Qualitative and quantitative analysis of Arnebiae Radix and Dictamni Cortex and efficacy study of herbal extracts on allergic contact dermatitis using 3D human reconstructed epidermis. Chin. Herb. Med. 2021;13:556–564. doi: 10.1016/j.chmed.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo X.P., Zhang X.Y., Zhang S.D. Clinical trial on the effects of shikonin mixture on later stage lung cancer. Chin. J. Mod. Dev. Tradit. Med. 1991;11:580+598–599. [PubMed] [Google Scholar]
- 10.Rajasekar S., Park D.J., Park C., Park S., Park Y.H., Kim S.T., Choi Y.H., Choi Y.W. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J. Ethnopharmacol. 2012;144:335–345. doi: 10.1016/j.jep.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 11.Chen X., Yang L., Oppenheim J.J., Howard M.Z. Cellular pharmacology studies of shikonin derivatives. Phytother. Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 12.Shen C.C., Syu W.J., Li S.Y., Lin C.H., Lee G.H., Sun C.M. Antimicrobial activities of naphthazarins from Arnebia euchroma. J. Nat. Prod. 2002;65:1857–1862. doi: 10.1021/np010599w. [DOI] [PubMed] [Google Scholar]
- 13.Asghari F., Ghanbari H., Moghimi H., Takzaree N., Nekounam H., Faridi-Majidi R. Study on the Chemistry Identification and Assessment of Antioxidant and Antibacterial Activity of the Biologically Active Constituents from the Roots of Arnebia Euchroma for Promising Application in Nanomedicine and Pharmaceutical. Biointerface Res. Appl. Chem. 2021;12:3735–3751. doi: 10.33263/BRIAC123.37353751. [DOI] [Google Scholar]
- 14.Qian X., Li H.T., Zeng W.X., Zhou Q. Research Progress on Chemical Constituents, Pharmacological Effects and Product Application of Gromwell Root. Chin. Wild Plant Resour. 2021;40:52–56+69. [Google Scholar]
- 15.Liao M.C., Yao Y., Chen F., Zhang Y.X. Studies on chemical constituents from arnebia guttata bunge. J. South-Cent. Univ. Natl. (Nat. Sci. Ed.) 2018;37:58–60. [Google Scholar]
- 16.Yuan Z.C., Hu B. Mass Spectrometry-Based Human Breath Analysis: Towards COVID-19 Diagnosis and Research. J. Anal. Test. 2021;5:287–297. doi: 10.1007/s41664-021-00194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Long N.P., Park S., Anh N.H., Kim S.J., Kim H.M., Yoon S.J., Lim J., Kwon S.W. Advances in Liquid Chromatography–Mass Spectrometry-Based Lipidomics: A Look Ahead. J. Anal. Test. 2020;4:183–197. doi: 10.1007/s41664-020-00135-y. [DOI] [Google Scholar]
- 18.Satheeshkumar N., Shantikumar S., Komali M., Silva B. Identification and Quantification of Aldose Reductase Inhibitory Flavonoids in Herbal Formulation and Extract of Gymnema sylvestre Using HPLC-PDA and LC-MS/MS. Chromatogr. Res. Int. 2014;2014:518175. doi: 10.1155/2014/518175. [DOI] [Google Scholar]
- 19.Cai W., Guan Y., Zhou Y., Wang Y., Ji H., Liu Z. Detection and characterization of the metabolites of rutaecarpine in rats based on ultra-high-performance liquid chromatography with linear ion trap-Orbitrap mass spectrometer. Pharm. Biol. 2017;55:294–298. doi: 10.1080/13880209.2016.1236392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stavrianidi A. A classification of liquid chromatography mass spectrometry techniques for evaluation of chemical composition and quality control of traditional medicines. J. Chromatogr. A. 2020;1609:460501. doi: 10.1016/j.chroma.2019.460501. [DOI] [PubMed] [Google Scholar]
- 21.Klont F., Jahn S., Grivet C., König S., Bonner R., Hopfgartner G. SWATH data independent acquisition mass spectrometry for screening of xenobiotics in biological fluids: Opportunities and challenges for data processing. Talanta. 2020;211:120747. doi: 10.1016/j.talanta.2020.120747. [DOI] [PubMed] [Google Scholar]
- 22.Cai W., Li K.L., Xiong P., Gong K.Y., Zhu L., Yang J.B., Wu W.H. A systematic strategy for rapid identification of chlorogenic acids derivatives in Duhaldea nervosa using UHPLC-Q-Exactive Orbitrap mass spectrometry. Arab. J. Chem. 2020;13:3751–3761. doi: 10.1016/j.arabjc.2020.01.007. [DOI] [Google Scholar]
- 23.Xiong P., Qin S.H., Li K.L., Liu M.J., Zhu L., Peng J., Shi S.L., Tang S.N., Tian A.P., Cai W. Identification of the tannins in traditional Chinese medicine Paeoniae Radix Alba by UHPLC-Q-Exactive Orbitrap MS. Arab. J. Chem. 2021;14:103398. doi: 10.1016/j.arabjc.2021.103398. [DOI] [Google Scholar]
- 24.Sun X., Zhang Y., Chen S., Fu Y. Characterization and identification of the chemical constituents in the root of Lindera reflexa Hemsl. using ultra-high performance liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry. J. Pharm. Biomed. Anal. 2016;126:34–47. doi: 10.1016/j.jpba.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H., Zheng D., Li H.H., Wang H., Tan H.S., Xu H.X. Diagnostic filtering to screen polycyclic polyprenylated acylphloroglucinols from Garcinia oblongifolia by ultrahigh performance liquid chromatography coupled with ion mobility quadrupole time-of-flight mass spectrometry. Anal. Chim. Acta. 2016;912:85–96. doi: 10.1016/j.aca.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J.Y., Zhang Q., Li N., Wang Z.J., Lu J.Q., Qiao Y.J. Diagnostic fragment-ion-based and extension strategy coupled to DFIs intensity analysis for identification of chlorogenic acids isomers in Flos Lonicerae Japonicae by HPLC-ESI-MS(n) Talanta. 2013;104:1–9. doi: 10.1016/j.talanta.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Liao M., Li A., Chen C., Ouyang H., Zhang Y., Xu Y., Feng Y., Jiang H. Systematic identification of shikonins and shikonofurans in medicinal Zicao species using ultra-high performance liquid chromatography quadrupole time of flight tandem mass spectrometry combined with a data mining strategy. J. Chromatogr. A. 2015;1425:158–172. doi: 10.1016/j.chroma.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Feng J., Yu P., Zhou Q., Tian Z., Sun M., Li X., Wang X., Jiang H. An integrated data filtering and identification strategy for rapid profiling of chemical constituents, with Arnebiae Radix as an example. J. Chromatogr. A. 2020;1629:461496. doi: 10.1016/j.chroma.2020.461496. [DOI] [PubMed] [Google Scholar]
- 29.Liao M., Yan P., Liu X., Du Z., Jia S., Aybek R., Li A., Kaisa S., Jiang H. Spectrum-effect relationship for anti-tumor activity of shikonins and shikonofurans in medicinal Zicao by UHPLC-MS/MS and chemometric approaches. J. Chromatogr. B. 2020;1136:121924. doi: 10.1016/j.jchromb.2019.121924. [DOI] [PubMed] [Google Scholar]
- 30.Beretta G.L., Ribaudo G., Menegazzo I., Supino R., Capranico G., Zunino F., Zagotto G. Synthesis and Evaluation of New Naphthalene and Naphthoquinone Derivatives as Anticancer Agents. Arch. Pharm. (Weinhein) 2017;350:1–15. doi: 10.1002/ardp.201600286. [DOI] [PubMed] [Google Scholar]
- 31.Chen J.H., Zhang Y.X., Liu M.H., Ren Y., Zhou F.H. Chemical profiling of Danshen water extract by UPLC-Q-TOF-MS /MS. J. Guangdong Pharm. Univ. 2020;36:1–9. doi: 10.16809/j.cnki.2096-3653.2019111201. [DOI] [Google Scholar]
- 32.Yang N., Yang K.K., Li Y.B. UPLC-Q-TOF/MS combined with data post-processing to quickly classify and identify the components of Danshen. Lishizhen Med. Mater. Med. Res. 2019;30:2408–2412. [Google Scholar]
- 33.Zheng Y., Jiang Y., Zhang X., Huang L.F. Research on anti-influenza activity of Salvia miltiorrhiza based on UPLC-Q-TOF-MS and molecular docking technology. Chin. Tradit. Herb. Drugs. 2021;52:4487–4495. [Google Scholar]
- 34.Li Z.Z., Chang X.Y., Shi Y. Chemical Constituents Characterization of Hexue Zhiyang Formula Based on UPLC-QE-Orbitrap-MS. Mod. Chin. Med. 2021;23:1542–1553. doi: 10.13313/j.issn.1673-4890.20200907004. [DOI] [Google Scholar]
- 35.Zhang C., Zhang C.J., Liu L., Wang X.Y. UPLC-LTQ Orbitrap MS rapid characterization and analysis of multiple chemical components of Danshen. Acta Chin. Med. Pharmacol. 2018;46:14–21. doi: 10.19664/j.cnki.1002-2392.180072. [DOI] [Google Scholar]
- 36.Zhang B.Y., Jiang Z.Z., Yang F., Yu H.J. Analysis of chemical constituents in fresh, dried and prepared Rehmanniae Radix by UPLC /ESI-Q-TOF MS. Chin. Tradit. Pat. Med. 2016;38:1104–1108. [Google Scholar]
- 37.Xiong P., Li K.L., Wu W.H., Cai W. Identification of Chemical Constituents in the Kadsura Coccinea Fructus Based on UHPLC- Q-Exactive Orbitrap MS. China Pharm. 2021;30:55–60. [Google Scholar]
- 38.Chen Y., Ni J., Wu Y.J. Effects of shikonin on colon cancer xenografts in nude mice based on serum metabolomics. Acta Pharm. Sin. 2020;55:987–994. doi: 10.16438/j.0513-4870.2019-0819. [DOI] [Google Scholar]
- 39.Cai W., Li K., Qin S., Xiong P., Peng J., Shi S., Zhang Z. Rapid Identification and Systematic Mechanism of Flavonoids from Potentilla freyniana Bornm. Based on UHPLC-Q-Exactive Orbitrap Mass Spectrometry and Network Pharmacology. Int. J. Anal. Chem. 2021;2021:1–9. doi: 10.1155/2021/6619959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L.H., Peng J., Shi S.L., Li K.L., Xiong P., Cai W. Characterization of flavonoid constituents in stems of Lithocarpus litseifolius (Hance) Chun based on UHPLC-Q-Exactive Orbitrap MS. Curr. Anal. Chem. 2020;16:521–527. doi: 10.2174/1573411016999200619184952. [DOI] [Google Scholar]
- 41.Wang S., Sun X., An S., Sang F., Zhao Y., Yu Z. High-Throughput Identification of Organic Compounds from Polygoni Multiflori Radix Praeparata (Zhiheshouwu) by UHPLC-Q-Exactive Orbitrap-MS. Molecules. 2021;26:3977. doi: 10.3390/molecules26133977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J.H., Choi S.M., Shreatha R., Jeong G.S., Jeong T.C., Lee S. Deoxyshikonin reversibly inhibits cytochrome P450 2B6. Biopharm. Drug Dispos. 2020;41:221–225. doi: 10.1002/bdd.2230. [DOI] [PubMed] [Google Scholar]
- 43.Kyekyeku J., Adosraku R., Asare–Nkansah S. LC–HRESI–MS/MS Profiling of Flavonoids from Chlorophora regia (Moraceae) Br. J. Pharm. Res. 2017;16:1–9. doi: 10.9734/BJPR/2017/32893. [DOI] [Google Scholar]
- 44.Monique P., Christine V.S., Konstantinos P., Claire E., Jean-Paul S., Aymeric M., Denis B. ESI-MS/MS analysis of underivatised amino acids: A new tool for the diagnosis of inherited disorders of amino acid metabolism. Fragmentation study of 79 molecules of biological interest in positive and negative ionisation mode. Rapid Commun. Mass Spectrom. 2003;17:1297–1311. doi: 10.1002/rcm.1054. [DOI] [PubMed] [Google Scholar]
- 45.Han M., Xie M., Han J., Yuan D., Yang T., Xie Y. Development and validation of a rapid, selective, and sensitive LC-MS/MS method for simultaneous determination of D- and L-amino acids in human serum: Application to the study of hepatocellular carcinoma. Anal. Bioanal Chem. 2018;410:2517–2531. doi: 10.1007/s00216-018-0883-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided upon request.