Abstract
Degradation of dimethyl sulfide and methanethiol in slurries prepared from sediments of minerotrophic peatland ditches were studied under various conditions. Maximal aerobic dimethyl sulfide-degrading capacities (4.95 nmol per ml of sediment slurry · h−1), measured in bottles shaken under an air atmosphere, were 10-fold higher than the maximal anaerobic degrading capacities determined from bottles shaken under N2 or H2 atmosphere (0.37 and 0.32 nmol per ml of sediment slurry · h−1, respectively). Incubations under experimental conditions which mimic the in situ conditions (i.e., not shaken and with an air headspace), however, revealed that aerobic degradation of dimethyl sulfide and methanethiol in freshwater sediments is low due to oxygen limitation. Inhibition studies with bromoethanesulfonic acid and sodium tungstate demonstrated that the degradation of dimethyl sulfide and methanethiol in these incubations originated mainly from methanogenic activity. Prolonged incubation under a H2 atmosphere resulted in lower dimethyl sulfide degradation rates. Kinetic analysis of the data resulted in apparent Km values (6 to 8 μM) for aerobic dimethyl sulfide degradation which are comparable to those reported for Thiobacillus spp., Hyphomicrobium spp., and other methylotrophs. Apparent Km values determined for anaerobic degradation of dimethyl sulfide (3 to 8 μM) were of the same order of magnitude. The low apparent Km values obtained explain the low dimethyl sulfide and methanethiol concentrations in freshwater sediments that we reported previously. Our observations point to methanogenesis as the major mechanism of dimethyl sulfide and methanethiol consumption in freshwater sediments.
In anoxic freshwater sediments dimethyl sulfide (DMS) and methanethiol (MT) are generally considered to be the dominant volatile organic sulfur compounds (6, 7, 12, 15, 20, 21). Fluxes of DMS and MT from freshwater systems towards the atmosphere depend on the steady-state concentrations of the compounds in these compartments, which are the result of the balance between their formation and degradation. In contrast with marine and estuarine systems, DMS and MT formation in freshwater sediments originates mainly from the methylation of sulfide or from degradation of sulfur-containing amino acids (4, 5, 10, 12, 15, 22). The formation of MT and DMS appeared to be localized mainly in the sediment and depended on the sulfide concentration in the sediment and the rate of production of precursors.
The catabolism of DMS and MT has been ascribed to a variety of bacteria, including sulfur-oxidizing aerobes (chemolithotrophs and methylotrophs) (3, 18, 25, 29) and several types of anaerobes (anoxygenic phototrophs, sulfate-reducing bacteria, and methanogens) (13–17, 26, 32, 34, 35). Aerobic bacteria able to oxidize DMS and MT to sulfate (Thiobacillus spp. and Hyphomicrobium spp.) are the only organisms that have been isolated from freshwater systems (1, 9, 18, 23, 25). In addition, Zhang et al. (33) described a Pseudomonas strain capable of oxidizing DMS to dimethyl sulfoxide. Although methanogens were shown in 1978 to be at least partially responsible for the anaerobic degradation of methylated sulfur compounds in freshwater sediments (34, 35), no pure cultures of DMS- or MT-degrading methanogens have been obtained from these systems. Freshwater systems apparently have both aerobic and anaerobic DMS and MT conversion capacities. Aerobic degradation of MT and DMS is known to be energetically more favorable than anaerobic conversion. Anaerobic bacteria, however, do not depend on oxygen, which may be limiting in freshwater sediments rich in organic matter. The present study describes for the first time the potentials of both aerobic and anaerobic degradation of DMS and MT in freshwater sediment slurries. These results suggest that methanogenesis is the major mechanism for the degradation of these compounds in freshwater sediments in situ. In addition, the kinetic parameters of aerobic and anaerobic DMS (and MT) degradation were determined, and the results are discussed in relation to the in situ MT and DMS concentrations.
MATERIALS AND METHODS
Site description and sampling.
Sediment samples were taken from ditches of a minerotrophic peatland in De Bruuk, The Netherlands. “Minerotrophic” refers to systems which receive their major input of minerals from seepage or groundwater rather than from deposition by rainwater. This site has been described previously by Smolders et al. (24). Samples were taken by suction in anoxic bottles as described by Lomans et al. (15). The specific characteristics of the sediment of this site are as follows: pH, 6.6 to 7.0; organic matter content, 16 to 24% (dry weight) of sediment (of which 1 to 1.3% is N, 15 to 17% is C, and 0.7 to 0.9% is S); and redox potential, −225 to −325 mV. The characteristics of the pore water of this site are as follows: sulfate concentration, 700 to 800 μM; nitrate concentration, 0 to 5 μM; and alkalinity, 2 to 3 meq.
Slurry incubations.
After settling for 1 h, the sediment samples were adjusted to give a water/sediment ratio of 1:1 (vol/vol) by removing either sediment or pore water in an anaerobic cabinet. The adjusted samples were stirred, and aliquots (30 ml) of the homogeneous slurry were dispensed in 120-ml crimp top serum bottles sealed with grey butyl rubber stoppers which did not emit or absorb volatile organic sulfur compounds. The bottles were preincubated for 48 h at 30°C. Before the experiment was started, the headspaces of the bottles were flushed with either air, N2, N2-CO2 (80:20 [vol/vol]), or H2. DMS was added from stock solutions to a final concentration of 50 to 150 μM (duplicate incubations). Additions of bromoethanesulfonic acid (BES) (10 mM) and sodium tungstate (2 mM), inhibitors of methanogenic and sulfate-reducing bacteria, respectively, were made from neutralized stock solutions. The sediment slurries were incubated in the dark with (100 rpm) or without shaking at 30°C. Sterilized sediment slurries (121°C; 20 min) served as abiotic controls. For experiments performed to localize the DMS-degrading bacteria, sediment samples collected from a eutrophic lake (on the campus of Dekkerswald Institute, Nijmegen, The Netherlands) were allowed to settle for 15 min. After phase separation, aliquots (25 ml) of pore water were dispensed into 120-ml bottles. From the residual sample, a homogeneous slurry was prepared with a pore water/sediment ratio of 1:1 (vol/vol), which was dispensed as mentioned above. After the addition of DMS, the bottles (duplicates) containing either pore water or sediment slurry were incubated in the dark without shaking. The procedure of sampling and slurry distribution described above is highly reproducible, since differences between the dry weights, organic matter contents, methane formation rates, and MT and DMS degradation rates of the duplicates were smaller than 3%.
Analytical procedures.
Methane, MT, and DMS were analyzed on a Hewlett-Packard model 5890 gas chromatograph equipped with a flame ionization detector and a Porapak Q (80/100-mesh) column (8). Low concentrations of MT and DMS were analyzed on a Packard 438A gas chromatograph equipped with a flame photometric detector and a Carbopack B HT100 (40/60-mesh) column as described before (2, 15). The oxygen concentrations in the headspaces of aerobically incubated bottles were monitored by gas chromatography (Hewlett-Packard 5890). The oxygen concentrations in the headspaces of these bottles were maintained at 20% by the addition of pure oxygen.
Kinetic analysis.
Kinetic analyses were performed by making single additions of DMS to sediment slurries and following the change in the degradation rate of DMS (and MT) in relation to its actual concentration. DMS and MT degradation rates were calculated from various incubations. The values obtained were plotted against the actual DMS concentrations (Michaelis-Menten curves) and converted to Lineweaver-Burke plots (regression analyses).
RESULTS
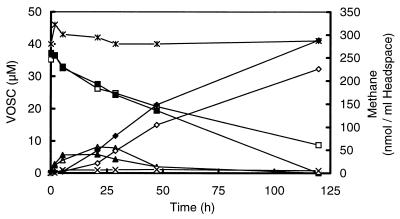
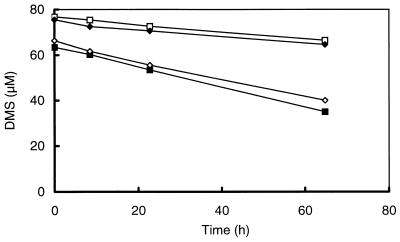
Sediment slurries were incubated under various conditions to study the degradation of MT and DMS in freshwater sediments. Incubations of DMS-amended slurries under N2-CO2 and N2 revealed that the rates of initial DMS degradation were similar (0.80 and 0.91 nmol per ml of sediment slurry · h−1, respectively). After prolonged incubation of the slurries, however, DMS degradation under N2-CO2 became significantly lower than that under N2 (0.39 and 0.64 nmol per ml of sediment slurry · h−1, respectively). Transient MT accumulation up to 5 to 8 μM was found under both atmospheres (Fig. 1). Under N2, methane formation, as well as DMS and MT degradation, was slightly higher; therefore, a N2 atmosphere was used in subsequent experiments. Measurements of the pHs of the slurries did not show significant differences (pH = 6.6 to 6.8). DMS did not disappear in sterilized controls, and no MT or methane was formed (Fig. 1).
FIG. 1.
Time courses of DMS (■ and □), MT (▴ and ▵), and methane (⧫ and ◊) of slurries prepared from the sediment of a ditch from a minerotrophic peatland (De Bruuk, Nijmegen, The Netherlands) after the addition of DMS to a final concentration of 40 μM. The slurries were incubated under N2 (solid symbols) and N2-CO2 (open symbols). No degradation of DMS ( ) and no accumulation of MT (×) or methane was found in heated slurries (121°C; 20 min) amended with DMS and incubated under N2 or N2-CO2. VOSC, volatile organic sulfur compounds (DMS or MT).
) and no accumulation of MT (×) or methane was found in heated slurries (121°C; 20 min) amended with DMS and incubated under N2 or N2-CO2. VOSC, volatile organic sulfur compounds (DMS or MT).
Aerobic versus anaerobic consumption of DMS.
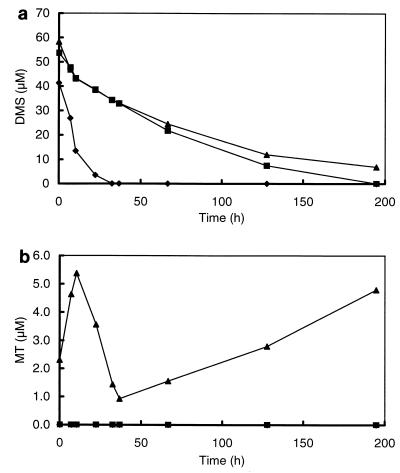
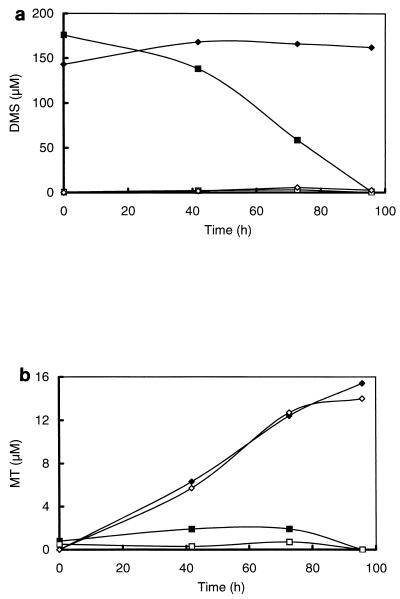
To elucidate the relevance and kinetics of both the aerobic and the anaerobic degradations of MT and DMS in freshwater sediments, sediment slurries were incubated under air, N2, and H2 atmospheres. Oxic conditions (air atmosphere with shaking at 100 rpm) resulted in rates of degradation of DMS (4.95 nmol of DMS per ml of sediment slurry · h−1) which were more than 10-fold higher than those under anoxic conditions (0.37 and 0.32 nmol of DMS per ml of sediment slurry · h−1 under N2 and H2 headspaces, respectively) (Fig. 2a and Table 1). Although DMS consumption was initially the same under H2 and N2 atmospheres, after prolonged incubation (>220 h) DMS consumption under H2 was almost completely inhibited. In contrast, the DMS consumption rates of sediment slurries incubated under N2 could easily be enhanced by four additions of DMS (50 μM each) up to 3.35 nmol per ml of sediment slurry · h−1 (Fig. 3).
FIG. 2.
Time courses of DMS (a) and MT (b) of slurries prepared from the sediment of a ditch from a minerotrophic peatland (De Bruuk, Nijmegen, The Netherlands) after the addition of DMS to a final concentration of 50 μM. The slurries were incubated under air (⧫), N2 (■), and H2 (▴).
TABLE 1.
DMS degradation rates of slurries prepared from the sediment of a ditch from a minerotrophic peatland (De Bruuk, Nijmegen, The Netherlands) incubated under aerobic (air) and anaerobic (N2 and H2) conditions, after the addition of 50 μM of DMS
| Exptl conditions | DMS degradation ratea |
|---|---|
| Air, not shaken | 0.44 |
| Air, shaken | 4.59–4.95 |
| N2, shaken | 0.37 |
| H2, shaken | 0.32 |
Degradation rates under anaerobic conditions were estimated from shaken incubations. Aerobic DMS degradation rates were estimated from both shaken (air; 100 rpm) and unshaken (air) incubations. The rates are given in nanomoles of DMS per milliliter of sediment per hour.
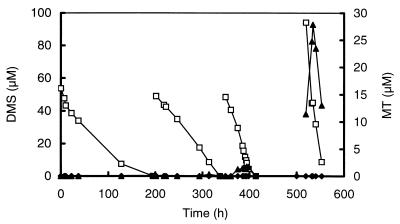
FIG. 3.
Degradation of added DMS by a slurry prepared from the sediment of a ditch from a minerotrophic peatland (De Bruuk, Nijmegen, The Netherlands). Four sequential additions of DMS were made to a final concentration of 50 to 100 μM. Notice the increasing rate of DMS consumption (□) and MT accumulation (▴). Control slurries without the addition of DMS (⧫) did not show accumulation of MT.
A striking difference between anaerobic and aerobic DMS degradations was that no MT was detected in the slurries incubated under air (with shaking) whereas in the slurries incubated under H2 there was a transient accumulation of MT (up to concentrations of 5.5 μM) upon addition of DMS (Fig. 2b). After an incubation period of 40 h, net MT consumption in the slurries under H2 decreased, which is reflected in an enhanced accumulation of MT. A second addition of DMS to these slurries caused an even stronger accumulation of MT (data not shown). The transient accumulation of MT in slurries incubated under N2 became especially significant after enhancement of the DMS degradation by pulsewise addition of DMS (Fig. 3). This accumulation was clearly caused by DMS degradation, since it was not observed in controls to which no DMS was added (Fig. 3). Although the incubation times exceeded the doubling times of bacterial populations, the enhancement of DMS consumption is most likely caused by the activation of bacterial populations. This is supported by initial enrichment experiments, which revealed that growth on DMS is very slow.
Effect of shaking on aerobic and anaerobic DMS degradation.
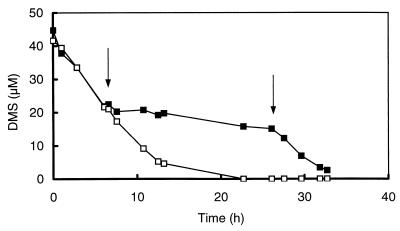
The remarkably high capacity of aerobic DMS degradation was studied in relation to oxygen availability (Fig. 4). Shaking of the aerobically incubated slurries was stopped (t = 6 h) to demonstrate the differences between the aerobic degradation capacity under optimal oxic conditions (continuous shaking and an aerobic headspace) and the capacity under conditions comparable to those in situ (an aerobic headspace without shaking). At the moment shaking of the aerobically incubated bottles was stopped, DMS degradation rates decreased from 4.59 to 0.44 nmol of DMS per ml of sediment slurry · h−1. The latter value is similar to the rates of anaerobic degradation (Fig. 2a and Table 1). When shaking was started again (t = 26 h), the rate of DMS degradation was restored to the original level. The degradation of DMS in unshaken, aerobically incubated sediment slurries decreased significantly if the slurries were preincubated under air with shaking (data not shown). Since the DMS degradation rate in aerobically incubated sediment slurries was dramatically affected by shaking, the impact of shaking on the anaerobically incubated sediment slurries was also studied. Methane formation was not affected by shaking the bottles; however, vigorous shaking of the unshaken incubated bottles before measurement appeared to be essential in order to release the methane captured in the slurry. Like those of methane, the degradation rates of MT and DMS were not affected by shaking the bottles. Unlike for methane measurement, however, shaking the slurries before measurement did not seem to be essential. This is probably due to the difference between the solubilities of methane and DMS or MT. The solubility and the distribution coefficient of DMS are 355 mM and 15; for MT the values are 813 mM and 11, respectively (reference 19 and our own data).
FIG. 4.
Effect of shaking on the degradation of DMS (50 μM) by slurries prepared from the sediment of a ditch from a minerotrophic peatland (De Bruuk, Nijmegen, The Netherlands). The slurries were incubated under an air atmosphere. Oxygen concentration was maintained at 20% (vol/vol) by the addition of pure oxygen to the bottles. The first arrow indicates the moment (6 h) at which shaking of the bottles (■) was stopped. At a later stage of the experiment (26 h), indicated by the second arrow, shaking of the bottles was started again. The control incubations (□) were shaken during the whole experiment.
Inhibition experiments.
The trophic groups that were responsible for DMS degradation in sediment slurries incubated under air without shaking were studied by the use of BES and tungstate, specific inhibitors of methanogenesis and sulfate reduction, respectively. BES addition effectively inhibited methanogenesis. A direct effect of tungstate on sulfate reduction could not be demonstrated, since after preincubation the sulfate became depleted. The DMS degradation rate of control slurries incubated under air atmosphere without shaking was similar to that of control slurries incubated anaerobically (Fig. 5). In slurries incubated under air headspace without shaking, inhibition of methanogenesis or both methanogenesis and sulfate reduction resulted in degradation rates which were only 36% of the rates of the control slurries (Fig. 5).
FIG. 5.
Degradation of DMS in slurries prepared from the sediment of a ditch from a minerotrophic peatland (De Bruuk, Nijmegen, The Netherlands) and amended with DMS. The slurries were incubated without shaking under N2 (◊), under air (■), under air and amended with BES (⧫), and under air and amended with BES plus tungstate (□).
Localization of degrading capacity.
In order to elucidate whether the DMS- and MT-degrading microorganisms are localized in the pore water or associated with the sediment particles, pore water and sediment slurry samples were incubated separately in the presence of DMS. In bottles containing sediment slurry, DMS disappeared completely within 100 h, whereas in the bottles with pore water, DMS was not degraded at all (Fig. 6a). Unlike the sediment slurry incubations, in which MT concentrations remained low, pore water samples showed MT accumulations to high levels (14 to 16 μM; accumulation rate, 225 pmol per ml of pore water · h−1) (Fig. 6b). This MT was formed from endogenous substrate (and not from DMS), since it accumulated in incubations with and without the addition of DMS.
FIG. 6.
Time courses of DMS (a) and MT (b) of pore water and sediment slurry samples from a eutrophic lake (campus of Dekkerswald Institute, Nijmegen, The Netherlands) incubated with or without addition of DMS. Pore water with DMS (⧫), pore water without the addition of DMS (◊), sediment slurry with DMS (■), and sediment slurry without the addition of DMS (□) are shown.
Kinetics of anaerobic and aerobic DMS degradation.
Kinetic parameters of the sediment slurries, such as apparent Km and threshold values, were studied under oxic and anoxic conditions to explain the low in situ steady-state DMS and MT concentrations observed in a previous study (15). As was shown above, DMS was degraded both aerobically and anaerobically to concentrations below its detection limit (0.06 nmol/ml of headspace, which corresponds to 0.85 μM for the slurry). From all available data, estimations were made for apparent Km values of the sediment slurry for anaerobic and aerobic degradation (shaken conditions) of both MT and DMS, using Michaelis-Menten curves and Lineweaver-Burke plots. These analyses (correlation coefficients, >0.96) revealed that the apparent Km value of the sediment slurry for aerobic DMS degradation (Km = 7 ± 1 μM; n = 2) was slightly higher than the apparent Km value of the anaerobic DMS degradation (Km = 5.6 ± 2.7 μM; n = 7). The apparent Km value of the slurry for anaerobic MT degradation was of the same order of magnitude (2.2 μM [single measurement]).
DISCUSSION
The data presented in this study demonstrate that estimated DMS degradation rates are highly dependent on incubation conditions. The initial rate of DMS degradation was the same for slurries incubated under N2 and N2-CO2. Prolonged incubation under N2-CO2, however, showed an inhibition of DMS degradation compared to that under N2. The differences between DMS consumption under N2 and N2-CO2 were not due to pH shifts caused by the introduction of CO2, since pH measurements of the slurries did not show significant differences. As far as we know, similar differences caused by the atmosphere applied have not been reported. Degradation of DMS was similar under H2 and N2 atmospheres in short-term incubations, although in incubations with H2, MT accumulated to higher levels. After prolonged incubation under H2, however, degradation of DMS and MT decreased dramatically. The reason for the higher MT accumulation and the inhibition of DMS degradation in long-term incubations under H2 remains unclear and is currently being investigated. Contrary to the results of this study, Zinder and Brock (35) mentioned that incubation of sediments under a H2 atmosphere greatly increased the ratio of [14C]methane to [14C]carbon dioxide and caused greater overall metabolism of [14C]MT.
Comparison of the capacity for both aerobic and anaerobic DMS consumption in sediment slurries led to some very striking results. Surprisingly, slurries prepared from an anoxic sediment showed high aerobic DMS consumption rates (4.59 to 4.95 nmol of DMS per ml of sediment slurry · h−1, estimated from shaken incubations under air). These values were 10-fold higher than the anaerobic consumption rates (0.37 and 0.32 nmol of DMS per ml of sediment slurry · h−1, estimated from slurries incubated under N2 and H2 headspaces). DMS consumption rates (0.44 nmol of DMS per ml of sediment slurry · h−1) estimated from slurries incubated under conditions comparable to those in situ (i.e., an aerobic headspace without shaking), however, were of the same order of magnitude as the anaerobic ones. The difference between the DMS degradation rates determined from slurries incubated under optimal oxic conditions (i.e., with shaking and an air headspace) and under conditions comparable to those in situ (i.e., an air headspace without shaking) is likely to be the result of oxygen limitation in the sediment slurry of the unshaken incubations. The limitation of oxygen is caused by oxygen consumption in abiotic processes and by the activity of aerobic and microaerophilic microorganisms. This results in a steep oxygen gradient in the interface between the air atmosphere and the top of the sediment slurry, as was also found in microbial mats and sediments of various origins (28). The anoxic character of sediment in situ, from which the slurries were prepared, was demonstrated by concentration profiles of volatile sulfur compounds showing high H2S concentrations in and just above the sediment (15). To our knowledge, this study is the first in which anaerobic and aerobic degradations of MT and DMS in freshwater sediments are compared. Other authors (3, 30) have measured DMS consumption rates of sediment slurries of estuarine and marine origin incubated under a N2 or an air atmosphere without shaking. Rates under both conditions were similar. Kiene (11) also mentioned similar consumption rates under an N2 or air atmosphere; however, the aerobic incubations were shaken. The comparison of DMS degradation under aerobic conditions (i.e., an air headspace with shaking) with that under conditions representative of the in situ situation (i.e., an air headspace without shaking) clearly demonstrates the relevance of the use of adequate experimental conditions for slurry incubations.
The results of our inhibition studies (with BES and tungstate) of slurries incubated under air without shaking clearly demonstrated that although freshwater sediments can have a very high potential of aerobic DMS consumption, DMS is mainly (64%) degraded by methanogenic activity due to oxygen limitation. This was further confirmed by the sensitivity of DMS consumption in these incubations (under air without shaking) to oxygen, as was indicated by its decrease, caused by prolonged shaking under oxic conditions during preincubation. In addition, the degradation of DMS and MT in these freshwater sediments is unlikely to originate from denitrifying bacteria or anoxygenic phototrophs, since nitrate concentrations in these sediments are usually low (<10 μM) and the sediment surfaces are normally strongly light limited due to the water column and vegetation (e.g., duckweed). The ecological niche for aerobic, microaerophilic, and denitrifying DMS-degrading microorganisms in freshwater sediments probably lies in a minimal DMS conversion at the oxygen- and nitrate-limited sediment-water column interface. Temporal variations of the in situ sediment conditions, like aeration due to aridification in summer and physical mixing of the sediment caused by strong currents of the water column, can enlarge the input of oxygen or nitrate and thereby stimulate the aerobic or nitrate-driven DMS conversion. In contrast to freshwater sediments, in microbial mats and salt marsh sediments aerobic DMS oxidizers are thought to be important in the regulation of the fluxes of DMS to the atmosphere (27, 28, 31). In these systems, however, aerobic bacteria encounter more-favorable conditions for DMS oxidation due to high diel fluctuations of oxygen, resulting in the coinciding presence of oxygen and reduced sulfur compounds.
Comparison of the anaerobic DMS degradation capacity of sediment slurries with that of sediment pore water revealed that the DMS-degrading microorganisms appeared to be associated with the sediment particles. The absence of MT-degrading microorganisms in the pore water was illustrated by the accumulation of MT in pore water incubations with and without the addition of DMS. These results are strong evidence for the fact that potential anaerobic DMS-degrading bacteria (methanogens and sulfate- and nitrate-reducing bacteria) are associated with the sediment particles. This is consistent with the results of fluorescence microscopy showing that most of the blue fluorescence specific for methanogens was associated with the sediment particles. The association of bacteria with the sediment particles increases the retention time of these bacteria in the system in spite of low metabolic and growth rates. Close association in aggregates or sediment particles also ensures that anaerobic bacteria have better protection against oxygen poisoning.
Microorganisms in the sediment had high affinities for DMS (and MT) under both oxic and anoxic conditions (apparent Km, 5 to 7 μM). This clarifies why DMS and MT concentrations in situ are often under or just above the detection limit (0.85 μM) (15). The apparent Km values determined in this study are comparable to those reported for Thiobacillus spp., Hyphomicrobium spp., and other methylotrophs capable of aerobic degradation of DMS (3, 18, 25). We are currently investigating Ks values for DMS in pure cultures of methanogens.
In conclusion, this study provides strong evidence that, although freshwater sediments rich in organic matter have very high aerobic consumption capacities, DMS is mainly converted anaerobically by methanogens due to oxygen limitation. As a consequence, in vitro aerobic consumption rates of substrates or most probable numbers of aerobic microorganisms used as evidence for the relevance of aerobic metabolism in vivo should be determined under appropriate conditions and interpreted with great care.
REFERENCES
- 1.De Bont J A M, van Dijken J P, Harder W. Dimethyl sulphoxide and dimethyl sulphide as a carbon, sulphur and energy source for growth of Hyphomicrobium S. J Gen Microbiol. 1981;127:315–323. [Google Scholar]
- 2.Derikx P J L, Op den Camp H J M, van der Drift C, Van Griensven L J L D, Vogels G D. Odorous sulfur compounds emitted during production of compost used as a substrate in mushroom cultivation. Appl Environ Microbiol. 1990;56:176–180. doi: 10.1128/aem.56.1.176-180.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Zwart J M M, Nelisse P N, Kuenen J G. Isolation and characterization of Methylophaga sulfidovorans sp. nov.: an obligate methylotrophic aerobic, DMS oxidizing bacterium from a microbial mat. FEMS Microbiol Ecol. 1996;20:261–271. [Google Scholar]
- 4.Drotar A, Burton G A, Jr, Tavernier J E, Fall R. Widespread occurrence of bacterial thiol methyltransferases and the biogenic emission of methylated sulfur gases. Appl Environ Microbiol. 1987;53:1626–1631. doi: 10.1128/aem.53.7.1626-1631.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finster K, King G M, Bak F. Formation of methyl mercaptan and dimethyl sulfide from methoxylated aromatic compounds in anoxic marine and freshwater sediments. FEMS Microbiol Ecol. 1990;74:295–302. [Google Scholar]
- 6.Henatsch J J, Jüttner F. Capillary gas chromatographic analysis of low-boiling organic sulphur compounds in anoxic lake-water by cryo-adsorption. J Chromatogr. 1988;445:97–105. [Google Scholar]
- 7.Henatsch J J, Jüttner F. Occurrence and distribution of methane thiol and other volatile organic sulphur compounds in a stratified lake with anoxic hypolimnion. Arch Hydrobiol. 1990;119:315–323. [Google Scholar]
- 8.Hutten T J, de Jong M H, Peeters B P H, van der Drift C, Vogels G D. Coenzyme M derivatives and their effects on methane formation from carbon dioxide and methanol by cell extracts of Methanosarcina barkeri. J Bacteriol. 1981;145:27–34. doi: 10.1128/jb.145.1.27-34.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanagawa T, Dazai M, Fukuoka S. Degradation of o,o-dimethylphosphorodithionate by Thiobacillus thioparus TK-1 and Pseudomonas AK-2. Agric Biol Chem. 1982;46:2571–2578. [Google Scholar]
- 10.Kelly D P, Smith N A. Organic sulfur compounds in the environment: biochemistry, microbiology and ecological aspects. Adv Microb Ecol. 1990;11:345–385. [Google Scholar]
- 11.Kiene R P. Dimethyl sulfide metabolism in salt marsh sediments. FEMS Microbiol Ecol. 1988;53:71–78. [Google Scholar]
- 12.Kiene R P, Hines M E. Microbial formation of dimethyl sulfide in anoxic Sphagnum peat. Appl Environ Microbiol. 1995;61:2720–2726. doi: 10.1128/aem.61.7.2720-2726.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiene R P, Oremland R S, Catena A, Miller L G, Capone D G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986;52:1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Boon D R, Choy C. Methanohalophilus oregonense, sp. nov., a methylotrophic methanogen from an alkaline, saline aquifer. Int J Syst Bacteriol. 1990;40:111–116. [Google Scholar]
- 15.Lomans B P, Smolders A J P, Intven L, Pol A, Op den Camp H J M, van der Drift C. Formation of dimethyl sulfide and methanethiol in anoxic freshwater sediments. Appl Environ Microbiol. 1997;63:4741–4747. doi: 10.1128/aem.63.12.4741-4747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathrani I M, Boone D R, Mah R A, Fox G E, Lau P P. Methanohalophilus zhilinae sp. nov., an alkaliphilic, halophilic, methylotrophic methanogen. Int J Syst Bacteriol. 1988;38:139–142. doi: 10.1099/00207713-38-2-139. [DOI] [PubMed] [Google Scholar]
- 17.Ni S, Boone D R. Isolation and characterization of a dimethyl sulfide degrading methanogen, Methanolobus siciliae HI350, from an oil well, characterization of M. siciliae T4/MT, and emendation of M. siciliae. Int J Syst Bacteriol. 1991;41:410–416. doi: 10.1099/00207713-41-3-410. [DOI] [PubMed] [Google Scholar]
- 18.Pol A, Op den Camp H J M, Mees S G M, Kersten M A S H, van der Drift C. Isolation of a dimethyl sulfide utilizing Hyphomicrobium species and its application in biofiltration of polluted air. Biodegradation. 1994;5:105–112. doi: 10.1007/BF00700635. [DOI] [PubMed] [Google Scholar]
- 19.Przyjazny A, Janicki W, Chrzanowski W, Staszewski R. Headspace gas chromato-graphic determination of distribution coefficients of selected organosulphur compounds and their dependence on some parameters. J Chromatogr. 1983;280:249–260. [Google Scholar]
- 20.Richards S R, Kelly C A, Rudd J W H. Organic volatile sulfur in lakes of the Canadian Shield and its loss to the atmosphere. Limnol Oceanogr. 1991;36:468–482. [Google Scholar]
- 21.Richards S R, Rudd J W M, Kelly C A. Organic volatile sulfur in lakes ranging in sulfate and dissolved salt concentration over five orders of magnitude. Limnol Oceanogr. 1994;39:562–572. [Google Scholar]
- 22.Segal W, Starkey R L. Microbial decomposition of methionine and identity of resulting sulfur compounds. J Bacteriol. 1969;98:908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith N A, Kelly D P. Isolation and physiological characterization of autotrophic sulphur bacteria oxidizing dimethyl disulphide as sole source of energy. J Gen Microbiol. 1988;134:1407–1417. [Google Scholar]
- 24.Smolders A J P, Nijboer R C, Roelofs J G M. Prevention of sulphide accumulation and phosphate mobilization by the addition of iron(II) chloride to a reduced sediment: an enclosure experiment. Freshwater Biol. 1995;34:559–568. [Google Scholar]
- 25.Suylen G M H. Microbial metabolism of dimethyl sulphide and related compounds. Ph.D. thesis. Delft, The Netherlands: University of Technology Delft; 1988. [Google Scholar]
- 26.Tanimoto Y, Bak F. Anaerobic degradation of methylmercaptan and dimethyl sulfide by newly isolated sulfate-reducing bacteria. Appl Environ Microbiol. 1994;60:2450–2455. doi: 10.1128/aem.60.7.2450-2455.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Gemerden H, Tughan C S, de Wit R, Herbert R A. Laminated microbial ecosystems on sheltered beaches in Scapa Flow, Orkney Islands. FEMS Microbiol Ecol. 1989;62:87–102. [Google Scholar]
- 28.Visscher P T, Quist P, van Gemerden H. Methylated sulfur compounds in microbial mats: in situ concentrations and metabolism by a colorless sulfur bacterium. Appl Environ Microbiol. 1991;57:1758–1763. doi: 10.1128/aem.57.6.1758-1763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Visscher P T, Taylor B F. A new mechanism for the aerobic catabolism of dimethyl sulfide. Appl Environ Microbiol. 1993;59:3784–3789. doi: 10.1128/aem.59.11.3784-3789.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visscher P T, Taylor B F, Kiene R P. Microbial consumption of dimethyl sulfide and methanethiol in coastal marine sediments. FEMS Microbiol Ecol. 1995;18:145–154. [Google Scholar]
- 31.Visscher P T, van Gemerden H. Production and consumption of dimethylsulfoniopropionate in marine microbial mats. Appl Environ Microbiol. 1991;57:3237–3242. doi: 10.1128/aem.57.11.3237-3242.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeyer J, Eicher P, Wakeham S G, Schwarzenbach R P. Oxidation of dimethyl sulfide to dimethylsulfoxide by phototrophic purple bacteria. Appl Environ Microbiol. 1987;53:2026–2032. doi: 10.1128/aem.53.9.2026-2032.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Kuniyoshi I, Hirai M, Shoda M. Oxidation of dimethyl sulfide by Pseudomonas acidovorans DMR-11 isolated from peat biofilter. Biotechnol Lett. 1991;13:223–228. [Google Scholar]
- 34.Zinder S H, Brock T D. Methane, carbon dioxide, and hydrogen sulfide production from the terminal methiol group of methionine by anaerobic lake sediments. Appl Environ Microbiol. 1978;35:344–352. doi: 10.1128/aem.35.2.344-352.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zinder S H, Brock T D. Production of methane and carbon dioxide from methane thiol and dimethyl sulfide by anaerobic lake sediments. Nature. 1978;273:226–228. doi: 10.1128/aem.35.2.344-352.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]