Abstract
Disrupted brain gamma-Aminobutyric acid (GABA)/glutamate homeostasis is a promising target for pharmacological intervention in co-occurring bipolar disorder (BD) and cannabis use disorder (CUD). Gabapentin is a safe and well-tolerated medication, FDA-approved to treat other neurological diseases, that restores GABA/glutamate homeostasis, with treatment studies supporting efficacy in treating CUD, as well as anxiety and sleep disorders that are common to both BD and CUD. The present manuscript represents the primary report of a randomized, double-blind, placebo-controlled, crossover (1-week/condition), multimodal-MRI (proton-MR spectroscopy, functional MRI) pilot study of gabapentin (1200mg/day) in BD+CUD (n=22). Primary analyses revealed that, A) gabapentin was well-tolerated, adherence and retention were high, B) gabapentin increased dorsal anterior cingulate cortex (dACC) and right basal ganglia (rBG) glutamate levels, and C) gabapentin increased activation to visual cannabis cues in the posterior midcingulate cortex (pMCC, a region involved in response inhibition to rewarding stimuli). Exploratory evaluation of clinical outcomes further found that, in participants taking gabapentin versus placebo: 1) elevations of dACC GABA levels were associated with lower manic/mixed and depressive symptoms and 2) elevations of rBG glutamate levels and pMCC activation to cannabis cues were associated with lower cannabis use. Though promising, the findings from this study should be interpreted with caution due to observed randomization order effects on dACC glutamate levels, and identification of statistical moderators that differed by randomization order (i.e., cigarette-smoking status on rBG glutamate levels and pMCC cue-activation). Nonetheless, they provide the necessary foundation for a more robustly-designed (urn-randomized, parallel-group) future study of adjuvant gabapentin for BD+CUD.
INTRODUCTION
There is an 8-fold increase in the prevalence of cannabis use disorder (CUD) in individuals with bipolar disorder (BD)1 relative to the general population2. Co-occurring BD and CUD (BD+CUD; relative to BD alone) is associated with more frequent mood cycling, mixed manic and depressive symptoms, poorer quality of life, elevated risk of cigarette-smoking and psychosis, and greater rates of disability, hospitalization, and suicide1,3–5. Treatment response to mood-stabilizing medications FDA-approved for BD is poor in individuals with BD+CUD6,7. Overall, little is known about optimal treatment of BD+CUD, as there have been no randomized controlled trials (RCTs) in this population to date.
Convergent evidence supports dysfunctional brain gamma-Aminobutyric acid (GABA) and glutamate neurotransmission as promising targets for pharmacological intervention in CUD and BD8,9. The reorganization of reward circuitry in CUD to preferentially respond to drug cues, manifesting clinically as drug craving/seeking, is due to drug-induced neuroplasticity mediated by glutamate and GABA10,11. Delta-9-tetrahydrocannabinol (Δ9-THC, the psychoactive component of cannabis) activates pre-synaptic cannabinoid type-1 (CB1) receptors that are densely distributed in frontostriatal brain regions, facilitating release (and reducing astrocytic uptake) of glutamate resulting in accumulated extracellular glutamate, and inhibiting release of GABA resulting in disinhibition of mesolimbic dopaminergic cells critical to the development of CUD8. Repeated Δ9-THC administration induces down-regulation and internalization of CB1 and glutamate receptors, and suppresses activity of glutamic-acid decarboxylase and glutamine synthetase, resulting in reduced synaptic glutamate and GABA transmission12.
Consistent with preclinical findings, Δ9-THC significantly increased glutamate levels, measured using proton magnetic resonance spectroscopy (1H-MRS), in the left caudate head (part of the striatum/basal ganglia) of healthy volunteers13. Chronic cannabis use and CUD have been, in turn, associated with, decreased anterior cingulate cortex (ACC, located in the medial-frontal lobe)14,15 and right basal ganglia (rBG)16,17 glutamate levels, decreased ACC GABA levels15, and heightened activation to cannabis cues in many of the same frontostriatal brain regions, which underlie reward, attention, motivation, and goal-directed behavior (e.g., ACC/medial-prefrontal cortex [mPFC], striatum/basal ganglia)18,19.
In contrast, 1H-MRS studies of BD have consistently demonstrated elevated glutamate levels across mood states and brain regions9. Investigations of GABA using the MEGA-PRESS acquisition technique have consistently found abnormal ACC and occipital cortex GABA concentrations in BD20–22, although the direction of disturbance has not been consistent. Though there have been no published studies of brain glutamate and GABA levels in BD+CUD, CUD was associated with reduced mPFC glutamate levels in individuals with early psychosis (subsuming BD and schizoaffective disorder, bipolar type)23. We similarly found that individuals with co-occurring BD and alcohol use disorder (AUD; with/without co-occurring CUD) had significantly lower dorsal ACC (dACC) levels of both GABA and glutamate relative to individuals with BD alone, AUD alone, or healthy volunteers, and that lower dACC GABA levels were associated with elevated alcohol craving and impulsivity24. Together, these studies suggest that although BD is associated with elevated glutamate levels9, BD appears to act like a “multiplier” to the impact of Substance Use Disorder (SUD) on lowering glutamate levels, reducing them well below levels associated with SUD alone23,24.
Gabapentin, a safe and well-tolerated medication that is FDA-approved to treat post-herpetic neuralgia, partial seizures, and restless-leg syndrome, holds promise as an adjuvant medication for normalizing brain GABA and glutamate transmission in individuals with BD+CUD. Gabapentin is known to modulate GABA and glutamate transmission via selective blockade of presynaptic voltage-gated calcium channels that contain the α2δ−1 subunit25. More recently, additional mechanisms have been identified, including activation of potassium channels26, increased expression of postsynaptic δ-subunit-containing GABAA receptors27, and reduced spontaneous synaptic-glutamate release dependent on α2δ−1-linked N-methyl-D-aspartate (NMDA) receptors28. In 1H-MRS studies, gabapentin significantly increased occipital GABA levels 1–6 hours following a single dose (900–1200mg) in healthy volunteer29,30 and patients with epilepsy31. Longer-term gabapentin dosing (i.e., ≥ 2-week) has also been shown to significantly increase occipital GABA, in a dose-dependent manner, in healthy volunteers (2400mg/day)30 and patients with epilepsy (1200–3600mg/day)31,32; though, ≥ 1-week of daily (M = 1,600mg/day) gabapentin was not associated with altered cortical GABA levels in a convenience sample of alcohol-dependent individuals during short-term abstincence33.
In neurobehavioral studies, gabapentin reduced cannabis use and withdrawal symptoms in cannabis-dependent adults34, distinguishing it as one of few promising medications for CUD warranting further research35. Gabapentin substitutes for Δ9-THC-discriminative stimuli in cannabis users, suggesting that it may reduce cannabis use by producing interoceptive effects that may replace those of Δ9-THC36. Furthermore, the gabapentinoid, pregabalin, blocked motor signs and anxiety behaviors associated with cannabis withdrawal in mice37. Animal studies focused on other SUDs found decreased self-administration38,39, reinstatement38, and conditioned place preference40 with gabapentin, mediated, in part, by normalization of GABAergic transmission in central amygdala and elevation of α2δ−1 subunit of voltage-gated calcium channels39,40. Although RCTs in treatment-refractory BD failed to support gabapentin for resolving acute mood episodes, one RCT demonstrated a prophylactic effect of gabapentin in BD41, and a long history of positive reports from open-label studies support the use of gabapentin for BD patients suffering from anxiety and sleep disturbance42.
The present study represents the primary report of an NIH/NIDA-funded (R21DA043917), randomized, double-blind, placebo-controlled, crossover, multimodal-MRI pilot study of gabapentin (1200mg/day) as an adjunctive medication for BD+CUD that evaluated the following hypotheses: 1) gabapentin will increase dACC and rBG GABA and glutamate levels (1H-MRS), and 2) gabapentin will decrease brain activity to visual cannabis cues (functional MRI, fMRI). Associations of changes in GABA and glutamate levels with cannabis use and mood symptoms were also explored.
SUBJECTS AND METHODS
Participants.
Twenty-two individuals, ages 18–65, who met DSM-5 criteria for BD-I or -II and current (within the past 3-months) moderate-to-severe CUD43, and who provided a positive urine cannabinoid screen at baseline, were recruited from clinical settings and advertisements and enrolled into the study across an 18-month period (see Table 1 for participant characteristics by Randomization Order [RO] and Supplemental Figure 1 for CONSORT diagram). Three participants diagnosed with Bipolar-I Disorder (n=2 in RO#1, n=1 in RO#2) were given a provisional diagnosis of Schizoaffective Disorder, Bipolar Type, following retrospective reports of hallucinations/delusions in the absence of a clear Mood Episode. Participants were required to document daily use of ≥1 FDA-approved mood-stabilizing medication for BD (lithium, lamotrigine, divalproex sodium, carbamazepine, 2nd-generation antipsychotic), as restricting the study to medication-naïve individuals would have represented a safety hazard, severely limited recruitment44, and would have been inconsistent with the purpose of the study (to evaluate gabapentin as an adjuvant medication). To minimize the impact of medications on results, participants with additions, discontinuations, or dose changes of >20%, <2-weeks prior to testing were excluded (Swann, 2009). Other exclusion criteria included history of significant medical illness, traumatic brain injury, or non-affective psychotic disorder (e.g., Schizophrenia); severe mood disturbance (Montgomery-Asberg Depression Rating Scale [MADRS]45 >35 and/or Young Mania Rating Scale [YMRS]46 >25) or suicidal/homicidal ideation; meeting DSM-5 criteria for moderate-to-severe SUD other than cannabis or tobacco within 60-days of evaluation; opioid or benzodiazepine use, as indicated by positive urine drug screen and/or self-report; pregnancy; and presence of non-MRI-safe implants or claustrophobia.
Table 1.
Baseline participant characteristics and comparison by randomization
| Overall Sample (n = 22) | Randomization 1 (n = 11) | Randomization 2 (n = 11) | p | |
|---|---|---|---|---|
| Age (in years), M (SD) | 37.59(11.91) | 38.55(11.41) | 36.63(12.86) | 0.717 |
| Sex, n (%) | 11(50%) | 5(45.5%) | 6(54.5%) | 0.670 |
| Smoking status, n (%) | 13(59.1%) | 4(36.4%) | 9(81.8%) | 0.080† |
| Anxiety Disorder, n (%) | 7(31.8%) | 0(0.0%) | 7(63.6%) | 0.004* |
| BD sub-type, n (%) I | 12(54.5%) | 6(54.5%) | 6(54.5%) | 1.000 |
| YMRS, M (SD) | 4.00(3.98) | 2.91(3.33) | 5.09(4.41) | 0.206 |
| MADRS, M (SD) | 8.27(7.86) | 5.36(4.43) | 11.18(9.56) | 0.082† |
| Cannabis Withdrawal Scale, M (SD) | 36.73(32.99) | 36.45(37.37) | 37.00(29.81) | 0.970 |
| Marijuana Craving Quest., M (SD) | 37.41(17.07) | 36.18(15.38) | 38.64(19.29) | 0.745 |
| Cannabis use (grams/day), M (SD) | 3.00(3.41) | 1.47(2.32) | 4.52(3.72) | 0.032* |
| Medication (%) | ||||
| Lithium | 4(18.2%) | 2(18.2%) | 2(18.2%) | 1.000 |
| Antipsychotic | 18(81.8%) | 8(72.7%) | 10(90.9%) | 0.586 |
| Anticonvulsant | 9(40.9%) | 4(36.4%) | 5(45.5%) | 1.000 |
| Antidepressant | 9(40.9%) | 4(36.4%) | 5(45.5%) | 1.000 |
χ2 (categorical variables) tests, using Fisher’s Exact when applicable, and independent samples t-tests (continuous variables) were performed.
BD = bipolar disorder; YMRS, Young Mania Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; Quest. = Questionnaire.
p < 0.10;
p < 0.05.
A target sample size (≥18 study-completers) was chosen to provide ≥80% power to detect a ≥25% increase in GABA, as past gabapentin studies reported GABA increases of 25–50%. Our most recent evaluation of the test-retest reliability of dACC GABA levels in 10 healthy volunteers, acquired via MEGA-PRESS with echo times of 68ms or 80ms, produced within-subject coefficient of variation (CVws) estimates of approximately 8% (i.e., irrespective of echo time) (unpublished data), which is in-line with47 or lower than48,49 published literature values. Similarly, our published50,51 and unpublished estimates of the test-retest reliability of dACC glutamate levels have been consistent across samples and acquisition methods (e.g., PRESS, MEGA-PRESS [i.e., Glx], 2d J-resolved PRESS), converging on a mean CVws of approximately 6.5%, which is also in line with15 or lower than52 published values. In sum, the present study was adequately powered and sensitive to detect hypothesized gabapentin effects on GABA and glutamate levels.
Procedure.
Written informed consent was obtained from every participant at a baseline evaluation appointment. Participants were screened for eligibility using the Structured Clinical Interview for DSM-553, and past 90-day cannabis use was assessed using the Timeline Followback (TLFB)54 method. Cannabis use was recorded in times used/day, as well as quantity (e.g., grams, number of blunts/joints) to standardize for different types of cannabis use. Participants were asked to quantify cannabis use by weighing out amounts of an inert cannabis surrogate and reporting on that amount’s potency through dollar-value estimates55. Cannabis use was then expressed in grams per day for statistical analyses. Cannabis craving was measured using the 12-item, Marijuana Craving Questionnaire (MCQ)56, and withdrawal symptoms were assessed using the Cannabis Withdrawal Scale (CWS)57. Mood symptoms were assessed using the YMRS/MADRS. Participants completed a history and physical examination and provided samples for blood chemistries and urine drug and pregnancy testing.
Following assessment, eligible participants completed two, 1-week experimental conditions (gabapentin, placebo) in randomized order. Each condition consisted of an in-person visit for assessment (repeating non-diagnostic baseline measures) and medication dispensation (Day 1), titration to maximum dose (i.e., 1200mg/day) (Days 1–5), assessment and MRI (Day 5; morning), immediately followed by medication-washout (Day 5 [afternoon]-Day 7) (see Supplemental Figure 2 for design schematic). Across each dosing period, 9 doses of gabapentin (doses 1–3=300mg, 4–9=600mg) or matched placebo were administered, with the first and final doses observed by study staff to ensure compliance, and with unused study medications returned for pill counts. A 5-day dosing period was chosen to minimize participant dropout while allowing sufficient time for participants to reach steady state concentrations of the target dose of gabapentin, given the medication’s 6–7-hour elimination half-life58. Medications were packaged and dispensed by our Investigational Drug Service (IDS), a centralized research pharmacy that compounds medications. IDS oversaw blinding procedures for the study and maintained treatment-assignment records.
Participants were asked to abstain from cannabis and alcohol ≥12-hours prior to each MRI appointment. Participants who smoked cigarettes were allowed to have their last cigarette immediately prior to taking their final medication dose (approximately 1-hour pre-MRI). During structural and 1H-MRS scanning, participants viewed scenic images via a mirror mounted to their 32-channel head-coil. Next, the Cannabis Cue Reactivity (CCR) task was administered19. Total scan time was 60–75-minutes in a Siemens 3.0T PrismaFit with actively-shielded magnet and high-performance gradients (80 mT/m, 200T/m-sec). The final medication dose was taken in the morning/early-afternoon of day 5, providing approximately 11 elimination half-lives prior to starting the subsequent condition58.
1H-MRS Acquisition and Processing.
A structural scan was taken for voxel placement and tissue segmentation (256 sagittal slices; 1mm thick/50% gap). 1H-MRS data were acquired from dACC and rBG, which form an important frontostriatal reward circuit59. The dACC voxel was placed on midsagittal T1-weighted images, posterior to the genu of the corpus callosum, with the ventral edge of the voxel aligned with the dorsal edge of the callosum60. An rBG voxel was placed on an axial T1-weighted slice about 1-cm above the genu, between the Sylvian fissure and the lateral ventricles including corpus striatum61. Each voxel was 2.5×2.5×3cm3 to ensure adequate signal-to-noise. See Figure 1 for voxel locations and sample spectra. Following placement of saturation bands 1-cm away from voxel faces and shimming via FASTESTMAP, single-voxel water-suppressed 1H-MRS spectra were acquired using MEGA-PRESS (TR=2000ms; TE=68ms; number of averages=256) with editing-pulse frequencies symmetric with respect to water (1.9ppm and 7.5ppm)47, and a PRESS sequence sensitive to glutamate (TR=2000ms; TE=40ms; number of averages=128)62. Unsuppressed water spectra were co-acquired for each sequence. MEGA-PRESS (GABA) data were processed using the Gannet MATLAB toolbox63. PRESS (glutamate) data were processed using LCModel 6.364. Metabolites with fitting uncertainties <20% were retained. Water was quantified from a Gaussian-Lorentzian fit to the non-water-suppressed data. Within-voxel tissue-fractions of gray and white matter and cerebrospinal fluid (CSF) were calculated based on automated segmentation in Statistical Parametric Mapping 12 (SPM12, Wellcome Department of Cognitive Neurology) using a volume mask generated in Gannet65. Metabolite concentrations were normalized to unsuppressed water and corrected for within-voxel CSF fraction.
Figure 1.
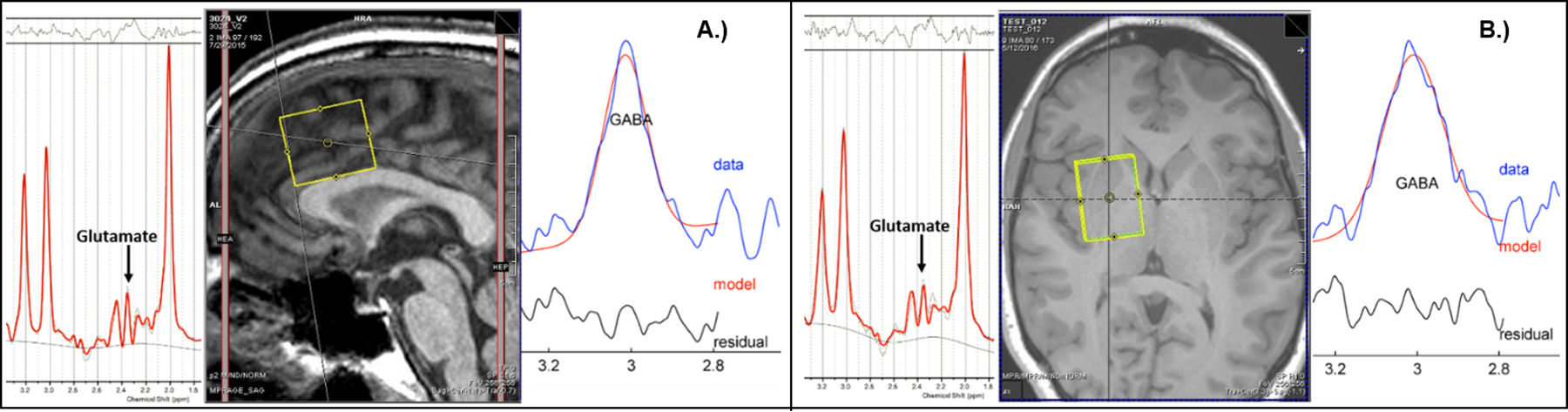
A.) Sample dACC voxel (center), fitted PRESS glutamate spectrum (left), fitted MEGA-PRESS GABA spectrum (right). B.) Sample rBG voxel (center), fitted PRESS glutamate spectrum (left), fitted MEGA-PRESS GABA spectrum (right).
fMRI Acquisition and Processing.
During the CCR task19, participants were shown pseudorandomly-interspersed cannabis (cannabis plant, paraphernalia), neutral (e.g., pine cone, trumpet), and fixation-cross images. Cannabis and neutral images were matched by color, hue, and complexity. Stimuli were presented in six 90-s epochs, each consisting of three 24-s blocks (one block each of cannabis, neutral, and fixation). Participants rated their “urge to use marijuana” for 6-s after each block from 0 (“none”) to 4 (“severe”) via handpad. See Supplemental Figure 3a for task schematic. A Simultaneous Multi-Slice EPI sequence was acquired (parameters: # of simultaneously acquired slices=3; TR/TE=1200/30ms; flip angle=65°; field of view=213×213mm; voxel size=2.8×2.8mm; 51 contiguous 2.8-mm-thick slices). The main contrast of interest was activation during cannabis vs. neutral image blocks. fMRI analysis was completed in SPM12. Standard preprocessing including realignment, normalization, and smoothing was performed. Volumes were censored for motion correction and/or abrupt changes in global signal intensity using DVARS66. Both censored volumes and realignment parameters were included as nuisance regressors in 1st-level models which included cannabis, neutral, and hand-pad rating conditions with implicit baseline. Preprocessed data were analyzed within a general linear model mixed-effects framework. Following 1st-level analysis, subject-specific spatially-normalized contrast maps were entered into 2nd-level, whole-brain random-effects analyses. Because the validity of the CCR fMRI task in BD+CUD had not been previously demonstrated, we first estimated whole-brain activation of cannabis vs. neutral images across participants while on placebo. Condition parameter maps were cluster thresholded at z >2.58, family-wise-error (FWE, p<0.05) corrected for multiple comparisons.
Data Analysis.
General linear mixed-effects models, which used all available data per analysis, accounted for the potential effects of condition order (i.e., Order #1 – gabapentin first vs. Order #2 – placebo first) via the interaction between treatment condition (gabapentin vs. placebo) and scan number (1st scan vs. 2nd scan)67. Significant effects were followed up by pairwise comparisons where indicated. Baseline participant characteristics that differed between Randomization Orders (p<0.10) were tested as potential moderators of associations between treatment condition and MRI dependent variables (DVs). Additional exploratory moderators, specified a priori in our proposal to examine associations of gabapentin-induced changes in GABA and glutamate with clinical outcomes, included cannabis use and YMRS and MADRS scores assessed at each visit. This analytic plan was executed for the primary DVs, dACC glutamate, GABA, rBG glutamate, GABA levels (1H-MRS), as well as for the secondary DV, whole-brain activation to cannabis-minus-neutral images (fMRI). Correlations of activation in significant cue-activation clusters with cannabis use were estimated to evaluate clinical relevance. A nominal α of 0.05 was used to evaluate each test in this preliminary study.
RESULTS
Participant Retention, Adherence, and Adverse Events
Although 21 participants (95.5% of enrolled) completed the study, all 22 enrolled participants completed at least one MRI and were therefore evaluable for analysis. Medication adherence, determined via pill counts, was ≥94%. Gabapentin was very well-tolerated, with participants reporting more Adverse Events (AEs) while on placebo than gabapentin (11 vs. 7 AEs), and no participants reported Serious AEs or having to leave the study due to AEs. See Supplemental Table 1 for AEs by condition.
Baseline Participant Characteristics by Randomization Order
Evaluation of baseline participant characteristics by RO# (Table 1) revealed that participants in RO#2 had higher prevalence of anxiety disorder (p<0.01) and cigarette-smoking (≥10 cigarettes/day68; p=0.08), along with elevated MADRS scores (p=0.08) and cannabis use in the 90-days preceding baseline (p=0.03) relative to participants in RO#1. As a result, baseline smoking status, MADRS, and cannabis use were evaluated as potential moderators of associations between treatment condition and MRI DVs. Because RO#2 contained 100% of individuals with anxiety disorders, evaluating this variable as a moderator was not possible. However, anxiety disorder was associated with, and may have been responsible for, the elevated MADRS scores (ps=0.07–0.09) and cannabis use (p=0.05) observed in RO#2.
Primary Outcomes: 1H-MRS Glutamate and GABA Levels
Quality-control evaluation of 1H-MRS spectra (blind to condition) resulted in some data loss, making the number of cases available for analysis (by brain region/metabolite): dACC glutamate n=22, GABA n=21, rBG glutamate n=19, GABA n=20. Gabapentin increased dACC glutamate levels, but only in RO#1 (Figure 2); a significant interaction of treatment condition with scan number was found (F=9.42, p<0.01). Gabapentin increased rBG glutamate levels, but only in cigarette-smoking participants (Figure 3a); significant interaction of treatment condition and cigarette-smoking status was found (F=4.31, p=0.05). This interaction was likely due to the lower glutamate levels observed in cigarette-smoking participants (n=11) relative to non-cigarette-smoking participants (n=7; p=0.13, Cohen’s d=0.75) while on placebo. Gabapentin failed to increase GABA levels across participants, as there were no significant main effects of treatment condition, nor interactions of condition with scan number, found for dACC or rBG GABA levels. No further interactions of condition with baseline moderators (smoking status, MADRS, cannabis use) were found in statistical models of dACC GABA or glutamate levels (ps>0.10).
Figure 2.
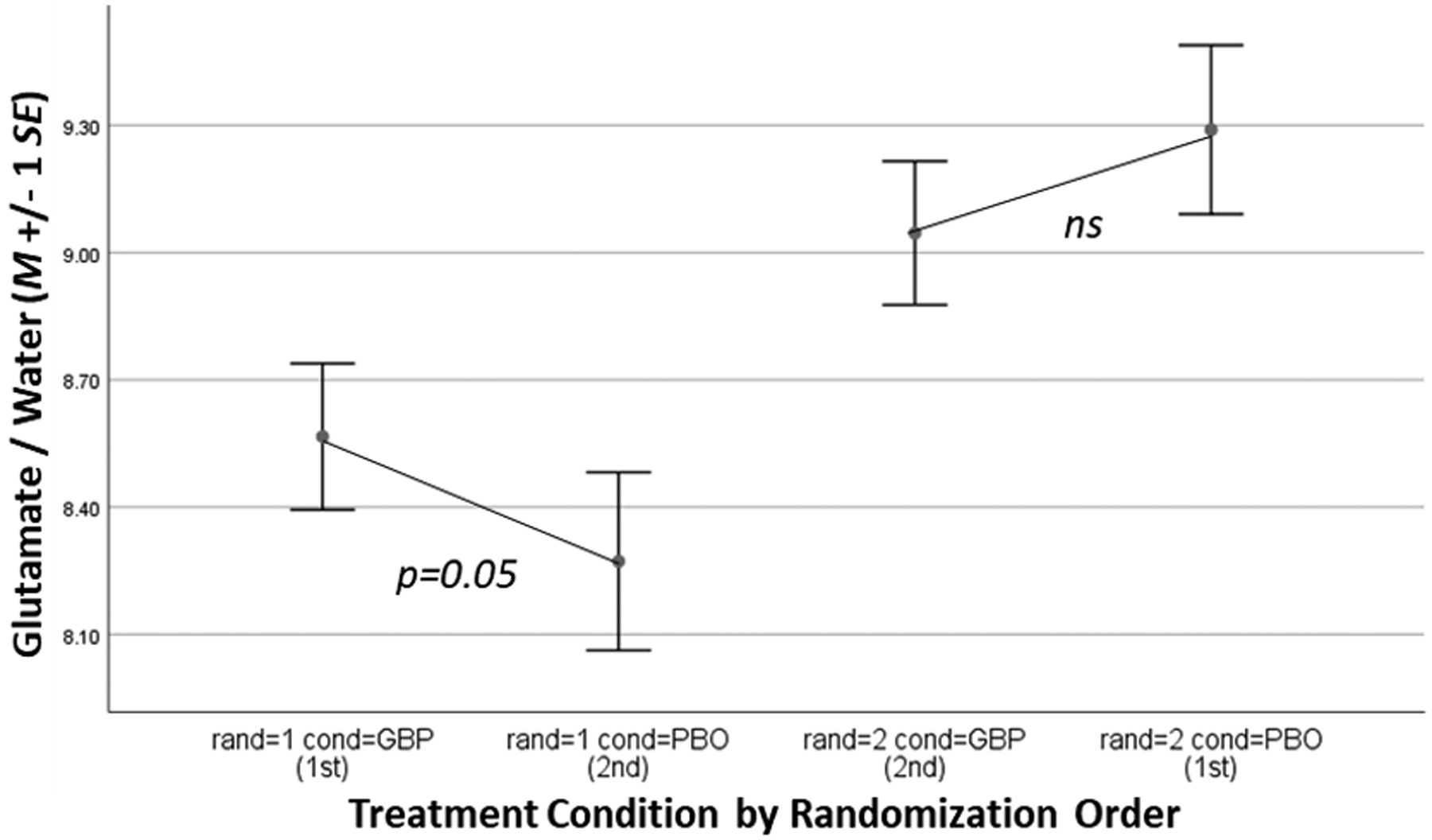
dACC glutamate levels by treatment condition (GBP=gabapentin, PBO = placebo) and randomization order (rand).
Figure 3.
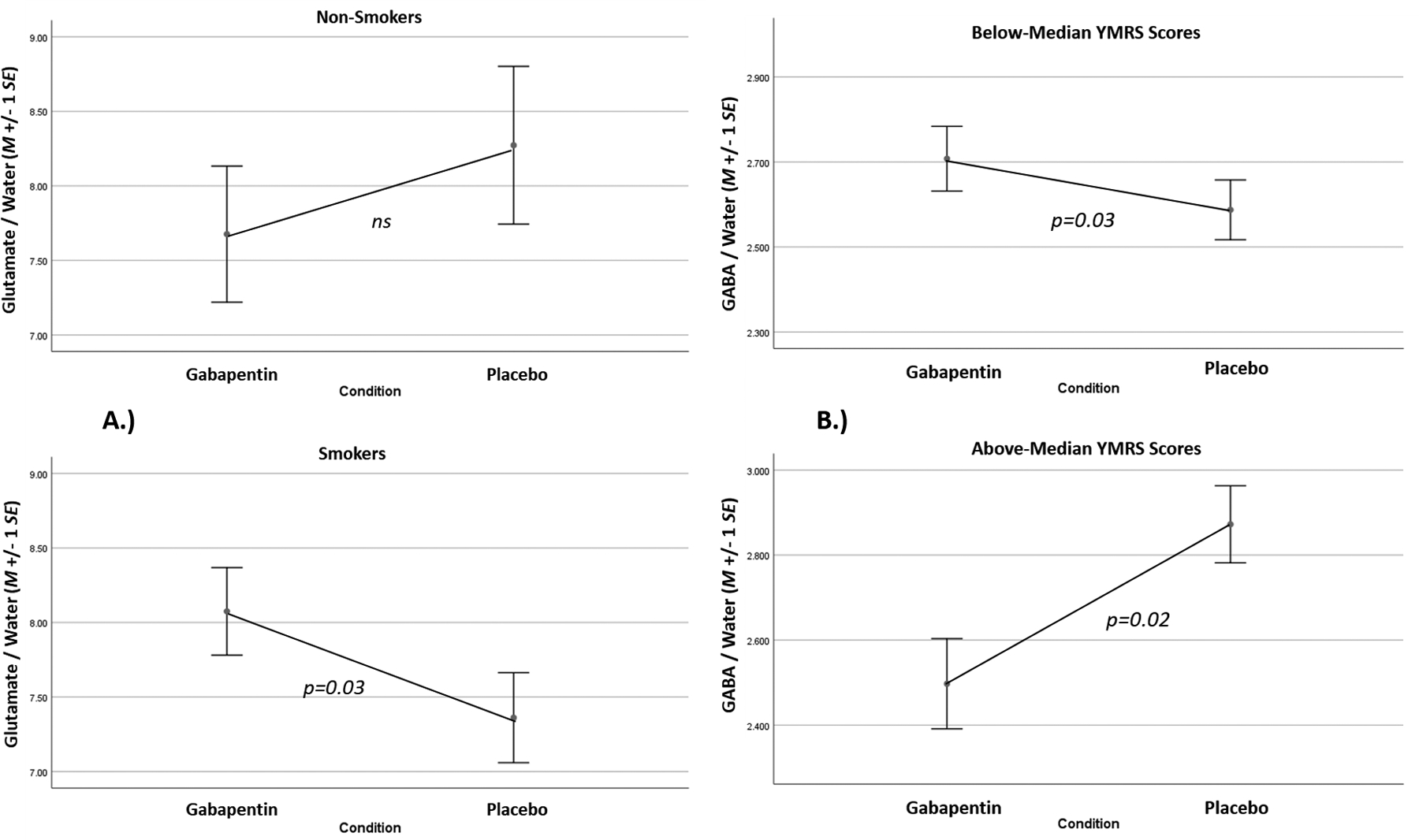
A.) rBG glutamate levels by treatment condition and smoking status (top=non-smokers, bottom=smokers). B.) dACC GABA levels by treatment condition and YMRS scores during the study (top=below-median YMRS, bottom=above-median YMRS).
Secondary Outcome: fMRI Activation to Cannabis Cues
fMRI data were not collected for 5 participants (insufficient time n=3, claustrophobia/discomfort n=2), leaving n=17 evaluable for fMRI analyses. When participants were on placebo, cannabis vs. neutral images were associated with activation in a number of brain regions associated with drug-cue reactivity (basal ganglia, posterior cingulate, thalamus, middle frontal gyrus, z > 2.58, FWE-corrected p < 0.05; Supplemental Figure 3b). Gabapentin increased cannabis-cue activation in the posterior midcingulate cortex (pMCC), relative to placebo, but only in cigarette-smoking participants (Figure 4); significant interaction of treatment condition with cigarette-smoking status was found in a cluster subsuming pMCC (z > 2.58, FWE-corrected p < 0.05, k=887, peak: x=3, y=−1, z=41). Across participants and conditions, increased cannabis-cue activation in pMCC was significantly associated with decreased cannabis use (r = −.38, p=0.03), demonstrating the potential clinical relevance of this finding. Since gabapentin was also found to increase rBG glutamate levels in cigarette-smoking participants, we evaluated the correlation of pMCC activation to cannabis cues with rBG glutamate and GABA levels by cigarette-smoking status, and found a positive association of pMCC cue activation with both rBG glutamate (r = 0.44, p = 0.08) and GABA (r = 0.66, p < 0.01) levels in cigarette-smoking, but not in non-cigarette-smoking (ps > 0.20), participants.
Figure 4.
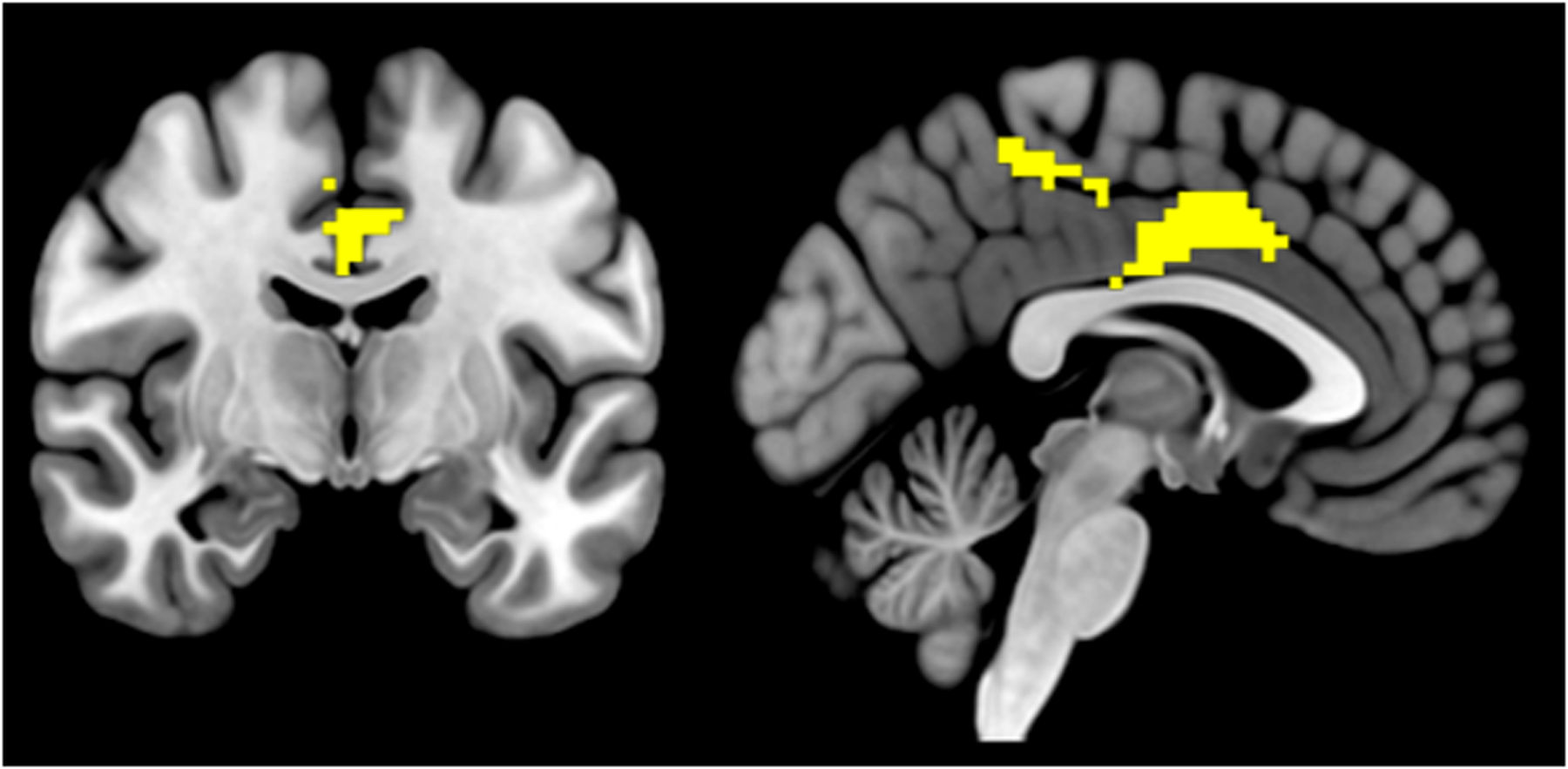
Posterior midcingulate cluster, in which gabapentin increased activation to cannabis cues, but only in cigarette-smoking participants (z > 2.58, FWE < 0.05, k = 640 voxels, center [x,y,z] = 1.9, −16.6, 42.2).
Exploratory Analysis of Associations of Gabapentin-induced Changes in dACC and rBG GABA and glutamate levels with Cannabis Use and Mood Symptoms During the Study
Though gabapentin failed to increase brain GABA levels across participants, elevations of dACC GABA in participants while on gabapentin versus placebo were associated with lower manic/mixed symptoms during the study, and vice versa (Figure 3b); significant interaction of treatment condition with YMRS scores was found (F=11.09, p<0.01). Elevations of dACC GABA levels in gabapentin-treated versus placebo-treated participants were also associated with lower depressive symptoms during the study, but only in RO#2 (Supplemental Figure 4a; significant 3-way interaction of condition, scan number, and MADRS scores was found, F=5.45, p=0.03). Furthermore, elevations of rBG glutamate levels in participants while on gabapentin versus placebo were associated with lower cannabis use during the study, but only in RO#2 (Supplemental Figure 4b; significant 3-way interaction of treatment condition, scan number, and cannabis use was found, F=6.81, p=0.02).
DISCUSSION
Results from this preliminary, randomized, double-blind, placebo-controlled, crossover, multimodal-MRI study of gabapentin for BD+CUD demonstrate that, a) gabapentin was well-tolerated with high adherence and strong retention, b) gabapentin increased dACC glutamate levels in participants with lower levels of substance use and mood symptoms, c) gabapentin increased rBG glutamate levels and pMCC activation to cannabis cues in cigarette-smoking participants, and d) elevated rBG glutamate and dACC GABA levels in participants while on gabapentin were associated with decreased cannabis use and mood symptoms in those with more severe substance use and mood symptoms. Together, these preliminary findings provide foundational support for gabapentin as a candidate adjuvant medication to therapeutically modulate brain GABA/glutamate levels in BD+CUD warranting further investigation.
Though promising, these findings must be interpreted with caution due to three interrelated limitations – relatively small sample size, randomization-order effects, and between-randomization-order differences in baseline characteristics. Although order effects may have genuinely reflected the effect of receiving gabapentin 1st versus 2nd on study outcomes, they more likely reflected the failure of simple randomization, due in part to small sample size, to balance condition orders on highly-impactful baseline characteristics. Cigarette-smoking status and anxiety disorder diagnosis, though specified a priori as moderators-of-interest in our proposal, were also evaluated due to significant between-randomization-order differences on these variables. Their impact on gabapentin-induced changes in brain GABA and/or glutamate levels was predicted because both have been associated with disturbances in GABAergic and/or glutamatergic transmission that are purported to be central to their phenomenology69,70, as well as worse clinical outcomes in individuals with BD+CUD relative to those who do not smoke cigarettes71 and do not have anxiety disorders72. Going forward, urn-randomization73 by smoking status and anxiety disorder in a larger sample, in conjunction with a parallel-group (between-subject) study design to rule out potentially-genuine order effects, will be critical to overcoming the interpretational challenges presented by the findings of this preliminary study.
In addition to these limitations, we did not predict that effects of gabapentin on brain metabolites would be specific to glutamate and not GABA29–32. However, studies that reported an effect of chronic gabapentin dosing on increasing brain GABA levels have generally evaluated a higher dose of gabapentin (≥1800mg/day vs. 1200mg/day) over a longer period of time (≥2-weeks vs. 5-days). The excellent tolerability of gabapentin in the present study suggests that we could safely increase gabapentin dosing from 1200mg/day to 1800mg/day. Likewise, our high participant-retention rate (95.5%), combined with a parallel-group design, suggests we could increase the dosing duration from 5- to 14-days without suffering significantly-more participant dropout. These changes would arguably increase our chances of observing a gabapentin effect on GABA levels. Alternatively, gabapentin-related changes in brain GABA levels may have been masked by co-edited macromolecular signals or unaccounted for menstrual-cycle-related variability in female participants47. These potential confounds could also be addressed in future studies. Provided these combined changes, if gabapentin still failed to increase GABA levels in BD+CUD (e.g., due to the concurrent effects of ongoing cannabis use), we may more-confidently conclude that observed neurobehavioral effects of gabapentin on BD+CUD are better explained by changes in glutamate transmission33.
Results from the CCR fMRI task added richness to the findings of the present study. In cigarette-smoking participants, gabapentin increased pMCC activation to cannabis cues which was, in turn, associated with decreased cannabis use during the study. Though interesting, we did not anticipate the effect of gabapentin on cannabis-cue brain activation to be facilitatory nor localized to the pMCC, as this region has not been identified as part of the cannabis-cue activation network by this study or others19. Instead, pMCC is central to recruitment of attentional-control circuitry to guide body orientation and reflexive movements in response to sensory stimuli, including rewards74,75. Nonetheless, behavioral manifestations of pMCC function include successful response inhibition while viewing emotionally-valenced images76, and people with BD (relative to healthy control subjects) exhibit significantly-less pMCC activation to cognitive-interference demands when exposed to similar experimental conditions77. As such, the observed effect of gabapentin on increasing pMCC activation to cues, and inverse association of increased pMCC cue-activation and decreased cannabis use, may represent gabapentin-induced suppression of involuntary drug-seeking motor behaviors (i.e., disrupting the sequence of events) that typically culminate in cannabis use67. That gabapentin increased pMCC cannabis-cue activation in parallel with increased rBG glutamate levels, may further indicate a restorative balance of subcortical (and cortical) reward-circuitry function, mediated in part by glutamatergic projections from pMCC to rBG78, in cigarette-smoking participants which could result in more adaptive behaviors. Finally, that these combined effects were relatively more pronounced in cigarette-smoking participants may reflect their substantially lower glutamate levels while on placebo, relative to non-cigarette-smoking participants, as observed in the present study13,29,31. Of course, this speculative interpretation requires empirical confirmation.
In addition to employing a more-robust study design, future studies of gabapentin in BD+CUD should evaluate a wider array of clinically-relevant phenomena, as gabapentin may indirectly reduce cannabis use by providing relief to symptoms of anxiety and sleep disturbance that drive persistent cannabis use79,80. Clinical trials of gabapentin for CUD34 or AUD81 have demonstrated that gabapentin may reduce mood and sleep disturbances, along with reducing drug use, in individuals with SUD. Furthermore, gabapentin has demonstrated efficacy in treating anxiety disorders82–84, which are prevalent and impairing in individuals with BD+SUD72,85, as well as sleep disturbance that occurs in the context of medical illness86; important because sleep disturbance is central symptom of, and potential trigger of, BD Mood Episodes87, as well as an impairing symptom of protracted cannabis withdrawal88. Along with evaluating more clinical-symptom measures, future studies should also evaluate participants’ motivation to reduce cannabis use. Most people with CUD do not seek treatment, with lack of motivation given as the predominant reason for not getting help89. However, participant-reported motivation to reduce/quit, and reasons for, cannabis use in BD+CUD individuals have never been reported. This information will be needed to successfully design and evaluate therapeutic interventions of any kind in this challenging clinical population.
In conclusion, despite the dire need for safe and efficacious treatments for BD+CUD, little is known about optimal treatment in this population. Convergent evidence supports disrupted brain GABA/glutamate homeostasis as a promising interventional target. Results from the present study support gabapentin as a candidate adjuvant medication to therapeutically engage that target, thereby reducing cannabis use, mood symptoms, and perhaps anxiety and sleep disturbance, in individuals with BD+CUD. Results from this study may guide the development and execution of larger, urn-randomized, parallel-group RCTs needed to further realize the potential therapeutic promise of gabapentin for BD+CUD. These results also add to the literature on associations of brain GABA and glutamate levels with constructs related to BD and CUD (including cannabis-cue reactivity and use, cigarette-smoking, and mood symptomatology), and provide foundational demonstration of a neurobehavioral, multimodal-MRI platform for evaluating glutamatergic/GABAergic drugs for BD+CUD and other conditions marked by dysfunction of GABAergic and/or glutamatergic transmission (e.g., schizophrenia, anxiety disorders).
Supplementary Material
ACKNOWLEDGMENTS
The Authors would like to thank Raymond Anton, MD, Truman Brown, PhD, and Aimee McCrae-Clark, PharmD, for their helpful feedback on early drafts of the study’s funding application. We would also like to thank Brecken Cornely for her invaluable contributions to study initiation. Finally, we would like to thank the clinicians (in particular, the attending physicians of the Institute of Psychiatry at MUSC) and their patients for their enthusiastic willingness to engage in this research.
The present study was funded by an R21 grant from NIH/NIDA to Dr. Prisciandaro (R21DA043917). In the past 3 years, Dr. Prisciandaro has been a consultant for, and received grant funding from, Laboratorio Farmaceutico CT. The study has been listed on clinicaltrials.gov (NCT03334721). The Authors have no competing financial interests to disclose.
REFERENCES
- 1.Pinto JV, Medeiros LS, Santana da Rosa G, Santana de Oliveira CE, Crippa JAdS, Passos IC, et al. The prevalence and clinical correlates of cannabis use and cannabis use disorder among patients with bipolar disorder: A systematic review with meta-analysis and meta-regression. Neuroscience & Biobehavioral Reviews. 2019;101:78–84. [DOI] [PubMed] [Google Scholar]
- 2.Hasin DS. US Epidemiology of Cannabis Use and Associated Problems. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2018;43(1):195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartoli F, Crocamo C, Carrà G. Cannabis use disorder and suicide attempts in bipolar disorder: A meta-analysis. Neuroscience and biobehavioral reviews. 2019;103:14–20. [DOI] [PubMed] [Google Scholar]
- 4.Lev-Ran S, Le Foll B, McKenzie K, George TP, Rehm J. Bipolar disorder and co-occurring cannabis use disorders: characteristics, co-morbidities and clinical correlates. Psychiatry research. 2013;209(3):459–465. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock LM, Gaudiano BA, Wenze SJ, Epstein-Lubow G, Miller IW. Demographic and clinical characteristics associated with comorbid cannabis use disorders (CUDs) in hospitalized patients with bipolar I disorder. Comprehensive psychiatry. 2016;65:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S-W, Dodd S, Berk L, Kulkarni J, de Castella A, Fitzgerald PB, et al. Impact of Cannabis Use on Long-Term Remission in Bipolar I and Schizoaffective Disorder. Psychiatry Investig. 2015;12(3):349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Rossum I, Boomsma M, Tenback D, Reed C, van Os J. Does cannabis use affect treatment outcome in bipolar disorder? A longitudinal analysis. The Journal of nervous and mental disease. 2009;197(1):35–40. [DOI] [PubMed] [Google Scholar]
- 8.Cohen K, Weizman A, Weinstein A. Modulatory effects of cannabinoids on brain neurotransmission. The European journal of neuroscience. 2019;50(3):2322–2345. [DOI] [PubMed] [Google Scholar]
- 9.Gigante AD, Bond DJ, Lafer B, Lam RW, Young LT, Yatham LN. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar disorders. 2012;14(5):478–487. [DOI] [PubMed] [Google Scholar]
- 10.Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10(8):561–572. [DOI] [PubMed] [Google Scholar]
- 11.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. The lancet Psychiatry. 2016;3(8):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colizzi M, McGuire P, Pertwee RG, Bhattacharyya S. Effect of cannabis on glutamate signalling in the brain: A systematic review of human and animal evidence. Neuroscience and biobehavioral reviews. 2016;64:359–381. [DOI] [PubMed] [Google Scholar]
- 13.Colizzi M, Weltens N, McGuire P, Lythgoe D, Williams S, Van Oudenhove L, et al. Delta-9-tetrahydrocannabinol increases striatal glutamate levels in healthy individuals: implications for psychosis. Molecular psychiatry. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. NeuroImage. 2011;57(1):69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prescot AP, Renshaw PF. Two-dimensional J-resolved proton MR spectroscopy and prior knowledge fitting (ProFit) in the frontal and parietal lobes of healthy volunteers: assessment of metabolite discrimination and general reproducibility. Journal of magnetic resonance imaging : JMRI. 2013;37(3):642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2006;1(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muetzel RL, Marjanska M, Collins PF, Becker MP, Valabregue R, Auerbach EJ, et al. In vivo H magnetic resonance spectroscopy in young-adult daily marijuana users. NeuroImage Clinical. 2013;2:581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cousijn J, Goudriaan AE, Ridderinkhof KR, van den Brink W, Veltman DJ, Wiers RW. Neural responses associated with cue-reactivity in frequent cannabis users. Addiction biology. 2013;18(3):570–580. [DOI] [PubMed] [Google Scholar]
- 19.Karoly HC, Schacht JP, Meredith LR, Jacobus J, Tapert SF, Gray KM, et al. Investigating a novel fMRI cannabis cue reactivity task in youth. Addictive behaviors. 2019;89:20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhagwagar Z, Wylezinska M, Jezzard P, Evans J, Ashworth F, Sule A, et al. Reduction in occipital cortex gamma-aminobutyric acid concentrations in medication-free recovered unipolar depressed and bipolar subjects. Biological psychiatry. 2007;61(6):806–812. [DOI] [PubMed] [Google Scholar]
- 21.Brady RO Jr., McCarthy JM, Prescot AP, Jensen JE, Cooper AJ, Cohen BM, et al. Brain gamma-aminobutyric acid (GABA) abnormalities in bipolar disorder. Bipolar disorders. 2013;15(4):434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang PW, Sailasuta N, Chandler RA, Ketter TA. Magnetic resonance spectroscopic measurement of cerebral gamma-aminobutyric acid concentrations in patients with bipolar disorders. Acta Neuropsychiatrica. 2006;18(2):120–126. [DOI] [PubMed] [Google Scholar]
- 23.Rigucci S, Xin L, Klauser P, Baumann PS, Alameda L, Cleusix M, et al. Cannabis use in early psychosis is associated with reduced glutamate levels in the prefrontal cortex. Psychopharmacology. 2018;235(1):13–22. [DOI] [PubMed] [Google Scholar]
- 24.Prisciandaro JJ, Tolliver BK, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Translational Psychiatry. 2017;7(7):e1163–e1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sills GJ. The mechanisms of action of gabapentin and pregabalin. Current opinion in pharmacology. 2006;6(1):108–113. [DOI] [PubMed] [Google Scholar]
- 26.Manville RW, Abbott GW. Gabapentin Is a Potent Activator of KCNQ3 and KCNQ5 Potassium Channels. Mol Pharmacol. 2018;94(4):1155–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu J, Wang DS, Bonin RP, Penna A, Alavian-Ghavanini A, Zurek AA, et al. Gabapentin increases expression of δ subunit-containing GABA(A) receptors. EBioMedicine. 2019;42:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taylor CP, Harris EW. Analgesia with Gabapentin and Pregabalin May Involve NMDA Receptors, Neurexins and Thrombospondins. Journal of Pharmacology and Experimental Therapeutics. 2020:jpet.120.266056. [DOI] [PubMed] [Google Scholar]
- 29.Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T (1)H-MRS study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(13):2764–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuzniecky R, Ho S, Pan J, Martin R, Gilliam F, Faught E, et al. Modulation of cerebral GABA by topiramate, lamotrigine, and gabapentin in healthy adults. Neurology. 2002;58(3):368–372. [DOI] [PubMed] [Google Scholar]
- 31.Petroff OA, Hyder F, Rothman DL, Mattson RH. Effects of gabapentin on brain GABA, homocarnosine, and pyrrolidinone in epilepsy patients. Epilepsia. 2000;41(6):675–680. [DOI] [PubMed] [Google Scholar]
- 32.Petroff OA, Rothman DL, Behar KL, Lamoureux D, Mattson RH. The effect of gabapentin on brain gamma-aminobutyric acid in patients with epilepsy. Annals of neurology. 1996;39(1):95–99. [DOI] [PubMed] [Google Scholar]
- 33.Meyerhoff DJ, Murray DE, Durazzo TC, Pennington DL. Brain GABA and Glutamate Concentrations Following Chronic Gabapentin Administration: A Convenience Sample Studied During Early Abstinence From Alcohol. Front Psychiatry. 2018;9:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(7):1689–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nielsen S, Gowing L, Sabioni P, Le Foll B. Pharmacotherapies for cannabis dependence. Cochrane Database of Systematic Reviews. 2019(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lile JA, Wesley MJ, Kelly TH, Hays LR. Separate and combined effects of gabapentin and [INCREMENT]9-tetrahydrocannabinol in humans discriminating [INCREMENT]9-tetrahydrocannabinol. Behav Pharmacol. 2016;27(2–3 Spec Issue):215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aracil-Fernández A, Almela P, Manzanares J. Pregabalin and topiramate regulate behavioural and brain gene transcription changes induced by spontaneous cannabinoid withdrawal in mice. Addiction biology. 2013;18(2):252–262. [DOI] [PubMed] [Google Scholar]
- 38.de Guglielmo G, Cippitelli A, Somaini L, Gerra G, Li H, Stopponi S, et al. Pregabalin reduces cocaine self-administration and relapse to cocaine seeking in the rat. Addiction biology. 2013;18(4):644–653. [DOI] [PubMed] [Google Scholar]
- 39.Roberto M, Gilpin NW, O’Dell LE, Cruz MT, Morse AC, Siggins GR, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5762–5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurokawa K, Shibasaki M, Mizuno K, Ohkuma S. Gabapentin blocks methamphetamine-induced sensitization and conditioned place preference via inhibition of α₂/δ−1 subunits of the voltage-gated calcium channels. Neuroscience. 2011;176:328–335. [DOI] [PubMed] [Google Scholar]
- 41.Vieta E, Manuel Goikolea J, Martinez-Aran A, Comes M, Verger K, Masramon X, et al. A double-blind, randomized, placebo-controlled, prophylaxis study of adjunctive gabapentin for bipolar disorder. The Journal of clinical psychiatry. 2006;67(3):473–477. [DOI] [PubMed] [Google Scholar]
- 42.Houghton KT, Forrest A, Awad A, Atkinson LZ, Stockton S, Harrison PJ, et al. Biological rationale and potential clinical use of gabapentin and pregabalin in bipolar disorder, insomnia and anxiety: protocol for a systematic review and meta-analysis. BMJ Open. 2017;7(3):e013433–e013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Association AP. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC2013. [Google Scholar]
- 44.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. The American journal of psychiatry. 2008;165(3):313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. The British journal of psychiatry : the journal of mental science. 1979;134:382–389. [DOI] [PubMed] [Google Scholar]
- 46.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. The British journal of psychiatry : the journal of mental science. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 47.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. NeuroImage. 2014;86:43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baeshen A, Wyss PO, Henning A, O’Gorman RL, Piccirelli M, Kollias S, et al. Test–Retest Reliability of the Brain Metabolites GABA and Glx With JPRESS, PRESS, and MEGA-PRESS MRS Sequences in vivo at 3T. Journal of Magnetic Resonance Imaging. 2020;51(4):1181–1191. [DOI] [PubMed] [Google Scholar]
- 49.Mikkelsen M, Singh KD, Sumner P, Evans CJ. Comparison of the repeatability of GABA-edited magnetic resonance spectroscopy with and without macromolecule suppression. Magnetic resonance in medicine. 2016;75(3):946–953. [DOI] [PubMed] [Google Scholar]
- 50.Prisciandaro JJ, Mikkelsen M, Saleh MG, Edden RAE. An evaluation of the reproducibility of (1)H-MRS GABA and GSH levels acquired in healthy volunteers with J-difference editing sequences at varying echo times. Magnetic resonance imaging. 2020;65:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prisciandaro JJ, Schacht JP, Prescot AP, Brenner HM, Renshaw PF, Brown TR, et al. Intraindividual changes in brain GABA, glutamate, and glutamine during monitored abstinence from alcohol in treatment-naive individuals with alcohol use disorder. Addiction biology. 2020;25(6):e12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Geurts JJG, Barkhof F, Castelijns JA, Uitdehaag BMJ, Polman CH, Pouwels PJW. Quantitative 1H-MRS of healthy human cortex, hippocampus, and thalamus: Metabolite concentrations, quantification precision, and reproducibility. Journal of Magnetic Resonance Imaging. 2004;20(3):366–371. [DOI] [PubMed] [Google Scholar]
- 53.First MB, Williams JBW, Karg RS, Spitzer RL. Structured Clinical Interview for DSM-5. Arlington, VA: American Psychiatric Association; 2015. [Google Scholar]
- 54.Sobell LC, Sobell MB. Alcohol Timeline Followback Users’ Manual. Toronto, Canada: Addiction Research Foundation; 1995. [Google Scholar]
- 55.Mariani JJ, Brooks D, Haney M, Levin FR. Quantification and comparison of marijuana smoking practices: blunts, joints, and pipes. Drug and alcohol dependence. 2011;113(2–3):249–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heishman SJ, Evans RJ, Singleton EG, Levin KH, Copersino ML, Gorelick DA. Reliability and validity of a short form of the Marijuana Craving Questionnaire. Drug and alcohol dependence. 2009;102(1–3):35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug and alcohol dependence. 2011;119(1–2):123–129. [DOI] [PubMed] [Google Scholar]
- 58.Katzung BG, Masters SB, Trevor AJ. Basic & Clinical Pharmacology. 12th ed. USA: McGraw-Hill Medical; 2012. [Google Scholar]
- 59.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biological psychiatry. 2012;71(11):1015–1021. [DOI] [PubMed] [Google Scholar]
- 61.Liu B, Wang G, Gao D, Gao F, Zhao B, Qiao M, et al. Alterations of GABA and glutamate-glutamine levels in premenstrual dysphoric disorder: a 3T proton magnetic resonance spectroscopy study. Psychiatry research. 2015;231(1):64–70. [DOI] [PubMed] [Google Scholar]
- 62.Mullins PG, Chen H, Xu J, Caprihan A, Gasparovic C. Comparative reliability of proton spectroscopy techniques designed to improve detection of J-coupled metabolites. Magnetic resonance in medicine. 2008;60(4):964–969. [DOI] [PubMed] [Google Scholar]
- 63.Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. Journal of magnetic resonance imaging : JMRI. 2014;40(6):1445–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magnetic resonance in medicine. 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 65.Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. Journal of magnetic resonance imaging : JMRI. 2015;42(5):1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu K. AB/BA design in continuous data. In: Crossover Designs.2016:7–29. [Google Scholar]
- 68.Lipari RN, Van Horn S. Smoking and Mental Illness Among Adults in the United States. In: The CBHSQ Report. Rockville (MD): Substance Abuse and Mental Health Services Administration (US); 2013:1–. [PubMed] [Google Scholar]
- 69.Alasmari F, Al-Rejaie SS, AlSharari SD, Sari Y. Targeting glutamate homeostasis for potential treatment of nicotine dependence. Brain Res Bull. 2016;121:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bandelow B, Michaelis S, Wedekind D. Treatment of anxiety disorders. Dialogues Clin Neurosci. 2017;19(2):93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Heffner JL, Anthenelli RM, Adler CM, Strakowski SM, Beavers J, DelBello MP. Prevalence and correlates of heavy smoking and nicotine dependence in adolescents with bipolar and cannabis use disorders. Psychiatry research. 2013;210(3):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prisciandaro JJ, Mellick W, Mitaro E, Tolliver BK. An evaluation of the impact of co-occurring anxiety and substance use disorder on bipolar disorder illness outcomes in STEP-BD. Journal of affective disorders. 2019;246:794–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9(4):345–364. [DOI] [PubMed] [Google Scholar]
- 74.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8(11):1458–1463. [DOI] [PubMed] [Google Scholar]
- 75.Vogt BA, Berger GR, Derbyshire SW. Structural and functional dichotomy of human midcingulate cortex. The European journal of neuroscience. 2003;18(11):3134–3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schulz KP, Clerkin SM, Halperin JM, Newcorn JH, Tang CY, Fan J. Dissociable neural effects of stimulus valence and preceding context during the inhibition of responses to emotional faces. Human brain mapping. 2009;30(9):2821–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ellard KK, Gosai AK, Felicione JM, Peters AT, Shea CV, Sylvia LG, et al. Deficits in frontoparietal activation and anterior insula functional connectivity during regulation of cognitive-affective interference in bipolar disorder. Bipolar disorders. 2019;21(3):244–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kunishio K, Haber SN. Primate cingulostriatal projection: limbic striatal versus sensorimotor striatal input. J Comp Neurol. 1994;350(3):337–356. [DOI] [PubMed] [Google Scholar]
- 79.Farris SG, Metrik J, Bonn-Miller MO, Kahler CW, Zvolensky MJ. Anxiety Sensitivity and Distress Intolerance as Predictors of Cannabis Dependence Symptoms, Problems, and Craving: The Mediating Role of Coping Motives. J Stud Alcohol Drugs. 2016;77(6):889–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sarvet AL, Wall MM, Keyes KM, Olfson M, Cerdá M, Hasin DS. Self-medication of mood and anxiety disorders with marijuana: Higher in states with medical marijuana laws. Drug and alcohol dependence. 2018;186:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA internal medicine. 2014;174(1):70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmed S, Bachu R, Kotapati P, Adnan M, Ahmed R, Farooq U, et al. Use of Gabapentin in the Treatment of Substance Use and Psychiatric Disorders: A Systematic Review. Front Psychiatry. 2019;10:228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de-Paris F, Sant’Anna MK, Vianna MR, Barichello T, Busnello JV, Kapczinski F, et al. Effects of gabapentin on anxiety induced by simulated public speaking. J Psychopharmacol. 2003;17(2):184–188. [DOI] [PubMed] [Google Scholar]
- 84.Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, et al. Placebo-controlled study of gabapentin treatment of panic disorder. Journal of clinical psychopharmacology. 2000;20(4):467–471. [DOI] [PubMed] [Google Scholar]
- 85.Prisciandaro JJ, Brown DG, Brady KT, Tolliver BK. Comorbid anxiety disorders and baseline medication regimens predict clinical outcomes in individuals with co-occurring bipolar disorder and alcohol dependence: Results of a randomized controlled trial. Psychiatry research. 2011;188(3):361–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu GJ, Karim MR, Xu LL, Wang SL, Yang C, Ding L, et al. Efficacy and Tolerability of Gabapentin in Adults with Sleep Disturbance in Medical Illness: A Systematic Review and Meta-analysis. Front Neurol. 2017;8:316–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pancheri C, Verdolini N, Pacchiarotti I, Samalin L, Delle Chiaie R, Biondi M, et al. A systematic review on sleep alterations anticipating the onset of bipolar disorder. European Psychiatry. 2019;58:45–53. [DOI] [PubMed] [Google Scholar]
- 88.Gates P, Albertella L, Copeland J. Cannabis withdrawal and sleep: A systematic review of human studies. Substance Abuse. 2016;37(1):255–269. [DOI] [PubMed] [Google Scholar]
- 89.Khan SS, Secades-Villa R, Okuda M, Wang S, Pérez-Fuentes G, Kerridge BT, et al. Gender differences in cannabis use disorders: results from the National Epidemiologic Survey of Alcohol and Related Conditions. Drug and alcohol dependence. 2013;130(1–3):101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


