Abstract
Background:
Callous-unemotional (CU) behaviors are important for identifying severe patterns of conduct problems (CP). One major fiber tract implicated in the development of CP is the uncinate fasciculus (UF), which connects amygdala and orbitofrontal cortex (OFC). The goals of the current study were to (a) explore differences in the white matter microstructure in the UF and other major fiber tracks between young typically developing (TD) children and those with a disruptive behavior disorder (DBD) and (b) explore, within the DBD group, whether individual differences in these white matter tracts relate to co-occurring CP and CU behaviors.
Methods:
Participants included 198 young children (69% boys, Mage = 5.66 years; 80% Latinx; 48.5% TD). CU behaviors and CP were measured via a combination of teacher/parent ratings. Non-invasive diffusion-weighted imaging (DWI) was used to measure fractional anisotropy (FA), an indirect indicator of white matter properties.
Results:
Relative to TD children, children in the DBD group had reduced FA on four out of the five fiber tracks we examined (except for cingulum and right ILF), even after accounting for whole brain FA, sex, movement, parental income, and IQ. Within the DBD group, no associations were found between CP and reduced white matter integrity across any of the fiber tracks examined. However, we found that even after accounting for CP, ADHD symptomology, and a host of covariates (whole brain FA, sex, movement, parental income, and IQ), CU behaviors were independently related to reduced FA in bilateral UF and left inferior fronto-occipital fasciculus (IFOF) in the DBD group, but this was not the case for TD children.
Conclusions:
Alterations in the white matter microstructure within bilateral UF and left IFOF may be biomarkers of CU behaviors, even in very young children.
Keywords: Callous-unemotional behaviors, conduct problems, preschool, DTI, imaging, uncinate fasciculus
Introduction
Young children exhibiting early signs of conduct problems (CP), typically represented by disruptive behavior disorder (DBD) diagnoses such as attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and/or conduct disorder (CD), represent the most common referrals to mental health clinics (Perou et al., 2013; Polanczyk, Willcutt, Salum, Kieling, & Rohde, 2014). A significant factor identified as contributing to the heterogeneity present in the manifestation of early CP is callous-unemotional (CU) traits, which refer to low levels of guilt, empathy, and caring for others (Frick, Ray, Thornton, & Kahn, 2014). CU traits or behaviors,1 a more developmentally appropriate term to refer to the CU construct in early childhood, can be reliability identified in the preschool period (Ezpeleta, de la Osa, Granero, Penelo, & Domenech, 2013; Waller, Hyde, Grabell, Alves, & Olson, 2015) and these have been an important construct for identifying the most pervasive, severe, and aggressive patterns of CP and later antisocial behavior (Frick et al., 2014). Not surprisingly, emerging research has examined the neural signatures of CU behaviors, both at the structural and functional level, with the current study focusing on the potential individual differences in connectivity between brain regions as a way to understand the development of CP and/or CU behaviors.
Connectomic differences associated with CP/CU
The fiber pathways comprising the structural connectome among extended limbic, frontal, and temporal regions have been the main subject of inquiry as it relates to CP/CU. Diffusion-weighted imaging (DWI), a non-invasive MRI technique that measures the diffusion of water molecules along anisotropic fiber bundles (Beaulieu, 2002), has been the method of choice for investigating the structural network of fiber pathways. Most studies have focused on differences in fractional anisotropy (FA; Winston, 2012). Higher FA values index a greater anisotropic (directional) water diffusion within axonal fibers, which is taken as a general index of fiber integrity (Soares, Marques, Alves, & Sousa, 2013; Thomason & Thompson, 2011).
Using this technique, researchers have attempted to determine whether individual differences in connectivity between brain regions are associated with the development of CP and CU behaviors (see Waller, Dotterer, Murray, Maxwell, & Hyde, 2017 for review). For example, some researchers have suggested that individual differences in connectivity between amygdala and prefrontal cortex are associated with the development of CP and CU behaviors (Blair, 2007), contributing specifically to the underlying cognitive, reward, and emotional processing mechanisms related to CP/CU (Raine, 2018). Fronto-amygdala connectivity is accomplished in part via the uncinate fasciculus (UF). This fiber pathway has rostral terminations in orbital and lateral frontal cortex, frontal pole, and anterior cingulate gyrus. The posterior termination in the temporal lobe includes projections through amygdala (de Schotten, Dell’Acqua, Valabregue, & Catani, 2012; Holl et al., 2011; Von Der Heide, Skipper, Klobusicky, & Olson, 2013).
Several studies have found reduced FA in the UF among adult samples exhibiting high levels of CP (Craig et al., 2009; Motzkin, Newman, Kiehl, & Koenigs, 2011; Sobhani, Baker, Martins, Tuvblad, & Aziz-Zadeh, 2015). The only studies of youth have been conducted in adolescents (Waller et al., 2017). In these cases, reduced FA in UF is associated with increased CU behaviors (Breeden, Cardinale, Lozier, VanMeter, & Marsh, 2015), increased psychopathy (Maurer, Paul, Anderson, Nyalakanti, & Kiehl, 2020), and diagnosis of CD (González-Madruga et al., 2020). Of note, some studies reported findings in the opposite direction (i.e., higher FA) as it relates to CU behaviors (Sarkar et al., 2013) and CD (Passamonti et al., 2012). Other abnormalities of the fiber pathways supporting extended limbic, frontal, and temporal regions have also been reported (Waller et al., 2017). In particular, fiber pathways of the ventral temporal lobe, namely the inferior longitudinal fasciculus (ILF) and inferior fronto-occipital fasciculus (IFOF), have been associated with psychopathic traits and CD in adolescents (Haney-Caron, Caprihan, & Stevens, 2014; Pape et al., 2015). The ILF courses in the ventral white matter of the temporal lobe, originating posteriorly in extrastriate areas of the occipital lobe, and ending with rostral terminations in the middle and inferior temporal gyri, the temporal pole, parahippocampal gyrus, hippocampus, and amygdala (Catani, Jones, Donato, & Ffytche, 2003). The IFOF runs medial to the ILF, originates in the inferior and medial occipital lobe, travels through the temporal stem dorsal to the UF, and projects to the inferior frontal gyrus, the medial and orbital frontal cortex, and the frontal pole (Catani et al., 2003; Martino, Brogna, Robles, Vergani, & Duffau, 2010; Martino, Vergani, Robles, & Duffau, 2010; Sarubbo, De Benedictis, Maldonado, Basso, & Duffau, 2013). These two pathways connect a number of limbic, frontal, and temporal regions associated with CP/ CU, and thus, these findings are predictable in that context. Finally, mixed findings in adolescents have been reported for the cingulum (González-Madruga et al., 2020; Waller et al., 2017), which is a collection of smaller short association fiber systems that course in the white matter under the cingulate gyrus, supporting connections to/from lateral and dorsal prefrontal cortex, medial prefrontal and anterior cingulate, insula, parahippocampal gyrus, subiculum, and amygdala. The structure and function of these regions, especially insula, amygdala, and anterior cingulate, have been associated with CP/CU. However, only a couple of studies have reported any association in adolescents (Haney-Caron et al., 2014; Pape et al., 2015), including González-Madruga et al. (2020), who found lower FA in the cingulum in male adolescents with CD relative to typically developing adolescents.
Although these are promising findings, the literature remains inadequate for understanding the development of CP/CU in very young children. One critical measurement issue when studying CP/CU in very young children is accounting for high comorbidity rates of ADHD and ODD/CD (Bendiksen et al., 2017). Comorbidity rates between ADHD and ODD/CD during the preschool period in community/population-based samples tend to be between 30 and 40% (Bendiksen et al., 2017; Wichstrøm et al., 2012) but significantly higher in clinically referred samples ranging from 42% to 70% (Bunte, Schoemaker, Hessen, van der Heijden, & Matthys, 2014; Forehand et al., 2016; Hare, Garcia, Hart, & Graziano, 2021). Children with comorbid diagnoses of ADHD and ODD/CD also experience significantly worse behavioral outcomes than children with either disorder alone (Waschbusch, 2002) and are at a higher risk for ‘fledgling psychopathy’ and criminal careers in adulthood (DeLisi, Drury, & Elbert, 2020; Gresham, Lane, & Lambros, 2000; Lynam, 1998). As pointed out by Waller et al. (2017), often comorbid ADHD is not measured among brain imaging studies, and therefore, it is unclear whether white matter microstructure findings are really due to CP/CU or unmeasured ADHD symptomology. More focused dissociation of CP with and without high levels of CU behaviors is also needed. To maximize our understanding of CP and CU behaviors, it is important to also include young children with only ADHD, given that this group of children are at a much higher risk for developing future CP (Mannuzza, Klein, Abikoff, & Moulton Iii, 2004) and can also exhibit CU behaviors that are independent from CP (Graziano & Garcia, 2016; Haas et al., 2011). Thus, to further our understanding of the neurobiology of CP, more pediatric connectivity studies are needed that take into account CP, CU behaviors, and high comorbidity of ADHD.
Goals of the current study
The overarching goal of the current study was to examine the white matter microstructure in the UF along with other major fiber tracks (ILF, IFOF, and cingulum; see Figure 1) among young typically developing (TD) children and those diagnosed with a DBD. As indicated in the previous section, to maximize the variability in our measurement of CP and CU behaviors, our DBD group consisted of children with an initial diagnosis of ADHD with or without comorbid ODD/CD diagnoses. Our goals were to (a) explore differences in these white matter connections between young TD children and those with a DBD, and 2) explore, within the DBD group evidencing sufficient variability in CP and CU behaviors, whether individual differences in white matter microstructure in these tracts relate to co-occurring CP and CU behaviors, even after accounting for ADHD symptomology. Based on prior work with older youth/adults (Breeden et al., 2015; Waller et al., 2017), we expected children in the DBD group to have lower FA across the examined fiber pathways. More specificity in white matter disruption was expected when examining only the DBD group, as we expected reduced white matter integrity in the UF to be associated with CU behaviors, above and beyond CP.
Figure 1.
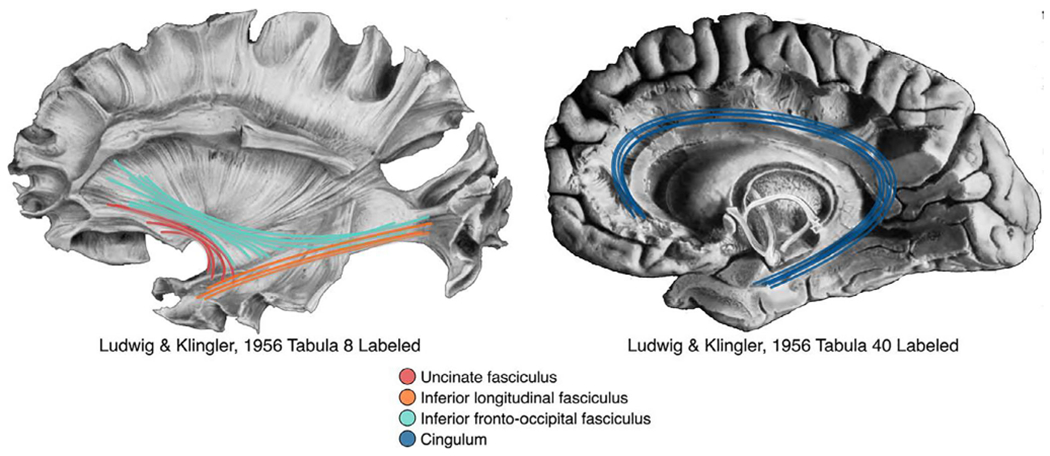
The four fiber pathways of interest are shown overlaid on fiber dissection tabula from Ludwig, E., & Klingler, J. (1956). Atlas cerebri humani. Boston and Toronto: Little, Brown, and Company
Method
Participants and recruitment
The study took place in a large urban southeastern city in the United States with a large Latinx population. Children and their caregivers were recruited from local preschools and mental health agencies via brochures, radio and newspaper ads, and open houses/parent workshops. For the DBD sample, parents and children were invited to participate in an assessment to determine study eligibility if the parent (a) endorsed his or her child as having clinically significant levels of ADHD symptoms, (b) indicated that his or her child was currently displaying clinically significant academic, behavioral, or social impairments as measured by a score of three or higher on a seven-point impairment rating scale (Fabiano et al., 2006), and (c) indicated that his or her child was not taking any psychotropic medication. For the TD sample, if the parent endorsed his or her child as having (a) less than 4 ADHD symptoms (across either Inattention or Hyperactivity/Impulsivity according to the DSM-5), (b) less than 4 ODD symptoms, and (c) indicated no clinically significant impairment, the parent and child were invited to participate in an assessment to determine study eligibility. Participants were also required to be enrolled in school during the previous year, have an estimated IQ of 70 based on the WPPSI-IV (Wechsler, 2012), have no confirmed history of an Autism Spectrum Disorder, and for only for the DBD sample, be able to attend an 8-week summer treatment program (STP-PreK; Graziano, Slavec, Hart, Garcia, & Pelham, 2014) prior to the start of the next school year.
ADHD diagnosis and comorbid disruptive behavior disorders were assessed through a combination of parent structured interview (Computerized-Diagnostic Interview Schedule for Children [C-DISC]; Shaffer, Fisher, Lucas, Dulcan, & Schwab-Stone, 2000) and parent and teacher ratings of symptoms and impairment (Disruptive Behavior Disorders Rating Scale, Impairment Rating Scale; Fabiano et al., 2006; Pelham, Gnagy, Greenslade, & Milich, 1992), as is recommended practice. Dual Ph.D. level clinician review was used to determine diagnosis and eligibility.
The final participating sample consisted of 198 young children (Mage = 5.66, SD = 0.87, and 69% male; 48.5% TD). Eighty percent of the children were identified by parents as Hispanic/Latino White, 12% as Non-Hispanic/Latino White, 6% as Non-Hispanic/Latino Black, and 2% as Hispanic/Latino Black. We also measured maternal education; 7.1% of mothers had a high school degree or less, 14.8% had some college, 13.1% had associates degrees, 32.2% had bachelor’s degrees, and 32.8% had an advanced degree. A diverse range of yearly parental income was also reported (16% = less than $20k, 32% = between $20k and $50k, 22% = between $50k and $80k, 15% = between $80k to $110k, and 15% = greater than $110k). Of the whole sample, 48.5% were TD (n = 96) while the remaining 51.5% met diagnostic criteria for ADHD (n = 102). In terms of comorbidity, 68.62% of children in the ADHD group also met diagnostic criteria for ODD/CD (n = 70). Of note, to maximize our variability in the continuous measurement of CP and CU behaviors, we included children with only an ADHD diagnosis (but no ODD/CD) in our analyses. We also re-ran the analyses with these children excluded and noted in the results section any differences.
Study design and procedure
This study was approved by the university’s Institutional Review Board. As part of the baseline assessment, children completed a series of tasks in the laboratory and participated in an MRI scanning session. Parents also completed various questionnaires regarding their children’s emotional, behavioral, and cognitive functioning. Families of children in the DBD group received the intervention (STP-PreK) at either no cost via a federal grant or at a subsidized cost via a local grant, and all families received compensation ($100 gift card for completing the assessment). Similar questionnaires were also obtained from children’s school teachers. TD children received a $100 gift card, academic and intellectual functioning feedback, study t-shirt, and a small gift from the study ‘treasure chest’.
Measures
CP.
Parents and teachers completed the Disruptive Behavior Disorders Rating Scale (DBD; Pelham et al., 1992), adapted for DSM-5 terminology, which assess for symptoms of ADHD, ODD, and CD on a four-point scale with respect to the frequency of occurrence. For the purposes of this study, we obtained an average score for the ODD and CD symptoms (α’s = .71–.88) as a measure of CP, given their significant correlations, rs = .73 (parent report) and .75 (teacher report), ps < .001. Consistent with prior work using the ‘and/or’ algorithm (Piacentini, Cohen, & Cohen, 1992), the highest score among parent and teacher reports was used. To control for ADHD symptom severity, we also examined the hyperactivity/impulsivity and inattention symptoms.
Callous-unemotional (CU) behaviors.
Parents (α = .83) and teachers (α = .72) completed a 12-item abbreviated version of the Inventory of Callous-Unemotional Traits (ICU) (Frick, 2004; Hawes et al., 2014). We first computed an overall CU composite by separately obtaining the average for the parent-report and teacher-report versions. To maximize our detection of CU behaviors and consistent with prior work (Sarkar et al., 2013), the highest composite score among parent and teacher reports was used.
MRI acquisition and processing:
All imaging was performed using a research-dedicated 3 Tesla Siemens MAGNETOM Prisma MRI scanner (V11C) with a 32-channel coil located on the university campus. Children first completed a preparatory phase using a realistic mock scanner. In the magnet, children watched a child-friendly movie of their choice. Ear protection was used, and sound was presented through MRI-compatible headphones.
We collected multi-shell high-angular diffusion-weighted imaging (HARDI) data according to the Adolescent Brain and Cognitive Development (ABCD) protocol (Hagler et al., 2019). These scans were collected with a 1.7 mm isotropic voxel size, using multiband imaging echo planar imaging (EPI; acceleration factor = 3). The acquisition consisted of ninety-six diffusion directions, six b = 0 frames, and four b-values (102 diffusion directions; 6 b = 500, 15 b = 1,000, 15 b = 2,000, and 60 b = 3,000).
Diffusion-weighted imaging post-processing:
Initial post-processing was accomplished with DTIPrep v1.2.8 (Oguz et al., 2014), TORTOISE DIFFPREP v3.1.0 (Irfanoglu, Nayak, Jenkins, & Pierpaoli, 2017; Pierpaoli et al., 2010), FSL v6.0.1 topup (Andersson, Skare, & Ashburner, 2003; Smith et al., 2004), and DSI Studio (v. June 2020; Yeh, Wedeen, & Tseng, 2010). We also implemented a pre- and post-analysis quality check assessing signal-to-noise of each diffusion b-value (Roalf et al., 2016).
Initial quality control was accomplished in DTIPrep to complete the following steps: (a) image/diffusion information check; (b) padding/cropping of data; (c) Rician noise removal; and (d) slice-wise, interlace-wise, and gradient-wise intensity and motion checking. The number of acquisitions removed was used as a proxy for movement/bad data quality and was included as a covariate in subsequent regression analyses. TORTOISE DIFFPREP was used to accomplish motion and eddy current correction. We implemented calculation of the diffusion tensor model in DSI Studio to estimate the eigenvalues reflecting diffusion parallel and perpendicular to each of the fibers along three axes (x, y, z). The resulting eigenvalues were then used to compute indices of FA, radial diffusivity (RD), and axial diffusivity (AD; Basser, Mattiello, & LeBihan, 1994; Hasan & Narayana, 2006). FA is an index for the amount of diffusion asymmetry within a voxel, normalized to take values from 0 (isotropic diffusion) to 1 (anisotropic diffusion). This value can be decomposed into AD, measuring the parallel eigenvalue (λ1), and RD, measuring the average of the secondary and tertiary perpendicular eigenvalues (λ2 + λ3]/2). AD and RD quantifications are sensitive to axon integrity and myelin integrity, respectively (Basser et al., 1994; Winston, 2012).
In addition to calculating the more familiar diffusion metrics (FA, AD, RD), we also reconstructed the data using higher-order HARDI generalized q-sampling imaging (GQI) technique (Yeh et al., 2010), implemented in DSI Studio. We calculated three additional metrics: Quantitative Anisotropy (QA) of the primary peak of the spin distribution function (SDF), Normalized QA (nQA), and Generalized Fractional Anisotropy (GFA). QA is the spin population in a specific direction, with multiple overlapping directions defined on the SDF. QA can be defined for each peak, and we report the result for the primary peak (QA0). nQA is normalized so that the QA0 can be meaningfully interpreted across participants. GFA can be thought of as a higher-order generalization of FA (Tuch, 2004). Like the traditional FA metric from DTI, the GFA values range from 0 to 1.
Fiber tract identification:
Tractography was conducted using DSI Studio’s built-in tractography atlas (Yeh, 2017). The atlas was originally created from 840 healthy adults in the HCP840 dataset and defines white matter regions of interest (ROIs) in the MNI space. The atlas is then non-linearly warped to the native participant space (Yeh et al., 2018). Because we are analyzing a pediatric dataset, each ROI was visually inspected to ensure that warping did not introduce inaccuracies (see Figure S1 for example participants). The following tracts were analyzed: UF, ILF, IFOF, cingulum, and corticospinal tract (CST; see Figure 1). As a final step, for each fiber pathway of interest, for each hemisphere, and for each subject, DTI FA, RD, AD, and GQI QA, nQA, and GFA statistics were exported and averaged across the whole fiber bundle for further analysis.
Brain-behavior data analyses
Analyses were conducted using R v.3.5.3. As an initial step, data were inspected for missingness. Only 2% of all data were missing. We corrected for this missingness using multiple imputation, with 20 imputation data sets (using R package Multivariate Imputation by Chained Equations; mice). We also examined whether there were significant group differences when it came to movement in the scanner. Out of 102 directions, the DBD sample moved more frequently and lost more directions (M = 83.96 directions kept, SD = 12.53) compared with TD (M = 88.74, SD = 9.29; t(196) = 3.03, p = .0027). Because of this, we included the number of retained diffusion directions as a covariate in all subsequent models, as a proxy for subject movement. In these regression models, we used robust regression (R function rlm; Wright & London, 2009) to mitigate the influence of outlying values (Wilcox, 2012). We also improved the estimation of the reliability of the parameter estimate by using the bootstrap method (Efron, 1981, 1987) to calculate the standard errors and 95% Confidence Intervals (CIs).
Correction for multiple comparisons
We focused on a small number of fiber pathways based on our review of the literature, but the number of comparisons necessitates statistical correction to control for Type I error. We employed the False Discovery Rate (FDR) correction (Benjamini & Hochberg, 1995) at three different nominal levels (q = .05, .10, .25), which defines the proportion of errors committed by falsely rejecting null hypotheses. Family was defined within a hemisphere for each measure (e.g., five left hemisphere ROIs for FA). We interpret results in the context of these FDR proportions, and in the context of effect sizes considered against their associated 95% CIs.
Results
In Table S1, we provide the intercorrelations among the behavioral measures for the full sample as well as the DBD and TD groups. First, we examined TD vs. DBD group differences in behavioral measures, and in the diffusion metrics across the five fiber tracts (UF, IFOF, cingulum, ILF, and CST). As expected and seen in Table 1, children in the DBD sample had significantly higher rates of CP (t (196) = 15.83, d = 2.23, p < .0001) and CU behaviors (t(196) = 11.10, d = 1.56, p < .0001) compared with TD children. There was also a significant group difference on IQ (t(194) = −4.28, d = −0.61, p < .0001), with the DBD group (M = 96.48, SD = 13.16) scoring lower than the TD group (M = 103.89, SD = 11.17).
Table 1.
Results comparing DBD and TD groups on behavioral measures and fiber pathway microstructure
| Group differences | DBD mean (SE) | TD mean (SE) | t | Cohen’s d | p |
|---|---|---|---|---|---|
| Behavior | |||||
| Conduct problems | 1.03 (0.049) | 0.18 (0.022) | 15.83 | 2.23 | <.001*** +++ |
| CU behaviors | 1.33 (0.052) | 0.67 (0.028) | 11.10 | 1.56 | <.001*** +++ |
| Full Scale IQ | 96.480 (1.303) | 103.893 (1.140) | −4.28 | 0.61 | <.001*** +++ |
| DBD-Hyperactivity/Impulsivity | 2.412 (0.051) | 0.590 (0.048) | 25.88 | 3.69 | <.001*** +++ |
| DBD-Inattention | 2.284 (0.059) | 0.404 (0.043) | 25.89 | 3.65 | <.001*** +++ |
| Microstructure | |||||
| Whole Brain FA | 0.325 (0.002) | 0.326 (0.002) | 0.20 | −0.11 | .85 |
| Whole Brain AD | 0.738 (0.005) | 0.728 (0.005) | 0.75 | 0.2 | .45 |
| Whole Brain RD | 0.491 (0.004) | 0.482 (0.004) | 0.46 | 0.25 | .69 |
| Left hemisphere | |||||
| UF FA | 0.220 (0.003) | 0.238 (0.002) | −3.39 | −0.65 | <.001*** +++ |
| Cingulum FA | 0.228 (0.003) | 0.227 (0.002) | 0.50 | 0.04 | .62 |
| ILF FA | 0.267 (0.003) | 0.283 (0.002) | −3.18 | −0.69 | .0017** +++ |
| IFOF FA | 0.264 (0.002) | 0.277 (0.002) | −3.34 | −0.59 | <.001*** +++ |
| CST FA | 0.347 (0.005) | 0.378 (0.003) | −3.63 | −0.83 | <.001*** +++ |
| UF AD | 0.909 (0.002) | 0.907 (0.002) | 0.01 | 0.09 | .99 |
| Cingulum AD | 0.867 (0.003) | 0.858 (0.001) | 1.61 | 0.41 | .11 |
| ILF AD | 0.890 (0.003) | 0.895 (0.002) | −1.81 | 0.18 | .07 |
| IFOF AD | 0.872 (0.002) | 0.872 (0.002) | −0.66 | 0.01 | .51 |
| CST AD | 0.852 (0.002) | 0.854 (0.002) | −2.04 | 0.09 | .043* + |
| UF RD | 0.653 (0.003) | 0.632 (0.003) | 3.91 | 0.69 | <.001*** +++ |
| Cingulum RD | 0.615 (0.003) | 0.611 (0.002) | 0.03 | 0.19 | .98 |
| ILF RD | 0.592 (0.002) | 0.582 (0.002) | 2.37 | 0.43 | .019** ++ |
| IFOF RD | 0.582 (0.002) | 0.569 (0.002) | 3.23 | 0.6 | .001*** +++ |
| CST RD | 0.496 (0.004) | 0.468 (0.003) | 2.86 | 0.75 | .005** +++ |
| Right hemisphere | |||||
| UF FA | 0.237 (0.003) | 0.255 (0.003) | −2.86 | −0.4 | 0.005 ** +++ |
| Cingulum FA | 0.227 (0.003) | 0.227 (0.002) | 0.28 | 0.004 | 0.782 |
| ILF FA | 0.267 (0.004) | 0.283 (0.003) | −1.85 | −0.5 | 0.067 |
| IFOF FA | 0.257 (0.003) | 0.270 (0.002) | −2.93 | −0.55 | <.001 *** +++ |
| CST FA | 0.329 (0.005) | 0.355 (0.003) | −3.07 | −0.63 | 0.003 ** +++ |
| UF AD | 0.907 (0.002) | 0.907 (0.002) | −0.47 | 0.02 | 0.638 |
| Cingulum AD | 0.863 (0.003) | 0.854 (0.002) | 1.96 | 0.38 | 0.051 |
| ILF AD | 0.861 (0.003) | 0.865 (0.002) | −1.49 | 0.15 | 0.137 |
| IFOF AD | 0.867 (0.002) | 0.865 (0.002) | −0.05 | 0.07 | 0.957 |
| CST AD | 0.842 (0.002) | 0.841 (0.002) | −0.5 | 0.08 | 0.612 |
| UF RD | 0.626 (0.003) | 0.607 (0.003) | 2.96 | 0.61 | 0.004 ** +++ |
| Cingulum RD | 0.565 (0.002) | 0.560 (0.002) | 0.39 | 0.21 | 0.699 |
| ILF RD | 0.573 (0.003) | 0.561 (0.002) | 3.15 | 0.45 | 0.141 |
| IFOF RD | 0.585 (0.002) | 0.572 (0.002) | 4.03 | 0.58 | <.001 *** +++ |
| CST RD | 0.506 (0.005) | 0.480 (0.003) | 2.18 | 0.68 | 0.031 * + |
AD, axial diffusivity; CST, corticospinal tract; DBD, disruptive behavior disorder; FA, fractional anisotropy; IFOF, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; RD, radial diffusivity; TD, typically developing; UF, uncinate fasciculus. Statistical tests of group differences controlled for sex, whole brain FA, movement, parental income, and IQ.
p < .05 (uncorrected).
p < .01 (uncorrected).
p < .001 (uncorrected).
FDR Correction:
q = .25;
q = .10;
q = .05.
Because FA is calculated using information contained in the other metrics (e.g. RD and AD), and because it is the most commonly reported summary metric for DWI, we focus on FA differences in our results for these initial group comparisons. We found no significant group differences for whole brain FA (t (192) = 0.20, d = −0.11, p = .85), nor for bilateral cingulum (t(191) = 0.50, d = 0.04, p = .62 for left; t(191) = 0.28, d = 0.0, p = .78 for right) or right ILF (t(191) = −1.85, d = −0.5, p = .07). However, all other pathway differences for FA were statistically significant (all p < .01; see Table 1 which also includes AD and RD values), even after controlling for sex, whole brain diffusion, movement, parental income, and IQ. Figure 2 shows bar plots of the FA group differences for each of the pathways, and the whole brain. Table S2 shows the results for GFA, QA, and nQA metrics.
Figure 2.
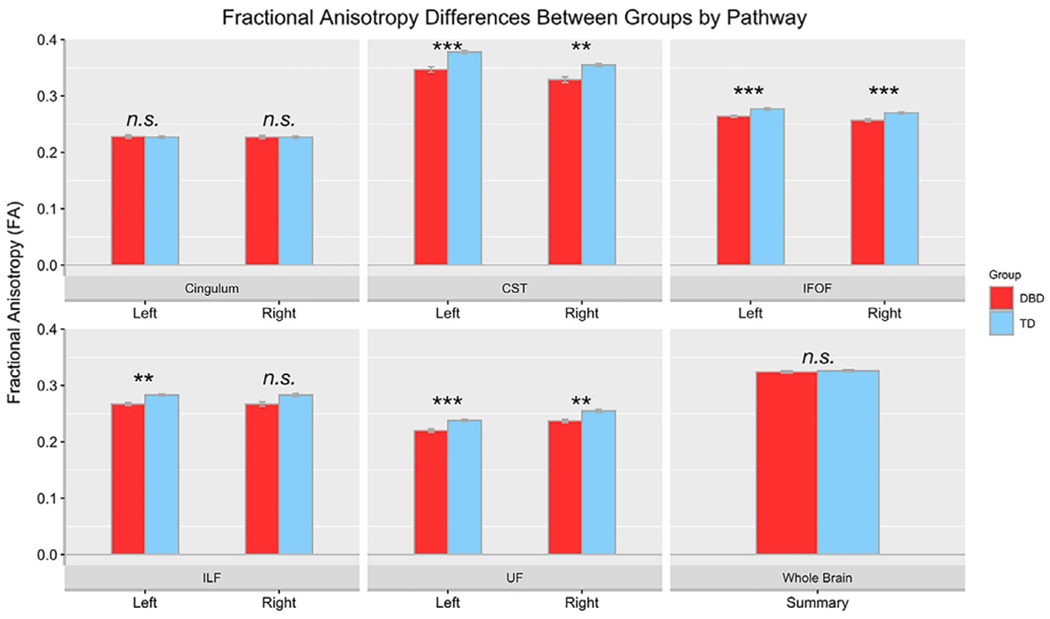
Fractional anisotropy (FA) mean differences are plotted by group, for each pathway and for each hemisphere. CST, Corticospinal Tract; DBD, Disruptive Behavior Disorder; IFOF, Inferior fronto-occipital fasciculus; ILF, Inferior longitudinal fasciculus; TD, typically developing children; UF, Uncinate fasciculus. The average of the whole brain FA is also plotted. n.s. = non-significant. ***p < .001. Statistical tests of group differences controlled for sex, whole brain FA, movement, parental income, and IQ
The next two analyses focused on associations between the fiber pathway metrics and CP and CU behaviors. In the second analysis, using robust regression we examined whether any of the examined fiber pathways were associated with CP, and whether these associations were moderated by diagnostic group (i.e. a pathway by group interaction). Again, we focus on FA, but to be comprehensive these models were run for AD and RD diffusion metrics as well. We also ran these analyses for GFA, QA, and nQA metrics (see Tables S3 and S4). These analyses controlled for sex, whole brain diffusion (e.g. for FA, AD, RD, QA, nQA, and GFA we controlled for whole brain FA, AD, RD, QA, nQA, and GFA, respectively), movement, parental income, IQ, and diagnostic group. Results are reported in Table 2 and Table S3, and show no significant associations between FA of any of the fiber pathways (i.e. no main effects) and CP symptoms, and no significant group by pathway interactions.
Table 2.
Results of robust regressions examining the association between tract diffusivity metric and conduct problems (CP)
| Group × Tract -> Conduct Problems (CP) | B ( SE) | β | t | p | CI 95% |
|---|---|---|---|---|---|
| Diagnostic Group × Pathway Interaction | |||||
| Left hemisphere | |||||
| Group × UF FA | −0.47 (1.98) | −.02 | 0.814 | .81 | −4.34, 3.41 |
| Group × Cingulum FA | −0.70 (2.44) | −.03 | −0.29 | .78 | −5.48, 4.08 |
| Group × ILF FA | 1.51 (2.14) | .07 | 0.71 | .48 | −2.68, 5.70 |
| Group × IFOF FA | −1.63 (2.48) | −.06 | −0.66 | .51 | −6.49, 3.23 |
| Group × CST FA | 1.04 (1.49) | .08 | 0.70 | .48 | −1.87, 3.96 |
| Right hemisphere | |||||
| Group × UF FA | 0.36 (1.89) | .02 | 0.19 | .85 | −3.34, 4.06 |
| Group × Cingulum FA | 0.57 (2.25) | .02 | 0.25 | .80 | −3.83, 4.97 |
| Group × ILF FA | 1.33 (1.91) | .08 | 0.70 | .49 | −2.40, 5.07 |
| Group × IFOF FA | −1.55 (2.40) | −.06 | −0.64 | .52 | −6.26, 3.16 |
| Group × CST FA | 1.90 (1.90) | .14 | 1.26 | .21 | −1.05, 4.84 |
| Simple effect within DBD Group | |||||
| Left hemisphere | |||||
| UF FA | −0.95 (1.39) | −.06 | −0.68 | .50 | −3.66, 1.77 |
| Cingulum FA | 0.09 (1.77) | .01 | 0.05 | .96 | −3.39, 3.56 |
| ILF FA | −0.89 (1.67) | −.05 | −0.53 | .59 | −4.16, 2.38 |
| IFOF FA | −2.59 (1.87) | −.13 | −1.39 | .17 | −6.24, 1.07 |
| CST FA | 0.09 (0.90) | .01 | 0.10 | .92 | −1.67, 1.84 |
| UF AD | −1.03 (2.13) | −.05 | −0.49 | .63 | −5.20, 3.14 |
| Cingulum AD | 0.69 (1.86) | .04 | 0.37 | .71 | −2.96, 4.34 |
| ILF AD | −0.58 (1.52) | −.04 | −0.38 | .70 | −3.56, 2.40 |
| IFOF AD | 0.83 (2.38) | .04 | 0.35 | .73 | −3.83, 5.49 |
| CST AD | 0.33 (2.58) | .01 | 0.13 | .90 | −4.74, 5.39 |
| UF RD | 0.15 (1.42) | .01 | 0.11 | .92 | −2.64, 2.94 |
| Cingulum RD | −1.62 (2.35) | −.09 | −0.69 | .49 | −6.23, 3.00 |
| ILF RD | −1.16 (2.81) | −.06 | −0.41 | .68 | −6.67, 4.36 |
| IFOF RD | 2.87 (2.11) | .14 | 1.30 | .20 | −1.47, 7.20 |
| CST RD | −0.16 (1.00) | −.01 | −0.16 | .87 | −2.12, 1.80 |
| Right hemisphere | |||||
| UF FA | −0.77 (1.36) | −.05 | −0.56 | .57 | −3.44, 1.91 |
| Cingulum FA | 0.48 (1.81) | .03 | 0.27 | .79 | −3.07, 4.04 |
| ILF FA | 0.38 (1.26) | .03 | 0.30 | .76 | −2.09, 2.85 |
| IFOF FA | −2.54 (1.74) | −.14 | −1.46 | .15 | −5.95, 0.87 |
| CST FA | 0.47 (0.94) | .05 | 0.50 | .62 | −1.37, 2.30 |
| UF AD | 1.11 (2.55) | .04 | 0.43 | .67 | −3.89, 6.10 |
| Cingulum AD | 0.30 (1.83) | .02 | 0.16 | .87 | −3.29, 3.89 |
| ILF AD | 1.08 (1.72) | .06 | 0.63 | .53 | −2.29, 4.44 |
| IFOF AD | −1.15 (2.49) | −.05 | −0.46 | .65 | −6.04, 3.74 |
| CST AD | 3.17 (2.48) | .13 | 1.28 | .21 | −1.70, 8.04 |
| UF RD | 0.55 (1.43) | .04 | 0.39 | .70 | −2.25, 3.36 |
| Cingulum RD | −2.50 (2.38) | −.12 | −1.05 | .30 | −7.17, 2.17 |
| ILF RD | −0.42 (1.89) | −.02 | −0.22 | .82 | −4.12, 3.27 |
| IFOF RD | 2.03 (2.34) | .10 | 0.87 | .39 | −2.55, 6.61 |
| CST RD | −0.30 (0.97) | −.03 | −0.31 | .76 | −2.19, 1.60 |
All regressions controlled for the following: sex, whole brain diffusion (either FA, AD, or RD depending on predictor of interest), movement in the scanner, parental income, and IQ. Analyses within the DBD group controlled for hyperactivity/impulsivity and inattention in place of Diagnostic Group. AD, axial diffusivity; CI, Confidence Interval; CST, corticospinal tract; FA, fractional anisotropy; IFOF, Inferior fronto-occipital fasciculus; ILF, Inferior longitudinal fasciculus; RD, radial diffusivity; UF, Uncinate fasciculus.
In the third analysis, the same models were run, but the ICU composite measuring CU behaviors was substituted for the outcome variable, and CP symptoms were entered as an additional covariate. Table 3 shows significant group by pathway interactions for the FA of the bilateral UF and left IFOF. Decomposing these interactions shows that FA in these pathways is negatively associated with CU behaviors, but only for the DBD group. No significant associations were revealed for the typically developing group. Figure 3 shows these effects plotted for FA for the left and right UF, and left IFOF. We explored these associations further within the DBD group, this time controlling for hyperactivity/impulsivity and inattention (and removing the group categorical variable). Controlling for these symptoms in the model did not attenuate the association between fiber pathway FA and CU behaviors, which remained significant for the bilateral UF and left IFOF (see Table 3). Looking more closely at AD and RD within the DBD group, we found that AD in bilateral UF was negatively associated with CU behaviors. This suggests that, at least for these pathways, the finding for FA is driven mainly by the longitudinal component of the diffusion tensor. Table S4 reports findings for GFA, which is in general agreement with the findings for FA, with the exception that the left UF finding does not meet the nominal statistical significance level for the interaction effect (p = .068). Taken together, these results show that, even when controlling for whole brain diffusion differences, movement, demographic effects, IQ, ADHD symptom severity, and CP, reduced directional diffusion (as measured by FA) within bilateral UF and left IFOF fiber pathways is significantly associated with increased CU behaviors.
Table 3.
Results of robust regressions examining the association between tract diffusivity metric and callous-unemotional (CU) behaviors
| Group × Tract -> CU Behaviors | B (SE) | β | t | p | CI 95% |
|---|---|---|---|---|---|
| Diagnostic Group × Pathway Interaction | |||||
| Left hemisphere | |||||
| Group × UF FA | −3.51 (1.75) | −.19 | −2.01 | .046* + | −6.94, −0.08 |
| Group × Cingulum FA | 0.03 (2.16) | .00 | 0.01 | .99 | −4.21, 4.26 |
| Group × ILF FA | −3.19 (1.90) | −.16 | −1.68 | .10 | −6.92, 0.54 |
| Group × IFOF FA | −5.70 (2.24) | −.24 | −2.54 | .01** ++ | −10.1, −1.31 |
| Group × CST FA | −1.1 (1.3) | −.09 | −0.85 | .40 | −3.64, 1.44 |
| Right hemisphere | |||||
| Group × UF FA | −4.22 (1.64) | −.24 | −2.58 | .01** ++ | −7.43, −1.02 |
| Group × Cingulum FA | −1.50 (1.95) | −.07 | −0.77 | .44 | −5.31, 2.32 |
| Group × ILF FA | −2.12 (1.63) | −.13 | −1.30 | .20 | −5.31, 1.07 |
| Group × IFOF FA | −2.74 (2.17) | −.12 | −1.26 | .21 | −6.98, 1.51 |
| Group × CST FA | −1.15 (1.33) | −.09 | −0.86 | .39 | −3.75, 1.46 |
| Simple effect within DBD Group | |||||
| Left hemisphere | |||||
| UF FA | −2.94 (−1.13) | −.18 | −2.27 | .026* + | −5.48, −0.40 |
| Cingulum FA | 2.18 (−1.78) | .12 | 1.23 | .22 | −1.31, 5.67 |
| ILF FA | −2.21 (−1.64) | −.11 | −1.30 | .20 | −5.34, −0.77 |
| IFOF FA | −4.25 (−1.78) | −.20 | −2.40 | .018* ++ | −7.73, −0.77 |
| CST FA | −0.37 (−0.09) | −.04 | −0.40 | .69 | −2.16, 1.42 |
| UF AD | −4.12 (−2.03) | −.17 | −2.03 | .045* + | −8.10, −0.14 |
| Cingulum AD | 2.17 (−1.89) | .11 | 1.15 | .25 | −1.54, 5.87 |
| ILF AD | −2.77 (−1.41) | −.17 | −1.97 | .05 | −5.53, −0.02 |
| IFOF AD | −3.92 (−2.31) | −.15 | −1.69 | .09 | −8.45, 0.62 |
| CST AD | 0.09 (−2.63) | .00 | 0.04 | .97 | −5.07, 5.26 |
| UF RD | 1.91 (−1.43) | .12 | 1.34 | .18 | −0.89, 4.70 |
| Cingulum RD | −1.76 (−2.45) | −.09 | −0.72 | .47 | −6.56, 3.04 |
| ILF RD | −0.94 (−2.81) | −.04 | −0.34 | .74 | −6.46, 4.57 |
| IFOF RD | 3.16 (−2.22) | .15 | 1.43 | .16 | −1.18, 7.51 |
| CST RD | 0.48 (−1.04) | .04 | 0.46 | .64 | −1.56, 2.52 |
| Right hemisphere | |||||
| UF FA | −2.70 (−1.30) | −.17 | −2.08 | .040* + | −5.25, −0.15 |
| Cingulum FA | 0.86 (−1.88) | .05 | 0.46 | .65 | −2.82, 4.55 |
| ILF FA | −1.51 (−1.24) | −.11 | −1.22 | .23 | −3.95, 0.92 |
| IFOF FA | −2.49 (−1.75) | −.13 | −1.43 | .16 | −5.91, 0.94 |
| CST FA | −0.26 (−0.94) | −.28 | −0.28 | .78 | −2.10, 1.58 |
| UF AD | −6.83 (−2.26) | −.24 | −3.03 | .003** +++ | −11.26, −2.41 |
| Cingulum AD | 2.30 (−1.85) | .12 | 1.24 | .22 | −1.33, 5.92 |
| ILF AD | −1.54 (−1.72) | −.09 | −0.90 | .37 | −4.91, 1.82 |
| IFOF AD | −0.35 (−2.55) | −.01 | −0.14 | .89 | −5.35, 4.65 |
| CST AD | −1.71 (−2.58) | −.07 | −0.66 | .51 | −6.76, 3.34 |
| UF RD | 1.31 (−1.44) | .08 | 0.90 | .37 | −1.53, 4.13 |
| Cingulum RD | 0.66 (−2.44) | .03 | 0.27 | .79 | −4.13, 5.44 |
| ILF RD | 1.52 (−1.94) | .08 | 0.78 | .44 | −2.28, 5.31 |
| IFOF RD | 3.37 (−2.21) | .16 | 1.52 | .13 | −0.96, 7.70 |
| CST RD | 0.27 (−1.00) | .02 | 0.27 | .79 | −1.69, 2.24 |
All regressions controlled for the following: sex, whole brain diffusion (either FA, AD, or RD depending on predictor of interest), movement in the scanner, parental income, IQ, and conduct problems (CP). Analyses within the DBD group controlled for hyperactivity/impulsivity and inattention in place of Diagnostic Group. AD, axial diffusivity; CI, confidence interval; CST, corticospinal tract; FA, Fractional Anisotropy; IFOF, Inferior fronto-occipital fasciculus; ILF, Inferior longitudinal fasciculus; RD, Radial Diffusivity; UF, Uncinate fasciculus.
p < .05 (uncorrected).
p < .01 (uncorrected).
FDR Correction:
q = .25;
q = .10;
q = .05.
Figure 3.
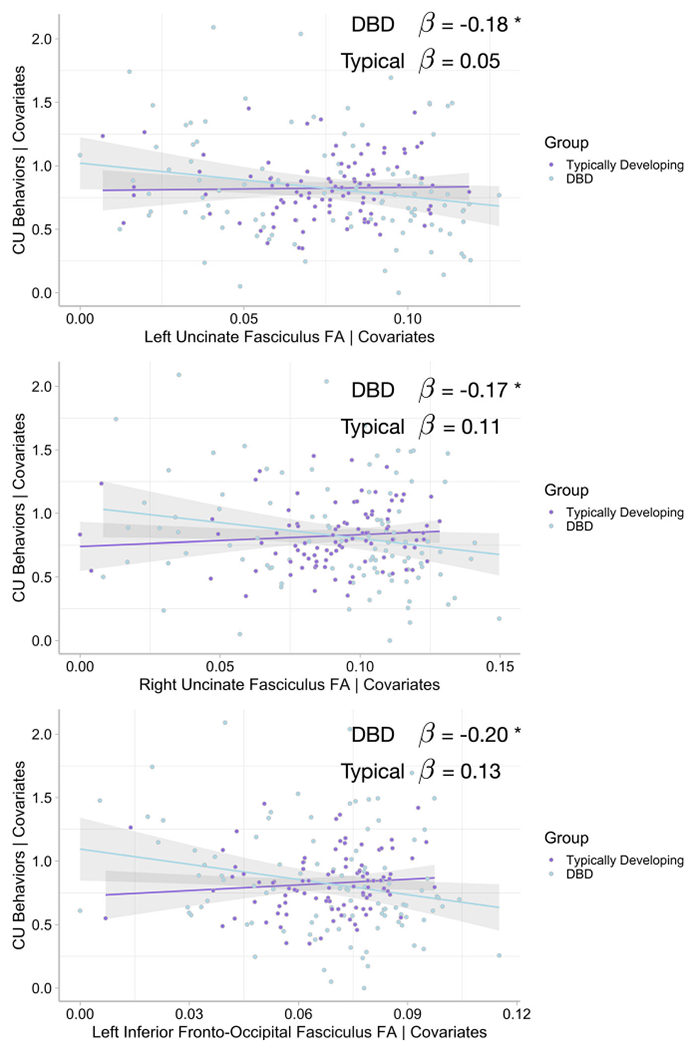
Added-variable plots of the Group × Pathway Interaction show negative associations between bilateral uncinate fasciculus and left inferior fronto-occipital fasciculus fractional anisotropy (FA) and callous-unemotional (CU) behaviors, controlling for the following covariates: group status (also entered as a moderator), sex, whole brain FA, movement in the scanner, parental income, intelligence, and conduct problems (CP). DBD. Disruptive Behavior Disorder. β = standardized regression slope parameter. Each point shows an individual child. Shading = 95% confidence intervals. *p < .05
We also re-ran these analyses removing children who only had a diagnosis of ADHD (no ODD/CD, n = 32). None of the findings for CP changed (no significant associations were revealed). In addition, for the CU analyses, all statistically significant results remained significant. Only two results became significant: right IFOF FA and RD (β = −.26, p = .0128, and β = .275, p = .0406, respectively). In effect, these findings reinforce the involvement of fibers coursing through the temporal stem via the UF and IFOF with respect to CU behaviors, regardless of comorbidity.
As a final analysis, we examined whether associations between fiber pathways and behaviors might only occur on the extremes of one behavior. For example, in a large sample of adults, Dotterer et al. (2019) reported no associations between antisocial behaviors and CU behaviors on a continuum. However, they did find a moderating effect such that only the combination of high antisocial behaviors and high levels of CU traits significantly related to lower FA across several of the fiber pathways we explored here. We thus examined our data for the same possibility. To do this, we explored the interaction of CP and CU behaviors as predictors in the model, with diffusion metrics of each tract of interest entered as the dependent measure. In our data, we found no evidence of such an interaction that reached the nominal level of significance, although the finding was approaching significance for the right IFOF (highest t value for the interaction slope, t(93) = 1.96, 95% CI −0.00002 to 0.037, beta = .36).
Discussion
In a recent review, Waller et al. (2017) found that reduced FA in the fiber tracks connecting the extended limbic, frontal, and temporal regions (namely UF, cingulum, IFOF, and ILF tracks investigated here) is associated with antisocial behavior in adults. In adolescents, they showed that the results were more mixed, with some studies showing reduced FA in these tracks, and others showing increased FA. In our study of younger children, the first to our knowledge with a large predominantly pre-kindergarten sample (Mage = 5.66), we found that relative to TD children, children with a DBD had reduced FA across the IFOF, ILF, UF, and CST. Of note, although the CST was included as a control tract, previous studies have documented that children with ADHD have reduced FA in this pathway as well, relative to TD children (D’Agati, Casarelli, Pitzianti, & Pasini, 2010; Hamilton et al., 2008). This reduced integrity of the CST may be associated with fine and gross motor difficulties, consistent hallmarks of ADHD (Mokobane, Pillay, & Meyer, 2019). Thus, our results replicate, in younger children, well-established findings regarding the group differences between youth diagnosed with ADHD and TD youth (e.g. see Svatkova et al., 2016; Wu et al., 2020).
Notably, we did not find a difference in general FA across the whole brain, nor did we find a group difference in FA in the cingulum. Differences in FA in cingulum have been found previously in studies of older children with DBDs such as ADHD and CD (González-Madruga et al., 2020; Svatkova et al., 2016; Wu et al., 2020), but not in all such studies (Ashtari et al., 2005; Davenport, Karatekin, White, & Lim, 2010), and not in children with CU behaviors (Pape et al., 2015) or in some studies of children with CD (Finger et al., 2012; Haney-Caron et al., 2014). It is important to point out, though, that these studies showing differences have tended to be small sample studies (e.g. n < 30), increasing the possibility that such effects are spurious (although Pape et al., 2015 and González-Madruga et al., 2020 are notable exceptions). Our study is a comparatively large sample, and thus, it is reasonable to conclude that, at this age, cingulum FA is not different between DBD and TD groups. But it is also notable that AD of the cingulum was significantly higher in the DBD group, although there was no significant difference for RD. We interpret the lack of a difference in FA as an important potential null finding as it relates to understanding the broader circuit dysfunction in youth with DBDs. However, it is possible FA differences might arise later in development, as fiber pathways continue to show maturational change well beyond the preschool and early school-age period that we studied here. For example, the larger-sample study conducted by González-Madruga et al. (2020) examines older children and may indicate a reliable difference in the cingulum (especially the retrosplenial portion) between TD adolescents and those with diagnosed CD. The small but significant difference in AD in cingulum that we found also suggests that structural differences might become more pronounced with development.
Turning to the analysis of associations with CP and CU behaviors, we found no group by pathway interactions for CP behaviors, but when examining CU behaviors, we did find reliable interaction effects for the bilateral UF and left IFOF. Caution is warranted, as while these interaction effects were significant at the nominal level, they survived statistical correction only at slightly more relaxed FDR levels (e.g. q = .10 and .25). Special caution is warranted for interpreting the left UF interaction effect, as the 95% CI for the slope estimate is appreciably close to zero. The findings are stronger, however, for the within DBD group analyses for CU behaviors, indicating a consistent negative association between bilateral UF and left IFOF and such behaviors in the DBD group. Notably, though, only for the UF are the effects also apparent when examining the longitudinal component of the diffusion tensor (i.e. AD). This was not present for the left IFOF, and thus, some caution in interpreting this finding is warranted.
Turning first to the null findings for CP, there are a number of obvious methodological differences with our study and prior studies. First, we examined a very young group of children, and previous work has mostly focused on adolescents. Second, within the clinical DBD group, all children in our study were diagnosed with ADHD, which may contribute to the mixed pattern of findings in the literature (Waller et al., 2017). More work is clearly needed examining the white matter microstructure within the frontal-temporal-extended limbic system taking into account ADHD comorbidity.
Our most noteworthy finding within the DBD group is that even after accounting for ADHD symptom severity, CP, demographic variables (parental income, sex, IQ), movement, and whole brain FA, CU behaviors were independently related to reduced FA in bilateral UF and left IFOF. Examination of higher-order DWI reconstruction metrics (e.g. GFA) showed that these associations were most prominent in the left UF and IFOF, although they remained trending for the right UF. Both of these fiber pathways support connections of temporal lobe and limbic structures with orbitofrontal cortex, and both pathways have been associated with CU behaviors in adolescents (e.g. Breeden et al., 2015; Pape et al., 2015; Sarkar et al., 2013) and psychopathy in adults (see Waller et al., 2017 for review). Both pathways have also been associated with emotion regulation. The UF supports extensive connectivity with amygdala and orbitofrontal cortex, and not surprisingly it has been implicated in the recognition of facial expressions of emotions (Philippi, Mehta, Grabowski, Adolphs, & Rudrauf, 2009). A more recent review of the UF further highlights its role in not only basic social-emotional processing but also, via temporal lobe-based stimulus associations, in assigning value (rewards/punishment) to stored representations, thus impacting decision making and behavior (Von Der Heide et al., 2013). Indeed, emotion processing deficits that include not only reduced amygdala response to fearful faces, but also general emotion recognition deficits at the behavioral level, have been consistently associated with CU behaviors (Dadds, Kimonis, Schollar-Root, Moul, & Hawes, 2018; Marsh et al., 2008). Hyposensitivity to punishment and social reward processing deficits are also associated with CU behaviors (Blair, Veroude, & Buitelaar, 2018; Huang et al., 2019). The IFOF is a far more extensive fiber pathway that passes just dorsal to the UF in its anterior course, but it also supports emotion recognition (Unger, Alm, Collins, O’Leary, & Olson, 2016). Disrupted emotional responsiveness is potentially a core feature in at least a subset of children displaying CU behaviors (Frick & Viding, 2009; Northam & Dadds, 2020). Our results suggest that disruption of the main fiber pathways supporting emotional processing might be a contributing factor to the development of CU behaviors in such children. Further, these differences can be detected reasonably early in development (i.e., in the preschool/early school-age period). The results thus add an additional level of analysis on which to advance causal theories for the development of CU behaviors (Frick & Viding, 2009).
It is important to note that the findings with respect to bilateral UF were mainly driven by the longitudinal component of the diffusion tensor (i.e. AD). While speculative, as we do not have access to the specific microstructural properties of the brain tissue, it is the case that AD is more sensitive to disruptions of axonal integrity and packing density, while RD quantifications are more sensitive myelin integrity (Basser et al., 1994; Winston, 2012). This may suggest that the UF of children with CU behaviors is characterized by less coherent longitudinal fiber orientation rather than reduced or delayed myelination, although such a possibility is speculative and would need additional verification. Regardless, our findings add to the extant pediatric literature highlighting the importance of the connectivity between amygdala and orbitofrontal cortex as it relates specifically to CU traits/behaviors (Blair, 2007).
Limitations
Some limitations to the current study include the fact that we did not have a pure CP group, as our clinical DBD sample had a primary diagnosis of ADHD. Given the high comorbidity of CP and ADHD in young children (Bendiksen et al., 2017), our approach was to isolate the CP component by statistically controlling for ADHD severity. However, we acknowledge the limitations of statistically covarying versus obtaining a pure CP group, although some evidence indicates that nearly all children with CP also meet criteria for ADHD (Loeber, Green, Keenan, & Lahey, 1995). Nevertheless, we cannot rule out the fact that the widespread disruption of multiple fiber tracts that we found in our DBD sample may not be similar within a ‘pure’ CP sample. Second, while we focused on several major fiber tracts related to network of extended limbic, frontal, and temporal regions given their theoretical and empirical associations with the development of CP/CU and associated impairments, it will be important in the future to also examine the fronto-striatal-cerebellar neurocircuitry given its link to ADHD (van Ewijk, Heslenfeld, Zwiers, Buitelaar, & Oosterlaan, 2012). Lastly, another limitation of the current study is the homogeneity of the sample, which was largely Latinx (80%) due to the study’s geographical location. The homogeneity of the sample limits the generalizability of these findings, but can also be viewed as a strength, as Latinx children represent the fastest growing group in the United States, but are understudied in child psychopathology research (La Greca, Silverman, & Lochman, 2009).
It is also important to point out that we used an abbreviated version (12 items) of the ICU to measure CU behaviors rather than the full 24-item ICU which prevents us from examining potential differences in how certain subscales of the ICU relate to the fiber tracks we examined. However, a significant strength of our study was our integration of multiple reporters of the abbreviated ICU (in our case parents and teachers) as most prior DWI studies relied solely on one source to measure CU behaviors (Dotterer et al., 2019; Maurer et al., 2020; Pape et al., 2015; Puzzo et al., 2018). Our approach in taking the highest score between reporters was consistent with the few prior DWI studies that measured CU behaviors in adolescents via multiple sources (self-report and parent-report; Breeden et al., 2015; Sarkar et al., 2013). From our perspective, given the young age of our sample, utilizing both teacher and parent reports (rather than self-report) is crucial toward maximizing our detection of early CU behaviors to ultimately understand their neurobiology.
Finally, it is important to note that, due to the nature of research on fiber pathways, a large number of statistical comparisons were conducted. We employed FDR corrections at three levels (q = .25, .10, and .05) because we wanted to present a full picture of the results. However, this means that some parameter estimates are much more reliable than others. The strongest findings are for the bilateral UF, which were revealed in both hemispheres and across two diffusion metrics (FA and AD). The IFOF finding was only apparent for the left hemisphere, and only for FA, and thus, extra caution is recommended in interpreting this finding. Both findings should be replicated in larger samples in order to increase the confidence in the results. At the same time, there is some consistency here. Both pathways (UF and IFOF) are comprised of fibers traversing through the extreme capsule from the temporal lobe to the frontal lobe, with the IFOF running only slightly superior to the UF. The resolution of DWI is insufficient to dissociate axonal projections at the microscopic level across the two fiber tracts. In future work, it may be beneficial to more precisely delineate the anterior projections of the IFOF to see whether the findings remain when only the anterior temporal-frontal component of the tract is examined, as the posterior component of the tract may be involved in very different behaviors. Furthermore, examination of hemispheric differences may be beneficial in future work. Separating the hemispheres in the analysis inherently increases the number of statistical comparisons, and hemispheric specialization is well established for some domains (e.g. language, visuo-spatial processing), but it is not known whether hemispheric specialization is a consistent feature of the neurobiology of CU behaviors.
In sum, relative to TD children, children with a DBD diagnosis (primarily ADHD with high comorbidity rates with ODD/CD) were found to have white matter disruption on four out of the five fiber tracks we examined (except for cingulum and right ILF). We also did not find any associations between CP and reduced white matter integrity in either group. However, we did find that, only for the DBD group, CU behaviors were associated with reduced FA in bilateral UF and left IFOF, even after accounting for CP and ADHD symptomology. Consistent with the adult and limited adolescent literature, our results suggest that alterations in white matter microstructure of these pathways may be biomarkers of CU behaviors/traits even in very young children. Such individual differences within the frontal/limbic network may map onto the emotional processing deficits, including lack of empathy, that are the core features of CU behaviors. Moving forward it will be important to identify multiple biomarkers (i.e. a ‘biosignature’) which may help guide the development of more targeted treatment options for young children with CP who display elevated levels of CU behaviors.
Supplementary Material
Table S1. Correlations among behavioral measures for the whole sample and within group.
Table S2. Results comparing DBD and TD groups on behavioral measures and fiber pathway microstructure.
Table S3. Results of robust regressions examining the association between tract diffusivity metric and conduct problems (CP).
Table S4. Results of robust regressions examining the association between tract diffusivity metric and callous-unemotional (CU) behaviors.
Figure S1. Sample participants showing successful application of the HCP atlas to the individual participants, for each fiber pathway region of interest.
Key points.
Disrupted connectivity between amygdala and prefrontal cortex is thought to be related to the development of CP and CU behaviors.
Our study of younger children, the first to our knowledge with a large predominantly pre-kindergarten sample (Mage = 5.66), shows that relative to TD children, children with DBD were found to have white matter disruption on four out of the five fiber tracks we examined.
Within the DBD group, we did find that CU behaviors (but not general CP) were associated with reduced white matter integrity in bilateral UF and left IFOF.
Consistent with the adult and limited adolescent literature, our results suggest that these pathways may be biomarkers of CU behaviors/traits even in very young children with disruptive behavior problems.
Acknowledgements
The authors would like to acknowledge the support of Miami-Dade County Public Schools and thank the families and dedicated staff who participated in the study. This work was supported by grants from the National Institute of Mental Health (R01MH112588) and the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK119814) to P.A.G. and A.S.D. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Given the young age of our sample and to facilitate consistency in our terminology when reviewing the literature, we used the term CU behaviors throughout the paper although we acknowledge that in older samples the term CU traits is also frequently used.
References
- Andersson JL, Skare S, & Ashburner J (2003). How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20, 870–888. [DOI] [PubMed] [Google Scholar]
- Ashtari M, Kumra S, Bhaskar SL, Clarke T, Thaden E, Cervellione KL, … & Ardekani BA (2005). Attention-deficit/hyperactivity disorder: a preliminary diffusion tensor imaging study. Biological Psychiatry, 57, 448–455. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, & LeBihan D (1994). Estimation of the effective self-diffusion tensor from the NMR spin echo. Journal of Magnetic Resonance, Series B, 103, 247–254. [DOI] [PubMed] [Google Scholar]
- Beaulieu C (2002). The basis of anisotropic water diffusion in the nervous system – A technical review. NMR in Biomedicine, 15, 435–455. [DOI] [PubMed] [Google Scholar]
- Bendiksen B, Svensson E, Aase H, Reichborn-Kjennerud T, Friis S, Myhre AM, & Zeiner P (2017). Co-occurrence of ODD and CD in preschool children with symptoms of ADHD. Journal of Attention Disorders, 21, 741–752. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B (Methodological), 57, 289–300. [Google Scholar]
- Blair RJ (2007). The amygdala and ventromedial prefrontal cortex in morality and psychopathy. Trends in Cognitive Sciences, 11, 387–392. [DOI] [PubMed] [Google Scholar]
- Blair R, Veroude K, & Buitelaar J (2018). Neuro-cognitive system dysfunction and symptom sets: A review of fMRI studies in youth with conduct problems. Neuroscience & Biobehavioral Reviews, 91, 69–90. [DOI] [PubMed] [Google Scholar]
- Breeden AL, Cardinale EM, Lozier LM, VanMeter JW, & Marsh AA (2015). Callous-unemotional traits drive reduced white-matter integrity in youths with conduct problems. Psychological Medicine, 45, 3033–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunte TL, Schoemaker K, Hessen DJ, van der Heijden PG, & Matthys W (2014). Stability and change of ODD, CD and ADHD diagnosis in referred preschool children. Journal of Abnormal Child Psychology, 42, 1213–1224. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, & Ffytche DH (2003). Occipito-temporal connections in the human brain. Brain, 126(Pt 9), 2093–2107. [DOI] [PubMed] [Google Scholar]
- Craig MC, Catani M, Deeley Q, Latham R, Daly E, Kanaan R, … & Murphy DGM. (2009). Altered connections on the road to psychopathy. Molecular Psychiatry, 14, 946–953, 907. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Kimonis ER, Schollar-Root O, Moul C, & Hawes DJ (2018). Are impairments in emotion recognition a core feature of callous-unemotional traits? Testing the primary versus secondary variants model in children. Developmental Psychopathology, 30, 67–77. [DOI] [PubMed] [Google Scholar]
- D’Agati E, Casarelli L, Pitzianti MB, & Pasini A (2010). Overflow movements and white matter abnormalities in ADHD. Progress in Neuro-psychopharmacolical and Biological Psychiatry, 34, 441–445. [DOI] [PubMed] [Google Scholar]
- Davenport ND, Karatekin C, White T, & Lim KO (2010). Differential fractional anisotropy abnormalities in adolescents with ADHD or schizophrenia. Psychiatry Research, 181, 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Schotten MT, Dell’Acqua F, Valabregue R, & Catani M (2012). Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex, 48, 82–96. [DOI] [PubMed] [Google Scholar]
- DeLisi M, Drury AJ, & Elbert MJ (2020). Fledgling psychopaths at midlife: Forensic features, criminal careers, and coextensive psychopathology. Forensic Science International: Mind and Law, 1, 100006. [Google Scholar]
- Dotterer HL, Waller R, Shaw DS, Plass J, Brang D, Forbes EE, & Hyde LW (2019). Antisocial behavior with callous-unemotional traits is associated with widespread disruptions to white matter structural connectivity among low-income, urban males. Neuroimage: Clinical, 23, 101836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B (1981). Nonparametric estimates of standard error: The jackknife, the bootstrap and other methods. Biometrika, 68, 589–599. [Google Scholar]
- Efron B (1987). Better bootstrap confidence intervals. Journal of the American Statistical Association, 82, 171–185. [Google Scholar]
- Ezpeleta L, de la Osa N, Granero R, Penelo E, & Domenech JM (2013). Inventory of callous-unemotional traits in a community sample of preschoolers. Journal of Clinical Child & Adolescent Psychology, 42, 91–105. [DOI] [PubMed] [Google Scholar]
- Fabiano GA, Pelham WE Jr., Waschbusch DA, Gnagy EM, Lahey BB, Chronis AM, … & Burrows-MacLean L (2006). A practical measure of impairment: Psychometric properties of the impairment rating scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. Journal of Clinical Child & Adolescent Psychology, 35, 369–385. [DOI] [PubMed] [Google Scholar]
- Finger EC, Marsh A, Blair KS, Majestic C, Evangelou I, Gupta K, … & Blair RJ (2012). Impaired functional but preserved structural connectivity in limbic white matter tracts in youth with conduct disorder or oppositional defiant disorder plus psychopathic traits. Psychiatry Research, 202, 239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forehand R, Parent J, Sonuga-Barke E, Peisch VD, Long N, & Abikoff HB (2016). Which type of parent training works best for preschoolers with comorbid ADHD and ODD? A secondary analysis of a randomized controlled trial comparing generic and specialized programs. Journal of Abnormal Child Psychology, 44, 1503–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ (2004). The inventory of callous-unemotional traits. Unpublished rating scale. [DOI] [PubMed]
- Frick PJ, Ray JV, Thornton LC, & Kahn RE (2014). Can callous-unemotional traits enhance the understanding, diagnosis, and treatment of serious conduct problems in children and adolescents? A comprehensive review. Psychological Bulletin, 140, 1–57. [DOI] [PubMed] [Google Scholar]
- Frick PJ, & Viding E (2009). Antisocial behavior from a developmental psychopathology perspective. Developmental Psychopathology, 21, 1111–1131. [DOI] [PubMed] [Google Scholar]
- González-Madruga K, Rogers J, Toschi N, Riccelli R, Smaragdi A, Puzzo I, … & Fairchild G (2020). White matter microstructure of the extended limbic system in male and female youth with conduct disorder. Psychological Medicine, 50, 58–67. [DOI] [PubMed] [Google Scholar]
- Graziano PA, & Garcia A (2016). Attention-deficit hyperactivity disorder and children’s emotion dysregulation: A meta-analysis. Clinical Psychology Review, 46, 106–123. [DOI] [PubMed] [Google Scholar]
- Graziano PA, Slavec J, Hart K, Garcia A, & Pelham WE (2014). Improving school readiness in preschoolers with behavior problems: Results from a summer treatment program. Journal of Psychopathology and Behavioral Assessment, 36, 555–569. [Google Scholar]
- Gresham FM, Lane KL, & Lambros KM (2000). Comorbidity of conduct problems and ADHD: Identification of “fledgling psychopaths”. Journal of Emotional and Behavioral Disorders, 8, 83–93. [Google Scholar]
- Haas SM, Waschbusch DA, Pelham WE, King S, Andrade BF, & Carrey NJ (2011). Treatment response in CP/ADHD children with callous/unemotional traits. Journal of Abnormal Child Psychology, 39, 541–552. [DOI] [PubMed] [Google Scholar]
- Hagler DJ Jr., Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS … & Dale AM (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. NeuroImage, 202, 116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton LS, Levitt JG, O’Neill J, Alger JR, Luders E, Phillips OR, … & Narr KL (2008). Reduced white matter integrity in attention-deficit hyperactivity disorder. NeuroReport, 19, 1705–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney-Caron E, Caprihan A, & Stevens MC (2014). DTI-measured white matter abnormalities in adolescents with Conduct Disorder. Journal of Psychiatric Research, 48, 111–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare MM, Garcia AM, Hart KC, & Graziano PA (2021). Intervention response among preschoolers with ADHD: The role of emotion understanding. Journal of School Psychology, 84, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, & Narayana PA (2006). Retrospective measurement of the diffusion tensor eigenvalues from diffusion anisotropy and mean diffusivity in DTI. Magnetic Resonance in Medicine, 56, 130–137. [DOI] [PubMed] [Google Scholar]
- Hawes SW, Byrd AL, Henderson CE, Gazda RL, Burke JD, Loeber R, & Pardini DA (2014). Refining the parent-reported Inventory of Callous-Unemotional Traits in boys with conduct problems. Psychological Assessment, 26, 256. [DOI] [PubMed] [Google Scholar]
- Holl N, Noblet V, Rodrigo S, Dietemann JL, Mekhbi MB, Kehrli P, … & Kremer S (2011). Temporal lobe association fiber tractography as compared to histology and dissection. Surgical and Radiologic Anatomy, 33, 713–722. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wu T, Gao YU, Luo Y, Wu Z, Fagan S, … & Li X (2019). The impact of callous-unemotional traits and externalizing tendencies on neural responsivity to reward and punishment in healthy adolescents. Frontiers in Neuroscience, 13, 1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irfanoglu MO, Nayak A, Jenkins J, & Pierpaoli C (2017). TORTOISE v3: Improvements and new features of the NIH diffusion MRI processing pipeline. Paper presented at the ISMRM Annual Meeting, Hawaii, HI. [Google Scholar]
- La Greca AM, Silverman WK, & Lochman JE (2009). Moving beyond efficacy and effectiveness in child and adolescent intervention research. Journal of Consulting and Clinical Psychology, 77, 373. [DOI] [PubMed] [Google Scholar]
- Loeber R, Green SM, Keenan K, & Lahey BB (1995). Which boys will fare worse? Early predictors of the onset of conduct disorder in a six-year longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry, 34, 499–509. [PubMed] [Google Scholar]
- Lynam DR (1998). Early identification of the fledgling psychopath: Locating the psychopathic child in the current nomenclature. Journal of Abnormal Psychology, 107, 566. [DOI] [PubMed] [Google Scholar]
- Mannuzza S, Klein RG, Abikoff H, & Moulton Iii JL (2004). Significance of childhood conduct problems to later development of conduct disorder among children with ADHD: A prospective follow-up study. Journal of Abnormal Child Psychology, 32, 565–573. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, Reid ME, Sims C, Kosson DS, … & Blair RJ. (2008). Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. American Journal of Psychiatry, 165, 712–720. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, & Duffau H (2010). Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex, 46, 691–699. [DOI] [PubMed] [Google Scholar]
- Martino J, Vergani F, Robles SG, & Duffau H (2010). New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery, 66(3 Suppl Operative), 4–12. [DOI] [PubMed] [Google Scholar]
- Maurer JM, Paul S, Anderson NE, Nyalakanti PK, & Kiehl KA (2020). Youth with elevated psychopathic traits exhibit structural integrity deficits in the uncinate fasciculus. NeuroImage Clinical, 26, 102236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokobane M, Pillay BJ, & Meyer A (2019). Fine motor deficits and attention deficit hyperactivity disorder in primary school children. South African Journal of Psychiatry, 25, 1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, & Koenigs M (2011). Reduced prefrontal connectivity in psychopathy. Journal of Neuroscience, 31, 17348–17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northam JC, & Dadds MR (2020). Is callous always cold? A critical review of the literature on emotion and the development of callous-unemotional traits in children. Clinical Child and Family Psychology Review, 23, 265–283. [DOI] [PubMed] [Google Scholar]
- Oguz I, Farzinfar M, Matsui J, Budin F, Liu Z, Gerig G, … Styner M (2014). DTIPrep: quality control of diffusion-weighted images. Frontiers in Neuroinformatics, 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape LE, Cohn MD, Caan MW, van Wingen G, van den Brink W, Veltman DJ, & Popma A (2015). Psychopathic traits in adolescents are associated with higher structural connectivity. Psychiatry Research, 233, 474–480. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, & Calder AJ (2012). Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PLoS One, 7, e48789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham WE, Gnagy EM, Greenslade KE, & Milich R (1992). Teacher ratings of DSM-III-R symptoms for the disruptive behavior disorders. Journal of the American Academy of Child & Adolescent Psychiatry, 31, 210–218. [DOI] [PubMed] [Google Scholar]
- Perou R, Bitsko RH, Blumberg SJ, Pastor P, Ghandour RM, Gfroerer JC, … & Schieve LA (2013). Mental health surveillance among children—United States, 2005–2011. MMWR Surveillance Summaries, 62(Suppl 2), 1–35. [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, & Rudrauf D (2009). Damage to association fiber tracts impairs recognition of the facial expression of emotion. Journal of Neuroscience, 29, 15089–15099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piacentini JC, Cohen P, & Cohen J (1992). Combining discrepant diagnostic information from multiple sources: Are complex algorithms better than simple ones? Journal of Abnormal Child Psychology, 20, 51–63. [DOI] [PubMed] [Google Scholar]
- Pierpaoli C, Walker L, Irfanoglu MO, Barnett A, Basser P, Chang L, … & Wu M. (2010). TORTOISE: an integrated software package for processing of diffusion MRI data. Paper presented at the ISMRM 18th annual meeting, Stockholm, Sweden. [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, & Rohde LA (2014). ADHD prevalence estimates across three decades: an updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43, 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puzzo I, Seunarine K, Sully K, Darekar A, Clark C, Sonuga-Barke EJ, & Fairchild G (2018). Altered white-matter microstructure in conduct disorder is specifically associated with elevated callous-unemotional traits. Journal of Abnormal Child Psychology, 46, 1451–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A (2018). Antisocial personality as a neurodevelopmental disorder. Annual Review of Clinical Psychology, 14, 259–289. [DOI] [PubMed] [Google Scholar]
- Roalf DR, Quarmley M, Elliott MA, Satterthwaite TD, Vandekar SN, Ruparel K, … & Gur RE. (2016). The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. NeuroImage, 125,903–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Craig MC, Catani M, Dell’acqua F, Fahy T, Deeley Q, & Murphy DG (2013). Frontotemporal white-matter microstructural abnormalities in adolescents with conduct disorder: A diffusion tensor imaging study. Psychological Medicine, 43, 401–411. [DOI] [PubMed] [Google Scholar]
- Sarubbo S, De Benedictis A, Maldonado IL, Basso G, & Duffau H (2013). Frontal terminations for the inferior fronto-occipital fascicle: anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Structure and Function, 218, 21–37. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, & Schwab-Stone ME (2000). NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry, 39, 28–38. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … & Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage, 23(Suppl 1), S208–S219. [DOI] [PubMed] [Google Scholar]
- Soares JM, Marques P, Alves V, & Sousa N (2013). A hitchhiker’s guide to diffusion tensor imaging. Frontiers Neuroscience, 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani M, Baker L, Martins B, Tuvblad C, & Aziz-Zadeh L (2015). Psychopathic traits modulate microstructural integrity of right uncinate fasciculus in a community population. Neuroimage Clinical, 8, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatkova A, Nestrasil I, Rudser K, Goldenring Fine J, Bledsoe J, & Semrud-Clikeman M (2016). Unique white matter microstructural patterns in ADHD presentations-a diffusion tensor imaging study. Human Brain Mapping, 37, 3323–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomason M, & Thompson P (2011). Diffusion imaging, white matter, and psychopathology. Annual Review of Clinical Psychology, 7, 63–85. [DOI] [PubMed] [Google Scholar]
- Tuch DS (2004). Q-ball imaging. Magnetic Resonance in Medicine, 52, 1358–1372. [DOI] [PubMed] [Google Scholar]
- Unger A, Alm KH, Collins JA, O’Leary JM, & Olson IR (2016). Variation in white matter connectivity predicts the ability to remember faces and discriminate their emotions. Journal of the International Neuropsychological Society, 22, 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ewijk H, Heslenfeld DJ, Zwiers MP, Buitelaar JK, & Oosterlaan J (2012). Diffusion tensor imaging in attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 36, 1093–1106. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, & Olson IR (2013). Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain, 136(Pt 6), 1692–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Dotterer HL, Murray L, Maxwell AM, & Hyde LW (2017). White-matter tract abnormalities and antisocial behavior: A systematic review of diffusion tensor imaging studies across development. Neuroimage Clinical, 14, 201–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller R, Hyde LW, Grabell AS, Alves ML, & Olson SL (2015). Differential associations of early callous-unemotional, oppositional, and ADHD behaviors: Multiple domains within early-starting conduct problems? Journal of Child Psychology and Psychiatry, 56, 657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschbusch DA (2002). A meta-analytic examination of comorbid hyperactive-impulsive-attention problems and conduct problems. Psychological Bulletin, 128, 118. [DOI] [PubMed] [Google Scholar]
- Wechsler D (2012). Wechsler Preschool and Primary Scale of Intelligence—Fourth edition technical and interpretive manual. San Antonio, TX: Pearson. [Google Scholar]
- Wichstrøm L, Berg-Nielsen TS, Angold A, Egger HL, Solheim E, & Sveen TH (2012). Prevalence of psychiatric disorders in preschoolers. Journal of Child Psychology and Psychiatry, 53, 695–705. [DOI] [PubMed] [Google Scholar]
- Wilcox RR (2012). Introduction to robust estimation and hypothesis testing (3rd edn). Waltham, MA: Academic Press. [Google Scholar]
- Winston GP (2012). The physical and biological basis of quantitative parameters derived from diffusion MRI. Quantitative Imaging in Medicine and Surgery, 2, 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright D, & London K (2009). Modern regression techniques using R: A practical guide. Sage. [Google Scholar]
- Wu W, McAnulty G, Hamoda HM, Sarill K, Karmacharya S, Gagoski B, … & Rathi Y (2020). Detecting microstructural white matter abnormalities of frontal pathways in children with ADHD using advanced diffusion models. Brain Imaging and Behavior, 14, 981–997. [DOI] [PubMed] [Google Scholar]
- Yeh FC (2017). Diffusion MRI Reconstruction in DSI Studio. Advanced Biomedical MRI Lab, National Taiwan University Hospital. Available from: http://dsi-studio.labsolver.org/Manual/Reconstruction#TOC-Q-Space-Diffeomorphic-Reconstruction-QSDR [Google Scholar]
- Yeh F-C, Panesar S, Fernandes D, Meola A, Yoshino M, Fernandez-Miranda JC, … & Verstynen T. (2018). Population-averaged atlas of the macroscale human structural connectome and its network topology. NeuroImage, 178, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh FC, Wedeen VJ, & Tseng WY (2010). Generalized q-sampling imaging. IEEE Transactions on Medical Imaging, 29, 1626–1635. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Correlations among behavioral measures for the whole sample and within group.
Table S2. Results comparing DBD and TD groups on behavioral measures and fiber pathway microstructure.
Table S3. Results of robust regressions examining the association between tract diffusivity metric and conduct problems (CP).
Table S4. Results of robust regressions examining the association between tract diffusivity metric and callous-unemotional (CU) behaviors.
Figure S1. Sample participants showing successful application of the HCP atlas to the individual participants, for each fiber pathway region of interest.


