Abstract
Background: Iodine is needed for the production of thyroid hormones, which are essential for infant growth and development. Given that there are wide variations in breast milk iodine concentration (BMIC) and urinary iodine concentration (UIC), it is unclear if BMIC is associated with UIC in populations residing in iodine sufficient or deficient areas. Aim: To investigate if BMIC can be used as a biomarker for iodine status in lactating women and children <2 years of age. Methods: Electronic databases; PubMed, Web of Science and Scopus were searched until year 2021, for studies investigating the relationship between BMIC and UIC. Studies were reviewed for eligibility, according to inclusion and exclusion criteria, followed by data extraction, according to the PRISMA guidelines. Results: Overall, 51 studies met the criteria for inclusion in the systematic review. BMIC ranged from 18 to 1153 µg/L. In iodine-deficient and iodine-sufficient lactating women, BMIC ranged from 26 to 185 µg/L and 15 to 1006 µg/L, respectively. In most studies, the categorisation of iodine status assessed by median UIC was consistent with the categorisation of iodine status assessed by median BMIC cut off of ≥100 µg/L, to determine iodine sufficiency in lactating women and children <2 years of age. Conclusions: The systematic review indicated that BMIC is a promising biomarker of iodine status in lactating women and children <2 years of age. However, these data need to be interpreted cautiously, given the study limitations in the included studies. Future studies should consider investigating the optimal median BMIC, as there is a lack of high-quality observational and intervention studies in lactating women and infants.
Keywords: breast milk iodine concentration, breast milk, lactation, maternal, infant
1. Introduction
Iodine is needed by the thyroid gland to produce thyroid hormones [1]. Thyroid hormones have several important functions in the human body, including maintaining thyroid function and body metabolism [2]. Iodine deficiency is one of the most common micronutrient deficiencies worldwide, affecting 30% of the population. During pregnancy, the dietary requirement for iodine increases by 50% (i.e., 250 µg/d), because of the increased production of thyroid hormones, required for both pregnant women and their fetus [3]. The prevalence of iodine deficiency in pregnant women has been reported to range between 16.1 and 84.0% [4]. When pregnant women have insufficient iodine intake (below the recommended iodine intake), the thyroid gland is unable to produce sufficient amounts of thyroid hormones [3]. As a result, low levels of thyroid hormones can cause a number of adverse effects, particularly on brain growth and development, which are collectively known as iodine deficiency disorders (IDD) [5].
Currently, median urinary iodine concentration (UIC) is the recommended biomarker of iodine status in populations [5,6]. However, UIC only measures recent dietary iodine intake and has high intra- and inter-individual variation. WHO/ICCIDD/UNICEF have proposed a median UIC cut off of ≥100 µg/L, to indicate adequate iodine status in lactating women, despite having the same iodine intake requirement as pregnant women [5]. This is because iodine is excreted in the breast milk of lactating women. In lactating women and breast-fed infants, breast milk iodine concentration (BMIC) has been proposed to be a better biomarker of iodine status. Studies have reported that in iodine-sufficient areas (as indicated by median infant UIC and adults ≥100 µg/L), median BMIC ranged between 150 and 180 µg/L [7,8,9]. Therefore, if pregnant or lactating women are iodine deficient, their infants may be at risk of iodine deficiency, which can lead to increased risk of developing cognitive and psychomotor impairments [10]. This is because infants are sensitive to maternal iodine intake. Exclusively breast-fed infants depend entirely on their mother’s BMIC for thyroid hormone synthesis, because they do not have considerable thyroxine stores compared with adults [11].
One of the research priorities recommended by the World Health Organization (WHO) is the need for more studies measuring BMIC [12]. Since there are wide variations in BMIC, it is unclear if BMIC is associated with the UIC of lactating women and children <2 years of age, residing in iodine-sufficient or -deficient regions. In addition, there are no accepted BMIC cut offs to categorise iodine sufficiency in lactating women and children <2 years of age. Therefore, the systematic review will firstly report the analytical methods used to measure BMIC and UIC, followed by observational studies and intervention studies, measuring both the BMIC and UIC of lactating women and children <2 years of age.
2. Methods
The systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines [13]. The full protocol of the systematic review was registered at PROSPERO (at https://www.crd.york.ac.uk/prospero/ (accessed on 13 October 2021), as CRD42021231711. The systematic review does not contain any studies with human participants or animals performed by any of the authors listed and is entirely based on previously conducted studies.
2.1. Search Strategy and Selection Criteria
The literature reporting results from studies examining the relationship between BMIC and UIC were reviewed. Three online electronic databases (PubMed, Web of Science and Scopus) were used to do the selection of articles until year 2021. Other relevant databases and search engines including Cochrane Library CENTRAL and Google Scholar were also searched. In addition, additional studies from references were located in the retrieved articles.
The major key search terms used included: “iodine”, “milk” and “urine”. Other term combinations were used as the searching strategy, such as the term ‘BMIC’ or ‘breast milk iodine concentration’ plus ‘UIC’ or ‘urinary iodine concentration’ plus related terms ‘maternal iodine status’, ‘human milk’, ‘colostrum’, ‘mature milk’, ‘lactation’, ‘lactating’ ‘postpartum’, ‘infants’, ‘newborns’, ‘offspring’ and ‘neonates’. The two databases PubMed and Web of Science can be found on the Endnote website, so the searching results from the third database Scopus were imported into the Endnote website to do the screening. Duplications in the primarily articles were removed between all databases. Title screening was then performed to exclude articles not relevant to BMIC and UIC by title. The inclusion and exclusion criteria were applied to screen the abstracts and full texts of the remaining articles.
Inclusion criteria were: original articles published until year 2021; exposures of the study most relevant to maternal iodine status; outcomes of the study must report BMIC and urinary iodine (UIC and/or I/Cr ratio); studies must focus on healthy women; studies must have either one of the following: BMIC and urinary iodine (UIC and/or I/Cr ratio) of lactating women, BMIC of lactating women and urinary iodine (UIC and/or I/Cr ratio) of children <2 years of age [5]; studies must be reported in the English language. For the purpose of the systematic review, the term ‘children’ was used to include neonates, infants and toddlers <2 years of age where appropriate. Exclusion criteria were: studies in animals; exposures of the study are not relevant to maternal iodine status; exposures of the study focus on not only iodine; outcomes of the study are not relevant to BMIC and UIC; studies reported just on single case (e.g., letters and case reports); reviews, rather than original research articles.
2.2. Data Extraction
Two investigators (SL and ZFM) independently extracted the following data from the selected studies included in the systematic review: the name of the first author, the type of the study, the year of the study published, the setting of the study, sample size, the characteristics of participants (including age and gestation weeks), and primary outcomes (i.e., BMIC and UIC), secondary outcomes (including clinical outcomes). Where necessary, further data or explanation of data analyses was sought from the authors of the studies. There was a high agreement between both investigators (SL and ZFM). Any identified discrepancies were discussed and resolved by consensus. The following data were extracted: first author, year of publication, country or location of study, study design, number of mothers and infants, infant age or time of postpartum, median or mean BMIC and UIC data of lactating women or infants, methods to assess BMIC and UIC.
2.3. Quality Assessment
Two scales were used to assess the quality of these studies depending on the types of studies. The Jadad scale was used to evaluate the quality of the randomised clinical trials, while the Newcastle–Ottawa scale was used to evaluate the quality of non-randomised and observational studies.
Quality of interventional studies was assessed using the Jadad scale [14], using a five-point checklist with yes/no answers to questions relating to methodology (Supplementary Tables S1–S3). This is a proven report quality indicator which is specifically designed for randomised intervention studies [14]. Points could be earned if the study was described as randomised (+1); the method of randomisation was described, and appropriate (+1); the study was performed double-blind (+1); the method of blinding was described and appropriate (+1); there was a description of withdrawals and subject dropouts (+1) [14]. There was no point awarded if the study was not described as randomised (+0); if the study was not performed double-blind (+0); if there was no description of withdrawals and subject dropouts (+0). Points were deducted if the method of randomisation was described and was inappropriate (−1); if the method of blinding was described and was inappropriate (−1) [14].
An adapted version of the Newcastle–Ottawa scale was used to assess the quality of the cohort studies [15]. The Newcastle–Ottawa scale has been recommended for assessing the quality of non-randomised studies, categorized into three dimensions including (1) selection, (2) comparability, and (3) assessment [16]. High-quality characteristics within each item according to these three dimensions were awarded a star, a maximum of five stars was awarded for selection, a maximum of four stars was awarded for comparability and a maximum of four stars was awarded for assessment [15]. A good point of this assessment tool is the avoidance of reporting the summary scores, which is difficult to interpret and can be considered unreliable [17].
The study quality was assessed using the criteria in Supplementary Tables S2 and S3. For descriptive purposes, scores of 0–4, 5–8, and 9–13 were used to indicate a low, moderate and high quality, respectively, for Newcastle–Ottawa scale articles. For Jadad scale articles, scores of 0–1, 2–3, and 4–5 were used to indicate a low, moderate and high quality, respectively.
2.4. Definitions and Outcomes
Currently, to our knowledge, there is no consensus regarding the suitable BMIC cut off indicative of iodine sufficiency in lactating women. Therefore, for the purpose of the systematic review, a median BMIC cut off of ≥100 µg/L was used to determine iodine sufficiency. This is because a full-term infant is considered to need 15 µg iodine/kg/day for maintaining normal thyroid metabolism [7,18,19]. A median UIC cut off ≥100 µg/L is used to define adequate iodine intake in lactating women and children <2 years of age [5].
3. Results
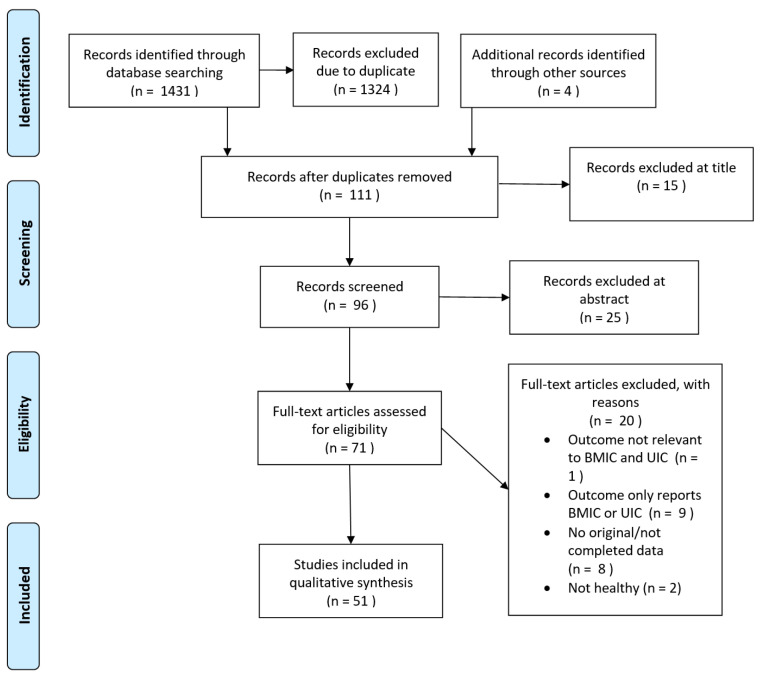
Figure 1 shows the flow chart for this review. In total, 1431 papers were identified in three online electronic databases (PubMed, Web of Science and Scopus). After removing duplications and adding 4 articles from the other sources (e.g., references list or online), 111 appeared potentially relevant. Then, the title and abstract screening were conducted according to the inclusion and exclusion criteria. Of these, 71 publications appeared potentially relevant and were assessed as full-text papers for inclusion. There were 20 studies that were excluded due to the lack of inclusion criteria; of these, most (n = 9) failed to report data of both BMIC and UIC, followed by the reason of not original/completed data paper (n = 8), not healthy women (n = 2) and not relevant to BMIC and UIC (n = 1). A total of 51 studies fulfilled the inclusion criteria and were included in the present systematic review. All included articles were published between 1992 and 2021.
Figure 1.
PRISMA 2009 Flow Diagram. Adapted from Moher et al. [13].
These 51 studies, including 29 countries on 5 continents, cover a wide geographical and socioeconomic spectrum, which could partially represent the BMIC and UIC situation in the world. The range of the sample size was from 10 to 2554. The studies were performed in China (n = 7), Iran (n = 6), Denmark (n = 3), Thailand (n = 3), Turkey (n = 3), New Zealand (n = 2), Morocco (n = 3), Algeria (n = 2), Australia (n = 2), Croatia (n = 2), South Africa (n = 2), United States (n = 2), Azerbaijan (n = 1), Brazil (n = 1), Ethiopia (n = 1), Gambia (n = 1), Germany (n = 1), Hungary (n = 1), Iceland (n = 1), India (n = 1), Italy (n = 1), Korea (n = 1), Nepal (n = 1), Norway (n = 1), Portugal (n = 1), Philippines (n = 1), Spain (n = 1), Switzerland (n = 1), and the Netherlands (n = 1). Of these, 50 studies focused on just one country, while only one study was performed in several countries, namely, China, Philippines, Croatia and Morocco [20]. The quality scores of the studies included in the systematic review ranged from 6 to 12 for observational or non-randomised intervention studies and 2 to 5 for randomised intervention studies (Table 1). There were 31 observational or non-randomised intervention studies and 2 randomised intervention studies that received the highest scores.
Table 1.
Summary of the association between BMIC and UIC and study scores for quality criteria.
| Reference | Adapted Newcastle–Ottawa Scale | ||||
|---|---|---|---|---|---|
| Association between BMIC and UIC |
Selection
(Maximum 5 *) |
Comparability
(Maximum 4 *) |
Assessment
(Maximum 4 *) |
Total Scores
(Maximum 13 *) 1 |
|
| Observational/Non-randomised intervention studies | |||||
| Aakre et al., 2015 [21] | + 2 | *** | ** | ** | 7 |
| Aakre et al., 2016 [22] | + 3 | *** | ** | *** | 8 |
| Anderson et al., 2014 [23] | + 2 | **** | *** | ** | 9 |
| Anderson et al., 2010 [24] | + | **** | ** | ** | 8 |
| Azizi, 2007 [25] | − 2 | **** | **** | 8 | |
| Bazrafshan et al., 2005 [26] | − 2 | **** | ** | 6 | |
| Böhles et al., 1993 [27] | − 2 | **** | **** | 8 | |
| Budak et al., 2009 [28] | + | **** | **** | 8 | |
| Chan et al., 2003 [29] | + 2 | **** | * | **** | 9 |
| Chen et al., 2020 [30] | + | **** | ** | *** | 9 |
| Chung et al., 2009 [31] | + 3 | **** | *** | 7 | |
| Costeira et al., 2009 [32] | + | **** | ** | *** | 9 |
| de Lima et al., 2013 [33] | + 3 | **** | ** | **** | 10 |
| Dold et al., 2017 [20] | + | **** | *** | *** | 10 |
| Dumrongwongsiri et al., 2018 [34] | + | **** | *** | **** | 11 |
| Groufh-Jacobsen et al., 2020 [35] | + 2 | **** | **** | *** | 11 |
| Gutierrez-Repiso et al., 2014 [36] | + 3 | **** | * | **** | 9 |
| Henjum et al., 2016 [37] | + 2 | **** | * | *** | 8 |
| Huynh et al., 2017 [38] | + | **** | *** | *** | 10 |
| Isiklar Ozberk et al., 2018 [39] | + | **** | ** | **** | 10 |
| Jin et al., 2021 [40] | + | **** | ** | **** | 10 |
| Kart et al., 2021 [41] | + | **** | *** | **** | 11 |
| Kirk et al., 2012 [42] | − 3 | **** | ** | **** | 10 |
| Kurtoglu et al., 2004 [43] | + | **** | * | **** | 9 |
| Laurberg et al., 2004 [44] | + 2 | **** | ** | *** | 9 |
| Liu et al., 2015 [45] | + | **** | ** | **** | 10 |
| Liu et al., 2020 [46] | + | **** | * | **** | 9 |
| Mobasseri et al., 2014 [47] | − | **** | * | *** | 8 |
| Nazeri et al., 2018 [48] | − | **** | *** | **** | 11 |
| Nøhr et al., 1994 [49] | + 3 | **** | * | *** | 8 |
| Ordookhani et al., 2007 [50] | + | **** | * | **** | 9 |
| Osei et al., 2016 [51] | + | **** | **** | **** | 12 |
| Osei et al., 2017 [52] | + | **** | ** | *** | 9 |
| Pal et al., 2018 [53] | + | **** | ** | **** | 10 |
| Pearce et al., 2007 [54] | + 2 | **** | * | **** | 9 |
| Petersen et al., 2020 [55] | − 2 | **** | *** | **** | 11 |
| Pongpaew et al., 1999 [56] | + 2 | **** | * | **** | 9 |
| Prpić et al., 2021 [57] | − | **** | *** | **** | 11 |
| Samson et al., 2021 [58] | − 2 | **** | **** | *** | 11 |
| Skeaff et al., 2005 [59] | + 3 | **** | ** | *** | 9 |
| Stinca et al., 2017 [60] | + | **** | ** | **** | 10 |
| Sukkhojaiwaratkul et al., 2014 [61] | − 2 | **** | *** | 7 | |
| Vermiglio et al., 1992 [62] | + | **** | **** | 8 | |
| Wang et al., 2018 [63] | + | **** | ** | *** | 9 |
| Wang et al., 2009 [64] | + | **** | **** | 8 | |
| Yan et al., 2005 [65] | − | **** | *** | 7 | |
| Jadad Scale | |||||
| The Jadad Scores (Maximum 5) | |||||
| Randomised interventional studies | |||||
| Bouhouch et al., 2014 [66] | + | 5 | |||
| Eriksen et al., 2020 [67] | + 2 | 4 | |||
| Gebreegziabher et al., 2017 [68] | + | 3 | |||
| Nazeri et al., 2017 [69] | − | 3 | |||
| Stoutjesdijk et al., 2018 [70] | + 2 | 2 | |||
+, BMIC has a positive association with UIC; −, BMIC has a negative association with UIC. 1 The asterisk denotes the score(s) for each criterion. Each asterisk denotes a score of 1. 2 Association of BMIC and UIC of lactating women. 3 Association of BMIC and UIC of infant. Newcastle–Ottawa scale. Overall Quality Assessment Rating; High-quality characteristics within each category were awarded a star, up to a maximum of five stars for selection, four stars for comparability and four stars for assessment. Jadad scale. Overall Quality Assessment Rating; High-quality characteristics within each category were awarded 1 score, 0 or −1 score was awarded if not met the characteristics, up to a maximum of 5 scores in total.
The inductively coupled plasma mass spectrometer method (ICP-MS) and Sandell–Kolthoff reaction are obviously the most commonly used methods among all the methods for detecting BMIC (98%) and UIC (98%) (Table 2). Only one study (2%) used reversed-phase high-performance liquid chromatography (HPLC). The detection methods of BMIC, ICP-MS and Sandell–Kolthoff reaction account for 47% and 51%, respectively. Of the types of biochemical methods used to assess UIC, a majority of the studies chose the Sandell–Kolthoff reaction (78%), followed by ICP-MS (20%) and HPLC (2%). The majority of studies (73%) employed the same method to assess both BMIC and UIC; only 14 studies (27%) used two different types of methods to assess BMIC and UIC.
Table 2.
Types of BMIC and UIC methods in studies assessing BMIC and UIC in lactating women and their infants.
| Studies | Year | BMIC Method | UIC Method |
|---|---|---|---|
| Vermiglio et al. [62] | 1992 | S-K 2 | S-K 2 |
| Böhles et al. [27] | 1993 | S-K 1 | S-K 3 |
| Nøhr et al. [49] | 1994 | S-K 3 | S-K 3 |
| Pongpaew et al. [56] | 1999 | S-K | S-K |
| Chan et al. [29] | 2003 | ICP-MS | ICP-MS |
| Kurtoglu et al. [43] | 2004 | HPLC | HPLC |
| Laurberg et al. [44] | 2004 | S-K | S-K |
| Bazrafshan et al. [26] | 2005 | S-K | S-K |
| Skeaff et al. [59] | 2005 | S-K | S-K |
| Yan et al. [65] | 2005 | S-K | S-K |
| Azizi [25] | 2007 | S-K | S-K |
| Ordookhani et al. [50] | 2007 | S-K | S-K |
| Pearce et al. [54] | 2007 | S-K | S-K |
| Budak et al. [28] | 2009 | S-K | S-K |
| Costeira et al. [32] | 2009 | S-K 2 | S-K |
| Chung et al. [31] | 2009 | S-K | S-K |
| Wang et al. [64] | 2009 | S-K | S-K |
| Anderson et al. [24] | 2010 | ICP-MS | S-K |
| Kirk et al. [42] | 2012 | ICP-MS | ICP-MS |
| de Lima et al. [33] | 2013 | ICP-MS | ICP-MS |
| Anderson et al. [23] | 2014 | S-K 3 | S-K 3 |
| Bouhouch et al. [66] | 2014 | ICP-MS | S-K |
| Gutierrez-Repiso et al. [36] | 2014 | S-K 3 | S-K 3 |
| Mobasseri et al. [47] | 2014 | S-K | S-K |
| Sukkhojaiwaratkul et al. [61] | 2014 | S-K | S-K |
| Aakre et al. [22] | 2015 | ICP-MS | S-K |
| Liu et al. [37] | 2015 | S-K 3 | S-K 3 |
| Aakre et al. [22] | 2016 | ICP-MS | S-K |
| Henjum et al. [37] | 2016 | ICP-MS | S-K |
| Osei et al. [51] | 2016 | ICP-MS | S-K |
| Osei et al. [52] | 2016 | ICP-MS | S-K |
| Dold et al. [20] | 2017 | ICP-MS | S-K |
| Gebreegziabher et al. [68] | 2017 | ICP-MS | ICP-MS |
| Huynh et al. [38] | 2017 | ICP-MS | S-K |
| Nazeri et al. [69] | 2017 | S-K | S-K |
| Pal et al. [53] | 2017 | S-K 3 | S-K 3 |
| Stinca et al. [60] | 2017 | ICP-MS | S-K |
| Dumrongwongsiri et al. [34] | 2018 | ICP-MS | S-K |
| Isiklar Ozberk et al. [39] | 2018 | S-K | S-K |
| Nazeri et al. [48] | 2018 | S-K | S-K |
| Stoutjesdijk et al. [70] | 2018 | ICP-MS | ICP-MS |
| Wang et al. [63] | 2018 | ICP-MS | S-K |
| Chen et al. [30] | 2020 | ICP-MS | S-K |
| Eriksen et al. [67] | 2020 | ICP-MS | ICP-MS |
| Groufh-Jacobsen et al. [35] | 2020 | ICP-MS 4 | ICP-MS 4 |
| Petersen et al. [55] | 2020 | ICP-MS | ICP-MS |
| Liu et al. [46] | 2020 | S-K 3 | S-K 3 |
| Jin et al. [40] | 2021 | ICP-MS | ICP-MS |
| Kart et al. [41] | 2021 | S-K | S-K |
| Prpić et al. [57] | 2021 | ICP-MS | S-K |
| Samson et al. [58] | 2021 | ICP-MS | ICP-MS |
S-K, Sandell–Kolthoff reaction; ICP-MS, Inductively Coupled Plasma Mass Spectrometer; HPLC, reversed-phase high-performance liquid chromatography, 1 acid digestion by a mixture of H2SO4, HClO4 and HNO3; 2 using the chloric acid digestion method, 3 Ce/As, arsenic–cerium catalytic spectrophotometry; 4 ICP-QQQ, Triple Quadruple Inductively Coupled Plasma Mass Spectrometer.
3.1. Studies Measuring Both UIC and BMIC of Lactating Women
BMIC Cut Off of ≥100 µg/L to Indicate Iodine Sufficiency
Thirty-eight observational studies (as indicated by the number of references), measuring both the UIC and BMIC of lactating women, were identified (Table 3). Fourteen studies reported that iodine-deficient lactating women (median UIC < 100 µg/L) had a median BMIC <100 µg/L, while only 4 reported that iodine-deficient lactating women (median UIC <100 µg/L) had a median BMIC ≥ 100 µg/L. On the other hand, 16 studies reported that iodine-sufficient lactating women (median UIC ≥ 100 µg/L) had a median BMIC ≥ 100 µg/L, while 7 studies reported that iodine-sufficient lactating women (median UIC ≥ 100 µg/L) had a median BMIC < 100 µg/L.
Table 3.
BMIC and UIC of lactating women.
| Author, Year | Country | Sample Size of Lactating Women | Time of Postpartum (Days/Weeks/Months) | BMIC 1 (µg/L) | UIC 1 (µg/L) | Comments | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational study | |||||||||||||
| Aakre et al., 2015 [21] | Algeria | 111 | 0–7 months |
479 | 350 | ||||||||
| Anderson et al., 2014 [23] | Denmark | 127 | 31 days 1 | Total | 83 | Total | 72 | ||||||
| Iodine-supplemented | 112 | Iodine-supplemented | 83 | ||||||||||
| Non-supplemented | 72 | Non-supplemented | 65 | ||||||||||
| Anderson et al., 2010 [24] | Switzerland | 507 | 6–12 months | 49 | 67 | ||||||||
| Azizi, 2007 [25] | Iran | 100 | NS | 93 | 259 | ||||||||
| Bazrafshan et al., 2005 [26] | Iran | 100 | 30–180 days | 94 | 259 | ||||||||
| Böhles et al., 1993 [27] | Germany | 10 | 5–7 days | 55 2 | 134 3 | Small sample size | |||||||
| Budak et al., 2009 [28] | Turkey | 35 | 18 days | 33 2 | 70 | ||||||||
| Chan et al., 2003 [29] | Australia | 50 | 4 days | 84 | 47 | ||||||||
| Chen et al., 2020 [30] | China | 634 | 1–24 weeks | 165 | 122 | ||||||||
| Costeira et al., 2009 [32] | Portugal | 140 | 3 months | 3 days | 95 | 3 days | 35 | ||||||
| 3 months | 70 | 3 months | 50 | ||||||||||
| Dold et al., 2017 [20] | China, Philippines, Croatia and Morocco | 866 | 3 month | China (n = 298) | 170 | China (n = 298) | 107 | ||||||
| Philippines (n = 281) | 185 | Philippines (n = 281) | 89 | ||||||||||
| Croatia (n = 73) | 124 | Croatia (n = 73) | 35 | ||||||||||
| Morocco (n = 74) | 30 | Morocco (n = 74) | 33 | ||||||||||
| Dumrongwongsiri et al., 2018 [34] | Thailand | 71 | NS | 255 | 149 | ||||||||
| Groufh-Jacobsen et al., 2020 [35] | Norway | 133 | 1–12 months | 71 | 80 | ||||||||
| Henjum et al., 2016 [37] | Nepal | 500 | 2–12 months | 250 | 230 | ||||||||
| Huynh et al., 2017 [38] | Australia | 696 | 3 months | 127 | 125 | ||||||||
| Isiklar Ozberk et al., 2018 [39] | Turkey | 107 | NS | 97 | 135 | ||||||||
| Jin et al., 2021 [40] | New Zealand | 87 | 3 months | 69 | 82 | ||||||||
| Kart et al., 2021 [41] | Turkey | 334 | 4–6 days | 138 | 125 | ||||||||
| Kurtoglu et al., 2004 [43] | Turkey | 70 | 5 days | 73 | 30 | ||||||||
| Laurberg et al., 2004 [44] | Denmark | 140 | 5 days | Smokers (n = 50) |
26 2 | Smokers (n = 50) |
41 2 | ||||||
| Non-smokers (n = 90) |
54 2 | Non-smokers (n = 90) |
40 2 | ||||||||||
| Liu et al., 2015 [45] | China | 343 | 1 year | Beihai (I-deficient areas) (n = 103) |
41 | Beihai (I-deficient areas) (n = 113) |
51 | ||||||
| Yangcheng and Jiajiazhuang (I-sufficient areas) (n = 91) |
346 | Yangcheng and Jiajiazhuang (I-sufficient areas) (n = 98) |
282 | ||||||||||
| Pingyao and Jicun (I-excess areas) (n = 99) |
942 | Pingyao and Jicun (I-excess areas) (n = 125) |
823 | ||||||||||
| Liu et al., 2020 [46] | China | 218 | 0–12 months | Suitable water iodine content areas (n = 97) | 312 | Suitable water iodine content areas (n = 97) | 284 | ||||||
| High water iodine content areas (n = 121) | 1006 | High water iodine content areas (n = 121) | 823 | ||||||||||
| Mobasseri et al., 2014 [47] | Azerbaijan | 106 | NS | 58 | 142 | ||||||||
| Nazeri et al., 2018 [48] | Iran | 124 | <3 months | 100 | 78 | ||||||||
| Ordookhani et al., 2007 [50] | Iran | 48 | 37 to 42 weeks | 148 | 107 | ||||||||
| Osei et al., 2016 [51] | South Africa | 100 | 2–4 months | 179 | 118 | ||||||||
| Osei et al., 2016 [52] | South Africa | 371 | 6 months | 180 | 128 | ||||||||
| Pal et al., 2017 [53] | India | 128 | 1–3 months | 230 | 185 | ||||||||
| Pearce et al., 2007 [54] | United States | 57 | 48 days 1 | 155 | 114 | ||||||||
| Petersen et al., 2020 [55] | Iceland | 60 | 25 weeks | 84 | 152 | ||||||||
| Pongpaew et al., 1999 [56] | Thailand | 75 | 233 days 1 | 51 | 90 | ||||||||
| Prpić et al., 2021 [57] | Croatia | 133 | 2–96 weeks | 121 | 75 | ||||||||
| Samson et al., 2021 [58] | Hungary | 100 | NS | 188 | 49 | ||||||||
| Stinca et al., 2017 [60] | Morocco | 239 | ≤8 weeks | 42 | 35 | ||||||||
| Vermiglio et al., 1992 [62] | Italy | 27 | 5–7 days | Endemic group (n = 11) | 33 2 | Endemic group (n = 11) | 12 2 | Small sample size | |||||
| Control group (n = 16) | 43 2 | Control group (n = 16) | 63 2 | ||||||||||
| Wang et al., 2018 [63] | China | 106 | 4–12 weeks | 4 weeks | 222 2 | 4 weeks | 152 | ||||||
| 8 weeks | 175 2 | 8 weeks | 112 | ||||||||||
| 12 weeks | 148 2 | 12 weeks | 109 | ||||||||||
| Wang et al., 2009 [64] | China | 100 | 0–1 year | 163 | 136 | ||||||||
| Yan et al., 2005 [65] | China | 2554 | 0–2 year | Urban | 136 | Urban | 189 | Huge study in 11 provinces of China | |||||
| Rural | 158 | Rural | 192 | ||||||||||
| Interventional study | |||||||||||||
| Bouhouch et al., 2014 [66] | Morocco | 241 | 0–9 months | Indirect infant supplementation | Direct infant supplementation | Indirect infant supplementation | Direct infant supplementation | One dose of 400 mg iodine as oral iodised oil soon after delivery | |||||
| Baseline | 41 | 43 | Baseline | 37 | 30 | ||||||||
| 3-month | 61 | 33 | 3-month | 58 | 34 | ||||||||
| 6-month | 49 | 36 | 6-month | 67 | 44 | ||||||||
| 9-month | 39 | 26 | 9-month | 58 | 39 | ||||||||
| Eriksen et al., 2020 [67] | The Gambia | 219 | 12 weeks | Baseline (<20 weeks of gestation) | - | Baseline (<20 weeks of gestation) | 51 | A daily supplement of multiple micronutrient containing 300 µg of iodine was taken starting from baseline (<20 weeks of gestation) until delivery. Only both BMIC and UIC data of lactating women at 12 weeks were available. |
|||||
| 12 weeks | 51 | 12 weeks | 39 | ||||||||||
| Gebreegziabher et al., 2017 [68] | Ethiopia | 101 | 6 month | Capsule group | I-salt group | Capsule group | I-salt group | 225 μg iodine as potassium iodide capsule daily for 6 months or 450 g of appropriately iodized salt (30–40 μg I as KIO3/g of salt) weekly for household consumption for 6 months |
|||||
| Baseline | 149 | 157 | Baseline | 136 | 95 | ||||||||
| 6 months | 104 | 111 | 6 months | 150 | 110 | ||||||||
| Nazeri et al., 2017 [69] | Iran | 84 | 1 month | Iodine fortified milk group (n = 40) | Control group (n = 40) | Iodine fortified milk group (n = 40) | Control group (n = 40) | 200 mL iodine fortified milk of which provided 150 µg iodine/day, started at the sixth day postpartum and lasted for four weeks |
|||||
| 3–5 days (baseline) | 176 | 215 | 3–5 days (baseline) | 70 | 97 | ||||||||
| 7 days | 191 | 176 | 7 days | 119 | 51 | ||||||||
| 10 days | 217 | 162 | 10 days | 131 | 103 | ||||||||
| 14 days | 242 | 160 | 14 days | 123 | 48 | ||||||||
| 1 month | 210 | 142 | 1 month | 104 | 41 | ||||||||
| Stoutjesdijk et al., 2018 [70] | Netherlands | 36 | 4 weeks | 20 gestational weeks (baseline) | - | 20 gestational weeks (baseline) | 102 | Multivitamin supplement containing 150 μg/day of iodine were given during 20 gestational weeks | |||||
| 4 weeks | 152 | 4 weeks | 112 | ||||||||||
| Sukkhojaiwaratkul et al., 2014 [61] | Thailand | 87 | 2 months | 3rd trimesters (baseline) | - | 3rd trimesters (baseline) | 204 | Multivitamin supplement containing 200 μg/day of iodine were given during 2-month postpartum | |||||
| Total | 91 | Total | 138 | ||||||||||
| Iodine-supplemented (200 µg) | 109 | Iodine-supplemented (200 µg) | 199 | ||||||||||
| non-supplemented | 70 | non-supplemented | 120 | ||||||||||
1 Median used unless mean reported, 2 mean (µg/L), 3 mean (µg/g), NS, not stated. References no. [20] and [45] were counted more than once in Section 3.1 because they reported findings in both iodine deficient and sufficient populations.
Six intervention studies, measuring both the UIC and BMIC of lactating women, were identified (Table 3). The longest duration of invention was 9 months. In a study by Bouhouch et al., despite the supplementation of one dose of 400 mg iodine as oral iodised oil, the women remained iodine deficient (both UIC and BMIC) throughout the intervention period. Although the study by Eriksen et al. supplemented women with 300 µg iodine, containing a prenatal multiple micronutrient supplement, the median BMIC of lactating women was <100 µg/L at 12 weeks postpartum. Another study, by Gebreegziabher et al., reported that the median BMIC values of women either receiving 225 µg iodine as a potassium iodide capsule daily or 450 g of iodized salt (30–40 µg iodine as KIO3/g of salt) weekly for 6 months was ≥100 µg/L at 6 months postpartum. The study by Nazeri et al. reported that both median BMIC values of women receiving iodine-fortified milk and control group were ≥100 µg/L at 1 month postpartum; women receiving iodine-fortified milk had a significantly higher median UIC than the control group (p < 0.001). The study by Stoutjesdijk et al. reported that Dutch women supplemented with 150 μg iodine at 20 weeks of gestation had both median BMIC and UIC values ≥ 100 µg/L at 4th week of postpartum, indicating iodine sufficiency. A study by Sukkhojaiwaratkul et al. reported that, despite the fact that both women receiving 200 µg iodine table daily and women in the non-supplemented group were iodine sufficient at 2 months postpartum, the median BMIC of women in the supplemented group was higher than the non-supplemented group, suggesting the importance of maternal iodine supplementation in the improving iodine status of breast-fed infants.
3.2. Studies Measuring UIC of Infants and BMIC of Lactating Women
BMIC Cut Off of ≥100 µg/L to Indicate Iodine Sufficiency
Twenty-nine observational studies (as indicated by the number of references), measuring both the UIC of infants and BMIC of lactating women, were identified (Table 4). Eight studies reported iodine-deficient infants (median UIC < 100 µg/L) born to lactating women with a median BMIC < 100 µg/L, while no studies reported iodine-deficient infants (median UIC < 100 µg/L) born to lactating women with a median BMIC ≥ 100 µg/L. On the other hand, 19 studies reported iodine-sufficient infants (median UIC ≥ 100 µg/L) born to lactating women with a median BMIC ≥ 100 µg/L, while 3 studies reported iodine-sufficient infants (median UIC ≥ 100 µg/L) born to lactating women with a median BMIC < 100 µg/L.
Table 4.
BMIC of lactating women and UIC of infants.
| Author, Year | Country | Sample Size of Infants | Time of Postpartum (Days/Weeks/Months) | BMIC 1 (µg/L) | UIC 1 (µg/L) | Comments | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Observational study | |||||||||||
| Aakre et al., 2016 [22] | Algeria | 289 | 31.4 days 1 | 479 | 722 | ||||||
| Anderson et al., 2010 [24] | Switzerland | 875 | 6–12 months | 49 | 82 | ||||||
| Budak et al., 2009 [28] | Turkey | 35 | 5–28 days | 33 2 | 100 | ||||||
| Chen et al., 2020 [30] | China | 634 | 24 weeks | 165 | 216 | ||||||
| Costeira et al., 2009 [32] | Portugal | 142 | 3 months | 3 days | 95 | 3 days | 65 | ||||
| 3 months | 70 | 3 months | 96 | ||||||||
| Chung et al., 2009 [31] | Korea | 31 | 6 weeks | 3rd week | 1153 | 3rd week | 1651 | Small sample size | |||
| 6th week | 822 | 6th week | 1832 | ||||||||
| de Lima et al., 2013 [33] | Brazil | 33 | ≤6 months | 206 | 293 | Small sample size | |||||
| Dold et al., 2017 [20] | China, Philippines and Croatia |
866 | 3 months | China (n = 298) | 170 | China (n = 298) | 278 | ||||
| Philippines (n = 281) | 185 | Philippines (n = 281) | 352 | ||||||||
| Croatia (n = 73) | 124 | Croatia (n = 73) | 239 | ||||||||
| Dumrongwongsiri et al., 2018 [34] | Thailand | 71 | NS | 255 | 282 | ||||||
| Huynh et al., 2017 [38] | Australia | 696 | 3 months | 127 | 198 | ||||||
| Isiklar Ozberk et al., 2018 [39] | Turkey | 107 | NS | 97 | 95 | ||||||
| Jin et al., 2021 [40] | New Zealand | 87 | 3 months | 69 | 115 | ||||||
| Kart et al., 2021 [41] | Turkey | 334 | 4–6 days | 138 | 142 | ||||||
| Kurtoglu et al., 2004 [43] | Turkey | 70 | 5 days | 73 | 24 | ||||||
| Liu et al., 2015 [45] | China | 343 | 1 year | Beihai (I-deficient areas) (n = 103) |
41 | Beihai (I-deficient areas) (n = 28) |
65 | ||||
| Yangcheng and Jiajiazhuang (I-sufficient areas) (n = 91) |
346 | Yangcheng and Jiajiazhuang (I-sufficient areas) (n = 90) |
427 | ||||||||
| Pingyao and Jicun (I-excess areas) (n = 99) |
942 | Pingyao and Jicun (I-excess areas) (n = 124) |
1222 | ||||||||
| Liu et al., 2020 [46] | China | 218 | 0–12 months | Suitable water iodine content areas (n = 97) | 312 | Suitable water iodine content areas (n = 97) | 427 | ||||
| High water iodine content areas (n = 121) | 1006 | High water iodine content areas (n = 121) | 1222 | ||||||||
| Mobasseri et al., 2014 [47] | Azerbaijan | 106 | NS | 58 | 307 | ||||||
| Nazeri et al., 2018 [48] | Iran | 124 | <3 months | 100 | 183 | ||||||
| Ordookhani et al., 2007 [50] | Iran | 27 | 37 to 42 weeks | 148 | 271 | Small sample size | |||||
| Osei et al., 2016 [51] | South Africa | 100 | 2–4 months | 179 | 373 | ||||||
| Osei et al., 2016 [52] | South Africa | 386 | 6 months | 180 | 345 | ||||||
| Pal et al., 2017 [53] | India | 128 | 1–3 months | 230 | 250 | ||||||
| Prpić et al., 2021 [57] | Croatia | 133 | 2–96 weeks | 121 | 2–26 weeks (n = 101) | 234 | |||||
| 27–96 weeks (n = 32) | 209 | ||||||||||
| Skeaff et al., 2005 [59] | New Zealand | 230 | 6–24-month | 22 | 67 | ||||||
| Stinca et al., 2017 [60] | Morocco | 239 | ≤8 weeks | 42 | 73 | ||||||
| Vermiglio et al., 1992 [62] | Italy | 27 | 5–7 days | Endemic group (n = 11) | 33 2 | Endemic group (n = 11) | 34 2 | Small sample size | |||
| Control group (n = 16) | 43 2 | Control group (n = 16) | 43 2 | ||||||||
| Wang et al., 2018 [63] | China | 106 | 4–12 weeks | 4-week | 222 2 | 4-week | 251 | ||||
| 8-week | 175 2 | 8-week | 183 | ||||||||
| 12-week | 148 2 | 12-week | 164 | ||||||||
| Wang et al., 2009 [64] | China | 61 | 0–1 year | 163 | 233 | ||||||
| Yan et al., 2005 [65] | China | 2537 | 0–2 years | Urban | 136 | Urban | 236 | Huge study in 11 provinces of China | |||
| Rural | 158 | Rural | 247 | ||||||||
| Interventional study | |||||||||||
| Bouhouch et al., 2014 [66] | Morocco | 241 | 0–9 months | Indirect infant supplementation | Direct infant supplementation | Indirect infant supplementation | Direct infant supplementation | One dose of 400 mg iodine as oral iodised oil soon after delivery | |||
| Baseline | 41 | 43 | Baseline | 73 | 74 | ||||||
| 3-month | 61 | 33 | 3-month | 132 | 99 | ||||||
| 6-month | 49 | 36 | 6-month | 142 | 122 | ||||||
| 9-month | 39 | 26 | 9-month | 97 | 90 | ||||||
| Gebreegziabher et al., 2017 [68] | Ethiopia | 101 | 6 months | Capsule group | I-salt group | Capsule group | I-salt group | 225 μg iodine as potassium iodide capsule daily for 6 months or 450 g of appropriately iodized salt (30–40 μg I as KIO3/g of salt) weekly for household consumption for 6 months |
|||
| Baseline | 149 | 157 | Baseline | 234 | 193 | ||||||
| 6-month | 104 | 111 | 6-month | 254 | 195 | ||||||
| Gutierrez-Repiso et al., 2014 [36] | Spain | 88 | NS | Control group (n = 21) | 109 | Control group (n = 21) | 112 | 300 µg of iodide (in the form of KI) were given from the first trimester of pregnancy (300 group) |
|||
| 300 group (n = 67) (300 µg) | 178 | 300 group (n = 67) (300 µg) | 215 | ||||||||
| Kirk et al., 2012 [42] | United States | 13 | 1–8 months | Pre supplementation | 53 2 | Pre supplementation | 239 | Small sample size | |||
| PM supplementation | 57 2 | PM supplementation | 379 | ||||||||
| AM supplementation | 57 2 | AM supplementation | 324 | ||||||||
| Nazeri et al., 2017 [69] | Iran | 84 | 1 month | Iodine fortified milk group (n = 40) | Control group (n = 40) | Iodine fortified milk group (n = 40) | Control group (n = 40) | 200 mL iodine fortified milk of which provided 150 µg iodine/day, started at the sixth day postpartum and lasted for four weeks |
|||
| 3–5 days (baseline) | 176 | 215 | 3–5 days (baseline) | 231 | 193 | ||||||
| 7 days | 191 | 176 | 7 days | 169 | 120 | ||||||
| 10 days | 217 | 162 | 10 days | 219 | 138 | ||||||
| 14 days | 242 | 160 | 14 days | 194 | 116 | ||||||
| 1 month | 210 | 142 | 1 month | 230 | 110 | ||||||
| Nøhr et al., 1994 [49] | Denmark | 147 | 5 days | Baseline | 34 | Baseline | 32 | Vitamin-mineral preparations containing iodine (with a declared iodine content of 150 µg/tablet). |
|||
| Not received iodine supplementation group (n = 94) | 34 | Not received iodine supplementation group (n = 94) | 32 | ||||||||
| Tablets containing iodine group (n = 53) | 57 | Tablets containing iodine group (n = 53) | 61 | ||||||||
1 Median used unless mean reported, 2 mean, NS, not stated. Reference no. [45] was counted more than once in Section 3.2 because it reported findings in both iodine deficient and sufficient populations.
Six intervention studies, measuring both the UIC of infants and BMIC of lactating women, were identified (Table 4). The longest duration of infants supplemented with iodine was 9 months. One study, by Bouhouch et al., reported that at 6 months, infants were iodine sufficient based on infant UIC, but iodine deficient based on BMIC. Three months later, infant UIC decreased to <100 µg/L and BMIC remained at <100 µg/L. Another study, by Gebreegziabher et al., reported that the median UIC of infants born to women either receiving 225 µg iodine as a potassium iodide capsule daily or 450 g of iodized salt (30–40 µg iodine as KIO3/g of salt) weekly for 6 months was ≥100 µg/L at 6 months postpartum. A study by Gutierrez-Repiso et al. reported that both women and their infants in the 300 and control group were iodine sufficient, based on median BMIC and infant UIC values (≥100 µg/L). A study by Kirk et al. demonstrated that, although median infant UIC was ≥100 µg/L, single-dose iodine supplements were not effective in improving BMIC values. Another study, by Nøhr et al., reported that both groups, one receiving tablets containing iodine and one not receiving iodine supplementation, had both median BMIC and UIC values < 100 µg/L. A study by Nazeri et al. reported that both median BMIC values of women and infants in the iodine-fortified milk and control groups were ≥100 µg/L at 1 month postpartum.
4. Discussion
The systematic review reveals that BMIC ranged from 26 to 185 µg/L and 15 to 1006 µg/L in iodine-deficient and iodine-sufficient lactating women, respectively. Only few studies on BMIC were from excessive iodine areas (median UIC ≥ 300 µg/L based on non-pregnant adult populations). The majority of the studies on BMIC findings were conducted in both iodine-deficiency and iodine-sufficiency areas. However, most studies were cross-sectional studies and did not clearly indicate if the infants were breast-fed. The dose of iodine supplementation ranged between 30 µg and 400 mg iodine. In terms of the dose of iodine that the infants received, there was a variation in the duration of iodine supplementation, the amount and form of the iodine supplemented to the lactating women. Therefore, high-quality data on the BMIC and UIC of lactating women, with different iodine status (iodine deficiency, iodine sufficiency, and excessive iodine) with breast-fed infants, are generally lacking.
Over the past two decades, Sandell–Kolthoff reaction, which is a traditional colorimetric method, has been commonly used to detect UIC. One of the possible reasons is because, according to the WHO/ICCIDD/UNICEF, Sandell–Kolthoff reaction, using ammonium persulfate as the digestion method, has been recommended, which is also known as method A [5]. There is also another method, called method B; the only difference is the digestion step, as method A uses the ammonium persulfate to digest urine samples, while method B digests with chloric acid [5]. However, chloric acid has potential hazards and it is more toxic as the digestant, so using ammonium persulfate is currently recommended by WHO/ICCIDD/UNICEF, and the method has been modified from the previous method [5,71]. The current recommended Sandell–Kolthoff reaction has simple, convenient and economic advantages [5].
Currently, there are no recommended methods for analysing BMIC. Spectrophotometric Sandell–Kolthoff, HPLC, and ICP-MS-based methods have been used to measure BMIC. Therefore, one of the challenges in comparing BMIC across different studies is due to the differences in the analytical methods used and lack of method standardisation across different analytical methods [72]. In addition, studies on BMIC were based on spot samples at different sampling times and stages of lactation. Therefore, the inconsistencies in these findings on nutrient compositions may be due to the different stages of lactation, sampling time, time of the day, maternal iodine status, and individual variation. However, BMIC does not seem to be affected by the sampling methods (i.e., time of day, before or after the lactation session, and left or right breast) [23,73].
4.1. Factors Influencing BMIC
BMIC may be affected by physiological fluctuations [74]. For example, median BMIC gradually increases with time, from birth up to 12 months [37]. A study by Etling et al. reported that BMIC was reported to increase during the first month of the lactation period [75]. Another study reported that BMIC decreased during the first 6 months of the lactation period [18], which may be due to suboptimal iodine status in lactating women. However, another study reported that BMIC varied from day to day [76]. These inconsistent findings should be confirmed in larger longitudinal studies of lactating women.
Several studies have reported that the nutrient content of breast milk differs significantly between different stages of the lactation period, suggesting that BMIC follows a similar pattern to that of other nutrients [77]. Higher BMIC has been observed during the first few days of the lactation period, followed by a decreasing trend over time, which may be because the colostrum is gradually changed into mature breast milk. However, there is no difference in BMIC between colostrum and mature breast milk in iodine-deficient lactating women [48].
4.2. BMIC as a Biomarker to Assess Iodine Status in Lactating Women and Children <2 Years of Age
A UIC cut off of ≥100 has been proposed to indicate iodine sufficiency in children aged <2 years [5]. Iodine intake of breast-fed infants corresponds to BMIC, because the dietary iodine sources of breast-fed infants depend entirely on the mothers’ iodine intake. Therefore, BMIC is also an important biomarker of iodine status for breast-fed infants [20]. In non-lactating women, absorbed iodine is partly transported to the thyroid gland and the remaining iodine (~90%) is cleared by passive renal glomerular filtration [78]. However, in lactating women, absorbed iodine is also transported to the mammary gland by NIS (sodium iodide symporter) [78]. Therefore, UIC is consequently lower, and the median UIC, indicating iodine sufficiency in lactating women (who are breastfeeding), is similar to non-pregnant individuals (≥100 µg/L), although lactating women (who are breastfeeding) have higher iodine requirements [5,7]. In iodine-sufficient lactating women (median UIC ≥100 µg/L), higher fractional iodine is excreted into breast milk at a lower range of daily maternal iodine intake and, consequently, renal fractional iodine excretion is decreased [20]. Even in non-lactating women, lower UIC is reported during lactation than in pregnancy, which might be due to the higher clearance of circulating iodine to the restoration of the depleted thyroid gland for the restoration of the depleted thyroid gland [79]. Therefore, BMIC is considered a more reliable biomarker of iodine status in lactating women than UIC.
However, there have been some studies reporting discrepancies between the UIC of lactating women and their BMIC, suggesting that BMIC may not be able to accurately reflect infant iodine status. Therefore, there is a need to further explore the reliability of BMIC as a biomarker of iodine status in infants.
4.3. Why Did Some Lactating Women Classified as Iodine Sufficient by UIC Have a BMIC Less Than the Proposed BMIC Cut Offs (i.e., BMIC Considered Iodine Deficient)?
This is probably as a result of the recent maternal dietary iodine intake and duration of the lactation period [36,74,80]. Future studies are needed to investigate other factors, such as the genetic variations in the SLC5A5 gene in relation to BMIC, which has been reported to play an important role in the iodine transfer into breast milk [81].
4.4. Why Did Some Lactating Women Classified as Iodine Deficient by UIC Have a BMIC Equivalent or Higher Than the Proposed BMIC Cut Offs (i.e., BMIC Considered Iodine Sufficient)?
In the systematic review, some studies reported that iodine-deficient lactating women (median UIC < 100 µg/L) had a median BMIC ≥ 100 µg/L. In iodine-deficient regions, since the mammary gland can concentrate iodine, iodine supply to the infants may be maintained via breast milk, even if the mothers are iodine deficient [8,62]. This may help explain why, in iodine-deficient regions, lactating women were classified as iodine deficient based on median UIC, but were iodine sufficient according to their median BMIC [7,8].
4.5. What Is an Appropriate BMIC Cut Off to Categorise Iodine Sufficiency in Lactating Women and Children <2 Years of Age?
Currently, there are no official guidelines on the median BMIC cut off to indicate iodine sufficiency in lactating women and children <2 years of age. However, there have been some median BMIC cut offs proposed to indicate iodine sufficiency (i.e., 50, 75, 80, 92, and 100 µg/L) [7,26,73,82,83]. A median BMIC cut off of >75 µg/L was suggested by Azizi and Smyth [73]; however, another higher median BMIC cut off of ≥100 µg/L has also been proposed [7,83]. This is because a full-term infant is considered to need 15 µg iodine/kg/day for maintaining normal thyroid metabolism [7,18,19]. Semba and Delange concluded that BMIC should be between 100 and 200 µg/L to meet the recommendations of the Food and Nutrition Board (FNB) of the Institute of Medicine (IoM) [7]. A study by Dold et al. suggested that, in iodine-sufficient regions, a BMIC reference range of 60–465 µg/kg can be used to suggest iodine sufficiency in lactating mothers and breast-fed infants [20]. However, it is unclear whether the similar BMIC reference range can be applied in iodine-deficient regions.
There is a wide range of median or mean BMIC across different studies and regions [7,8,73]. In the USA, the median BMIC of lactating women ranges from 35 to 155 µg/L [84]. Several possible reasons might have contributed to this phenomenon, which include: lack of standardisation of breast milk collection methods, physiological mechanisms during pregnancy and lactation, iodine status during pre-pregnancy or pregnancy, dietary intake, and the region where the study was conducted (i.e., iodine deficient or iodine sufficient). A study by Leung et al. reported an increase in BMIC following acute maternal dietary iodine intake, suggesting that BMIC can be influenced by physiological mechanisms [74].
The main strength of the systematic review is the inclusion of the BMIC and UIC of lactating women and infants. In addition, the analytical methods of BMIC and UIC were reviewed. The limitations of the included studies were as follows: cross-sectional studies did not clearly indicate if the infants were breast-fed; further, high-quality data on the BMIC and UIC of lactating women with different iodine status with breast-fed infants are generally lacking. Concerning BMIC, differences in the analytical methods used and lack of method standardization across different analytical methods is an important limitation. Given the limited numbers of studies (n = 6) that have assessed BMIC across subgroups of lactating women, at different stages of the lactation period, these findings should be interpreted cautiously. Studies reporting whether BMIC changes with time of lactation period are inconsistent [8]. It is unclear if BMIC varies with regard to the collection time of the day, fore or hind milk, or left or right breast [23,59]. There is only one study, by Andersen et al., that collected breast milk samples from one and both breasts of breastfeeding women [23]. The authors reported no difference in median BMIC between one and both breasts (83 vs. 83 µg/L). In addition, breast-milk sampling, performed before or after breastfeeding, did not influence median BMIC (82 vs. 78 µg/L) [23,85].
In conclusion, this systematic review revealed that, although BMIC can be used to assess iodine status in lactating women and children <2 years of age, it is associated with some limitations, including an optimal BMIC cut off used to indicate iodine sufficiency. Therefore, it is difficult and challenging to draw a firm conclusion regarding the usefulness of BMIC as a biomarker of iodine status based on these studies. More well-designed, large-scale studies are needed to examine the usefulness and feasibility of BMIC in assessing iodine status in lactating women and children <2 years of age, with different levels of iodine intake.
Acknowledgments
We would like to thank Kate Navaratnam and Sarah Donegan for assistance with the systematic review protocol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14091691/s1, Table S1. The Jadad scale for assessment of study quality for intervention studies (Jadad et al., 1996), Table S2. The Jadad scores of included studies, Table S3. Assessment of quality for a cohort study; adapted from the Newcastle-Ottawa scale. Stars were awarded if the criteria shown in italics were met. The number of stars awarded are indicated at the end of each statement. Maximum of 13 stars*.
Author Contributions
Conceptualization, S.L. and Z.F.M.; methodology, S.L., A.S., E.V., and Z.F.M.; formal analysis, S.L.; writing—original draft preparation, S.L.; writing—review and editing, S.L., A.S., E.V., and Z.F.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zimmermann M.B., Boelaert K. Iodine deficiency and thyroid disorders. Lancet Diabetes Endocrinol. 2015;3:286–295. doi: 10.1016/S2213-8587(14)70225-6. [DOI] [PubMed] [Google Scholar]
- 2.Zimmermann M.B. The role of iodine in human growth and development. Semin. Cell Dev. Biol. 2011;22:645–652. doi: 10.1016/j.semcdb.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Pearce E.N., Lazarus J.H., Moreno-Reyes R., Zimmermann M.B. Consequences of iodine deficiency and excess in pregnant women: An overview of current knowns and unknowns. Am. J. Clin. Nutr. 2016;104:918S–923S. doi: 10.3945/ajcn.115.110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candido A.C., Morais N.D.S.D., Dutra L.V., Pinto C.A., Franceschini S.D.C., Alfenas R.D.C.G. Insufficient iodine intake in pregnant women in different regions of the world: A systematic review. Arch. Endocrinol. Metab. 2019;63:306–311. doi: 10.20945/2359-3997000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Assessment of Iodine Deficiency Disorders And monitoring Their Elimination: A Guide for Programme Managers. World Health Organization (WHO); Geneva, Switzerland: 2007. [Google Scholar]
- 6.Ma Z.F., Skeaff S.A. Iodine Deficiency Disorders and Their Elimination. Springer; Berlin/Heidelberg, Germany: 2017. Assessment of population iodine status; pp. 15–28. [Google Scholar]
- 7.Semba R.D., Delange F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001;59:269–278. doi: 10.1111/j.1753-4887.2001.tb05512.x. [DOI] [PubMed] [Google Scholar]
- 8.Dorea J.G. Iodine nutrition and breast feeding. J. Trace Elem. Med. Biol. 2002;16:207–220. doi: 10.1016/S0946-672X(02)80047-5. [DOI] [PubMed] [Google Scholar]
- 9.Delange F. Iodine requirements during pregnancy, lactation and the neonatal period and indicators of optimal iodine nutrition. Public Health Nutr. 2007;10:1571–1580. doi: 10.1017/S1368980007360941. [DOI] [PubMed] [Google Scholar]
- 10.Redman K., Ruffman T., Fitzgerald P., Skeaff S. Iodine deficiency and the brain: Effects and mechanisms. Crit. Rev. Food Sci. Nutr. 2016;56:2695–2713. doi: 10.1080/10408398.2014.922042. [DOI] [PubMed] [Google Scholar]
- 11.van den Hove M.F., Beckers C., Devlieger H., de Zegher F., De Nayer P. Hormone synthesis and storage in the thyroid of human preterm and term newborns: Effect of thyroxine treatment. Biochimie. 1999;81:563–570. doi: 10.1016/S0300-9084(99)80111-4. [DOI] [PubMed] [Google Scholar]
- 12.Andersson M., De Benoist B., Delange F., Zupan J. Prevention and control of iodine deficiency in pregnant and lactating women and in children less than 2-years-old: Conclusions and recommendations of the Technical Consultation. Public Health Nutr. 2007;10:1606–1611. doi: 10.1017/S1368980007361004. [DOI] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jadad A.R., Moore R.A., Carroll D., Jenkinson C., Reynolds D.J.M., Gavaghan D.J., McQuay H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 7 June 2021)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm.
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 17.Jüni P., Witschi A., Bloch R., Egger M. The hazards of scoring the quality of clinical trials for meta-analysis. JAMA. 1999;282:1054–1060. doi: 10.1001/jama.282.11.1054. [DOI] [PubMed] [Google Scholar]
- 18.Mulrine H.M., Skeaff S.A., Ferguson E.L., Gray A.R., Valeix P. Breast-milk iodine concentration declines over the first 6 mo postpartum in iodine-deficient women. Am. J. Clin. Nutr. 2010;92:849–856. doi: 10.3945/ajcn.2010.29630. [DOI] [PubMed] [Google Scholar]
- 19.Henjum S., Lilleengen A.M., Aakre I., Dudareva A., Gjengedal E.L.F., Meltzer H.M., Brantsæter A.L. Suboptimal iodine concentration in breastmilk and inadequate iodine intake among lactating women in Norway. Nutrients. 2017;9:643. doi: 10.3390/nu9070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dold S., Zimmermann M.B., Aboussad A., Cherkaoui M., Jia Q., Jukic T., Kusic Z., Quirino A., Sang Z., San Luis T.O. Breast milk iodine concentration is a more accurate biomarker of iodine status than urinary iodine concentration in exclusively breastfeeding women. J. Nutr. 2017;147:528–537. doi: 10.3945/jn.116.242560. [DOI] [PubMed] [Google Scholar]
- 21.Aakre I., Bjøro T., Norheim I., Strand T.A., Barikmo I., Henjum S. Excessive iodine intake and thyroid dysfunction among lactating Saharawi women. J. Trace Elem. Med. Biol. 2015;31:279–284. doi: 10.1016/j.jtemb.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Aakre I., Strand T.A., Bjøro T., Norheim I., Barikmo I., Ares S., Alcorta M.D., Henjum S. Thyroid function among breastfed children with chronically excessive iodine intakes. Nutrients. 2016;8:398. doi: 10.3390/nu8070398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersen S.L., Møller M., Laurberg P. Iodine concentrations in milk and in urine during breastfeeding are differently affected by maternal fluid intake. Thyroid. 2014;24:764–772. doi: 10.1089/thy.2013.0541. [DOI] [PubMed] [Google Scholar]
- 24.Andersson M., Aeberli I., Wüst N., Piacenza A.M., Bucher T., Henschen I., Haldimann M., Zimmermann M.B. The Swiss iodized salt program provides adequate iodine for school children and pregnant women, but weaning infants not receiving iodine-containing complementary foods as well as their mothers are iodine deficient. J. Clin. Endocrinol. Metab. 2010;95:5217–5224. doi: 10.1210/jc.2010-0975. [DOI] [PubMed] [Google Scholar]
- 25.Azizi F. Iodine nutrition in pregnancy and lactation in Iran. Public Health Nutr. 2007;10:1596–1599. doi: 10.1017/S1368980007360977. [DOI] [PubMed] [Google Scholar]
- 26.Bazrafshan H.R., Mohammadian S., Ordookhani A., Abedini A., Davoudy R., Pearce E.N., Hedayati M., Azizi F., Braverman L.E. An assessment of urinary and breast milk iodine concentrations in lactating mothers from Gorgan, Iran, 2003. Thyroid. 2005;15:1165–1168. doi: 10.1089/thy.2005.15.1165. [DOI] [PubMed] [Google Scholar]
- 27.Böhles H., Aschenbrenner M., Roth M., Loewenich V.V., Usadel K.H. Development of thyroid gland volume during the first 3 months of life in breast-fed versus iodine-supplemented and iodine-free formula-fed infants. Clin. Investig. 1993;71:13. doi: 10.1007/BF00210957. [DOI] [PubMed] [Google Scholar]
- 28.Budak N., Şahin U., Kurtoğlu S., Ülgen A., Akçakuş M., Kurtoğlu S., Patıroğlu T. Nutritional iodine status of breast-feeding mothers and their neonates in Kayseri. Erciyes Med. J. 2009;31:208–212. [Google Scholar]
- 29.Chan S.S., Hams G., Wiley V., Wilcken B., McElduff A. Postpartum maternal iodine status and the relationship to neonatal thyroid function. Thyroid. 2003;13:873–876. doi: 10.1089/105072503322401078. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y., Gao M., Bai Y., Hao Y., Chen W., Cui T., Guo W., Pan Z., Lin L., Wang C., et al. Variation of iodine concentration in breast milk and urine in exclusively breastfeeding women and their infants during the first 24 wk after childbirth. Nutrition. 2020;71:110599. doi: 10.1016/j.nut.2019.110599. [DOI] [PubMed] [Google Scholar]
- 31.Chung H.R., Shin C.H., Yang S.W., Choi C.W., Kim B.I. Subclinical hypothyroidism in Korean preterm infants associated with high levels of iodine in breast milk. J. Clin. Endocrinol. Metab. 2009;94:4444–4447. doi: 10.1210/jc.2009-0632. [DOI] [PubMed] [Google Scholar]
- 32.Costeira M.J., Oliveira P., Ares S., de Escobar G.M., Palha J.A. Iodine status of pregnant women and their progeny in the Minho Region of Portugal. Thyroid. 2009;19:157–163. doi: 10.1089/thy.2008.0249. [DOI] [PubMed] [Google Scholar]
- 33.de Lima L.F., Barbosa F., Jr., Navarro A.M. Excess iodinuria in infants and its relation to the iodine in maternal milk. J. Trace Elem. Med. Biol. 2013;27:221–225. doi: 10.1016/j.jtemb.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Dumrongwongsiri O., Chatvutinun S., Phoonlabdacha P., Sangcakul A., Chailurkit L.O., Siripinyanond A., Suthutvoravut U., Chongviriyaphan N. High Urinary Iodine Concentration Among Breastfed Infants and the Factors Associated with Iodine Content in Breast Milk. Biol. Trace Elem. Res. 2018;186:106–113. doi: 10.1007/s12011-018-1303-4. [DOI] [PubMed] [Google Scholar]
- 35.Groufh-Jacobsen S., Mosand L.M., Bakken K.S., Solvik B.S., Oma I., Gjengedal E.L.F., Brantsæter A.L., Strand T.A., Henjum S. Mild to moderate iodine deficiency and inadequate iodine intake in lactating women in the inland area of Norway. Nutrients. 2020;12:630. doi: 10.3390/nu12030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gutiérrez-Repiso C., Velasco I., Garcia-Escobar E., Garcia-Serrano S., Rodríguez-Pacheco F., Linares F., Ruiz de Adana M.S., Rubio-Martin E., Garrido-Sanchez L., Cobos-Bravo J.F., et al. Does dietary iodine regulate oxidative stress and adiponectin levels in human breast milk? Antioxid. Redox Signal. 2014;20:847–853. doi: 10.1089/ars.2013.5554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henjum S., Kjellevold M., Ulak M., Chandyo R.K., Shrestha P.S., Frøyland L., Strydom E.E., Dhansay M.A., Strand T.A. Iodine concentration in breastmilk and urine among lactating women of Bhaktapur, Nepal. Nutrients. 2016;8:255. doi: 10.3390/nu8050255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huynh D., Condo D., Gibson R., Muhlhausler B., Ryan P., Skeaff S., Makrides M., Zhou S.J. Iodine status of postpartum women and their infants in Australia after the introduction of mandatory iodine fortification. Br. J. Nutr. 2017;117:1656–1662. doi: 10.1017/S0007114517001775. [DOI] [PubMed] [Google Scholar]
- 39.Isiklar Ozberk D., Kutlu R., Kilinc I., Kilicaslan A.O. Effects of mandatory salt iodization on breast milk, urinary iodine concentrations, and thyroid hormones: Is iodine deficiency still a continuing problem? J. Endocrinol. Investig. 2019;42:411–418. doi: 10.1007/s40618-018-0930-0. [DOI] [PubMed] [Google Scholar]
- 40.Jin Y., Coad J., Zhou S.J., Skeaff S., Benn C., Brough L. Use of Iodine Supplements by Breastfeeding Mothers Is Associated with Better Maternal and Infant Iodine Status. Biol. Trace Elem. Res. 2021;199:2893–2903. doi: 10.1007/s12011-020-02438-8. [DOI] [PubMed] [Google Scholar]
- 41.Kart P., Türkmen M.K., Anık A., Anık A., Ünüvar T. The association of lactating mothers’ urinary and breast milk iodine levels with iodine nutrition status and thyroid hormone levels of newborns. Turk. Arch. Pediatr. 2021;56:207–212. doi: 10.5152/TurkArchPediatr.2021.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kirk A.B., Kroll M., Dyke J.V., Ohira S., Dias R.A., Dasgupta P.K. Perchlorate, iodine supplements, iodized salt and breast milk iodine content. Sci. Total Environ. 2012;420:73–78. doi: 10.1016/j.scitotenv.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 43.Kurtoglu S., Akcakus M., Kocaoglu C., Gunes T., Budak N., Atabek M.E., Karakucuk I., Delange F. Iodine status remains critical in mother and infant in Central Anatolia (Kayseri) of Turkey. Eur. J. Nutr. 2004;43:297–303. doi: 10.1007/s00394-004-0474-2. [DOI] [PubMed] [Google Scholar]
- 44.Laurberg P., Nøhr S.B., Pedersen K.M., Fuglsang E. Iodine nutrition in breast-fed infants is impaired by maternal smoking. J. Clin. Endocrinol. Metab. 2004;89:181–187. doi: 10.1210/jc.2003-030829. [DOI] [PubMed] [Google Scholar]
- 45.Liu L., Wang D., Liu P., Meng F., Wen D., Jia Q., Liu J., Zhang X., Jiang P., Shen H. The relationship between iodine nutrition and thyroid disease in lactating women with different iodine intakes. Br. J. Nutr. 2015;114:1487–1495. doi: 10.1017/S0007114515003128. [DOI] [PubMed] [Google Scholar]
- 46.Liu L., Liu J., Wang D., Shen H., Jia Q. Effect of Urinary Iodine Concentration in Pregnant and Lactating Women, and in Their Infants Residing in Areas with Excessive Iodine in Drinking Water in Shanxi Province, China. Biol. Trace Elem. Res. 2020;193:326–333. doi: 10.1007/s12011-019-01716-4. [DOI] [PubMed] [Google Scholar]
- 47.Mobasseri M., Roshanravan N., Mesri Alamdari N., Ostadrahimi A., Asghari Jafarabadi M., Anari F., Hedayati M. Urinary and Milk Iodine Status in Neonates and Their Mothers during Congenital Hypothyroidism Screening Program in Eastern Azerbaijan: A Pilot Study. Iran J. Public Health. 2014;43:1380–1384. [PMC free article] [PubMed] [Google Scholar]
- 48.Nazeri P., Dalili H., Mehrabi Y., Hedayati M., Mirmiran P., Azizi F. Is there any difference between the iodine statuses of breast-fed and formula-fed infants and their mothers in an area with iodine deficiency? Br. J. Nutr. 2018;119:1012–1018. doi: 10.1017/S0007114518000351. [DOI] [PubMed] [Google Scholar]
- 49.Nøhr S.B., Lawberg P., Børlum K.G., Pedersen K.M., Johannesen P.L., Damm P., Fuglsang E., Johansen A. Iodine status in neonates in Denmark: Regional variations and dependency on maternal iodine supplementation. Acta Paediatr. 1994;83:578–582. doi: 10.1111/j.1651-2227.1994.tb13085.x. [DOI] [PubMed] [Google Scholar]
- 50.Ordookhani A., Pearce E.N., Hedayati M., Mirmiran P., Salimi S., Azizi F., Braverman L.E. Assessment of thyroid function and urinary and breast milk iodine concentrations in healthy newborns and their mothers in Tehran. Clin. Endocrinol. 2007;67:175–179. doi: 10.1111/j.1365-2265.2007.02857.x. [DOI] [PubMed] [Google Scholar]
- 51.Osei J., Andersson M., van der Reijden O., Dold S., Smuts C.M., Baumgartner J. Breast-milk iodine concentrations, iodine status, and thyroid function of breastfed infants aged 2–4 months and their mothers residing in a south African township. J. Clin. Res. Pediatr. Endocrinol. 2016;8:381. doi: 10.4274/jcrpe.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Osei J., Baumgartner J., Rothman M., Matsungo T.M., Covic N., Faber M., Smuts C.M. Iodine status and associations with feeding practices and psychomotor milestone development in six-month-old South African infants. Matern. Child. Nutr. 2017;13:e12408. doi: 10.1111/mcn.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pal N., Samanta S.K., Chakraborty A., Chandra N.K., Chandra A.K. Interrelationship between iodine nutritional status of lactating mothers and their absolutely breast-fed infants in coastal districts of Gangetic West Bengal in India. Eur. J. Pediatr. 2018;177:39–45. doi: 10.1007/s00431-017-3025-6. [DOI] [PubMed] [Google Scholar]
- 54.Pearce E.N., Leung A.M., Blount B.C., Bazrafshan H.R., He X., Pino S., Valentin-Blasini L., Braverman L.E. Breast milk iodine and perchlorate concentrations in lactating Boston-area women. J. Clin. Endocrinol. Metab. 2007;92:1673–1677. doi: 10.1210/jc.2006-2738. [DOI] [PubMed] [Google Scholar]
- 55.Petersen E., Thorisdottir B., Thorsdottir I., Gunnlaugsson G., Arohonka P., Erlund I., Gunnarsdottir I. Iodine status of breastfed infants and their mothers’ breast milk iodine concentration. Matern. Child. Nutr. 2020;16:e12993. doi: 10.1111/mcn.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pongpaew P., Supawan V., Tungtrongchitr R., Phonrat B., Vudhivai N., Chantaranipapong Y., Kitjaroentham A., Jintaridhi P., Intarakhao C., Mahaweerawat U. Urinary iodine excretion as a predictor of the iodine content of breast milk. J. Med. Assoc. Thail. 1999;82:284–289. [PubMed] [Google Scholar]
- 57.Prpić M., Franceschi M., Vidranski V., Andersson M., Zimmermann M.B., Hunziker S., Milošević M., Kusić Z., Jukić T. Iodine status and thyroid function in lactating women and infants—A survey in the Zagreb area, Croatia. Acta Clin. Croat. 2021;60:259–266. doi: 10.20471/acc.2021.60.02.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Samson L., Hircsu I., Katko M., Bodor M., Nagy E.V. Lower educational status interferes with maternal iodine intake during both pregnancy and lactation. Endocr. Connect. 2021;10:742–749. doi: 10.1530/EC-21-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skeaff S.A., Ferguson E.L., McKenzie J.E., Valeix P., Gibson R.S., Thomson C.D. Are breast-fed infants and toddlers in New Zealand at risk of iodine deficiency? Nutrition. 2005;21:325–331. doi: 10.1016/j.nut.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Stinca S., Andersson M., Herter-Aeberli I., Chabaa L., Cherkaoui M., El Ansari N., Aboussad A., Weibel S., Zimmermann M.B.J. Moderate-to-severe iodine deficiency in the “first 1000 days” causes more thyroid hypofunction in infants than in pregnant or lactating women. J. Nutr. 2017;147:589–595. doi: 10.3945/jn.116.244665. [DOI] [PubMed] [Google Scholar]
- 61.Sukkhojaiwaratkul D., Mahachoklertwattana P., Poomthavorn P., Panburana P., Chailurkit L.O., Khlairit P., Pongratanakul S. Effects of maternal iodine supplementation during pregnancy and lactation on iodine status and neonatal thyroid-stimulating hormone. J. Perinatol. 2014;34:594–598. doi: 10.1038/jp.2014.62. [DOI] [PubMed] [Google Scholar]
- 62.Vermiglio F., Presti V.L., Finocchiaro M., Battiato S., Grasso L., Ardita R., Mancuso A., Trimarchi F. Enhanced iodine concentrating capacity by the mammary gland in iodine deficient lactating women of an endemic goiter region in Sicily. J. Endocrinol. Investig. 1992;15:137–142. doi: 10.1007/BF03348681. [DOI] [PubMed] [Google Scholar]
- 63.Wang W., Sun Y., Zhang M., Zhang Y., Chen W., Tan L., Shen J., Zhao Z., Lan S., Zhang W. Breast milk and infant iodine status during the first 12 weeks of lactation in Tianjin City, China. Asia Pac. J. Clin. Nutr. 2018;27:393–398. doi: 10.6133/apjcn.062017.03. [DOI] [PubMed] [Google Scholar]
- 64.Wang Y., Zhang Z., Ge P., Wang Y., Wang S. Iodine status and thyroid function of pregnant, lactating women and infants (0–1 yr) residing in areas with an effective Universal Salt Iodization program. Asia Pac. J. Clin. Nutr. 2009;18:34–40. [PubMed] [Google Scholar]
- 65.Yan Y.Q., Chen Z.P., Yang X.M., Liu H. Attention to the hiding iodine deficiency in pregnant and lactating women after universal salt iodization: A multi-community study in China. J. Endocrinol. Investig. 2005;28:547–553. doi: 10.1007/BF03347244. [DOI] [PubMed] [Google Scholar]
- 66.Bouhouch R.R., Bouhouch S., Cherkaoui M., Aboussad A., Stinca S., Haldimann M., Andersson M., Zimmermann M.B. Direct iodine supplementation of infants versus supplementation of their breastfeeding mothers: A double-blind, randomised, placebo-controlled trial. Lancet Diabetes Endocrinol. 2014;2:197–209. doi: 10.1016/S2213-8587(13)70155-4. [DOI] [PubMed] [Google Scholar]
- 67.Eriksen K.G., Andersson M., Hunziker S., Zimmermann M.B., Moore S.E. Effects of an Iodine-Containing Prenatal Multiple Micronutrient on Maternal and Infant Iodine Status and Thyroid Function: A Randomized Trial in the Gambia. Thyroid. 2020;30:1355–1365. doi: 10.1089/thy.2019.0789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gebreegziabher T., Stoecker B.J. Comparison of two sources of iodine delivery on breast milk iodine and maternal and infant urinary iodine concentrations in southern Ethiopia: A randomized trial. Food Sci. Nutr. 2017;5:921–928. doi: 10.1002/fsn3.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nazeri P., Mirmiran P., Tahmasebinejad Z., Hedayati M., Delshad H., Azizi F. The effects of iodine fortified milk on the iodine status of lactating mothers and infants in an area with a successful salt iodization program: A randomized controlled trial. Nutrients. 2017;9:180. doi: 10.3390/nu9020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stoutjesdijk E., Schaafsma A., Dijck-Brouwer D.A.J., Muskiet F.A.J. Iodine status during pregnancy and lactation: A pilot study in the Netherlands. Neth. J. Med. 2018;76:210–217. [PubMed] [Google Scholar]
- 71.Dunn J.T., Crutchfield H.E., Gutekunst R., Dunn A. Methods for Measuring Iodine in Urine. Thyroid. 1993;3:119–123. doi: 10.1089/thy.1993.3.119. [DOI] [PubMed] [Google Scholar]
- 72.Dold S., Baumgartner J., Zeder C., Krzystek A., Osei J., Haldimann M., Zimmermann M.B., Andersson M. Optimization of a new mass spectrometry method for measurement of breast milk iodine concentrations and an assessment of the effect of analytic method and timing of within-feed sample collection on breast milk iodine concentrations. Thyroid. 2016;26:287–295. doi: 10.1089/thy.2015.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azizi F., Smyth P. Breastfeeding and maternal and infant iodine nutrition. Clin. Endocrinol. 2009;70:803–809. doi: 10.1111/j.1365-2265.2008.03442.x. [DOI] [PubMed] [Google Scholar]
- 74.Leung A.M., Braverman L.E., He X., Heeren T., Pearce E.N. Breastmilk iodine concentrations following acute dietary iodine intake. Thyroid. 2012;22:1176–1180. doi: 10.1089/thy.2012.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Etling N., Padovani E., Fouque F., Tato L. First-month variations in total iodine content of human breast milks. Early Hum. Dev. 1986;13:81–85. doi: 10.1016/0378-3782(86)90101-5. [DOI] [PubMed] [Google Scholar]
- 76.Kirk A.B., Dyke J.V., Martin C.F., Dasgupta P.K. Temporal patterns in perchlorate, thiocyanate, and iodide excretion in human milk. Environ. Health Perspect. 2007;115:182–186. doi: 10.1289/ehp.9558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao A., Ning Y., Zhang Y., Yang X., Wang J., Li W., Wang P. Mineral compositions in breast milk of healthy Chinese lactating women in urban areas and its associated factors. Chin. Med. J. 2014;127:2643–2648. [PubMed] [Google Scholar]
- 78.Dohan O., De la Vieja A., Paroder V., Riedel C., Artani M., Reed M., Ginter C.S., Carrasco N. The sodium/iodide symporter (NIS): Characterization, regulation, and medical significance. Endocr. Rev. 2003;24:48–77. doi: 10.1210/er.2001-0029. [DOI] [PubMed] [Google Scholar]
- 79.Manousou S., Augustin H., Eggertsen R., Hulthén L., Filipsson Nyström H. Inadequate iodine intake in lactating women in Sweden: A pilot 1-year, prospective, observational study. Acta Obstet. Gynecol. Scand. 2021;100:48–57. doi: 10.1111/aogs.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nazeri P., Kabir A., Dalili H., Mirmiran P., Azizi F. Breast-milk iodine concentrations and iodine levels of infants according to the iodine status of the country of residence: A systematic review and meta-analysis. Thyroid. 2018;28:124–138. doi: 10.1089/thy.2017.0403. [DOI] [PubMed] [Google Scholar]
- 81.Siro S.S., Baumgartner J., Schoonen M., Ngounda J., Malan L., Symington E.A., Smuts C.M., Zandberg L. Characterization of genetic variants in the SLC5A5 gene and associations with breast milk iodine concentration in lactating women of African descent: The NUPED Study. Front. Nutr. 2021;8:692504. doi: 10.3389/fnut.2021.692504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zimmermann M., Braverman L., Cooper S. Werner Ingbar’s the Thyroid: A Fundamental Clinical Text. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2012. Iodine deficiency and endemic cretinism; pp. 217–241. [Google Scholar]
- 83.Fisher W., Wang J., George N.I., Gearhart J.M., McLanahan E.D. Dietary iodine sufficiency and moderate insufficiency in the lactating mother and nursing infant: A computational perspective. PLoS ONE. 2016;11:e0149300. doi: 10.1371/journal.pone.0149300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leung A.M., Pearce E.N., Braverman L.E. Iodine nutrition in pregnancy and lactation. Endocrinol. Metab. Clin. 2011;40:765–777. doi: 10.1016/j.ecl.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bruhn J.C., Franke A.A. Iodine in human milk. J. Dairy Sci. 1983;66:1396–1398. doi: 10.3168/jds.S0022-0302(83)81950-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.