Abstract
Maltose and maltotriose are the two most abundant fermentable sugars in brewer’s wort, and the rate of uptake of these sugars by brewer’s yeast can have a major impact on fermentation performance. In spite of this, no information is currently available on the genetics of maltose and maltotriose uptake in brewing strains of yeast. In this work, we studied 30 brewing strains of yeast (5 ale strains and 25 lager strains) with the aim of examining the alleles of maltose and maltotriose transporter genes contained by them. To do this, we hybridized gene probes to chromosome blots. Studies performed with laboratory strains have shown that maltose utilization is conferred by any one of five unlinked but highly homologous MAL loci (MAL1 to MAL4 and MAL6). Gene 1 at each locus encodes a maltose transporter. All of the strains of brewer’s yeast examined except two were found to contain MAL11 and MAL31 sequences, and only one of these strains lacked MAL41. MAL21 was not present in the five ale strains and 12 of the lager strains. MAL61 was not found in any of the yeast strains. In three of the lager strains, there was evidence that MAL transporter gene sequences occurred on chromosomes other than those known to carry MAL loci. Sequences corresponding to the AGT1 gene, which encodes a transporter of several α-glucosides, including maltose and maltotriose, were detected in all but one of the yeast strains. Homologues of AGT1 were identified in three of the lager strains, and two of these homologues were mapped, one to chromosome II and the other to chromosome XI. AGT1 appears to be a member of a family of closely related genes, which may have arisen in brewer’s yeast in response to selective pressure.
The three major fermentable sugars found in brewer’s wort are glucose and the α-glucosides maltose and maltotriose. Maltose is by far the most abundant of these sugars, typically accounting for 50 to 60% of the total fermentable sugar in an all-malt wort (8); glucose and maltotriose account for 10 to 15 and 15 to 20%, respectively. Sucrose and fructose are also found in wort but at much lower levels (1 to 2%) (8). Of the three major wort sugars, glucose is preferentially utilized by brewing strains of yeast (Saccharomyces cerevisiae or Saccharomyces pastorianus) (23), but efficient fermentation requires rapid and complete utilization of both maltose and maltotriose. Gene dosage studies performed with laboratory strains of yeast have shown that the transport of maltose into the cell may be the rate-limiting step in the utilization of this sugar (12). In addition, constitutive expression of a maltose transporter gene in a lager strain of yeast has been found to accelerate the fermentation of maltose during high-gravity brewing (17). Information on the maltose and maltotriose transporter genes present in brewer’s yeast may therefore be of some value in selecting suitable strains and in predicting fermentation performance.
Brewing strains of yeast are polyploid, aneuploid, or, in the case of lager strains, alloploid (reviewed in references 13 and 16). Such strains sporulate poorly, and even when spores can be obtained, they are frequently not viable. In rare cases, spores may germinate, but the vegetative cells lack the ability to mate. Consequently, genetic analysis of brewing strains of yeast by classical methods has been severely hampered. Advances in the molecular genetics of yeast, including the complete sequencing of the genome (10, 11), have provided an opportunity to examine in more detail the genetic constitution of brewing strains of yeast.
Maltose utilization in yeast is conferred by any one of five MAL loci, MAL1 to MAL4 and MAL6 (reviewed in reference 26). Each locus consists of three genes; gene 1 encodes a maltose transporter, gene 2 encodes a maltase (α-glucosidase), and gene 3 encodes a transcriptional activator of the other two genes. Thus, for example, the maltose transporter gene at the MAL6 locus is designated MAL61. The five MAL loci each map to a different yeast chromosome, as follows: MAL1, chromosome VII; MAL2, chromosome III; MAL3, chromosome II; MAL4, chromosome XI; and MAL6, chromosome VIII. The MAL loci exhibit a very high degree of homology and are telomere linked, suggesting that they evolved by translocation from telomeric regions of different chromosomes (18). Since a fully functional or partial allele of the MAL1 locus is found in all strains of S. cerevisiae, this locus has been proposed as the progenitor of the other MAL loci (5).
Han et al. (14) have described a yeast gene, AGT1, which encodes a general α-glucoside transporter that is capable of taking up maltotriose, isomaltose, α-methylglucoside, palatinose, trehalose, and melezitose in addition to maltose (and turanose). AGT1 is an allele of MAL11 on chromosome VII. The AGT1 protein is 57% identical to the MAL61-encoded maltose transporter MAL61 and thus far is the only S. cerevisiae protein that has been demonstrated to be a transporter of maltotriose.
Sequencing of the yeast genome has revealed two other open reading frames (ORFs) that encode products with strong homology to MAL61. These ORFs are YDL247w and YJR160c, which are located on chromosomes IV and X, respectively (20). Although no results of a functional analysis of the genes or their products have been published, it is quite possible that YDL247w and YJR160c could play some role in α-glucoside transport.
In this work, we surveyed brewing strains of yeast for the presence of maltose and maltotriose transporter gene sequences. In addition to identifying alleles of known α-glucoside transporter genes, we also obtained evidence that related sequences map to other chromosomes in some of the genomes examined.
MATERIALS AND METHODS
Yeast strains.
Five ale strains of yeast (S. cerevisiae KVL011 to KVL015) and 25 lager strains (S. pastorianus KVL001 to KVL010 and KVL016 to KVL030) were included in this work. All of these strains are currently used in beer production at a number of different brewery sites. Three of the lager strains (KVL028 to KVL030) originated from the same brewery, but their relationship to one another is not known. KVL025 is a single-colony isolate obtained from KVL007; these are the only two strains used in this study that are known to be very closely related or possibly identical.
PFGE.
Yeast strains were propagated in yeast extract-peptone-glucose broth containing (per liter of distilled water) 10 g of yeast extract (Difco), 20 g of Bacto Peptone (Difco), and 40 g of glucose. For preparation of chromosomes, strains were initially grown with shaking at 25°C for 48 h in 100 ml of broth and then propagated twice (24 h each) in the same broth. Cells of each strain were harvested by centrifugation at 3,000 × g for 5 min and washed once with 8 ml of spheroplasting buffer (1.2 M sorbitol, 10 mM Tris-HCl, 10 mM CaCl2; pH 7.5) before they were resuspended in 6 ml of the same buffer. Following addition of Zymolyase 100-T (0.2 ml of a 5-mg ml−1 suspension; Seikagaku America, Ijamsville, Md.), the yeast cells were incubated for 1 h at 37°C. An aliquot (1 ml) of the spheroplast suspension was mixed with an equal volume of 1.5% (wt/vol) low-melting-point agarose (Sigma) dissolved in TES buffer (10 mM Tris-HCl [pH 7.5], 10 mM NaCl, 1 mM EDTA) containing 10.3% (wt/vol) sucrose. This mixture was dispensed into a mold (Pharmacia Biotech) in order to produce small blocks that were used in pulsed-field gel electrophoresis (PFGE) and allowed to solidify. The blocks were then immersed in a protease solution (5 mg of pronase E [Sigma] per ml, 1% [wt/vol] N-laurylsarcosine, 500 mM EDTA; pH 8) and incubated at 45°C overnight. After the blocks were washed twice (1 h each) at 50°C with TE buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA), between one-quarter and one-half of each block was transferred to a 1.2% (wt/vol) NA-agarose (Pharmacia Biotech) gel. PFGE was performed at 10°C in TBE buffer (45 mM Tris base, 44 mM boric acid, 1 mM EDTA; pH 7.5) by using a Gene Navigator pulsed-field system (Pharmacia Biotech) with the following settings: 100 to 120 mA; 165 V; and a 90-s pulse for 14 h, a 105-s pulse for 12 h, and a 120-s pulse for 14 h. Yeast DNA PFGE markers (Pharmacia Biotech) were used for molecular weight calibration. Following electrophoresis, the gel was stained with ethidium bromide in TBE buffer and photographed.
Chromosome blotting and hybridization.
The chromosomes separated by PFGE were transferred to a nylon membrane (Boehringer Mannheim) by using a VacuGene XL blotting system (Pharmacia Biotech) as recommended by the manufacturer. Following this transfer, the DNA was UV cross-linked to the nylon membrane. For hybridization, the membrane was initially incubated for 1 h at 67°C in a solution containing 5× SSC, 0.1% (wt/vol) N-laurylsarcosine, 0.02% (wt/vol) sodium dodecyl sulfate (SDS), and 2% (wt/vol) blocking reagent (Boehringer Mannheim) (1× SSC is 0.15 M NaCl plus 15 mM sodium citrate). The denatured, digoxigenin (DIG)-labeled probe (10 ng) was then added to the solution, and the preparation was incubated at 67°C overnight. The membrane was washed twice (5 min each) at room temperature with 2× SSC–0.1% (wt/vol) SDS and then twice (15 min each) at 67°C with 0.1× SSC–0.1% (wt/vol) SDS. Chemiluminescent detection of DIG hybrids on the membrane was performed by using CSPD (Boehringer Mannheim) as directed by the manufacturer. X-ray film (Kodak BioMax MR) was exposed to the membrane for 1 to 3 h before it was developed.
Preparation of DIG-labeled probes.
DIG-labeled probes were prepared by PCR as described previously (27). Genomic DNA from yeast strain 1403-7A (MATa MAL4 MGL3 gal3 gal4 ura3; Yeast Genetic Stock Center, Berkeley, Calif.) was generally used as the template for PCR. This strain has been shown to contain the AGT1 gene (14). For production of a probe hybridizing to the MAL-encoded maltose transporter gene, plasmid pSC138 containing MAL61 (4) was used as the template. The primers used for amplification of DNA by PCR (Table 1) were designed by using the program Primer3 (http://www.genome.wi.mit.edu/cgi-bin/primer/primer3.cgi) and specifying a product length of 750 to 1,000 bp. Probes were stored at −20°C and were denatured immediately prior to use by diluting them to a volume of 0.1 ml with distilled water and heating them at 100°C for 10 min.
TABLE 1.
PCR primers used in this work
| Gene and/or ORF | Primer
|
Nucleotide sequence positions detected by probeb | |
|---|---|---|---|
| Orientationa | Sequence | ||
| MAL61c | F | 5′-GGAGCTTTCTATGCCCTGC-3′ | 361 to 1140 |
| R | 5′-TAATGATGCACCACAGGAGC-3′ | ||
| AGT1 (YGR289c) | F | 5′-TTGCTTTACAATGGATTTGGC-3′ | 842 to 1828 |
| R | 5′-CTCGCTGTTTTATGCTTGAGG-3′ | ||
| YJR160c and YDL247w | F | 5′-ATGGAAGGGTACGACACAGC-3′ | 328 to 1263 |
| R | 5′-ATACTTTGACGCCCACCAAG-3′ | ||
| BGL2 (YGR282c)d | F | 5′-TTCACAGCCTCCCAAGTTTC-3′ | 46 to 898 |
| R | 5′-AAGTGAAAACACCCCAGTGC-3′ | ||
| LYS2 (YBR115c)d | F | 5′-TTGGACAATGGCGAGGAT-3′ | 1162 to 2081 |
| R | 5′-CATTCACAGTCACCGTTTGG-3′ | ||
| SER2 (YGR208w)d | F | 5′-TCTCCCAAAAGAAACCATCG-3′ | 42 to 845 |
| R | 5′-GCAGCTTTCTGCACCTTTG-3′ | ||
| TRP3 (YKL211c)d | F | 5′-GTGCACGCTGCAACAAAC-3′ | 7 to 956 |
| R | 5′-TGTAACGAACCGTGAAACCA-3′ | ||
F, forward; R, reverse.
The numbering is from the first nucleotide of the translational start (position 1).
MAL61 was not found in the chromosome used for systematic sequencing of chromosome VIII (15) and therefore was not given an ORF designation.
The genes are located on the following chromosomes: BGL2, chromosome VII; LYS2, chromosome II; SER2, chromosome VII; and TRP3, chromosome XI.
Other methods.
Nucleotide sequences of yeast genes or ORFs were obtained from the Saccharomyces Genome Database (http://genome-www.stanford.edu/Saccharomyces/). Updates on the sizes of yeast chromosomes were obtained from the Munich Information Center for Protein Sequences (http://speedy.mips.biochem.mpg.de/mips/yeast/index.htmlx).
RESULTS
Maltose transporter genes in brewing strains of yeast.
Following separation by PFGE, chromosomes from each of the yeast strains were blotted onto nylon membranes and hybridized with the MAL61 probe. Representative results are shown in Fig. 1, and the complete results obtained for all 30 strains are summarized in Table 2. It is quite possible that some of the MAL alleles detected in the yeast strains are mutated and therefore do not encode functional products. Indeed, naturally occurring mutations at the MAL loci, in particular at MAL1, have been reported previously (19). For this reason, the genotypes shown in Table 2 should be considered tentative. Nevertheless, brewing strains of yeast must be able to take up maltose efficiently, and so it is reasonable to suppose that the majority of the alleles detected with the MAL61 probe do in fact encode a functional transporter.
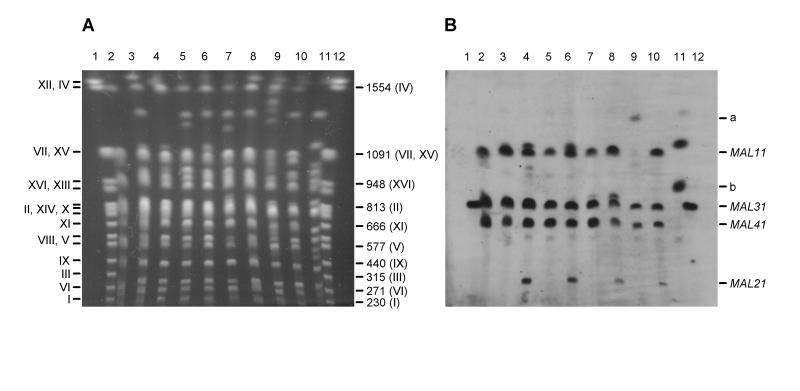
FIG. 1.
Detection of MAL transporter genes in brewing strains of yeast. (A) Separation of chromosomes of yeast strains by PFGE. (B) Detection of MAL transporter genes after chromosomes were blotted onto a nylon membrane and hybridized with a probe for MAL61. Images were obtained by scanning with a Color OneScanner 600/27 scanner operated from an Apple Macintosh and were annotated in Adobe Photoshop 3.0 (Macintosh version). The Roman numerals on the left in panel A are the chromosome numbers, in order of decreasing size where more than one numeral is given on a line. The values on the right in panel A are the sizes (in kilobase pairs) of selected chromosomes. The positions of chromosomes carrying MAL11, MAL21, MAL31, and MAL41 are indicated on the right in panel B. Bands a and b are bands for specific chromosomes, as described in the text. Lanes 1 and 12, markers; lanes 2, KVL011; lanes 3, KVL012; lanes 4, KVL001; lanes 5, KVL004; lanes 6, KVL005; lanes 7, KVL006; lanes 8, KVL018; lanes 9, KVL021; lanes 10, KVL024; lanes 11, KVL026.
TABLE 2.
MAL transporter genes in brewing strains of yeast
| Yeast strainsa
|
Genotype | |
|---|---|---|
| Category | Designation(s) | |
| Ale | KVL011, KVL012, KVL013, KVL014, KVL015 | MAL11 MAL31 MAL41 |
| Lager | KVL001, KVL002, KVL005, KVL008, KVL010, KVL017, KVL018, KVL019, KVL020, KVL024, KVL027, KVL029, KVL030 | MAL11 MAL21 MAL31 MAL41 |
| KVL003, KVL004, KVL006, KVL007, KVL009, KVL016, KVL022, KVL025, KVL028 | MAL11 MAL31 MAL41 | |
| KVL023 | MAL11 MAL31 | |
| KVL021b | MAL31 MAL41 | |
Two chromosomes that hybridized to MAL61 (at 900 and 1,150 kbp) were detected in KVL026, but the identities of these chromosomes are not known.
Strain KVL021 also contained a 1,350-kbp chromosome that hybridized to the MAL61 probe.
MAL11, MAL31, and MAL41 sequences were detected in all of the brewing strains examined except the lager yeast strains KVL021 (which lacked MAL11) and KVL026 (which lacked all three sequences) (see below). Although all of the ale strains lacked MAL21 (Fig. 1B, lanes 2 and 3), this gene was found in 13 of the 25 lager strains (Table 2). For many of these strains, the signal corresponding to MAL21 was weak (Fig. 1B, lanes 8 and 10). One explanation for this could be mutation which resulted in the loss of homology to the MAL61 probe. Alternatively, there may be fewer copies of MAL21 in the genome compared to the number of MAL11, MAL31, and MAL41 copies. We favor the second of these possibilities, as there is no obvious reason why MAL21 should have a higher level of mutation than the other MAL transporter genes have. Brewing strains of yeast are polyploid or, in the case of lager strains, alloploid (16), and different copies of the same chromosome are not necessarily expected to be identical in structure and to contain the full complement of genes. MAL61 (on chromosome VIII) was not detected in any of the strains examined.
In general, the sizes of the different chromosomes carrying the MAL genes were highly conserved in different yeast strains, although chromosome length polymorphisms were evident in some of the strains. Size doublets were clearly observed for chromosome VII (carrying MAL11) of KVL001 and KVL005 (Fig. 1B, lanes 4 and 6). In KVL001, the same doublet was detected with a probe for BGL2 (another gene mapping to chromosome VII) (results not shown), which confirmed that both chromosomes were chromosome VII. A similar test was not carried out with KVL005, but it is very likely that in this strain the doublet also resulted from two copies of chromosome VII that were different sizes (as observed for KVL001). Similarly, a doublet that may have corresponded to two copies of chromosome II (which contains MAL31) was obtained for KVL018 (Fig. 1B, lane 8). However, only the smaller chromosome in the doublet hybridized to a probe for LYS2 (which maps to chromosome II) (results not shown). The identity of the larger chromosome (length, approximately 850 kbp) is therefore unclear.
KVL021 and KVL026 were significantly different from the other lager strains with respect to the patterns of hybridization of their chromosomes to the MAL61 probe. In the case of KVL021, a chromosome with an estimated size of 1,350 kbp exhibited relatively weak hybridization to the MAL61 probe (Fig. 1B, lane 9, band a). At first, we thought that this chromosome probably corresponded to chromosome VII, which is the largest chromosome (length, 1,091 kbp) (25) known to carry a MAL locus. Even so, a substantial addition of DNA (250 kbp) would have been necessary to account for the size of the 1,350-kbp chromosome in KVL021. However, this chromosome could not be detected with probes for the BGL2 or SER2 genes, both of which map to chromosome VII (Table 1). Recently, Tamai et al. (24) identified a 1,350-kbp chromosome in S. pastorianus that appeared to originate from the non-S. cerevisiae parent (namely, Saccharomyces bayanus) (22). Translocation of MAL to the 1,350-kbp chromosome would account for the hybridization pattern which we observed. With KVL021 there was no strong hybridization at the expected position for chromosome VII (1,091 kbp) (25).
The pattern obtained for KVL026 was very different from the patterns obtained for all of the other yeast strains included in this study, both ale and lager, when chromosomes were probed with MAL61. Although the MAL61 probe detected a chromosome at 1,150 kbp (Fig. 1B, lane 11) that was thought most likely to be chromosome VII, the BGL2 probe specific for chromosome VII failed to detect this chromosome (results not shown). Another KVL026 chromosome that hybridized to the MAL61 probe migrated at approximately 900 kbp (Fig. 1B, lane 11, band b). The size of this chromosome is about 85 kbp greater than the size of chromosome II (9), the closest size match for a chromosome that carries a known MAL locus (in this case, MAL3). However, a probe for LYS2 (which maps to chromosome II) (Table 1) failed to detect the 900-kbp chromosome.
Detection of the general α-glucoside transporter gene AGT1.
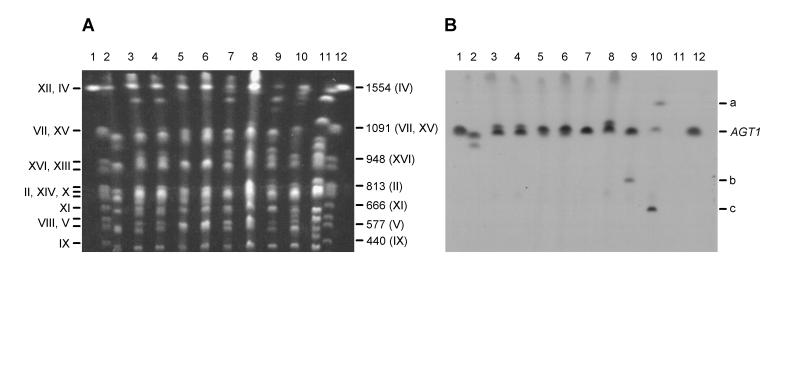
DNA that hybridized to the AGT1 probe was found in all of the yeast strains except KVL026. The results obtained for some of the strains are shown in Fig. 2. For the majority of the strains that exhibited hybridization to the AGT1 probe, the signal mapped, as expected, to chromosome VII. In the case of the five ale strains (KVL011 to KVL015) (Fig. 2B, lanes 2 to 6), two bands that migrated close together were observed. These bands were probably chromosome length polymorphisms of chromosome VII, as demonstrated for KVL001 with the BGL2 probe (see above). A similar pattern was also observed for some of the lager strains, including KVL001 (Fig. 2B, lane 8). For KVL021, a chromosome that hybridized to the AGT1 probe was detected at 1,350 kbp (Fig. 2B, lane 10, band a), as was the case for the MAL61 probe. In contrast to the findings obtained with the MAL61 probe, a copy of chromosome VII that hybridized to the AGT1 probe was also detected in KVL021 at the expected position, about 1,100 kbp (Fig. 2B, lane 10). Two of the lager strains in addition to KVL021 showed evidence of AGT1-related sequences located on other chromosomes. For KVL006, a signal was observed at approximately 860 kbp (Fig. 2B, lane 9, band b); this signal may have corresponded to either chromosome II (813 kbp) or chromosome XIII (924 kbp) (2, 9). Similarly, strong hybridization to a chromosome migrating at 670 kbp (tentatively identified as chromosome XI at 666 kbp) (6) was observed for KVL021 (Fig. 2B, lane 10, band c).
FIG. 2.
Detection of the AGT1 gene and homologues in brewing strains of yeast. (A) Separation of chromosomes of yeast strains by PFGE. (B) Detection of AGT1 after chromosomes were blotted onto a nylon membrane and hybridized with a probe for AGT1. Images were obtained by scanning with a Color OneScanner 600/27 scanner operated from an Apple Macintosh and were annotated in Adobe Photoshop 3.0 (Macintosh version). The Roman numerals on the left in panel A are the chromosome numbers, in order of decreasing size where more than one numeral is given on a line. The values on the right in panel A are the sizes (in kilobase pairs) of selected chromosomes. The position of chromosome VII carrying AGT1 is indicated on the right in panel B; bands a, b, and c are bands for specific chromosomes, as described in the text. Lanes 1 and 12, markers; lanes 2, KVL011; lanes 3, KVL012; lanes 4, KVL013; lanes 5, KVL014; lanes 6, KVL015; lanes 7, KVL024; lanes 8, KVL001; lanes 9, KVL006; lanes 10, KVL021; lanes 11, KVL026.
Detection of the maltose transporter gene homologues YDL247w and YJR160c.
YDL247w (chromosome IV) and YJR160c (chromosome X) are two ORFs that encode products that exhibit 96% identity with each other, 74% identity with MAL61, and 53% identity with AGT1. The high levels of homology exhibited by the products of YDL247w and YJR160c to MAL61 and AGT1 suggest that these proteins play a role in α-glucoside transport. The 5′ noncoding regions of YDL247w and YJR160c are more than 99% identical (for at least 2,000 nucleotides upstream of the translational start), but they lack any significant similarity to the 5′ noncoding regions of MAL61 and AGT1. This suggests that YDL247w and YJR160c may be regulated rather differently than MAL61 and AGT1 are.
Hybridization of the YDL247w-YJR160c probe to chromosome blots revealed that whereas most of the yeast strains contained nucleotide sequences corresponding to YDL247w, none contained YJR160c. Figure 3 shows the results obtained for 10 of the strains examined. As only YDL247w was detected, it is likely that this ORF represents the ancestral sequence. Only 4 of the 30 strains that were investigated (ale strains KVL011 [Fig. 3B, lane 2], KVL014, and KVL015 and lager strain KVL026 [Fig. 3B, lane 11]) lacked YDL247w.
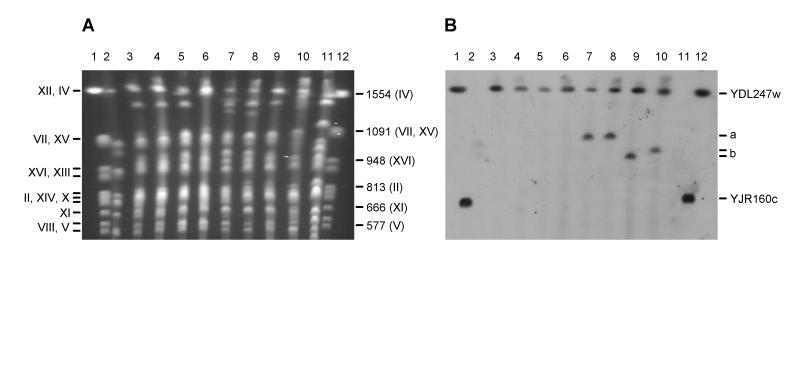
FIG. 3.
Detection of YDL247w in brewing strains of yeast. (A) Separation of chromosomes of yeast strains by PFGE. (B) Detection of MAL transporter genes after chromosomes were blotted onto a nylon membrane and hybridized with a probe for YDL247w and YJR160c. Images were obtained by scanning with a Color OneScanner 600/27 scanner operated from an Apple Macintosh and were annotated in Adobe Photoshop 3.0 (Macintosh version). The Roman numerals on the left in panel A are the chromosome numbers, in order of decreasing size where more than one numeral is given on a line. The values on the right in panel A are the sizes (in kilobase pairs) of selected chromosomes. The positions of chromosomes carrying YDL247w (and, for the markers, YJR160c) are indicated on the right in panel B; bands a and b are bands for specific chromosomes, as described in the text. Lanes 1 and 12, markers; lanes 2, KVL011; lanes 3, KVL012; lanes 4, KVL013; lanes 5, KVL024; lanes 6, KVL001; lanes 7, KVL004; lanes 8, KVL006; lanes 9, KVL018; lanes 10, KVL021; lanes 11, KVL026.
Evidence that other chromosomes exhibited strong hybridization to the YDL247w-YJR160c probe was obtained for four of the lager strains examined (KVL004, KVL006, KVL018, and KVL021) (Fig. 3B, lanes 7 to 10). In two of these strains, KVL004 and KVL006, the chromosome containing the homologous sequence is about 1,050 kbp long (Fig. 3B, band a). This chromosome most likely corresponds to either chromosome VII or chromosome XV (both of which are 1,091 kbp long) (7, 25). In KVL018 and KVL021, the chromosomes containing sequences homologous to YDL247w and YJR160c sequences are 960 and 990 kbp long, respectively (Fig. 3B, band b). The closest match to these chromosomes is chromosome XVI (length, 948 kbp) (3).
Mapping of AGT1 homologues to chromosomes.
Additional blotting and hybridization experiments were carried out to map two of the homologues identified with the AGT1 probe. Chromosomes from yeast strains KVL006 and KVL021 were subjected to PFGE in duplicate and blotted. One blot of each duplicate was hybridized with the AGT1 probe, and the other was hybridized with a gene probe that hybridized to a known chromosome (Fig. 4).
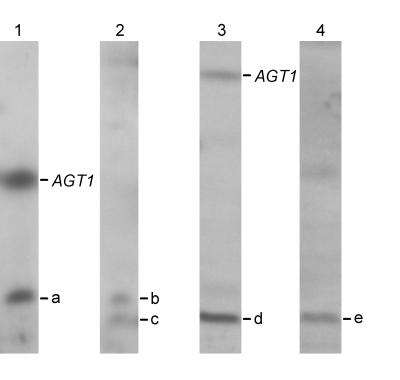
FIG. 4.
Mapping of the AGT1 homologues found in yeast strains KVL006 and KVL021. Lane 1, chromosome blot of KVL006 hybridized with a probe for AGT1; lane 2, same as lane 1 except that the blot was hybridized with a LYS2 probe; lane 3, chromosome blot of KVL021 hybridized with a probe for AGT1; lane 4, same as lane 3 except that the blot was hybridized with a TRP3 probe. Images were obtained by scanning with a Color OneScanner 600/27 scanner operated from an Apple Macintosh and were annotated in Adobe Photoshop 3.0 (Macintosh version). The position of chromosome VII containing AGT1 is indicated on the right in lanes 1 and 3. Band a, KVL006 chromosome containing a homologue of AGT1; bands b and c, KVL006 chromosome II (containing LYS2); band d, KVL021 chromosome containing a homologue of AGT1; band e, KVL021 chromosome XI (containing TRP3).
For KVL006, chromosome blots were separately hybridized with the AGT1 probe and a probe for LYS2 (a gene that is located on chromosome II). The LYS2 probe detected two copies of chromosome II whose sizes differed by about 60 kbp (Fig. 4, lane 2, bands b and c). The larger copy comigrated with the chromosome carrying the homologue of AGT1 (Fig. 4, lane 1, band a), confirming that this homologue does indeed lie on chromosome II. A similar experiment was carried out in order to map the AGT1 homologue found in KVL021 to chromosome XI. A probe for TRP3, which hybridized to chromosome XI (length, 666 kbp) (6), produced a single band on the developed blot (Fig. 4, lane 4, band e). This band comigrated with the chromosome carrying the AGT1 homologue in KVL021 (Fig. 4, lane 3, band d).
DISCUSSION
It is likely that because of the demands of brewery fermentation, brewing strains of yeast have been subjected to selection for more efficient fermentation of maltose and maltotriose. The finding that most strains examined in this study contained sequences corresponding to the maltose transporter gene at three or four different MAL loci is therefore not surprising. The complete absence of MAL61 was more unexpected. However, in a study of 28 strains of S. cerevisiae isolated from natural sources, Naumov et al. (19) identified only 1 strain that carried the MAL6 locus. Similarly, Oda and Tonomura (21) found that only one of seven baking strains of S. cerevisiae which they examined contained MAL6. These findings and the results of this study suggest that the evolution of MAL6 may have been a comparatively recent event.
A majority of the yeast strains used in this study, both ale and lager, contained MAL transporter gene sequences that mapped to chromosomes previously shown to carry MAL loci. For three of the lager strains, KVL018, KVL021, and KVL026, there is evidence that MAL transporter genes occur on other chromosomes. In the case of the 1,350-kbp chromosome of KVL021 that hybridizes to the MAL61 probe, the best size match is with a chromosome from S. bayanus rather than S. cerevisiae. Telomeric translocation of a MAL locus from a chromosome of S. cerevisiae would account for this. The most likely source of the translocated DNA is chromosome VII, since the MAL1 locus on this chromosome is thought to be the progenitor of the other MAL loci (5). The 1,350-kbp chromosome could not be detected with a probe for BGL2, which is located about 18 kbp from the MAL1 locus (23). This suggests that either the translocated DNA sequence is relatively short or it was derived from a chromosome other than chromosome VII.
With the exception of KVL026, all of the yeast strains examined in this study contained chromosomes that hybridized to the AGT1 probe. Most of these strains contained both AGT1 and MAL11 sequences on chromosome VII, and there are three possible explanations for this. One is that different copies of chromosome VII in the same strain carry either MAL11 or AGT1. Since brewing strains of yeast are polyploid or aneuploid, this is quite plausible. Alternatively, AGT1 and MAL11 may be closely linked on the same chromosome. This seems less likely since AGT1 has been shown (at least in laboratory strains of yeast) to be an allele of MAL11 (14). A third possibility, the least likely possibility, is that hybrid genes with homology to both the AGT1 and MAL61 probes are present. Cloning and sequencing of the MAL11 and AGT1 alleles and adjacent DNA would allow these possibilities to be explored.
Homologues of AGT1 were identified in two of the lager strains of yeast (KVL006 and KVL021), and these homologues were separately mapped to chromosomes II and XI. Interestingly, each of these chromosomes carries a copy of the MAL locus (MAL3 on chromosome II and MAL4 on chromosome XI). Since AGT1 is an allele of MAL11 (on chromosome VII), it is quite possible that the homologues of AGT1 identified on chromosomes II and XI are themselves alleles of MAL31 and MAL41, respectively. Cloning of the AGT1 homologues followed by DNA sequencing would have to be carried out to investigate this further.
Han et al. (14) proposed that AGT1 arose from two recombination events. The first of these events was the translocation of AGT1 sequences to the telomere of chromosome VII, and the second event brought AGT1 into position within the MAL locus (MAL1) on that chromosome. A similar set of events may have been responsible for the origin of the AGT1 homologues found on chromosomes II and XI. Alternatively, the translocations that gave rise to the spread of MAL loci from the ancestral locus on chromosome VII may also have been responsible for the dispersal of AGT1 sequences to chromosomes II and XI. Han et al. (14) speculated that AGT1 might be just one member of a gene family in Saccharomyces spp., and the findings reported here provide the first evidence that such a family of genes does indeed exist. Naumov et al. (19) were not able to detect AGT1 sequences in the yeast strains which they examined, but this is probably because they used a MAL61 probe in their hybridization analysis. As shown here, under stringent conditions there is no detectable hybridization between AGT1 and MAL-encoded transporter genes. Nevertheless, it remains to be seen how widespread AGT1 is among nonbrewing Saccharomyces strains. The presence of maltotriose in brewer’s wort may have imposed selection for AGT1 and other closely related genes in brewing strains of yeast, and such selection should not apply to many nonbrewing strains, such as wine yeast strains.
An analysis of YDL247w and YJR160c sequences in brewing strains of yeast was included in this study because it has been predicted that both of these ORFs encode products that are highly homologous to the MAL-encoded transporter. The fact that YDL247w and YJR160c were discovered not through genetic analysis of maltose utilization but by genome sequencing suggests that their products do not play a major role in maltose transport. One possible function of the proteins encoded by YDL247w and YJR160c might be in low-affinity transport of maltose, although this phenomenon has been ruled out in one study as an artifact (1). The finding that YJR160c was absent from all of the yeast strains surveyed in this study leads to the conclusion that YDL247w, which was found in all but four strains, is the progenitor sequence. Both YDL247w and YJR160c are subtelomeric, suggesting that a mechanism involving translocation of telomeric sequences (as proposed for the MAL loci) may have given rise to this gene family. The YDL247w and YJR160c homologues identified in some of the brewing strains which we examined (Fig. 3) are therefore also likely to be telomere associated.
This work was undertaken to obtain information on maltose and maltotriose transporter genes in brewing strains of yeast, because the products of these genes are expected to have a major influence on yeast fermentation performance in brewing. As expected, MAL transporter gene sequences were widespread. We also found evidence that there is a family of genes related to AGT1, which may contribute to efficient fermentation of maltotriose. Additional work involving a functional analysis of the AGT1-related sequences will be necessary to investigate this possibility.
ACKNOWLEDGMENTS
This work was supported by the FØTEK program, which was sponsored by the Danish Ministry of Education through LMC (the Centre for Advanced Food Studies) and Alfred Jørgensen Laboratory Ltd. (Copenhagen, Denmark).
REFERENCES
- 1.Benito B, Lagunas R. The low-affinity component of Saccharomyces cerevisiae maltose transport is an artifact. J Bacteriol. 1992;174:3065–3069. doi: 10.1128/jb.174.9.3065-3069.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowman, S., C. Churcher, K. Badcock, D. Brown, et al. 1997. The nucleotide sequence of Saccharomyces cerevisiae chromosome XIII. Nature 387(Suppl.):90–93. [PubMed]
- 3.Bussey, H., R. K. Storms, A. Ahmed, K. Albermann, et al. 1997. The nucleotide sequence of Saccharomyces cerevisiae chromosome XVI. Nature 387(Suppl.):103–105. [PubMed]
- 4.Chang Y S, Dubin R A, Perkins E, Michels C A, Needleman R B. Identification and characterization of the maltose permease in a genetically defined Saccharomyces strain. J Bacteriol. 1989;171:6148–6154. doi: 10.1128/jb.171.11.6148-6154.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow T, Goldenthal M J, Cohen J D, Hegde M, Marmur J. Identification and physical characterization of yeast maltase structural genes. Mol Gen Genet. 1983;191:366–371. doi: 10.1007/BF00425747. [DOI] [PubMed] [Google Scholar]
- 6.Dujon B, Alexandraki D, André B, Ansorge W, et al. Complete DNA sequence of yeast chromosome XI. Nature. 1994;369:371–378. doi: 10.1038/369371a0. [DOI] [PubMed] [Google Scholar]
- 7.Dujon, B., K. Albermann, M. Aldea, D. Alexandraki, et al. 1997. The complete nucleotide sequence of yeast chromosome XV. Nature 387(Suppl.):98–102. [PubMed]
- 8.Ernandes J R, Williams J W, Russell I, Stewart G G. Effect of yeast adaptation to maltose utilization on sugar uptake during the fermentation of brewer’s wort. J Inst Brew. 1993;99:67–71. [Google Scholar]
- 9.Feldmann H, Aigle M, Aljinovic G, André B, et al. Complete DNA sequence of yeast chromosome II. EMBO J. 1994;13:5795–5809. doi: 10.1002/j.1460-2075.1994.tb06923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goffeau A, Barrell B G, Bussey H, Davis R W, Dujon B, Feldmann H, Galibert F, Hoheisel J D, Jacq C, Johnston M, Louis E J, Mewes H W, Murakami Y, Philippsen P, Tettelin H, Oliver S G. Life with 6000 genes. Science. 1996;274:546–567. doi: 10.1126/science.274.5287.546. [DOI] [PubMed] [Google Scholar]
- 11.Goffeau, A., R. Aert, M. L. Agostini-Carbone, A. Ahmed, et al. 1997. The Yeast Genome Directory. Nature 387(Suppl.):1–105.
- 12.Goldenthal M J, Vanoni M, Buchferer B, Marmur J. Regulation of MAL gene expression in yeast: gene dosage effects. Mol Gen Genet. 1987;209:508–517. doi: 10.1007/BF00331157. [DOI] [PubMed] [Google Scholar]
- 13.Hammond J R M. Brewer’s yeasts. In: Rose A H, Harrison J S, editors. The yeasts. 5. Yeast technology. London, United Kingdom: Academic Press; 1993. pp. 7–67. [Google Scholar]
- 14.Han E-K, Cotty F, Sottas C, Jiang H, Michels C A. Characterization of AGT1 encoding a general α-glucoside transporter from Saccharomyces. Mol Microbiol. 1995;17:1093–1107. doi: 10.1111/j.1365-2958.1995.mmi_17061093.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnston M, Andrews S, Brinkman R, Cooper J, et al. Complete nucleotide sequence of Saccharomyces cerevisiae chromosome VIII. Science. 1994;265:2077–2082. doi: 10.1126/science.8091229. [DOI] [PubMed] [Google Scholar]
- 16.Kielland-Brandt M C, Nilsson-Tillgren T, Gjermansen C, Holmberg S, Pedersen M B. Genetics of brewing yeasts. In: Rose A H, Wheals A E, Harrison J S, editors. The yeasts. 6. Yeast genetics. London, United Kingdom: Academic Press; 1995. pp. 223–254. [Google Scholar]
- 17.Kodama Y, Fukui N, Ashikari T, Shibano Y, Morioka-Fujimoto K, Hiraki Y, Nakatani K. Improvement of maltose fermentation efficiency: constitutive expression of MAL genes in brewing yeasts. J Am Soc Brew Chem. 1995;53:24–29. [Google Scholar]
- 18.Michels C A, Read E, Nat K, Charron M J. The telomere-associated MAL3 locus of Saccharomyces is a tandem array of repeated genes. Yeast. 1992;8:655–665. doi: 10.1002/yea.320080809. [DOI] [PubMed] [Google Scholar]
- 19.Naumov G I, Naumova E S, Michels C A. Genetic variation of the repeated MAL loci in natural populations of Saccharomyces cerevisiae and Saccharomyces paradoxus. Genetics. 1994;136:803–812. doi: 10.1093/genetics/136.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelissen B, De Wachter R, Goffeau A. Classification of all putative permeases and other membrane multispanners of the major facilitator superfamily encoded by the complete genome of Saccharomyces cerevisiae. FEMS Microbiol Rev. 1997;21:113–134. doi: 10.1111/j.1574-6976.1997.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 21.Oda Y, Tonomura K. Detection of maltose fermentation genes in the baking yeast strains of Saccharomyces cerevisiae. Lett Appl Microbiol. 1996;23:266–268. doi: 10.1111/j.1472-765x.1996.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 22.Ryu S-L, Murooka Y, Kaneko Y. Genomic reorganization between two sibling yeast species, Saccharomyces bayanus and Saccharomyces cerevisiae. Yeast. 1996;12:757–764. doi: 10.1002/(sici)1097-0061(19960630)12:8<757::aid-yea970>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 23.Stewart G G, Erratt J, Garrison I, Goring T, Hancock I. Studies on the utilization of wort carbohydrates by brewer’s yeast strains. Tech Q Master Brew Assoc Am. 1979;16:1–7. [Google Scholar]
- 24.Tamai Y, Momma T, Yoshimoto H, Kaneko Y. Co-existence of two types of chromosomes in the bottom fermenting yeast, Saccharomyces pastorianus. Yeast. 1998;14:923–933. doi: 10.1002/(SICI)1097-0061(199807)14:10<923::AID-YEA298>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Tettelin, H., M. L. Agostoni-Carbone, K. Albermann, M. Albers, et al. 1997. The nucleotide sequence of Saccharomyces cerevisiae chromosome VII. Nature 387(Suppl.):81–84. [PubMed]
- 26.Vanoni M, Sollitti P, Goldenthal M, Marmur J. Structure and regulation of the multigene family controlling maltose fermentation in budding yeast. Prog Nucleic Acid Res Mol Biol. 1989;37:281–322. doi: 10.1016/s0079-6603(08)60701-1. [DOI] [PubMed] [Google Scholar]
- 27.Wightman P, Quain D E, Meaden P G. Analysis of production brewing strains of yeast by DNA fingerprinting. Lett Appl Microbiol. 1996;22:90–94. doi: 10.1111/j.1472-765x.1996.tb01115.x. [DOI] [PubMed] [Google Scholar]