Summary
Introduction
Surveillance of health care-associated infections (HAIs) is an essential part of an efficient healthcare system. This study is an update on incidence and mortality rates of HAIs in Iran in 2018.
Methods
Almost all hospitals across the country (940 hospitals) entered the data of HAIs and denominators to the Iranian Nosocomial Infections Surveillance (INIS) software. Statistics were derived from INIS.
Results
From 9,607,213 hospitalized patients, 127,953 suffered from HAI, 15.65% of whom died. The incidence rate of HAI was calculated as 4.2 per 1000 patient-days. Considering relative frequencies among HAIs, Pneumonia (29.1%) and UTIs (25.6%) were the most common types of infection. Ventilator-associated pneumonia (VAP) was the most frequent device-associated infection (DAI) 25.66 per 1000 ventilator-days, and had the highest mortality rate (43.08%). Incidence density of other DAIs was 5.43 for catheter-associated UTI and 2.86 for catheter-associated BSI per 1000 device-days. Medical ICUs had the highest incidence and percentage of deaths (15.35% and 37.63%, respectively). The most causative organisms were Escherichia coli, Acinetobacter baumannii, and Klebsiella pneumonia. The rate of methicillin-resistance Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria was about 49%, 57%, and 58% respectively.
Conclusion
This study provided an overview of HAIs in Iran and indicated that HAIs required special attention both in detection/reporting and in infection control measures. Future studies could be done on adherence rate of DAIs’ preventive bundles, interventions via multimodal strategies, evaluating the effect of training, and effect of antibiotic stewardship programs.
Key words: Nosocomial infections, Resistance, Surveillance, Mortality, Infection Control
Introduction
Health care-associated infections (HAIs) are amongst the major patient safety problems, which cause significant morbidity, mortality, prolonged hospitalization, and increased health care costs [1]. HAIs are infections that are acquired after admission to a hospital or during the process of care in a health care facility [2]. The prevalence of HAIs varies from 5-7% in Europe and North America to 6-20% in Sub-Saharan Africa, Latin America and parts of Asia [3-10]. Among HAIs, the upmost important and problematic ones are device-associated infections (DAIs) including ventilator-associated pneumonia (VAP), central-line bloodstream infection (CLA-BSI), and catheter-associated urinary tract infection (CA-UTI). Statistics of these infections shows an incidence density of about 5 VAPs, less than 1 CLA-BSI, and 0.5-5 CA-UTI per 1000 device-days in well controlled ICUs of developed countries vs. 20-50 VAPs, 4-12 CLA-BSIs, and about 3-8 CA-UTIs per 1000 device-days in ICUs of some developing countries [7-10].
HAIs’ causative organisms vary in different infection types and in different locations (wards, hospitals, states, and countries). Overall, the most important gram negative bacilli are Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Escherichia coli, and other Enterobacteriaceae; among gram positive cocci the most prevalent ones are Staphylococcus aureus, Staphylococcus epidermidis, and Enterococcus Spp. and at the top of fungi list are Candida albicans and non-albicans Candida (NAC) species. Antimicrobial resistance has been developed and increased gradually in these organisms during recent years and lead to a global threat [4, 5, 8-11].
Due to the complicated and multifactorial nature of HAIs, without information about the incidence of HAIs and causative organisms, effective programming for infection prevention and control (IPC) is almost impossible. In March 2007, first national nosocomial surveillance guideline was specifically established by the Iranian Center for Communicable Disease Control (ICDC) to report and control HAIs and a software named Iranian Nosocomial Infection Surveillance (INIS) was designed to facilitate data registry and management [6, 12-15]. Under- and over-reporting of HAIs are main challenges in Iran. The results of a blinded retrospective review of ICUs’ medical records in Iran revealed 57.3%-82.2% under-reporting and 8%-15% over-reporting of four types of HAIs [14, 16].
In order to prevent HAIs, it is crucial that physicians, healthcare providers, scientists, and health care authorities have access to information on data at the country level, which can help to develop newer guidelines, make better decisions and necessary modifications. Therefore, we aimed to report the updated data on national surveillance of HAIs regarding the incidence and mortality in Iran.
Methods
Almost all hospitals in Iran (940 hospitals) participated in this study and entered the data of HAIs and denominators to the Iranian Nosocomial Infections Surveillance (INIS) software. This national software has been used by ICDC since the first year of implementation of the surveillance program in 2007 (last update: 2017). Registry is performed on a monthly basis upon completion of individual hospital forms. The forms are completed by a trained infection control nurse who has been assigned to the program in each hospital. The criteria for diagnosis of HAIs are per advice of the ICDC guidelines which are based on the CDC/NHSN case-definitions criteria.
DEFINITIONS
The HAIs’ diagnostic criteria were as follows [12]. BSI was defined as having a positive blood culture of a known BSI pathogen in one or more blood samples such that the growing organism was not related to infection in another site; or at least one of the following signs or symptoms (fever, chills, or hypotension [Aged ≤1 year: fever, hypothermia, apnea, or bradycardia]). In addition, common commensals were cultured from two or more blood samples drawn on separate occasions.
Symptomatic UTI was defined as fever (T >38C), urgency, frequency, dysuria, suprapubic tenderness, or costovertebral angle pain/tenderness with a positive urine culture of ≥105 CFU/mL of no more than two isolated species. Asymptomatic bacteremic UTI was defined as no urinary symptoms and both urine culture and blood culture were positive with an uropathogen.
SSI was defined as purulent discharge from the surgical incision, organisms isolated from an aseptically obtained culture, an abscess involving the deep incision which is found on direct examination, during reoperation, or by histopathologic or radiologic examination; or one of signs or symptoms of infection (pain or tenderness, swelling, redness, or heat), and diagnosis of SSI by the surgeon or attending physician within 30 days of the surgery, or within 90 days for some specific surgeries including ones involving implants.
For Clinically Defined Pneumonia, chest radiographs with one of the following findings (new or progressive and persistent infiltrates, consolidation, cavitation, or pneumatocele [Aged ≤1 year]) are needed plus one of the following (fever, leukopenia or leukocytosis, or altered mental status [Aged >70 years]) and at least two of the following: purulent sputum, or change in character of sputum, or increased respiratory secretions, cough, or dyspnea, or tachypnea, rales or bronchial breath sounds, or worsening gas exchange. Furthermore, for Pneumonia with Specific Laboratory Findings, there needed to be a positive culture (from respiratory secretions, pleural fluid, lung tissue, or blood) or histopathologic evidence of infection such as abscess formation or foci of consolidation with polymorphonuclear cell accumulation in bronchioles and alveoli. For Pneumonia in Immunocompromised Patients, in addition to the radiologic findings and signs/symptoms mentioned above, hemoptysis or pleuritic chest pain were also considered as symptoms; and additional criteria were matching positive blood and sputum cultures with Candida spp, or evidence of fungi or Pneumocystis carinii from pulmonary-derived specimen.
And finally, the Ventilator-Associated Event defined as at least 20% increase in the minFiO2 or a minimum increase of 3 cm-H2O in the PEEP (positive end-expiratory pressure) to maintain oxygenation for a sustained period of more than 2 days (VAC: Ventilator-associated condition). And it happened in the setting of an infection (fever, leukocytosis, etc.) and antibiotics are instituted for a minimum of 4 days (IVAC: Infection related VAC). And the detection of respiratory pathogens on cultures or by equivalent techniques (PVAP: Possible VAP).
DATA ACQUISITION AND STATISTICAL ANALYSIS
We obtained our data, including demographic features (such as age, gender, ward, date of admission, and date of discharge/death), diagnoses, devices data, microbiologic studies, by using the standard checklists which then entered to INIS system. We acquired items of interest that included the number of hospitalizations, diagnosed HAIs, and deaths. All retrieved forms were finally analyzed by the Iranian Center for Communicable Disease Control (ICDC).
ETHICAL CONSIDERATIONS
This study was approved by the ICDC Research Council and all data were registered under the supervision of the Iran Ministry of Health and Medical Education.
Results
12-month HAIs surveillance reported from 940 hospitals (out of 999 hospitals in total [94% coverage]) were registered in the INIS system during the year 2018. From 9,607,213 hospitalized patients, 127,953 were diagnosed with HAI (cumulative incidence = 1.33%) which was 13.4% higher compared to 2017. This number varied amongst different medical universities (0.14%-3.41%), hospitals (0.01%-33.45%), and departments (0.15%-15.35%). In addition, 30,559,894 patient-days were registered and incidence rate of HAI was calculated as 4.2 per 1000 patient-days (Tab. I).
Tab. I.
Summary of health care-associated infections (HAIs) in Iran, 2018.
| Pneumonia* | UTI | BSI | SSI | Others | Total | |
|---|---|---|---|---|---|---|
| Frequency (Number) | 37234 | 32756 | 14843 | 27894 | 15226 | 127953 |
| Relative Frequency (% of total infections) | 29.1 | 25.6 | 11.6 | 21.8 | 11.9 | 100 |
| Incidence (% in 100 admissions) | 0.38 | 0.34 | 0.15 | 0.29 | 0.16 | 1.33 |
| Incidence (in 1000 patient-days) | 1.2 | 1.1 | 0.5 | 0.9 | 0.5 | 4.2 |
| Crude Mortality Rate (%) | 28.6 | 12.7 | 20.5 | 3.6 | 8.0 | 15.65 |
* Pneumonia: including ventilator-associated pneumonia (VAP) and non-VAP pneumonia. UTI: Urinary Tract Infection; BSI: Blood stream infection; SSI: Surgical site infection; Others: Other than 4 major infections.
Considering relative frequencies among HAIs, the most common was pneumonia (29.1%) followed by UTIs (25.6%), SSIs (21.8%), and BSIs (11.6%). Although pneumonia was the most frequent HAI, age-adjusted rates revealed that SSI was the most common HAI in ages 5-44. Among device-associated infections, 25.66 ventilator-associated pneumonias (VAPs), 5.43 catheter-associated urinary tract infections (CA-UTIs), and 2.86 catheter-associated bloodstream infections (CA-BSI) per 1000 device-days were identified. The highest incidence of HAI were reported from medical ICUs (15.35%) followed by burn units (11.98%), general ICUs (10.89%) and transplant units (8.85%). Additional information is shown in Figure 1.
Fig. 1.
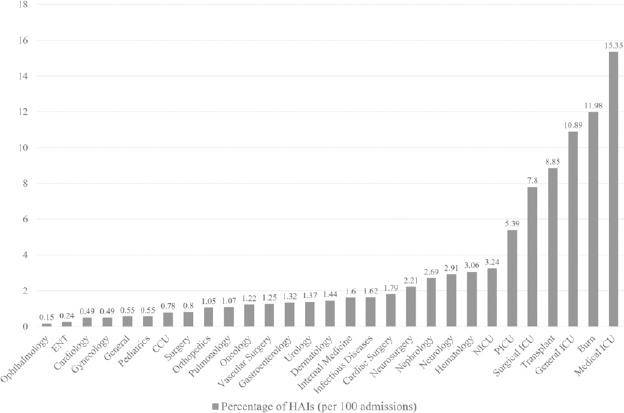
Incidence of health care-associated infections (HAIs) in different wards in Iran, 2018.
In 28% of cases, infection-causing pathogen was not identified (Fig. 2). This number was 58% in SSIs. The most frequent microorganisms reported in cultures were Escherichia coli, Acinetobacter baumannii and Klebsiella pneumonia (15.29%, 9.47%, and 6.91%, respectively). The most common germs of the four main infections separately were: Pneumonia/VAP (Acinetobacter baumannii, Klebsiella pneumonia, and Pseudomonas aeruginosa), UTI (Escherichia coli, Klebsiella pneumonia, and Candida Spp.), BSI (Staphylococcus epidermidis, Staphylococcus aureus, and Escherichia coli), and SSI (Escherichia coli, Staphylococcus aureus, and Acinetobacter baumannii)
Fig. 2.
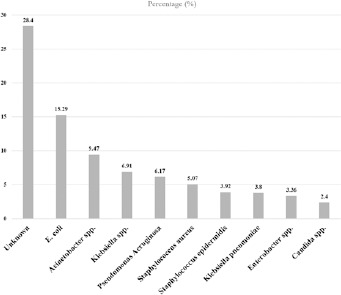
Microorganisms in health care-associated infections (HAIs) in Iran, 2018.
Antimicrobial resistance pattern was shown in Table II. The rate of methicillin-resistance Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus (VRE), and Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria was about 49%, 57%, and 58% respectively. Extended spectrum beta-lactamase (ESBL)-producing gram negative bacilli were reported with a wide range of 35-95% from different centers.
Tab. II.
Antimicrobial resistance pattern of main microorganisms in HAIs in Iran, 2018.
| Microorganism | Antibiotic | Resistance (%) | Interpretation |
|---|---|---|---|
| Staphylococcus aureus | Oxacillin / Cefoxitin | 49.29 | MRSA |
| Clindamycin | 59.77 | ||
| Vancomycin | 0.04 | ||
| Enterococcus spp. | Ampicillin | 55.88 | |
| Vancomycin | 56.56 | VRE | |
| Linezolid | 0.76 | ||
| Klebsiella pneumonia | 3rd or 4th generation cephalosporin | 80.41 | ESBL-producing |
| Fluoroquinolone | 68.19 | ||
| Beta-lactamase inhibitor | 71.63 | ||
| Carbapenem | 57.83 | KPC-producing | |
| Escherichia coli | 3rd or 4th generation cephalosporin | 70.16 | ESBL |
| Fluoroquinolone | 62.69 | ||
| Beta-lactamase inhibitor | 33.96 | ||
| Carbapenem | 21.45 | ||
| Pseudomonas Aeruginosa | Ceftazidime | 57.75 | |
| Fluoroquinolone | 56.57 | ||
| Aminoglycoside | 54.97 | ||
| Piperacillin/Tazobactam | 54.55 | ||
| Carbapenem | 60.06 | ||
| Acinetobacter baumannii | Ceftazidime | 93.76 | |
| Fluoroquinolone | 92.82 | ||
| Aminoglycoside | 89.18 | ||
| Ampicillin/Sulbactam | 68.05 | ||
| Carbapenem | 93.02 | ||
| Colistin | 3.81 |
HAI: Health care associated infection; MRSA: Methicillin-resistance Staphylococcus aureus; VRE: Vancomycin-resistant Enterococcus; ESBL: Extended spectrum beta-lactamase; KPC: Klebsiella pneumoniae carbapenemase.
Overall crude mortality rate due to HAIs was 15.65%. The highest percentage of deaths was reported from medical ICUs (37.63%), general ICU (31%) and Surgical ICUs (30%).
Discussion
The incidence of health care-associated infections was measured at 940 hospitals in the Islamic Republic of Iran in 2018, with an average reported 1.33% nationwide. Calculation and evaluation the incidence density of HAIs per 1000 patient-days in Iran is also carried out following the recommendation of the WHO, which has been reported 4.2 per 1000 patient-days during the mentioned period of time. Although pneumonia was more common overall followed by UTIs, surveys of infections by age groups showed that SSI is the most prevalent infection at ages 5 to 44 years. In addition, the most common devices-related infections were ventilator and urinary catheter associated infections, respectively. Escherichia coli, Acinetobacter baumannii and Klebsiella pneumonia were known as the most common causative agents. As we expected, ICUs had the highest incidence of HAIs and the highest death rate.
HAI is one of the most important threatening factors to patient safety that lead to important complications, including increased mortality, delay in surgical wound healing, occupation of hospital beds, increased hospitalization time, increased costs, increased use of antibiotics, development of antimicrobial resistance, and adverse psychological effects on patients and their families [17].
According to the WHO report in 2011, the prevalence of nosocomial infections in Iran (1990-2010) expected to be 8.8% [18] that was confirmed by well-designed studies in the country [19-21]. In the Iranian CDC national report in 2015, the nationwide incidence rate was reported to be 1.18% [16], likewise 1.33% in the current report. According to the study conducted on the accuracy of the routine surveillance system in Iran, these low estimates may be due to the weakness of system in post-discharge surveillance, insufficient personnel training, misinterpretation of HAIs’ definitions, high workload of infection control nurses, and low-activity of infection control link-nurses in the wards [14].
Pneumonia in this study (accounted for 29.1% of HAIs) was the most common infection; a finding in contrast to previous national study and a number of other studies in Iran that represented UTI was the most prevalent [16, 20]. The reason for the increase in pneumonia rank in Iran can be the decrease in adherence to pneumonia/VAP preventive bundle in most hospitals (compared to urinary-catheter and CV-line) in recent years. Therefore, the evaluation of nationwide adherence to DAIs’ preventive bundles can be a good topic for future research.
On device-associated infections (DAIs), Tab. III shows a comparison among this national study, CDC/NHSN [7], European CDC [8], International Nosocomial Infection Control Consortium (INICC) [10], and a multi-center study at Tehran University of Medical Sciences (TUMS) [21].
Tab. III.
Comparison of DAIs’ Incidence density per 1000 device-days among this study, CDC/NHSN. ECDC, INICC, and TUMS multi-center study.
| This study 2018 | CDC/NHSN 2019 | ECDC 2017 | INICC 2012-2017 | TUMS 2014 | |
|---|---|---|---|---|---|
| CLA-BSI | 2.86 | 0.84 | 3.7 | 5.05 | 10.20 |
| VAP | 25.66 | 4.49 | 9.5 | 14.1 | 21.08 |
| CA-UTI | 5.43 | 0.78 | 3.6 | 5.1 | 7.42 |
DAI: Device-associated Infection; CDC/NHSN: Centers for Disease Control and Prevention, National Healthcare Safety Network; ECDC: European Centre for Disease Prevention and Control; INICC: International Nosocomial Infection Control Consortium; TUMS: Tehran University of Medical Sciences; CLA-BSI: Central-line Bloodstream Infection; VAP: Ventilator-associated Pneumonia; CA-UTI: Catheter-associated Urinary Tract Infection.
The first thing that comes to mind by the above table is that the rate of VAP was much higher in Iran than developed countries and even INICC report [7, 8, 10]. As mentioned earlier, the reason might be poor adherence to VAP preventive bundle in most hospitals; and it states that more attention should be paid to the implementation of the pneumonia preventive bundle by using multimodal strategies includes training ICUs’ staff, supervising, and etc. About CA-UTI, the rate in Iran was like other developing countries as the summarized report of the INICC showed [10]; however, more than developed countries as expected [7, 8]. Although the rate of CLA-BSI seemed relatively low in Iran, the authors of this article believed that there was an under-detection of BSI across the country because of negative blood cultures in a significant number of patients despite clinical sepsis. This could be due to prescribing antibiotics before taking the blood sample, technical errors in sampling, or improper culture of blood samples. We know, according to CDC/NHSN case-definitions, BSI can only be reported when the blood culture is positive.
In microbiological study of this research, Enterobacteriaceae were the most common isolated pathogens. The frequency pattern of reported pathogens in the four major infections is different, as in VAPs, Acinetobacter baumannii; in UTIs and SSIs, Escherichia coli; and in BSIs, Staphylococcus epidermidis were the most abundant. Comparing this study with INICC, NHSN, and ECDC report for DAIs’ causative agents, the organisms almost were the same with a bolder role of Pseudomonas Aeruginosa in these reports [7-10]. Antimicrobial resistance (AMR) patterns in HAIs-related organisms were less resistance in developed countries for gram negative bacilli e.g. NHSN reported resistance to carbapenems about 15%, 60%, and 25% for Klebsiella pneumonia (KP), Acinetobacter baumannii (AB), and Pseudomonas Aeruginosa (PA), respectively [9]; resistance to carbapenems was higher in INICC report: about 35%, 80%, and 40% for KP, AB, and PA respectively [10]; and in the current study, 58%, 93%, and 60% for KP, AB, and PA respectively. The rate of being methicillin-resistant (MRSA) among staphylococcus aureus isolates was almost similar in the above studies (about 50%) [8-10]. Our interpretation of increasing resistance rate in gram negative bacilli from developed countries to less-developed countries including Iran was overuse and inappropriate use of antibiotics. The solution is antibiotic stewardship program (ASP) and implementation of infection control principles.
In the current study, the causative agents of a significant number of infections (28.4%) were unknown (Fig. 2). The possible reasons were tendency to use the clinical criteria more than the culture-based criteria to diagnose some infections especially for surgical site infection, lack of appropriate access to the microbiology laboratory for some centers, and financial limitations to perform microbiological cultures for suspected patients at some hospitals.
The limitations of this survey are the inclusion of mostly four major HAIs (although some hospitals had a more partial categorization), relative failure to perform post-discharge surveillance, lack of documentation of imaging results, and low sensitivity of routine surveillance [8].
Conclusions
Despite the limitations mentioned earlier, this study provides a general overview of health care-associated infections in Iran, such as incidence percentage, incidence density per 1000 patient-days, device-associated infections rates (per 1000 device-days), rates in different wards, the pathogens and their epidemiology, and antimicrobial resistance patterns; which led health care authorities and practitioners to make better decisions. The findings also indicate that HAIs in Iran require special attention both in detection/reporting HAIs and in IPC measures. Future studies could be done on adherence rate of HAIs’ preventive bundles, making interventions via multimodal strategies and assay their efficacy on HAIs rates, evaluating the effect of training on more accurate detection of HAIs, design antibiotic stewardship programs and review the results on reducing antimicrobial resistance., and etc.
Acknowledgement
Special appreciation to infection control nurses (supervisors) and physicians in all hospitals, health centers, and medical universities across the country.
Figures and tables
Footnotes
Funding
This study did not receive any specific grant.
Ethics
This study was approved by the ICDC Research Council and all data were registered under the supervision of the Iran Ministry of Health and Medical Education.
Conflict of interest statement
None to declare.
Authors’ contributions
MM and GMM: senior supervisors of the study from ICDC; PZ and EB: data extraction from INIS database and data analysis; ASH: preparing the manuscript draft and revising the paper; FMR: revising and confirming the microbiological data; SA: designing the study, final analysis and revising the paper.
References
- [1].Ferreira E, Pina E, Sousa-Uva M, Sousa-Uva A. Risk factors for health care-associated infections: From better knowledge to better prevention. Am J Infect Control 2017;45:e103-7. http://dx.doi.org/10.1016/j.ajic.2017.03.036 10.1016/j.ajic.2017.03.036 [DOI] [PubMed] [Google Scholar]
- [2].Shang J, Stone P, Larson E. Studies on nurse staffing and health care-associated infection: methodologic challenges and potential solutions. Am J Infect Control 2015;43:581-8. https://dx.doi.org/10.1016/j.ajic.2015.03.029 10.1016/j.ajic.2015.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].World Health Organization. Health care-associated infections FACT SHEET. Available from https://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf (Accessed: May 26, 2020).
- [4].Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, Jans B, Hopkins S, Hansen S, Lyytikäinen O, Reilly J, Deptula A, Zingg W, Plachouras D, Monnet DL. The Healthcare-Associated Infections Prevalence Study Group. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill 2018;23:1800516. https://doi.org/10.2807/1560-7917.ES.2018.23.46.1800516 10.2807/1560-7917.ES.2018.23.46.1800516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections - an overview. Infect Drug Resist 2018;11:2321-33. https://doi.org/10.2147/IDR.S177247 10.2147/IDR.S177247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zahraei SM, Eshrati B, Masoumi Asl H, Pezeshki Z. Epidemiology of four main nosocomial infections in Iran during March 2007 - March 2008 based on the findings of a routine surveillance system. Arch Iran Med 2012;15:764-6. https://pubmed.ncbi.nlm.nih.gov/23199249/ [PubMed] [Google Scholar]
- [7].Centers for Disease Control and Prevention, National Healthcare Safety Network (CDC/NHSN). 2019 National and State Healthcare-Associated Infections Progress Report. Available from https://www.cdc.gov/hai/excel/hai-progress-report/2019-SIR-ACH.xlsx (Accessed: July 18, 2021)
- [8].European Centre for Disease Prevention and Control. Healthcare-associated infections acquired in intensive care units. In: ECDC. Annual epidemiological report for 2017. Stockholm: ECDC; 2019. Available from https://www.ecdc.europa.eu/sites/default/files/documents/AER_for_2017-HAI.pdf (Accessed: July 18, 2021). [Google Scholar]
- [9].Dudeck MA, Weiner LM, Allen-Bridson K, Malpiedi PJ, Peterson KD, Pollock DA, Sievert DM, Edwards JR. National Healthcare Safety Network (NHSN) report, data summary for 2012, Device-associated module. Am J Infect Control. 2013;41:1148-66. https://doi.org/10.1016/j.ajic.2013.09.002 10.1016/j.ajic.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rosenthal VD, Bat-Erdene I, Gupta D, Belkebir S, Rajhans P, Zand F, Myatra SN, Afeef M, Tanzi VL, Muralidharan S, Gurskis V, Al-Abdely HM, El-Kholy A, AlKhawaja SAA, Sen S, Mehta Y, Rai V, Hung NV, Sayed AF, Guerrero-Toapanta FM, Elahi N, Morfin-Otero MDR, Somabutr S, De-Carvalho BM, Magdarao MS, Velinova VA, Quesada-Mora AM, Anguseva T, Ikram A, Aguilar-de-Moros D, Duszynska W, Mejia N, Horhat FG, Belskiy V, Mioljevic V, Di-Silvestre G, Furova K, Gamar-Elanbya MO, Gupta U, Abidi K, Raka L, Guo X, Luque-Torres MT, Jayatilleke K, Ben-Jaballah N, Gikas A, Sandoval-Castillo HR, Trotter A, Valderrama-Beltrán SL, Leblebicioglu H; International Nosocomial Infection Control Consortium. International Nosocomial Infection Control Consortium (INICC) report, data summary of 45 countries for 2012-2017: Device-associated module. Am J Infect Control 2020;48:423-32. https://doi.org/10.1016/j.ajic.2019.08.023 10.1016/j.ajic.2019.08.023 [DOI] [PubMed] [Google Scholar]
- [11].AhmedKhan H, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed 2017;7:478-82. https://doi.org/10.1016/j.apjtb.2017.01.019 10.1016/j.apjtb.2017.01.019 [DOI] [Google Scholar]
- [12].Iranian Center for Communicable Diseases (ICDC). National Nosocomial Infections Surveillance System. Tehran: MOHME Press; 2007. Available from https://www.who.int/patientsafety/events/media/iran_infection_surv.pdf?ua=1 [Google Scholar]
- [13].Masoumi Asl H. The National Nosocomial Infections Surveillance in Iran. A 4 years report. BMC Proc 2011;5:P243. https://dx.doi.org/10.1186/1753-6561-5-S6-P243 10.1186/1753-6561-5-S6-P243 [DOI] [Google Scholar]
- [14].Seifi A, Dehghan-Nayeri N, Rostamnia L, Varaei S, Akbari Sari A, Haghani H, Ghanbari V. Health care-associated infection surveillance system in Iran: Reporting and accuracy. Am J Infect Control 2019;47:951-5. https://doi.org/10.1016/j.ajic.2018.12.028 10.1016/j.ajic.2018.12.028 [DOI] [PubMed] [Google Scholar]
- [15].Iranian CDC. Iran Nosocomial Infections Surveillance Software (INIS) [Software]. Available from http://inis.health.gov.ir (Accessed: May 26, 2020).
- [16].Eshrati B, Masoumi Asl H, Afhami S, Pezeshki Z, Seifi A. Health care-associated infections in Iran: A national update for the year 2015. Am J Infect Control 2018;46:663-7. https://doi.org/10.1016/j.ajic.2017.11.017 10.1016/j.ajic.2017.11.017 [DOI] [PubMed] [Google Scholar]
- [17].Jay SJ. Nosocomial infections. Med Clin North Am 1983;67:1251-77. https://doi.org/10.1016/s0025-7125(16)31152-x 10.1016/s0025-7125(16)31152-x [DOI] [PubMed] [Google Scholar]
- [18].World Health Organization. Report on the burden of endemic health care-associated infection worldwide. 2011. Available from https://apps.who.int/iris/handle/10665/80135 (Accessed: May 26, 2020).
- [19].Lahsaeizadeh S, Jafari H, Askarian M. Healthcare-associated infection in Shiraz, Iran 2004-2005. J Hosp Infect 2008;69:283-7. https://doi.org/10.1016/j.jhin.2008.05.006 10.1016/j.jhin.2008.05.006 [DOI] [PubMed] [Google Scholar]
- [20].Jahani-Sherafat S, Razaghi M, Rosenthal VD, Tajeddin E, Seyedjavadi S, Rashidan M, Alebouyeh M, Rostampour M, Haghi A, Sayarbayat M, Farazmandian S, Yarmohammadi T, Arshadi FK, Mansouri N, Sarbazi MR, Vilar M, Zali MR. Device-associated infection rates and bacterial resistance in six academic teaching hospitals of Iran: Findings from the International Nocosomial Infection Control Consortium (INICC). J Infect Public Health 2015;8:553-61. https://doi.org/10.1016/j.jiph.2015.04.028 10.1016/j.jiph.2015.04.028 [DOI] [PubMed] [Google Scholar]
- [21].Afhami S, Seifi A, Hajiabdolbaghi M, Bazaz NE, Hadadi A, Hasibi M, Rezaie P, Mohamadnejad E, Ghahan A, Hajinoori M, Veyceh F, Adinehkharrat S, Hojjati Z, Azimbeik Z. Assessment of device-associated infection rates in four teaching hospitals in Islamic Republic of Iran. East Mediterr Health J 2019;25:90-7. https://doi.org/10.26719/emhj.18.015 10.26719/emhj.18.015 [DOI] [PubMed] [Google Scholar]


