Summary
Background
We conducted a population-based cohort study to estimate mortality before, during and after the COVID-19 peak and to compare mortality in 2020 with rates reported in previous years, with a view to helping decision makers to apply containment measures for high-risk groups.
Methods
All deaths were collected between 2015 and 2020 from municipal registry database. In 2020, weeks 1-26 were stratified in three periods: before, during and after the COVID mortality peak. The Poisson Generalized Linear regression Model showed the “harvesting effect”. Three logistic regressions for 8 dependent variables (age and comorbidities) and a t-test of differences described all-cause mortality risk factors in 2019 and 2020 and differences between COVID and non-COVID patients.
Results
A total of 47,876 deaths were collected. All-cause deaths increased by 38.5% during the COVID peak and decreased by 18% during the post-peak period in comparison with the average registered during the control period (2015-19), with significant mortality displacement in 2020. Except for chronic renal injuries in subjects aged 45-64 years, diabetes and chronic cardiovascular diseases in those aged 65-84 years, and neuropathies in those aged > 84 years, the weight of comorbidities in deaths was similar or lower in COVID subjects than in non-COVID subjects.
Discussions
Surprisingly, the weight of comorbidities in death, compared to weight in non-COVID subjects allows you to highlight some surprising results such as COPD, IBD and Cancer. The excess mortality that we observed in the entire period were modest in comparison with initial estimates during the peak, owing to the mild influenza season and the harvesting effect starting from the second half of May.
Keywords: COVID-19, SARS-CoV-2, Harvesting effect, Mortality displacement, Comorbidities, Risk factor
Introduction
The harvesting effect is the compensatory reduction in mortality following a temporary increase in the number of deaths of older individuals, frail subjects and patients with comorbidities. This effect is usually observed after environmental phenomena, such as heat waves or cold spells, but is sometimes seen after epidemics and pandemics, such as unusually virulent seasonal influenza pandemics [1-3]. Mortality displacement was first described as a short-term effect within days or weeks after heat waves [4]. Subsequent studies reported major long-term mortality displacement, showing, for example, that the winter mortality rate significantly modified the estimated effect of temperature on mortality in the following summer [5, 8].
Mortality displacement due to the COVID-19 pandemic, and the weight of factors potentially related to death, i.e. underlying health conditions, have been scantly investigated, despite their importance in delineating the epidemiological picture and in guiding public health measures to reduce the impact of the disease.
From the start of the pandemic to 5th July 2020, almost 35,000 deaths due to SARS-CoV-2 were recorded in Italy, the third highest mortality rate, behind Belgium and Spain. The estimated case-fatality rate was 14%, which peaked at 20% among people aged 80 years and older [9].
In Liguria, a region of North-Western Italy with 1,550,640 inhabitants, the impact of COVID-19 was heavy, with cumulative incidences above 6 cases and 1 death per 1,000 residents. SARS-CoV-2 circulated intensely between weeks 11 and 18 and the epidemic curve reached its peak in week 14 [10]. The demographic structure of the region affected COVID-19-related mortality, as Liguria is the Italian region with the oldest population, with 28.5% (442,279) of residents aged ≥64 years and 5.1% (80,229) aged ≥ 84 years [11].
In order to estimate mortality before, during and after the COVID-19 peak, and to compare mortality in 2020 with the rates reported in previous years, we carried out a population-based cohort study of the Ligurian population. Our objective was to provide relevant parameters, such as COVID-19-related excess mortality and risks according to underlying health conditions at different ages [12]. These parameters constitute valuable aids to understanding the real burden of disease and assisting decision-makers in applying and prioritizing the most appropriate physical distancing and other strategies focused on high-risk groups [13].
Materials and methods
Raw data on deaths and demographic information, such as age and municipality of residence, were obtained from municipal registry databases. These data were subsequently linked to and integrated with the central platform of electronic health records of the Ligurian Health System, which includes data on primary care and hospital care; comorbidities were estimated through the chronic condition data warehouse by using validated and standardized definitions of several hundred underlying conditions [14-16].
Confirmed cases were diagnosed on nasopharyngeal swabs, by means of real-time polymerase chain reaction testing and applying the World Health Organization (WHO) case definition.
The study population consisted of a dynamic cohort of about 940,000 > 44-year-old residents in the Liguria region, from 2015 to 2020. The analysis considered the total number of deaths during weeks 1-26 each year from 2015 to 2020 among residents in cities with more than 10,000 inhabitants; this was done in order to avoid the possibility of delayed notification by the regional registry office. The study population included patients who had received a diagnosis of COVID-19 from February 26th (first case in Liguria) to June 14th and who had died up to July 5th 2020. Excess mortality was calculated as the difference between the mortality rates observed and the all-cause mortality rates registered in the last five years. We considered three different periods according to the pattern of SARS-CoV-2 circulation: weeks 1-10 (Pre-COVID), weeks 11-18 (COVID peak), weeks 19-26 (Post-peak). Poisson time-series Generalized Linear regression Model (GLM) analysis was used to assess the difference between years, and a value of p < 0.05 was taken to indicate statistical significance[17-19]. To estimate the weight of underlying health conditions, multiple logistic regressions were used to assess associations between death and comorbidities, i.e. chronic cardiovascular disease, chronic obstructive pulmonary disease (COPD), chronic renal injuries (CRI), diabetes, neoplasia, chronic inflammatory bowel disease (IBD) and neuropathies, in 3 age-groups (45-64, 65-84, > 84 years) and in COVID-19 patients and non-COVID-19 patients in 2019 and 2020. Odds ratios (ORs) were used to assess the magnitude of associations, and 95% confidence intervals (95% CI) are reported. To compare the impact that risk factors had on death in COVID-19 patients and in non-COVID-19 patients in 2019 and 2020, the ratio between Odds Ratios was used.
Statistical significance in this case was obtained from the standard normal distribution of z-value, for the ratio 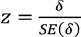 with
with 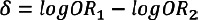 with the standard error pooled SE (δ) resulting from SE (δ)=
with the standard error pooled SE (δ) resulting from SE (δ)=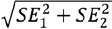 [18]. Statistical analyses were conducted by means of JMP v.15 software.
[18]. Statistical analyses were conducted by means of JMP v.15 software.
Results
A total of 47,876 deaths were registered from 2015 to 2020 during weeks 1-26 in cities of Liguria with 10,000 inhabitants or more, accounting for more than 70% of all deaths in the Region. In the first 26 weeks of each year from 2015 to 2019, the number of deaths ranged between 7,330 and 8,146, while during the same period in 2020, 8,371 deaths were recorded; 1,070 (12.8%) were in individuals positive for SARS-Cov2. In this last group, the M:F ratio was 1.42:1 and the median age was 82 years (IQR 76-88; min 35; max 102).
In the first 26 weeks of 2020, cumulative mortality due to COVID-19 increased with age from 1.9 deaths/10,000 in the 45-64-year age-group to 21.2/10,000 and 80.3/10,000 in the 65-84-year and > 84-year classes, respectively. Cumulative non-COVID-19-related mortality increased with age from 14.2 deaths/10,000 in the 45-64-year age-group to 113.7/10,000 and 675.1/10,000 in the 65-84-year and > 84-year age-classes, respectively.
In the 2015-19 period, the M:F ratio was 0.86:1 and the median age was 85 years (IQR 77-90). Yearly cumulative mortality increased with age from 17.7 deaths/10,000 in the 45-64-year age-group to 125.9/10,000 and 727.7/10,000 in the 65-84-year and > 84-year age-classes, respectively.
Figure 1 illustrates the number of deaths in each week of the first semester from 2015 to 2020. During the 2015-19 period, the number of weekly deaths ranged between 217 and 446 (mean 338.6, median 338, IQR 309-359) in weeks 1-10, between 243 and 348 (mean 288.9, median 290, IQR 271.8-305) in weeks 11-18, and between 232 and 335 (mean 272.8, median 274, IQR 259-283) in weeks 19-26. In 2020, the number of weakly deaths ranged between 219 and 315 (mean 277.2, median 276, IQR 268-299.7) in the pre-COVID period, between 325 and 615 (mean 470.1, median 468, IQR 401.5-550.8) during the COVID-19 peak and between 179 and 268 (mean 229.8, median 237, IQR 219.3-248.5) in the post-peak period. In comparison with the average number of all-cause deaths registered during the control period (2015-19), an increase of 63.3% (+ 1449.6, 95% CI 55.5-72.5%) during the COVID peak and a decrease of 14% (- 314.6, 95% CI 8.5-19.2) during the post-peak period were observed. Deaths in SARS-CoV-2-positive patients accounted for 31% (n. 908) and 13% (n. 160) of all deaths in the peak and post-peak periods, respectively.
Fig. 1.
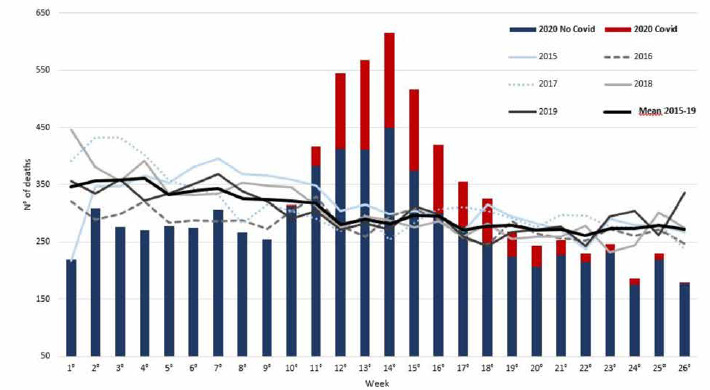
Deaths in the Liguria region in 2015-2020 in cities with > 10,000 inhabitants, from week 1 to 26.
Table I shows the mean number of weekly deaths, the regression coefficient and intercept estimates over the 6 years considered, as calculated by means of Poisson distributed time-series regression GLM.
Tab. I.
Analysis of yearly variation of mean number of weekly deaths, by Poisson distributed time-series regression of generalized linear model (GLM).
| N° weekly deaths | 05% Cl | Regression coefficients | 95% Cl | P value | |
|---|---|---|---|---|---|
| WEEKS 1-10 (mild seasons in 2016 and 2020) | |||||
| Intercept | 330.15 | 325.6-334.8 | - | - | <.0001° |
| 2020 | 277.2 | 266.9-287.5 | -52.9 | -62.5; -43.3 | <.0001° |
| 2019 | 337.3 | 325.9-348.7 | 7.15 | -3.13-17.6 | 0.1741 |
| 2018 | 361.8 | 350-373.6 | 31.7 | 21.1-42.2 | <.0001° |
| 2017 | 359.5 | 347.8-371.3 | 29.4 | 18.8-40.1 | <.0001° |
| 2016 | 295.3 | 284.7-305.9 | -34.9 | -44.6; -24.9 | <.0001° |
| 2015 | 349.8 | 338.2-361.4 | - | - | - |
| WEEKS 11-18 (excess mortality in 2020) | |||||
| Intercept | 319.12 | 314.1-324.2 | - | - | <.0001° |
| 2020 | 470.1 | 455.1-485.2 | 151.0 | 137.8-164.4 | <.0001° |
| 2019 | 280.8 | 269.1-292.4 | -38.4 | -49.01; -27.5 | <.0001° |
| 2018 | 284.6 | 272.9-296.3 | -34 5 | -45.2-23.6 | <.0001° |
| 2017 | 289.9 | 278.1-301.7 | -29.3 | -40.0; -18.3 | <.0001° |
| 2016 | 282.8 | 271.1-294.4 | -36.4 | -47.1; -25.5 | <.0001° |
| 2015 | 306.6 | 294.7-319 | - | - | - |
| WEEKS 19 26 (mortality displacement in 2020) | |||||
| Intercept | 265.60 | 261-270.2 | - | - | <.0001° |
| 2020 | 229.8 | 219.3-240.3 | -35.9 | -45.5; -26.0 | <.0001° |
| 2019 | 281.9 | 270.2-293.5 | 16.3 | 5.8-26.9 | 0.0021° |
| 2018 | 263.1 | 251.9-274.4 | -2.5 | -12.6-7.9 | 0.637 |
| 2017 | 279.1 | 267.6-290.7 | 13.5 | 3.1-24.1 | 0.0107° |
| 2016 | 264.4 | 253.1-275.6 | -1.2 | -11.4-9.2 | 0.8151 |
| 2015 | 275.4 | 263.9-286.9 | - | - | - |
During the 2015-19 period, weeks 1-10 showed a high variation of regression coefficients, ranging between -34.9 and 31.7, while during weeks 11-18 and weeks 19-26 the coefficients displayed lower ranges (between -38.4 and -29.3 and between -2.5 and 16.3). In 2020, 151 (95% CI 137.8-164.4) excess deaths per week were registered during the COVID-19 peak, and a significant mortality displacement occurred in the following weeks (-35.9, 95% CI -45.5-26.0).
Age, COPD, CRI, diabetes, chronic cardiovascular disease, neoplasia (except in those aged > 84 years), and neurological syndromes were significantly related to increased mortality in both COVID-19 patients and non-COVID-19 patients who died in 2019 and 2020; IBD was a significant risk factor in non-COVID-19 patients who died in 2019 and 2020.
The probability of dying of COVID-19 per year of age (Y.O.A.) in people aged > 44 yrs increased by 8.2% (OR 1.082, 95%CI 1.077-1.088), with values not statistically different from the OR in non-COVID patients in the same period and in 2019 (1.11, 95% CI 1.107-1.113 and 1.106, 95% CI 1.102-1.108, respectively).
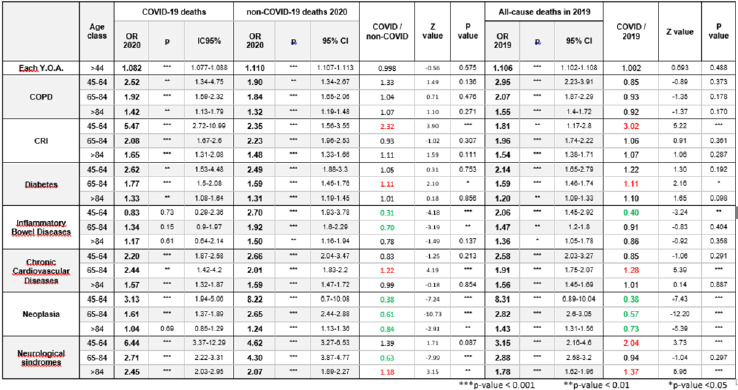
Multivariate logistic regressions of comorbidities associated to all-cause deaths in 2019, and COVID-19 and non-COVID-19 deaths in 2020, in the 3 age-classes, are shown in Table II.
Tab. II.
Impact of age and risk factors on death in COVID-19 patients and non-COVID-19 patients in 2019 and 2020 in adults aged > 44 years.
|
The ORs for underlying conditions according to age-group were not significantly different between COVID-19 patients and non-COVID-19 patients who died in 2019 and 2020, with some exceptions. Patients with CRI in the 45-64-year age-group (OR1/OR2 of 2.32 in 2020 and 3.02 in 2019), and patients aged 65-84 years with diabetes (OR1/OR2 of 1.11 in 2020 and 1.11 in 2019) and chronic cardiovascular disease (OR1/OR2 of 1.22 in 2020 and 1.28 in 2019) showed a higher risk of dying of COVID-19 than of dying of other causes in 2019 and 2020). Neurological syndromes showed higher OR in patients who died with COVID-19 in 45-64 years age group (OR1/OR2 of 2.04) compared with patients died in 2019 and in > 84 year adults compared with non-COVID-19 patient died in 2019 and 2020 (OR1/OR2 of 1.18 in 2020 and 1.37 in 2019).
IBD displayed a lower OR in COVID-19 patients aged 45-64 years who died than in non-COVID-19 patients who died in 2019 and 2020 (OR1/OR2 of 0.31 in 2020 and 0.40 in 2019); neoplasia showed a lower OR in all age-groups (OR1/OR2 ranged from 0.84 to 0.38 in 2020, and from 0.73 to 0.38, in 2019, see table 2 for further details).
Discussion and conclusion
Estimating mortality attributable to the COVID-19 epidemic and the role played by risk factors for mortality are fundamental to understanding the real burden of the disease and to identifying risk-based preventive strategies. The present population-based study provided an estimate of excess COVID-19-related mortality in Liguria during the epidemic peak; it also estimated the short-term mortality displacement and calculated the weight of risk factors for mortality in COVID-19 patients and non-COVID-19 patients in 2019 and 2020 in different age-groups.
During the epidemic peak, a 63.3% increase in all-cause deaths was observed in comparison with the average registered during the control period (2015-19); however, the decrease in mortality observed in the following 8 weeks softened this impact by 22%. Indeed, Poisson GNL indicated a short-term mortality displacement equal to 24% of the amount of excess mortality observed during the peak. The harvesting effect can reasonably be entirely ascribed to the extra mortality due to COVID-19, as the 2019-20 influenza season was extraordinarily mild, being globally similar in terms of mortality to the 2015-2016 seasons (WHO, EUROMOMO, Italian Ministry of Health) [20-23].
The global effect of the mild influenza season in January and February, the vigorous circulation of SARS-CoV-2 in March and April and the harvesting effect starting from the second half of May determined a mild/reduced increase in mortality in the first 26 weeks of 2020, amounting to 7% of expected deaths.
Since the start of the pandemic, age has been indicated as the key determinant of prognosis in COVID-19 patients. In our study, age was identified as an independent predictor of mortality in patients with COVID-19, and the magnitude of the odds ratio per year of increase was similar in SARS-CoV-2-positive patients and non-COVID-19 patients who died in 2019 and 2020. We observed an increased risk with an OR of 1.08 per year of age in COVID-19 patients; this largely overlaps with the ORs registered in other populations with different characteristics during the pandemic [24].
Our study confirmed that COVID-19 patients with various comorbidities, including chronic lung disease, CRI, neoplasia, chronic cardiovascular disease, neurological syndromes and diabetes, have a higher likelihood of complications and death [25-30]. The weight of these underlying conditions in COVID-19 patients in comparison with non-COVID patients, both during and before the spread of SARS-CoV-2, showed different patterns; for the majority of comorbidities, however, the risk proved comparable in COVID-19 and non-COVID-19 patients.
In the literature, the reported effect of comorbidities on the outcomes of patients with COVID-19 are conflicting. In our study, CRI in COVID-19 patients aged 44-64 years, diabetes and chronic cardiovascular disease in those aged 65-84 years and neurological syndromes in those aged > 84 years were found to exert more influence than in non- COVID individuals.
Several studies have found that CRI, diabetes, chronic cardiovascular disease and neurological syndromes are associated with severe outcomes. However, the present study is the first to compare these independent predictors of death in SARS-CoV-2-positive and -negative patients during the pandemic and in previous years. Various immunological and molecular mechanisms, including chronic inflammation, proinflammatory cytokine storms, increased coagulation activity, impaired immune response, elevated expression of ACE-2 and potential direct pancreatic damage by SARS-CoV-2, may explain these associations. These effects are counterbalanced or increased by the asymmetric distribution of some underlying diseases, of social mixing behaviour, of compliance with social isolation or of living in high-risk settings (e.g. nursing homes), etc. The role played by each single immunological, molecular or behavioural factor is difficult to estimate.
Surprisingly, the weight of cancer in influencing death in COVID subjects proved minimal in those aged > 84 years; moreover, it was much lower than in non-COVID patients in 2020 and 2019 in all age-groups. The results of a recent meta-analysis by Vassilis G. et al. showed that all-cause mortality in the elderly (aged 64 years) was comparable between individuals with cancer and those without (RR, 1.06; 95%CI, 0.79 to 1.41; P = .71), as found in our study in individuals aged > 84 years. The absence of an increased mortality risk in older individuals implies that the presence of cancer does not further affect the already burdened prognosis among older individuals [31]. In cancer patients, immunosuppression may dampen the so-called “cytokine storm”, the most dangerous and potentially life-threatening event related to COVID-19, because of their down-regulated immune response. Furthermore, cancer patients are more likely to implement self-isolation, social and physical distancing and personal health measures, which may explain the lower incidence of COVID-19 [32-34]. The reduced weight of neoplasia in COVID-19 patients during the first wave is only the short-term effect of the pandemic, but substantial increases in the number of avoidable cancer deaths are to be expected in the near future as a result of diagnostic delays due to the COVID-19 pandemic [35]
IBD seems not to influence the likelihood of death in COVID-19 patients; the ORs were significantly lower in this group than in non-COVID patients in 2020 and 2019. These results could suggest a protective role of immunosuppressive drugs; indeed, some therapies that are frequently administered in patients with IBD, such as anti-TNFα, anti-IL-6 and JAK inhibitors, may have a beneficial role in attenuating severe COVID-19 disease, although more evidence is needed [36, 37]. In our population, about 10% of patients with IBD were on immunosuppressive drugs.
This study has some limitations. The analysis considered the total number of deaths in residents in cities with more than 10,000 inhabitants (above 70% of the total population) to avoid the possibility of delayed notification by the regional registry office. This sample was therefore not exactly representative of the total population; this may have introduced the bias of the possible different access to treatment in small towns or the different spread of the virus in less populated areas. However, as we compared different outcomes and risk factors in infected and non-infected residents in cities with > 10,000 inhabitants in different periods of time, this bias does not affect, or only minimally affects, the results.
To compare the impact of risk factors on death in COVID-19 patients and non-COVID-19 patients in 2019 and 2020, we used the ratio between Odds Ratios, evaluated by the difference between logOR and pooled SE, by means of Student’s T test. Some epidemiologists argue that, in order to compare two odds ratios, it is enough that the OR confidence intervals overlap. However, we needed a finer method in order to obtain an indicator of results that was comparable between the various categories.
In conclusion, the evaluation of the weight of comorbidities in COVID-19 patients yielded some surprising results, such as the low/minimal weight of cancer in influencing death in older COVID-19 patients and the comparable weight of COPD and chronic cardiovascular disease (except in the 65-84-year age-group) in COVID-19 and non-COVID subjects. The excess mortality that we observed in the first semester of 2020 was modest, in comparison with the initial estimate during the peak, owing to the mild influenza season and the harvesting effect starting from the second half of May.
Acknowledgements
Funding sources: this research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Figures and tables
Footnotes
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
FA, GI and MA conceived the study. MA, FT, CP and FA verified the analytical methods. SS and DG provided informatics data. All authors contributed to data acquisition and data quality control. MA, FT, CP, DA, MFA, AO, GI, DP, IA and MP contributed to the interpretation of the results. MA, FA and FT wrote the manuscript, with input from all authors. All authors have read and agreed to the published version of the manuscript.
References
- [1].Lytras T, Pantavou K, Mouratidou E, Tsiodras S. Mortality attributable to seasonal influenza in Greece, 2013 to 2017: variation by type/subtype and age, and a possible harvesting effect. Euro Surveill 2019;24:1800118. https://doi.org/10.2807/1560-7917.ES.2019.24.14.1800118 10.2807/1560-7917.ES.2019.24.14.1800118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kinney PL, Schwartz J, et al. Winter season mortality: will climate warming bring benefits? Environ Res Lett 2015;10:064016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Baccini M, Kosatsky T, Biggeri A. Impact of summer heat on urban population mortality in Europe during the 1990s: an evaluation of years of life lost adjusted for harvesting. PLoS One 2013;8:e69638. https://doi.org/10.1371/journal.pone.0069638 10.1371/journal.pone.0069638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hajat S, Armstrong BG, Gouveia N, Wilkinson P. Mortality displacement of heat-related deaths: a comparison of Delhi, São Paulo, and London. Epidemiology 2005;16:613-20. https://doi.org/10.1097/01.ede.0000164559.41092.2a 10.1097/01.ede.0000164559.41092.2a [DOI] [PubMed] [Google Scholar]
- [5].Braga AL, Zanobetti A, Schwartz J. The time course of weather-related deaths. Epidemiology 2001;12:662-7. https://doi.org/10.1097/00001648-200111000-00014 10.1097/00001648-200111000-00014 [DOI] [PubMed] [Google Scholar]
- [6].Rocklöv J, Forsberg B. The effect of high ambient temperature on the elderly population in three regions of Sweden. Int J Environ Res Public Health 2010;7:2607-19. https://doi.org/10.3390/ijerph7062607 10.3390/ijerph7062607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hajat S, Armstrong B, Baccini M, Biggeri A, Bisanti L, Russo A, Paldy A, Menne B, Kosatsky T. Impact of high temperatures on mortality: is there an added heat wave effect? Epidemiology 2006;17:632-8. https://doi.org/10.1097/01.ede.0000239688.70829.63 10.1097/01.ede.0000239688.70829.63 [DOI] [PubMed] [Google Scholar]
- [8].Analitis A, Katsouyanni K, Biggeri A, Baccini M, Forsberg B, Bisanti L, Kirchmayer U, Ballester F, Cadum E, Goodman PG, Hojs A, Sunyer J, Tiittanen P, Michelozzi P. Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol 2008;168:1397-408. https://doi.org/10.1093/aje/kwn266 10.1093/aje/kwn266 [DOI] [PubMed] [Google Scholar]
- [9].World of Meters. Available at: https://www.worldometers.info/coronavirus (Accessed on: 09/07/2020).
- [10].Epicentro, Italian Superior Institute (ISS) (Accessed on: 09/07/2020). [Google Scholar]
- [11].Italian National Statistic’s Institute (ISTAT), web archive: https://www.istat.it/it/archivio (Accessed on: 10/07/2020).
- [12].Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, Noursadeghi M, Pillay D, Sebire N, Holmes C, Pagel C, Wong WK, Langenberg C, Williams B, Denaxas S, Hemingway H. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet 2020;395(10238):1715-25. https://doi.org/10.1016/S0140-6736(20)30854-0 10.1016/S0140-6736(20)30854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Spiegelhalter D. How much ‘normal’ risk does Covid represent? Medium, March 21, 2020. Available at: https://medium.com/wintoncentre/how-much-normal-risk-does-covid-represent-4539118e1196 (Accessed on: April 16, 2020).
- [14].Sylvestre E, Bouzillé G, Chazard E, His-Mahier C, Riou C, Cuggia M. Combining information from a clinical data warehouse and a pharmaceutical database to generate a framework to detect comorbidities in electronic health records. BMC Med Inform Decis Mak 2018;18:9. https://doi.org/10.1186/s12911-018-0586-x 10.1186/s12911-018-0586-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rattanaumpawan P, Wongkamhla T, Thamlikitkul V. Accuracy of ICD-10 coding system for identifying comorbidities and infectious conditions using data from a Thai University Hospital Administrative Database. J Med Assoc Thai 2016;99:368-73. [PubMed] [Google Scholar]
- [16].Chen Y, Zivkovic M, Wang T, Su S, Lee J, Bortnichak EA. A Systematic review of coding systems used in pharmacoepidemiology and database research. Methods Inf Med 2018;57:1-42. https://doi.org/10.3414/ME17-05-0006 10.3414/ME17-05-0006 [DOI] [PubMed] [Google Scholar]
- [17].Dushoff J, Plotkin JB, Viboud C, Earn DJ, Simonsen L. Mortality due to influenza in the United States--an annualized regression approach using multiple-cause mortality data. Am J Epidemiol 2006;163:181-7. https://doi.org/10.1093/aje/kwj024 10.1093/aje/kwj024 [DOI] [PubMed] [Google Scholar]
- [18].Hajat S, Armstrong BG, Gouveia N, Wilkinson P. Mortality displacement of heat-related deaths: a comparison of Delhi, São Paulo, and London. Epidemiology 2005;16:613-20. https://doi.org/10.1097/01.ede.0000164559.41092.2a 10.1097/01.ede.0000164559.41092.2a [DOI] [PubMed] [Google Scholar]
- [19].Bramness JG, Walby FA, Morken G, Røislien J. analyzing seasonal variations in suicide with fourier poisson time-series regression: a registry-based study from Norway, 1969-2007. Am J Epidemiol 2015;182:244-54. https://doi.org/10.1093/aje/kwv064 10.1093/aje/kwv064 [DOI] [PubMed] [Google Scholar]
- [20].Stephen Politzer-Ahles, The Hong Kong Polytechnic University, Researchgate.net 2 september 2016. Avaailable at https://www.researchgate.net/post/Comparing_two_odds_ratios_for_statistical_significant_difference (Accessed on: 10 July 2020).
- [21].WHO: report interactive charts online. Available at: https://apps.who.int/flumart/Default?ReportNo=10 (Accessed on: 30 july 2020).
- [22].EUROMOMO: excess mortality 2016-2020. Available at: https://www.euromomo.eu/bulletins/2020-32 (Accessed on: 30 july 2020).
- [23].Italian Ministry of Health - Mortality surveillance system of Regione Lazio. Available at: http://www.salute.gov.it/portale/caldo/SISMG_sintesi_ULTIMO.pdf
- [24].Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054-1062. https://doi.org/10.1016/S0140-6736(20)30566-3. 10.1016/S0140-6736(20)30566-3 Erratum in: Lancet 2020;395:1038. Erratum in: Lancet 2020;395:1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].[CDC] People with medical condition need extra precaution. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html (Accessed to 30 July 2020).
- [26].Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020;97:829-38. https://doi.org/10.1016/j.kint.2020.03.005 10.1016/j.kint.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Mantovani A, Byrne CD, Zheng MH, Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr Metab Cardiovasc Dis 2020;30:1236-48. https://doi.org/10.1016/j.numecd.2020.05.014 10.1016/j.numecd.2020.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mehra MR, Desai SS, Kuy S, Henry TD, Patel AN. cardiovascular disease, drug therapy, and mortality in Covid-19. N Engl J Med 2020;382:e102. https://doi.org/10.1056/NEJMoa2007621 10.1056/NEJMoa2007621 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [29].Wang B, Li R, Lu Z, Huang Y. Does comorbidity increase the risk of patients with COVID-19: evidence from meta-analysis. Aging (Albany NY) 2020;12:6049-57. https://doi.org/10.18632/aging.103000 10.18632/aging.103000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis 2020;94:91-5. https://doi.org/10.1016/j.ijid.2020.03.017 10.1016/j.ijid.2020.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].WHO - Risk Communication and Community Engagement (RCCE) Available at: https://www.who.int/publications/i/item/risk-communication-and-community-engagement-(rcce)-action-plan-guidance (Accessed on: 10 august 2020).
- [32].Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, Rachet B, Aggarwal A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023-34. https://doi.org/10.1016/S1470-2045(20)30388-0. 10.1016/S1470-2045(20)30388-0 Erratum in: Lancet Oncol 2021;22:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Giannakoulis VG, Papoutsi E, Siempos II. Effect of cancer on clinical outcomes of patients with COVID-19: a meta-analysis of patient data. JCO Glob Oncol 2020;6:799-808. https://doi.org/10.1200/GO.20.00225 10.1200/GO.20.00225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Joharatnam-Hogan N, Hochhauser D, Shiu KK, Rush H, Crolley V, Wilson W, Sharma A, Muhammad A, Anwar M, Vasdev N, Goldstein R, Kantser G, Saha A, Raja F, Bridgewater J, Khan K. Outcomes of the 2019 el coronavirus in patients with or without a history of cancer: a multi-centre North London experience. Ther Adv Med Oncol 2020;12:1758835920956803. https://doi.org/10.1177/1758835920956803 10.1177/1758835920956803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020;8:420-422. https://doi.org/10.1016/S2213-2600(20)30076-X. Erratum in: Lancet Respir Med 2020 Feb 25. 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ling KL, Hilmi I, Raja Ali RA, Leong RWL, Leung WK, Ng SC, Wu KC, Chen MH, Ran ZH, Hisamatsu T, Ahuja V, Makharia GK, Banerjee R, Wei SC, Wu DC, Pisespongsa P, Ye BD, Sollano J, Simadibrata M, Chuah SW, Ooi CJ. Asian Pacific Association of Gastroenterology (APAGE) Inflammatory Bowel Disease (IBD) Working Party guidelines on IBD management during the COVID-19 pandemic. JGH Open 2020;4:320-3. https://doi.org/10.1002/jgh3.12362 10.1002/jgh3.12362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bezzio C, Saibeni S, Variola A, Allocca M, Massari A, Gerardi V, Casini V, Ricci C, Zingone F, Amato A, Caprioli F, Lenti MV, Viganò C, Ascolani M, Bossa F, Castiglione F, Cortelezzi C, Grossi L, Milla M, Morganti D, Pastorelli L, Ribaldone DG, Sartini A, Soriano A, Manes G, Danese S, Fantini MC, Armuzzi A, Daperno M, Fiorino G; Italian Group for the Study of Inflammatory Bowel Disease (IG-IBD). Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut 2020;69:1213-7. https://doi.org/10.1136/gutjnl-2020-321411 10.1136/gutjnl-2020-321411 [DOI] [PubMed] [Google Scholar]


