Summary
Background
Gestational diabetes mellitus (GDM) is one of the most common metabolic disorders during pregnancy that significantly affects perinatal outcomes.
Objective
The aim of this study was to determine the prevalence of GDM and its relation with the incidence of stillbirth, preterm birth, macrosomia, abortion and cesarean section (C-section) delivery in pregnant women.
Methods
This cross-sectional study was conducted on 3675 pregnant women in 11 provinces across Iran. Cluster sampling was used to select samples from mothers covered by health plans in 11 provinces of Iran. Prevalence of adverse pregnancy outcomes, including preterm delivery, type of delivery, macrosomic preterm birth, miscarriage, stillbirth, infant death, and birth weight were measured, using family record and face-to-face interviews. Data were analyzed by logistic regression, using STATA14.2 software.
Results
About four percent of Iranian pregnant women had GDM during pregnancy. Prevalence of C-section was significantly higher in diabetic women than in the non-diabetic ones (53.19 vs 46.81, respectively, P < 0.001). Abortion in diabetic mothers was more than twice that of the non-diabetic mothers (P < 0.001). In the adjusted logistic regression model, the odds of stillbirth in mothers with GDM were 1.8 (95% CI: 1.11, 2.91, P = 0.018) times higher than that of the non-diabetics. The odds of macrosomia in diabetic women was about 7 times higher than the non-diabetic women (95% CI: 2.81, 17.14, P < 0.001). The odds of GDM had an increasing trend according to the BMI (p < 0.001). The risk of GDM were significantly lower, according to the daily physical activity (PA) (p < 0.001).
Conclusion
The GDM prevalence has a decreasing trend in Iran. It increases the adverse pregnancy outcomes such as stillbirth, neonatal deaths, macrosomia, preterm birth, abortion and C-section delivery. As, some of these consequences like macrosomia are not treatable, thus early prevention is very crucial.
Keywords: Gestational diabetes mellitus, Macrosomia, Stillbirth, Abortion
Background
Gestational diabetes mellitus (GDM) is one of the most common metabolic disorders during pregnancy that significantly affects perinatal outcomes [1]. The prevalence of GDM is different in recent studies, worldwide. The prevalence of GDM is reported to be 5.4% in Europe, 14% in Africa and 0.7 to 51% in Asia [2-6]. Timely diagnosis and appropriate treatment of GDM are very vital in preventing maternal and fetal complications [7]. GDM occurs in approximately 2-5% of pregnancies and has short- and long-term consequences for the mother, infant, and fetus [8, 9].
Preterm delivery, macrosomia, abortion, respiratory distress, stillbirth, neonatal deaths, and increased caesarean section (C-section) delivery are among the outcomes of GDM. In addition, GDM not only increases the risk of type 2 diabetes and hypertension disorders in mothers [10, 11] but also increases the risk of congenital malformations, especially obstructive urinary tract disorders, renal agenesis, and cardiovascular disorders by 1.2 times in fetus [12].
Preterm labor and increased cesarean delivery are other major complications of GDM that can lead to stillbirth and infant mortality. For example, a case-control study reported that the risks of C-section delivery in diabetic pregnant women were twice that of non-diabetic mothers [13]. In addition, in a cohort study, the relative risk of C-section delivery for diabetic mothers was 1.4 times (95% CI: 1.04-2.02) more than the non-diabetic mothers [10].
Furthermore, a study on the consequences of GDM has shown that perinatal events are higher in diabetic mothers than in the non-diabetic patients. The results of this study reported a 3-fold higher prenatal mortality in neonates born in diabetic mothers, 9-fold higher rates of first-year mortality, 3-fold more congenital anomalies, and 3.6-fold higher incidence of large for gestational age birth (LGA) in neonates of diabetic mothers compared to the non-diabetic mothers [11]. Another study has shown that pregnant women with a higher glucose challenge test (GCT) have a significantly higher chance of preterm delivery and perinatal events [14]. Another adverse of GDM is the increased risk of diabetes type 1 in children. Despite therapeutic advances such as stem cell therapies for the treatment of diabetes type 1, a feasible and safe clinical approach still remain for this purpose [15, 16].
Given that the consequences of GDM can endanger the health of the mother and baby and even some of these consequences like macrosomia are not treatable, therefore, comprehensive research is needed on this area, involving different population. Understanding the adverse consequences of GDM can help us to better plan for the prevention and control of GDM. So far, several studies individually have investigated the prevalence and complications of GDM in several cities of Iran, but the present study was conducted in a more comprehensive way, at the national level in 11 provinces of Iran. The aim of this study was to determine the prevalence of GDM and its relationship with the occurrence of stillbirth, preterm birth, macrosomia, abortion and C-section in pregnant women.
Materials and methods
STUDY DESIGN AND POPULATION
This cross-sectional study was performed on 3,675 pregnant mothers in 11 provinces of Iran in 2019. The present study was conducted, using data from a population-based national case-control study conducted in the year 2018 to determine the factors associated with stillbirth and neonatal death. At the beginning of the study, participants were provided with comprehensive information on the study objectives, and questionnaires were filled with informed consent. In Iran, health centers provide primary health cares and general medical services to the residences who are living in their defined geographical areas. Mother and child’s health cares are among the most important health services, which are provided by the health centers under the supervision of the Iranian ministry of health. The services include maternity and pregnancy cares, vaccination and monitoring child’s growth and development [17].
Samples were selected by cluster random sampling from different regions of Iran, so that from all health centers in Fars, Golestan, Kohkiluyeh and Boyer Ahmad, Yazd, Kermanshah, Hamadan, Hormozgan, Chaharmahal and Bakhtiari and South Khorasan provinces, as well as health centers in Mashhad and Zahedan cities. Four cities in each province were selected by cluster sampling from different geographic regions of the North, South, East and West, and in each of these areas, two health centers, one urban and one rural, were randomly selected. Pregnant women with a history of pre-gestational diabetes, use of drugs that affect glucose metabolism, such as steroids and chronic liver disease, endocrine disorders and connective tissue disorders were excluded from the study.
Diagnosis of GDM is based on the latest nationwide guidelines on GDM screening and diagnosis. Women with GDM were identified by a GCT test by taking 50 g of glucose and the blood glucose was measured one hour later. The test result, as low as 130 mg/dL were considered negative and disease free, but equal to or greater than 130 mg/dLwere deemed to be positive in this program, and OGTT testing with 100 g of glucose was performed for those subjects. Finally, the diagnosis was based on Carpenter’s criteria [17], so that if at least two of the glucose tests were positive, the GDM was confirmed.
OUTCOMES OF PREGNANCY
Adverse pregnancy outcomes: Maternal and neonatal outcomes included preterm delivery, type of delivery (vaginal or C-section), and neonatal preterm birth. Fetal/neonatal outcomes included macrosomia, abortion, stillbirth, neonatal death, and low birth weight. Preterm delivery and preterm birth were defined as delivery, or birth before 37 weeks of gestation. Stillbirth was defined as infant death at 22 weeks of gestation or after, and abortion was defined as neonatal birth before 22 weeks of gestation. Weight less than 2500 grams at birth, was defined as low birth weight (LBW). Birth weight of 4000 g or more was considered as macrosomia of the newborn [4].
DATA COLLECTION
Required data were collected based on the family records of pregnant women in health centers, and by in-person interviews to complete the questionnaires, using trained individuals in health centers in the designated provinces. The questionnaire included demographic characteristics such as maternal age, place of residence, education, ethnic, domestic violence during pregnancy and the information on the outcomes of GDM, including type of delivery, preterm birth, macrosomia, abortion, stillbirth, neonatal death, and infant birth weight.
Weight gain during pregnancy was defined according to the recommended weight gain by the World Health Organization (WHO) guidelines and it was adjusted by BMI categories [18]. We used five questions regarding domestic violence during pregnancy to determine the experience of it during pregnancy. It was defined as a binary variable for analysis.
STATISTICAL ANALYSIS
Statistical analysis of data was performed at 95% CI, using STATA 14.2 software (StataCorp, College Station, TX, USA). Descriptive statistics were reported in frequency and percentage. Chi-square test was used to compare the frequency of pregnancy outcomes between diabetic and non-diabetic pregnant mothers. Univariate and multiple logistic regression analysis was used to evaluate the relationship between variables and GDM.
Results
In this study, 3,675 pregnant mothers from 11 provinces of Iran with mean age of 27.2 ± 6.0 years were participated. Overall, four percent of Iranian pregnant women had GDM during pregnancy. Most pregnancies were in the age group of 26-35 years (46.78%) and the highest prevalence of GDM was in the age group of 26-35 years (50.71%). The proportion of GDM was higher in urban mothers compared to the rural mothers. The prevalence of C- section was significantly higher in diabetic patients than in the non-diabetic women (53.19 vs 46.81, P < 0.001). Abortion in diabetic mothers was more than twice that of the non-diabetic mothers (P < 0.001) (Tab. I).
Tab. I.
Descriptive characteristics of participants by gestational diabetes.
| Variables | Categories | Total | Gestational diabetes | |
|---|---|---|---|---|
| Yes | No | |||
| No. (%) | No. (%) | |||
| Mother’s age | < 20 | 469 (13.19) | 9 (6.43) | 460 (13.46) |
| 21-25 | 1,090 (30.64) | 19 (13.57) | 1,071 (31.34) | |
| 26-35 | 1,664 (46.78) | 71 (50.71) | 1,593 (46.62) | |
| 36-40 | 253 (7.11) | 30 (21.43) | 223 (6.53) | |
| > 41 | 81 (2.28) | 11 (7.86) | 70 (2.05) | |
| P-value* | < 0.001 | |||
| Living location | Urban | 1,528 (45.34) | 71 (51.82) | 1,599 (45.59) |
| Rural | 1,842 (54.66) | 66 (48.18) | 1,908 (54.41) | |
| P-value | 0.135 | |||
| Education | Illiterate | 192 (5.560 | 12 (8.51) | 204 (5.67) |
| Under diploma | 1,676 (48.51) | 69 (48.94) | 1,745 (48.53) | |
| Diploma | 1,251 (36.21) | 38 (26.95) | 1,289 (35.85) | |
| Academic | 336 (9/73) | 22 (15.60) | 358 (9.96) | |
| P-value | 0.018 | |||
| Type of delivery | Vaginal | 2,340 (68.18) | 75 (53.19) | 2,415 (67.59) |
| Cesarean | 1,092 (31.82) | 66 (46.81) | 1,158 (32.41) | |
| P value | < 0.001 | |||
| Birth weight | < 2,500 | 1,336 (63.30) | 33 (47.83) | 1,303 (66.96) |
| 2,500-4,000 | 637 (31.61) | 30 (43.48) | 607 (31.19) | |
| > 4,000 | 42 (2.08) | 6 (8.70) | 36 (1.85) | |
| P-value | < 0.001 | |||
| Weight gain during pregnancy | 0-11.5 kg | 332 (10.61) | 9 (7.89) | 323 (10.72) |
| 12-14 kg | 1,447 (46.26) | 64 (56.14) | 1,383 (45.89) | |
| > 14 kg | 1,349 (43.13) | 41 (35.96) | 1,308 (43.40) | |
| P-value | 0.094 | |||
| Gestational age | > 37 week | 1,174 (60.64) | 27 (42.19) | 1,147 (61.27) |
| < 37 week | 762 (39.36) | 37 (57.81) | 725 (38.73) | |
| P-value | 0.002 | |||
| Abortion | Yes | 475 (13.18) | 41 (29.08) | 434 (12.54) |
| No | 3,128 (86.82) | 100 (70.92) | 3,028 (87.46) | |
| P-value | < 0.001 | |||
| Birth outcome | Live birth | 1,016 (28.20) | 27 (10.15) | 989 (28.57) |
| Stillbirth | 1,438 (39.91) | 65 (46.10) | 1,373 (39.66) | |
| Infant death | 1,149 (31.89) | 49 (34.75) | 1,100 (31.77) | |
| P-value | 0.049 | |||
* P value < 0.05, using the Chi-squared test.
Table II presents the association between gestational diabetes and adverse pregnancy outcomes. The independent variable is GDM that was included into the logistic regression model with adverse pregnancy outcomes. The variables of age, education, place of residence, ethnicity and physical violence were considered as confounding factors in the logistic regression analysis.
Tab. II.
Crude and adjusted odds ratios (95% CI) of the association between gestational diabetes and adverse pregnancy outcomes.
| Variables | Categories | Unavailable Odds Ratio (95% CI) | P-value | Adjusted Odds Ratio (95% CI) | P-value |
|---|---|---|---|---|---|
| Birth outcome | Live birth | Ref. | - | Ref. | - |
| Stillbirth | 1.73 (1.09, 2.73) | 0.018 | 1.80 (1.11, 2.91) | 0.018 | |
| Infant’s death | 1.63 (1.01, 2.63) | 0.044 | 1.72 (1.03, 2.85) | 0.036 | |
| Type delivery | Vaginal | Ref. | - | Ref. | - |
| Cesarean | 1.88 (1.34, 2.64) | < 0.001 | 1.53 (1.07, 2.19) | 0.02 | |
| Birth weight | Normal | Ref. | - | Ref. | - |
| Macrosomia (> 4,000 gr) | 6.58 (2.59, 16.69) | < 0.001 | 6.94 (2.81, 17.14) | < 0.001 | |
| Weight gain during pregnancy | 12-14 kg | Ref. | - | Ref. | - |
| > 14 kg | 1.66 (0.81, 3.37) | 0.160 | 1.20 (0.57, 2.53) | 0.636 | |
| Preterm birth | No | Ref. | - | Ref. | - |
| Yes (< 37 weeks) | 2.16 (1.30, 3.59) | 0.003 | 1.82 (1.07, 3.09) | 0.028 | |
| Abortion History | No | Ref. | - | Ref. | |
| Yes | 2.86 (1.96, 4.17) | < 0.001 | 1.84 (1.21, 2.78) | 0.004 |
* Adjusted for age, place of residency, ethnic, physical activity.
GDM increases the adverse outcomes of pregnancy, with a 63% higher risk of neonatal death in diabetic mothers than the non-diabetic mothers (95% CI: 1.01, 2.63, P = 0.044). Abortion in pregnant women with GDM was 2.86 times more than the non-diabetic mothers (95% CI: 1.96, 4.17, P < 0.001).
Adjusted logistic regression model showed that the chance of stillbirth in women with GDM was 80% higher than in the non-diabetic women (95% CI: 1.11, 2.91, P = 0.018). The chance of macrosomia in diabetic women was about 7 times higher, compared to the non-diabetic women (95% CI: 2.81, 17.14, P < 0.001). The chances of preterm birth were about two times higher in women with GDM than in women without GDM. GDM in women with history of C-section had a higher chance of 50% than women who did not have C-section (95% CI: 1.07, 2.19, P = 0.02).
The chance of overweight pregnancy (over 14 kg) were about 20% higher in women with GDM than in the non-diabetic women. A significant increasing trend was found in the odds of having GDM in terms of body mass index (P < 0.001). There was also a significant decrease in the odds of GDM, according to the daily physical activity (PA) (P < 0.001) (Fig. 1).
Fig. 1.
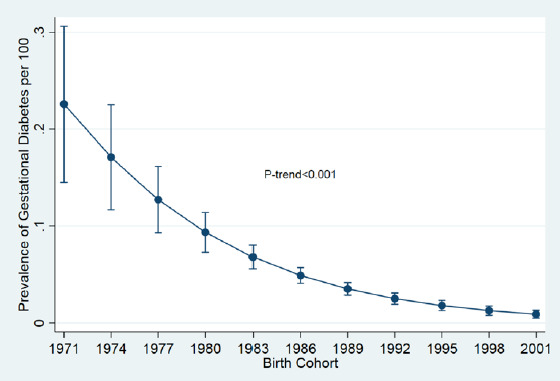
Prevalence trend of GDM during pregnancy in Iran from 1971 to 2001.
Discussion
The findings of the present study, conducted in 11 provinces of Iran, showed that GDM increased the adverse outcomes of pregnancy such as stillbirth, neonatal death, macrosomia, preterm birth, abortion, and cesarean delivery. A significant decreasing trend in the GDM was found by birth cohort in the pregnant Iranian women. In diabetic mothers, the odds of macrosomia, miscarriage, and preterm birth were 6.9, 1.84 and 1.82 times of the non-diabetic mothers, respectively. These consequences can decrease the quality of life of the mother and infant in the short term or until the end of life. Some of these outcomes like macrosomia are not treatable, so early prevention is very important.
However, multifactorial congenital anomalies and their causes are largely unknown, but meanwhile, several non-hereditary factors including maternal age, pre- and intra-pregnancy diabetes, maternal obesity, and folic acid deficiency play an important role in their development [19, 20]. The findings of this study indicate that GDM is one of the important and changeable factors that increases the chance of congenital anomalies. The macrosomia was 7 times higher in mothers with GDM than the non-diabetic mothers. The results of a study conducted in Canada during the years 2002–2012 is consistent with the results of the present study, and shows an increasing trend of macrosomia in diabetic mothers.
The percentage of population attributable risk (PAR%) of congenital malformations associated with diabetes mellitus increased from 0.6 to 1.2% in pregnant women. PAR% was increased from 2.3 to 4.2 and 0.8 to 1.4, in congenital cardiovascular malformations and gastrointestinal deficiency, respectively [21]. Numerous studies have reported a considerable high incidence of congenital malformations in diabetic mothers than the non-diabetic mothers [12, 19]. The study of Nelson et al. has also shown that GDM increases the risk of congenital malformations by 1.2 times, especially obstructive urinary tract disorders, renal agenesis, cardiovascular disorders, and multiple congenital abnormalities [12]. The cost of treatment of these outcomes is very high and on the other hand, complete improvement is often not feasible and may affect on quality of life. Accordingly, there is a necessity for better implementation of primary care and screening before pregnancy.
We found that the chance of stillbirth and neonatal death in women with GDM was about 80% higher than that of the non-diabetics. Previous studies have also reported that GDM is associated with stillbirth and neonatal death. In a study, the infant mortality rate (death rate per 1,000 or relative risk) in diabetic mothers was 15.5 vs 2.8, stillbirths in diabetic mothers, 9.7 vs 4, compared to the non-diabetic women, and both outcomes were significantly greater in the diabetic mothers. Perinatal deaths in diabetic mothers were more than 3 times higher than in the non-diabetic mothers [11]. Given the adverse physical and psychological effects of this outcome for mothers, it is recommended that pre-pregnancy health care be provided at counseling clinics to reduce these unpleasant outcomes. Control and regulation of blood sugar before pregnancy can reduce the neonatal deaths from GDM.
Having a body mass index (BMI) less than or above the normal range is considered as a high-risk pregnancy [22]. Maternal obesity during pregnancy increases premature birth and the risk of C-section delivery which is associated with GDM, preeclampsia, intrauterine growth restriction (IUGR) and thromboembolic events [23]. Numerous studies have reported a relationship between high BMI during pregnancy and increased risk of GDM and insulin resistance [24, 25]. In the present study, the chance of weight gain (over 14 kg) in mothers with GDM was about 20% higher, compared to the non-diabetic mothers.
A significant increasing trend in the odds of GDM was found in terms of BMI in the results. Since there is a non-linear dose-response relationship between concomitant increases in gestational BMI and GDM [25, 26], the incidence of GDM also increases with increasing BMI levels, such as in those with low birth weight is 5.5% and in obese individuals is 14.6% [25]. Therefore, high BMI at the beginning of pregnancy is a serious alarm for GDM that should be considered in prenatal care. In fact, GDM increases the risk of type 2 diabetes, but the results of various previous studies have shown that the risk of type 2 diabetes decreases with weight loss, and increased by PA during pregnancy and postpartum as well [27]. Based on the similar results of various studies and the importance of weight gain during pregnancy, it can be said that BMI can predict the occurrence of GDM in subsequent pregnancy [25].
In the present study, the chance of GDM in women with a history of C- section was about 50% higher, compared to women without a history of C- section. The association between GDM and increased risk of cesarean delivery has been reported in numerous studies [10, 13]. C-section in mothers with diabetes is approximately twice than that of the non-diabetic mothers [13]. Gorgal et al. in a study to determine whether GDM is associated with non-elective cesarean sectionshowed that the rate of non-selective C-section in diabetic mothers was 19.5% and in non-diabetic mothers 13.5% with a relative risk of 1.4 [10]. In another study, cesarean delivery was also significantly higher in diabetic mothers than in the non-diabetic mothers (50.8 vs 31.8%) [13]. Therefore, GDM is a risk factor for C-section and its associated complications, and timely diagnosis and control can reduce cesarean delivery and its complications.
The present study also revealed that the chance of preterm birth in mothers with GDM is about twice as high as those of the non-diabetic mothers. Other similar studies have also reported an increased chance of preterm delivery and preterm birth in diabetic mothers [28]. Another study has shown that preterm labor in diabetic mothers is not different from the non-diabetic mothers, but controlling blood glucose and bringing glucose levels to a normal level, reduce preterm birth rates in diabetic mothers [29].
Our study showed that the risk of GDM in mothers with a history of abortion was about 80% higher than women who had no history of abortion. The results of national and international studies are in line with our findings [30]. In the study of Jiang et al. the history of abortion in mothers with GDM was significantly higher than in the non-diabetic mothers (39.8 vs 30.5%) [31]. A study by Feleke et alalso showed the association of GDM with a history of abortion, and reported that abortion increases the risk of GDM in the future pregnancies. In his study, the chances of GDM in women with a history of abortion were 5 times higher [32], which may be due to the effect of abortion on impaired normal insulin metabolism in women [33].
Previous epidemiological studies have reported an inverse relationship between the amount of PA during pregnancy and GDM, and women with high levels of PA were significantly less likely to develop GDM [5]. The results of the present study also show a significant decrease in the chance of GDM in terms of daily PA. Increasing PA levels can reduce the risk of GDM in various approaches. First, PA can compensate for the defect in the insulin signaling pathway [34]. Second, PA may alter adipokine profile levels, including adiponectin, leptin, resistin, and Visfatin, which may lead to a decreased insulin resistance [35]. Third, PA by controlling the secretion and activity of inflammatory markers such as TNF-α and IL-6 can decrease the level of inflammation and insulin resistance inhibiting factor [36]. Fourth, PA can decrease insulin resistance in GDM by increasing levels of antioxidants such as superoxide dismutase, catalase and glutathione peroxidase, oxidative stress and the pathogenesis of insulin resistance in GDM [37]. Therefore, the importance of adequate PA during pregnancy is clearly understood.
Geographical extent and large sample size are the strengths of the present study. One of the limitations of the study is its cross-sectional design that does not indicate any causal relationships. Future cohort studies and clinical trials can provide more definitive conclusions by better controlling the confounding factors.
Conclusions
The results revealed that GDM could be considered as a predictive factor which increases the adverse pregnancy outcomes such as stillbirth, neonatal deaths, macrosomia, preterm birth, abortion and C-section delivery which are related with both maternal and fetus. Given that these adverse consequences are preventable and treatable, hence, early diagnosis of pregnant women at high-risk for GDM is suggested to implementation the educational program and to better prevent of the adverse pregnancy outcomes.
Acknowledgements
Funding sources: this national work was funded and supported by the Research Deputy of Shiraz, Hormozgan, and Kermanshah University of Medical Sciences.
The authors would like to thank the Clinical Research Development Center of Imam Reza Hospital for their wise advices.
Figures and tables
Footnotes
Ethical approval
This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (IR.KUMS.REC.1400.385).
Conflict of interest statement
The authors declare no conflict of interest.
Authors’ contributions
Conception and design was done by MH, SRe, and TV; collection and assembly of data was done by HY, MS, MH, NT, and TV; data analysis and interpretation was done by SRe, MDa, KE and MDi; manuscript writing was done by SRe, MDa, and MDi; final approval of manuscript was done by all authors.
References
- [1].Erjavec K, Poljičanin T, Matijević R. Impact of the implementation of new WHO diagnostic criteria for gestational diabetes mellitus on prevalence and perinatal outcomes: a population-based study. J Pregnancy 2016;2016:2670912. https://doi.org/10.1155/2016/2670912 10.1155/2016/2670912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eades CE, Cameron DM, Evans JM. Prevalence of gestational diabetes mellitus in Europe: a meta-analysis. Diabetes Res Clin Pract 2017;129:173-81. [DOI] [PubMed] [Google Scholar]
- [3].Mwanri AW, Kinabo J, Ramaiya K, et al. Gestational diabetes mellitus in sub-Saharan Africa: systematic review and metaregression on prevalence and risk factors. Trop Med Int Health 2015;20:983-1002. https://doi.org/10.1111/tmi.12521 10.1111/tmi.12521 [DOI] [PubMed] [Google Scholar]
- [4].Alfadhli EM, Osman EN, Basri TH, et al. Gestational diabetes among Saudi women: prevalence, risk factors and pregnancy outcomes. Ann Saudi Med 2015;35:222-30. https://doi.org/10.5144/0256-4947.2015.222 10.5144/0256-4947.2015.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nguyen CL, Pham NM, Binns CW, et al. Prevalence of gestational diabetes mellitus in eastern and southeastern Asia: a systematic review and meta-analysis. J Diabetes Res 2018;2018:6536974. https://doi.org/10.1155/2018/6536974 10.1155/2018/6536974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wahi P, Dogra V, Jandial K, et al. Prevalence of gestational diabetes mellitus (GDM) and its outcomes in Jammu region. J Assoc Physicians India 2011;59:227-30. [PubMed] [Google Scholar]
- [7].Alwan N, Tuffnell DJ, West J. Treatments for gestational diabetes. Cochrane Database Syst Rev 2009;2009:CD003395. https://doi.org/10.1002/14651858.CD003395.pub2 10.1002/14651858.CD003395.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30(Suppl 2):S251-60. https://doi.org/10.2337/dc07-s225 10.2337/dc07-s225 [DOI] [PubMed] [Google Scholar]
- [9].Ashwal E, Hod M. Gestational diabetes mellitus: where are we now? Clin Chim Acta 2015;451:14-20. https://doi.org/10.1016/j.cca.2015.01.021 10.1016/j.cca.2015.01.021 [DOI] [PubMed] [Google Scholar]
- [10].Gorgal R, Gonçalves E, Barros M, et al. Gestational diabetes mellitus: a risk factor for non-elective cesarean section. J Obstet Gynaecol Res 2012;38:154-9. https://doi.org/10.1111/j.1447-0756.2011.01659.x 10.1111/j.1447-0756.2011.01659.x [DOI] [PubMed] [Google Scholar]
- [11].Yang J, Cummings EA, O’Connell C, et al. Fetal and neonatal outcomes of diabetic pregnancies. Obstet Gynecol 2006;108:644-50. https://doi.org/10.1097/01.AOG.0000231688.08263.47 10.1097/01.AOG.0000231688.08263.47 [DOI] [PubMed] [Google Scholar]
- [12].Nielsen GL, Norgard B, Puho E, et al. Risk of specific congenital abnormalities in offspring of women with diabetes. Diabet Med 2005;22:693-6. https://doi.org/10.1111/j.1464-5491.2005.01477.x 10.1111/j.1464-5491.2005.01477.x [DOI] [PubMed] [Google Scholar]
- [13].Aviram A, Guy L, Ashwal E, Hiersch L, Yogev Y, Hadar E. Pregnancy outcome in pregnancies complicated with gestational diabetes mellitus and late preterm birth. Diabetes Res Clin Pract 2016;113:198-203. https://doi.org/10.1016/j.diabres.2015.12.018 10.1016/j.diabres.2015.12.018 [DOI] [PubMed] [Google Scholar]
- [14].Sit D, Luther J, Dills JL, Eng H, Wisniewski S, Wisner KL. Abnormal screening for gestational diabetes, maternal mood disorder, and preterm birth. Bipolar Disord 2014;16:308-17. https://doi.org/10.1111/bdi.12129 10.1111/bdi.12129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Frumento D, Ben Nasr M, El Essawy B, D’Addio F, Zuccotti GV, Fiorina P. Immunotherapy for type 1 diabetes. J Endocrinol Invest 2017;40:803-14. https://doi.org/10.1007/s40618-017-0641-y 10.1007/s40618-017-0641-y [DOI] [PubMed] [Google Scholar]
- [16].Ben Nasr M, Frumento D, Fiorina P. Adipose Stem Cell Therapy for Chronic Pancreatitis. Mol Ther 2017;25:2438-9. https://doi.org/10.1016/j.ymthe.2017.10.007 10.1016/j.ymthe.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fatemi MJ, Fararouei M, Moravej H, Dianatinasab M. Stunting and its associated factors among 6-7-year-old children in southern Iran: a nested case-control study. Public Health Nutr 2018. Oct 15:1-8. https://doi.org/10.1017/S136898001800263X 10.1017/S136898001800263X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].World Health Organization. Global database on body mass index: an interactive surveillance tool for monitoring nutrition transition Geneva: WHO 2009. Available from: http://www.who.int/bmi/index.jsp
- [19].American Diabetes Association. Classification and diagnosis of diabetes mellitus. Diabetes Care 2006;29(Suppl 1):S4-S7. https://doi.org/10.2337/dc21-S002 10.2337/dc21-S002 [DOI] [PubMed] [Google Scholar]
- [20].Lisowski LA, Verheijen PM, Copel JA, Kleinman CS, Wassink S, Visser GH, Meijboom EJ. Congenital heart disease in pregnancies complicated by maternal diabetes mellitus. An international clinical collaboration, literature review, and meta-analysis. Herz 2010;35:19-26. https://doi.org/10.1007/s00059-010-3244-3 10.1007/s00059-010-3244-3 [DOI] [PubMed] [Google Scholar]
- [21].Macumber I, Schwartz S, Leca N. Maternal obesity is associated with congenital anomalies of the kidney and urinary tract in offspring. Pediatr Nephrol 2017;32:635-42. https://doi.org/10.1007/s00467-016-3543-x 10.1007/s00467-016-3543-x [DOI] [PubMed] [Google Scholar]
- [22].Liu S, Rouleau J, León JA, Sauve R, Joseph KS, Ray JG; Canadian Perinatal Surveillance System. Impact of pre-pregnancy diabetes mellitus on congenital anomalies, Canada, 2002-2012. Health Promot Chronic Dis Prev Can 2015;35:79-84. https://doi.org/10.24095/hpcdp.35.5.01 10.24095/hpcdp.35.5.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Nagle C, Skouteris H, Hotchin A, Bruce L, Patterson D, Teale G. Continuity of midwifery care and gestational weight gain in obese women: a randomised controlled trial. BMC Public Health 2011;11:174. https://doi.org/10.1186/1471-2458-11-174 10.1186/1471-2458-11-174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Basraon SK, Mele L, Myatt L, Roberts JM, Hauth JC, Leveno KJ, Varner MW, Wapner RJ, Thorp JM, Jr, Peaceman AM, Ramin SM, Sciscione A, Tolosa JE, Sorokin Y; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal–Fetal Medicine Units Network. Relationship of Early Pregnancy Waist-to-Hip Ratio versus Body Mass Index with Gestational Diabetes Mellitus and Insulin Resistance. Am J Perinatol 2016;33:114-21. https://doi.org/10.1055/s-0035-1562928 10.1055/s-0035-1562928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Makgoba M, Savvidou MD, Steer PJ. An analysis of the interrelationship between maternal age, body mass index and racial origin in the development of gestational diabetes mellitus. BJOG 2012;119:276-82. https://doi.org/10.1111/j.1471-0528.2011.03156.x 10.1111/j.1471-0528.2011.03156.x [DOI] [PubMed] [Google Scholar]
- [26].Hashemi-Nazari SS, Najafi F, Rahimi MA, Izadi N, Heydarpour F, Forooghirad H. Estimation of gestational diabetes mellitus and dose-response association of BMI with the occurrence of diabetes mellitus in pregnant women of the west of Iran. Health Care Women Int 2020;41:121-30. https://doi.org/10.1080/07399332.2018.1521812 10.1080/07399332.2018.1521812 [DOI] [PubMed] [Google Scholar]
- [27].Rana JS, Li TY, Manson JE, Hu FB. Adiposity compared with physical inactivity and risk of type 2 diabetes in women. Diabetes Care 2007;30:53-8. https://doi.org/10.2337/dc06-1456 10.2337/dc06-1456 [DOI] [PubMed] [Google Scholar]
- [28].Boriboonhirunsarn D, Waiyanikorn R. Emergency cesarean section rate between women with gestational diabetes and normal pregnant women. Taiwan J Obstet Gynecol 2016;55:64-7. https://doi.org/10.1016/j.tjog.2015.08.024 10.1016/j.tjog.2015.08.024 [DOI] [PubMed] [Google Scholar]
- [29].Deryabina EG, Yakornova GV, Pestryaeva LA, Sandyreva ND. Perinatal outcome in pregnancies complicated with gestational diabetes mellitus and very preterm birth: case-control study. Gynecol Endocrinol 2016;32(Suppl 2):52-5. https://doi.org/10.1080/09513590.2016.1232215 10.1080/09513590.2016.1232215 [DOI] [PubMed] [Google Scholar]
- [30].[Yogev Y, Langer O. Spontaneous preterm delivery and gestational diabetes: the impact of glycemic control. Arch Gynecol Obstet 2007;276:361-5. https://doi.org/10.1007/s00404-007-0359-8 10.1007/s00404-007-0359-8 [DOI] [PubMed] [Google Scholar]
- [31].Garshasbi A, Faghihzadeh S, Naghizadeh MM, Ghavam M. Prevalence and risk factors for gestational diabetes mellitus in Tehran. J Family Reprod Health 2008;2:75-80. [Google Scholar]
- [32].Jiang TT, Zhao L, Lin Y, Zhou D, Wang L, Sun GQ, Xiao M. Effects of gestational diabetes mellitus on time to delivery and pregnancy outcomes in full-term pregnancies with dinoprostone labor induction. Clin Exp Hypertens 2019;41:44-8. https://doi.org/10.1080/10641963.2018.1441859 10.1080/10641963.2018.1441859 [DOI] [PubMed] [Google Scholar]
- [33].Feleke BE. Determinants of gestational diabetes mellitus: a case-control study. J Matern Fetal Neonatal Med 2018;31:2584-9. https://doi.org/10.1080/14767058.2017.1347923 10.1080/14767058.2017.1347923 [DOI] [PubMed] [Google Scholar]
- [34].Leng J, Liu G, Zhang C, Xin S, Chen F, Li B, Tian H, Yu Z, Tuomilehto J, Hu G, Yang X. Physical activity, sedentary behaviors and risk of gestational diabetes mellitus: a population-based cross-sectional study in Tianjin, China. Eur J Endocrinol 2016;174:763-73. https://doi.org/10.1530/EJE-15-1103 10.1530/EJE-15-1103 [DOI] [PubMed] [Google Scholar]
- [35].Golbidi S, Laher I. Potential mechanisms of exercise in gestational diabetes. J Nutr Metab 2013;2013:285948. https://doi.org/10.1155/2013/285948 10.1155/2013/285948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Daniele G, Guardado Mendoza R, Winnier D, Fiorentino TV, Pengou Z, Cornell J, Andreozzi F, Jenkinson C, Cersosimo E, Federici M, Tripathy D, Folli F. The inflammatory status score including IL-6, TNF-α, osteopontin, fractalkine, MCP-1 and adiponectin underlies whole-body insulin resistance and hyperglycemia in type 2 diabetes mellitus. Acta Diabetol 2014;51:123-31. https://doi.org/10.1007/s00592-013-0543-1 10.1007/s00592-013-0543-1 [DOI] [PubMed] [Google Scholar]
- [37].Hayashino Y, Jackson JL, Hirata T, Fukumori N, Nakamura F, Fukuhara S, Tsujii S, Ishii H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism 2014;63:431-40. https://doi.org/10.1016/j.metabol.2013.08.018 10.1016/j.metabol.2013.08.018 [DOI] [PubMed] [Google Scholar]


