Abstract
The basidiomycetous yeast Rhodosporidium toruloides (anamorph, Rhodotorula glutinis) is a common phylloplane epiphyte with biocontrol potential. To understand how R. toruloides adheres to plant surfaces, we obtained nonadherent fungal mutants after chemical mutagenesis with methane-sulfonic acid ethyl ester. Sixteen attachment-minus (Att−) mutants were identified by three methods: (i) screening capsule-minus colonies for loss of adhesive ability; (ii) enrichment for mutants unable to attach to polystyrene; and (iii) selection for reduced fluorescence of fluorescein isothiocyanate-concanavalin A (Con A)-stained cells by fluorescence-activated cell sorting. None of the 16 mutants attached to polystyrene or barley leaves. The lectin Con A eliminated adhesion in all of the wild-type isolates tested. Hapten competition assays indicated that Con A bound to mannose residues on the cell surface. Adhesion of wild-type R. toruloides was transient; nonadhesive cells subsequently became adhesive, with bud development. All Att− mutants and nonattaching wild-type cells lacked polar regions that stained intensely with fluorescein isothiocyanate-Con A and India ink. Lectin, enzyme, and chemical treatments showed that the polar regions consisted of alkali-soluble materials, including mannose residues. Tunicamycin treatment reduced wild-type adhesion, indicating that the mannose residues could be associated with glycoproteins. We concluded that compounds, including mannose residues, that are localized at sites of bud development mediate adhesion of R. toruloides to both polystyrene and barley leaf surfaces.
Attachment of microorganisms to surfaces presumably has survival value and may be required for colonization (28). Most of the data available on the adhesion of fungi to plant surfaces concerns preinfection stages of filamentous fungal pathogens (for reviews see references 5, 17, and 37). Leaf surfaces are colonized by members of several genera of saprophytic yeasts that provide a natural buffer against plant pathogens (20). It has been shown that phylloplane yeasts have biological control potential (7, 18, 20), yet virtually nothing is known about how these organisms adhere to plants. Yeast attachment to various synthetic surfaces has been studied with the human pathogens Candida albicans and Cryptococcus neoformans (13, 27).
Many fungal adhesives appear to be cell surface glycoproteins. Incubation of fungal cells with the lectin concanavalin A (Con A), which binds to glycoproteins, reduces adhesion of Colletotrichum graminicola to polystyrene or dimethyldichlorosilane-coated glass (34), adhesion of Magnaporthe grisea to teflon (23), and adhesion of Nectria haematococca to polystyrene (30). Glycoproteins mediate the attachment of C. albicans to plastic (41) or acrylic (33) and the adherence of Puccinia sorghi to plastic and glass (8). Bipolaris sorokiniana adheres to glass with surface polysaccharides composed of galactosaminoglycans (38). Not all putative adhesives are glycoproteins or polysaccharides; attachment of Colletotrichum musae to banana fruit (40) and attachment of Uromyces appendiculatis to bean leaves (16) is mediated by cell surface proteins.
Formation of an extracellular polysaccharide (EPS) capsule is a common in vitro characteristic of leaf surface yeasts (9), and yeasts appear to produce slime on the phylloplane (3). The role of the capsule in attachment of yeasts to leaf surfaces is unclear. It has been speculated that yeast capsules promote adhesion to leaves, thereby preventing cells from being dislodged by wind and rain (10). EPS apparently plays a role in adhesion of the yeastlike fungus Aureobasidium pullulans to cellulose membranes and apple leaves (1). However, Pertsovskaya and Golubev observed that the presence of capsules on yeast cells decreased the adhesive strength severalfold, as reported by Golubev (22).
To investigate the possible adhesion mechanism(s) of leaf surface yeasts, we chose as a model system the basidiomycetous yeast Rhodosporidium toruloides Banno (anamorph, Rhodotorula glutinis [Fresenius] Harrison), a common component of phylloplane communities (9). Unlike A. pullulans, about which little is known genetically, R. toruloides is haploid and amenable to mutagenesis (42). It has exhibited biological control potential against Botrytis cinerea on the phylloplanes of tomato and bean (15) and on apple fruit surfaces during postharvest storage (18). The objectives of this study were to produce and characterize mutants that were not able to attach and to determine how R. toruloides adheres to barley leaves. We present evidence that adhesion of R. toruloides to polystyrene and leaves is not mediated by the EPS capsule directly but is mediated by a region of material that probably includes mannoproteins localized at the poles of cells. Attachment appears to be transient and most pronounced in actively dividing cells.
MATERIALS AND METHODS
Yeast strains and inoculum.
Wild-type cultures of R. toruloides NRRL Y-1588, NRRL Y-1091, NRRL Y-6672, and NRRL Y-17902 were obtained from the Agricultural Research Service Culture Collection, Peoria, Ill. R. toruloides Rg1 and G27 were provided by D. Becher, Ernst-Moritz-Arndt-Universität, Greifswald, Germany. Stock cultures were stored in 15% glycerol at −80°C. Working cultures were maintained on potato-carrot agar at 4°C. To prepare inocula, plates containing yeast nitrogen base (YNB) (Difco Laboratories, Detroit, Mich.) supplemented with 2% glucose were streaked and incubated at 28°C for 2 to 3 days. We used cells obtained from a single colony to inoculate a 50-ml flask containing liquid YNB, which was incubated at 26°C for 24 h with agitation (100 rpm). Portions (100 μl) of the resulting 24-h culture were used as inocula for 50-ml YNB cultures, which were the final inocula. Unless stated otherwise, cells were grown to the mid-log phase (approximately 107 cells ml−1), harvested by centrifugation (3,000 × g, 10 min), and washed twice with the appropriate test buffer.
Plant growth conditions.
Barley (Hordeum vulgare cv. Hazen) was provided by the Wisconsin Foundation Seed Program, Madison. Barley seeds were sown in Redi-Earth Peat Lite Mix (Scotts-Sierra Horticultural Products Co., Marysville, Ohio) and incubated at 24°C with a light regimen consisting of 12 h of light (approximately 180 microeinsteins s−1 m−2 at pot level) and 12 h of darkness. The first fully expanded leaf of individual 9- to 11-day-old plants was used for the adhesion assays.
Adhesion assay.
Adhesion was expressed in terms of the number of yeast cells removed from test surfaces by agitation compared to the original inoculum density that was applied (30). Briefly, cells were suspended in 10 mM sodium phosphate buffer (pH 7.0) at a concentration of 3.5 × 106 cells ml−1 and applied in 150- or 175-μl drops onto either 3-cm-long barley leaf segments or 1.5-cm2 polystyrene squares (Ward’s Plastics, Rochester, N.Y.), and the preparations were incubated in a moist chamber for either 1 h (polystyrene) or 2 h (barley). The drops and test surfaces were placed into 2-ml silicon-coated microcentrifuge tubes (Sigma Chemical Co., St. Louis, Mo.) along with 1 ml of buffer and agitated with a Vortex Genie agitator (Scientific Products, McGaw Park, Ill.) for 10 s at setting 3. The test surfaces were removed, and the yeast cells in the remaining solution were counted with an electronic particle counter equipped with a 76-μm orifice and a 500-μm sample tube (Elzone model 80 XY; Particle Data Inc., Elmhurst, Ill.). We determined the initial inoculum size by placing a 150- or 175-μl drop of the yeast suspension and 1 ml of buffer into a comparable tube without a test surface and counting the cells as described above. Prior to enumeration, the yeast cells were killed by adding a 1-mg ml−1 thimerosal (Spectrum Chemical Mfg. Corp., Gardena, Calif.) solution to each tube (final concentration, 100 μg ml−1). The level of adhesion was determined as follows: (number of cells recovered after incubation on test surface/initial number in inoculum) × 100%. All experiments were performed at least twice.
Production of R. toruloides attachment-minus mutants.
R. toruloides NRRL Y-1588 was mutagenized with methane-sulfonic acid ethyl ester (EMS) (31). Unless stated otherwise, EMS treatment killed approximately 50% of the initial population. Three methods were used to select for R. toruloides attachment-minus (Att−) mutants. First, mutagenized cells were plated directly onto capsule-inducing agar (yeast carbon base [Difco Laboratories] containing 3% glucose), and the nonencapsulated colonies were screened for loss of adhesive ability by the assay described above. Second, Att− mutants were obtained by a polystyrene enrichment procedure (26). Following mutagenesis, the cells were divided into separate populations and grown for 24 h in 50 ml of YPD (1% yeast extract, 2% peptone, 2% glucose). Each culture containing mutagenized cells was pelleted by centrifugation (3,000 × g, 10 min) and washed with 50 mM potassium phosphate buffer (pH 7.0). Aliquots (9 ml) of each cell population (106 cells ml−1) were placed in 9-cm polystyrene petri dishes and incubated for 90 min at room temperature. Nonattached cells were resuspended by agitating the plates gently (100 rpm) for 3 min and were transferred to new petri dishes. This process was repeated through three passages. The final enriched, nonadhesive fraction was pelleted, resuspended in phosphate buffer, and plated onto YPD amended with 50 μg of chloramphenicol per ml. From each plate representing a separate initial population of mutagenized cells, 12 to 18 colonies were retested for the loss of adhesive ability as described above. Third, potential Att− mutants were obtained by fluorescence activated cell sorting (FACS) by selecting for fluorescein isothiocyanate (FITC)-Con A (Vector Laboratories, Burlingame, Calif.)-stained cells that exhibited 10- to 100-fold less fluorescence than nonmutagenized cells. Mutagenized cells were grown for 24 h in 50 ml of YPD broth. The cells were pelleted, washed with lectin buffer (10 mM HEPES [pH 7.5], 0.15 M NaCl, 0.1 mM CaCl2, 0.01 mM MnCl2), and incubated with 50 μg of FITC-Con A per ml for 30 min at 22°C. The cells were sorted with an EPICS Elite flow cytometer (Coulter Corporation, Miami, Fla.) equipped with a 488-nm argon ion laser tuned to 15 mW. FITC fluorescence was detected by using a 525-nm band pass filter. Cells that exhibited reduced FITC-Con A fluorescence were collected individually by using the AUTOCLONE adaptation with 96-well microtiter plates containing (per well) 250 μl of YPD containing 50 μg of chloramphenicol per ml and were incubated at 28°C for 2 to 3 days. Putative Att− colonies were tested for loss of adhesive ability as described above.
All Att− mutants were tested for presence of auxotrophic mutations, and the growth rates in YNB were determined. Mutants that were markedly debilitated (e.g., had reduced growth rates) were discarded.
Determination of cell surface polysaccharides and effect of lectins on adhesion.
The presence of specific cell surface polysaccharides on R. toruloides was determined by using FITC-labeled lectins with different sugar affinities (Fluorescein Lectin Kit I; Vector Laboratories). Cells were pelleted, washed in lectin buffer, and incubated with 200 μg of FITC-labeled lectins per ml for 30 min at 22°C, and the presence of a fluorescent signal was assessed by fluorescent microscopy. Lectins that bound to the cell surface, which indicated that the corresponding sugar or hapten was present, were tested to determine their effects on adhesion. Yeast cells were incubated with wheat germ agglutinin (WGA), Ulex europaeus agglutinin I (UEAI), or Con A at a concentration of 200 μg ml−1 for 30 min at 22°C and then applied to polystyrene or barley. The adhesion and FITC-Con A fluorescence patterns of Att− mutants were assessed as described above. We tested a lectin(s) that interfered with adhesion by using five additional wild-type R. toruloides isolates to ensure that the effects observed were not isolate specific.
To determine to which sugar moiety Con A bound on the cell surface of R. toruloides, we used a hapten competition assay (21). Various haptens (50 mM methyl mannoside, 50 mM methyl glucoside, 50 mM mannose, 50 mM glucose, 50 mM N-acetyl-d-glucosamine, 50 mM sucrose, and 50 mM phenyl glucoside) in 10 mM HEPES (pH 7.5) were incubated for 30 min at 22°C with 50 μg of FITC-Con A per ml before yeast cells were added. The compounds were assessed for their abilities to block FITC-Con A staining of R. toruloides cells.
Enrichment for nonattaching wild-type cells and cell surface staining patterns.
Nonattaching Y-1588 cells were enriched from mid-log-phase cultures by using carboxymethyl (CM)-Sephadex C-25 cation-exchange beads (Sigma). Adherent cells attached to CM beads, while nonadherent cells remained in the buffer solution. The beads were washed six times in 50 ml of 10 mM sodium phosphate buffer (pH 7.0) and then added to mid-log-phase cultures to give a final bead-to-volume ratio of 1:4 (beads were present in approximately 25% of the buffer volume). The bead-cell mixtures were incubated for 1 h at 22°C with gentle agitation (75 rpm). The beads and attached cells were allowed to settle and were discarded; nonattached cells in the supernatant were collected by vacuum filtration with 8.0-μm-pore-size filters and were resuspended in buffer. Adhesion to polystyrene and barley leaves was determined as described above.
We used India ink as a positive stain (29) with wild-type cells and Att− mutants to determine cell surface staining patterns and as a negative stain to detect EPS capsules.
Characterization of a potential adhesive(s) with enzyme and chemical treatments of wild-type R. toruloides.
Nonadhesive wild-type cells were obtained by CM-Sephadex enrichment as described above and were resuspended in conditioned medium in order to obtain relatively uninterrupted growth without a lag phase in the new medium (36). Conditioned medium was prepared by centrifugation of mid-log-phase Y-1588 cultures and filter sterilization (pore size, 0.22 μm) of the supernatants. To determine if glycoprotein synthesis was involved in adhesion, we incubated cells for 9 h on an orbital shaker (250 rpm, 22°C) with or without tunicamycin (5 μg ml−1). Treated and control cells were collected by vacuum filtration, washed, and resuspended in phosphate buffer, and the level of adhesion was determined.
Our chemical characterization of potential adhesive materials included the use of the following enzymes (all obtained from Sigma). Sulfatase (25 U ml−1), α-mannosidase (2 U ml−1), crude glucuronidase (type HP-2; crude solution from Helix pomatia; 3,300 U ml−1), and purified glucuronidase (type VII-A; 3,125 U ml−1) were incubated with cells (107 cells ml−1) in 50 mM sodium acetate buffer (pH 4.8). The crude glucuronidase solution contained up to 5,000 U of sulfatase activity per ml according to the manufacturer. The purified glucuronidase solution contained less than 0.05% β-galactosidase, β-N-acetyl glucosamidase, α-galactosidase, or α-l-fucosidase activity according to the manufacturer. Chitinase (2.5 mg ml−1) was incubated with cells in 50 mM sodium acetate buffer (pH 5.5). Pronase E (2.5 mg ml−1) and protease (type XIII; 2.5 mg ml−1) with and without 35 mM β-mercaptoethanol were suspended in 25 mM HEPES (pH 7.0). Glucosidase (20 U ml−1) and 35 mM β-mercaptoethanol were suspended in 25 mM HEPES (pH 7.0). The controls contained no enzyme and heat-denatured enzymes (enzymes boiled for 10 min). Cells and enzymes were incubated for 3 h, pelleted, and washed twice in the appropriate buffer, and the level of adhesion was determined.
The chemical treatments used included treatments with alkali (1.0 N NaOH), acid (1.0 N HCl), and hot ethanol (final ethanol concentration, 75%). The cells were incubated for 1 h at 30°C (acid or alkali) or at 70°C (ethanol), pelleted, and washed twice in the appropriate buffer, and the level of adhesion to polystyrene was determined.
Microscopy and image analysis.
The images were averages of six frames obtained with a model B60 microscope (Olympus America Inc., Lake Success, N.Y.) equipped with a model DEI-470 cooled charge-coupled device camera (Optronics Engineering, Goleta, Calif.) controlled by Optimas 6.2 software (Optimas Corp., Bothell, Wash.). The images were subjected to postacquisition processing as follows: background subtraction was performed to eliminate noise and field artifacts, and the contrast was adjusted with Adobe Photoshop 5.0 (Adobe Systems, Mountain View, Calif.).
RESULTS
Isolation of Att− mutants.
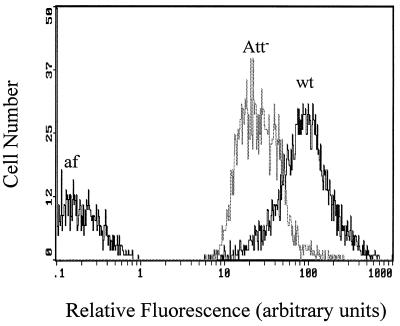
Sixteen independent Att− mutants were isolated by the three methods used, as follows: 4 mutants were isolated by the capsule screen method, 11 mutants were isolated by the polystyrene enrichment method, and 1 mutant was isolated by FACS. The mutants isolated by the capsule screen method were obtained in two experiments. In one experiment, EMS treatment killed approximately 69% of the cells. Three Att− mutants (5d, 15d, and 26d) were obtained from 22 capsule-minus mutants obtained from 12,000 colonies. In a second experiment, EMS treatment killed approximately 55% of the cells. One additional stable Att− mutant (38d) was obtained from the 37 capsule-minus mutants obtained from the 17,000 colonies examined. The fact that capsules were absent was confirmed by negative staining and microscopy. Two of the 11 independent Att− mutants obtained by enrichment with polystyrene exhibited a clumping phenotype (cells grew in aggregates and fell out of solution), but all of the mutants isolated by the enrichment method produced a capsule (Table 1). One Att− mutant (IID2) was obtained from 709 colonies sorted by FACS based on reduced FITC-Con A fluorescence intensity compared to the fluorescence intensity of nonmutagenized cells (Table 1). The total fluorescence intensity of FITC-Con A-stained Att− mutant IID2 cells, as determined by flow cytometry, was less than the total fluorescence intensity of stained wild-type cells (Fig. 1).
TABLE 1.
Growth phenotypes, presence of polar FITC-Con A and India ink staining patterns, and capsule production in R. toruloides wild-type parent Y-1588 and Att− mutants
| Isolate | Isolation methoda | Growth phenotypeb | Strong polar FITC-Con A staining pattern | Strong polar India ink staining pattern | Capsule productionc |
|---|---|---|---|---|---|
| Parent strain Y-1588 | wt | Yes | Yes | Yes | |
| Mutants | |||||
| 5d | caps | clumping | No | No | No |
| 15d | caps | clumping | No | No | No |
| 26d | caps | clumping | No | No | No |
| 38d | caps | clumping | No | No | No |
| 2f | poly | clumping | No | No | Yes |
| 4a | poly | clumping | No | No | Yes |
| 5-11 | poly | wt | No | No | Yes |
| 1-12 | poly | wt | No | No | Yes |
| 2d | poly | wt | No | No | Yes |
| 1n | poly | wt | No | No | Yes |
| 4e | poly | wt | No | No | Yes |
| 3-3 | poly | wt | No | No | Yes |
| 5a | poly | wt | No | No | Yes |
| 3k | poly | wt | No | No | Yes |
| 2i | poly | wt | No | No | Yes |
| IID2 | FACS | wt | No | No | Yes |
caps, loss of capsule production; poly, enrichment from polystyrene; FACS, flow cytometry and cell sorting (see text).
wt, growth phenotype similar to the growth phenotype of the wild-type parent; clumping, clumps of cells readily fell out of solution.
Determined by negative staining with India ink.
FIG. 1.
Relative fluorescence intensities of FITC-Con A-stained cell populations (5,000 cells per isolate) of R. toruloides wild-type strain Y-1588 (wt) and Att− mutant IID2 (Att−) and wild-type autofluorescence (af), as determined by flow cytometry.
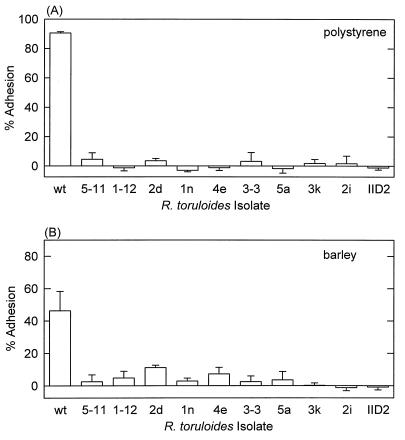
The adhesion of the nonclumping Att− mutants to polystyrene and barley was significantly less than the adhesion of wild-type strain Y-1588 (Fig. 2). The six clumping Att− mutants also did not adhere to either test surface (data not shown). Twelve of the 16 Att− mutants produced an EPS capsule on agar plates or in liquid cultures (Table 1), as determined by negative staining with India ink and microscopy. Capsule-minus, Att− mutants all exhibited an altered, clumping growth phenotype in liquid culture, although clumping also occurred with two capsule-positive, Att− mutants (isolates 2f and 4a).
FIG. 2.
Attachment of wild-type strain R. toruloides Y-1588 and Att− mutants to polystyrene (A) and barley leaf segments (B). The data are the means and standard deviations for four polystyrene replicates and six barley replicates.
Characterization of cell surface polysaccharides.
Four of the seven FITC-lectin preparations tested, the preparations containing Con A, WGA, UEAI, and Ricinus communis agglutinin I, labeled the cell surfaces of both wild-type R. toruloides strains, indicating that chitin, fucose, and galactose were present (Table 2). Con A binds to α-mannose, α-glucose, and N-acetyl-glucosamine in decreasing order of affinity (21). Preincubation of FITC-Con A with the hapten α-d-methyl mannopyranoside (but not with glucose or glucosamine) greatly reduced the subsequent fluorescent labeling of yeast cells, indicating that the lectin bound to mannose residues on the yeast (data not shown).
TABLE 2.
Cell surface polysaccharides of R. toruloides wild-type isolates as determined by FITC-lectin staining
| Lectin | Sugar affinitya | Fluorescence signal on cell surface
|
|
|---|---|---|---|
| R. toruloides Y-1588 | R. toruloides Y-1091 | ||
| Con A | Mannose > glucose > N-acetylglucosamine | +b | + |
| WGA | N-Acetylglucosamine | + | + |
| UEAI | α-l-Fucose | + | + |
| Ricinus communis agglutinin I | Galactose | + | + |
| Dolichos biflorus agglutinin | α-Linked N-acetylgalactos-amine | − | − |
| Soybean agglutinin | Terminal N-acetylgalactos-amine | − | − |
| Peanut agglutinin | Galactosyl (β-1,3) N-acetyl-galactosamine | − | − |
| Lectin buffer | NAc | − | − |
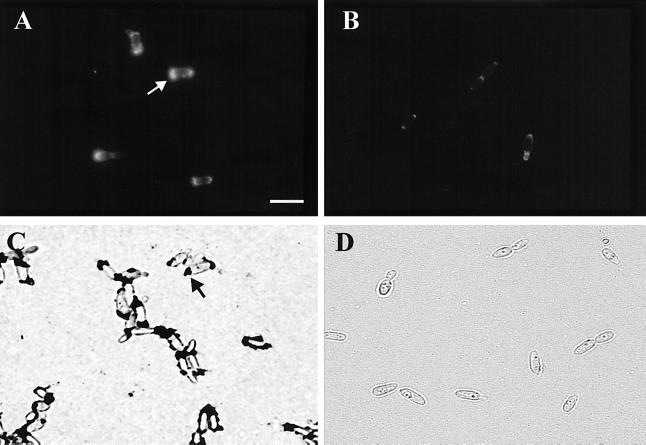
The cell surface FITC staining patterns obtained with the various lectins were different. With wild-type R. toruloides cells FITC-Con A fluorescence occurred over the entire cell surface and was very intense at the poles of some cells (Fig. 3A). The intense polar staining was variable; cells were stained at one pole, at both poles, or at neither pole (data not shown). The strong polar FITC-Con A staining patterns were not observed with any of the Att− mutants (a representative mutant is shown in Fig. 3B), and the staining was less intense in the nonadhesive wild-type fraction. FITC-WGA fluorescence covered the entire wild-type cell surface, and there was no polar pattern similar to the FITC-Con A fluorescence pattern observed with Att− mutants (Fig. 3B). The staining obtained with FITC-UEAI was weak but covered the entire cell surface. FITC-R. communis agglutinin I fluorescence was stronger than UEAI fluorescence, but the distribution over the cell surface was patchy (data not shown).
FIG. 3.
FITC-Con A fluorescence staining patterns for R. toruloides Y-1588 cells (A) and Att− mutant IID2 cells (B) and India ink staining patterns (dark blotches) for R. toruloides Y-1588 cells (C) and Att− mutant IID2 cells (D). Note the strongly stained polar regions on the wild-type strain Y-1588 cells obtained with both FITC-Con A and India ink (arrows) and the pronounced reduction in the mutant. Bar = 10 μm.
Effect of lectins on adhesion of R. toruloides.
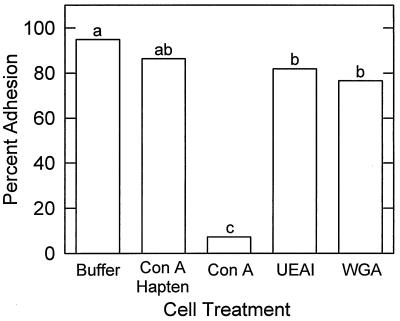
Con A was the only lectin tested that eliminated attachment of R. toruloides to both polystyrene and barley leaf segments, suggesting that mannose residues are involved in adhesion (Fig. 4). Preincubation of Con A with the hapten α-d-methyl mannopyranoside significantly (P = 0.01) reduced the Con A inhibition of adhesion of R. toruloides (Fig. 4). Con A significantly reduced the attachment to polystyrene of all of the wild-type isolates of R. toruloides tested, indicating that the effect on adhesion was not isolate specific.
FIG. 4.
Effects of the lectins Con A, WGA, and UEAI on adhesion of R. toruloides Y-1588 to polystyrene. Lectins (200 μg ml−1) and cells (3 × 106 cells ml−1) were preincubated for 45 min and then applied to polystyrene for 60 min, and the levels of adhesion were determined. The controls included Con A preincubated with the hapten α-d-methyl mannopyranoside (200 mM) for 45 min and lectin buffer containing no lectin. The data are the means for five replicates. The bars with different letters are significantly different, as determined by Fisher’s least significant difference test (P = 0.01).
Dual attachment phenotype and differential India ink staining patterns of R. toruloides.
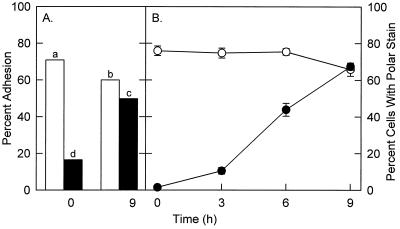
Wild-type populations of R. toruloides cells were composed of attachment-competent and attachment-incompetent cells; enrichment for nonattaching wild-type cells with CM-Sephadex beads resulted in a significant decrease in the observed attachment to polystyrene and barley (Fig. 5A). The ability of the cells to adhere increased with growth (9 h of incubation) (Fig. 5A). Loss of attachment was correlated with a loss of the polar staining pattern obtained with the positive stain India ink (Fig. 5B). India ink stained the poles of cells at sites of bud development, and the pattern was similar to the pattern observed with FITC-Con A (Fig. 3A and C). Preincubation of wild-type R. toruloides with Con A eliminated subsequent polar staining with India ink (data not shown). Wild-type cells that were not able to attach and all of the Att− mutants did not exhibit polar staining patterns with either India ink or FITC-Con A, suggesting that the localized, stained areas were involved in adhesion (Fig. 3 and Table 1). Wild-type R. toruloides cells often associated in small clumps consisting of three or more cells; none of the Att− mutant cells appeared to clump (Fig. 3). The polar India ink staining patterns were observed with all of the wild-type R. toruloides isolates and therefore were not isolate specific. In general, the proportion of wild-type cells that stained with India ink was correlated with the adhesion observed (data not shown).
FIG. 5.
(A) Adhesion of the nonattaching fractions of CM-Sephadex-enriched cell populations (solid bars) and nonenriched cells (open bars) of R. toruloides Y-1588 to polystyrene at zero time and after 9 h of incubation in conditioned growth medium. The data are the means for five replicates. Bars with different letters are significantly different, as determined by Fisher’s least significant difference test (P = 0.01). (B) Proportion of CM-Sephadex-enriched cells (•) and nonenriched cells (○) stained with India ink and exhibiting polar staining. The data are means and standard deviations for three samples, each containing at least 250 cells.
Effect of enzyme and chemical treatments on adhesion.
Treatment of the cells with tunicamycin, which disrupts glycoprotein synthesis, resulted in a significant decrease in the ability of the cells to attach to polystyrene (Table 3) and barley (data not shown) compared to untreated control cells. Partial cell wall digestion with crude or purified glucuronidase significantly reduced adhesion. Enzymatic digestion by mannosidase reduced but did not eliminate adhesion. Treatments that are used to disrupt cell surface proteins (proteolytic enzymes or β-mercaptoethanol) had no effect on adhesion. Alkali and ethanol treatment eliminated adhesion of R. toruloides Y-1588.
TABLE 3.
Effects of various enzyme and chemical treatments on adhesion of R. toruloides to polystyrene
| Enzyme or chemical | Adhesiona |
|---|---|
| Snail gut enzyme | ++ |
| Purified glucuronidase | +++ |
| Sulfatase | ++++ |
| Mannosidase | +++ |
| Glucosidase | ++++ |
| Chitinase | ++++ |
| Protease | ++++ |
| Pronase E | ++++ |
| β-Mercaptoethanol | ++++ |
| Tunicamycin | + |
| NaOH (1 N) | − |
| HCl (1 N) | ++++ |
| Ethanol | − |
| Buffers | ++++ |
−, no adhesion; +, >60% reduction; ++, 30 to 60% reduction; +++, <30% reduction; ++++, no effect. The reduction in adhesion was significant at P = 0.05.
DISCUSSION
The main significance of our work is the finding that R. toruloides attaches to leaf surfaces by means of a localized region consisting of adhesive material that apparently is produced transiently at sites of bud growth. This region contains mannose residues (possibly mannoproteins). The yeast capsule does not directly mediate adhesion of R. toruloides to barley leaf segments or polystyrene. The evidence which led to these conclusions is discussed below.
Con A eliminated attachment of R. toruloides, presumably by attaching to mannose residues, and this lectin blocks adhesion in several other fungal systems (23, 30, 34, 39). The following two lines of evidence support the hypothesis that mannose residues are involved in the observed adhesion of R. toruloides: the results of hapten competition assays and the reduction in adhesion resulting from mannosidase digestion but not from glucosidase digestion. However, the data do not imply that mannose residues alone are directly responsible for adhesion; Con A could also physically block access of other adhesive compounds (e.g., proteins).
The evidence that glycoproteins are involved in the adhesion of R. toruloides includes the fact that adhesion is reduced by tunicamycin. This drug blocks glycoprotein synthesis in yeast cells (2). Tunicamycin reduced adhesion of C. albicans to human buccal epithelial cells (14) but had no effect on adhesion of N. haematococca conidia to polystyrene (25). However, proteolytic enzymes or mercaptoethanol had no effect on adhesion of R. toruloides. These data suggest that if glycoproteins are involved in adhesion, the protein moiety might be physically protected from enzymatic digestion. Disruption of cell wall integrity by glucuronidase reduced the adhesion observed, possibly by releasing glycoproteins.
Mannoproteins are extremely common on the surfaces of yeast cells and account for 56% of the total surface glycoproteins of R. toruloides (6). Mannoproteins are commonly isolated from yeast cell walls by alkali extraction and are precipitated by ethanol (19). Both alkali and hot ethanol eliminated adhesion, which supported the hypothesis that glycoproteins are potential adhesives.
The strong localized staining patterns obtained with FITC-Con A were presumably due to the presence of large amounts of mannose residues at the poles of adhesive cells. Positive staining with India ink produced similar staining patterns. This probably reflected the ink’s affinity for proteins with positively charged and/or hydrophobic regions (29). We are currently investigating the role of cell surface charge or hydrophobic interactions in the observed adhesion of R. toruloides to leaves. The localized staining patterns occurred at sites of bud initiation. Budding in R. toruloides is enteroblastic (phialidic); i.e., the first bud leaves an opening in the cell wall through which subsequent buds develop (43). Localized degradation of surface mannoproteins associated with sites of germination is involved in the adhesion of C. albicans to plastic (41). Cell wall changes at the early germ tube emergence stage are believed to be responsible for the strong adhesion of Botrytis cinerea conidia (12). Marchant and Smith (32) observed that mother cells of R. glutinis produced localized mucilage which surrounded the developing daughter cells. They hypothesized that this mucilage physically protects the developing buds. We propose that a similar pattern of mucilage deposition could also be involved in the adhesion of this yeast to leaves.
The EPS capsule does not appear to mediate adhesion of R. toruloides to leaves or polystyrene. Twelve of our 16 Att− mutants produced a capsule, and most capsule-minus isolates attached to polystyrene. Nonencapsulated strains of C. neoformans are three times more adherent to glial cells than encapsulated strains are (35). Merkel and Scofield (35) hypothesized that capsular material may actually block adhesins present on the yeast cell surface. Coating an acapsular mutant of C. neoformans with cryptococcal glucuronoxylomannan decreases adhesion to endothelial cells (24). Unlike C. neoformans, addition of soluble EPS to blastospores of A. pullulans promotes adhesion of the blastospores to leaves (1). Clearly, the role of the EPS capsule in adhesion differs in different yeast species, possibly due to differences in capsular chemical composition.
The Att− mutants probably have multiple mutations due to the broad activity of the chemical mutagen used. Currently, there is no efficient insertional mutagenesis technique (e.g., restriction enzyme-mediated integration) available for R. toruloides. Such a technique would be desirable as it would result in nonspecific damage to the genome that is less extensive than the damage caused by chemical mutagenesis. However, the common phenotypes of the Att− mutants (loss of attachment and polar staining patterns), the large number of mutants, the relatively low kill rates, and the fact that three different methods produced mutants suggest that a single mutation is responsible for the phenotype.
Finally, R. toruloides cells were intrinsically either adhesive or not adhesive. Bud development appeared to be a prerequisite for adhesion; adhesive cells exhibited strong polar staining patterns with both India ink and FITC-Con A that were absent in nonadhesive wild-type cells and the Att− mutants. Adhesion to surfaces was observed to occur at the polar regions (unpublished data). The transient nature of adhesion competence in R. toruloides could influence colonization of plant surfaces and the biocontrol activity that has been observed by other workers (15, 18). Culture conditions that promote cell division (e.g., mid-log-phase growth) should dramatically affect the initial adhesion of yeast cells applied to aerial plant surfaces. Environmental conditions (including the availability of exogenous nutrients and humidity) which are conducive to the growth of yeasts on the phylloplane (4, 11) could promote attachment of R. toruloides by acting on bud development and the associated polar mucilage. We are currently investigating the role of adhesion in the colonization of barley leaf surfaces by R. toruloides.
ACKNOWLEDGMENTS
This research was supported by United States Department of Agriculture Hatch grant 142-3995.
We thank Russ Spear for discussions and help with the photomicrograph and Jo Handelsman and Gary Roberts for suggestions for improving the manuscript.
REFERENCES
- 1.Andrews J H, Harris R F, Spear R N, Lau G W, Nordheim E V. Morphogenesis and adhesion of Aureobasidium pullulans. Can J Microbiol. 1994;40:6–17. [Google Scholar]
- 2.Arnold E, Tanner W. An obligatory role of protein glycosylation in the life cycle of yeast cells. FEBS Lett. 1982;148:49–53. doi: 10.1016/0014-5793(82)81240-4. [DOI] [PubMed] [Google Scholar]
- 3.Bashi E, Fokkema N J. Scanning electron microscopy of Sporobolomyces roseus on wheat leaves. Trans Br Mycol Soc. 1976;67:500–506. [Google Scholar]
- 4.Bashi E, Fokkema N J. Environmental factors limiting growth of Sporobolomyces roseus, an antagonist of Cochliobolus sativus, on wheat leaves. Trans Br Mycol Soc. 1977;68:17–25. [Google Scholar]
- 5.Braun E J, Howard R J. Adhesion of fungal spores and germlings to host plant surfaces. Protoplasma. 1994;181:202–212. [Google Scholar]
- 6.Breierová E, Kocková-Kratochvílová A. Cryoprotective effects of yeast extracellular polysaccharides and glycoproteins. Cryobiology. 1992;29:385–390. doi: 10.1016/0011-2240(92)90039-5. [DOI] [PubMed] [Google Scholar]
- 7.Chand-Goyal T, Spotts R A. Postharvest biological control of blue mold of apple and brown rot of sweet cherry by natural saprophytic yeasts alone or in combination with low doses of fungicides. Biol Control. 1996;6:253–259. [Google Scholar]
- 8.Chaubal R, Wilmot V A, Wynn W K. Visualization, adhesiveness, and cytochemistry of the extracellular matrix produced by urediniospore germ tubes of Puccinia sorghi. Can J Bot. 1991;69:2044–2054. [Google Scholar]
- 9.di Menna M E. Yeasts from the leaves of pasture plants. N Z J Agric Res. 1959;2:394–405. [Google Scholar]
- 10.Dickinson C H. Adaptations of micro-organisms to climatic conditions affecting aerial plant surfaces. In: Fokkema N J, van den Heuvel J, editors. Microbiology of the phyllosphere. New York, N.Y: Cambridge University Press; 1986. pp. 77–100. [Google Scholar]
- 11.Dik A J, Fokkema N J, van Pelt J A. Influence of climatic and nutritional factors on yeast population dynamics in the phyllosphere of wheat. Microb Ecol. 1992;23:41–52. doi: 10.1007/BF00165906. [DOI] [PubMed] [Google Scholar]
- 12.Doss R P, Potter S W, Soeldner A H, Christian J K, Fukunaga L E. Adhesion of germlings of Botrytis cinerea. Appl Environ Microbiol. 1995;61:260–265. doi: 10.1128/aem.61.1.260-265.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Douglas L J. Adhesion to surfaces. In: Rose A H, Harrison J S, editors. The yeasts. Vol. 2. London, United Kingdom: Academic Press; 1987. pp. 238–280. [Google Scholar]
- 14.Douglas L J, McCourtie J. Effect of tunicamycin treatment on the adherence of Candida albicans to human buccal epithelial cells. FEMS Microbiol Lett. 1983;16:199–202. [Google Scholar]
- 15.Elad Y, Köhl J, Fokkema N J. Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic yeasts. Phytopathology. 1994;84:1193–1200. [Google Scholar]
- 16.Epstein L, Laccetti L B, Staples R C, Hoch H C. Cell-substratum adhesive protein involved in surface contact responses of the bean rust fungus. Physiol Mol Plant Pathol. 1987;30:373–388. [Google Scholar]
- 17.Epstein L, Nicholson R L. Adhesion of spores and hyphae to plant surfaces. In: Carroll G, Tudzynski P, editors. The mycota. V. Plant relationships. New York, N.Y: Springer-Verlag; 1997. pp. 11–25. [Google Scholar]
- 18.Filonow A B, Vishniac H S, Anderson J A, Janisiewicz W J. Biological control of Botrytis cinerea in apple by yeasts from various habitats and their putative mechanisms of antagonism. Biol Control. 1996;7:212–220. [Google Scholar]
- 19.Fleet G H. Cell walls. In: Rose A H, Harrison J S, editors. The yeasts. Vol. 4. London, United Kingdom: Academic Press; 1991. pp. 199–278. [Google Scholar]
- 20.Fokkema N J, den Houter J G, Kosterman Y J C, Nelis A L. Manipulation of yeasts on field-grown wheat leaves and their antagonistic effect on Cochliobolus sativus and Septoria nodorum. Trans Br Mycol Soc. 1979;72:19–29. [Google Scholar]
- 21.Goldstein I J, Poretz R D. Isolation, physicochemical characterization, and carbohydrate-binding specificity of lectins. In: Liener I E, Sharon N, Goldstein I J, editors. The lectins. New York, N.Y: Academic Press, Inc.; 1986. pp. 33–247. [Google Scholar]
- 22.Golubev W I. Capsules. In: Rose A H, Harrison J S, editors. The yeasts. Vol. 4. London, United Kingdom: Academic Press; 1991. pp. 239–280. [Google Scholar]
- 23.Hamer J E, Howard R J, Chumley F G, Valent B. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science. 1988;239:288–290. doi: 10.1126/science.239.4837.288. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim A S, Filler S G, Alcouloumre M S, Kozel T R, Edwards J E, Jr, Ghannoum M A. Adherence to and damage of endothelial cells by Cryptococcus neoformans in vitro: role of the capsule. Infect Immun. 1995;63:4368–4374. doi: 10.1128/iai.63.11.4368-4374.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones M J, Epstein L. Adhesion of Nectria haematococca macroconidia. Physiol Mol Plant Pathol. 1989;35:453–461. [Google Scholar]
- 26.Jones M J, Epstein L. Adhesion of macroconidia to the plant surface and virulence of Nectria haematococca. Appl Environ Microbiol. 1990;56:3772–3778. doi: 10.1128/aem.56.12.3772-3778.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kennedy M J. Adhesion and association mechanisms of Candida albicans. In: McGinnis M R, editor. Current topics in medical mycology. Vol. 2. New York, N.Y: Springer-Verlag; 1988. pp. 73–169. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy M J. Models for studying the role of fungal attachment in colonization and pathogenesis. Mycopathologia. 1990;109:123–138. doi: 10.1007/BF00436792. [DOI] [PubMed] [Google Scholar]
- 29.Kuo K, Hoch H C. Visualization of the extracellular matrix surrounding pycnidiospores, germlings, and appressoria of Phyllosticta ampelicida. Mycologia. 1995;87:759–771. [Google Scholar]
- 30.Kwon Y H, Epstein L. A 90-kDa glycoprotein associated with adhesion of Nectria haematococca macroconidia to substrata. Mol Plant Microbe Interact. 1993;6:481–487. [Google Scholar]
- 31.Lawrence C W. Classical mutagenesis techniques. Methods Enzymol. 1991;194:273–281. doi: 10.1016/0076-6879(91)94021-4. [DOI] [PubMed] [Google Scholar]
- 32.Marchant R, Smith D G. Wall structure and bud formation in Rhodotorula glutinis. Arch Mikrobiol. 1967;58:248–256. doi: 10.1007/BF00408807. [DOI] [PubMed] [Google Scholar]
- 33.McCourtie J, Douglas L J. Extracellular polymer of Candida albicans: isolation, analysis and role in adhesion. J Gen Microbiol. 1985;131:495–503. doi: 10.1099/00221287-131-3-495. [DOI] [PubMed] [Google Scholar]
- 34.Mercure E W, Leite B, Nicholson R L. Adhesion of ungerminated conidia of Colletotrichum graminicola to artificial hydrophobic surfaces. Physiol Mol Plant Pathol. 1994;45:421–440. [Google Scholar]
- 35.Merkel G J, Scofield B A. Comparisons between in vitro glial cell adherence and internalization of non-encapsulated and encapsulated strains of Cryptococcus neoformans. J Med Vet Mycol. 1994;32:361–372. [PubMed] [Google Scholar]
- 36.Mitchison J M, Carter B L A. Cell cycle analysis. Methods Cell Biol. 1975;11:201–219. [PubMed] [Google Scholar]
- 37.Nicholson R L, Epstein L. Adhesion of fungi to the plant surface: prerequisite for pathogenesis. In: Cole G T, Hoch H C, editors. The fungal spore and disease initiation in plants and animals. New York, N.Y: Plenum Press; 1991. pp. 3–23. [Google Scholar]
- 38.Pringle R B. Nonspecific adhesion of Bipolaris sorokiniana sporelings. Can J Plant Pathol. 1981;3:9–11. [Google Scholar]
- 39.Sandin R L, Rogers A L, Patterson R J, Beneke E S. Evidence for mannose-mediated adherence of Candida albicans to human buccal cells in vitro. Infect Immun. 1982;35:79–85. doi: 10.1128/iai.35.1.79-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sela-Burrlage M B, Epstein L, Rodriguez R J. Adhesion of ungerminated Colletotrichum musae conidia. Physiol Mol Plant Physiol. 1991;39:345–352. [Google Scholar]
- 41.Tronchin G, Bouchara J, Robert R. Dynamic changes of the cell wall surface of Candida albicans associated with germination and adherence. Eur J Cell Biol. 1989;50:285–290. [PubMed] [Google Scholar]
- 42.Tully M. Enrichment of mutants of Rhodosporidium toruloides by the use of inositol starvation. J Basic Microbiol. 1985;25:683–686. [Google Scholar]
- 43.von Arx J A, Weijman A C M. Conidiation and carbohydrate composition in some Candida and Torulopsis species. Antonie Leeuwenhoek. 1979;45:547–555. doi: 10.1007/BF00403654. [DOI] [PubMed] [Google Scholar]